Abstract
Context:
Assessment of comorbid burden of depression and associated factors among adult people living with epilepsy (PWE) has the potential to inform clinical evaluation and management to improve outcomes.
Aim:
The aim of this study is to determine frequency of depressive symptoms and factors associated with it among PWE attending a tertiary care hospital.
Setting and Design:
This was a cross-sectional observational study conducted in the outpatient clinic of a tertiary care center.
Subjects and Methods:
One hundred and fifty patients with epilepsy were recruited between May 2016 and August 2016. For assessing depression, Tamil validated version of Patient Health Questionnaire-12 was used. Frequency of depression and its association with various sociodemographic and clinical factors were assessed.
Statistical Analysis Used:
Student t-test and Chi-square test for univariate analysis and logistic regression for multivariate analysis were used for statistical analysis.
Results:
Of the 150 subjects, 89 (59.3%) were males. Generalized tonic–clonic seizures were present in 131 people (87.3%). Genetic and unknown epilepsies contributed higher proportion (44.7%) as compared to structural epilepsies (37.3%). Majority (88%) were on one or two antiepileptic drugs. Thirty-one people (20.3%) had less than one seizure per year. Depressive symptoms were present in 95 patients (63.3%). In multivariate regression analysis, secondary generalized seizures and seizure frequency >1/month emerged as significant predictors of depression ([OR]: 5.48 [95% (CI): 1.35–22.28] and OR: 2.53 [95% CI: 1.11–5.75], respectively).
Conclusion:
Depression is prevalent in a majority of adult PWE. Hence, a routine screening for depression as part of comprehensive epilepsy care is advisable for PWE attending the outpatient clinic.
KEYWORDS: Comorbidity, depression, epilepsy, mood disorders, psychiatric disorders, psychiatry
INTRODUCTION
Epilepsy is a common, chronic, and disabling neurological condition. One-eighth of the total epilepsy patients in the world reside in India, which comes to about 55,00,000 persons.[1] According to the World Health Organization, depression is one of the leading causes of disability-adjusted life years worldwide.[2] Depression, in the context of epilepsy, is associated with poor treatment adherence, poor quality of life, unemployment, lower educational status, and increased burden on health-care services.[3] In a population-based study, coexistence of epilepsy and affective disorder was found to increase the risk of suicide by 32 times.[4]
A few studies have highlighted the bidirectional interaction between epilepsy and depression. A cross-sectional study revealed that up to 60% of epileptic patients had associated depression and such individuals carried elevated risk for further seizures.[5] Frequency of depression was found to be significantly high in people living with epilepsy (PWE) as compared to people suffering from other diseases such as diabetes, bronchial asthma, and multiple sclerosis.[6,7] However, previous Indian studies had shown lower prevalence of depression in PWE as compared to those in other countries.[8,9] These variations may be explained by the different study settings, criteria, and instruments used to diagnose depression. Some of these studies were done on selected epilepsy population such as drug-resistant epilepsy or on small patient population.[10]
The pathomechanisms that link depression and epilepsy are also poorly understood. Culturally determined factors, including but not limited to familial support and coping styles, have been suggested to play a role in determining the risk of depression in PWE.[11] However, there have also been negative studies in this regard.[12] Hence, more data about depression in epilepsy are needed, from across cultures and settings, and have the potential to inform clinical evaluation and management of epilepsy. It would also improve our understanding about the factors leading to depression in epilepsy or vice versa, thus paving the way for newer treatment targets in both conditions. As Friedman et al. noted, assessment of depression in epilepsy in a busy clinical setting is improved using a systematic screening method.[13] This will help not only in identifying depression but also in the treatment of this comorbidity. In addition, we wanted to assess whether the magnitude of depression in PWE is low as reported by some Indian studies or high similar to reports from other countries.[8,9] To address this knowledge gap, we carried out the present exploratory study to determine the frequency of depression and factors associated with it among adult PWE attending a tertiary care hospital using a validated screening instrument.
SUBJECTS AND METHODS
Design and setting
This observational, cross-sectional study was conducted in the epilepsy outpatient clinic of the Department of Neurology of a tertiary care hospital in Puducherry, South India, over a period of 3 months from May 2016 to August 2016. The institution provides highly subsidized treatment to most of the patients and mainly draws its clientele from Puducherry as well as adjoining districts of Tamil Nadu. The Department of Neurology offers both outpatient and inpatient services including dedicated epilepsy clinic in its premises. Epilepsy clinic functions on a weekly basis, with an average attendance of 2500 per month. Patients are initially evaluated by a neurology senior resident (undergoing superspecialty training) and discussed with consultant neurologist, following which patients diagnosed to have epilepsy are initiated on appropriate antiepileptic agents and treated either on outpatient or inpatient basis. All diagnoses of epilepsy are made as per definitions and classification of the International League Against Epilepsy (ILAE), 1981 and its modification 2010.[14,15] All patients are subsequently followed up in the epilepsy clinic monthly. This specialty clinic has been in existence for more than two decades and is populated by epilepsy patients who come regularly for their monthly drug refills. For the present study, patients were selected from this clinic using a convenience sampling method and the inclusion criteria were as follows. all patients diagnosed to have epilepsy as per definitions and classification of ILAE 1981,[14] aged ≥18 years, and who could read and write Tamil. They were included after obtaining informed consent. We excluded those who could not read or write Tamil as the instrument we used was a self-reported questionnaire validated in the Tamil language.[16] Those with acute symptomatic seizures and history of intellectual impairment were excluded. The study was conducted after obtaining approval from Institute Ethics Committee.
Using a semi-structured pro forma, we collected relevant demographic and clinical details including birth and developmental history, educational status, occupation, age of onset and duration of epilepsy, type and frequency of seizures, epilepsy syndrome, antiepileptic drugs used, and its compliance. Computed tomography/magnetic resonance imaging findings and electroencephalography findings, wherever available, were recorded.
Assessment tool
For assessing depression, Tamil validated version of Patient Health Questionnaire-12 (PHQ-12) was used.[16] It is a modification of PHQ-9 questionnaire which assesses the actual nine criteria for major depressive disorder as per Diagnostic and Statistical Manual Fourth Edition. Three items in the PHQ-9 were split to make it culturally compatible for Indian setting, thus leading to a total of 12 items in PHQ-12. Response categories in PHQ-12 are dichotomous (yes or no) [Table 1]. The questionnaire was administered to each patient after making them sit in a comfortable room. They were asked to respond to the items with time reference frame of the last 2 weeks. Each “yes” response scores one point while “no” scores zero. Total scores were obtained by summing the individual items, and a score of more than 4 was taken as positive for depression as suggested by the authors as this cutoff afforded the highest sensitivity (92.0%) and specificity (90.7%). The scale has robust psychometric properties with excellent internal consistency (Cronbach's alpha 0.88) and positive and negative predictive values for depression being 76.7% and 97.1%, respectively. The PHQ was preferred over other questionnaires such as the Hamilton Depression Rating Scale[17] to assess depression as it is a short, convenient instrument validated in the Indian setting and because our primary aim was to assess frequency of depressive symptoms and not rate the clinical severity of depression.
Table 1.
Patient health questionnaire-12

Statistical analysis
Sample size was calculated as 148 using EpiInfo 7 Stat Calc software (Centers for Disease Control and Prevention (CDC), Atlanta, Georgia (USA) with an expected frequency of depression of 30%, type I error of 5%, and power of 80%. Data are presented as mean and standard deviation or median and range, as appropriate, for continuous variables. For categorical variables, frequency and proportions are used to depict results. For comparison of continuous data, Student's t-test, and for categorical data, Chi-square or Fisher's exact test were used. Binary logistic regression yielding odds ratio (OR) with 95% confidence interval (CI) was used to identify the factors independently associated with depression. Analysis was done using SPSS Statistics for Windows, Version 17.0 (Chicago, Illinois, USA: SPSS Inc). A P < 0.05 was considered as statistically significant.
RESULTS
A total of 160 adult people were screened during this period, and 10 were excluded due to coexisting chronic illness or inability to read and write Tamil. A total of 150 people were included in the study, of which 59.3% (n = 89) were males. One hundred and thirty-one people (87.3%) had either primary or secondary generalized tonic–clonic seizures. Majority (88%) were on one or two antiepileptic drugs. Thirty-one people (20.3%) had infrequent seizures with frequency of <1 seizure per year. Other baseline characteristics are shown in Table 2.
Table 2.
Baseline demographic and clinical variables
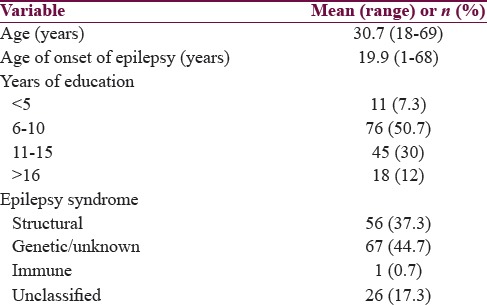
Ninety-five people scored >4 on PHQ 12 putting the frequency of depression at 63.3%. On univariate analysis, seizure type (P < 0.04) and seizure frequency (P < 0.02) were the factors associated with depression, whereas age, gender, age of onset of epilepsy, duration of epilepsy, educational status, epilepsy syndrome, and antiepileptic drug numbers or type were not found to have any association with depression [Tables 3 and 4].
Table 3.
Comparison of demographic factors with depression groups in epilepsy
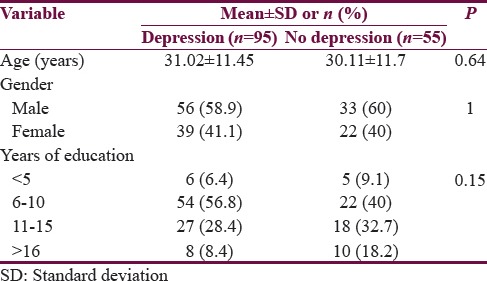
Table 4.
Comparison of clinical parameters of epilepsy with depression groups
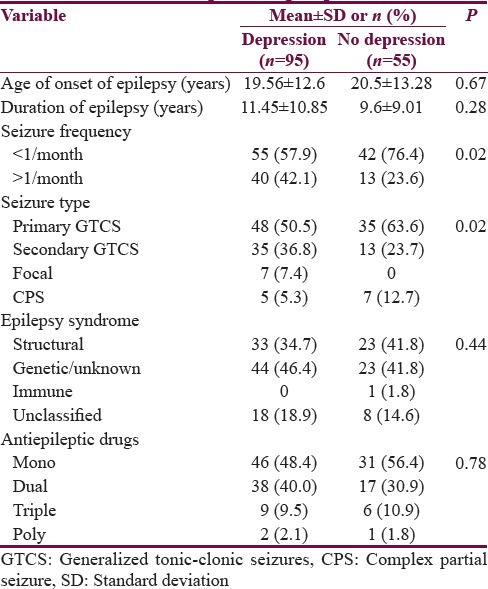
Multivariate logistic regression analysis was carried out with depression (yes/no) as the outcome variable. Covariates entered in the model included age, gender, duration of epilepsy, seizure type and seizure frequency, and number of antiepileptics. It was noted that seizure type and seizure frequency emerged as factors independently associated with depression. Seizures with secondary generalization showed OR: 5.48 [95% C1: 1.35–22.28] (P < 0.02), and seizure frequency of >1/month showed OR: 2.53 (95% CI: 1.11–5.75) (P < 0.03) for having depression [Figure 1]. In other words, those with secondary generalized seizures had 5.48 times increased risk of having depression while the risk of having depression was 2.5 times more in those with high seizure frequency as compared to those with lower seizure frequency.
Figure 1.
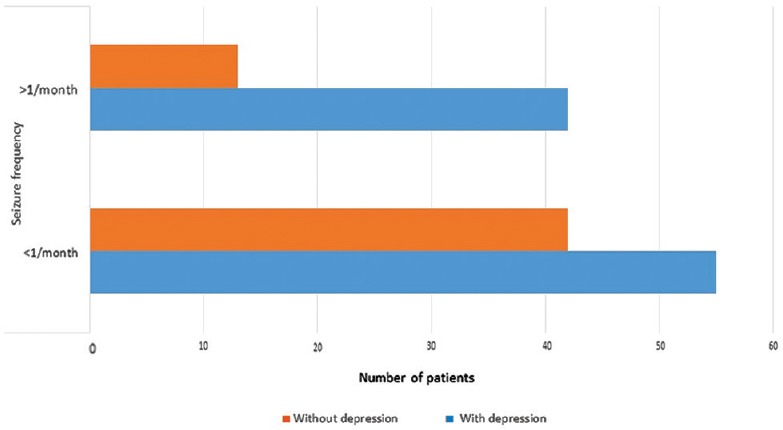
Association of depression with frequency of seizures
DISCUSSION
The study showed that 63% of adult PWE who seek medical care have features suggestive of depression. Such a high coexistence of depression, if unrecognized, can have a negative impact on core outcomes in epilepsy such as inadequate seizure control, quality of life, and increased rates of suicide.[18,19] Psychological assessment is not usually a part of a routine epilepsy consultation, and therefore, it is possible that many cases of coexisting depression may be missed. Our findings highlight the need for a comprehensive and multidisciplinary epilepsy care program involving psychiatry. Psychological assessment using simple screening tools such as PHQ-12 may be made a routine component of comprehensive epilepsy care.
Majority of the previous studies on this topic have reported a lower frequency of depression in epilepsy in the range of 5%–28%.[9,10,20] Similar frequency (60%) was reported in South Indian population in a recent study conducted to find out the association of depression with adverse drug reactions of antiepileptic drugs.[21] These variations may be attributed to the heterogeneity of study population, socioeconomic factors, or difference in the screening tools used. A high frequency of depression among PWE has also been reported from countries such as Pakistan (60%), Korea (62%), and Nigeria (85%).[8,22,23]
The factors associated with depression in this study were type of seizures and seizure frequency. Seizures with secondary generalization were associated with roughly five times higher risk for depressive symptoms over those with dyscognitive or complex partial seizures. This has not been reported previously. Overall, there is scanty information for risk factors for depression in epilepsy. Some authors have previously postulated shared pathogenic mechanism between epilepsy and depression involving decreased neurotransmitter activity.[24,25] Neural networks involved in the genesis of dyscognitive seizures and tonic–clonic seizures differ. Animal experiments have shown that amygdala kindling results in severe motor seizures and anxiety–depression-like behavior. This may be the probable reason for differential association of depression with types of seizures.[26,27] In addition, one cannot disregard the role of potential psychosocial mediators such as social support and self-efficacy that has been shown to be relevant in epilepsy.[28] One possible explanation that may reconcile our findings is that secondary generalized epilepsy is, often, more dramatic with violent movements and associated urinary incontinence which may have negative impact on self-efficacy and thus lead onto depression. Future studies should evaluate this theory by studying psychosocial correlates as potential mediators between depression and epilepsy.
This study also shows that seizure frequency of >1 per month is associated with 2.5 times increased risk of depression over those with lesser number of seizures. This is in consensus with the previous studies.[29,30] A few studies have assessed the relationship between uncontrolled epilepsy and depression. While some of them showed that uncontrolled and recurrent seizures were associated with twofold higher depression scores,[31,32] one longitudinal study showed that seizure frequency and depression shared a bidirectional relationship.[33] The latter finding only underscores the importance of early diagnosis and prompt management of depression as it may affect the clinical course of epilepsy. Preliminary evidence that treating depression with antidepressants may enhance seizure control is available from a small uncontrolled study,[34] and this is worth investigating further in controlled studies.
Contrary to the previous reports,[35,36] use of multiple antiepileptic drugs was not associated with an increased risk of depression. However, majority of our patients were using only one or two antiepileptic drugs and only 12% patients were using >2 drugs. Thus, our findings in this regard may be due to inadequate numbers in this subgroup. Furthermore, drugs such as levetiracetam, phenobarbitone, and clobazam were not associated with depression in isolation or combination which may have implications in the antiepileptic drug management of Indian patients with epilepsy.
Bifftu et al. have shown that depression is linked to low educational status and earlier age of onset of epilepsy.[37] Both these factors did not show any association with depression in the current study. The frequency of patients with low education (7.3%) and those with onset of epilepsy <10 years (18%) was comparatively low in our study, which might have contributed to the finding of lack of association.
A variety of treatment strategies, both pharmacological and psychosocial, have shown encouraging results in the treatment of depression in PWE. A recent international consensus guidelines statement supports the use of selective serotonin reuptake inhibitors, which have relatively low epileptogenic potential, and cognitive-behavior therapy for the treatment of depression.[38] The use of novel internet-based interventions to manage depression in PWE is gaining research attention and the ongoing ENCODE trial by Meyer et al. is a case in point.[39]
The instrument that we have used (PHQ-12) is a screening tool for depressive symptoms. Hence, a comparatively higher rate of prevalence can be expected in the present study and other related studies that have used similar measures as opposed to studies that use tools such as Hamilton Depression Rating Scale.[17] PHQ-12 was preferred in this study as our primary aim was to assess frequency of depressive symptoms and not to make a diagnosis of major depressive disorder or rate the clinical severity of depression.
Limitations of the present study include its cross-sectional nature, and therefore, inferences about the direction of relationship between epilepsy and depression cannot be made. Further, some of the features of depression such as cognitive impairment may overlap with that of epilepsy. Due to low numbers who were taking multiple antiepileptic drugs, its confounding effects could not be studied adequately though we did include it as a covariate in the regression model. We have not assessed possible relationships between medications, other than anti-epileptics, that the patient may have been taking and depression. We have not assessed the confounding variables in psychological domain such as family history of depression, other significant life events, history of use of psychotropic drugs, or coexisting illnesses such as anxiety or psychosis. The study has been done in a dedicated center for epilepsy, and the results may not necessarily generalize to other settings. The strengths of the study are that we have studied a wide range of sociodemographic and clinical factors associated with depression among a sample of adult patients with epilepsy. We have also used a tool for measuring depression that is reliable and validated in the local language.
CONCLUSION
Depression is common in a substantial proportion of PWE visiting the hospital. Uncontrolled seizures and seizures with secondary generalization appear to be risk factors for depression in the Indian setting. No association was noted between depression and either demographic factors such as age and gender or other types of seizures, epilepsy syndromes, number and type of antiepileptic agents, or duration of illness. Given the high morbidity of depression and its consequences in epilepsy, we recommend a routine screening for depression among PWE attending the outpatient clinic. Further community-based studies with longitudinal designs would help unravel the causal relationship between epilepsy and depression and the possible mediators in this association.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sridharan R, Murthy BN. Prevalence and pattern of epilepsy in India. Epilepsia. 1999;40:631–6. doi: 10.1111/j.1528-1157.1999.tb05566.x. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD, Jamison DT. The global burden of disease in 1990: Summary results, sensitivity analysis and future directions. Bull World Health Organ. 1994;72:495–509. [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer JA, Blum D, Fanning K, Reed M Epilepsy Impact Project Group. The impact of comorbid depression on health resource utilization in a community sample of people with epilepsy. Epilepsy Behav. 2004;5:337–42. doi: 10.1016/j.yebeh.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Christensen J, Vestergaard M, Mortensen PB, Sidenius P, Agerbo E. Epilepsy and risk of suicide: A population-based case-control study. Lancet Neurol. 2007;6:693–8. doi: 10.1016/S1474-4422(07)70175-8. [DOI] [PubMed] [Google Scholar]
- 5.Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000;47:246–9. [PubMed] [Google Scholar]
- 6.Alsaadi T, El Hammasi K, Shahrour TM, Shakra M, Turkawi L, Nasreddine W, et al. Depression and Anxiety among Patients with Epilepsy and Multiple Sclerosis: UAE Comparative Study. Behav Neurol. 2015;2015:196373. doi: 10.1155/2015/196373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettinger A, Reed M, Cramer J Epilepsy Impact Project Group. Depression and comorbidity in community-based patients with epilepsy or asthma. Neurology. 2004;63:1008–14. doi: 10.1212/01.wnl.0000138430.11829.61. [DOI] [PubMed] [Google Scholar]
- 8.Onwuekwe I, Ekenze O, Bzeala-Adikaibe, Ejekwu J. Depression in patients with epilepsy: A study from Enugu, South East Nigeria. Ann Med Health Sci Res. 2012;2:10–3. doi: 10.4103/2141-9248.96929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babu CS, Satishchandra P, Sinha S, Subbakrishna DK. Co-morbidities in people living with epilepsy: Hospital based case-control study from a resource-poor setting. Epilepsy Res. 2009;86:146–52. doi: 10.1016/j.eplepsyres.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Desai S, Shukla G, Goyal V, Srivastava A, Srivastava MV, Tripathi M, et al. Changes in psychiatric comorbidity during early postsurgical period in patients operated for medically refractory epilepsy – A MINI-based follow-up study. Epilepsy Behav. 2014;32:29–33. doi: 10.1016/j.yebeh.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Hesdorffer DC, Lee P. Health, wealth, and culture as predominant factors in psychosocial morbidity. Epilepsy Behav. 2009;15(Suppl 1):S36–40. doi: 10.1016/j.yebeh.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz EB, Robertson MM, Trimble MR. Depression and schizophrenia in epilepsy: Social and biological risk factors. Epilepsy Res. 1999;35:59–68. doi: 10.1016/s0920-1211(98)00129-6. [DOI] [PubMed] [Google Scholar]
- 13.Friedman DE, Kung DH, Laowattana S, Kass JS, Hrachovy RA, Levin HS. Identifying depression in epilepsy in a busy clinical setting is enhanced with systematic screening. Seizure. 2009;18:429–33. doi: 10.1016/j.seizure.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League against Epilepsy. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 15.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 16.Poongothai S, Pradeepa R, Ganesan A, Mohan V. Reliability and validity of a modified PHQ-9 item inventory (PHQ-12) as a screening instrument for assessing depression in Asian Indians (CURES-65) J Assoc Physicians India. 2009;57:147–52. [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mainio A, Alamäki K, Karvonen K, Hakko H, Särkioja T, Räsänen P. Depression and suicide in epileptic victims: A population-based study of suicide victims during the years 1988-2002 in Northern Finland. Epilepsy Behav. 2007;11:389–93. doi: 10.1016/j.yebeh.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Mehta S, Tyagi A, Tripathi R, Kumar M. Study of inter-relationship of depression, seizure frequency and quality of life of people with epilepsy in India. Ment Illn. 2014;6:5169. doi: 10.4081/mi.2014.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seshadri V, Thomas J, Murthy J, Raju C. Prevalence of depression and its effect on quality of life in patients with epilepsy: A community based study and a comprehensive rural epilepsy study in South India (CRESSI) Neurol Asia. 2004;9:139. [Google Scholar]
- 21.Pandian GB, Mohan A. Study on prevalence of depression and adverse drug reactions among South Indian epileptic patients. Asian J Pharm Clin Res. 2015;8:73–6. [Google Scholar]
- 22.Lee SA, Lee SM, No YJ. Factors contributing to depression in patients with epilepsy. Epilepsia. 2010;51:1305–8. doi: 10.1111/j.1528-1167.2009.02387.x. [DOI] [PubMed] [Google Scholar]
- 23.Yousafzai AU, Yousafzai AW, Taj R. Frequency of depression in epilepsy: A hospital based study. J Ayub Med Coll Abbottabad. 2009;21:73–5. [PubMed] [Google Scholar]
- 24.Kanner AM, Balabanov A. Depression and epilepsy: How closely related are they? Neurology. 2002;58(8 Suppl 5):S27–39. doi: 10.1212/wnl.58.8_suppl_5.s27. [DOI] [PubMed] [Google Scholar]
- 25.Kanner AM. Depression in epilepsy: A neurobiologic perspective. Epilepsy Curr. 2005;5:21–7. doi: 10.1111/j.1535-7597.2005.05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazarati A, Shin D, Auvin S, Caplan R, Sankar R. Kindling epileptogenesis in immature rats leads to persistent depressive behavior. Epilepsy Behav. 2007;10:377–83. doi: 10.1016/j.yebeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen SD, Wang YL, Liang SF, Shaw FZ. Rapid amygdala kindling causes motor seizure and comorbidity of anxiety- and depression-like behaviors in rats. Front Behav Neurosci. 2016;10:129. doi: 10.3389/fnbeh.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amir M, Roziner I, Knoll A, Neufeld MY. Self-efficacy and social support as mediators in the relation between disease severity and quality of life in patients with epilepsy. Epilepsia. 1999;40:216–24. doi: 10.1111/j.1528-1157.1999.tb02078.x. [DOI] [PubMed] [Google Scholar]
- 29.Tegegne MT, Mossie TB, Awoke AA, Assaye AM, Gebrie BT, Eshetu DA. Depression and anxiety disorder among epileptic people at Amanuel Specialized Mental Hospital, Addis Ababa, Ethiopia. BMC Psychiatry. 2015;15:210. doi: 10.1186/s12888-015-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogunrin OA, Obiabo YO. Depressive symptoms in patients with epilepsy: Analysis of self-rating and physician's assessment. Neurol India. 2010;58:565–70. doi: 10.4103/0028-3886.68679. [DOI] [PubMed] [Google Scholar]
- 31.Dias R, Bateman LM, Farias ST, Li CS, Lin TC, Jorgensen J, et al. Depression in epilepsy is associated with lack of seizure control. Epilepsy Behav. 2010;19:445–7. doi: 10.1016/j.yebeh.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Jacoby A, Baker GA, Steen N, Potts P, Chadwick DW. The clinical course of epilepsy and its psychosocial correlates: Findings from a U.K. Community study. Epilepsia. 1996;37:148–61. doi: 10.1111/j.1528-1157.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 33.Thapar A, Roland M, Harold G. Do depression symptoms predict seizure frequency – Or vice versa? J Psychosom Res. 2005;59:269–74. doi: 10.1016/j.jpsychores.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Ojemann LM, Friel PN, Trejo WJ, Dudley DL. Effect of doxepin on seizure frequency in depressed epileptic patients. Neurology. 1983;33:646–8. doi: 10.1212/wnl.33.5.646. [DOI] [PubMed] [Google Scholar]
- 35.Mula M, Sander JW. Negative effects of antiepileptic drugs on mood in patients with epilepsy. Drug Saf. 2007;30:555–67. doi: 10.2165/00002018-200730070-00001. [DOI] [PubMed] [Google Scholar]
- 36.Trimble MR, Rüsch N, Betts T, Crawford PM. Psychiatric symptoms after therapy with new antiepileptic drugs: Psychopathological and seizure related variables. Seizure. 2000;9:249–54. doi: 10.1053/seiz.2000.0405. [DOI] [PubMed] [Google Scholar]
- 37.Bifftu BB, Dachew BA, Tiruneh BT, Birhan Tebeje N. Depression among people with epilepsy in Northwest Ethiopia: A cross-sectional institution based study. BMC Res Notes. 2015;8:585. doi: 10.1186/s13104-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr MP, Mensah S, Besag F, de Toffol B, Ettinger A, Kanemoto K, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia. 2011;52:2133–8. doi: 10.1111/j.1528-1167.2011.03276.x. [DOI] [PubMed] [Google Scholar]
- 39.Meyer B, Weiss M, Holtkamp M, Arnold S, Brückner K, Schröder J, et al. Protocol for the ENCODE trial: Evaluating a novel online depression intervention for persons with epilepsy. BMC Psychiatry. 2017;17:55. doi: 10.1186/s12888-017-1229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


