Abstract
Objective:
This study was to assess the usefulness of newer three-dimensional (3D)-T1 sampling perfection with application optimized contrast using different flip-angle evolutions (SPACE) and 3D-T2 fluid-attenuated inversion recovery (FLAIR) sequences in evaluation of meningeal abnormalities.
Methods:
78 patients who presented with high suspicion of meningeal abnormalities were evaluated using post-contrast 3D-T2-FLAIR, 3D-T1 magnetization-prepared rapid gradient-echo (MPRAGE) and 3D-T1-SPACE sequences. The images were evaluated independently by two radiologists for cortical gyral, sulcal space, basal cisterns and dural enhancement. The diagnoses were confirmed by further investigations including histopathology.
Results:
Post-contrast 3D-T1-SPACE and 3D-T2-FLAIR images yielded significantly more information than MPRAGE images (p < 0.05 for both SPACE and FLAIR images) in detection of meningeal abnormalities. SPACE images best demonstrated abnormalities in dural and sulcal spaces, whereas FLAIR was useful for basal cisterns enhancement. Both SPACE and FLAIR performed equally well in detection of gyral enhancement. In all 10 patients, where both SPACE and T2-FLAIR images failed to demonstrate any abnormality, further analysis was also negative.
Conclusion:
The 3D-T1-SPACE sequence best demonstrated abnormalities in dural and sulcal spaces, whereas FLAIR was useful for abnormalities in basal cisterns. Both SPACE and FLAIR performed holds good for detection of gyral enhancement. Post-contrast SPACE and FLAIR sequences are superior to the MPRAGE sequence for evaluation of meningeal abnormalities and when used in combination have the maximum sensitivity for leptomeningeal abnormalities. The negative-predictive value is nearly 100%, where no leptomeningeal abnormality was detected on these sequences.
Advances in knowledge:
Post-contrast 3D-T1-SPACE and 3D-T2-FLAIR images are more useful than 3D-T1-MPRAGE images in evaluation of meningeal abnormalities.
INTRODUCTION
Detecting meningeal abnormalities is vital because untreated malignant leptomeningeal abnormality decreases the survival rate time to less than 3 months.1–3 A delay of 4–6 h in the administration of antibiotics after presentation has also been shown to cause about 8.4-fold greater risk of death from meningitis.4
Even with the advent of technological advancements, there is still a considerable number of meningeal abnormalities and meningitis which could not be well demonstrated in MR images. The leptomeningeal and dural enhancements are due to contrast leakage from vessels into the cerebrospinal fluid (CSF) because of either increase in permeability of meningeal vessels or due to the breakdown of the blood–brain, blood–CSF or blood–nerve barriers.5–7
Despite newer sequences being developed, protocols for post-contrast imaging are largely restricted to pre- and post-contrast T1 sequences. In the past decade, three-dimensional (3D)-T1 magnetization-prepared rapid gradient-echo (MPRAGE) dominated the post-contrast T1 images.8,9 Several studies have shown that small lesions and leptomeningeal abnormalities with contrast enhancement may go undetected in routine post-contrast MPRAGE images. Recently, several studies have shown excellent results with post-contrast 3D-T1 sampling perfection with application optimized contrast using different flip-angle evolutions (SPACE).10,11 The post-contrast T2 sequence is not commonly used as a routine sequence to detect brain lesions.
But there are several recent studies which have shown that post-contrast T2-FLAIR images are just as useful and sometimes better than post-contrast T1 images. This is due to the fact that unlike T1 weighted images, post-contrast 3D-T2-FLAIR images do not usually demonstrate contrast-enhanced normal vessels.12–15 Since these normal sulcal vascular enhancement can be mistaken for leptomeningeal enhancement. Some studies have shown the value of post-contrast FLAIR in differentiating leptomeningeal abnormalities from normal leptomeningeal vascular enhancement which is not seen on FLAIR. It has been also proved that post-gadolinium effects were visible on FLAIR images at concentrations four times lower than that on T1 weighted images.5 To the best of our knowledge no study comparing post-contrast 3D-T2-FLAIR, T1-MPRAGE and T1-SPACE images for the evaluation of brain and leptomeningeal abnormalities has yet been published. 3D-T2-FLAIR sequences can be acquired in a clinically acceptable time with a non-selective inversion pulse by using the variable flip-angle technique in a 3D turbo spin-echo (SE) sequence.
Aim
Our study aims for comparison of post-contrast 3D-T1-MPRAGE, 3D-T1-SPACE and 3D-T2-FLAIR MR images in evaluation of meningeal abnormalities.
Objectives
(1) To evaluate the usefulness of post-contrast 3D-T2-FLAIR in evaluation of meningeal abnormalities and comparing the information obtained by 3D-T2-FLAIR with that of post-contrast 3D-T1-MPRAGE and 3D-T1-SPACE
(2) to evaluate the different meningeal enhancement patterns
(3) gyral enhancement
(4) sulcal space enhancement
(5) basal cisterns enhancement and
(6) dural enhancement.
and to find out which MRI sequence is best for demonstrating each abnormal enhancement patterns.
Review of literature
MRI is a revolutionary modality in detecting neurological disorders in modern medicine. However, not all lesions are conspicuous on CT and non-contrast MRI. The expertise of the radiologist lies in tailoring the MR sequences and detecting small lesions. Most of the brain lesions and meningeal abnormalities require contrast imaging for better evaluation, and post-gadolinium contrast-enhanced images have proved to be essential in the evaluation of primary brain tumours and metastases.16
The basis of imaging in leptomeningeal abnormality is post-contrast enhancement of the meninges. The leptomeningeal and dural enhancements are due to contrast leakage from vessels into the CSF. The contrast enhancement is due to the combination of two primary processes: vascular and interstitial (extravascular) enhancement.17–19 The leptomeningeal enhancement is due to contrast leakage from the vessels into the CSF due to an increase in permeability of the meningeal vessels due to breakdown of the blood–brain, blood–CSF or blood–nerve barrier. Although MPRAGE images were reported to be more helpful than contrast-enhanced two-dimensional (2D) SE images to detect brain metastases at 3 T;20,21 however, some small lesions with contrast enhancement have been missed in post-contrast MPRAGE images.21,22
Post-contrast SPACE sequence
In quest for developing better MRI sequence, Mugler et al23 developed the SPACE sequence. SPACE is a 3D fast SE sequence with variable flip-angle refocusing pulses available at 3 T. Post-contrast SPACE imaging was developed which scores over the post-contrast T1-MPRAGE in detection and evaluation of brain or meningeal metastasis as it suppresses normal blood vessel signals in the brain surface.
Komada et al10 compared the detectability of brain metastases on contrast-enhanced images among SPACE and MPRAGE and stated that SPACE might be useful for detecting small brain metastasis. They also reported that the contrast-to-noise ratio of brain metastasis on SPACE was significantly higher than that on MPRAGE at 3 T.
Kato et al11 in addition stated that post-contrast 3D-T1-SPACE was significantly better in detecting leptomeningeal metastasis than post-contrast 3D-T1-MPRAGE. They reported that the high detectability of SPACE is due to non-visualization of normal vessels, minimized partial volume effect and greater magnetization transfer (MT) effect. They concluded that conspicuity and delectability of brain metastases were better with contrast-enhanced SPACE than with contrast-enhanced MPRAGE.
Post-contrast FLAIR sequences
In the first ever study on post-contrast FLAIR sequences, Mathews et al12 showed that post-contrast T2-FLAIR images can be useful for detecting superficial abnormalities and meningeal disease. Contrast-enhanced FLAIR imaging is highly sensitive for detecting subarachnoid space disease.
However, it was found that CSF pulsation artefacts on 2D T2-FLAIR images may render the detection of lesions in the CSF spaces difficult,24,25 and although post-contrast 2D T2-FLAIR images were useful for assessing leptomeningeal abnormalities, concerns regarding its usefulness were raised.25,26
Consequently, 3D-T2-FLAIR imaging of high spatial resolution has been developed which has significant advantage in detecting small brain lesions and abnormal leptomeningeal enhancement by avoiding partial volume effect26,27 and by reducing CSF flow artefacts.28,29
3D-T2-FLAIR sequences can be acquired in a clinically acceptable time with a non-selective inversion pulse by using the variable flip-angle technique in a 3D turbo SE sequence.26–29
The amount of contrast administered also plays a vital role in demonstrating the abnormal enhancement. The intravenous injection of gadolinium at a dose of 0.1 mmol kg−1 can detect brain lesions effectively. The maximum concentration of gadolinium in the blood after 10 to 60 s on intravenous injection of gadolinium at 0.1 mmol kg−1 body weight is 2.0 ± 1.2 mmol l−1 for the aorta and 0.6 ± 0.3 mmol l−1 for the inferior vena cava.30 Therefore, administration of post-contrast at a dose of 0.1 mmol kg−1 is ideal.
The other advantage of 3D-T2-FLAIR sequence is that it also requires lower contrast dosage as it is highly sensitive to low gadolinium concentrations than T1 weighted or MPRAGE sequences. This phenomenon is due to the increase in T2 effect caused by the sequence.31 But since our study mainly concentrates for comparison with the other two sequences for qualitative demonstration of abnormal enhancement, we administered the ideal dosage, i.e. 0.1 mmol kg−1, for all cases. A comparison of various studies in evaluation of meningeal abnormalities has been depicted in Table 1.
Table 1.
Comparison of various studies in evaluation of meningeal abnormalities
| Studies | Post-contrast T1-MPRAGE | Post-contrast T2-FLAIR | Post-contrast T1-SPACE |
|---|---|---|---|
| Splendiani et al15 2005 | T1-MPRA had low sensitivity (50%) in detecting infectious meningitis | Had 100% sensitivity in detecting meningitis | – |
| Allesandra et al 2006 | Less sensitive in detecting meningitis | More sensitive | – |
| Ercan et al32 2004 | Post-contrast T1W imaging is essential | Post-contrast FLAIR is a valuable adjunct to post-contrast T1W for diagnosing meningitis | – |
| Vaswani et al33 2015 | Less sensitive | More sensitive in detecting meningitis | – |
| Fukuoka et al34 2010 | Less sensitive | Provide more information than T1-MPRAGE images in leptomeningeal enhancement | – |
| Kato et al11 2009 | Less conspicuous | – | More conspicuous and high detectability in brain metastases |
| Komada et al10 2008 | Low CNR | – | High CNR and superior contrast rate than T1-MPRAGE |
CNR, contrast-to-noise ratio; FLAIR, 3D-T2 fluid-attenuated inversion recovery; MPRAGE, 3D-T1 magnetization-prepared rapid gradient-echo; SPACE, sampling perfection with application optimized contrast using different flip-angle evolutions; T1W, T1 weighted imaging.
Thus our study aims to compare the usefulness of post-contrast 3D-T2-FLAIR with post-contrast 3D-T1-SPACE and post-contrast 3D-T1-MPRAGE.
METHODS AND MATERIALS
Between the period of April 2014 to March 2016, 78 patients with high clinical suspicion of meningitis and meningeal abnormalities were evaluated in the study.
Study site
The study was carried out in Kovai medical centre and Hospital, in India. The study was approved by the institutional review board of our hospital and local ethical committee. Prior written informed consent was obtained from the patients who had to undergo MRI. The consent form was in compliance with the institutional review board at our hospital.
Study design
The study was a prospective clinical study designed to compare the effectiveness of post-contrast 3D-T2-FLAIR over post-contrast T1-MPRAGE and T1-SPACE in detecting and evaluating the meningeal abnormalities. 48 patients (26 males and 22 females) fulfilled the inclusion criteria and underwent pre- and post-contrast 3D-T2-FLAIR, post-contrast T1-MPRAGE and T1-SPACE imaging. All lesions detected by post-contrast 3D sequences were compared, and any additional information that was gained by 3D-T2-FLAIR imaging was documented.
Inclusion criteria
All patients with
(1) high clinical suspicion for meningeal lesions
(2) abnormal CSF findings within 10 days before MRI of the brain
(3) initial 2D FLAIR images showing suspicious meningeal abnormalities.
Exclusion criteria
(1) Patients who were unable to provide consent;
(2) Patients who were unable to undergo MRI because of a pacemaker, an aneurysm clip or a metallic foreign body in or near the eye;
(3) Patients who were already treated with surgery/chemotherapy/radiotherapy.
Imaging techniques
3D-T2-FLAIR with a section thickness of 0.9 mm and isotropic information was initially performed in all patients, followed by intravenous contrast gadopentetate dimeglumine (Magnevist®; Bayer-Schering, Berlin, Germany) administered with a dose of 0.1 mmol kg−1.20
MR studies were started about 60–120 s after the contrast material injection. The post-contrast 3D-T2-FLAIR, 3D-T1-MPRAGE and 3D-T1-SPACE images were then acquired (Figures 1–7).
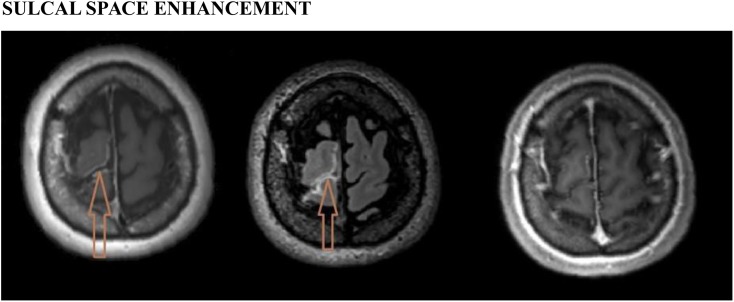
Figure 1.
Sulcal space enhancement: left, post-contrast three-dimensional (3D)-T1-sampling perfection with application optimized contrast using different flip-angle evolutions image showing abnormal sulcal space enhancement (arrow) in the right frontal lobe in a patient with pneumococcal meningitis; middle, post-contrast T2-fluid-attenuated inversion recovery image showing the enhancement (arrow); and right, post-contrast 3D-T1-magnetization-prepared rapid gradient-echo image failed to show the abnormal enhancement.
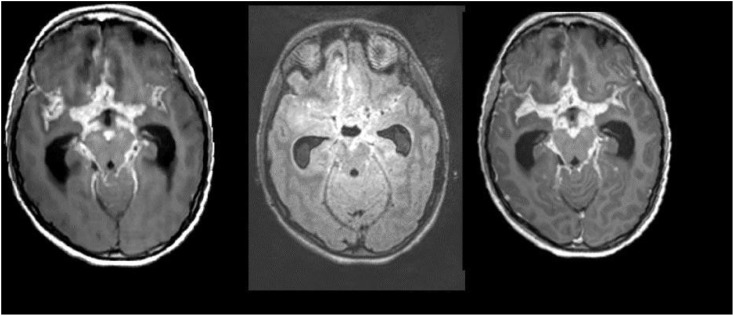
Figure 7.
A case of tubercular meningitis; post-contrast three-dimensional (3D)-T1 sampling perfection with application optimized contrast using different flip-angle evolutions (left), 3D-T2 fluid-attenuated inversion recovery (middle) and 3D-T1 magnetization-prepared rapid gradient-echo (right) images show enhancing exudates in the basal cisterns leading to hydrocephalous.
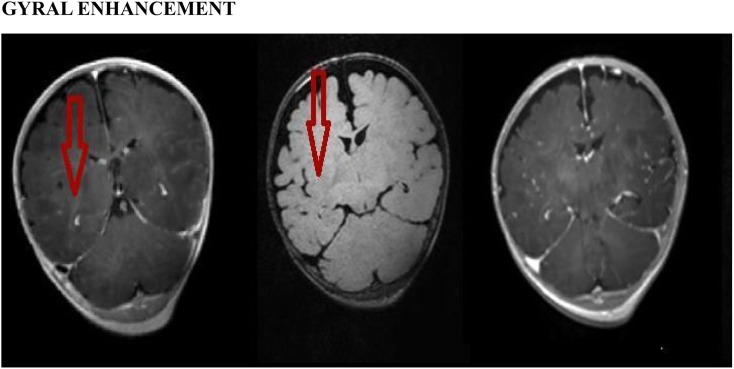
Figure 2.
Gyral enhancement: from the left, post-contrast three-dimensional (3D)-T1 sampling perfection with application optimized contrast using different flip-angle evolutions (SPACE) (left), 3D-T2 fluid-attenuated inversion recovery (FLAIR) (middle) and 3D-T1 magnetization-prepared rapid gradient-echo (MPRAGE) (right) images. The SPACE image shows abnormal gyral enhancement (arrow) in the right lentiform nucleus region in a patient with viral meningitis. The post-contrast T2-FLAIR image shows subtle enhancement (arrow), and in the post-contrast 3D-T1-MPRAGE image, the enhancement is barely visible.
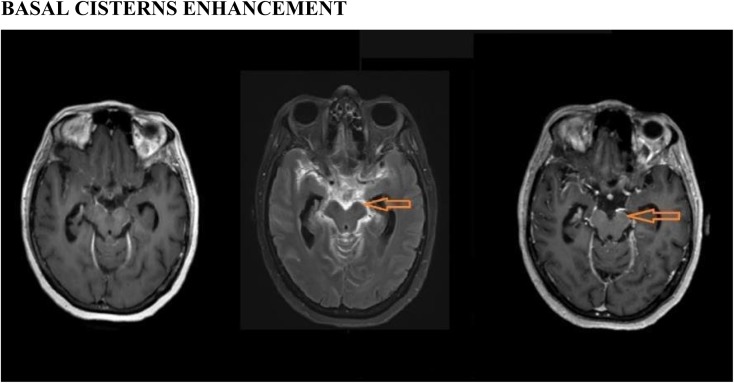
Figure 3.
Basal cisterns enhancement: a patient who is a known case of tubercular meningitis with enhancing exudates, post-contrast fluid-attenuated inversion recovery image (middle) shows the enhancing exudates (arrows) with better conspicuity and superior contrast, whereas both post-contrast three-dimensional (3D)-T1 sampling perfection with application optimized contrast using different flip-angle evolutions (left) and 3D-T1 magnetization-prepared rapid gradient-echo (right) images failed to show the enhancement.
Figure 4.
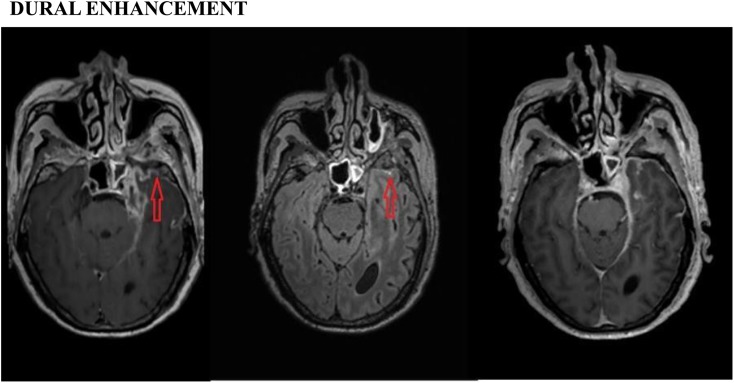
Dural enhancement: post-contrast three-dimensional (3D)-T1 sampling perfection with application optimized contrast using different flip-angle evolutions (SPACE) (left), 3D-T2 fluid-attenuated inversion recovery (FLAIR) (middle) and 3D-T1 magnetization-prepared rapid gradient-echo (MPRAGE) (right) images showing abnormal dural enhancement (arrows) in the left temporal region in a patient with tubercular meningitis. However, the post-contrast T1-SPACE image is much more conspicuous than the post-contrast 3D-T1-MPRAGE and 3D-T2-FLAIR images.
Figure 5.
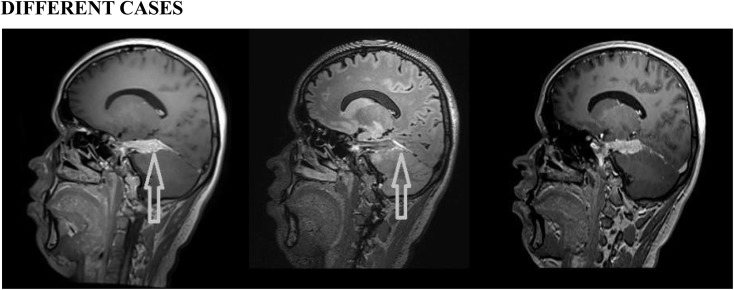
Different cases: post-contrast three-dimensional (3D)-T1 sampling perfection with application optimized contrast using different flip-angle evolutions (left), 3D-T2 fluid-attenuated inversion recovery (middle) and 3D-T1 magnetization-prepared rapid gradient-echo (right) images showing abnormal thickening with enhancement (arrows) of the dura in the right tentorial leaflet. Histopathology proved it to be en plaque meningioma.
Figure 6.
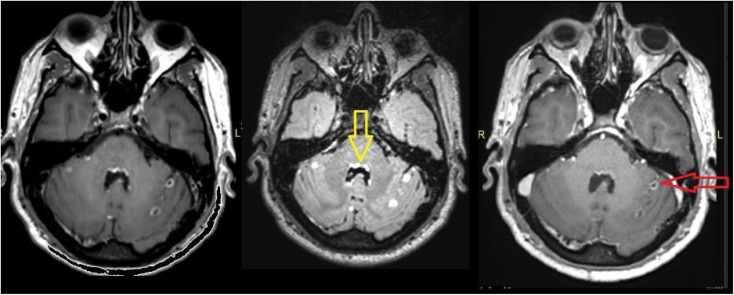
Post-contrast three-dimensional (3D)-T1 sampling perfection with application optimized contrast using different flip-angle evolutions (left), 3D-T2 fluid-attenuated inversion recovery (FLAIR) (middle) and 3D-T1 magnetization-prepared rapid gradient-echo (right) images showing nodular sulcal spaces enhancement (arrows) in the bilateral cerebellar hemisphere. Post-contrast 3D-T2-FLAIR shows better leptomeningeal enhancement and the ependymal enhancement in the fourth ventricle. The patient is a known case of pineoblastoma.
To avoid timing bias after contrast injection, the order of the three 3D sequences were alternated in the patients by rotation.35
Standard MR protocol
Localizer in all planes. The 3D MRI studies were performed by using the following parameters:
(1) repetition time (TR), 6000 ms; effective echo time (TE eff), 421 ms; inversion time, 2000 ms; echo-train length, 284; imaging time, 4 min 14 s; field of view (FOV), 240 × 240 mm; matrix, 256 × 256; and 0.9-mm-thick sections for 3D-T2-FLAIR.
(2) TR, 1740 ms; TE eff, 2.49 ms; inversion time, 900 ms; imaging time, 3 min 34 s; FOV, 240 × 240 mm; matrix, 256 × 256; and 0.9-mm-thick sections for 3D-T1-MPRAGE.
(3) TR, 500 ms; TE eff, 12.0 ms; imaging time, 4 min 01 s; FOV, 250 × 250 mm; matrix, 256 × 256; and 0.9-mm-thick sections for 3D-T1-SPACE.
Image evaluation
Two senior radiologists assessed the meningeal abnormalities and compared the post-contrast 3D-T2-FLAIR with post-contrast 3D-T1-MPRAGE and 3D-T1-SPACE images with pre-contrast 3D-T2-FLAIR images simultaneously reviewed as the reference. The radiologists were blinded to the clinical history and cytological results.
All the images were assessed on GE picture archiving and communication system workstation. Image evaluation was performed using the multiplanar reconstruction of 3D MR images.
The presence of additional information on post-contrast 3D MR images was compared with post-contrast 3D-T1-MPRAGE and 3D-T1-SPACE images. The conspicuity and additional information were evaluated with a four-point scoring system.
Score 3: post-contrast 3D-T2-FLAIR images provided additional information of a lesion with definite abnormal meningeal enhancement clearly discriminated from leptomeningeal vessels
Score 2: probable abnormal meningeal enhancement that seemed to be different from leptomeningeal vessels
Score 1: possible abnormal meningeal enhancement that was not discriminated from leptomeningeal vessels and
Score 0: no additional information about abnormal meningeal enhancement.
The presence or absence of additional information that was detected in post-contrast 3D-T2-FLAIR on comparison with post-contrast 3D-T1-MPRAGE and in post-contrast 3D-T1-SPACE was also recorded.
The investigators documented the information scores for each of the different meningeal enhancement patterns.34
(a) gyral enhancement
(b) sulcal space enhancement
(c) basal cisterns enhancement and
(d) dural enhancement.
And the informations gained by the post-contrast 3D-T2-FLAIR, 3D-T1-MPRAGE and 3D-T1-SPACE images.
Final diagnoses were correlated with microbiological CSF examination, biochemical CSF evaluatory methods and pathological examinations, with the gold standard examination being the polymerase chain reaction examination of the CSF.
RESULTS
On pre-contrast 3D-T2-FLAIR images, a slightly high signal intensity relative to the CSF was seen in the sulci and in basal cisterns in 12 patients. Abnormal leptomeningeal contrast enhancement on post-contrast MR images was observed in 38 patients but not observed in 10 patients. Contrast enhancement of the cortical veins was seen on post-contrast 3D-T1-MPRAGE images but not on post-contrast 3D-T2-FLAIR and post-contrast 3D-T2-FLAIR images in all patients.
Our results of patients showing different enhancement patterns and semi-quantitative comparison of additional information yielded on 3D-T2-FLAIR, 3D-T1-SPACE and 3D-T1-MPRAGE are depicted in Tables 2 and 3, respectively. Demographics of the study and diseases causing meningeal abnormalities are described in Tables 4 and 5, respectively.
Table 2.
Number of patients with each sequence showing different enhancement patterns
| Pattern of enhancement | Post-contrast 3D-T1-MPRAGE | Post-contrast 3D-T1-SPACE | Post-contrast 3D-T2-FLAIR |
|---|---|---|---|
| Gyral enhancement | 8 | 11 | 10 |
| Sulcal space enhancement | 15 | 26 | 21 |
| Basal cisterns enhancement | 9 | 13 | 18 |
| Dural enhancement | 10 | 12 | 11 |
3D, three-dimensional; FLAIR, 3D-T2 fluid-attenuated inversion recovery; MPRAGE, 3D-T1 magnetization-prepared rapid gradient-echo; SPACE, sampling perfection with application optimized contrast using different flip-angle evolutions.
Table 3.
Additional information of post-contrast three-dimensional (3D) MR images compared with post-contrast two-dimensional T1 weighted images for the conspicuity of leptomeningeal enhancement
| Pattern of enhancement with grade | 3D-T1-MPRAGE |
3D-T1-SPACE |
3D-T2-FLAIR |
|||
|---|---|---|---|---|---|---|
| Rad 1 | Rad 2 | Rad 1 | Rad 2 | Rad 1 | Rad 2 | |
| Gyral enhancement grade | ||||||
| 3 | 0 | 1 | 5 | 5 | 4 | 4 |
| 2 | 3 | 2 | 3 | 4 | 2 | 2 |
| 1 | 5 | 5 | 3 | 3 | 4 | 4 |
| 0 | 3 | 3 | 0 | 1 | 1 | 1 |
| Sulcal space enhancement grade | ||||||
| 3 | 2 | 2 | 17 | 19 | 14 | 13 |
| 2 | 8 | 9 | 4 | 2 | 5 | 6 |
| 1 | 5 | 5 | 1 | 1 | 6 | 6 |
| 0 | 11 | 10 | 0 | 0 | 1 | 1 |
| Basal cisterns enhancement grade | ||||||
| 3 | 2 | 2 | 7 | 7 | 10 | 11 |
| 2 | 0 | 0 | 4 | 3 | 6 | 6 |
| 1 | 7 | 5 | 2 | 2 | 2 | 1 |
| 0 | 9 | 11 | 5 | 6 | 0 | 0 |
| Dural enhancement grade | ||||||
| 3 | 3 | 3 | 10 | 11 | 7 | 8 |
| 2 | 4 | 4 | 2 | 1 | 3 | 2 |
| 1 | 3 | 3 | 0 | 0 | 1 | 1 |
| 0 | 2 | 2 | 0 | 0 | 0 | 0 |
FLAIR, 3D-T2 fluid-attenuated inversion recovery; MPRAGE, 3D-T1 magnetization-prepared rapid gradient-echo; SPACE, sampling perfection with application optimized contrast using different flip-angle evolutions.
Table 4.
Demographic distribution of the study
| <10 years | 11–20 years | 31–40 years | 41–50 years | 51–60 years | 61–70 years | 71–80 years |
|---|---|---|---|---|---|---|
| 15% | 26% | 11% | 7% | 15% | 11% | 4% |
Table 5.
Distribution of final diagnoses
| Tuberculosis | 26.3% |
| Metastases | 15.8% |
| Viral meningitis | 13.2% |
| Pneumococcus | 10.5% |
| Group B streptococci | 10.5% |
| Autoimmune | 7.9% |
| Dural sinus thrombosis | 7.9% |
| Cyanotic CHD with Haemophilus influenzae | 5.3% |
| En plaque meningioma | 2.6% |
CHD, congenital heart disease.
Statistical methods
An interobserver reliability analysis using the kappa statistic was performed to determine consistency among observers (Table 6), with a kappa value of 0.648 suggesting substantial agreement. The statistical significance of differences scored on the four-point scale was determined by Wilcoxon signed rank test. Differences with p-value <0.05 were considered significant. Sensitivity values for each sequence are described in Table 7.
Table 6.
Number of abnormal meningeal enhancement detected by each sequences
| Positive cases by T1-MPRAGE | Positive cases by T2-FLAIR | Positive cases by T1-SPACE | Proven cases of meningeal abnormalities |
|---|---|---|---|
| 29 | 36 | 38 | 38 |
FLAIR, 3D-T2 fluid-attenuated inversion recovery; MPRAGE, 3D-T1 magnetization-prepared rapid gradient-echo; SPACE, sampling perfection with application optimized contrast using different flip-angle evolutions.
Table 7.
Sensitivity of each sequence to detect the meningeal abnormalities
| MPRAGE | 80.56% |
| T2-FLAIR | 94.73% |
| SPACE | 100% |
FLAIR, 3D-T2 fluid-attenuated inversion recovery; MPRAGE, 3D-T1 magnetization-prepared rapid gradient-echo; SPACE, sampling perfection with application optimized contrast using different flip-angle evolutions.
DISCUSSION
The results of our study indicated that both post-contrast 3D-T1-SPACE and post-contrast 3D-T2-FLAIR images add significantly more information than do post-contrast 3D-T1-MPRAGE images.
The ability of 3D-T1-SPACE and 3D-T2-FLAIR sequences to detect more leptomeningeal abnormalities is due to the fact that they do not show signals in the leptomeningeal vasculatures and cortical vessels at normal flow which is the usual cause for overlooking foci of leptomeningeal enhancement.
On comparing the post-contrast 3D-T1-SPACE MR images with post-contrast 3D-T2-FLAIR images for overall detection of meningeal abnormalities, we did not find any statistically significant difference between the two.
Gyral enhancement
Both the gyral and sulcal space enhancements are more specific for leptomeningeal metastases and viral meningitis. Leptomeningeal carcinomatosis is usually a small nodular leptomeningeal lesion ranging from 2 to 5 mm. Kato et al11 reported that post-contrast T1-SPACE had higher contrast-to-noise ratio and more conspicuity than post-contrast T1-MPRAGE in detection of leptomeningeal metastases. In our study, we found that the post-contrast SPACE images hold good for the demonstration of gyral enhancement than the post-contrast MPRAGE and T2-FLAIR images with a statistical significance of p < 0.05. This is in par with the Kato et al study.
Sulcal space enhancement
Our results of the semi-quantitative comparison of 3D-T2-FLAIR, SPACE and MPRAGE radiologists assigned overall score. The mean score of sulcal space enhancement by the radiologists are depicted in Table 8.
Table 8.
Mean score of sulcal space enhancement
| MPRAGE | FLAIR | SPACE |
|---|---|---|
| 2.17 ± 1.17 | 2.28 ± 1.08 | 2.52 ± 1.17 |
FLAIR, 3D-T2 fluid-attenuated inversion recovery; MPRAGE, 3D-T1 magnetization-prepared rapid gradient-echo; SPACE, sampling perfection with application optimized contrast using different flip-angle evolutions.
The assigned scores were significantly higher for post-contrast 3D-T1-SPACE than were for post-contrast 3D-T2-FLAIR and post-contrast 3D-T1-MPRAGE images (p < 0.05 and p < 0.01, respectively).
Dural enhancement
Dural thickening and enhancements are predominantly seen in cases of granulomatous diseases such as tuberculosis and sarcoidosis. Similarly for the dural enhancement, we found out that post-contrast 3D-T1-SPACE images are much superior to T1-MPRAGE and T2-FLAIR images (statistical significance p < 0.05).
Basal cisterns enhancement
Basal cisterns leptomeningeal enhancement is predominantly the feature of tubercular and pyogenic meningitis. The basal cisterns enhancing exudates more specifically favour tubercular meningitis. Interestingly, our study shows that the assigned scores for abnormal basal cisterns enhancement were significantly higher for post-contrast T2-FLAIR than for SPACE and MPRAGE post-contrast images (p < 0.05 for both).
There are some limitations in this study. First, our study population consisted of a small number of patients with meningeal abnormalities, especially patients with abnormal gyral and dural enhancements. Because our results were promising regardless of the small number of subjects, further clinical studies with large population should be performed. Second, we could not obtain histological confirmation of the metastatic lesions because patients with multiple brain metastases generally do not undergo surgery. We believe that careful observation of 69 lesions in leptomeningeal carcinomatosis, including follow-up imaging and also the evaluation conducted by two neuroradiologists, minimized contamination of our subjects with false positives and negatives. Finally, the neuroradiologists and readers of the study were not blinded as to which pulse sequence was being evaluated because the grey–white differentiation was so different between SPACE, T2-FLAIR and MPRAGE that they knew the sequence at a glance, and this could introduce bias.
CONCLUSION
Post-contrast 3D-T1-SPACE imaging adds significantly more information to post-contrast 3D-T2-FLAIR and 3D-T1 weighted MPRAGE imaging. Hence post-contrast 3D-T1-SPACE and 3D-T2-FLAIR images can replace the routine post-contrast T1-MPRAGE images.
In the evaluation of different enhancement patterns, post-contrast 3D-T1-SPACE imaging scored better than 3D-T2-FLAIR and 3D-T1-weighted MPRAGE images in evaluation of gyral, sulcal space and dural enhancements.
However, for evaluation of basal cisterns enhancement, our study revealed that post-contrast 3D-T2-FLAIR was better than post-contrast 3D-T1-SPACE and 3D-T1 weighted MPRAGE images. Hence, if there is a clinical suspicion for tubercular or pyogenic meningitis, post-contrast 3D-T2-FLAIR imaging is better than post-contrast 3D-T1-MPRAGE and post-contrast 3D-T1-SPACE imaging sequences.
Post-contrast 3D-T2-FLAIR and 3D-T1-SPACE imaging sequences should be performed for better detection and enhanced conspicuity of meningeal abnormalities.
A combination of both post-contrast 3D-T2-FLAIR and 3D-T1-SPACE images can lead to a 100% detection rate for meningeal abnormalities.
Further larger scale prospective studies with more number of meningeal abnormalities are required to validate the results of this study.
The preliminary results of the above study have been presented in the European Congress of Radiology (ECR) 2015 in Vienna, Austria, as a paper presentation.
Contributor Information
Balaji Jeevanandham, Email: bjradiologist@gmail.com.
Tejas Kalyanpur, Email: tejmedico@gmail.com.
Prashant Gupta, Email: drprashg@gmail.com.
Mathew Cherian, Email: dr.mathewcherian@gmail.com.
REFERENCES
- 1.Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev 1999; 25: 103–19. doi: https://doi.org/10.1053/ctrv.1999.0119 [DOI] [PubMed] [Google Scholar]
- 2.Balm M, Hammack J. Leptomeningeal carcinomatosis: presenting features and prognostic factors. Arch Neurol 1996; 53: 626–32. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain MC. Leptomeningeal metastases: a review of evaluation and treatment. J Neurooncol 1998; 37: 271–84. [DOI] [PubMed] [Google Scholar]
- 4.Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98: 291–8. doi: https://doi.org/10.1093/qjmed/hci047 [DOI] [PubMed] [Google Scholar]
- 5.Mamourian AC, Hoopes PJ, Lewis LD. Visualization of intravenously administered contrast material in the CSF on fluid-attenuated inversion-recovery MR images: an in vitro and animal-model investigation. AJNR Am J Neuroradiol 2000; 21: 105–11. [PMC free article] [PubMed] [Google Scholar]
- 6.Bozzao A, Floris R, Fasoli F, Fantozzi LM, Colonnese C, Simonetti G. Cerebrospinal fluid changes after intravenous injection of gadolinium chelate: assessment by FLAIR MR imaging. Eur Radiol 2003; 13: 592–7. [DOI] [PubMed] [Google Scholar]
- 7.Hirai T, Ando Y, Yamura M, Kitajima M, Hayashida Y, Korogi Y, et al. Transthyretin-related familial amyloid polyneuropathy: evaluation of CSF enhancement on serial T1-weighted and fluid-attenuated inversion recovery images following intravenous contrast administration. AJNR Am J Neuroradiol 2005; 26: 2043–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Mugler JP, 3rd, Brookeman JR. Rapid three dimensional T1 weighted MR imaging with the MP-RAGE sequence. J Magn Reson Imaging 1991; 1: 561–7. doi: https://doi.org/10.1002/jmri.1880010509 [DOI] [PubMed] [Google Scholar]
- 9.Brant-Zawadski M, Gillan GD, Nitz WR. MPRAGE: a three-dimensional, T1 weighted, gradient echo sequence-initial experience in the brain. Radiology 1992; 182: 769–75. [DOI] [PubMed] [Google Scholar]
- 10.Komada T, Naganawa S, Ogawa H, Matsushima M, Kubota S, Kawai H, et al. Contrast-enhanced MR imaging of metastatic brain tumor at 3 Tesla: utility of T(1)-weighted SPACE compared with 2D spin echo and 3D gradient echo sequence. Magn Reson Med Sci 2008; 7: 13–21. doi: https://doi.org/10.2463/mrms.7.13 [DOI] [PubMed] [Google Scholar]
- 11.Kato Y, Higano S, Tamura H. Usefulness of contrast-enhanced T1-SPACE imaging by using different flip angle evolutions in detection of small brain metastasis at 3T MR imaging comparison with MP-RAGE imaging. AJNR Am J Neuroradiol 2009; 30: 923–9. doi: https://doi.org/10.3174/ajnr.a1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews VP, Caldemeyer KS, Lowe MJ. Brain: gadolinium-enhanced fast fluid-attenuated inversion-recovery MR imaging. Radiology 1999; 211: 257–63. doi: https://doi.org/10.1148/radiology.211.1.r99mr25257 [DOI] [PubMed] [Google Scholar]
- 13.Tsuchiya K, Katase S, Yoshino A, Hachiya J. FLAIR MR imaging for diagnosing intracranial meningeal carcinomatosis. AJR Am J Roentgenol 2001; 176: 1585–8. doi: https://doi.org/10.2214/ajr.176.6.1761585 [DOI] [PubMed] [Google Scholar]
- 14.Griffiths PD, Coley SC, Romanowski CA, Hodgson T, Wilkinson ID. Contrast-enhanced fluid-attenuated inversion recovery imaging for leptomeningeal disease in children. AJNR Am J Neuroradiol 2003; 24: 719–23. [PMC free article] [PubMed] [Google Scholar]
- 15.Splendiani A, Puglielli E, Amicis RD, Necozione S, Masciocchi C, Gallucci M. Contrast-enhanced FLAIR in the early diagnosis of infectious meningitis. Neuroradiology 2005; 47: 591–8. doi: https://doi.org/10.1007/s00234-005-1383-7 [DOI] [PubMed] [Google Scholar]
- 16.Singh SK, Agris JM, Leeds NE, Ginsberg LE. Intracranial leptomeningeal metastases: comparison of depiction at FLAIR and contrast-enhanced MR imaging. Radiology 2000; 217: 50–3. doi: https://doi.org/10.1148/radiology.217.1.r00oc3550 [DOI] [PubMed] [Google Scholar]
- 17.Sage MR, Wilson AJ, Scroop R. Contrast media and the brain: the basis of CT and MR imaging enhancement. Neuroimaging Clin N Am 1998; 8: 695–707. [PubMed] [Google Scholar]
- 18.Provenzale JM, Mukundan S, Dewhirst M. The role of blood-brain barrier permeability in brain tumor imaging and therapeutics. AJR Am J Roentgenol 2005; 185: 763–7. doi: https://doi.org/10.2214/ajr.185.3.01850763 [DOI] [PubMed] [Google Scholar]
- 19.Smirniotopoulos JG, Murphy FM, Rushing EJ. Patterns of contrast enhancement in the brain and meninges. Radiographics 2007; 27: 525–51. doi: https://doi.org/10.1148/rg.272065155 [DOI] [PubMed] [Google Scholar]
- 20.Kakeda S, Korogi Y, Hiai Y. Detection of brain metastasis at 3T: comparison among SE, IR-FSE and 3D-GRE sequences. Eur Radiol 2007; 17: 2345–51. doi: https://doi.org/10.1007/s00330-007-0599-9 [DOI] [PubMed] [Google Scholar]
- 21.Furutani K, Harada M, Mawlan M, Nishitani H. Difference in enhancement between spin echo and 3-dimensional fast spoiled gradient recalled acquisition in steady state magnetic resonance imaging of brain metastasis at 3-T magnetic resonance imaging. J Comput Assist Tomogr 2008; 32: 313–19. doi: https://doi.org/10.1097/rct.0b013e318074fd9d [DOI] [PubMed] [Google Scholar]
- 22.Fishbach F, Bruhn H, Pech M, Neumann F, Ricke J, Felix R, et al. Efficacy of contrast medium use for neuroimaging at 3.0T: utility of IR-FSE compared to other T1-weighted pulse sequences. J Comput Assist Tomogr 2005; 29: 499–505. [DOI] [PubMed] [Google Scholar]
- 23.Mugler JP, 3rd, Bao S, Mulkern RV, Guttmann CR, Robertson RL, Jolesz FA, et al. Optimized single-slab three dimensional spin-echo MR imaging of the brain. Radiology 2000; 216: 891–9. doi: https://doi.org/10.1148/radiology.216.3.r00au46891 [DOI] [PubMed] [Google Scholar]
- 24.Singh SK, Leeds NE, Ginsberg LE. MR imaging of leptomeningeal metastases: comparison of three sequences. AJNR Am J Neuroradiol 2002; 23: 817–21. [PMC free article] [PubMed] [Google Scholar]
- 25.Galassi W, Phuttharak W, Hesselink JR, Healy JF, Dietrich RB, Imbesi SG. Intracranial meningeal disease: comparison of contrast-enhanced MR imaging with fluid-attenuated inversion recovery and fat-suppressed T1-weighted sequences. AJNR Am J Neuroradiol 2005; 26: 553–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Kallmes DF, Hui FK, Mugler JP, 3rd. Suppression of cerebrospinal fluid and blood flow artefacts in FLAIR MR imaging with a single-slab three-dimensional pulse sequence: initial experience. Radiology 2001; 221: 251–5. doi: https://doi.org/10.1148/radiol.2211001712 [DOI] [PubMed] [Google Scholar]
- 27.Bink A, Schmitt M, Gaa J. Detection of lesions in multiple sclerosis by 2D-FLAIR and single-slab 3D FLAIR sequences at 3.0 T: initial results. Eur Radiol 2006; 16: 1104–10. doi: https://doi.org/10.1007/s00330-005-0107-z [DOI] [PubMed] [Google Scholar]
- 28.Naganawa S, Koshikawa T, Nakamura T. Comparison of flow artifacts between 2D-FLAIR and 3D-FLAIR sequences at 3 T. Eur Radiol 2004; 14: 1901–8. doi: https://doi.org/10.1007/s00330-004-2372-7 [DOI] [PubMed] [Google Scholar]
- 29.Chagla GH, Busse RF, Sydnor R. Three-dimensional fluid attenuated inversion recovery imaging with isotropic resolution and nonselective adiabatic inversion provides improved three-dimensional visualization and cerebrospinal fluid suppression compared to two-dimensional FLAIR at 3 Tesla. Invest Radiol 2008; 43: 547–51. doi: https://doi.org/10.1097/rli.0b013e3181814d28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strouse PJ, Prince MR, Chenevert TL. Effect of the rate of gadopentetate dimeglumine administration on abdominal vascular and soft-tissue MR imaging enhancement patterns. Radiology 1996; 201: 809–16. doi: https://doi.org/10.1148/radiology.201.3.8939236 [DOI] [PubMed] [Google Scholar]
- 31.Essig M, Knopp MV, Schoenberg SO. Cerebral gliomas and metastases: assessment with contrast-enhanced fast fluid-attenuated inversion-recovery MR imaging. Radiology 1999; 210: 551–7. doi: https://doi.org/10.1148/radiology.210.2.r99ja22551 [DOI] [PubMed] [Google Scholar]
- 32.Ercan N, Gultekin S, Celik H, Tali TE, Oner YA, Erbas G. Diagnostic value of contrast-enhanced fluid-attenuated inversion recovery MR imaging of intracranial metastases. AJNR Am J Neuroradiol 2004; 25: 761–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Vaswani K, Nizamani M, Ali M. Contrast-enhanced FLAIR magnetic resonance imaging in diagnosis of meningitis correlated with CSF analysis. Radiology 2014; 2014: 578986. doi: https://doi.org/10.1155/2014/578986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuoka H, Hirai T, Okuda T, Shigematsu Y, Sasao A, Kimura E, et al. Comparison of the added value of contrast-enhanced 3D fluid-attenuated inversion recovery and magnetization-prepared rapid acquisition of gradient echo sequences in relation to conventional postcontrast T1-weighted images for the evaluation of leptomeningeal diseases at 3T. AJNR Am J Neuroradiol 2010; 31: 868–73. doi: https://doi.org/10.3174/ajnr.a1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schörner W, Laniado M, Niendorf HP, Schubert C, Felix R. Time dependent changes in image contrast in brain tumors after gadolinium-DTPA. AJNR Am J Neuroradiol 1986; 7: 1013–20. [PMC free article] [PubMed] [Google Scholar]