Abstract
Carbon monoxide (CO) inhalation is nowadays the most common cause of fatal poisoning worldwide. CO binds to haemoglobin 230–270 times more avidly than oxygen, thus leading to formation of carboxyhaemoglobin with subsequent reduction of tissue oxygenation. Brain is mainly affected due to its high oxygen requirement. Up to two-thirds of patients who survive the acute phase of this pathology present a delayed leukoencephalopathy, usually in a period ranging from two to 40 days. White matter damage closely relates to long-term prognosis of these patients. On the other hand CO seems to play a fundamental role as a possible neuro-protective agent in ischaemic stroke. Diagnostic imaging, with computed tomography and magnetic resonance imaging, especially magnetic resonance spectroscopy, is very useful to depict the presence and extension of this pathology, in both acute and late phase. Nevertheless, a correlation of imaging studies with clinical history and laboratory data is fundamental to perform the correct diagnosis. The purpose of this article is to highlight the imaging features of brain CO poisoning in acute and late phase, describing a case report of a 56-year-old man found unconscious at home.
Keywords: Brain CO poisoning, leukoencephalopathy, diffusion magnetic resonance imaging, magnetic resonance spectroscopy
Introduction
Carbon monoxide (CO) is a colourless, odourless and tasteless gas resulting from the incomplete burning of carbon-containing substances due to insufficient oxygen supply to enable complete oxidation to carbon dioxide. CO exposure represents an important health issue since this gas is toxic and it can kill in the case of exposure to high levels.
Accidental CO exposure is usually from car exhaust, boilers, central heating systems or cookers; other possible causes of CO exposure include blocked chimneys or burning fuel in an enclosed or unventilated space.
In Australia and in several Asian countries, however, CO accidental exposure accounts for less than 10% of emergency calls about CO poisoning, the wide majority resulting from voluntary suicidal exposure, usually by charcoal burning or car exhausting.
CO poisoning occurs in the case of inhalation of high levels of CO and it represents a common cause of poisoning worldwide with an estimated 50,000 annual exposures in the USA. Every year in the UK more than 200 people are admitted to hospital with carbon monoxide poisoning, which leads to about 50 deaths per year.
CO toxicity primarily depends on impaired oxygen transport by haemoglobin as CO binds to haemoglobin 230–270 times more avidly than oxygen, thus leading to formation of carboxyhaemoglobin (COHb); as COHb cannot carry oxygen, this causes tissue hypoxia.1
Moreover, CO binds to cytochrome c oxidase, resulting in impairment of adenosine triphosphate synthesis and inhibition of mitochondrial metabolism, leading to cellular respiratory dysfunction.
Other mechanisms of damage include platelet-to-neutrophil aggregation and neutrophil degranulation, leading to release of myeloperoxidase, resulting in oxidative stress and apoptosis.
A theory regarding catecholamine crisis was recently suggested as a novel underlying pathophysiological mechanism in acute CO poisoning. According to this theory, CO-associated hypoxia causes an increase of dopamine and a decrease of dopamine turnover rates.2 Dopamine excess, during the acute phase of CO intoxication, could be a major contributor to neurotoxicity.
CO presents an affinity for myoglobin about 60 times greater than that of oxygen; CO bound to myoglobin causes reduced cardiac output and hypotension, which may result in brain ischaemia.
Organs with the highest oxygen requirement are the most susceptible to damage; in fact brain and cardiac effects dominate clinical features.
Clinical presentation of CO poisoning strongly depends on levels of exposure. A tension-type headache is the most common symptom of mild poisoning; other symptoms include flu-like effects, dizziness, nausea and vomiting, confusion, stomach pain, shortness of breath and subtle cognitive impairment.
Long-term exposure to low levels of CO can also lead to neurological symptoms, such as difficulty in concentration and frequent emotional changes.
Patients with severe intoxication typically demonstrate impaired mental state and personality changes, vertigo, ataxia, tachycardia, chest pain caused by angina, seizures and also loss of consciousness, coma and death.
Some patients who survive the condition of acute CO intoxication may develop neuropsychiatric sequelae in the following weeks, usually within one month.
These sequelae vary widely and are estimated to occur in up to two-thirds of patients.3,4
Delayed neurological syndrome (DNS) is characterized by acute onset of neuropsychiatric, behavioural symptoms and motor dysfunction5 after a lucid interval that ranges from two to 40 days. Patients may present confusion, urinary incontinence, emotional disorders, gait disturbances and, in rare cases, Parkinsonism and dementia with subacute onset.6
DNS can follow any event causing a prolonged cerebral hypo-oxygenation and is usually preceded by a period of unconsciousness; nevertheless about 10% of cases associated with CO poisoning may not present loss of consciousness.
The mechanism responsible for neurological delayed effects seems to be related to chemical mediators leading to brain lipid peroxidation, which causes delayed reversible demyelinization of cerebral white matter, known as Grinker myelinopathy.4
Identification of patients at risk of developing DNS plays an important role in the setting of these patients. Today, the best identified risk factors for long-term neurological effects are early and obvious neurological damage and a sustained loss of consciousness during CO exposure.3
A retrospective cohort study divided 79 consecutive patients treated for CO poisoning after attempting suicide into a group of 66 patients who did not develop DNS and a group of 13 who did, comparing the two groups in terms of different items, in order to find predictors for developing DNS after CO poisoning.
Results showed that more severe consciousness disturbance at time of hospital admittance, positive computed tomography (CT) findings of diffuse white matter involvement, abnormally high CKMB and LDH enzyme levels and aging could be considered as risk factors for developing DNS.7
It is interesting to note that the non-DNS developing group had a higher mean COHb level than the other group.
Recently, neuron-specific enolase, a glycolytic enzyme localized primarily to the neuronal cytoplasm and S100B, a calcium-binding protein localized to astroglial cells, showed a promising role as a marker of brain injury. In particular a serum S100B of more than 0.165 µg/l predicted DNS with a sensitivity of 90% and a specificity of 87%.3,8
DNS can improve within 1–2 years in approximately 60–70% of patients;4 the possibility of full recovery appears to be inversely related with age.9
CO poisoning and cerebral ischaemia: pathophysiology and possible therapeutic approaches
A complex and, in a sense, controversial association is present between CO poisoning and brain ischaemia.
An increase in blood level of COHb, as we mentioned above, leads to hypoxia, brain oedema and neuronal cell death.
Moreover a recent study comparing a CO-poisoning cohort with a control cohort without CO exposure has demonstrated that long-term risk of ischaemic stroke is nearly 2.5-fold greater in the first group;10 however, the mechanisms are still widely unclear.
On the contrary, we now know that CO is produced in every cell of healthy subjects by enzymes known as the heme oxygenases, which act on heme substrate to originate ferrous iron, biliverdin and CO.
There are two isoforms of this enzyme: HO-1, inducible, presenting a low basal activity in brain but highly induced in condition of stress (i.e. ischaemia), and HO-2, constitutive and highly expressed in brain.
It has been demonstrated that, in mice, inhalation of low-concentration CO (250 ppm for 18 h immediately after middle cerebral artery occlusion) is neuroprotective in the early stages of reperfusion after ischaemic stroke, significantly reducing the infarct size, even if no significant effects on behavioural deficits are observed.10 This neuroprotective effect depends on the up-regulation of the Nrf2 pathway, Nrf2 being a transcriptional factor, considered to be a multi-organ protector for its antioxidant effects.
This protective effect was in fact absent in mice deficient in Nrf2.
The time window for CO administration appears to be a crucial aspect for its biological effect that still has to be elucidated.11–13
Nowadays, treatment of patients with CO poisoning is primarily supportive, with administration of high-flow oxygen. In the acute phase, the principal treatments for CO poisoning are high-flow oxygen treatment or hyperbaric oxygen therapy (HBOT); the latter is often recommended for patients showing loss of consciousness in severe poisoning. HBOT facilitates oxygen transport to tissues and it is currently known as the most common method for treating CO poisoning.14 HBOT is warranted for patients with serious intoxication, loss of consciousness, neurological deficits, significant metabolic acidosis and COHb levels higher than 25%. Nevertheless its use is controversial due to insufficient evidences of efficacy and, as hypoxic stress is not the only pathophysiologic mechanism in CO poisoning, its role is likely over-rated.15 Its therapeutic effect may be enhanced if combined with other treatments.
In recent years a therapeutic role of HBOT in ischaemic stroke has been postulated.
A prospective study, in particular, demonstrated a better clinical outcome in patients with mild disability after acute ischaemic stroke who received HBOT for two weeks, within 3–5 days after stroke onset, than in patients who received only conventional antiplatelet therapy.16 The therapeutic role of HBOT could depend on the improvement of cerebral blood flow, on the promotion of collateral circulation and on its anti-inflammatory and anti-apoptotic properties.
It is generally acknowledged that HBOT seems to be more efficient the sooner it is applied but currently its effectiveness in the treatment of acute ischaemic stroke still has to be elucidated.17
Targeted temperature management with mild therapeutic hypothermia seems to represent a promising treatment for CO poisoning. Also, based on the catecholamine crisis theory, administration of sympatholytics such as dexmethetomidine or remifentanil may be beneficial in preventing dopamine excess.15 Corticosteroid therapy plays a role in the treatment of delayed leukoencephalopathy as this phenomenon depends on ischaemic changes but also demyelination and inflammation involving myelin basic protein; therefore it is reasonable to administer corticosteroid pulse therapy.17
Treatment of CO poisoning, however, still remains controversial.
Role of neuroimaging
Neuroimaging plays an important role in the study of this entity both in the acute phase, to confirm the clinical diagnosis and provide prompt treatment, and in the chronic phase to evaluate possible neurological sequelae, since neuroimaging shows correlation with clinical outcome of these patients.
CT-scan of the brain is a very fast and widely available examination and, for these reasons, it represents in most cases the first examination performed in these patients.
CT-scan in the acute phase of CO poisoning typically demonstrates focal, bilateral and symmetrical hypodensities affecting the globus pallidus, as a result of necrosis of these structures. Selective damage of globus pallidus in CO poisoning could depend on the hypotensive effects of CO on the poor anastomotic vascular supply of these structures, or on a direct binding of CO to the heme iron in the globus pallidus, one of the regions of the brain with the highest iron content.4 In rare cases, the globus pallidus could be spared, and involvement of the caudate nucleus, putamen or thalamus could be observed. Involvement of the cerebellum or brainstem in the acute phase is rare and it seems to reflect a more severe poisoning, since these posterior structures are more resistant to hypoxia. Cortical involvement in the acute phase is far less frequent and, if present, usually involves the temporal lobe and hippocampus.
Magnetic resonance imaging (MRI) can be useful in the acute phase to depict the pathological findings of this disease, showing altered signal intensity areas involving the globus pallidus bilaterally, which will result hypointense in T1-w sequences and hyperintense in T2-w and FLAIR sequences.18 Diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) maps often show areas of restricted diffusivity in the globus pallidus, as for cytotoxic oedema.19
In a subsequent phase, CT examination may show diffuse hypodense areas in the bilateral periventricular white matter and centrum semiovale as a result of extensive demyelination. In more severe cases, this process can extend to involve the internal and external capsules, corpus callosum and subcortical white matter.
In this phase MRI can better depict the real extent of the demyelinating process, revealing areas of hypointensity in T1-w images and hyperintensity in T2-w and FLAIR sequences in the bilateral white matter, partially confluent. DWI and ADC maps usually reveal in these areas restriction in water diffusivity referable to cytotoxic oedema and demyelination.20
CT and MRI findings of chronic phase CO poisoning are represented by brain atrophy, depicted as areas of sulcal widening and increased ventricle-to-brain ratio.
Emergency management
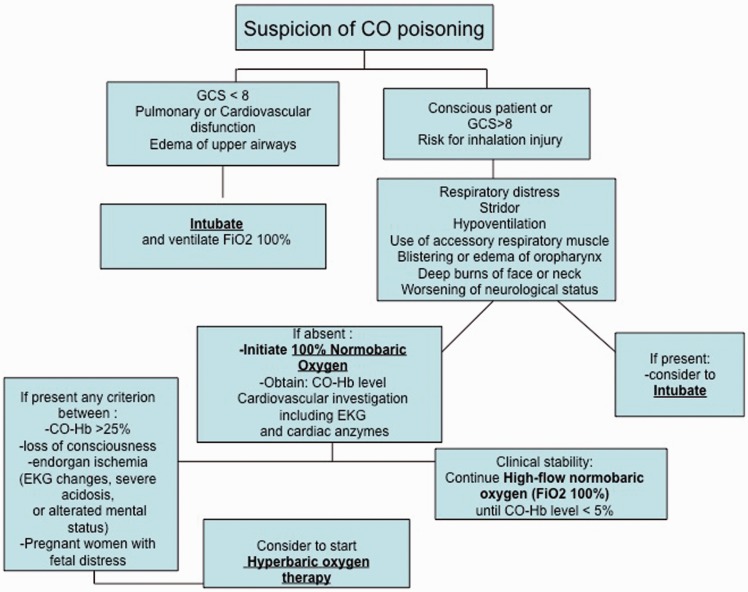
In the case of clinical suspicion of acute CO poisoning, immediate and direct assessment of the respiratory and circulatory status of the patient should be performed. Intubation should be considered if respiratory distress, stridor or hypoventilation are present, in order to obtain adequate airway protection and ventilation. CO exposure can cause cardiac injury, therefore cardiovascular investigations including electrocardiograms and measurement of cardiac enzymes (troponin and CK-MB) is required.21 COHb levels in arterial blood should be measured to confirm the diagnosis. If diagnosis is confirmed, treatment with normobaric or hyperbaric high-flow oxygen, mainly depending on COHb levels, should be started (Figure 1).
Figure 1.
Suggested emergency management of patients with suspicion of carbon monoxide poisoning.
CO: carbon monoxide; GCS: Glasgow coma scale; EKG: electrocardiogram; COHb: carboxyhaemoglobin
It is important to ensure that the patient is resting in order to decrease the oxygen demand.
However, the COHb level at which hyperbaric oxygen therapy should be performed is controversial.
Methodology
A comprehensive review of the literature was performed using Pubmed and Medline databases, typing the keywords ‘CO brain poisoning’, ‘CO brain neuroimaging’, ‘CO poisoning MRI’, ‘CO poisoning, ischaemic stroke’ and ‘CO poisoning therapy’.
Review articles from the last 15 years were preferred.
Discussion of issues
Most of these articles highlight the role of neuroimaging to perform prompt diagnosis of CO brain poisoning.
CT is considered as a first step technique in the evaluation of patients with suspicion of poisoning; it is particularly useful in the case of loss of consciousness.
Its main limitation depends on the difficulty of evaluating the real extent of the white matter impairment, which, according to many authors, correlates with prognosis in these patients.4
MRI is a second step technique that has been performed in the evaluation of this condition since the end of the 1980s. MRI is more sensitive than CT in assessing the extent of involvement of the white matter, which, according to many reports, closely relates with prognosis.
Moreover, DWI imaging with corresponding ADC maps represents nowadays a fundamental diagnostic tool to evaluate cytotoxic oedema and, in subacute and chronic phases, progressive demyelination, responsible for the development of delayed neurological sequelae.
It is important to highlight that DWI positivity persists even for some months differently from cytotoxic oedema, which usually resolves in about four weeks.
Functional MR techniques have been developed in recent years in order to obtain further diagnostic and prognostic information.
MR spectroscopy (MRS) enables non-invasive monitoring of metabolic changes in brain tissue. This technique could play an important role in the diagnosis and follow-up of this entity.
An increase in choline (Cho) levels and of the Cho/creatine (Cr) ratio reflects active membrane metabolism associated with several pathological conditions such as degeneration and gliosis. From three to four weeks after clinical onset, moreover, a decrease of the N-acetylaspartate (NAA)/Cr ratio can be observed as a result of neuronal loss. It is noteworthy that reductions in NAA levels have been proposed as a good prognostic factor, as the NAA/Cr ratio correlates with symptoms development.4 The presence of lactate represents anaerobic glycolysis under ischaemic or hypoxic conditions. Lactate began to appear at the earliest from one to two months after CO poisoning. Its presence is considered as a marker of irreversible brain damage because it has been observed in patients with severe chronic symptoms.
However, even though MRS provides valuable information on brain metabolism in patients with delayed encephalopathy after acute CO exposure, long-term outcome is still complicated since patients with severe, initial MRS measurements may show reversibility;22 hence a careful and accurate follow-up of these patients appears to be crucial.
Diffusion tensor imaging (DTI) has been applied to evaluate CO poisoning since 2005. DTI evaluates the directionality of water diffusion in cerebral tissue. Normal white matter tracts show a higher degree of fractional anisotropy since the tracts are highly directional. On the contrary, if myelination is compromised, fractional anisotropy values are expected to decrease.23 DTI can provide a quantitative assessment of the degree of demyelination and it could offer a predictor for the development of DNS. Moreover, longitudinal assessment of DTI parameters could be very useful to evaluate the efficacy of treatments.24
The main limitation of MRI is its being time-consuming and difficult to perform in uncooperative patients.
Case report
Informed consent was obtained from the patient.
A 56-year-old man was found unconscious at home by his wife. On arrival at the Emergency Department, the patient underwent endotracheal intubation for airway protection. Physical examination revealed normal blood pressure and heart rate; Glasgow coma scale score was 8. Laboratory examinations were unremarkable except for mild leukocytosis (13,800/mm3).
Haemogasanalysis revealed: pH 7.28, pCO2 25.4 mmHg, pO2 93.6 mmHg, Hb 16.4 g/dl, a level of COHb of 28.7 %, cLactate 91 mg/dl.
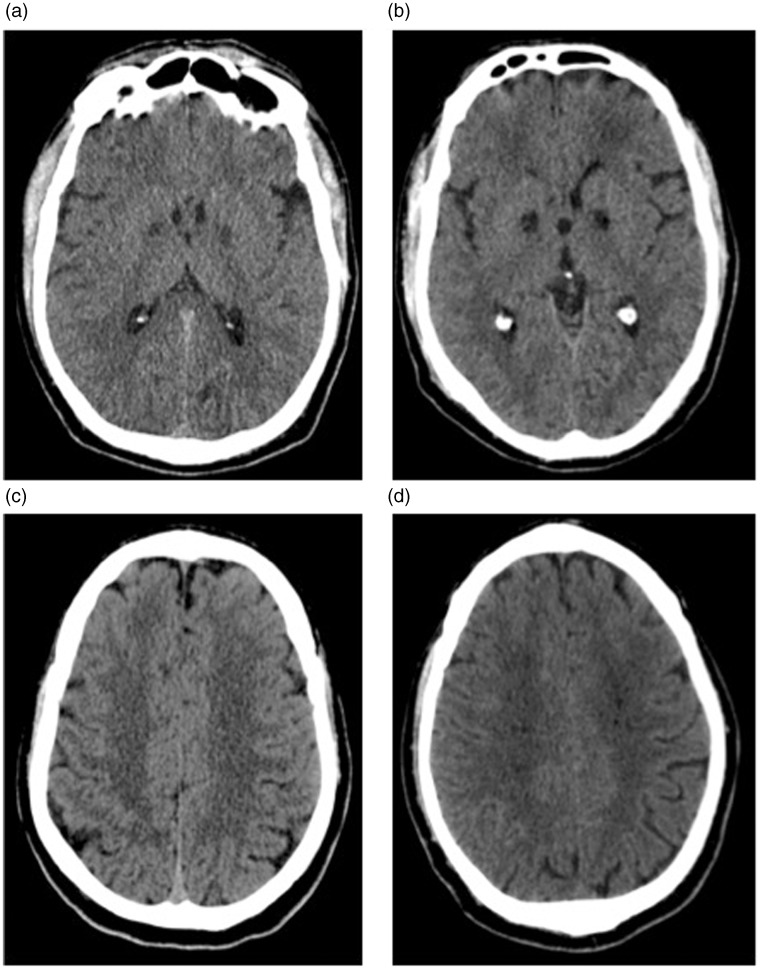
The patient underwent CT brain examination (Brilliance 16 CT scanner; Philips, Amsterdam, Netherlands) that showed bilateral and symmetrical hypodensities affecting the globus pallidus (Figure 2(a)). No other significant findings were observed (Figure 2(c)). Acute cerebral CO poisoning was diagnosed and high-flow oxygen therapy was immediately administered. The patient was then moved, on the same day, to another hospital in another town to perform hyperbaric oxygen therapy, which was not performed due to a reduction of COHb levels after high-flow oxygen therapy.
Figure 2.
(a) and (c): mildly hypodense areas bilaterally affecting globus pallidus; no relevant findings in the centrum semiovale. (b) and (d): Six weeks follow-up showing malacic evolution of the bilateral and symmetrical hypodensities in the globus pallidus. Appearance of microlacunar lesions bilaterally involving centrum semiovale.
The patient was finally discharged after six days, in relatively good physical condition, despite some neurological impairments.
About six weeks later, the patient returned to the Emergency Department of our hospital; he presented temporo-spatial disorientation with mild gait impairment characterized by steppage and tendency to retropulsion.
CT brain examination, on the same scanner, was then repeated.
CT examination showed malacic evolution of the hypodensities bilaterally affecting the globus pallidus (Figure 2(b)); moreover, some hypodensities bilaterally involving the white matter of the centrum semiovale, with microlacunar appearance, were observed (Figure 2(d)).
A brain MRI examination was then requested.
MRI examination was performed on a 3T MRI scan (GE Signa Excite, Healthcare Technologies, Milwaukee, Wisconsin) with acquisition of sequences in axial, sagittal and coronal planes.
In particular the following sequences were acquired: FLAIR CUBE (TR: 11000 ms, TE: 112 ms, slice thickness: 1.2 mm, NEX: 1, with a matrix of 512 × 512), axial FSE T2-weighted (TR: 6500 ms, TE: 106 ms, slice thickness: 4 mm, NEX: 2, with a matrix of 512 × 512), axial gradient-echo (TR: 640 ms, TE: 7 ms, slice thickness: 4 mm, NEX: 1, with a matrix of 512 × 512), axial BRAVO (TR: 450 ms, TE: 3.24 ms, slice thickness: 1 mm, NEX: 1, with a matrix of 512 × 512). b1000 DWI and ADC maps were further acquired; moreover, cerebral proton MRS was performed with multivoxel short echo-time (144 ms) PRESS sequence.
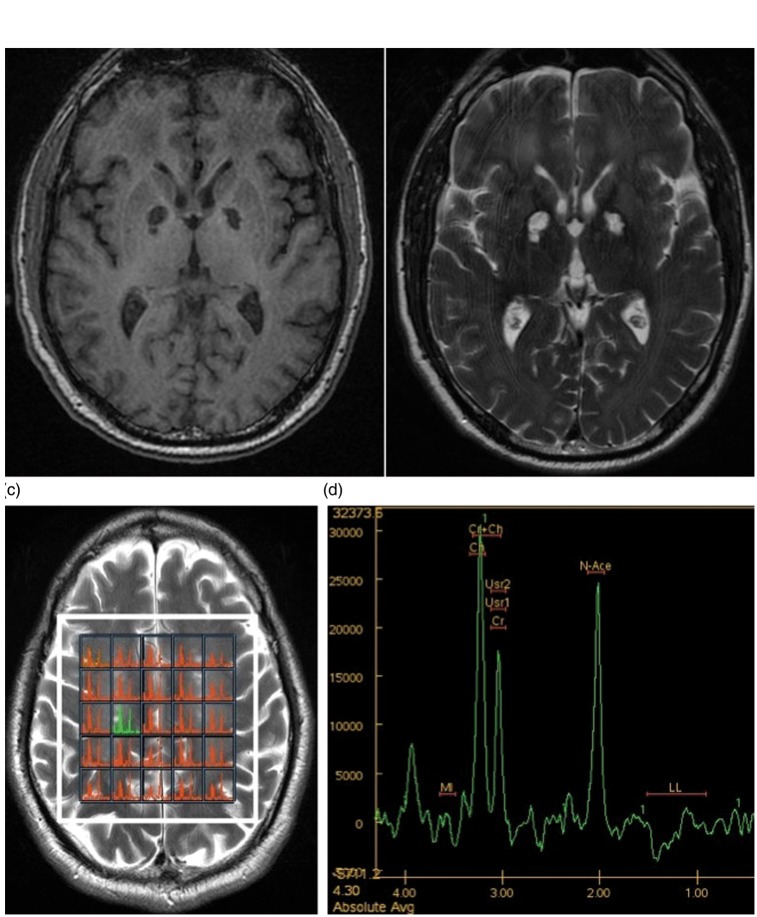
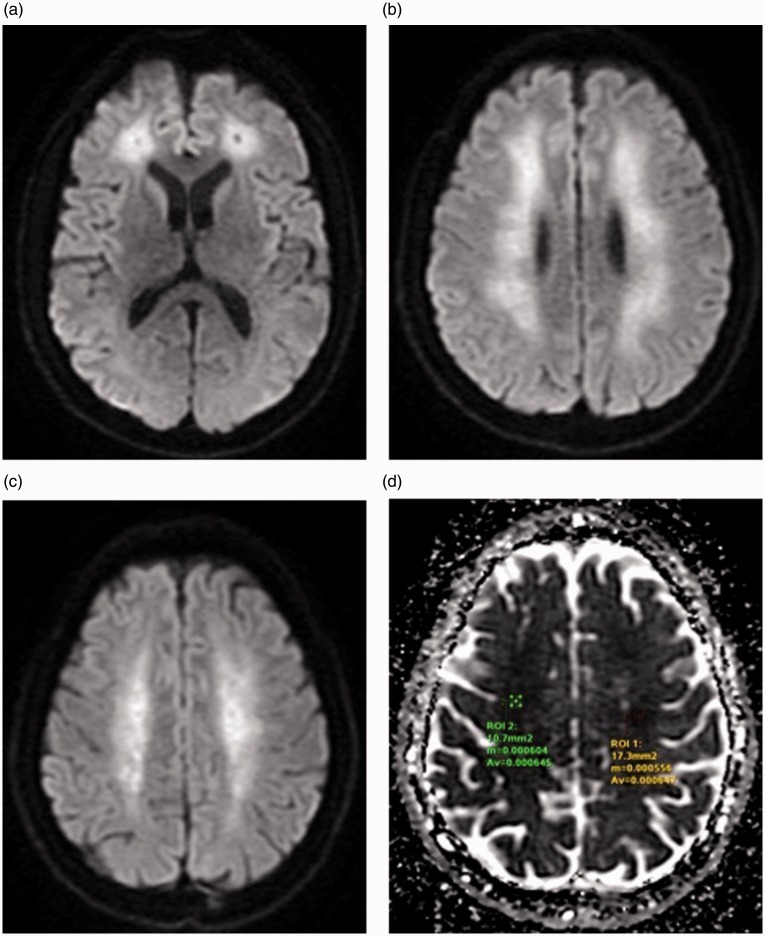
MRI examination showed multiple altered signal intensities areas, partially confluent, bilaterally involving periventricular white matter, the corona radiata and centrum semiovale. These lesions appeared mildly hypointense on T1-weighted sequences (Figure 3(a)) and hyperintense on T2-weighted (Figure 3(b)) and FLAIR sequences (Figure 4(a) to (d)).
Figure 3.
(a) and (b): T1- and T2-weighted sequences showing bilateral and symmetrical infarctions in the globus pallidus. (c) and (d): H-MR spectrum in the right centrum semiovale showing increase in choline/creatine ratio, slight reduction of N-acetylaspartate and evidence of lactate peak. These findings show demyelination, loss or degeneration of neurons and activation of anaerobic metabolism.
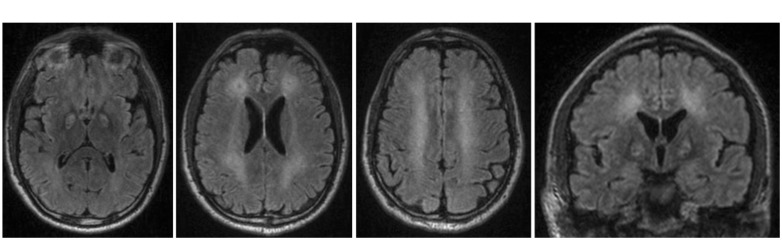
Figure 4.
(a) to (d): axial and coronal FLAIR sequences bilaterally showing infarction of nucleus pallidus and multiple lesions involving the periventricular white matter of corona radiata and centrum semiovale.
An axial DWI sequence depicted diffuse hyperintensities in these areas (Figure 5(a) to (c)) with corresponding low values on the ADC map, consisting with areas of restricted diffusion. In fact mean ADC values were 0.000645 and 0.000647 mm2/s respectively in white matter of right and left centrum semiovale (Figure 5(d)).
Figure 5.
(a) to (c): diffusion weighted images showing multiple, bilateral areas of restricted diffusivity involving the periventricular white matter and centrum semiovale as a result of cytotoxic oedema and progressive demyelination. (d): apparent diffusion coefficient map values bilaterally confirm the decreased diffusivity of these areas compared with normal white matter.
MRS showed an increase in the Cho/Cr ratio, a decrease in NAA and a mild increase in lactate (Figure 3(c) and (d)).
The patient currently shows slight improvements of his neurological condition, above all regarding the gait impairment; however, temporo-spatial disorientation is still present.
Imaging protocol
An adequate imaging protocol in a patient with suspicion of CO poisoning should start with CT brain examination, particularly if loss of consciousness is present.
After that, an MRI examination should be performed to confirm CT findings and to obtain a detailed assessment of the white matter. DWI with ADC maps represents nowadays a fundamental tool to clarify the extent of brain injury and to provide further diagnostic and prognostic information. MRS and DTI represent promising techniques to identify patients at higher risk of developing DNS or presenting more severe demyelination.
It is difficult to define a standardized follow-up imaging protocol since the pathogenesis of DNS has not been delineated fully.
However, a closer follow-up is recommended for patients presenting a more severe onset with prolonged loss of consciousness, who are elderly and with diffuse white matter damage, who are considered to be at higher risk of developing DNS.
Teaching points
CO poisoning is the most common cause of fatal poisoning worldwide.
Neuroimaging plays a fundamental role in the diagnosis and prognosis of this disease both in the acute and in the chronic phase.
A consistent number of patients presenting brain CO poisoning (up to two-thirds) will suffer a delayed leukoencephalopathy with acute clinical onset of neurological and neuropsychiatric symptoms after a lucid interval of 2–40 days.
A controversial association is present between CO poisoning and brain ischaemia since a recent study demonstrated a 2.5-fold greater incidence of ischaemic stroke in a CO-poisoning cohort than in a control cohort without CO exposure.
It is time to reconsider the new role of CO, since CO has been demonstrated to be a neuroprotective agent with a promising therapeutic role in ischaemic and haemorrhagic stroke.
It will be important in the future to clarify the promising role of HBOT in the treatment of DNS. Moreover it will be necessary to understand whether CO can fulfil an effective role in the treatment of ischaemic stroke as a fundamental cerebral mediator.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol 2015; 131: 191–203. [DOI] [PubMed] [Google Scholar]
- 2.Park EJ, Min YG, Kim GW, et al. Pathophysiology of brain injuries in acute carbon monoxide poisoning: A novel hypothesis. Med Hypotheses 2014; 83: 186–189. [DOI] [PubMed] [Google Scholar]
- 3.Chiew AL, Buckley NA. Carbon monoxide poisoning in the 21st century. Crit Care 2014; 18: 221. [Google Scholar]
- 4.Beppu T. The role of MR imaging in assessment of brain damage from carbon monoxide poisoning: A review of the literature. AJNR Am J Neuroradiol 2014; 35: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkins RO, Woon FL. Neuroimaging, cognitive, and neurobehavioural outcomes following carbon monoxide poisoning. Behav Cogn Neurosci Rev 2006; 5: 141–155. [DOI] [PubMed] [Google Scholar]
- 6.Mizuno Y, Sakurai Y, Sugimoto I, et al. Delayed leukoencephalopathy after carbon monoxide poisoning presenting as subacute dementia. Intern Med 2014; 53: 1441–1445. [DOI] [PubMed] [Google Scholar]
- 7.Kudo K, Otsuka K, Yagi J, et al. Predictors for delayed encephalopathy following acute carbon monoxide poisoning. BMC Emerg Med 2014; 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park E, Ahn J, Min YG, et al. The usefulness of the serum s100b protein for predicting delayed neurological sequelae in acute carbon monoxide poisoning. Clin Toxicol 2012; 50: 183–188. [DOI] [PubMed] [Google Scholar]
- 9.Shprecher D, Mehta L. The syndrome of delayed post-hypoxic leukoencephalopathy. Neurorehabilitation 2010; 26: 65–72. [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CW, Chen WK, Hung DZ, et al. Association between ischemic stroke and carbon monoxide poisoning: A population-based retrospective cohort analysis. Eur J Intern Med 2016; 29: 65–70. [DOI] [PubMed] [Google Scholar]
- 11.Queiroga CS, Vercelli A, Vieira HL. Carbon monoxide and the CNS: Challenges and achievements. Br J Pharmacol 2015; 172: 1533–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanafy KA, Oh J, Otterbein LE. Carbon monoxide and the brain: Time to rethink the dogma. Curr Pharm Des 2013; 19: 2771–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang B, Cao W, Biswal S, et al. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke 2011; 42: 2605–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver LK. Hyperbaric oxygen therapy for carbon monoxide poisoning. Undersea Hyperb Med 2014; 41: 339–354. [PubMed] [Google Scholar]
- 15.Sungho O, Sang-Cheon C. Acute carbon monoxide poisoning and delayed neurological sequelae: A potential neuroprotection bundle therapy. Neural Regen Res 2015; 10: 36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CH, Chen SY, Wang V, et al. Effects of repetitive hyperbaric oxygen treatment in patients with acute cerebral infarction: A pilot study. Scis World J 2012; 2012: 694703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Z, Tong WC, Lu X-X, et al. Hyperbaric oxygen therapy in acute ischemic stroke: A Review. Interv Neurol 2014; 2: 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedge AN, Mohan S, Lath N, et al. Differential diagnosis for bilateral abnormalities of the basal ganglia and thalamus. Radiographics 2011; 31: 5–30. [DOI] [PubMed] [Google Scholar]
- 19.Sener RN. Acute carbon monoxide poisoning: Diffusion MR imaging findings. AJNR Am J Neuroradiol 2003; 24: 1475–1477. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JH, Chang KH, Song IC, et al. Delayed encephalopathy of acute carbon monoxide intoxication: Diffusivity of cerebral white matter lesions. AJNR Am J Neuroradiol 2003; 24: 1592–1597. [PMC free article] [PubMed] [Google Scholar]
- 21.Tanizaki S. Assessing inhalation injury in the emergency room. Open Access Emerg Med 2015; 20: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen MB, Kondziella D, Danielsen ER, et al. Cerebral proton magnetic resonance spectroscopy demonstrates reversibility of N-acetylaspartate/creatine in gray matter after delayed encephalopathy due to carbon monoxide intoxication: A case report. J Med Case Rep 2014; 8: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YT, Chen SY, Lo CP, et al. Utilizing cerebral perfusion scan and diffusion-tensor MR imaging to evaluate the effect of hyperbaric oxygen therapy in carbon monoxide-induced delayed neuropsychiatric sequelae – a case report and literature review. Acta Neurol Taiwan 2015; 24: 57–62. [PubMed] [Google Scholar]
- 24.Kuroda H, Fujihara K, Takahashi S, et al. A case of delayed encephalopathy after carbon monoxide poisoning longitudinally monitored by diffusion tensor imaging. AJNR Am J Neuroradiol 2012; 33: E52–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]