Abstract
Objective:
The study was prospectively designed as a single-arm, single-institution prospective trial of pre-operative concomitant hyperfractionated radiotherapy (HART) with co-administration of chemotherapy based on 5-fluorouracil (5FU) in patients with T2/N+ or T3/any N resectable mid-low primary rectal cancer. The aim of the study was to assess the safety and efficacy of accelerated HART with concurrent 5FU-based chemotherapy in patients with locally advanced rectal cancer.
Methods:
Patients with resectable locally advanced (≥T3 or N+) rectal cancer were eligible. The patients received total dose 42 Gy in 28 fractions of 1.5 Gy, two times daily, with at least 8 h of interval, with concurrent chemotherapy: 325 mg m−2 of 5FU (bolus) on Days 1–3 and Days 16–18 (except for cN0 patients for whom only one cycle on Days 1–3 was prescribed). The primary end point included tolerance, post-operative complication rate and pathological response rate. The secondary end points included locoregional relapse-free survival, metastasis-free survival and overall survival.
Results:
Out of 53 enrolled patients; 2 did not undergo surgery. Of the 51 patients evaluable for pathological response, there were 8 (15.6%), 20 (39.3%), 18 (35.3%) and 5 (9.8%) patients with tumour regression grade 0, 1, 2 and 3, respectively. Downstaging of the primary tumour and lymph nodes was observed in 22 (43%) and 25 (49%) patients, respectively. The primary tumour ypCR (ypT0) rate was 15% (8/51). The nodal ypCR rate for cN+ patients was 60% (21/35). The total ypCR (ypT0N0M0) rate was 11% (6/51). Toxicity included: Grade 3 diarrhoea (4/51, 7.8%), Grade 2 diarrhoea (22/51, 43.1%), Grade 2 leukopenia (7/51, 13.7%), Grade 2 neutropenia (6/51, 11.7%) and Grade 1 thrombocytopenia (3/51, 5.9%). No Grade 4 toxicity was reported. Nine patients (18%) presented with post-operative complications (during the 3 months after surgery). There were 6 locoregional relapses (11.8%) and distant metastasis occurred in 11 patients (21.6%). The 2-year cumulative locoregional relapse-free survival, metastasis-free survival and overall survival was 87%, 79% and 89%, respectively.
Conclusion:
The proposed pre-operative HART with co-administration of 5FU had acceptable toxicity profile and provided satisfactory rate of ypCR. This created rationale to initiate a Phase III randomized study that was registered under ClinicalTrials.gov Identifier: NCT01814969.
Advances in knowledge:
The results of this research show that responders to pre-operative radiochemotherapy have favourable outcome. Tumour regression grade as prognostic clinical feature holds the promise of better classifying patients at high risk of local and systemic recurrence and this issue may be an interesting objective for future research.
INTRODUCTION
The biological rationale1 and clinical experience in locally advanced rectal cancer suggest that accelerated pre-operative hyperfractionated radiotherapy (HART) may provide a comparable local control but favourable tolerance compared with pre-operative treatment given in higher fraction doses.2–4
Although the rate of locoregional recurrences after adequate pre-operative radiotherapy and surgery is relatively low, there is apparent need for further improvement in local control, particularly in high-risk patients. Also, in spite of implementation of new surgical and radiotherapy strategies, the rate of distant metastases remains high. Several controversies exist over efficacy of combining pre-operative radiotherapy with concurrent and post-operative chemotherapy.5,6 These controversies are addressed in a growing number of studies on combined chemotherapy and HART.7–13 Most of the authors of these studies conclude that such combination is worth further evaluation.
The purpose of the present study is to assess the tolerance and effectiveness of the proposed combination of chemotherapy and HART. The schedule, if feasible, will be further evaluated in institutional Phase III trial.
METHODS AND MATERIALS
Eligibility criteria
All patients had histologically confirmed mid-low rectal adenocarcinoma (within 12 cm from the anal verge), with no evidence of distant metastasis. Only the patients with resectable T2/N+ or T3/any N tumors were enrolled. Resectability and staging were assessed by a multidisciplinary team based on clinical examination and CT imaging/MRI. The age at diagnosis was between 18 and 75 years. Patients were also required to have a World Health Organization performance status of 0 or 1 with adequate liver, kidney and bone marrow functions. Patients who had a history of chemotherapy or pelvic radiation therapy were excluded. Patients with a history of other malignancy within 5 years were also excluded. Other exclusion criteria included acute obstructive symptoms, unresectable disease or any serious comorbidities deemed not suitable for chemoradiotherapy.
Patients
The study was approved by the local Maria Skłodowska-Curie Memorial Cancer Center and Institute of Oncology Bioethical Committee in June 2012. All patients had given informed consent before recruitment. Pre-treatment evaluation included a complete history of physical examination, pre-operative staging and laboratory test. Pre-operative staging included colonoscopy, chest radiography, abdominal ultrasound and pelvic computed (CT) scans. Endoscopic ultrasound and/or pelvic magnetic imaging (MRI) were used to determine the clinical T/N classification. The seventh edition of the TNM staging standard of American Joint Committee on Cancer was used.14 Completed laboratory test included blood counts, liver and kidney function tests, gastrointestinal tumour markers and urine analysis.
Treatment
The study was prospectively designed as a single-arm, single-institution prospective trial of pre-operative concomitant accelerated HART with co-administration of two cycles of chemotherapy based on 5-fluorouracil (5FU) in patients with T2; N1-N2 or T3/any N resectable mid-low primary rectal cancer.
Patients underwent CT simulation with 5-mm slices with full bladder. The scan extended from L3 vertebral body to below the perineum. A thermoplastic pelvic immobilization in the prone position was used to minimize setup variability. Patients positioning was performed daily using skin markers and kilovoltage digital reconstructed radiograph portal verification before each fraction. The gross target volumes (GTVs) and clinical target volumes (CTVs) were contoured on axial CT scan slices. The GTV was defined as primary tumour and involved lymph nodes. The CTV was defined as primary tumour, mesorectal region, pre-sacral region and internal iliac lymph nodes. The external iliac lymph nodes were considered part of the CTV when there was a major tumour extension into the internal and external anal sphincter. The radiation dose was prescribed to the planning target volume (PTV). The PTV of the GTV and CTV were created by adding a 5-mm margin.
The total dose to be delivered to PTV was 42 Gy in 28 fractions of 1.5 Gy, two times daily with at least 8 h of interval. A three-field (with individualizing shields) or intensity-modulated radiation therapy (sliding window technique) was used; all fields were treated during each fraction. High-energy photon beams of 20 MV were used. Two cycles of chemotherapy were given concurrently with radiation therapy according to the scheme: 325 mg m−2 of 5FU (bolus) on Days 1–3 and 16–18 (last 3 days of radiotherapy). In concern of possible overtreatment the original protocol was, however, amended before the first recruitments, such that for cN0 patients, only one cycle of 5FU on Days 1–3 was prescribed. Also, it was allowed to withhold 5FU on Days 16–18 for patients with grade III or IV acute side effects, if observed at the first course.
Surgery was performed 6 weeks after completion of chemoradiotherapy. The choice between abdominoperineal resection and anterior resection was left to the judgment of the surgeon as the function of primary tumour location and response to chemoradiotherapy assessed before and during surgery. For node-positive patients, adjuvant 5Fu-based chemotherapy was recommended after surgery to the total of six cycles.
Pathology
For the evaluation of chemoradiation effect in residual cancer, the tumour regression grading (TRG) was used. The TRG scale is based on microscopic evaluation of the presence residual tumour cells in relation to extension of fibrosis using the following four-point scale: TRG0 (pCR) denoted no cancer cells; TRG1 was diagnosed when a few cancer foci had been seen in <10% of a tumour mass; TRG2 denoted cancer cells seen in 10–50% of tumour mass; in order to diagnose TRG3, cancer cells had to be seen in >50% of tumour mass.15 The pathology evaluation included assessment of ypT category, ypN category, grade of tumour malignancy, status of resection margins and TRG scale.
Study end points
The main end points were acute and late toxicity (Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer criteria), post-operative complications (within 90 days of surgery) and pathological complete response according to the TRG scale. The secondary end points included cumulative locoregional relapse-free survival, metastasis-free survival (MFS) and overall survival (OS).
Statistical analysis
The rates of acute and late toxicities, as well as the rates of pathological response were calculated as simple proportions. Locoregional failure was defined as recurrence of cancer in the irradiated area. Local recurrence was defined as relapse within the original location of the tumour, nodal recurrence of cancer was defined as failure within the lymphatic system of the pelvis. Distant metastases were defined as a relapse outside the irradiated area. The OS was calculated from the date of study entry to the date of death from any cause or to the date of last follow-up. Progression-free survival was calculated from the date of study entry until disease progression (recurrence, metastasis or death from any cause). The Kaplan–Meier method was used to estimate the OS and progression-free survival . The log-rank test was used to test the significance level, with a value of p < 0.05 considered to be statistically significant.
RESULTS
Between April 2012 and January 2014, a total of 53 eligible patients received pre-operative HART combined with intravenous bolus of 5Fu according to the protocol study. One patient withdrew from the trial after completion of chemoradiotherapy, and one died before planned surgery (traffic accident). Figure 1 shows the study profile, and Table 1 lists the patients' characteristics. The mean follow-up time was 2.3 years and the median was 2.2 years.
Figure 1.
Study profile. HART–CT, concomitant hyperfractionated radiotherapy with co-administration of two cycles of chemotherapy based on 5-fluorouracil.
Table 1.
Characteristics of the patients
| Parameter | (n = 51) subgroup (%) | 2-years DFS (%) | RR | p-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 25 (49) | 78 | 1.06 | 0.91 |
| Female | 26 (51) | 75 | ||
| Age [median (range)] (years) | 65 (34–79) | |||
| <63 | 25 (49) | 78 | 1.01 | 0.85 |
| ≥63 | 26 (51) | 62 | ||
| Zubrod index | ||||
| 0 | 37 (72) | 88 | 1.9 | 0.06 |
| 1 | 14 (28) | 58 | ||
| c Tumour stage | ||||
| c T2 | 0 | – | – | – |
| c T3 | 51 (100) | 76 | ||
| c Nodal stage | ||||
| c N0 | 16 (32) | 80 | 1.17 | 0.68 |
| c N1 | 23 (45) | 77 | ||
| c N2 | 12 (23) | 73 | ||
| Number of cycles of 5Fu during radiotherapy | ||||
| 1 | 24 (45) | 81 | 1.56 | 0.46 |
| 2 | 27 (55) | 73 | ||
| yp Tumour stage | ||||
| ypT0 | 8 (15) | 100 | 5.15 | 0.001a |
| ypT1 | 3 (6) | 100 | ||
| ypT2 | 12 (24) | 91 | ||
| ypT3 | 28 (55) | 65 | ||
| yp Nodal stage | ||||
| yp N0 | 29 (57) | 89 | 2.77 | 0.007a |
| yp N1 | 14 (27) | 61 | ||
| yp N2 | 8 (16) | 40 | ||
| TRG scale | ||||
| 0 | 8 (15) | 100 | 2.63 | 0.004a |
| 1 | 20 (40) | 89 | ||
| 2 | 18 (35) | 55 | ||
| 3 | 5 (10) | 60 | ||
| Post-operative chemotherapy | ||||
| No | 31 (61) | 100 | 4.04 | 0.0008a |
| Yes | 20 (39) | 58 | ||
| CEA before treatment | ||||
| ≤5 ng ml−1 | 29 (57%) | 93 | 4.93 | 0.006a |
| >5 ng ml−1 | 17 (33%) | 49 | ||
| Missing | 5 (10%) | – | ||
5FU, 5-fluorouracil; CEA, carcinoembryonic antigen; DFS, disease-free survival; RR, Relative Risk; TRG, tumour regression grade.
p < 0.05.
Toxicity of chemoradiation
Medical history of 53 patients was available for toxicity evaluation. Severe acute grade 3 toxicity included diarrhoea (4 patients, 8%), grade 2 diarrhoea (22 patients, 41%), grade 2 leukopenia (7 patients, 13%), grade 2 neutropenia (6 patients, 11%), grade 1 thrombocytopenia (3 patients, 6%). There was no grade 4 toxicity reported. All 53 patients completed the planned radiotherapy treatment. Because of bank holidays, radiotherapy was interrupted in three patients for a median of 3 days (range 1–4). No interruptions resulted from toxicity.
The second course of chemotherapy was not given in 24 patients: in 16 patients with cN0 stage, in 4 patients due to grade 3 toxicity after the first course of 5FU and in 4 patients due to other factors (3 patients—refusal, 1 patient—stenocardial event).
Surgery and post-operative complication
Surgery was performed at the median interval of 48 days (range 20–81 days). Although the recommended interval of surgery–radiotherapy was 6 weeks, six patients (11.8%) underwent surgery earlier or later mainly owing to patients' or surgeon's preference. Abdominoperineal resection was carried out in 22 cases (43%), anterior resection in 27 cases (53%), and low anterior resection with permanent colostomy in 1 patient, and in 1 case during operation, the tumour was found to be unresectable while 1 patient withdrew consent for surgery. The sphincter preservation rate for the whole analyzed group of rectal cancer patients was 55%. All primary tumour resections had negative proximal, distal and circumferential resection margins (R0 resections).
No particular complications were encountered intraoperatively. Nine patients (18%) presented with post-operative complications (during 3 months after surgery). Three patients with post-operative wound infections, two cases of anastomotic fistulae (required surgical interventions), one rectovaginal and one rectoperineal fistulas managed surgically, one case of small bowel obstruction (treated conservatively) and one case of urinary infection. There was no post-operative death in the analyzed group of patients.
Pathological response
Pathological analysis was performed on 51 eligible patients. According to the TRG scale system for pathological downstaging, there were 8, 20, 18 and 5 patients with TRG 0, 1, 2 and 3, respectively. Comparison of pre- chemoradiation clinical (c) stages with post-operative (yp) stages is presented in Table 1. Downstaging of the primary tumour and lymph nodes was observed in 22 (43%) and 25 (49%) patients. The primary tumour ypCR (ypT0) rate was 15% (8/51). The nodal ypCR rate for cN+ patients was 60% (21/35). The total ypCR (ypT0N0M0) rate was 12% (6/51).
Adjuvant chemotherapy
20 patients of the analyzed group (39%) received adjuvant chemotherapy. Of the 22 patients with pathologically confirmed positive lymph node (ypN+) disease, 16 (73%) received adjuvant 5FU-based chemotherapy.
Progression-free survival and overall survival
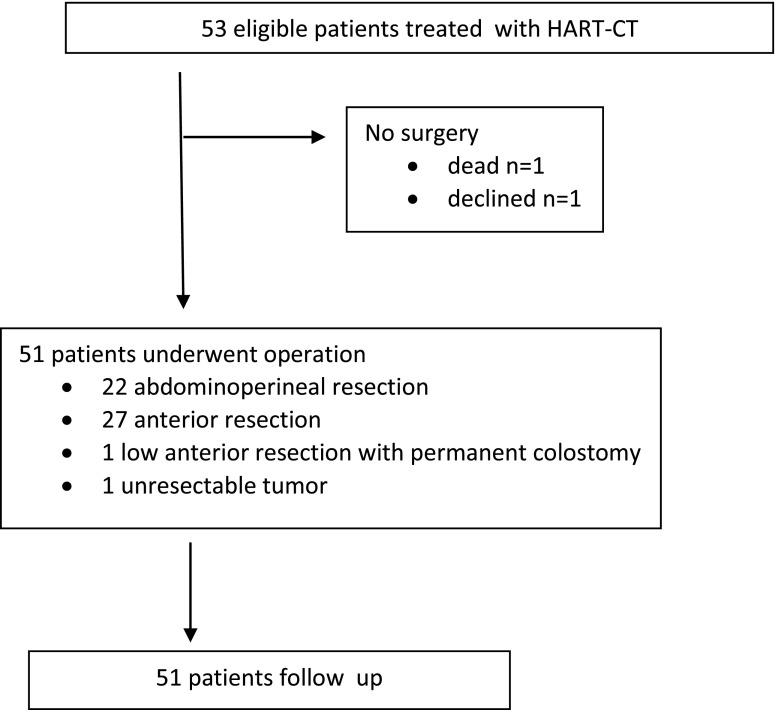
All 53 patients were included in the survival analysis on an intent-to-treat basis. 2 patients (1 died and 1 who completed radiochemaotherapy and refused the planned operation) were excluded from the study, leaving 51 patients (25 males and 26 females) eligible for further analysis. There were six locoregional relapse (12%). The 1- and 2-year cumulative locoregional relapse-free survival was 87%, respectively (Figure 2).
Figure 2.
Locoregional control (Kaplan–Meier).
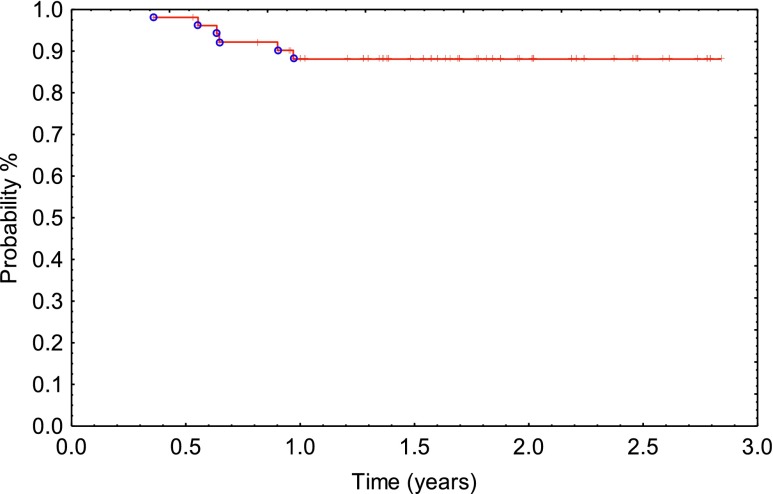
Distant metastasis occurred in 11 patients (21%). The liver was involved in 5 patients, the lung in 4, retroperitoneal lymph's nodes in 1 and brain in 1 patient. The 1- and 2-year cumulative MFS was 83% and 79%, respectively (Figure 3). The median time to systemic relapse was 1.63 years (0.18–2.84).
Figure 3.
Metastasis-free survival (Kaplan–Meier).
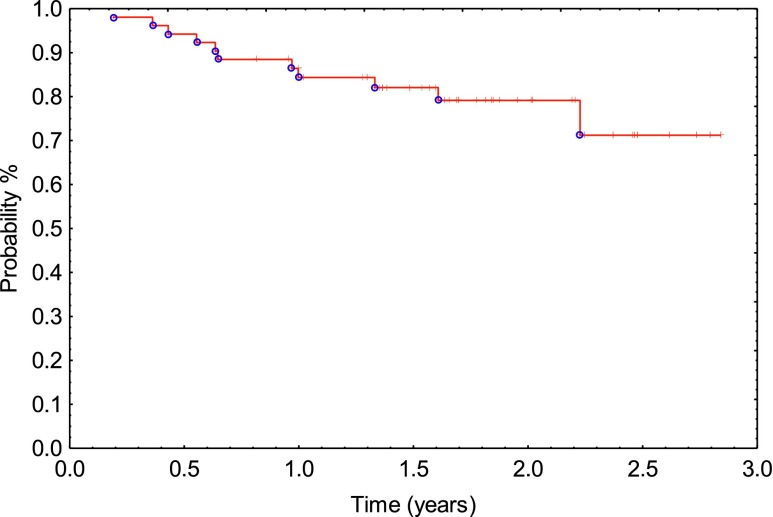
The 1- and 2-year actuarial survival rate for all patients was 96% and 89%, respectively (Figure 4).
Figure 4.
Overall survival (Kaplan–Meier).
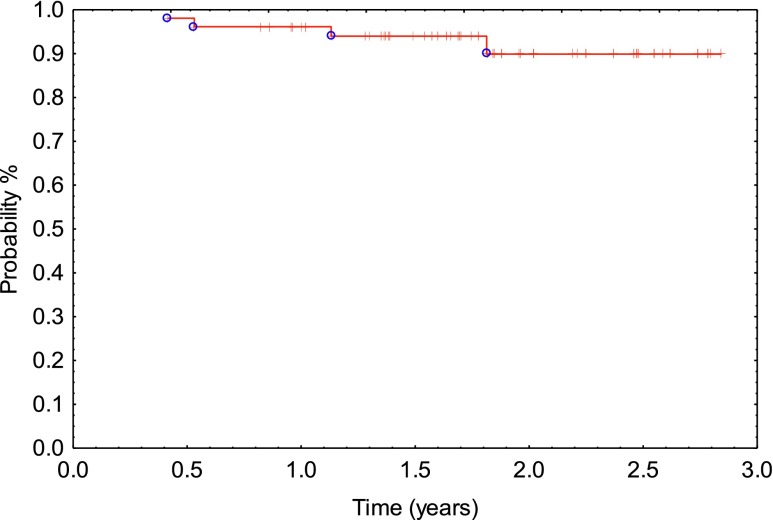
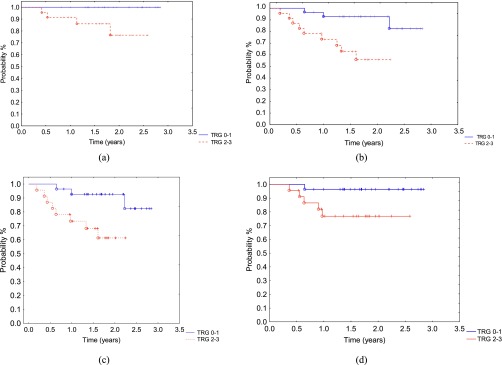
When TRG was combined into two groups (TRG 0–1 or TRG 2–3), there was a significant difference in outcomes. This included 2-year overall survival: TRG 0–1: 100% vs TRG 2–3: 76%; p = 0.019) (Figure 5a) and 2-year disease-free survival: TRG 0–1: 92% vs TRG 2–3: 55%; p = 0.007 (Figure 5b). Significant differences in 2-year MFS (TRG 0–1: 92% vs TRG 2–3 61%; p = 0.01 (Figure 5c) and locoregional tumour control (TRG 0–1: 96% vs TRG 2–3 76%; p = 0.04) were also found (Figure 5d).
Figure 5.
When tumour regression grade (TRG) was combined into two groups (TRG 0–1 or TRG 2–3), there was a significant difference in outcome: (a) 2-year overall survival: TRG 0–1: 100% vs TRG 2–3: 76%; p = 0.019; (b) 2-year disease-free survival: TRG 0–1: 92% vs TRG 2–3: 55%; p = 0.007; (c) 2-year metastasis-free survival: TRG 0–1: 92% vs TRG 2–3: 61%; p = 0.017; (d) 2-year locoregional control: TRG 0–1: 96% vs TRG 2–3: 76%; p = 0.04.
DISCUSSION
The results of the present study show that hyperfractionated chemoradiotherapy (42 Gy delivered in 1.5-Gy fractions over 18 days concurrently with 5Fu-based chemotherapy) is feasible and provides a satisfactory long-term outcome. The acute toxicity was acceptable, with radiotherapy compliance rate of 100% and occasional diarrhoea that did not necessitate a treatment brake. These data are similar with the toxicity profile associated with standard radiotherapy combined with chemotherapy.5,16–18
Tumour downstaging may be important for resectability of advanced disease and may affect the rate of sphincter-saving surgery. The rate of complete pathological response in the present series (15%) was comparable to that reported by Gerard et al18 (15–19%) using recent chemoradiotherapy protocols.19 According to the authors,20–22 the rate of sterilized operative specimen is close to 0% after short-course irradiation and immediate surgery. It is <10% with pre-operative radiotherapy alone and long interval before surgery5,19 and reaches 15–19% with recent chemoradiotherapy protocols.23,24 Several controversies, however, exist over translation of improved pathological responses into increase in the rate of sphincter-saving surgery, disease-free survival or overall survival.25 Clearly, now studies on this subject may allow to better address this issue.
The outcome of the present study suggests that, non-responders to pre-operative radiochemotherapy represent a distinct subgroup, with unfavourable long-term outcome. Both MFS and locoregional tumour control was significantly impaired in TRG 2–3, compared with TRG 0–1. It may suggest that non-responders should be treated differently, probably more aggressive, both with respect to local and systemic treatment. Recent studies suggest that addition of oxaliplatin to 5Fu chemotherapy may enhance distant control.26 Also, neoadjuvant chemotherapy is becoming a new field of clinical trials in this perspective.27 Identification of diagnostic modalities to predict histopathological response could be declared an interesting objective for the future.
Some questions may refer to local control reported in the present series, with 12% of local relapse being higher than in some other series.20,21 The largest Polish study on pre-operative radiotherapy for rectal cancer28 that compared short-course radiotherapy (5 fractions of 5 Gy delivered in 1 week) vs chemoradiation (25–30 fractions of 1.8 Gy) reported, however, local recurrence rate of 10.6% vs 15.6, it is similar to the present series. In our opinion, the rate of local relapses in the national series may reflect heterogeneity of patients within a given clinical stage, with predominance of more advanced cases in Polish population, compared with the data reported in Western Europe or USA. This may be related to impaired access to the modern diagnostic tools (particularly in the rural areas of Poland) and thus to delays in the diagnosis. Also, some differences in surgical procedures may contribute to this factor, in spite of national attempts to follow the best international standards. For the same reasons, sphincter preservation rate in the present series (55%) appears lower than in the best series from the literature.
From our point of view, the proposed schedule can reduce time of radiotherapy to 14 working days instead of 5–6 weeks for standard radiochemotherapy (25–30 fractions, once daily). On the other hand, prescription of bolus 5Fu given concurrently with radiotherapy may be considered equally effective to continuous infusion.29 From such perspective, the proposed treatment may appear attractive for patients living in a distance from radiotherapy facilities, because hotel stay of patients for treatment becomes relatively short. Very short hypofractionated radiotherapy alone is, perhaps, even more convenient, but at a price of possible increase in late toxicity and lack of concurrent systemic treatment. Clearly, however, concurrent chemotherapy and HART needs further studies before any broader implementation, considering, e.g. that only subgroups of patients may benefit from such therapy.
CONCLUSION
The outcome of the present pilot study indicates that the proposed treatment schedule is feasible and provides an acceptable outcome, although systemic treatment intensification in non-responders might be considered in future. A Phase III randomized study designed to formally compare the tolerance and effectiveness of pre-operative HART vs concurrent chemotherapy and HART was opened in May 2015 and registered under ClinicalTrials.gov Identifier: NCT01814969.
Contributor Information
Adam Idasiak, Email: aidasiak@op.pl.
Katarzyna Galwas-Kliber, Email: kasiagalwas@gmail.com.
Katarzyna Behrendt, Email: kbehrendt@io.gliwice.pl.
Iwona Wziętek, Email: iwonawzk@go2.pl.
Mariusz Kryj, Email: mhkryj@op.pl.
Ewa Stobiecka, Email: e.stobiecka@gmail.com.
Ewa Chmielik, Email: chmiel@mp.pl.
Rafał Suwiński, Email: rafals@io.gliwice.pl.
REFERENCES
- 1.Coucke P, Sartorelli B, Cuttat J, Jeanneret W, Gillet M, Mirimanoff RO, et al. The rationale to switch from postoperative hyperfractionated accelerated radiotherapy to preoperative hyperfractionated accelerated radiotherapy in rectal cancer. Int J Radiat Oncol Biol Phys 1995; 32: 181–8. doi: https://doi.org/10.1016/0360-3016(95)00549-e [DOI] [PubMed] [Google Scholar]
- 2.Coucke P, Notter M, Stamm B, Master M, Pasolini F, Schlumpf R, et al. Preoperative hyperfractionated accelerated radiotherapy (HART) in locally advanced rectal cancer (LARC) immediately followed by surgery. A prospective phase II trial. Radiother Oncol 2006; 79: 52–8. doi: https://doi.org/10.1016/j.radonc.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 3.Suwinski R, Wydmański J, Pwełczyk I, Starzewski J. A pilot study of accelerated preoperative hyperfractionated pelvic irradiation with or without low-dose preoperative prophylactic liver irradiation in patients with locally advanced rectal cancer. Radiother Oncol 2006; 80: 27–32. doi: https://doi.org/10.1016/j.radonc.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 4.Wzietek I, Kryj M, Idasiak A, Bialas M, Chmielarz A, Miszczyk L, et al. Randomized clinical trial on hyperfractionated versus hypofractionated preoperative radiotherapy for rectal cancer: long term outcomes including quality of life assessment. Int J Radiat Oncol Biol Phys 2014; 90: S21. doi: https://doi.org/10.1016/j.ijrobp.2014.05.114 [Google Scholar]
- 5.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 35: 1114–23. doi: https://doi.org/10.1056/NEJMoa060829 [DOI] [PubMed] [Google Scholar]
- 6.Bujko K, Glimelius B, Valentini V, Michalski W, Spalek M. Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: a meta-analysis of randomized trials comparing surgery ± a fluoropyrimidine and surgery + a fluoropyrimidine ± oxaliplatin. Eur J Surg Oncol 2015; 41: 713–23. doi: https://doi.org/10.1016/j.ejso.2015.03.233 [DOI] [PubMed] [Google Scholar]
- 7.Valentini V, Morganti AG, Gambacorta MA, Mohiuddin M, Doglietto GB, Coco C, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: a multicentric phase II study. Int J Radiat Oncol Biol Phys 2006; 64: 1129–39. doi: https://doi.org/10.1016/j.ijrobp.2005.09.017 [DOI] [PubMed] [Google Scholar]
- 8.Movsas B, Hanlon AL, Lanciano R, Scher RM, Weiner LM, Sigurdson ER, et al. Phase I dose escalating trial of hyperfractionated pre-operative chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 1998; 42: 43–50. [DOI] [PubMed] [Google Scholar]
- 9.Aboelnaga EM, Daoud MA, Eladl EI, Zaid AM. Induction FOLFOX followed by preoperative hyperfractionated radiotherapy plus bolus 5-fluorouracil in locally advanced rectal carcinoma: single arm phase I–II study. Med Oncol 2015; 32: 108. doi: https://doi.org/10.1007/s12032-015-0556-4 [DOI] [PubMed] [Google Scholar]
- 10.Doi H, Beppu N, Odawara S, Tanooka M, Takada Y, Niwa Y, et al. Neoadjuvant short-course hyperfractionated accelerated radiotherapy (SC-HART) combined with S-1 for locally advanced rectal cancer. J Radiat Res 2013; 54: 1118–24. doi: https://doi.org/10.1093/jrr/rrt058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsh RW, George TJ, Siddiqui T, Mendenhall WM, Zlotecki RA, Grobmyer S, et al. A phase II trial of neoadjuvant capecitabine combined with hyperfractionated accelerated radiation therapy in locally advanced rectal cancer. Am J Clin Oncol 2010; 33: 251–6. [DOI] [PubMed] [Google Scholar]
- 12.Voelter V, Zouhair A, Vuilleumier H, Matter M, Bouzourene H, Leyvraz S, et al. CPT-11 and concomitant hyperfractionated accelerated radiotherapy induce efficient local control in rectal cancer patients: results from a phase II. Br J Cancer 2006; 95: 710–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceelen W, Boterberg T, Pattyn P, van Eijkeren M, Gillardin JM, Demetter P, et al. Neoadjuvant chemoradiation versus hyperfractionated accelerated radiotherapy in locally advanced rectal cancer. Ann Surg Oncol 2007; 14: 424–31. [DOI] [PubMed] [Google Scholar]
- 14.Frederick L, Page DL, Fleming ID. Colon and rectum. In: AJCC cancer staging manual. 7th edn. [Accessed December 2009]. Available from: http://www.cancerstaging.net/ [Google Scholar]
- 15.Bujko K, Kołodziejczyk M, Nasierowska-Guttmejer A, Michalski W, Kepka L, Chmielik E, et al. Tumour regression grading in patients with residual rectal cancer after preoperative chemoradiation. Radiother Oncol 2010; 95: 298–302. [DOI] [PubMed] [Google Scholar]
- 16.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40. doi: https://doi.org/10.1056/nejmoa040694 [DOI] [PubMed] [Google Scholar]
- 17.Bosset JF, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results-EORTC 22921. J Clin Oncol 2005; 23: 5620–7. doi: https://doi.org/10.1200/jco.2005.02.113 [DOI] [PubMed] [Google Scholar]
- 18.Gerard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006; 24: 4620–5. doi: https://doi.org/10.1200/jco.2006.06.7629 [DOI] [PubMed] [Google Scholar]
- 19.Gerard JP, Rostom Y, Gal J, Benchimol D, Ortholan C, Aschele C, et al. Can we increase the chance of sphincter saving surgery in rectal cancer with neoadjuvant treatments: lessons from a systematic review of recent randomized trials. Crit Rev Oncol Hematol 2012; 81: 21–8. doi: https://doi.org/10.1016/j.critrevonc.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 20.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001; 345: 638–46. [DOI] [PubMed] [Google Scholar]
- 21.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009; 373: 811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cedermark B, Dahlberg M, Glimelius B; Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997; 336: 980–7. [DOI] [PubMed] [Google Scholar]
- 23.Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011; 10: 2773–80. doi: https://doi.org/10.1200/JCO.2010.34.4911 [DOI] [PubMed] [Google Scholar]
- 24.Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne P, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer. Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010; 28: 1638–44. [DOI] [PubMed] [Google Scholar]
- 25.Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2009; 1: CD006041. doi: https://doi.org/10.1002/14651858.CD006041.Review [DOI] [PubMed] [Google Scholar]
- 26.Cheeseman SL, Joel SP, Chester JD, Wilson G, Dent JT, Richards FJ, et al. A “modified de Gramont” regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer 2002; 87: 393–9. doi: https://doi.org/10.1038/sj.bjc.6600467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenhalgh TA, Dearman C, Sharma RA. Combination of novel agents with radiotherapy to treat rectal cancer. Clin Oncol (R Coll Radiol) 2016; 28: 116–39. doi: https://doi.org/10.1016/j.clon.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 28.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006; 93: 1215–23. doi: https://doi.org/10.1002/bjs.5506 [DOI] [PubMed] [Google Scholar]
- 29.Smalley SR, Benedetti JK, Williamson SK, Robertson JM, Estes NC, Maher T, et al. Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol 2006; 24: 3542–7. [DOI] [PubMed] [Google Scholar]