Abstract
Purpose:
To compare axial length (AL) and subfoveal choroidal thickness (SFCT) between individuals with age-related macular degeneration (AMD) and controls with no lesions.
Methods:
This was a case-control study. In total, 853 eyes of 484 patients (>65 years), including 397 eyes at various AMD stages and 456 eyes with no fundus lesions (controls) were recruited. Using color fundus photography, eyes were grouped according to AMD degree. AL was automatically measured using IOL Master and SFCT was manually measured by two independent observers. The associations among age, AL, SFCT, and each AMD grade were analyzed.
Results:
Out of 853 eyes, 456 had no lesions, 217 contained drusen only, 134 had early AMD, and 46 had late AMD. The eyes with late AMD were older (p = 0.007) and had longer AL (p ≥ 0.001) and thinner SFCT (p < 0.001) compared with groups of no fundus lesions, drusen only, and early AMD. SFCT in eyes with late AMD decreased by 19.20 μm (p = 0.049), 24.78 μm (p = 0.029), and 15.56 μm (p = 0.162) compared with groups of no fundus lesions, drusen only, and early AMD, respectively. SFCT decreased by 14.18 μm/mm increase in AL (p < 0.001). The odds ratio (OR) for late AMD by longer AL (≥25 mm) and thinner SFCT (<240 μm) was 4.54 (χ2 = 9.36; p = 0.002) and 4.86 (χ2 = 17.62; p < 0.001), respectively, and was 9.57 (χ2 = 18.07; p < 0.001) when both AL ≥ 25 ≥m and SFCT < 240 μm.
Conclusion:
Eyes with late AMD have distinct reduced SFCT and elongated AL. Eyes with thinner SFCT and longer AL showed high ORs for late AMD and even higher ORs when both factors were simultaneously present. These findings illustrate the crucial pathophysiological role of these two important ocular fac tors and arouse our attention to patients with both characteristics, especially in Asian countries where the prevalence of myopia are disturbingly high.
Keywords: age-related macular degeneration, axial length, risk factors, subfoveal choroidal thickness
1. Introduction
Age-related macular degeneration (AMD) is a leading cause of blindness in the elderly,1 and is the most common cause of legal blindness in the Western world and in industrialized nations.2,3,4,5,6,7 AMD can lead to loss of central vision; it can be associated with several environmental factors8,9 including cigarette smoking,3,10 higher body mass index,11,12 and dietary carotenoids.13,14 A ge netic effect was suggested on the basis of clinical observations, familial aggregation, and linkage studies,15,16 and has been confirmed with studies showing the associations between AMD and several genetic loci.17
Besides the genetic and environmental factors, previous population-based studies among Caucasians have identified the possible associations of various ocular factors with AMD.18,19,20 The Los Angeles Latino Eye Study also evaluated the associations between various ocular factors and AMD in Latinos. These factors included iris color, lens opacity, retinal pigment epithelium (RPE) depigmentation, and longer axial length (AL).21 Some early histological studies22,23 showed a decrease in the density of choroidal blood vessels and thinning of the choroid in AMD eyes. Some small scale image studies24,25 also confirmed decreased subfoveal choroidal thickness (SFCT) in exudative AMD eyes. The choroidal circulatory status seems to play an important role. For example, the findings of choroidal hypoperfusion were seen on indocyanine green angiography in eyes with neovascular AMD.26 It is reasonable to assume that the disruption of the integrity of the choroidal circulation is part of the pathophysiology of wet AMD. Choriocapillaris dysfunction and insufficient blood supply to the RPE cells may allow the RPE cells to be more prone to harmful oxidants or insults; this initiates the accumulation of waste within Bruch’s membrane, resulting in the progression of AMD.27,28
Although anatomical choroid thickness is not necessarily indicative of choroidal blood volume, flow, and function, measurement of SFCT is currently the most rational and accessible method to evaluate choroidal function. To the best of our knowledge, this study is the largest population-based survey comparing SFCT and AL both together in normal eyes and in eyes at different stages of AMD in an Asian population. We analyzed the relationship between age, AL, and SFCT in different groups. All of these factors may play a crucial pathophysiological role in the development of late AMD in aging people.
2. Methods
This study is a case-control observational study based on our prior population-based cross-sectional study, the Puzih Eye Study.29 From January 2010 to March 2012, a total of 708 volunteers (aged > 65 years) who lived in Puzih City participated in the Puzih Eye Study. Puzih is also the area with the highest concentration of aging population in Taiwan.
The study adhered to the Declaration of Helsinki, and ethics approval was obtained from the Institutional Review Board of Chang Gung Memorial Hospital at Chiayi, Taiwan. Written informed consent was obtained from all participants after a verbal explanation by study researchers.
2.1. Patient selection and grouping
Among the 708 volunteers, 680 (96.04%) had at least one fundus photograph of either eye, whereas 673 (95.05%) had recognizable fundus photographs. After excluding eyes with retinopathy or maculopathy other than AMD (e.g., diabetic retinopathy, retinal vessel occlusion disease, high myopic maculopathy, and polypoidal choroidal vasculopathy), 853 eyes from 484 patients using qualified optical coherence tomography (OCT) images were enrolled in our present study. The 853 eyes were grouped using color fundus photographs according to the different stages of AMD: no fundus lesions, drusen only, early AMD, and late AMD (Fig. 1).
Fig. 1.
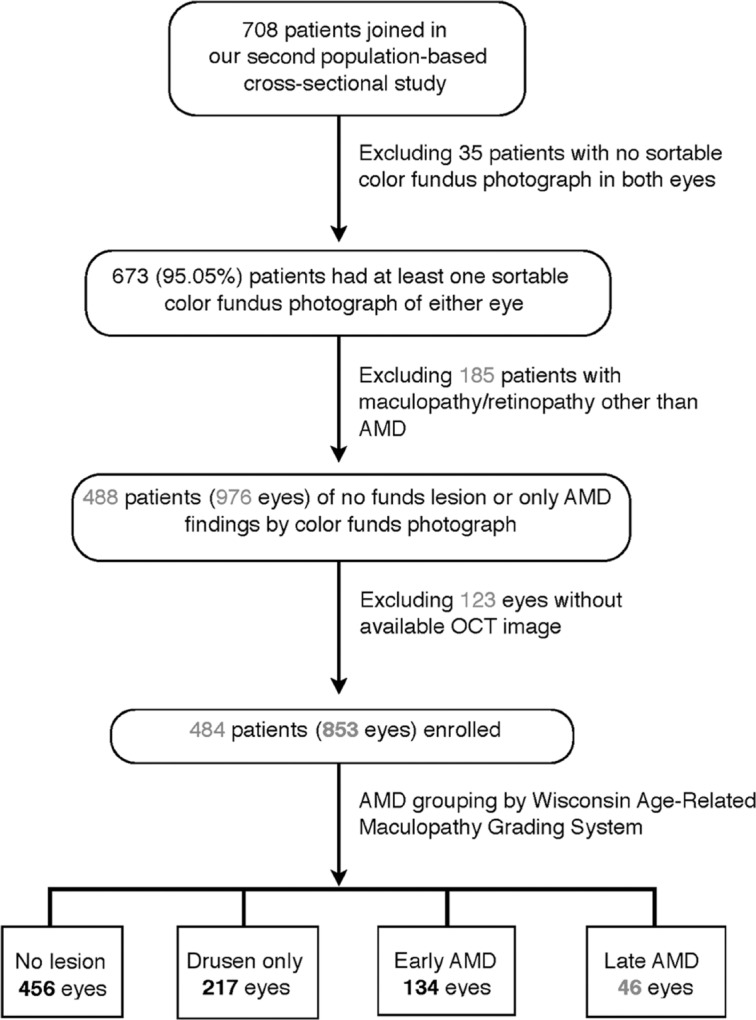
Flow chart of patient selection and grouping. AMD = age-related macular degeneration; OCT = optical coherence tomography.
The grading of the AMD stage was determined by two independent retinal specialists, according to the Wisconsin Age-Related Maculopathy Grading System,30 using the color fundus photograph that had been taken using a fundus camera (CF-60UD, Canon Inc, Tokyo, Japan). The two specialists were masked from all general data for the volunteers; all equivocal photos were reviewed together with senior retina specialists.
2.2. Parameters measurement
AL of each eye was measured using optical biometry (IOL Master; Zeiss, Jena, Germany) for each eye by averaging five measurements. SFCT was manually measured by two observers using 1-line raster scans OCT image (Stratus OCT, Carl Zeiss Meditec, Inc, Dublin, CA, USA) taken through the foveal center horizontally. The sub-foveal choroid was defined as the choroid beneath the concave central retinal depression 1500 μm in diameter. A single choroidal thickness measurement was obtained for the horizontal raster from the outer border of the RPE to the inner scleral border. The manual caliper was used to sequentially measure 250μm distances in both radial directions encompassing 1500 μm centered on the foveola by each observer and the average of these measurements were calculated as SFCT (Fig. 2). The observers were masked from patients’ clinical data. Intraobserver/interobserver repeatablility was also calculated.
Fig. 2.
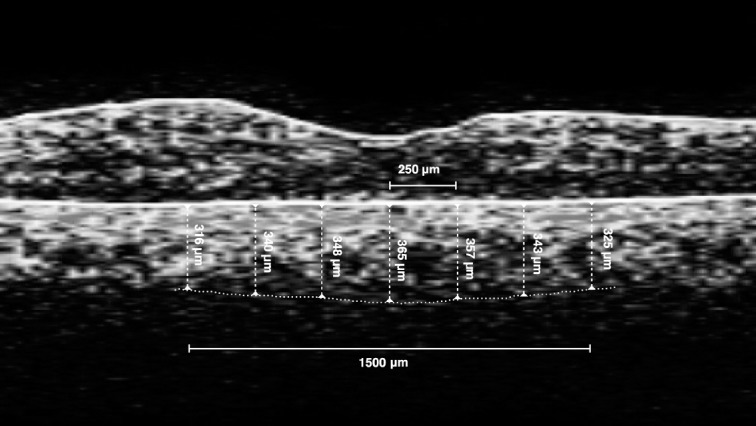
Example of measurement of subfoveal choroidal thickness. A one-line raster scan optical coherence tomography image was taken through the foveal center horizontally. The subfoveal choroid was defined as the choroid beneath the concave central retinal depression 1500 μm in diameter. A single choroidal thickness measurement was obtained for the horizontal raster from the outer border of the retinal pigment epithelium to the inner scleral border. The manual caliper was used to sequentially measure 250-μm distances in both radial directions (total of 7 measurements/patient) by two independent observers. The average of these measurements was calculated as subfoveal choroidal thickness. As shown in this patient, the subfoveal choroidal thickness was 342 μm.
2.3. Statistical analysis
One-way analysis of variance and post-hoc test (Scheffe’s procedure) were used to calculate and compare the age, AL, and SFCTof each AMD-grading group. The association between age and AL, age and SFCT, and AL and SFCT were analyzed using univariate regression, and the Pearson’s correlation coefficient was calculated. Using multivariate regression, the effect on SFCT by age, AL, and AMD grading was analyzed. Finally, the odds ratio (OR) for late AMD with a certain cut-off value (22 mm and 25 mm of AL, and 240 μm of SFCT) was calculated. The generalized estimating equation model was used to estimate the strength of the relationship between the outcome and the risk factors, while appropriately taking into account the magnitude of the correlation between fellow eyes.19,31 A p value ≤ 0.05 was considered statistically significant. All statistics were calculated using SPSS version 16.0 (SPSS Inc, Chicago, IL, USA).
3. Results
The study enrolled 853 eyes from 484 patients, including 456 eyes with no lesion, 217 with drusen only, 134 with early AMD, and 46 with late AMD. Four hundred and eighty-three eyes (56.6%) were of male participants. The mean age of the participants was 73.51 ± 6.02 years (range, 65–90 years). The mean AL and SFCT were 23.42 ± 1.22 mm and 282.7 ± 45.16 μm (Table 1).
Table 1.
The average age, axial length, and subfoveal choroidal thickness in each group.
| Total | No lesion | Drusen only | Early AMD | Late AMD | pa | |
|---|---|---|---|---|---|---|
| Eyes (n) | 853 | 456 | 217 | 134 | 46 | – |
| Age (y)b | 73.51 ± 6.02 | 73.09 ± 5.84 | 72.56 ± 5.78 | 73.84 ± 6.01 | 76.73 ± 6.32c | 0.007 |
| AL (mm)b | 23.42 ± 1.22 | 23.43 ± 1.04 | 23.32 ± 1.11 | 23.26 ± 0.9 | 24.32 ± 2.88c | 0.001 |
| SFCT (μm)b | 282.7 ± 45.2 | 282.2 ± 42.5 | 292.1 ± 44.1 | 280.5 ± 40.7 | 250.7 ± 68.2c | 0.001 |
AL = axial length; AMD = age-related macular degeneration; SFCT = subfoveal choroidal thickness.
a One-way analysis of variance between different age-related macular degeneration grading groups.
b Data are presented as the mean ± standard deviation.
c Group that was significantly different from other groups using a post-hoc test (Scheffe).
3.1. Analysis between age, AL, and SFCT
The single linear regression analysis of age, AL, and SFCT revealed no significant correlation between age and SFCT (r = −0.002; p = 0.967), a very weak negative correlation between age and AL (r =−0.077; p = 0.043; Figs. 3A and 3B), and a moderately negative correlation between AL and SFCT (r = −0.455; p < 0.001). The linear equation of AL versus SFCT showed that SFCT decreased by 16.15 μm for every mm increase in AL (Fig. 4).
Fig. 3.
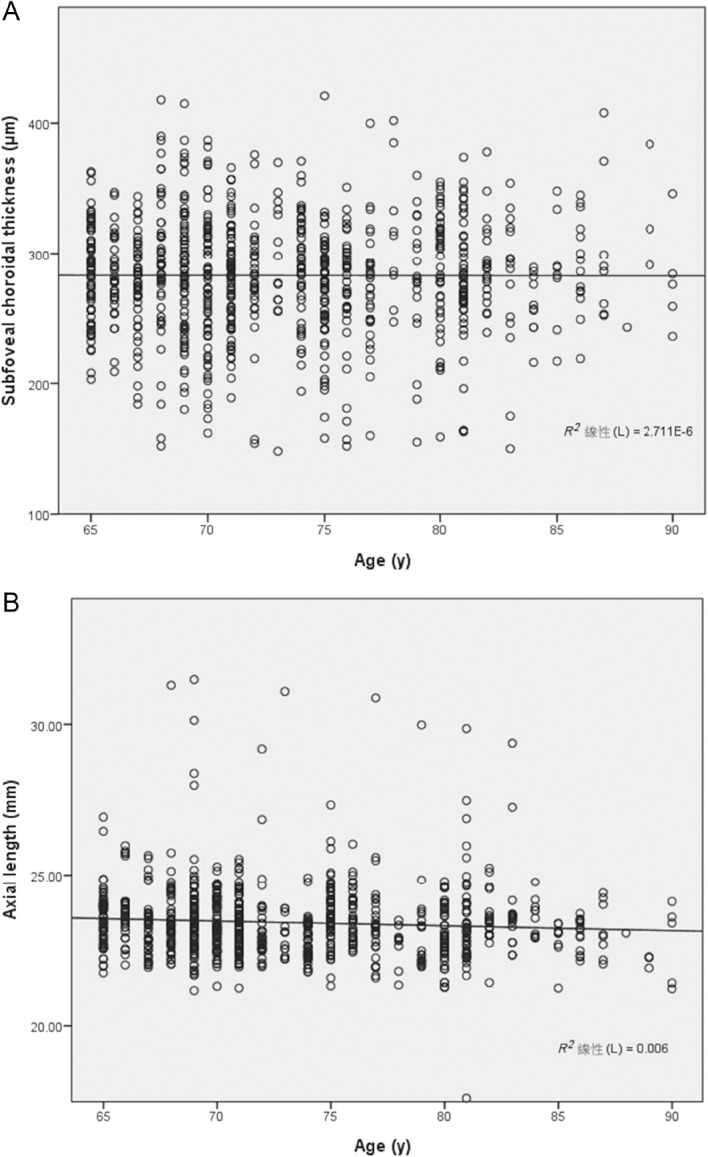
(A) Scatterplot of subfoveal choroidal thickness with age, showing the subfoveal choroidal thickness with age in this series of all eyes (r=−0.0016, p = 0.967); (B) scatterplot of axial length with age, showing the axial length with age in this series of all eyes (r = −0.0775, p = 0.043).
Fig. 4.
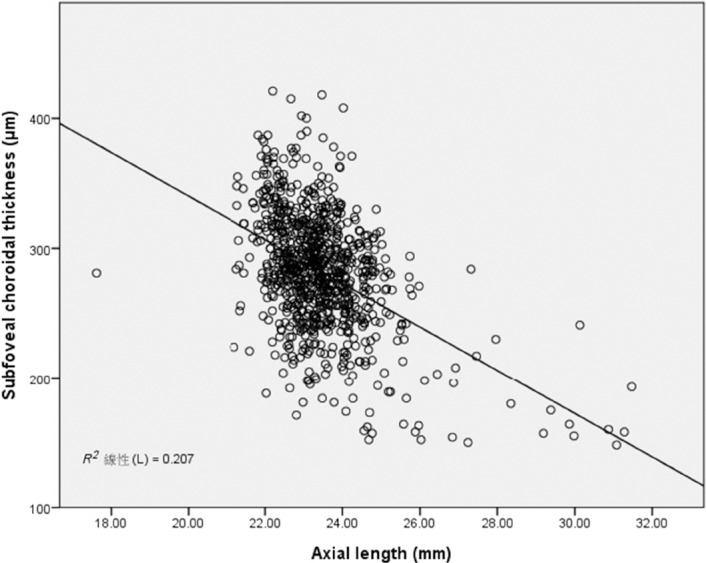
Scatterplot of axial length and subfoveal choroidal thickness of all eyes in this series shows a significant negative correlation (r = −0.455, p < 0.001; y = −16.15x + 549.7; R2 = 0.207).
In a stepwise multiple regression model analysis of the effect on SFCT, AL had the most effect on SFCT after adjustment for age and AMD grading; SFCT decreased by 14.18 μm for every mm increase in AL (p < 0.001). Age showed a nonsignificant effect, whereas SFCT increased 0.55 μm for each additional year of age (p = 0.082).
3.2. Age, AL, and SFCT of each subgroup
The mean age in the late AMD group was 76.73 ± 6.32 years, which was higher than that in the other groups (p = 0.007). The mean AL in the late AMD group (24.32 ± 2.88 mm) was longer than that in the other groups (p < 0.001). The mean SFCT in the late AMD group (250.7 ± 68.2 μm) was thinner than that in the other groups (p < 0.001). The mean SFCT in the no lesions, drusen only, and early AMD groups were 282.2 ± 42.5 μm, 292.1 ± 44.1 μm, and 280.5 ± 40.7 μm, respectively. There were no significant differences in age, AL, and SFCT among all the groups except for the late AMD group (Table 1).
3.3. Analysis of SFCT and AL between late AMD and other subgroups
After adjusting for age and AL, SFCT in the late AMD group decreased by 19.20 μm (p = 0.049), 24.78 μm (p = 0.029), and 15.56 μm (p = 0.162) compared with the no lesions, drusen only, and early AMD groups, respectively (Table 2).
Table 2.
Multivariate analysis of associations between subfoveal choroidal thickness and ocular and general parameters.
| Parameter | B factor | p | 95% CI |
|---|---|---|---|
| Age (y) | 0.55 | 0.082 | (−0.07, 1.18) |
| Axial length(mm) | −14.18 | < 0.001 | (−17.82, −10.54) |
| Disease stage (compared with late AMD group) | |||
| No lesion | 19.2 | 0.049 | 4.67, 39.09) |
| Drusen only | 24.78 | 0.029 | (6.59, 46.97) |
| EarlyAMD | 15.56 | 0.162 | (−2.238, (37.35) |
AMD = age-related macular degeneration; CI = confidence interval.
OR for late AMD by AL and SFCT was analyzed using a certain cut-off value. For analysis of shorter AL, we considered AL as the mean–1 standard deviation (SD) = 23.42–1.22 = 22.20 (mm), and we set the cut-off value at 22 mm for convenience. Likewise, we accepted the longer AL cut-off value of 25 mm as the mean + 1 SD = 24.67 (mm). The cut-off value of 240 μm for thinner SFCT was used because the mean–1SD = 282.7 -45.2 = 237.5 (μm). Using these cut-off values, OR for the late AMD group was 4.54 [χ2 = 9.36, p = 0.002; 95% confidence interval (CI), 1.72–11.99] when AL ≥ 25 mm and was 4.86 (χ2 = 17.62, p < 0.001; 95% CI, 2.32–10.18) when SFCT < 240 μm. OR was as high as 9.57 (χ2 = 18.07, p < 0.001; 95% CI, 3.38–27.12) when the patients had both AL ≥ 25 mm and SFCT < 240 μm. AL measurements that are <22 mm have a nonsignificant OR of 1.41 (χ2 = 0.193, p = 0.661; 95% CI, 0.30–6.58; Table 3).
Table 3.
Odds ratio of late age-related macular degeneration by axial length and subfoveal choroidal thickness.
| OR | X2 | p | 95% CI | |
|---|---|---|---|---|
| AL | ||||
| < 22 mma | 1.41 | 0.193 | 0.661 | (0.30, 6.58) |
| ≥ 22 mm | 1 | — | — | — |
| AL | ||||
| ≥ 25 mmb | 4.54 | 9.36 | 0.002 | (1.72, 11.99) |
| < 25 mm | 1 | — | — | — |
| SFCT | ||||
| < 240 μmc | 4.86 | 17.62 | <0.001 | (2.32, 10.18) |
| ≥ 240 μmc | 1 | — | — | — |
| AL ≥ 25 mm and SFCT < 240 μm | 9.57 | 18.07 | <0.001 | (3.38, 27.12) |
| AL < 25 mm or SFCT ≥ 240 μm | 1 | — | — | — |
AL = axial length; CI = confidence interval; OR = odds ratio; SD = standard deviation; SFCT = subfoveal choroidal thickness.
a Axial length below mean – 1 SD = 23.42 – 1.22 = 22.20 mm, use the cut off value of 22 for convenience.
bAxial length above mean + 1 SD = 23.42 + 1.25 = 24.67 mm, use the cut off value of 25 for convenience.
c Subfoveal choroidal thickness below mean – 1 SD = 282.7 – 45.2 = 237.5 μm, use the cut off value of 240 for convenience.
4. Discussion
In our study, the mean SFCT was 282.7 ± 45.2 μm for the no lesion group, which was similar to other studies. However, there is a remarkable variation among these studies. A mean SFCT from 261.93 ± 88.42 μm to 354 ± 111 μm in healthy controls were observed in different researches.32,33,34,35,36 The variation in choroidal thicknesses among different studies may be due to many factors, including different OCT models, different light source wavelengths, measurement software and processing, number of cases, ages, sex, races, patient groupings, refractive errors (REs), and ALs. The visibility of the choroidal-scleral interface (CSI) by time-domain OCT also has an unignorable influence in the measurement of choroidal thickness. Time-domain OCT has a slower scanning speed and lower resolution that make interpreting the CSI more difficult. As shown in Fig. 1, we excluded 123 eyes without available OCT images. Among these eyes, 29 eyes had no OCT image due to severe lens and media opacity and 94 (9.6%) eyes had ill-defined CSI. In our study, the intraobserver interclass coefficient was 0.853 and the interobserver/intraobserver interclass coefficient was 0.726, which were acceptable repeatability.
4.1. Age and choroidal thickness
In many studies, age had a significant negative correlation with choroid thickness. It is more prevalent in participants who have a wider range of age.
Using enhanced depth imaging-OCT, Ding et al32 measured choroidal thickness in a population of 210 healthy volunteers whose mean age was 49.73 ± 17.89 years (range, 20–85 years). The correlation analysis showed that SFCT negatively correlated with age (r = -0.474; p < 0.001). Ikuno et al’s36 study involving 43 young and healthy Japanese volunteers (mean age, 39.4 ± 16.0 years; range, 23–88 years) also showed a borderline significant negative correlation between age and SFCT (p = 0.07; y =-1.4x + 410.6; r2 = 0.04), i.e., a reduction of 14 μm every decade. Shin et al33 found a moderate negative correlation (r = -0.61; p < 0.001) in 34 patients (average age, 51.1 years; range, 22–78 years).
Our study showed a very weak negative and insignificant correlation between age and SFCT (r = -0.002; p = 0.967) and a borderline positive relationship in the stepwise analysis (coefficient B = 0.55; r = 0.082). Our population consisted of an older age group, who were all aged < 65 years within a narrow distribution (mean, 73.51 ± 6.02 years; range, 65–90 years). Therefore, the effect of aging in SFCT is less significant in our study population when it is not compared with younger groups. It is more meaningful when comparing the old with the young of the same group. However, the role of thinner SFCT and longer AL can be observed more clearly without the influence of age.
4.2. AL and choroidal thickness
AL was the most important factor influencing choroidal thickness as observed in our study. SFCT decreased as AL elongated as found by single variation linear regression. After adjustment for age and AMD grading using a stepwise multiple regression, SFCT decreased by 14.18 μm for each mm increase in AL (p < 0.001).
This result is similar to that of Flores-Moreno et al37 who investigated choroidal thickness in highly myopic eyes and healthy eyes. Choroidal thickness in highly myopic eyes was thinner (115.56 ± 85.3 μm) than that in controls (257.4 ± 99.3 μm)(p < 0.001, Mann-Whitney U-test); for each additional mm increase in AL, choroidal thickness decreased by 25.91 μm in highly myopic eyes. Other studies in Asia also have similar results.32,36 Besides age, AL is the single most important ocular factor that can affect choroidal thickness.
4.3. Choroidal thickness and AMD
Before OCT was available for clinic practice, in-vitro evidence of a thinner choroid in AMD patients was reported in 1976. Sarks22 conducted a histological study on 378 eyes from patients (age range, 43–97 years) whose maculae had presented with a variety of clinical appearances. He found that thinning was associated with increasing age and in those with all stages of AMD.
McLeod et al23 used an image analysis technique for quantifying pathological changes in RPE and choriocapillaris in the eyes of two AMD and one normal control donor. The study demonstrated a large area in the posterior pole, with very few RPE cells (90% loss of RPE); there was a 30–50% decrease in the density of choroidal blood vessels in this area in the eye of Case 1, and an even greater decrease in the eye of Case 2 (53% decrease in the submacular region) compared with that in the control eye.
These studies raise our speculation that eyes with longer ALs and, hence, thinner choroidal thicknesses are more susceptible to developing AMD with increasing age.
In our study, the mean SFCT of the late AMD group was significantly less than that of the other groups (p < 0.01). In a multiple regression model analysis after adjustment for age and AL, SFCT was also less when compared with the no lesion, drusen only, and early AMD groups, with a decrease of 19.20 μm, 24.78 μm, and 15.56 μm, respectively. The more significantly thinner choroidal thickness in the late AMD group and the less significant association between early AMD and normal controls were also observed in other small scale studies.24,25
Furthermore, as a population-based study, we also analyzed OR for late AMD between eyes with SFCT below the mean–1SD (<240 μm) and eyes with SFCT ≥ 240 μm; the results showed a significantly high OR of 4.86. In light of numerous histologic and in vivo evidence and a clear statistical relationship between choroidal thickness and AMD, it is reasonable to believe that thinner choroidal thickness played a large part in the development of late AMD.
4.4. AL and AMD
The association between AL and AMD has been inconclusive to date. Our present study revealed that the late AMD group has a longer mean AL (24.32 ± 2.88 mm; one-way analysis of variance: p < 0.001) compared with the other groups. The eyes with longer AL (≥25 mm) also have higher odds (OR: 4.54) of late AMD than eyes with AL < 25 mm.
Ikuno36 investigated the association of AL and age-related maculopathy/AMD in a study that included 663 randomly selected people aged > 65 years. There was no significant difference in the refractions or ALs among the groups with different stages of age-related maculopathy. The Los Angeles Latino Eye Study21 reported that longer AL (mm) was associated with decreased odds of soft drusen but increased odds of geographic atrophy [(OR = 0.8;95% CI, 0.7–0.9), (OR = 0.7; 95% CI, 0.5–0.9), respectively].
The role of AL in AMD is paradoxical and is not as predominant as it is in high myopic maculopathy as mentioned in current literature. However, as in our study, if we refine a study group by excluding the pathological myopic eyes which manifest maculopathy in early age, late stage AMD would show a strong positive association with longer AL and also high OR. It is probably because highly myopic eyes were excluded from our refined study group that there was a distinct increase in the proportion of late AMD in eyes with long AL. Besides, we defined long AL as >25 mm according to the mean + 1 SD distribution rather than using RE < −1.0 Diopter (D) (used in most cross-section population-based studies) because RE can be highly affected by other ocular parameters such as cataracts, lens elasticity, cornea curvature, media clarity, and the ability of ciliary muscle regulation. Our study supported that it is AL that indeed has a direct influence on choroidal thickness and hence on the choroidal circulatory function, resulting in the progress of late AMD.
Furthermore, the analysis of the combination of both long AL and thinner choroidal thickness showed even higher OR (9.57) for late AMD (χ2 = 18.07, p < 0.001; 95% CI, 3.38–27.12). This result gave us a hint that longer AL is not only directly influencing choroidal thickness but it also has an unignorable impact on the development of late AMD. Because high myopic maculopathy and late AMD share some similar clinical findings such as choroidal neovascularization, they may have similar pathophysiology involving subretinal structures. The stretching of ocular tissues in myopia affects the hemodynamics of the choroids. This results in diffuse choroidal thinning, and choroidal neovascularization is more likely to develop. The major difference is that high myopic maculopathy happens earlier in young adults. The long AL combing thinner choroids causing the less efficient choroidal blood circulation may explain why the pathological transformation occurs earlier in these highly myopic eyes. However, although having a long AL, eyes with a relative efficient choroidal blood supply could be sheltered from such pathological changes when they are young. However, they are more likely to develop AMD when the choroids become thinner and thinner and then circulation deteriorates as they age. It is especially important in Asian countries where the prevalence of myopia is disturbingly high.
5. Conclusion
This study was based on our previous study and the advantage of this study is that it is a near population-based case-control study of large scale.
Thinner SFCT is found to be related to the degree of AMD by others and our researches, and longer AL is regarded as an important factor of thinner choroidal thickness. Our study is the largest population-based study comparing both the effect of SFCT and AL together in Asian AMD patients. It is the first one to reveal the independent high OR of SFCT and AL for late AMD and an even higher OR when both come together (9.57, p < 0.001). The results gave us a hint that longer AL not only directly influences choroidal thickness but also has an unignorable impact on the development of late AMD. These findings illustrate the crucial pathophysiological role of these two factors and also arouse our attention to patients with both characteristics.
Being a cross-sectional study, however, it is still difficult to tell whether decreased choroidal thickness is an influential factor for the development of late AMD or if it is just a morphological change as a result of senile degeneration. Further long-term cohort studies comparing the progression and accumulative incidence of AMD in patients with different AL, choroidal thickness, and even choroidal circulation status using advanced devices, such as angiography OCT,38 with a standard and automatic measurement process is needed to clarify these issues.
Footnotes
Conflicts of interest: The authors have no conflicts of interest and no proprietary interests related to this article.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Tomany SC, Wang JJ, Van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 5.Wang JJ, Rochtchina E, Lee AJ, et al. Ten-year incidence and progression of age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2007;114:92–98. doi: 10.1016/j.ophtha.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Augood CA, Vingerling JR, de Jong PT, et al. Prevalence of age-related macul-opathy in older Europeans: the European Eye Study (EUREYE) Arch Oph-thalmol. 2006;124:529–535. doi: 10.1001/archopht.124.4.529. [DOI] [PubMed] [Google Scholar]
- 7.Chen SJ, Cheng CY, Peng KL. Prevalence and associated risk factors of age-related macular degeneration in an elderly Chinese population in Taiwan: the Shihpai Eye study. Invest Ophthalmol Vis Sci. 2008;49:3126–3133. doi: 10.1167/iovs.08-1803. [DOI] [PubMed] [Google Scholar]
- 8.Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age-related macular degeneration: a review of progress to date. Surv Ophthalmol. 2006;51:316–363. doi: 10.1016/j.survophthal.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Seddon JM, Reynolds R, Rosner B. Associations of smoking, body mass index, dietary lutein, and the LIPC genetic variant rs10468017 with advanced age-related macular degeneration. Mol Vis. 2010;16:2412–2424. [PMC free article] [PubMed] [Google Scholar]
- 10.Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996;276:1141–1146. [PubMed] [Google Scholar]
- 11.Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference and waist-hip ratio. Arch Ophthalmol. 2003;121:785–792. doi: 10.1001/archopht.121.6.785. [DOI] [PubMed] [Google Scholar]
- 12.Age-related Eye Disease Study Group. Risk factors associated with age-related macular degeneration. a case-control study in the Age-related Eye Disease Study. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA. 1994;272:1413–1420. [PubMed] [Google Scholar]
- 14.van Leeuwen R, Boekhoorn S, Vingerling JR, et al. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005;294:3101–3107. doi: 10.1001/jama.294.24.3101. [DOI] [PubMed] [Google Scholar]
- 15.Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age-related macul-opathy. Am J Ophthalmol. 1997;123:199–206. doi: 10.1016/s0002-9394(14)71036-0. [DOI] [PubMed] [Google Scholar]
- 16.Klaver CC, Wolfs RC, Assink JJ, van Duijn CM, Hofman A, de Jong PT. Genetic risk of age-related maculopathy: population-based familial aggregation study. Arch Ophthalmol. 1998;116:1646–1651. doi: 10.1001/archopht.116.12.1646. [DOI] [PubMed] [Google Scholar]
- 17.Seddon JM, Santangelo SL, Book K, Chong S, Cote J. A genomewide scan for age-related macular degeneration provides evidence for linkage to several chro mosomal regions. Am J Hum Genet. 2003;73:780–790. doi: 10.1086/378505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikram MK, van Leeuwen R, Vingerling JR, Hofman A, de Jong PT. Relationship between refraction and prevalent as well as incident age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2003;44:3778–3782. doi: 10.1167/iovs.03-0120. [DOI] [PubMed] [Google Scholar]
- 19.Wang JJ, Mitchell P, Smith W. Refractive error and age-related maculopathy: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 1998;39:2167–2171. [PubMed] [Google Scholar]
- 20.Klein R, Klein BE, Wang Q, Moss SE. Is age-related maculopathy associated with cataracts. Arch Ophthalmol? 1994;112:191–196. doi: 10.1001/archopht.1994.01090140067025. [DOI] [PubMed] [Google Scholar]
- 21.Fraser-Bell S, Choudhury F, Klein R, Azen S, Varma R Los Angeles Latino Eye Study Group. Ocular risk factors for age-related macular degeneration: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149:735–740. doi: 10.1016/j.ajo.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLeod DS, Taomoto M, Otsuji T, Green WR, Sunness JS, Lutty GA. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:1986–1993. [PubMed] [Google Scholar]
- 24.Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118:840–845. doi: 10.1016/j.ophtha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Manjunath V, Goren J, Fujimoto JG, Duker JS. Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;152:663–668. doi: 10.1016/j.ajo.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori K, Gehlbach PL, Ito YN, Yoneya S. Decreased arterial dye-filling and venous dilation in the macular choroid associated with age-related macular degeneration. Retina. 2005;25:430–437. doi: 10.1097/00006982-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Feeney-Burns L, Ellersieck MR. Age-related changes in the ultrastructure of Bruch’s membrane. Am J Ophthalmol. 1985;100:686–697. doi: 10.1016/0002-9394(85)90625-7. [DOI] [PubMed] [Google Scholar]
- 28.Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35. [PubMed] [Google Scholar]
- 29.Huang EJ, Wu SH, Lai CH, et al. Prevalence and risk factors for age-related macular degeneration in the elderly Chinese population in south-western Taiwan: the Puzih eye study. Eye (Lond) 2014;28:705–714. doi: 10.1038/eye.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 31.Katz J, Zeger S, Liang KY. Appropriate statistical methods to account for simi larities in binary outcomes between fellow eyes. Invest Ophthalmol Vis Sci. 1994;35:2461–2465. [PubMed] [Google Scholar]
- 32.Ding X, Li J, Zeng J, et al. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci. 2011;52:9555–9560. doi: 10.1167/iovs.11-8076. [DOI] [PubMed] [Google Scholar]
- 33.Shin JW, Shin YU, Cho HY, Lee BR. Measurement of choroidal thickness in normal eyes using 3D OCT-1000 spectral domain optical coherence tomogra phy. Korean J Ophthalmol. 2012;26:255–259. doi: 10.3341/kjo.2012.26.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150:325–329. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51:2173–2176. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 37.Flores-Moreno I, Lugo F, Duker JS, Ruiz-Moreno JM. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol. 2013;155:314–319. doi: 10.1016/j.ajo.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 38.de Carlo TE, Bonini Filho MA, Chin AT, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology. 2015;122:1228–1238. doi: 10.1016/j.ophtha.2015.01.029. [DOI] [PubMed] [Google Scholar]


