Abstract
BAFF is a B cell survival and maturation factor implicated in the pathogenesis of systemic lupus erythematosus (SLE). In this in vitro study, we describe that soluble BAFF in combination with IL-2 and IL-21 is a T cell contact-independent inducer of human B cell proliferation, plasmablast differentiation, and IgG secretion from circulating CD27+ memory and memory-like CD27−IgD− double-negative (DN) B cells, but not CD27−IgD+ naive B cells. In contrast, soluble CD40L in combination with IL-2 and IL-21 induces these activities in both memory and naive B cells. Blood from healthy donors and SLE patients have similar circulating levels of IL-2, whereas SLE patients exhibit elevated BAFF and DN B cells and reduced IL-21. B cell differentiation transcription factors in memory, DN, and naive B cells in SLE show elevated levels of Aiolos, whereas Ikaros levels are unchanged. Treatment with CC-220, a modulator of the cullin ring ligase 4-cereblon E3 ubiquitin ligase complex, reduces Aiolos and Ikaros protein levels and BAFF- and CD40L-induced proliferation, plasmablast differentiation, and IgG secretion. The observation that the soluble factors BAFF, IL-2, and IL-21 induce memory and DN B cell activation and differentiation has implications for extrafollicular plasmablast development within inflamed tissue. Inhibition of B cell plasmablast differentiation by reduction of Aiolos and Ikaros may have utility in the treatment of SLE, where elevated levels of BAFF and Aiolos may prime CD27+ memory and DN memory-like B cells to become Ab-producing plasmablasts in the presence of BAFF and proinflammatory cytokines.
Introduction
B cells play a major role in the development of the immune response to foreign pathogens by a complex network of activities including BCR Ag recognition, Ag presentation, cytokine secretion, and differentiation into Ab-producing plasmablasts and plasma cells. The development of B cells and Ag-induced maturation leading to Ab class selection and secretion has been well studied and is broadly characterized as T cell–dependent and –independent processes (1). In T cell–independent Ab development, naive B cells are activated in the absence of T cells by Ags such as polysaccharides that crosslink BCRs or by activation of TLRs in the extrafollicular regions of secondary lymphoid organs, where the activated B cells proliferate and differentiate into short-lived low-affinity Ab-producing plasmablasts. In T cell–dependent driven processes, naive B cells in the extrafollicular regions of secondary lymphoid organs bind Ags to the BCR, and internalize and process these Ags for MHC class II presentation to cognate Ag-recognizing TCRs that in turn induce CD40L expression on the T cell surface. Subsequent binding of CD40 on B cells to CD40L on T cell in the presence of continued Ag BCR stimulation can induce extrafollicular proliferation and short-lived plasmablast differentiation or induce migration to germinal centers, where they can undergo a variety of fates including differentiation into memory cells, affinity maturation by hypersomatic mutation, or differentiation into plasmablasts and long-lived plasma cells.
Peripheral circulating B cells represent the net balance of cells that are trafficking to and from the bone marrow, secondary lymphoid organs, and peripheral tissues at various stages of maturation, development, and activation, thus reflect ongoing homeostatic immune surveillance activity. Alterations in circulating memory B cells, plasmablasts, plasma cells, and Ab levels often accompany the pathology observed in autoimmune diseases. For example, changes in the ratios of circulating CD27+ memory B cells to CD27− naive B cells have been described for rheumatoid arthritis (RA) (2), systemic lupus erythematosus (SLE) (3–6), and Sjögren’s syndrome (7). Blood levels of CD27−IgD− double-negative (DN) B cells with memory-like cell characteristics are elevated in SLE (8–10) and RA (11, 12). Plasmablasts in the blood also have been described to be elevated in autoimmune disease including multiple sclerosis (13), RA (11, 12), and SLE (6, 14). In SLE, high levels of memory B cells, plasmablasts, and anti-dsDNA Ab reappearance after B cell–depleting therapy are correlated to increased rates of disease relapse (15, 16). The ramifications of these increased circulating autoreactive memory B cells and plasmablasts are that they can lead to their appearance in affected disease tissue, where they enhance local concentrations of Ab and immune complexes, such as observed in the inflamed kidney of a lupus nephritis mouse model (17). The observation that plasma cells appear in areas of T cell–B cell interaction in lupus nephritis kidneys suggests that components of a T cell–driven B cell activation and differentiation into Ab-secreting cells may take place locally (18).
Soluble factors that may play a role in B cell differentiation in the presence of T cells include IL-2, IL-21, and the B cell–stimulatory cytokine, BAFF. IL-21 is a member of the common γ-chain cytokine family shown to play a central role in plasmablast and plasma cell differentiation during T cell–dependent B cell responses (19, 20). In humans, IL-21 is mainly produced by activated peripheral CD4+ T cells and follicular Th cells (21, 22). IL-21 regulates B cell apoptosis, growth arrest, costimulation, and differentiation depending on the nature of the activation signals (23–25). For example, IL-21 induces maximum proliferation and produce Abs when costimulated with CD40L, but induces apoptosis when costimulated with anti-IgM in mice and human (19, 26). IL-21 and BAFF synergize in stimulating plasma cell differentiation from human splenic memory B cells (27). IL-2 is predominately a T cell–derived cytokine that promotes B cell proliferation and enhances the effects of IL-21 in inducing plasmablast by CD40L stimulation (19, 28, 29). The levels of both cytokines are altered in SLE and thought to play a role in pathogenesis (30–33).
BAFF is known to be a B cell survival and maturation factor and is expressed by a wide variety of cell types, including monocytes, activated neutrophils, T cells, and dendritic cells (34–36). BAFF is produced as type II transmembrane protein and cleaved into its soluble form at a furin protease site and released in a soluble form (36). BAFF binds to three different receptors: BAFF receptor (BAFF-R), transmembrane activator and Ca2+ modulator and cyclophilin ligand interactor (TACI), and B cell maturation Ag (BCMA), the functions of which are reviewed elsewhere (37, 38). Although BAFF may remain in an active membrane-bound form, soluble BAFF is required for B cell homeostasis and enhances the survival of plasmablasts (39, 40). BAFF is elevated in the blood of SLE patients and is associated with the pathogenesis of SLE (41–45). BAFF-transgenic mouse studies demonstrated that overexpression of BAFF results in development of SLE-like autoimmune pathology including increased autoantibody production (46–48). Belimumab (Benlysta), a specific inhibitor of BAFF, shows significant efficacy in evidence-based clinical trials for SLE, further supporting the role of BAFF in SLE pathology (49–54).
CC-220 is a modulator of the cullin ring ligase 4-cereblon (CRL4CRBN) E3 ubiquitin ligase complex, which induces ubiquitination of the CRBN substrates Aiolos (encoded by the gene IKZF3) and Ikaros (encoded by the gene IKZF1), resulting in their proteasomal degradation (55–57). Although thalidomide and related compounds bind both murine and human CRBN, only human CRBN induces ubiquitination and degradation of Aiolos and Ikaros (58). CC-220 has a similar binding mechanism and also does not induce murine aiolos and ikaros degradation (data not shown), precluding mouse cell or SLE disease model testing. Aiolos and Ikaros are key ikaros family zinc-finger transcription factors that regulate lymphoid and myeloid cell development and immune homeostasis (59). Aiolos and Ikaros are crucial for the function of mature B cells in the periphery and are required for the generation of high-affinity Ab-secreting bone marrow plasma cells (60–62). Studies suggest an essential role of Aiolos at later stages of B cell differentiation (61, 63). Numerous studies have identified polymorphisms in IKZF1 and IKZF3 to be associated with a risk for development of SLE (64–71). Thus, modulation of Aiolos and Ikaros expression has the potential to correct multiple aspects of the immune dysregulation mediated by B cells and differentiation into autoantibody-secreting cells associated with SLE.
We investigated soluble factors associated with SLE that may contribute to plasmablast differentiation and Ab secretion from circulating human memory, DN, and naive B cell subsets. Our data show that BAFF in the presence of IL-2 and IL-21 can induce plasmablast development from CD27+ memory B cells and CD27−IgD− DN B cells, whereas having no effect on naive B cells. Mimicking T cell–dependent processes by CD40L treatment of B cells demonstrate a broader capacity to induce B cell differentiation from memory, DN, and naive B cell populations. We found that Aiolos expression is increased in B cells from SLE patients along with increased levels of circulating DN B cells and BAFF, suggesting that memory and DN B cells may contribute to and be predisposed toward plasmablast differentiation in SLE. Plasmablast differentiation, whether induced by BAFF or CD40L, is prevented by modulation of CRBN activity with CC-220, which induces degradation of the B cell differentiation factors Aiolos and Ikaros. These findings provide a possible scenario whereby circulating memory and DN B cells are primed to differentiate into Ab-secreting plasmablasts by overexpression of Aiolos, and that the soluble factors BAFF, IL-2, and IL-21 have the potential for inducing plasmablast and Ab production from memory B cells in nonsecondary lymphoid organ niches and in inflamed tissue. Downregulation of these B cell differentiation processes by CC-220 modulation of CRBN provides a new therapeutic approach to treating B cell–mediated pathology in SLE.
Materials and Methods
Human B cell isolation
Human buffy coats were obtained from The Blood Center of New Jersey (East Orange, NJ) or New York Blood Center (New York, NY). Leukopacks from healthy control donors (HCs) were from Biological Specialty (Colmar, PA). PBMCs were isolated by density gradient centrifugation using Ficoll (GE Healthcare) followed by EasySep Human Naive B Cell Enrichment Kit (Stemcell Technologies, Vancouver, BC, Canada) or combination of EasySep human B cell enrichment and memory B cell isolation kit (Miltenyi Biotec, San Diego, CA) according to the protocol provided by the manufacturers. Purity was >96% for naive B cells, >85% for memory B cells, and >90% for total B cells as confirmed by flow cytometry.
Patients and healthy individuals
PBMCs were freshly isolated from buffy coats of SLE patients and HCs, and used either fresh or frozen until use. Frozen PBMCs of SLE patients and HCs were thawed, cultured at 1 × 107/ml in 100 μl in 96-well plate, and stained for surface marker analysis by flow cytometry or cultured with DMSO or CC-220 (1, 10, and 100 nM) for 24 h or 5 d as indicated. SLE patient demographics and SLE activity index scores, when available, ranged from 5 to 17 (Supplemental Table I).
Cell culture, activation, and CellTrace labeling
Cells were cultured at 1 × 106/ml in 100 μl in 96-well plate using IMDM (Life Technologies, Carlsbad, CA) with 10% FCS, 1% penicillin/streptomycin, and 0.05% insulin. The cells were cultured with anti-IgM (5 μg/ml; Jackson ImmnoResearch Laboratories, West Grove, PA), soluble CD40L (50 ng/ml; R&D Systems, Minneapolis, MN), and anti-polyhistidine mAb (5 μg/ml; R&D Systems), or BAFF (200 ng/ml; R&D Systems), IL-2 (20 U/ml; R&D Systems), and IL-21 (100 ng/ml; Life Technologies), as indicated in each figure. In some experiments, cells were labeled with CellTrace Violet (Life Technologies) according to the manufacturer’s instruction to assess proliferation. In brief, purified B cells were washed and resuspended at a concentration of 2.5 × 106 cells/ml in PBS with 1 μM CellTrace Violet (Life Technologies), incubated at 37°C for 10 min, quenched with 10% FBS-containing media, and washed two times before culture.
Reagents
The Abs used for surface B cell markers and p-STAT3 were from BD Biosciences (San Jose, CA): anti–CD19-PE-CF594 (clone H1B19), anti–CD20-PerCP-Cy5.5 (clone L27), anti–CD21-allophycocyanin (clone B-ly4), anti–CD23-Alexa Fluor 700 (clone M-L233), anti–CD27-BV786 (clone L128), anti–CD27-allophycocyanin (clone L128), anti–CD38-PE-Cy7 (clone HB7), anti-CD95 FITC (clone DX2), anti–IL-21R-PE (clone 17A12), anti–IgD-allophycocyanin-H7 (clone IA6-2), anti–IgM-BV510 (clone G20-127), anti–IgG-BUV395 (clone G18-145), anti–CD45-V450 (clone HI30), anti–CD183-Alexa Fluor 700 (clone 1C6/CXCR3), anti–Aiolos-Alexa Fluor 488 (clone S50-895), anti–Ikaros-Alexa Fluor 647 (clone R32-1149), anti-STAT3 (pY705) PE (clone CF594). Polyclonal rabbit anti-Aiolos was from Santa Cruz Biotechnology (Dallas, TX), and rabbit anti-Ikaros (clone EPR9342) was from Abcam (Cambridge, MA). Live/Dead Fixable Blue stain (Life Technologies) was used to exclude dead cells. Cells were pretreated with DMSO (Sigma) or 1, 10, and 100 nM Celgene compound CC-220 for 1 h at 37°C before stimulating under the indicated conditions.
Flow cytometry
Multicolor flow cytometry was performed using an LSRII Fortessa and FACSDiva 8.0 (Becton Dickinson, San Jose, CA). In brief, all cells were harvested from 96-well plates on day 5 and stained for Live/Dead stain, then surface markers for B cells for 30 min at room temperature, and then fixed, permeabilized using a Transcription Factor Buffer Set (BD) for Aiolos or Ikaros staining. Cells were acquired with CountBright (Life Technologies) to calculate the number per microliters and analyzed by FlowJo (Tree Star).
Cell sorting
Four-way sort was performed using a BD FACSAria Cell Sorter (Becton Dickinson) based on CD19, CD20, CD27, IgD, and IgG expression. B cells were negatively enriched before sorting. Sorted cells were pretreated with CC-220 at 1, 10, or 100 nM for 1 h at 37°C and cultured at 2 × 105/ml in 100 μl volume in 96-well culture plate for 5 d using IMDM with 10% FCS, 1% penicillin/streptomycin, and 0.05% insulin. The culture conditions are as follows: media control, CD40L (50 ng/ml) and anti-polyhistidine mAb (5 μg/ml), or BAFF (200 ng/ml), with IL-2 (20 U/ml) and IL-21 (100 ng/ml), as indicated in each figure.
ELISA
Secreted IgM and IgG in the culture supernatant and BAFF in normal and SLE donor serum were quantified by ELISA using human IgM and IgG ELISA kits or Human BAFF ELISA Kit from Abcam, according to the manufacturer’s recommendations, and read at 450 nm.
MagPix
The frozen SLE (n = 10) and normal (n = 8) serum patient samples were analyzed in triplicate for cytokine production in a magnetic multiplex bead format using the MagPix instrument (Millipore, Billerica, MA). The following analytes were tested in a Human Cytokine Multi-Plex Panel: IFN-γ, IL-2, IL-17A, IL-21, and TNF-α (EMD Millipore). Data analysis was performed using Milliplex Analyst Software (Millipore). All of the data were graphed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA).
Real-time quantitative RT-PCR
On day 5, naive or memory B cells were harvested, and total RNA was isolated with RNeasy Mini kits using QIA Cube system (Qiagen) or QIA HT System (Qiagen). Reverse transcription was performed using the high-capacity cDNA kit (Life Technologies). Real-time quantitative RT-PCR with 25–100 ng of total RNA was performed using the reverse transcription kit and TaqMan PCR probes specific for the genes of interest according to standard methods using Viia7 (Applied Biosystems). The quantity was normalized to GAPDH as the endogenous housekeeping gene. Fold increase of gene expression was calculated using the comparative threshold cycle method (2−ΔΔCt). Primers used are as follows: IRF4 (Hs01056533_m1), XBP1 (Hs00231936_m1), IgJ (Hs00376160_m1), PRDM1 (Hs00153357_m1), and AICDA Hs00757808_m1 (Life Technologies).
Western blot
A total of 1 × 106 cells was treated with DMSO or CC-220 (1, 10, or 100 nM) in IMDM for 15 min, 2 h, or 24 h at 37°C. The cells were centrifuged and the cell pellet lysed in 100 μl of RIPA buffer (Cell Signaling Technologies) and incubated on ice for 30 min. The lysates were then spun through a QIAshredder to break up DNA in the lysate. Lysates were stored at −80°C until use. Lysates were diluted with a premade 5× SDS loading buffer (made by mixing 0.5 ml of 0.5 M Tris/HCl + 0.4 ml 2-ME + 2 ml glycerol + 1.6 ml 10% SDS + 0.4 ml 0.5% bromophenol blue + 3.1 ml water) and boiled for 10 min before loading the gel. The lysates were then run on 12% Tris-HCl precast gels (Invitrogen, Grand Island, NY). The gels were run at 150 V until the protein of interest reached two-thirds of the gel. The proteins were then transferred to nitrocellulose membrane (Invitrogen) using the Invitrogen I-blot system. The membranes were blocked using Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature on a shaker. After 1 h, the membranes were probed with Ab diluted in Odyssey Blocking Buffer (1:1000). The membranes were incubated overnight at 4°C with gentle shaking. The next day, the membranes were washed three times for 5 min each with a 1× mixture of TBST. LI-COR secondary Ab was added to each membrane. The secondary Ab was diluted 1:25,000 in Odyssey Blocking Buffer. Membranes were incubated for 1 h at room temperature in the dark. The blots were then washed three times for 5 min each in 1× TBST followed by washing 1× in PBS to remove Tween 20. Blots were analyzed for fluorescence intensity on the LI-COR Odyssey.
Statistical analysis
Statistical comparisons were made with the one-way ANOVA followed by Dunnett’s multiple comparison tests or Student t test when comparing two groups using GraphPad Prism 6.0. The p values <0.05 were considered significant.
Results
BAFF costimulation with IL-2 and IL-21 selectively induces plasmablast differentiation from CD27+ memory versus CD27− naive B cells
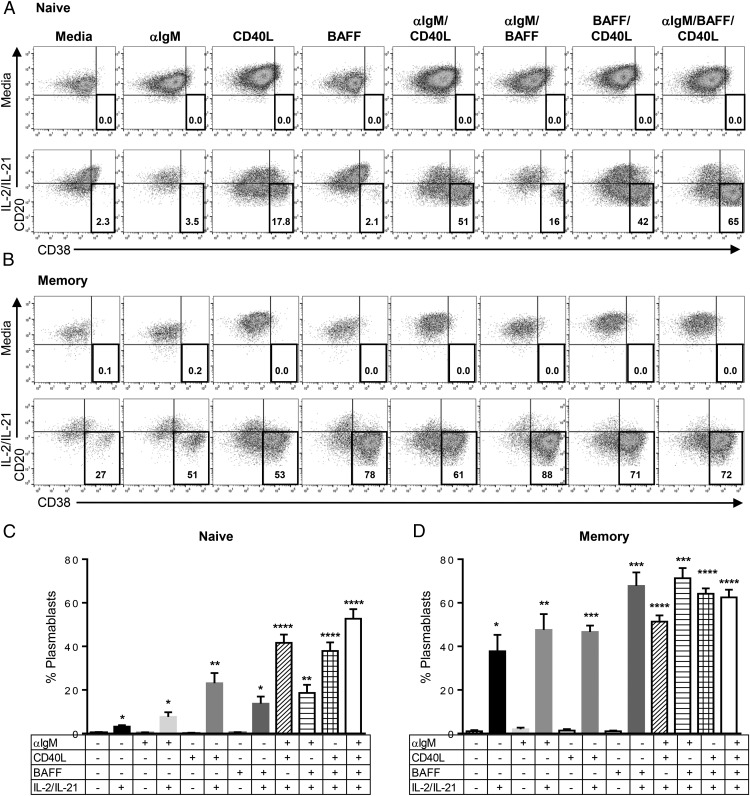
To examine the role of BAFF on B cell function, we investigated the differentiation potential of naive and memory B cells in three model systems of B cell activation using anti-IgM, CD40L, and BAFF. Naive and memory B cells were isolated from human PBMCs using surface CD27 expression, which is a general marker for somatically mutated B cells (72). Cells were broadly defined as CD27− naive B cells and CD27+ memory B cells. Plasmablasts were identified by the upregulation of CD38 and the downregulation of CD20 expression on their cell surface (73, 74). IL-2 and IL-21 were tested alone and in combination for their ability to induce differentiation from total B cells and were found to be corequired factors for optimal plasmablast differentiation (Supplemental Fig. 1). Therefore, all subsequent experiments with purified B cell subsets were tested in the absence and presence of a combination of IL-2 and IL-21. Corequirements of IL-2 and IL-21 optimal CD40L-induced naive B cell differentiation have been previously reported (19, 28). In this study, a combination of IL-2 and IL-21 in the absence of additional stimuli was found to have negligible levels of plasmablast differentiation (Figs. 1, 2), but enhanced cell survival effects on naive B cells (Supplemental Fig. 2). Memory B cell survival was greater than that of naive B cells in the absence of added factors and was not further enhanced by IL-2 and IL-21 (Supplemental Fig. 2). In the absence of IL-2 and IL-21, none of the stimuli tested (BAFF, anti-IgM, or CD40L), alone or in combination, were able to induce plasmablast differentiation from either naive or memory B cells (Fig. 1). Naive B cells in the presence of IL-2 and IL-21 did not respond to BAFF or anti-IgM, whereas CD40L induced significant proliferation (Fig. 2) and plasmablast development (Fig. 1). Further enhancement of naive B cell differentiation induced by CD40L was observed when additional stimuli from BAFF, anti-IgM, or BAFF and anti-IgM together were added. Thus, CD40L is the dominant driving agent of naive B cell differentiation when IL-2 and IL-21 are present, with the potential for synergy when other stimuli not normally sufficient for naive B cell differentiation are added, such as BAFF and anti-IgM.
FIGURE 1.
BAFF induces plasmablasts from memory B cells, but not from naive B cells. CD27− naive B cells (A and C) and CD27+ memory B cells (B and D) were isolated from human PBMCs, left untreated, or cultured with indicated stimuli for 5 d. (A and B) Representative dot plots in indicated conditions. Total CD19+ B cells [(A) naive and (B) memory] were gated and analyzed for differentiation by expression of CD20 and CD38; the number in the plot is the percent of plasmablast among CD19+ B cells. (C and D) Graphs show the percent of plasmablast among CD19+ B cells in the indicated conditions (mean ± SEM). Dot plots are representative of multiple donors. Statistical comparisons were made with one-way ANOVA followed by Dunnett’s multiple-comparison test. Naive B cells: n = 12; memory B cells: n = 6. *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
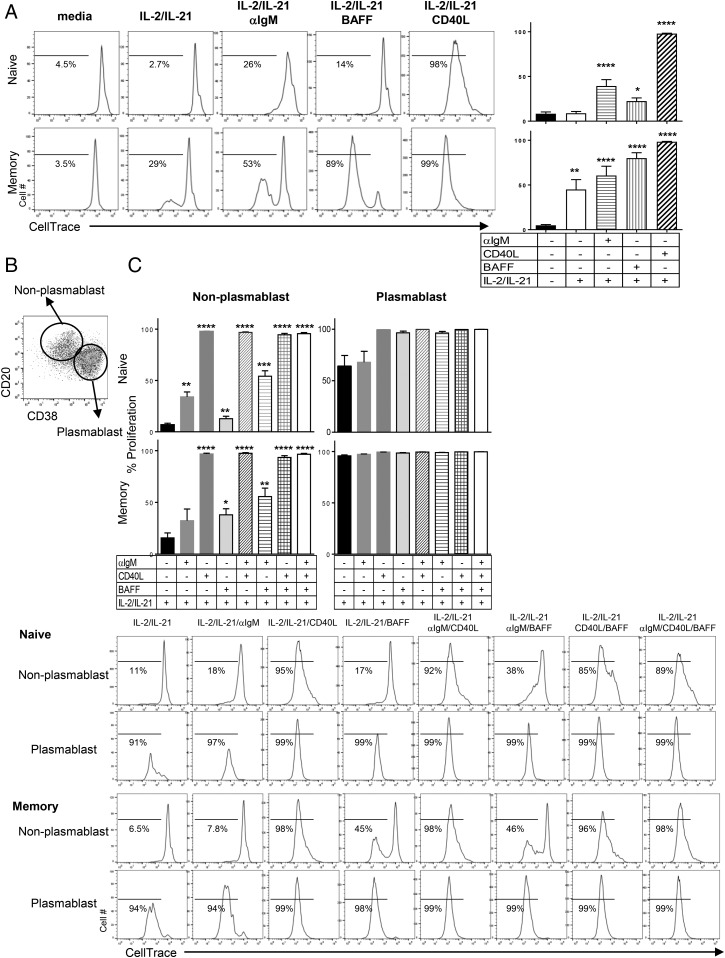
FIGURE 2.
Plasmablasts induced by anti-IgM, CD40L, or BAFF are actively dividing B cells. (A) Representative histogram for dividing cells by CellTrace dilution (left). The numbers in histograms are percent of dividing cells among CD19+ B cells. Graphs shown (right) are the mean percent ± SEM of the number of dividing cells in naive (top) and memory (bottom) B cells after 5 d stimulation with the indicated conditions. (B) Representative dot plot for gating of plasmablast and nonplasmablasts. CD19+ B cells were gated as plasmablast (CD20loCD38hi) and nonplasmablast (CD20hiCD38lo), and analyzed for proliferation by CellTrace dilution. (C) Graphs are shown as the mean percent ± SEM of dividing cells in nonplasmablast (left) and plasmablast (right) from naive (top) and memory (bottom) B cells under the indicated conditions on day 5. Representative histograms from the data are shown below the graphs. Statistical comparisons were made with one-way ANOVA followed by Dunnett’s multiple-comparison test. Naive B cells: n = 12; memory B cells: n = 6. *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
Memory B cell plasmablast differentiation responses displayed both similarities and differences to naive B cells. Similar to naive B cells, memory B cells did not become plasmablasts in the absence of IL-2 and IL-21, regardless of stimuli (BAFF, anti-IgM, or CD40L alone or in combination). Unlike naive B cells, IL-2 and IL-21 alone could induce low levels of plasmablast differentiation from memory cells (Fig. 1). Moreover, differentiation of memory B cells by IL-2 and IL-21 was greatly enhanced by BAFF and anti-IgM, which had not been observed in naive cells. CD40L in the presence of IL-2 and IL-21 significantly induced memory cell differentiation, as was observed for CD40L effects on naive B cells. Notably, CD40L-induced plasmablasts showed a wider range of CD38 expression compared with the high expression of CD38 observed in BAFF-treated plasmablasts (Fig. 1). A similar CD38 expression phenomenon was reported in studies using the two-step in vitro B cell differentiation culture system with IL-2, IL-10, CD40L, and BAFF in other studies (40, 75).
Interestingly, when BCR stimulation by anti-IgM is combined with IL-21, it reduced the number of naive B cells significantly because of cell death, but not of memory B cells. Because BCR activation and IL-21 were previously shown to cause cell death in total B cell populations (19, 76), we assessed the survival of naive and memory B cells after stimulation with anti-IgM and IL-21. Anti-IgM or IL-21 alone increased the survival of naive B cells compared with media control. The combination of anti-IgM and IL-21, however, increased cell death in naive B cells. In contrast, anti-IgM stimulation of memory B cells was resistant to IL-21 cell death effects. This IL-21–induced cell death was specific for anti-IgM because no increased cell death was observed by IL-21 in the presence of either BAFF or CD40L (Supplement Fig. 2).
To assess the correlation of differentiation and proliferation, we assessed cell division in plasmablast (CD20loCD38hi) and nonplasmablast (CD20hiCD38lo) gated populations after 5 d of culture treatments (Fig. 2). In both naive and memory B cells, all the plasmablasts were dividing, whereas nonplasmablasts were in a mixed state of dividing and nondividing cells depending on the stimulus. This would suggest that proliferation precedes or at least is corequired for plasmablast differentiation in the systems tested in this study. In BAFF, anti-IgM, or a combination of BAFF and anti-IgM–treated cells, there were dividing and nondividing cell populations. In contrast, CD40L alone or in combination with other stimuli induced proliferation in all of these nonplasmablast cells. Thus, similar to plasmablast differentiation, the effects of CD40L on proliferation are greater than BAFF or anti-IgM on both naive and memory B cells. Once plasmablast differentiation occurs, however, the proliferation level is indistinguishable from the stimuli that generated them under the conditions tested.
Because it has been reported that IL-21 and BAFF can induce plasmablast cells from a unique population of splenic memory B cells referred to as human splenic marginal zone analog (MZA) B cells (27), we profiled the peripheral blood–derived memory cells used in this study to determine whether there were similarities between MZA and circulating CD27+ memory B cells. MZA B cells have been defined as CD21hiCD23−CD27+IgD+/−IgM+/−IgG+/−TACIhiBCMA−BAFF-RhiCD95hiB220hi, whereas follicular B cells are CD21+/−CD23hiCD27−IgDhiIgMloIgG−TACI−BCMA−BAFF-R+CD95+B220lo (27). CD27+ memory B cells in PBMCs are CD21+CD23+CD27+CD95hiIgDlowIgMloIgGhiB220hiTACI+BCMA+BAFF-Rhi, and CD27− naive B cells in PBMCs are CD21+CD23+CD95hiIgDloIgMloIgGhiB220hiTACI+BCMA+BAFF-RhiCD21hiCD23+ (data not shown). Thus, we conclude that the circulating CD27+ memory B cells we used in this study are a distinct population not derived from recirculating splenic MZA B cells.
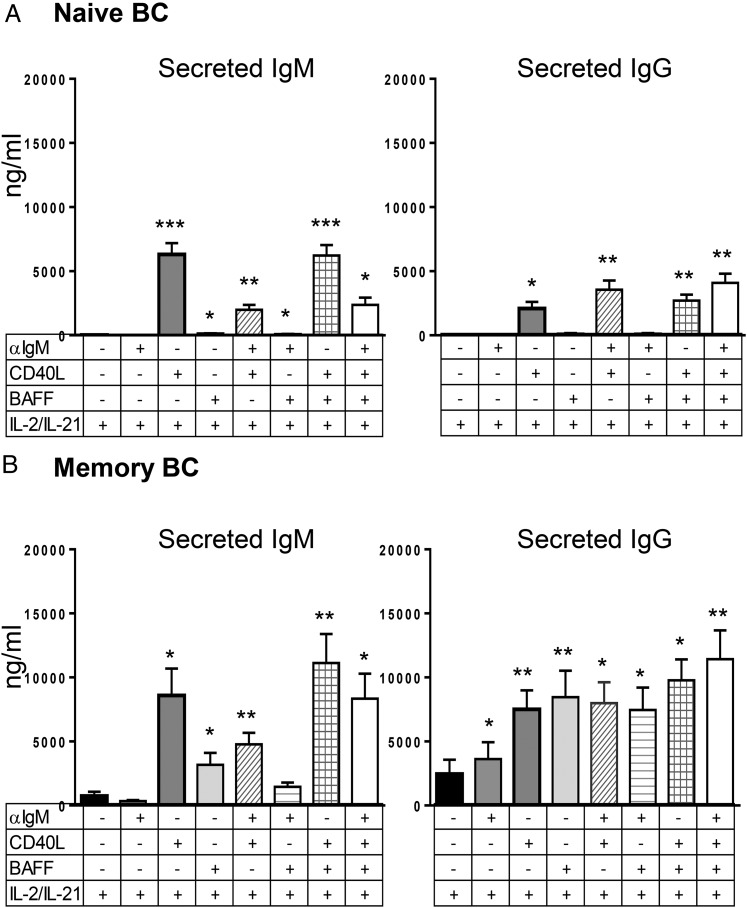
Because Ab production is a key functional feature of plasmablasts, we evaluated IgM and IgG in the culture supernatants of cells treated with IL-2 and IL-21 in the absence and presence of combinations of BAFF, anti-IgM, and CD40L (Fig. 3). Levels of secreted Ab reflected the degree of plasmablast differentiation. As discussed earlier with proliferation and plasmablast differentiation experiments on naive B cells, CD40L was required for optimal secretion of IgM and IgG from these cells in the presence of IL-2 and IL-21, with little to no IgM or IgG secretion induced by BAFF and anti-IgM (Fig. 3). In the absence of IL-2 and IL-21, little IgM or IgG was secreted by CD40L, BAFF, or anti-IgM stimulation of naive B cells (data not shown). Memory B cells produced large amounts of IgM and IgG by all three stimuli (BAFF, anti-IgM, and CD40L) in the presence of IL-2 and IL-21, with IL-2 and IL-21 stimulation alone inducing lower levels of Ab secretion (Table I).
FIGURE 3.
BAFF induces IgM and IgG production from memory, but not naive, B cells. Naive (A) and memory (B) B cells were cultured as described in Fig.1. IgM and IgG in culture supernatant from indicated conditions were measured by ELISA on day 5. Graphs are shown as mean concentration ± SEM. n = 8 for naive, n = 9 for memory B cells. One-way ANOVA compared with IL-2/IL-21–only condition. *p < 0.05, **p < 0.005, ***p < 0.001.
Table I. BAFF and CD40L induction of IgM and IgG secretion from naive and memory B cells with IL-2 and IL-21.
| + IL-2/IL-21 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Media | Media | Anti-IgM | CD40L | BAFF | Anti-IgM/CD40L | Anti-IgM/BAFF | BAFF/CD40L | Anti-IgM/CD40L/BAFF | |
| Naive IgM (ng/ml) | 7.7 ± 2.2 | 19.0 ± 5.3 | 22 ± 6.7 | 6306 ± 901.3*** | 135 ± 30.4* | 1996 ± 367.0** | 84 ± 18.7* | 6,225 ± 783.9*** | 2,381 ± 536.8* |
| Naive IgG (ng/ml) | 2.0 ± 1.0 | 7.7 ± 4.2 | 19 ± 7.4 | 2094 ± 505.3* | 125 ± 52.7 | 3565 ± 692.4** | 128 ± 42.8 | 2,707 ± 456.5** | 4,093 ± 692.7** |
| Memory IgM (ng/ml) | 25 ± 4.7 | 746 ± 324.0 | 345 ± 75.3 | 8595 ± 2045.3* | 3176 ± 919.3* | 4779 ± 888.7** | 1458 ± 323.3 | 11,127 ± 2215.0** | 8,343 ± 1,898.3* |
| Memory IgG (ng/ml) | 13 ± 4.3 | 2456 ± 1092.0 | 3619 ± 1286.7* | 7503 ± 1452.0** | 8465 ± 2023.7** | 7987 ± 2023.7* | 7463 ± 1734.0* | 9,783 ± 1633.0* | 11,428 ± 2,260.7** |
Naive and memory B cells were cultured under indicated conditions. IgM and IgG in culture supernatant were measured by ELISA on day 5. Numbers are mean concentration ± SEM. n = 8 for naive B cells, n = 9 for memory B cells.
p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001, one-way ANOVA compared with IL-2/IL-21–only condition.
CD27−IgD− DN B cells exhibit a memory-like phenotype and differentiate to IgG- and IgM-secreting plasmablasts in response to BAFF and CD40L
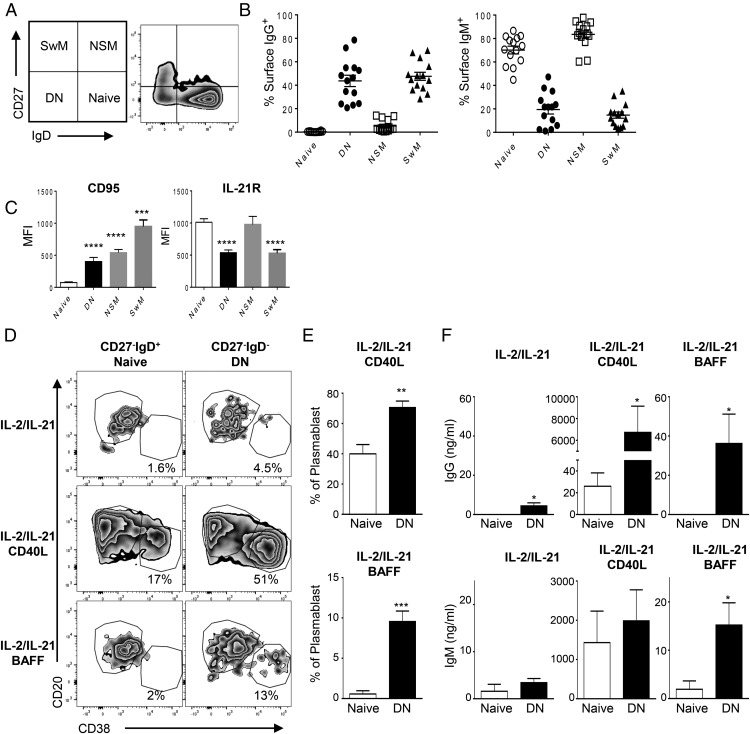
We further investigated a circulating CD27−IgD− DN B cell subset reported to be increased in SLE patients (8, 77). These cells are one of four subpopulations that can be defined using surface expression of CD27 and IgD (Fig. 4A) and are referred to as CD27+IgD− switched memory (SwM) B cells, CD27+IgD+ double-positive nonswitched memory (NSM) B cells, CD27−IgD+ mature naive B cells (naive), and CD27−IgD− DN B cells.
FIGURE 4.
DN B cells display memory phenotype responses to BAFF. Normal PBMCs were analyzed by flow cytometry to assess the difference between naive and DN B cells. (A) Gating scheme and the representative plots for the four subpopulations: DN, naive, SwM, and NSM. (B) Shown are mean percent ± SEM of B cells expressing IgG (left) or IgM (right) in each population (n = 10). (C) Mean fluorescence intensity (MFI) ± SEM of indicated surface molecules is shown. Statistical comparisons were made with one-way ANOVA followed by Dunnett’s multiple-comparison test (n = 10 donors). (D) CD27−IgD+ naive and DN B cells were sorted based on CD27 and IgD expression and cultured with differentiation mixture used in previous experiments for 5 d. Shown are representative plots on day 5. (E) The plasmablast differentiation by IL-2, IL-21, and CD40L (top) or by IL-2, IL-21, and BAFF (bottom) from naive or DN B cells is shown by percent ± SEM in each population. Student t test between naive and DN B cells (n = 5). (F) Culture supernatants were collected on day 5, and IgG and IgM secretion were measured by ELISA. Student t test between naive and DN B cells (n = 5). *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
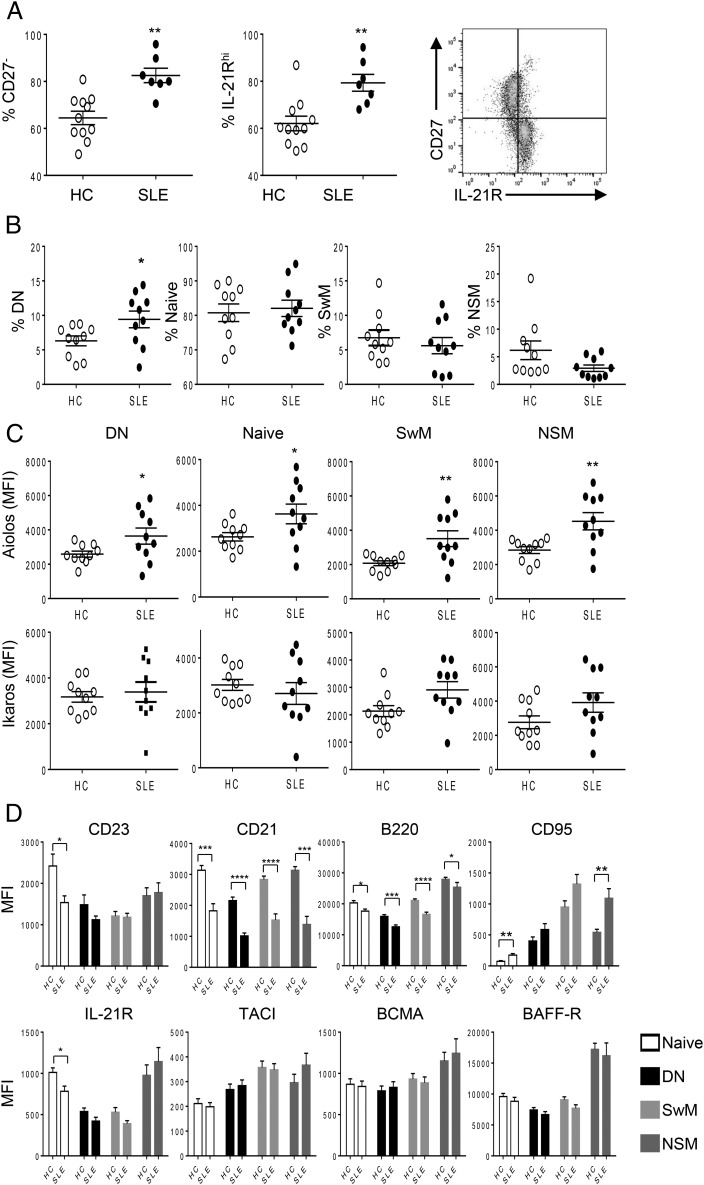
In agreement with prior reports, we find that circulating CD27−IgD− DN B cells are elevated in SLE (Fig. 5B). The identity of DN B cells and function is unknown; however, there is a correlation between the degree of DN B cell expansion and higher autoantibody titers in SLE that suggest that they are either directly or indirectly involved in autoantibody responses (10). To investigate the difference between these subpopulations, we characterized the phenotype and function of CD27−IgD+ and CD27−IgD− populations using normal PBMCs. As shown in Fig. 4B, CD27−IgD+ naive and NSM express no or very low-surface IgG and high-surface IgM, respectively, whereas DN and SwM highly expressed surface IgG and low or no IgM. DN B cells expressed increased CD95 and decreased IL-21R compared with IgD+ naive B cells, which mimics memory B cell characteristics (Fig. 4C) (8).
FIGURE 5.
B cell subpopulation alterations and Aiolos overexpression in SLE. (A) PBMCs were isolated from buffy coat of HC and SLE donors, and phenotype was analyzed by flow cytometry. The percent of CD27− B cells (left) and IL-21R+ B cells (middle) among B cells were shown as mean ± SEM. The correlation of CD27 and IL-21R expression is shown as a representative histogram (right). (B) PBMCs from HCs and SLE patients were analyzed by flow cytometry. The mean percent ± SEM of four subpopulations in HC and SLE are shown. (C) Aiolos and Ikaros expression in the indicated B cell subpopulations are shown as mean fluorescence intensity (MFI) ± SEM. (D) Expressions of indicated B cell markers in indicated populations are shown as MFI ± SEM. Student t test between HC and SLE B cells (n = 5): *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
We sorted B cell subpopulations based on CD27 and IgD expression, and tested the potential of CD27−IgD+ naive and DN B cells to differentiate into plasmablasts. The purity of sorted population was >93% for naive and 92% for DN B cell populations, with the contaminating cells being represented by overlapping low borderline detection of IgD+/− cells. No contaminating CD27+ cells were detected in either population. As shown in this study (Fig. 4D), CD27−IgD+ naive B cells differentiated into plasmablasts by IL-2, IL-21, and CD40L treatment, but not by IL-2, IL-21, and BAFF treatment, similar to the results observed when stimulating the broader CD27− naive B cell population (Figs. 1, 4D). In parallel with plasmablast differentiation, CD40L induced IgG secretion from CD27−IgD+ naive B cells, but BAFF was unable to induce IgG secretion (Fig. 4F). DN B cells were similar to CD27+ memory B cells in that they differentiated into plasmablasts when treated with IL-2, IL-21, and CD40L, as well as when treated with IL-2, IL-21, and BAFF (Figs. 1, 4D, 4E).
Plasmablast differentiation parallels Ab production where IL-2 and IL-21 in combination with either CD40L or BAFF induces IgG and IgM production from DN B cells, but with notable reduced BAFF potency relative to CD27+ memory B cells. In CD27+ B cells, BAFF in the presence of IL-2 and IL-21 induced high levels of secreted IgG and IgM (8.5 and 3.2 μg/ml, respectively; Fig. 3B), but in DN B cells, only low levels of secreted IgG and IgM (36 and 25 ng/ml, respectively; Fig. 4F) were observed. In comparison, CD40L stimulated CD27+ B cell secretion of IgG and IgM (7.5 and 8.6 μg/ml, respectively; Fig. 3B) to similar high levels as CD40L stimulated DN B cell secretion of IgG and IgM (6.7 and 2.0 μg/ml, respectively; Fig. 4F). Together, these results suggest that DN B cells phenotypically and functionally resemble memory B cells in the capacity to differentiate into plasmablasts and to secrete IgG and IgM when treated with either CD40L or BAFF in the presence of IL-2 and IL-21. Although CD40L and BAFF both induced DN B cell plasmablast differentiation, CD40L was more potent than BAFF at inducing IgG and IgM secretion.
Alterations of circulating B cell subtypes and overexpression of Aiolos in SLE
We investigated the levels of circulating B cell populations, B cell protein biomarkers, BAFF, and cytokine levels in SLE (Fig. 5, Supplemental Fig. 3). Although the overall level of circulating total CD19+ B cells was similar between HCs and SLE, there were differences in the ratio of subpopulations of B cells. CD27− naive B cell and IL-21Rhi B cell populations were both significantly increased in SLE samples; correspondingly, the CD27+ memory B cell population was reduced. On further investigation, the IL-21Rhi population was found to correspond to CD27− naive B cells, confirming that these two are the same populations (Fig. 5A). Further analysis with IgD expression revealed a significant increase of DN B cells in SLE patients among the CD27− B cell population (Fig. 5B). NSM was slightly, but not significantly, decreased in SLE patients, and there were no differences in the levels of CD27−IgD+ naive and SwM B cells.
The protein expression levels of Aiolos and Ikaros, transcription factors important for immune cell development and homeostasis that have been genetically linked to the risk for development of SLE, were measured in the subpopulations of B cells in HCs and SLE patients. In all four subpopulations tested (CD27+IgD− SwM, CD27+IgD+ NSM, CD27−IgD+ naive, and CD27−IgD− DN B cells), Aiolos protein levels were increased in SLE patients, whereas there was no difference in Ikaros protein levels (Fig. 5C).
We compared the phenotypes of four subpopulations of B cells by staining surface B cell markers used to determine B cell subtypes and activation state (Fig. 5D). In brief, CD23low and CD21low B cells are functionally more immature; correspondingly, the CD21low B cells produce more IgM compared with CD21hi B cells (78, 79). CD21 is a BCR coreceptor that is downregulated in activated B cells. Interestingly, decreased surface expression of CD21 and CD23 are observed in the lupus mice model and SLE patients in support of these cells being in an activated state (80–84). B220 identifies an isoform of the phosphatase CD45 present on naive B cells reduced in activation states and dysregulated in lymphoproliferative disorders (85). Lastly, CD95 (Fas, the receptor for the apoptosis-inducing Fas ligand) is upregulated on activated B cells (86, 87). An overall trend was evident in the four B cell subsets tested in this study that show SLE B cells to be in a more activated state (lower CD21, CD23, and B220 and higher CD95). In CD27−IgD− naive B cells, expression of CD21, CD23, B220, and IL-21R were decreased and CD95 was increased in SLE patients; in DN B cells, CD21 and B220 were significantly decreased and CD95 trended toward an increase in SLE patients; SwM and NSM B cells expressed decreased CD21 and B220 and increased CD95 as well (Fig. 5D). There were no significant differences in expression of the three BAFF receptors TACI, BCMA, and BAFF-R between normal and SLE donors in all B cell subpopulations.
BAFF, IL-2, and IL-21 were also measured in HCs and SLE patients. Circulating BAFF was significantly increased in SLE patients, with no change in IL-2 and a reduction in IL-21 being observed (Supplemental Fig. 3).
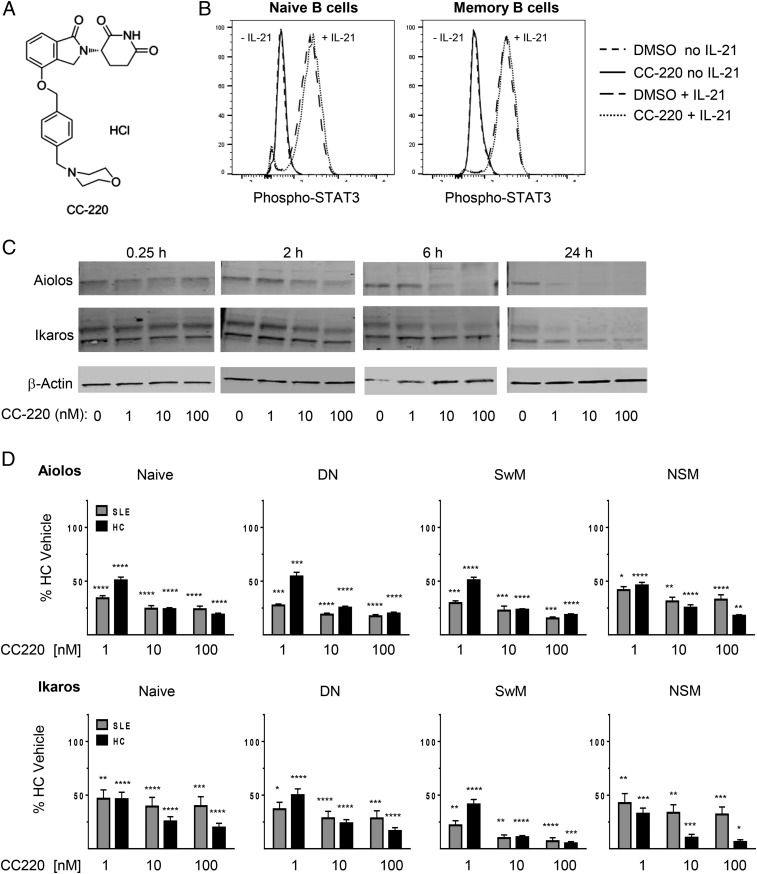
CC-220 reduces Aiolos and Ikaros in HCs and SLE patients independent of IL-21 STAT3 signaling
Results thus far show distinct plasmablast differentiation requirements of naive and memory B cells distinguished by BAFF, novel DN B cell BAFF-induced plasmablast differentiation and Ab secretion, and SLE-associated alterations in circulating B cell subpopulations and activation states, as well as elevations in the B cell differentiation transcription factor Aiolos. CC-220 is a modulator of the CRL4CRBN E3 ubiquitin ligase complex, which induces ubiquitination of the CRBN substrates Aiolos and Ikaros, resulting in their proteasomal degradation (55–57). CC-220 has the chemical name 2,6-piperidinedione, 3-[1,3-dihydro-4-([4-(4-morpholinylmethyl)phenyl]methoxy)-1-oxo-2H-isoindol-2-yl]-, (3S)-, hydrochloride (1:1), and an m.w. of 485.96 (Fig. 6A). We also investigated the effects that CC-220 might have on IL-21 signaling in B cells. IL-21 signals through IL-21R to induce B cell plasmablast differentiation through STAT3 (28, 88), with differential requirements in naive and memory B cells (89). IL-21 stimulation of B cells induced rapid STAT3 phosphorylation in both naive and memory B cells primed with CD40L and anti-IgM that was unaffected by pretreatment of cells with CC-220 (Fig. 6B), further supporting the specificity for Aiolos and Ikaros mechanisms proposed for inhibition of B cell differentiation. The effects of CC-220 on Aiolos and Ikaros protein levels and cellular function were examined in B cells from HCs and SLE patients. Treatment of B cells with CC-220 degrades Aiolos and Ikaros in normal B cells within 2 h of treatment (Fig. 6C). To further examine the effects of CC-220, we treated PBMCs from HCs and SLE patients with CC-220 (1, 10, and 100 nM) for 24 h and measured Aiolos and Ikaros protein levels in naive, DN, SwM, and NSM B cell subsets (Fig. 6D). CC-220 reduced Aiolos and Ikaros protein levels significantly in all four of the B cell subsets measured (naive, SwM, NSM, and DN) from both HCs and SLE patients within 24 h in a dose-dependent manner.
FIGURE 6.
CC-220 reduces B cell Aiolos and Ikaros expression in HC and SLE patients independent of IL-21 STAT3 signaling. (A) Chemical structure of CC-220. (B) B cells from HC were stimulated with CD40L and anti-IgM for 24 h. Cells were then treated with DMSO or CC-220 (10 nM) for 1 h at 37°C, then exposed to IL-21 for 15 min and p-STAT3 levels determined in CD19+CD27lo naive B cells (left) or CD19+CD27hi memory B cells (right) by flow cytometry. (C) B cells from HCs were treated with the indicated amounts of CC-220 for 0.25, 2, 6, and 24 h at 37°C, and expression of Aiolos and Ikaros were measured by Western blot. (D) Inhibition of Aiolos (top) and Ikaros (bottom) expression by CC-220 in indicated populations are shown as mean percent ± SEM relative to untreated HC Aiolos or Ikaros expression (n = 10). Statistical comparisons are by one-way ANOVA and Dunnett’s multiple-comparison test compared with HC vehicle control: *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
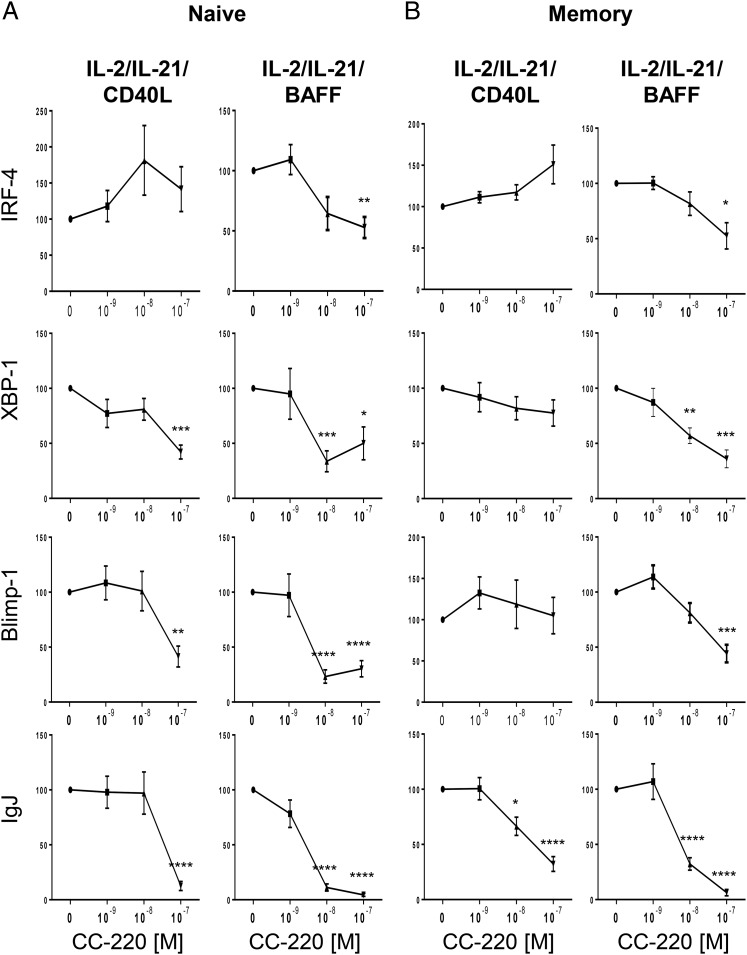
CC-220 reduced expression of transcription factor genes mediating B cell differentiation
To further investigate the mechanism of CC-220 action during plasmablast differentiation, we measured the expression of genes involved in B cell differentiation. CD27− naive and CD27+ memory B cells were treated with CC-220 with BAFF or CD40L stimulation and assessed on day 5 for expression of IFN regulatory factor 4 (IRF4), X-box-binding protein 1 (XBP1), B lymphocyte–induced maturation protein 1 (Blimp1), and Ig joining chain (IgJ). Blimp1, XBP1, and IRF4 are essential transcription factors upregulated during plasmablast differentiation, and IgJ is required for IgM and IgA secretion and similarly upregulated during plasmablast differentiation (90–92). In BAFF-induced plasmablast differentiation conditions, Blimp1, XBP1, IRF4, and IgJ were all decreased by CC-220 treatment whether in naive or memory-treated B cells (Fig. 7). Mixed effects were observed by CC-220 treatment of CD40L-stimulated naive and memory B cells. CC-220 increased IRF4 expression in both naive and memory B cells when treated with IL-2 and IL-21 and CD40L, and reduced the expression of XBP1, Blimp1, and IgJ in naive B cells, but only IgJ in memory B cells. XBP1 and Blimp1 were unchanged by CC-220 treatment of CD40L-stimulated memory B cells.
FIGURE 7.
CC-220 reduces mRNA levels of B cell differentiation transcription factors. Naive and memory B cells were cultured as described in Fig. 1. Cells were harvested on day 5, and mRNA was isolated for quantitation by quantitative RT-PCR. The effects of CC-220 on gene expression related to B cell differentiation in plasmablasts from naive (A) and memory (B) B cells are measured as relative expression to vehicle-treated samples. The graphs shown are mean relative expression to vehicle control ± SEM (n = 7–8), one-way ANOVA followed by Dunnett’s multiple-comparison test: *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
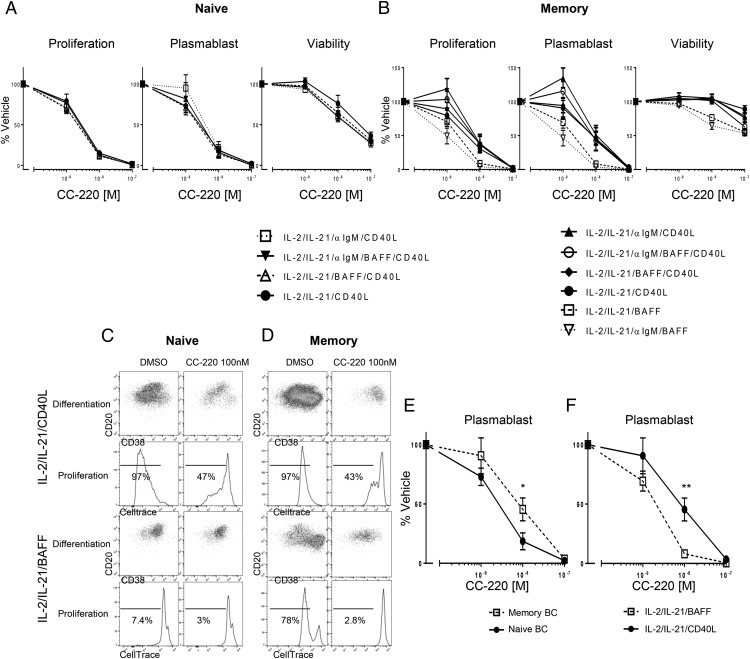
CC-220 differentially inhibits proliferation, differentiation, and Ab secretion from naive and memory B cells
Because in this study CC-220 reduced Aiolos and Ikaros protein levels in all B cell types tested, and at least one or more genes involved in B cell differentiation, we investigated the possibility that CC-220 would prevent BAFF- and CD40L-induced proliferation and plasmablast differentiation of CD27− naive and CD27+ memory B cells (Fig. 8). Because naive B cells do not respond to BAFF, but do respond to a combination of IL-2, IL-21, and CD40L (Fig. 1), only stimulus conditions including CD40L are shown for the effects of CC-220 on proliferation and differentiation for naive B cells (Fig. 8A). For naive B cells stimulated with IL-2 and IL-21 in the presence of CD40L alone or in combination anti-IgM, BAFF, or anti-IgM and BAFF together, inhibition of induced naive B cell proliferation (>85%) and plasmablast differentiation (>80%) was observed at 10 nM treatment with CC-220. For memory B cells stimulated with IL-2 and IL-21 in the presence of CD40L or BAFF alone or in the combinations indicated, inhibition of induced memory B cell proliferation (62–91% depending on stimulus) and plasmablast differentiation (55–96% depending on stimulus) was observed at 10 nM treatment with CC-220 (Fig. 8B). When compared under the same CD40L-stimulating condition, naive B cells are more sensitive to CC-220–inhibitory effects at 10 nM treatment than observed for memory B cells (Fig. 8E). This is consistent with the effects of CC-220 on B cell differentiation factor gene expression, where three genes measured (XBP1, BLIMP1, and IgJ) were reduced in naive cells after CD40L treatment, but in memory B cells, only IgJ was reduced (Fig. 7). For memory B cells, differential CC-220 effects were observed in that CC-220 was more efficacious at inhibiting BAFF-induced proliferation and plasmablast differentiation than observed for CD40L-stimulated cells (Fig. 8F). Interestingly, this correlates to the inhibitory effects observed for CC-220 on B cell differentiation genes where all four measured B cell differentiation gene factors (IRF4, XBP1, BLIMP1, and IgJ) were reduced in BAFF-treated memory B cells, but only IgJ inhibition was observed for CD40L-treated cells (Fig. 7).
FIGURE 8.
CC-220 inhibits B cell proliferation and plasmablast differentiation. Naive (A) and memory (B) B cells were treated with vehicle or CC-220 (1, 10, and 100 nM) for 1 h before adding indicated stimuli and cultured for 5 d. Proliferation, plasmablast differentiation, and cell viability were assessed 5 d later by flow cytometry. Effects of CC-220 are shown as percent of vehicle control, mean ± SEM. (C) Representative plasmablast differentiation and proliferation plots for naive B cells treated with CC-220. (D) Representative plasmablast differentiation and proliferation plots for memory B cells treated with CC-220. (E) Comparison of CC-220 effects on naive and memory B cell plasmablast differentiation induced by IL-2/IL-21/CD40L. Statistics were Student t test comparison of naive and memory B cells on plasmablast differentiation at the same concentration of CC-220 treatment. (F) Comparison of CC-220 effects on memory B cell plasmablast differentiation induced by IL-2/IL-21/CD40L or IL-2/IL-21/BAFF. Statistics were Student t test comparison of CC-220 effects on plasmablast differentiation at each concentration between IL-2/IL-21/CD40L and IL-2/IL-21/BAFF treatment. (E and F) n = 6. *p < 0.05, **p < 0.005.
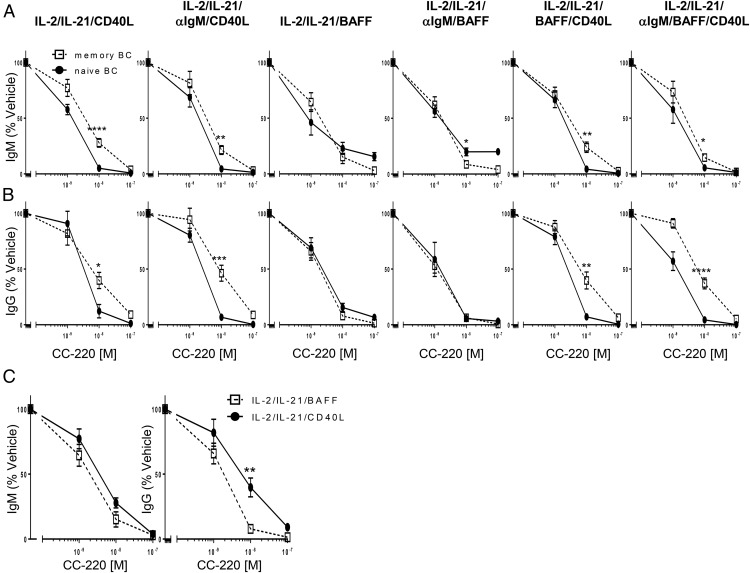
We further investigated the effects of CC-220 on the secretion of IgM and IgG in naive and memory B cells under the same BAFF, CD40L, and anti-IgM stimulation conditions used in the proliferation and plasmablast differentiation cultures. Naive and memory B cells were treated with vehicle or CC-220, stimulated and cultured for 5 d as described earlier, and IgM and IgG in the culture supernatant were measured by ELISA. CC-220, as observed for inhibition of proliferation and plasmablast differentiation (Fig. 8), inhibited both IgM and IgG secretion under each stimulus condition in a dose-dependent manner (Fig. 9). Consistent with the prior differential effects of CC-220 on cell type and stimulus described for proliferation and differentiation, CC-220 inhibition of CD40L-induced Ab secretion was found to be more potent on naive B cells relative to memory B cells (Fig. 9A, 9B). Also similar to the differentiation effects of CC-220 (Fig. 8), IgM and IgG production from memory B cells stimulated with BAFF were more sensitive to the inhibitory effects of CC-220 relative to memory B cells stimulated with CD40L (Fig. 9C).
FIGURE 9.
Differential potency effects of CC-220 on IgM and IgG production from naive and memory B cells. Naive and memory B cells were cultured for 5 d under the indicated stimulation conditions in the absence and presence of CC-220 and assessed for IgM (A) and IgG (B) production. Graphs shown are plotted as the mean percent of vehicle control (naive or memory B cells in the absence of CC-220) ± SEM. (C) CC-220 potency comparison between memory B cells stimulated with either IL-2/IL-21/BAFF or IL-2/IL-21/CD40L and production of IgM (left) and IgG (right) after 5 d of culture. Statistics were Student t test comparison of naive versus memory B cell IgM or IgG production at the same concentration of CC-220 treatment. n = 8 for naive, n = 9 for memory B cells. *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
BAFF induces isotype class switch through BAFF-R and TACI interactions (35, 93). An early stage of this process is via a class switch recombination initiated by activation-induced cytidine deaminase, which converts cytosines in S regions and Ig variable DNA regions to uracils by deamination leading to dsDNA nicking for recombination events to occur (94, 95). CC-220 did not reduce activation-induced cytidine deaminase expression in activated B cells, indicating that inhibition of class-switched IgG secretion occurs after this early stage of differentiation (data not shown).
CC-220 inhibits plasmablast differentiation from DN B cells
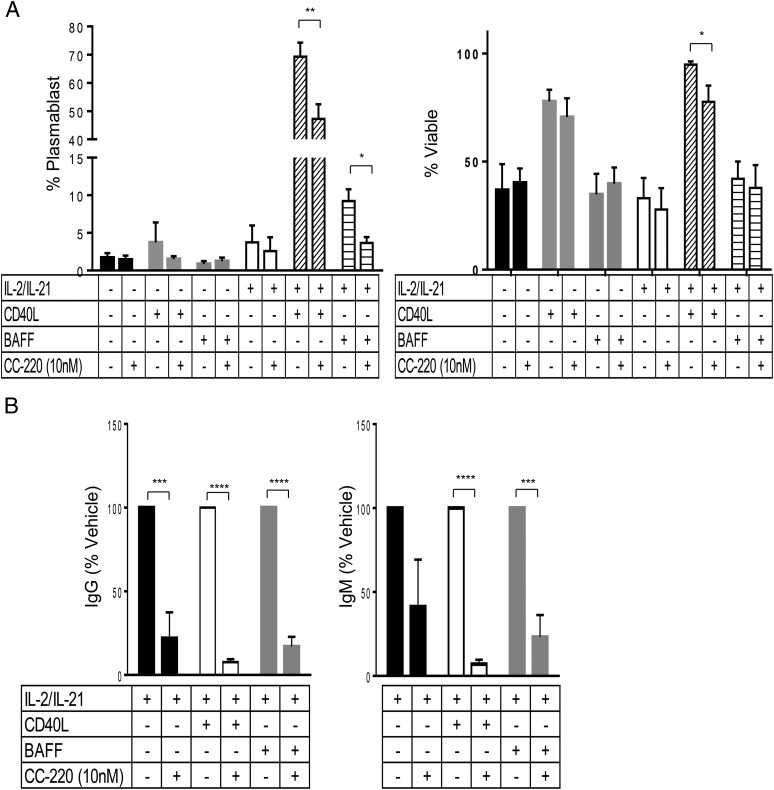
Our data demonstrate that DN B cells express memory-like surface protein markers, differentiate in response to BAFF or CD40L, and secrete IgG and IgM. The elevation of circulating DN B cells and their overexpression of Aiolos in SLE suggests that these cells can be a contributor to lupus disease pathology. We therefore tested CC-220 for the potential to modulate DN B cell differentiation and secretion of IgG and IgM after stimulation with BAFF or CD40L. Purified DN B cells were treated for 1 h with 10 nM CC-220 and incubated for 5 d in the BAFF or CD40L differentiation culture media followed by assessment of plasmablast and secreted Ab levels. As observed with the broader CD27− naive and CD27+ memory B cell populations, IL-2 and IL-21 were required cofactors for differentiation and Ab secretion (Fig. 10). Similar to CC-220 on the naive and memory B cells (Figs. 8, 9), CC-220 effectively reduced plasmablast differentiation (Fig. 10A) and Ab production (Fig. 10B) from DN B cells in both CD40L and BAFF stimulus conditions.
FIGURE 10.
CC-220 inhibits plasmablast differentiation and Ab production from DN B cells. DN B cells from HC PBMCs were sorted based on CD19+CD27− and IgD− expression, and treated with vehicle or CC-220 (10 nM) for 1 h at 37°C before adding the stimuli indicated and culturing for 5 d. (A) Data shown are the percent of plasmablasts and cell viability ± SEM relative to the total cells present after 5 d of treatment. (B) IgG (left) and IgM (right) production in the culture supernatant from DN cells were measured on day 5. Inhibition by CC-220 is shown as the percent of stimulated cells in the absence of CC-220 treatment ± SEM: IL-2/IL-21 only (black bars), IL-2/IL-21/CD40L (white bars), or IL-2/IL-21/BAFF (gray bars) (n = 5). Student t test: *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
CC-220 inhibits plasmablast differentiation and Ab production from SLE B cells
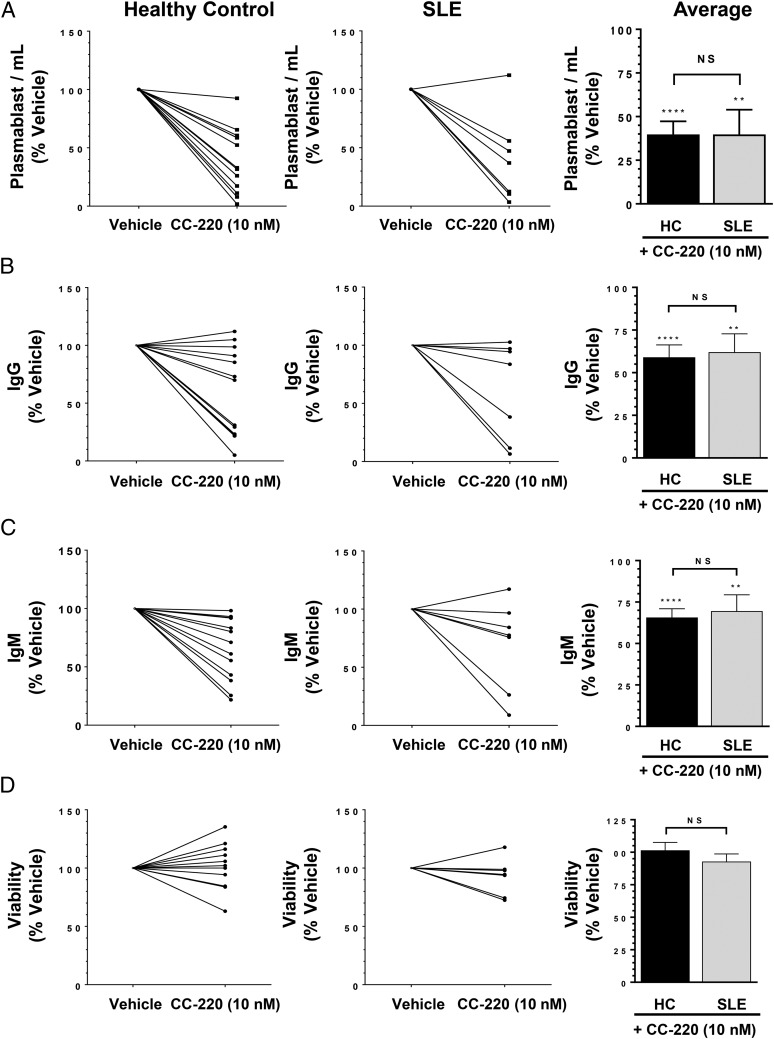
To test whether CC-220 has similar functional inhibitory effects on SLE B cells as was observed for HC B cells, we cultured total B cell cultures derived from HCs and SLE patients with 10 nM CC-220 for 1 h followed by IL-2/IL-21/BAFF treatment for 5 d, and tested for plasmablast differentiation and IgG and IgM production. Plasmablast differentiation in total B cell cultures was inhibited by 40% in both HC and SLE B cell cultures (Fig. 11A). IgG Ab production was inhibited by 41 and 38% in HC and SLE B cell cultures, respectively (Fig. 11B). IgM production was inhibited by 34 and 31% in HC and SLE B cell cultures, respectively (Fig. 11C), with no discernible effect on cell viability (Fig. 11D).
FIGURE 11.
CC-220 inhibits SLE plasmablast differentiation and Ab production from BAFF activated B cells. Total B cells isolated from HCs and SLE patients were treated with CC-220 (10 nM) for 1 h at 37°C before adding IL-2/IL-21/BAFF and culturing for 5 d. (A) Induced differentiated plasmablasts defined as percent CD20loCD38hi cells of total CD19+ B cells. IgG (B) and IgM (C) production after 5 d in culture. (D) Viability of cells posttreatment with CC-220 as a percent of the corresponding vehicle control (HC, SLE). Graphs shown are plotted as the mean percent of the corresponding vehicle control (HC or SLE B cells in the absence of CC-220) ± SEM. n = 13 HCs, n = 7 SLE patients. ** p < 0.005, ****p < 0.0001 for CC-220–treated cells versus their corresponding vehicle-treated cells (HC or SLE). NS, not significant comparing CC-220–treated HC versus CC-220–treated SLE cells.
Discussion
In B cell–mediated autoimmune diseases such as SLE, aberrant recognition of self-antigen and the secretion of autoantibodies can drive a cascade of immune responses leading to localized inflammation and pathogenic changes in affected tissues and organs. We show in this study that the soluble factors BAFF, IL-2, and IL-21 implicated in SLE pathogenesis (32, 37, 38, 76, 96–98) have the capacity to contribute to disease by inducing B cell proliferation, plasmablast differentiation, and Ab secretion from a select memory pool of B cells consisting of CD27+ memory and CD27−IgD− DN memory-like B cell subtypes. Naive B cells, in contrast, are incapable of responding to BAFF, IL-2, and IL-21 in the absence of CD40L, a signal most commonly provided by T cell contact in the germinal center within secondary lymphoid structures. The finding that BAFF induced plasmablast differentiation from a CD27−IgD− DN subpopulation represents a new functional response identified for these cells that exist in higher abundance in the blood of SLE patients. Prior studies have shown that MZA B cell subsets in the spleen, defined as postswitch IgG+ memory B cells, were IL-21 and BAFF responsive and could differentiate into plasmablasts (27). However, the CD27+ memory B cells in peripheral blood demonstrated in this study that we show are responsive to BAFF are phenotypically distinct from MZA B cells, excluding the possibility that they are recirculating MZA B cells (data not shown). The CRBN modulator CC-220 rapidly reduced Aiolos and Ikaros protein levels and inhibited both BAFF, IL-2, and IL-21–induced and CD40L, IL-2, and IL-21–induced proliferation, plasmablast differentiation, and Ab secretion from circulating naive, memory, and DN B cell subpopulations. Elevation of Aiolos expression levels in these circulating CD27+ memory and DN B cells of SLE patients suggests a possible predisposed condition of responsiveness to prodifferentiation stimuli such as BAFF and CD40L. The findings in this study provide further insight into the regulation of Ag-independent B cell differentiation mediated by soluble factors associated with inflammation. The reduction of Aiolos and Ikaros and subsequent inhibition of BAFF-induced B cell differentiation by CC-220 support the potential for it to be used in the therapeutic treatment of SLE, where elevations in BAFF and Aiolos expression may drive autoantibody-mediated disease pathology.
Alterations in circulating levels of BAFF, IL-2, and IL-21 have been reported in SLE, with differing results. BAFF elevation in the blood of SLE patients has been reported with consistency (41–45). IL-2 reports and its effects on immune function have varied. In SLE, T cells are reported to produce decreased amounts of IL-2 in active disease patients, leading to impaired regulatory T cell development (99). Although IL-2 plays an important role in the development and maintenance of regulatory T cells, SLE deficiency in quantity or quality of regulatory T cells has not been consistently reported (100). In other studies, a correlation between serum IL-2 levels and disease activity in SLE patients has been reported (32, 101). In this study, we observed no difference in circulating IL-2 when comparing SLE and HC samples (Supplemental Fig. 3). Differing reports on the levels of IL-21 in SLE patients also have been published; IL-21 levels in SLE patients are shown to be elevated in some studies (102, 103), but have no change in another study (104). We observed a small decrease in IL-21 in SLE and extensive overlap with HC levels (Supplemental Fig. 3). In mouse lupus models, elevated IL-21 expression drives pathologic autoantibody production (26, 105). The alterations in BAFF, IL-2, and IL-21 in SLE indicate a dysregulation of these factors. Our data in this study demonstrate the B cell differentiation–inducing potential of these factors on memory and DN B cells, which may have an impact in SLE where B cells are known to be in an activated state (106).
SLE patients have altered circulating levels of B cell populations, although discrepancies in the findings among investigators can be seen. In some reports, including this study, it has been shown that CD27+ memory phenotypes in circulation are decreased with a concomitant increase in the CD27− naive phenotype (4, 5). Conversely, other studies have shown increased CD27+ memory B cells and decreased naive B cells (3, 6, 10, 14). Further distinction of B cell subsets within CD27+ and CD27− cells may help to further define the relevance of these changes. For example, memory CD27+ B cells can be separated into IgD− isotype-switched and IgD+ NSM subsets, and CD27− B cells can be separated into IgD+ mature naive and IgD− SwM-like B cells (107). The importance of distinguishing the latter CD27−IgD− DN B cells that we and others have shown to be elevated in SLE patients (Fig. 5B) is underscored by their ability to differentiate into IgG Ab-secreting plasmablasts in response to BAFF (Fig. 4). This BAFF-induced plasmablast differentiation of DN B cells is of particular interest in SLE, where they have the potential to contribute to disease pathology. In support of this, DN B cells are associated with a higher disease activity index, history of nephritis, and higher level of disease-specific autoantibodies in SLE (8, 9, 16).
The CD27−IgD− DN subpopulation accounts for ∼5% of the peripheral B cell compartment in healthy individuals (108). The function of DN B cells, however, is poorly defined. DN B cells are elevated in the elderly and in inflammatory diseases including established RA, SLE, and Alzheimer’s disease, suggesting their contribution to pathology in disease and in immune changes associated with aging (8, 11, 109, 110). There are several hypotheses on the origin of DN B cells including that they are exhausted memory B cells that have downregulated CD27 (109), memory B cell precursors that have not acquired CD27 yet (111), or an entirely new B cell population that has undergone low levels of somatic hypermutation (111, 112). DN B cells show an activated phenotype where levels of these cells are associated with higher levels of circulating autoantibodies in SLE (8, 106) and have a proinflammatory phenotype in Alzheimer’s disease (110). Because of the phenotypic similarity to CD27+ memory B cells defined by cell surface markers such as CD95 shown in this study (Fig. 5) and by others, DN B cells are also described as CD27− memory B cells (8, 106, 109, 113). In this study, we showed that DN B cell differentiation into plasmablasts can occur when these cells are treated with either BAFF or by CD40L in the presence of IL-2 and IL-21. We further show that these cells have a memory phenotype because they have a differentiation response to BAFF, an effect not observed from naive B cells. To our knowledge, this is the first example that DN B cells can be assigned a functional differentiation and Ab secretion response to stimuli (BAFF) that can distinguish them from naive B cells.
We compared BAFF with two other commonly studied inducers of B cell differentiation, CD40L and anti-IgM BCR stimulation, and found significant functional response differences among these three B cell activation systems. CD40L induces proliferation in both naive and memory B cells without any costimulation requirements of IL-2 and IL-21, but does not induce plasmablast differentiation unless IL-21 is added (Fig. 1, Supplemental Fig. 1). As shown in this and other studies, CD40L costimulation with IL-21 induces robust plasmablast differentiation from both naive and memory B cells (19, 27, 114). Stimulation of naive B cells with BAFF or anti-IgM alone or each in the presence of IL-2 and IL-21, however, does not induce significant proliferation or differentiation. A major distinction between BAFF and anti-IgM on naive B cells is apparent with regard to cell death. Anti-IgM in the presence of IL-2 and IL-21 induces significant cell death in naive B cells (Supplemental Fig. 2), an effect not observed for BAFF treatment of naive B cells with IL-2 and IL-21. In contrast with naive B cells, memory B cells treated with anti-IgM, IL-2, and IL-21 do not undergo cell death. Levels of secreted IgG and IgM generally reflect the degree of plasmablast differentiation under each stimulus tested. In all conditions, CC-220 inhibited plasmablast differentiation and Ab production, with the efficacy dependent on the cell type or stimuli. In particular, CC-220 exhibited significantly more potent inhibitory effects on CD27− B cells compared with CD27+ memory B cells (Fig. 8C), which may be advantageous given that SLE patients have elevated autoreactive B cells in the CD27− B cell compartment (98, 115). In further support of SLE applications for CC-220, BAFF-induced plasmablast differentiation derived from CD27+ memory B cells is more sensitive to the inhibitory effects of CC-220 compared with CD40L-stimulated cells (Fig. 8D).
Little is known about the potential for B cell differentiation to occur in peripheral tissue locations independent of T cell contact. In CD40L T cell–dependent B cell responses in lymphoid follicles, Ag-activated cells differentiate into extrafollicular short-lived plasmablasts and plasma cells, germinal center–independent memory B cells, or germinal center–dependent memory B cells and plasmablast and plasma cells. We raise the possibility that when circulating memory and DN B cells encounter soluble inflammatory factors at sites of local inflammation, they may also differentiate into Ab-secreting cells in the absence of T cell contact by novel mechanisms not involving BCR or TLR activation. First, ectopic clusters contain both follicular Th cells that produce IL-21 and B cells that are present in the inflamed kidneys of patients with SLE (116), providing local increases in IL-21 available for BAFF-mediated T cell–independent B cells responses, as well for T cell–dependent responses. Second, BAFF is expressed locally in the inflamed tissue, as has been reported in the kidney in lupus nephritis (117). Lastly, plasma cell accumulation has been shown to accumulate and enhance the local concentrations of Ab and immunocomplex formation in inflamed kidneys in a mouse lupus model, demonstrating that local inflamed tissue environments can support plasmablast and plasma cell survival and disease-inducing Ab production (17). Taken together, the inflamed kidney environment in SLE contains the components required to induce local plasmablast differentiation and Ab secretion by T cell–independent mechanisms mediated by BAFF.
CC-220 is structurally related to lenalidomide and pomalidomide that show antiproliferative and immunomodulatory effects on B and T cells (118, 119). Similar to lenalidomide and pomalidomide, which bind to CRBN, a critical component of a ubiquitin E3 CRL4CRBN complex (120, 121), CC-220 binds to CRBN to promote recruitment of Ikaros and Aiolos to CRL4CRBN and the subsequent enhancement of the ubiquitination and degradation of these transcription factors (55–57). Ikaros and Aiolos are members of the Ikaros family of zinc-finger transcription factors crucial for the function of mature B cells in the periphery, and required for the generation and survival of high-affinity bone marrow plasma cells (60–62). We have shown in this study that CC-220 inhibits plasmablast differentiation, and Ab production is associated with reduction of Aiolos, Ikaros, and other transcription factors known to be required for plasmablast differentiation (Figs. 6, 7). The effect of CC-220 is independent of IL-21 signaling because IL-21R signaling is intact after CC-220 treatment (Fig. 6B). The effect of CC-220 is also independent of adenosine deaminase expression involved in IgG class-switch activity because mRNA upregulation in response to B cell stimulus still occurs in the presence of CC-220 (data not shown). The rapid reduction of Aiolos and Ikaros protein levels by CC-220 and the associated prevention of increased expression in the transcription factors IRF-4, XBP-1, and Blimp-1 required for plasmablast development suggest that CC-220 modulation of CRBN activity exerts its inhibitory effects at an early stage of B cell plasmablast differentiation.
SLE immunopathology is closely linked to alterations in B cell function and may be a common feature of this otherwise heterogeneous disease. In support of this concept, clinical benefit of direct B cell depletion (122) and indirect B cell modulation by Ab blockade of BAFF has been reported (123). Other factors such as IFN-α may also have a link to B cell–driven SLE pathology. Evidence of an elevation of IFN-α in some SLE patients (124) and an IFN gene signature in circulating cells in SLE patients (125) has led to the testing of IFN-modifying approaches in treating SLE (126). IFN-α, a product of activated plasmacytoid cells, is thought to exert its SLE effects in part by its ability to potentiate B cell differentiation and Ab secretion in vitro and in vivo to worsen B cell–mediated pathology in SLE mouse models (127). Further support of the role of B cells in SLE come from genetic studies where susceptibility loci in SLE have been described that are involved with B cell–related functions including MHC haplotype Ag presentation and transcription factors involved in B cell Ab production (128–130). Of particular importance is the genetic association of Aiolos (64) and Ikaros (65), which are essential factors in B cell differentiation and Ab secretion, and in the case of Aiolos, found to be overexpressed in the B cells of SLE patients (Fig. 5). Our data demonstrating increased Aiolos expression levels per cell in SLE B cells add to a prior report where it was shown that the percentage of B cells that express detectable Aiolos are similar in HCs and SLE, whereas the percentage of CD8 T cells and monocytes that express Aiolos are elevated (131). Taken together, the data support the hypothesis that regulating B cell differentiation and Ab production induced by factors that stimulate these processes in SLE may be key to treating lupus pathology.
In summary, the results from this study demonstrate a new function for BAFF in the induction of proliferation and differentiation of memory B cells into Ab-secreting plasmablasts by a T cell contact–independent mechanism. In addition, circulating CD27−IgD− DN B cells that are elevated in SLE also differentiate in response to BAFF in a T cell contact–independent manner, which further defines these DN B cells as memory-like B cells that can be induced to secrete IgG. Increased levels of BAFF and elevated expression of Aiolos in B cells of SLE patients may predispose B cells toward differentiation and Ab secretion. The multiple mechanisms by which B cells may become activated and secrete potentially pathogenic Abs in SLE support the therapeutic use of the CRBN-modulating drug CC-220 to regulate core mechanisms involved in B cell differentiation and Ig production.
Supplementary Material
The online version of this article contains supplemental material.
- BAFF-R
- BAFF receptor
- BCMA
- B cell maturation Ag
- Blimp1
- B lymphocyte–induced maturation protein 1
- CRL4CRBN
- cullin ring ligase 4-cereblon
- DN
- double-negative
- HC
- healthy control donor
- IgJ
- Ig joining chain
- IRF4
- IFN regulatory factor 4
- MZA
- marginal zone analog
- NSM
- nonswitched memory
- RA
- rheumatoid arthritis
- SLE
- systemic lupus erythematosus
- SwM
- switched memory
- TACI
- transmembrane activator and Ca2+ modulator and cyclophilin ligand interactor
- XBP1
- X-box-binding protein 1.
Disclosures
All authors are employees and have stock or equity interests in Celgene Corporation.
References
- 1.Zubler R. H. 2001. Naive and memory B cells in T-cell-dependent and T-independent responses. Springer Semin. Immunopathol. 23: 405–419. [DOI] [PubMed] [Google Scholar]
- 2.Souto-Carneiro M. M., Mahadevan V., Takada K., Fritsch-Stork R., Nanki T., Brown M., Fleisher T. A., Wilson M., Goldbach-Mansky R., Lipsky P. E. 2009. Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumour necrosis factor. Arthritis Res. Ther. 11: R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arce E., Jackson D. G., Gill M. A., Bennett L. B., Banchereau J., Pascual V. 2001. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J. Immunol. 167: 2361–2369. [DOI] [PubMed] [Google Scholar]
- 4.Chang N. H., McKenzie T., Bonventi G., Landolt-Marticorena C., Fortin P. R., Gladman D., Urowitz M., Wither J. E. 2008. Expanded population of activated antigen-engaged cells within the naive B cell compartment of patients with systemic lupus erythematosus. J. Immunol. 180: 1276–1284. [DOI] [PubMed] [Google Scholar]
- 5.Huang W., Sinha J., Newman J., Reddy B., Budhai L., Furie R., Vaishnaw A., Davidson A. 2002. The effect of anti-CD40 ligand antibody on B cells in human systemic lupus erythematosus. Arthritis Rheum. 46: 1554–1562. [DOI] [PubMed] [Google Scholar]
- 6.Odendahl M., Jacobi A., Hansen A., Feist E., Hiepe F., Burmester G. R., Lipsky P. E., Radbruch A., Dörner T. 2000. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 165: 5970–5979. [DOI] [PubMed] [Google Scholar]
- 7.Hansen A., Lipsky P. E., Dörner T. 2007. B cells in Sjögren’s syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res. Ther. 9: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei C., Anolik J., Cappione A., Zheng B., Pugh-Bernard A., Brooks J., Lee E. H., Milner E. C., Sanz I. 2007. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol. 178: 6624–6633. [DOI] [PubMed] [Google Scholar]
- 9.Le Coz C., Joublin A., Pasquali J. L., Korganow A. S., Dumortier H., Monneaux F. 2013. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One 8: e75319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anolik J. H., Barnard J., Cappione A., Pugh-Bernard A. E., Felgar R. E., Looney R. J., Sanz I. 2004. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 50: 3580–3590. [DOI] [PubMed] [Google Scholar]
- 11.Fedele A. L., Tolusso B., Gremese E., Bosello S. L., Carbonella A., Canestri S., Ferraccioli G. 2014. Memory B cell subsets and plasmablasts are lower in early than in long-standing rheumatoid arthritis. BMC Immunol. 15: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmood Z., Muhammad K., Schmalzing M., Roll P., Dörner T., Tony H. P. 2015. CD27-IgD- memory B cells are modulated by in vivo interleukin-6 receptor (IL-6R) blockade in rheumatoid arthritis. Arthritis Res. Ther. 17: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romme Christensen J., Börnsen L., Ratzer R., Piehl F., Khademi M., Olsson T., Sørensen P. S., Sellebjerg F. 2013. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One 8: e57820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobi A. M., Odendahl M., Reiter K., Bruns A., Burmester G. R., Radbruch A., Valet G., Lipsky P. E., Dörner T. 2003. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 48: 1332–1342. [DOI] [PubMed] [Google Scholar]
- 15.Vital E. M., Dass S., Buch M. H., Henshaw K., Pease C. T., Martin M. F., Ponchel F., Rawstron A. C., Emery P. 2011. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 63: 3038–3047. [DOI] [PubMed] [Google Scholar]
- 16.Lazarus M. N., Turner-Stokes T., Chavele K. M., Isenberg D. A., Ehrenstein M. R. 2012. B-cell numbers and phenotype at clinical relapse following rituximab therapy differ in SLE patients according to anti-dsDNA antibody levels. Rheumatology 51: 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassese G., Lindenau S., de Boer B., Arce S., Hauser A., Riemekasten G., Berek C., Hiepe F., Krenn V., Radbruch A., Manz R. A. 2001. Inflamed kidneys of NZB / W mice are a major site for the homeostasis of plasma cells. Eur. J. Immunol. 31: 2726–2732. [DOI] [PubMed] [Google Scholar]
- 18.Hutloff A., Büchner K., Reiter K., Baelde H. J., Odendahl M., Jacobi A., Dörner T., Kroczek R. A. 2004. Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis Rheum. 50: 3211–3220. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger R., Sims G. P., Fairhurst A. M., Robbins R., da Silva Y. S., Spolski R., Leonard W. J., Lipsky P. E. 2005. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 175: 7867–7879. [DOI] [PubMed] [Google Scholar]
- 20.Recher M., Berglund L. J., Avery D. T., Cowan M. J., Gennery A. R., Smart J., Peake J., Wong M., Pai S. Y., Baxi S., et al. 2011. IL-21 is the primary common γ chain-binding cytokine required for human B-cell differentiation in vivo. Blood 118: 6824–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chtanova T., Tangye S. G., Newton R., Frank N., Hodge M. R., Rolph M. S., Mackay C. R. 2004. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173: 68–78. [DOI] [PubMed] [Google Scholar]
- 22.Parrish-Novak J., Dillon S. R., Nelson A., Hammond A., Sprecher C., Gross J. A., Johnston J., Madden K., Xu W., West J., et al. 2000. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408: 57–63. [DOI] [PubMed] [Google Scholar]
- 23.Jin H., Carrio R., Yu A., Malek T. R. 2004. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J. Immunol. 173: 657–665. [DOI] [PubMed] [Google Scholar]
- 24.Mehta D. S., Wurster A. L., Whitters M. J., Young D. A., Collins M., Grusby M. J. 2003. IL-21 induces the apoptosis of resting and activated primary B cells. J. Immunol. 170: 4111–4118. [DOI] [PubMed] [Google Scholar]
- 25.Leonard W. J., Spolski R. 2005. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat. Rev. Immunol. 5: 688–698. [DOI] [PubMed] [Google Scholar]
- 26.Ozaki K., Spolski R., Ettinger R., Kim H. P., Wang G., Qi C. F., Hwu P., Shaffer D. J., Akilesh S., Roopenian D. C., et al. 2004. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 173: 5361–5371. [DOI] [PubMed] [Google Scholar]
- 27.Ettinger R., Sims G. P., Robbins R., Withers D., Fischer R. T., Grammer A. C., Kuchen S., Lipsky P. E. 2007. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J. Immunol. 178: 2872–2882. [DOI] [PubMed] [Google Scholar]
- 28.Berglund L. J., Avery D. T., Ma C. S., Moens L., Deenick E. K., Bustamante J., Boisson-Dupuis S., Wong M., Adelstein S., Arkwright P. D., et al. 2013. IL-21 signalling via STAT3 primes human naive B cells to respond to IL-2 to enhance their differentiation into plasmablasts. Blood 122: 3940–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mingari M. C., Gerosa F., Carra G., Accolla R. S., Moretta A., Zubler R. H., Waldmann T. A., Moretta L. 1984. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature 312: 641–643. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman L. A., Tsokos G. C. 2010. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J. Biomed. Biotechnol. 2010: 740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bubier J. A., Sproule T. J., Foreman O., Spolski R., Shaffer D. J., Morse H. C., III, Leonard W. J., Roopenian D. C. 2009. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl. Acad. Sci. USA 106: 1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sedighi S., Aghaei M., Musavi S., Nomali M. 2014. Relationship between serum level of interleukin-2 in patients with systemic lupus erythematosus and disease activity in comparison with control group. J. Clin. Diagn. Res. 8: MC16–MC18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawalha A. H., Kaufman K. M., Kelly J. A., Adler A. J., Aberle T., Kilpatrick J., Wakeland E. K., Li Q. Z., Wandstrat A. E., Karp D. R., et al. 2008. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann. Rheum. Dis. 67: 458–461. [DOI] [PubMed] [Google Scholar]
- 34.Lavie F., Miceli-Richard C., Quillard J., Roux S., Leclerc P., Mariette X. 2004. Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjögren’s syndrome. J. Pathol. 202: 496–502. [DOI] [PubMed] [Google Scholar]
- 35.Litinskiy M. B., Nardelli B., Hilbert D. M., He B., Schaffer A., Casali P., Cerutti A. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3: 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nardelli B., Belvedere O., Roschke V., Moore P. A., Olsen H. S., Migone T. S., Sosnovtseva S., Carrell J. A., Feng P., Giri J. G., Hilbert D. M. 2001. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97: 198–204. [DOI] [PubMed] [Google Scholar]
- 37.Vincent F. B., Morand E. F., Schneider P., Mackay F. 2014. The BAFF/APRIL system in SLE pathogenesis. Nat. Rev. Rheumatol. 10: 365–373. [DOI] [PubMed] [Google Scholar]
- 38.Vincent F. B., Saulep-Easton D., Figgett W. A., Fairfax K. A., Mackay F. 2013. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 24: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bossen C., Tardivel A., Willen L., Fletcher C. A., Perroud M., Beermann F., Rolink A. G., Scott M. L., Mackay F., Schneider P. 2011. Mutation of the BAFF furin cleavage site impairs B-cell homeostasis and antibody responses. Eur. J. Immunol. 41: 787–797. [DOI] [PubMed] [Google Scholar]
- 40.Avery D. T., Kalled S. L., Ellyard J. I., Ambrose C., Bixler S. A., Thien M., Brink R., Mackay F., Hodgkin P. D., Tangye S. G. 2003. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Invest. 112: 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter L. M., Isenberg D. A., Ehrenstein M. R. 2013. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum. 65: 2672–2679. [DOI] [PubMed] [Google Scholar]
- 42.Cheema G. S., Roschke V., Hilbert D. M., Stohl W. 2001. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 44: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 43.Eilertsen G. O., Van Ghelue M., Strand H., Nossent J. C. 2011. Increased levels of BAFF in patients with systemic lupus erythematosus are associated with acute-phase reactants, independent of BAFF genetics: a case-control study. Rheumatology (Oxford) 50: 2197–2205. [DOI] [PubMed] [Google Scholar]
- 44.Petri M., Stohl W., Chatham W., McCune W. J., Chevrier M., Ryel J., Recta V., Zhong J., Freimuth W. 2008. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 58: 2453–2459. [DOI] [PubMed] [Google Scholar]
- 45.Roschke V., Sosnovtseva S., Ward C. D., Hong J. S., Smith R., Albert V., Stohl W., Baker K. P., Ullrich S., Nardelli B., et al. 2002. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J. Immunol. 169: 4314–4321. [DOI] [PubMed] [Google Scholar]
- 46.Gross J. A., Johnston J., Mudri S., Enselman R., Dillon S. R., Madden K., Xu W., Parrish-Novak J., Foster D., Lofton-Day C., et al. 2000. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 404: 995–999. [DOI] [PubMed] [Google Scholar]
- 47.Khare S. D., Sarosi I., Xia X. Z., McCabe S., Miner K., Solovyev I., Hawkins N., Kelley M., Chang D., Van G., et al. 2000. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc. Natl. Acad. Sci. USA 97: 3370–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackay F., Woodcock S. A., Lawton P., Ambrose C., Baetscher M., Schneider P., Tschopp J., Browning J. L. 1999. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190: 1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furie R., Petri M., Zamani O., Cervera R., Wallace D. J., Tegzová D., Sanchez-Guerrero J., Schwarting A., Merrill J. T., Chatham W. W., et al. ; BLISS-76 Study Group. 2011. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63: 3918–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furie R. A., Leon G., Thomas M., Petri M. A., Chu A. D., Hislop C., Martin R. S., Scheinberg M. A., PEARL-SC Study. 2015. A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study. Ann. Rheum. Dis. 74: 1667–1675. [DOI] [PubMed] [Google Scholar]
- 51.Manzi S., Sánchez-Guerrero J., Merrill J. T., Furie R., Gladman D., Navarra S. V., Ginzler E. M., D’Cruz D. P., Doria A., Cooper S., et al. ; BLISS-52 and BLISS-76 Study Groups. 2012. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann. Rheum. Dis. 71: 1833–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navarra S. V., Guzmán R. M., Gallacher A. E., Hall S., Levy R. A., Jimenez R. E., Li E. K., Thomas M., Kim H. Y., León M. G., et al. ; BLISS-52 Study Group. 2011. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377: 721–731. [DOI] [PubMed] [Google Scholar]
- 53.van Vollenhoven R. F., Petri M. A., Cervera R., Roth D. A., Ji B. N., Kleoudis C. S., Zhong Z. J., Freimuth W. 2012. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann. Rheum. Dis. 71: 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiglesworth A. K., Ennis K. M., Kockler D. R. 2010. Belimumab: a BLyS-specific inhibitor for systemic lupus erythematosus. Ann. Pharmacother. 44: 1955–1961. [DOI] [PubMed] [Google Scholar]
- 55.Gandhi A. K., Kang J., Havens C. G., Conklin T., Ning Y., Wu L., Ito T., Ando H., Waldman M. F., Thakurta A., et al. 2014. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br. J. Haematol. 164: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krönke J., Udeshi N. D., Narla A., Grauman P., Hurst S. N., McConkey M., Svinkina T., Heckl D., Comer E., Li X., et al. 2014. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343: 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu G., Middleton R. E., Sun H., Naniong M., Ott C. J., Mitsiades C. S., Wong K. K., Bradner J. E., Kaelin W. G., Jr. 2014. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 343: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chamberlain P. P., Lopez-Girona A., Miller K., Carmel G., Pagarigan B., Chie-Leon B., Rychak E., Corral L. G., Ren Y. J., Wang M., et al. 2014. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol. 21: 803–809. [DOI] [PubMed] [Google Scholar]
- 59.John L. B., Ward A. C. 2011. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol. Immunol. 48: 1272–1278. [DOI] [PubMed] [Google Scholar]
- 60.Cortés M., Georgopoulos K. 2004. Aiolos is required for the generation of high affinity bone marrow plasma cells responsible for long-term immunity. J. Exp. Med. 199: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmitt C., Tonnelle C., Dalloul A., Chabannon C., Debré P., Rebollo A. 2002. Aiolos and Ikaros: regulators of lymphocyte development, homeostasis and lymphoproliferation. Apoptosis 7: 277–284. [DOI] [PubMed] [Google Scholar]
- 62.Wang J. H., Avitahl N., Cariappa A., Friedrich C., Ikeda T., Renold A., Andrikopoulos K., Liang L., Pillai S., Morgan B. A., Georgopoulos K. 1998. Aiolos regulates B cell activation and maturation to effector state. Immunity 9: 543–553. [DOI] [PubMed] [Google Scholar]
- 63.Morgan B., Sun L., Avitahl N., Andrikopoulos K., Ikeda T., Gonzales E., Wu P., Neben S., Georgopoulos K. 1997. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 16: 2004–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai X., Qiao Y., Diao C., Xu X., Chen Y., Du S., Liu X., Liu N., Yu S., Chen D., Jiang Y. 2014. Association between polymorphisms of the IKZF3 gene and systemic lupus erythematosus in a Chinese Han population. PLoS One 9: e108661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cunninghame Graham D. S., Morris D. L., Bhangale T. R., Criswell L. A., Syvänen A. C., Rönnblom L., Behrens T. W., Graham R. R., Vyse T. J. 2011. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. 7: e1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dang J., Shan S., Li J., Zhao H., Xin Q., Liu Y., Bian X., Liu Q. 2014. Gene-gene interactions of IRF5, STAT4, IKZF1 and ETS1 in systemic lupus erythematosus. Tissue Antigens 83: 401–408. [DOI] [PubMed] [Google Scholar]
- 67.Han J. W., Zheng H. F., Cui Y., Sun L. D., Ye D. Q., Hu Z., Xu J. H., Cai Z. M., Huang W., Zhao G. P., et al. 2009. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat. Genet. 41: 1234–1237. [DOI] [PubMed] [Google Scholar]
- 68.Hu S. J., Wen L. L., Hu X., Yin X. Y., Cui Y., Yang S., Zhang X. J. 2013. IKZF1: a critical role in the pathogenesis of systemic lupus erythematosus? Mod. Rheumatol. 23: 205–209. [DOI] [PubMed] [Google Scholar]
- 69.Lessard C. J., Adrianto I., Ice J. A., Wiley G. B., Kelly J. A., Glenn S. B., Adler A. J., Li H., Rasmussen A., Williams A. H., et al. ; BIOLUPUS Network; GENLES Network. 2012. Identification of IRF8, TMEM39A, and IKZF3-ZPBP2 as susceptibility loci for systemic lupus erythematosus in a large-scale multiracial replication study. Am. J. Hum. Genet. 90: 648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang C., Ahlford A., Järvinen T. M., Nordmark G., Eloranta M. L., Gunnarsson I., Svenungsson E., Padyukov L., Sturfelt G., Jönsen A., et al. 2013. Genes identified in Asian SLE GWASs are also associated with SLE in Caucasian populations. Eur. J. Hum. Genet. 21: 994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.You Y., Zhai Z. F., Chen F. R., Chen W., Hao F. 2015. Autoimmune risk loci of IL12RB2, IKZF1, XKR6, TMEM39A and CSK in Chinese patients with systemic lupus erythematosus. Tissue Antigens 85: 200–203. [DOI] [PubMed] [Google Scholar]
- 72.Klein U., Rajewsky K., Küppers R. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188: 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leo R., Boeker M., Peest D., Hein R., Bartl R., Gessner J. E., Selbach J., Wacker G., Deicher H. 1992. Multiparameter analyses of normal and malignant human plasma cells: CD38++, CD56+, CD54+, cIg+ is the common phenotype of myeloma cells. Ann. Hematol. 64: 132–139. [DOI] [PubMed] [Google Scholar]
- 74.Terstappen L. W., Johnsen S., Segers-Nolten I. M., Loken M. R. 1990. Identification and characterization of plasma cells in normal human bone marrow by high-resolution flow cytometry. Blood 76: 1739–1747. [PubMed] [Google Scholar]
- 75.Tangye S. G., Avery D. T., Hodgkin P. D. 2003. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory B cells. J. Immunol. 170: 261–269. [DOI] [PubMed] [Google Scholar]
- 76.Ettinger R., Kuchen S., Lipsky P. E. 2008. The role of IL-21 in regulating B-cell function in health and disease. Immunol. Rev. 223: 60–86. [DOI] [PubMed] [Google Scholar]
- 77.Dörner T., Jacobi A. M., Lee J., Lipsky P. E. 2011. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J. Immunol. Methods 363: 187–197. [DOI] [PubMed] [Google Scholar]
- 78.Suryani S., Fulcher D. A., Santner-Nanan B., Nanan R., Wong M., Shaw P. J., Gibson J., Williams A., Tangye S. G. 2010. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood 115: 519–529. [DOI] [PubMed] [Google Scholar]
- 79.Chung J. B., Sater R. A., Fields M. L., Erikson J., Monroe J. G. 2002. CD23 defines two distinct subsets of immature B cells which differ in their responses to T cell help signals. Int. Immunol. 14: 157–166. [DOI] [PubMed] [Google Scholar]
- 80.Mandik-Nayak L., Seo S. J., Sokol C., Potts K. M., Bui A., Erikson J. 1999. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J. Exp. Med. 189: 1799–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marquart H. V., Svendsen A., Rasmussen J. M., Nielsen C. H., Junker P., Svehag S. E., Leslie R. G. 1995. Complement receptor expression and activation of the complement cascade on B lymphocytes from patients with systemic lupus erythematosus (SLE). Clin. Exp. Immunol. 101: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reap E. A., Piecyk M. L., Oliver A., Sobel E. S., Waldschmidt T., Cohen P. L., Eisenberg R. A. 1996. Phenotypic abnormalities of splenic and bone marrow B cells in lpr and gld mice. Clin. Immunol. Immunopathol. 78: 21–29. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi K., Kozono Y., Waldschmidt T. J., Berthiaume D., Quigg R. J., Baron A., Holers V. M. 1997. Mouse complement receptors type 1 (CR1;CD35) and type 2 (CR2;CD21): expression on normal B cell subpopulations and decreased levels during the development of autoimmunity in MRL/lpr mice. J. Immunol. 159: 1557–1569. [PubMed] [Google Scholar]
- 84.Wilson J. G., Ratnoff W. D., Schur P. H., Fearon D. T. 1986. Decreased expression of the C3b/C4b receptor (CR1) and the C3d receptor (CR2) on B lymphocytes and of CR1 on neutrophils of patients with systemic lupus erythematosus. Arthritis Rheum. 29: 739–747. [DOI] [PubMed] [Google Scholar]
- 85.Rodig S. J., Shahsafaei A., Li B., Dorfman D. M. 2005. The CD45 isoform B220 identifies select subsets of human B cells and B-cell lymphoproliferative disorders. Hum. Pathol. 36: 51–57. [DOI] [PubMed] [Google Scholar]
- 86.Rathmell J. C., Townsend S. E., Xu J. C., Flavell R. A., Goodnow C. C. 1996. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell 87: 319–329. [DOI] [PubMed] [Google Scholar]
- 87.Wang J., Taniuchi I., Maekawa Y., Howard M., Cooper M. D., Watanabe T. 1996. Expression and function of Fas antigen on activated murine B cells. Eur. J. Immunol. 26: 92–96. [DOI] [PubMed] [Google Scholar]
- 88.Avery D. T., Deenick E. K., Ma C. S., Suryani S., Simpson N., Chew G. Y., Chan T. D., Palendira U., Bustamante J., Boisson-Dupuis S., et al. 2010. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207: 155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deenick E. K., Avery D. T., Chan A., Berglund L. J., Ives M. L., Moens L., Stoddard J. L., Bustamante J., Boisson-Dupuis S., Tsumura M., et al. 2013. Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. J. Exp. Med. 210: 2739–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koshland M. E. 1985. The coming of age of the immunoglobulin J chain. Annu. Rev. Immunol. 3: 425–453. [DOI] [PubMed] [Google Scholar]
- 91.Matthias P., Rolink A. G. 2005. Transcriptional networks in developing and mature B cells. Nat. Rev. Immunol. 5: 497–508. [DOI] [PubMed] [Google Scholar]
- 92.Calame K. L. 2001. Plasma cells: finding new light at the end of B cell development. Nat. Immunol. 2: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 93.Castigli E., Wilson S. A., Scott S., Dedeoglu F., Xu S., Lam K. P., Bram R. J., Jabara H., Geha R. S. 2005. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 201: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102: 553–563. [DOI] [PubMed] [Google Scholar]
- 95.Xu Z., Pone E. J., Al-Qahtani A., Park S. R., Zan H., Casali P. 2007. Regulation of aicda expression and AID activity: relevance to somatic hypermutation and class switch DNA recombination. Crit. Rev. Immunol. 27: 367–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakou M., Papadimitraki E. D., Fanouriakis A., Bertsias G. K., Choulaki C., Goulidaki N., Sidiropoulos P., Boumpas D. T. 2013. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to the generation of plasma B cells. Clin. Exp. Rheumatol. 31: 172–179. [PubMed] [Google Scholar]
- 97.Stohl W., Metyas S., Tan S. M., Cheema G. S., Oamar B., Xu D., Roschke V., Wu Y., Baker K. P., Hilbert D. M. 2003. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 48: 3475–3486. [DOI] [PubMed] [Google Scholar]
- 98.Yurasov S., Wardemann H., Hammersen J., Tsuiji M., Meffre E., Pascual V., Nussenzweig M. C. 2005. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alcocer-Varela J., Alarcón-Segovia D. 1982. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. J. Clin. Invest. 69: 1388–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.La Cava A. 2008. T-regulatory cells in systemic lupus erythematosus. Lupus 17: 421–425. [DOI] [PubMed] [Google Scholar]
- 101.Brugos B., Vincze Z., Sipka S., Szegedi G., Zeher M. 2012. Serum and urinary cytokine levels of SLE patients. Pharmazie 67: 411–413. [PubMed] [Google Scholar]
- 102.Lan Y., Luo B., Wang J. L., Jiang Y. W., Wei Y. S. 2014. The association of interleukin-21 polymorphisms with interleukin-21 serum levels and risk of systemic lupus erythematosus. Gene 538: 94–98. [DOI] [PubMed] [Google Scholar]
- 103.Wang X. F., Yuan S. L., Jiang L., Zhang X. L., Li S. F., Guo Y., Wu C. L., Chen J. J. 2007. Changes of serum BAFF and IL-21 levels in patients with systemic lupus erythematosus and their clinical significance. Xibao Yu Fenzi Mianyixue Zazhi 23: 1041–1042. [PubMed] [Google Scholar]
- 104.Kang K. Y., Kim H. O., Kwok S. K., Ju J. H., Park K. S., Sun D. I., Jhun J. Y., Oh H. J., Park S. H., Kim H. Y. 2011. Impact of interleukin-21 in the pathogenesis of primary Sjögren’s syndrome: increased serum levels of interleukin-21 and its expression in the labial salivary glands. Arthritis Res. Ther. 13: R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herber D., Brown T. P., Liang S., Young D. A., Collins M., Dunussi-Joannopoulos K. 2007. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J. Immunol. 178: 3822–3830. [DOI] [PubMed] [Google Scholar]
- 106.Jacobi A. M., Reiter K., Mackay M., Aranow C., Hiepe F., Radbruch A., Hansen A., Burmester G. R., Diamond B., Lipsky P. E., Dörner T. 2008. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum. 58: 1762–1773. [DOI] [PubMed] [Google Scholar]
- 107.Kaminski D. A., Wei C., Qian Y., Rosenberg A. F., Sanz I. 2012. Advances in human B cell phenotypic profiling. Front. Immunol. 3: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanz I., Wei C., Lee F. E., Anolik J. 2008. Phenotypic and functional heterogeneity of human memory B cells. Semin. Immunol. 20: 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Colonna-Romano G., Bulati M., Aquino A., Pellicanò M., Vitello S., Lio D., Candore G., Caruso C. 2009. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech. Ageing Dev. 130: 681–690. [DOI] [PubMed] [Google Scholar]
- 110.Bulati M., Buffa S., Martorana A., Gervasi F., Camarda C., Azzarello D. M., Monastero R., Caruso C., Colonna-Romano G. 2015. Double negative (IgG+IgD-CD27-) B cells are increased in a cohort of moderate-severe Alzheimer’s disease patients and show a pro-inflammatory trafficking receptor phenotype. J. Alzheimers Dis. 44: 1241–1251. [DOI] [PubMed] [Google Scholar]
- 111.Fecteau J. F., Côté G., Néron S. 2006. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J. Immunol. 177: 3728–3736. [DOI] [PubMed] [Google Scholar]
- 112.Berkowska M. A., Driessen G. J., Bikos V., Grosserichter-Wagener C., Stamatopoulos K., Cerutti A., He B., Biermann K., Lange J. F., van der Burg M., et al. 2011. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood 118: 2150–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu Y. C., Kipling D., Dunn-Walters D. K. 2011. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front. Immunol. 2: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuchen S., Robbins R., Sims G. P., Sheng C., Phillips T. M., Lipsky P. E., Ettinger R. 2007. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J. Immunol. 179: 5886–5896. [DOI] [PubMed] [Google Scholar]
- 115.Meffre E., Wardemann H. 2008. B-cell tolerance checkpoints in health and autoimmunity. Curr. Opin. Immunol. 20: 632–638. [DOI] [PubMed] [Google Scholar]
- 116.Liarski V. M., Kaverina N., Chang A., Brandt D., Yanez D., Talasnik L., Carlesso G., Herbst R., Utset T. O., Labno C., et al. 2014. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci. Transl. Med. 6: 230ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun C. Y., Shen Y., Chen X. W., Yan Y. C., Wu F. X., Dai M., Li T., Yang C. D. 2013. The characteristics and significance of locally infiltrating B cells in lupus nephritis and their association with local BAFF expression. [Published erratum appears in 2016 Int. J. Rheumatol. 2016: 7513892.] Int. J. Rheumatol. 2013: 954292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Corral L. G., Haslett P. A., Muller G. W., Chen R., Wong L. M., Ocampo C. J., Patterson R. T., Stirling D. I., Kaplan G. 1999. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J. Immunol. 163: 380–386. [PubMed] [Google Scholar]
- 119.Zhu Y. X., Braggio E., Shi C. X., Bruins L. A., Schmidt J. E., Van Wier S., Chang X. B., Bjorklund C. C., Fonseca R., Bergsagel P. L., et al. 2011. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 118: 4771–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ito T., Ando H., Suzuki T., Ogura T., Hotta K., Imamura Y., Yamaguchi Y., Handa H. 2010. Identification of a primary target of thalidomide teratogenicity. Science 327: 1345–1350. [DOI] [PubMed] [Google Scholar]
- 121.Lopez-Girona A., Mendy D., Ito T., Miller K., Gandhi A. K., Kang J., Karasawa S., Carmel G., Jackson P., Abbasian M., et al. 2012. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26: 2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aguiar R., Araújo C., Martins-Coelho G., Isenberg D. 2017. Use of rituximab in systemic lupus erythematosus: a single center experience over 14 years. Arthritis Care Res. (Hoboken) 69: 257–262. [DOI] [PubMed] [Google Scholar]
- 123.Iaccarino L., Bettio S., Reggia R., Zen M., Frassi M., Andreoli L., Gatto M., Piantoni S., Nalotto L., Franceschini F., Larosa M., et al. 2017. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res. (Hoboken) 69: 115–123. [DOI] [PubMed] [Google Scholar]
- 124.Dall’era M. C., Cardarelli P. M., Preston B. T., Witte A., Davis J. C., Jr. 2005. Type I interferon correlates with serological and clinical manifestations of SLE. Ann. Rheum. Dis. 64: 1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bennett L., Palucka A. K., Arce E., Cantrell V., Borvak J., Banchereau J., Pascual V. 2003. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oon S., Wilson N. J., Wicks I. 2016. Targeted therapeutics in SLE: emerging strategies to modulate the interferon pathway. Clin. Transl. Immunology 5: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kiefer K., Oropallo M. A., Cancro M. P., Marshak-Rothstein A. 2012. Role of type I interferons in the activation of autoreactive B cells. Immunol. Cell Biol. 90: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ceccarelli F., Perricone C., Borgiani P., Ciccacci C., Rufini S., Cipriano E., Alessandri C., Spinelli F. R., Sili Scavalli A., Novelli G., et al. 2015. Genetic factors in systemic lupus erythematosus: contribution to disease phenotype. J. Immunol. Res. 2015: 745647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mohan C., Putterman C. 2015. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 11: 329–341. [DOI] [PubMed] [Google Scholar]
- 130.Chung S. A., Taylor K. E., Graham R. R., Nititham J., Lee A. T., Ortmann W. A., Jacob C. O., Alarcón-Riquelme M. E., Tsao B. P., Harley J. B., et al. ; SLEGEN. 2011. Differential genetic associations for systemic lupus erythematosus based on anti-dsDNA autoantibody production. PLoS Genet. 7: e1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cai X., Liu X., Du S., Xu X., Liu A., Ge X., Qiao Y., Jiang Y. 2016. Overexpression of Aiolos in peripheral blood mononuclear cell subsets from patients with systemic lupus erythematosus and rheumatoid arthritis. Biochem. Genet. 54: 73–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.