Abstract
Recent studies have pointed out dysfunction and histopathological changes of the choroid plexuses (CPs) with aging. This paper reviews apparent diffusion coefficient (ADC) values of the CPs for age-related changes. All the brain MR images of the patients between January 2013 and June 2014 in our Radiology Department were retrospectively investigated. Patients with major cranial abnormalities (brain tumors, hyperacute or acute ischemia, developmental anomalies, hemorrhage, hydrocephaly) were excluded. Diffusion-weighted images were obtained at the parameter values of b = 1000 s/mm2 in the axial plane. The transverse diameters of the lateral ventricles (LVs) and ADC values of both CPs were measured. Brain MRIs of 202 individuals, 97 men (48%), 105 women (52%), were studied. There were statistically significant positive correlations between the ADC values of CP and patient ages. (Right CP: r = 0.623; p < 0.05. Left CP: r = 0.654; p < 0.05). There were positive correlations between LV diameters and age (r = 0.624, p < 0.05 for the right LV; r = 0.621, p < 0.05 for the left LV). The ADC values of age groups significantly differed (p < 0.05); the ≥61-year-old group was significantly higher compared to younger individuals. There is a progressive increase of water diffusivity in the CPs during aging. ADC values should be considered as a neuroimaging quantitative biomarker in normal aging-dementia syndromes.
Keywords: Aging, brain, choroid plexus, diffusion magnetic resonance imaging
Introduction
Choroid plexuses (CPs) and their secretion, cerebrospinal fluid (CSF), have an essential function in the central nervous system (CNS) physiology. CPs are strategically positioned in lateral ventricles (LVs), which are the major site for CSF secretion. The CP-CSF system plays an active role in the development, homeostasis, and repair of the CNS, providing physical protection of the brain, removing toxic metabolites, synthesizing trophic and angiogenic factors, carrier proteins, and chemorepellents, and acting as a nursery for neuronal and astrocytic progenitor cells. Proteins that have a specific role in brain homeostasis such as transthyretin, transferring, insulin-like growth factor II, and binding proteins are secreted from the CP to the CSF. The CP epithelium forms a physical barrier between blood and the CSF (the blood-CSF barrier, BCSFB) on the grounds of the complex tight junctions between adjacent epithelial cells.1
Human and animal studies have shown that the morphology and functions of CPs and CSF are altered during aging. Ventricular volume increases by 25%–30% in 50- to 80-year-old individuals compared to 20- to 40-year-old individuals. A significant reduction in CSF secretion,2 an increase in the resistance of CSF drainage, a decrease in CSF flow, and an increase in the protein content of the CSF are fundamental changes during aging.3 Histopathologically, the height of CP epithelial cells decreases about 10%–11% by age 88 years; the aged epithelial cell cytoplasm becomes rich with Biondi ring tangles and lipofuscin age-pigment deposits, and the nuclei appear irregular and flattened. The basement membrane becomes thicker, coarser, and more irregular. The stroma also thickens and contains collagen fibers, hyaline bodies, calcifications, and psammoma bodies, and the infiltrating arteries become thicker and fragmented.1,4,5
The histopathological transformations of the CP have been found to be correlated with CP functions of absorption, secretion, and filtration.6 The metabolic changes in the CP were demonstrated in experimental studies; total adenosine triphosphatase (ATPase) activity and sodium-potassium (Na/K) ATPase activity are lower in older rats compared to young rats.1 Recent studies showed a decline in the messenger RNA (mRNA) expression of Na/K ATPase along with water channel Aquaporin 1 protein and mRNA expression. Since the secretion of CSF depends on ion and water translocation, the rate of CSF secretion decreases with age.1,3,7 Along with decreased CSF turnover, transthyretin secretion and beta-amyloid secretion decrease.8 The decreased CP-CSF axis functions result from the increased risk of the development of age-related diseases (Alzheimer disease or normal-pressure hydrocephalus); nevertheless, the normal senescent changes of the CP-CSF axis changes and central nervous pathologies have some similarities.3
Currently, diffusion-weighted imaging (DWI) is a routine component of the brain magnetic resonance imaging (MRI) examination and is critical in the evaluation of stroke patients; DWI differentiates abscesses, infections, tumors, and degenerative and demyelinating disorders. The sequence is based on the random microscopic motion of water molecules in different tissues, providing unique information about the physiologic state of brain tissue. Therefore, DWI detects early or subtle changes within the brain before any visible abnormality can be seen on conventional morphologic imaging.9,10 There is a complex relationship between histology and diffusion; highly cellular tissue impedes the movement of water molecules. However, low cellularity or defective cells permit greater water molecule movement.11 CPs appear brighter on DWI in the elderly; this phenomenon was thought to be the consequence of gelatinous cystic changes with aging.12 We hypothesized that cellular and functional alterations of the CP may affect the diffusion characteristics of the tissue that could be demonstrated semiquantitatively via DWI MRI. The structural changes in CP apparent diffusion coefficients (ADCs) may be a sign of aging and related disorders. The aim of this research was to quantify the ADCs of human CPs as well as to reveal age-related changes.
Materials and methods
Imaging
In this cross-sectional study, all MRIs of the brains of patients who visited the Radiology Department of our University Hospital between January 2013 and June 2014 were retrospectively investigated. Ethics approval was obtained from the local ethics committee (YDU/2017/45-374). MRI scanning indications were various neurological symptoms such as headache, vertigo, epilepsy, intracranial mass, and ischemia. MRI scanning was performed on a 1.5 Tesla MRI device (Signa HDx; General Electric, Milwaukee, WI, USA). The standard brain MRI protocol included the following sequences: axial T2-weighted (T2W), T1-weighted (T1W), and fluid-attenuated inversion recovery (FLAIR), sagittal T1 FLAIR, coronal fat-saturated T2W, susceptibility WI sequence, and DWI with ADC maps. DWI was applied at the parameter values of b = 0 s/mm2 and b = 1000 s/mm2 in the axial plane. The other parameter values for DWI were as follows: sequence echo planar imaging (EPI), repetition time (TR)/echo time (TE): 8000 ms/99.3 ms (minimum TE), field of view (FOV): 22 mm, matrix: 128 × 128, slice thickness/interval: 5 mm/1 mm, number of excitations (NEX) 2, scan time 72 seconds. After acquisition, all data were transferred to a workstation (Advantage Windows 4.4, GE Healthcare, Milwaukee, WI, USA). All images were evaluated by the same radiology specialist (two years of experience, G.Y.) under the supervision of a senior radiology specialist (15 years of experience, B.A).
Exclusion criteria
Patients with major cranial abnormalities were excluded from the study (brain tumors, hyperacute or acute ischemia, developmental anomalies, hemorrhage, hydrocephaly); only normal or mild abnormal findings were included. ADC maps were generated. The ADCs were measured by a single observer.
Measurements
The CPs were identified in the atrium of the right and left lateral ventricles (LVs) on the DWI and/or ADC maps. Region of interest (ROI) was inserted on the CP to try to avoid CSF contamination. The ROI sizes varied between 14 mm2 and 37 mm2. Multiple ADC measurements were performed, and the minimum value was considered for each CP. The diameters of the LVs were measured at the level of the ventricular atriums on the T2W images.
Statistical analysis
Descriptive statistics for all variables were calculated and are provided in the text. Distribution properties were investigated with the Shapiro-Wilk test of normality. Since parametric assumptions were met, parametric hypothesis tests were applied. Multiple groups were compared with one-way analysis of variance (ANOVA) test and in case of statistical significance, pairwise comparisons were carried out with Tukey’s post hoc test. For paired groups, statistical comparisons were performed with the paired samples t test. Possible associations between study variables were analyzed with Pearson correlation analysis.
Regression analysis with least square smoothing was applied to see the age-related functional structures of ADC values of right and left CP values. Regression analysis was carried out for all patients, as well as for both genders separately. Analysis with all patient data was adjusted for gender. Third-order polynomial (cubic) functions were applied as the best fitting non-linear curves. GraphPad Prism (Demo Version 7.0) was used for regression analysis. All other statistical analysis and calculations were carried out with SPSS (Demo Version 23.0) statistical software. Level of significance was accepted to be 0.05.
Results
Study participants
The brain MRIs of a total of 202 patients, 97 men (48%) and 105 women (52%), were studied. The mean age of the patients was 44.1 ± 24.2 (range between 1.0 and 87.0) years old.
LV diameters
The mean diameter of the LV was 11.1 ± 3.5 (minimum: 6.0, maximum: 27.0) mm in the right; 10.7 ± 3.6 (minimum: 5.1, maximum: 23.0) mm in the left LV. The ADCs of the right and left CP were not significantly different (p > 0.05). The mean ADC of the CP was 1.81 ± 0.50 × 10−3 mm2/sec. The minimum ADC of the CP was 1 × 10−3 mm2/sec at the right side and 0.93 × 10−3 mm2/sec at the left side. The maximum ADC of the CP was 3.05 × 10−3 mm2/sec at the right side and 2.97 × 10−3 mm2/sec at the left side.
There were statistically significant positive correlations between the ADC of the CP and patient age (right CP: r = 0.623; p < 0.05. left CP: r = 0.654; p < 0.05). Similarly, there were positive correlations between the LV diameters and patient age (r = 0.624, p < 0.05 for the right LV; r = 0.621, p < 0.05 for the left LV).
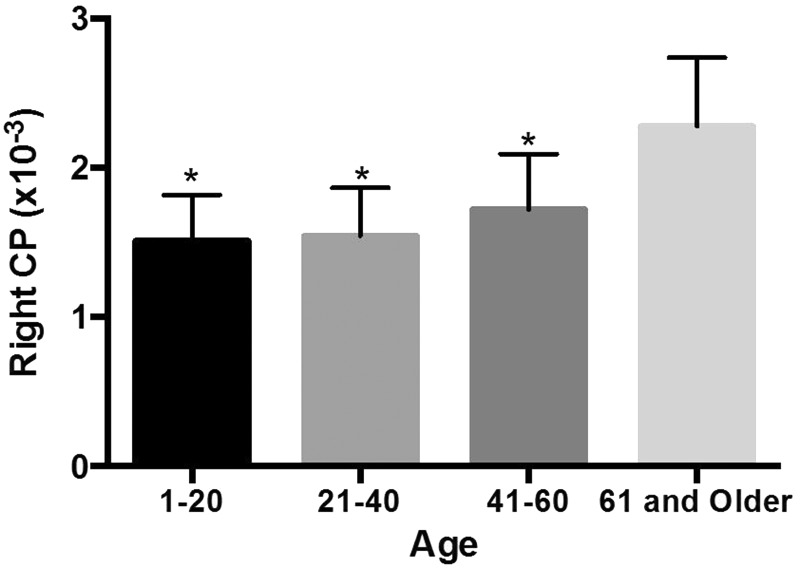
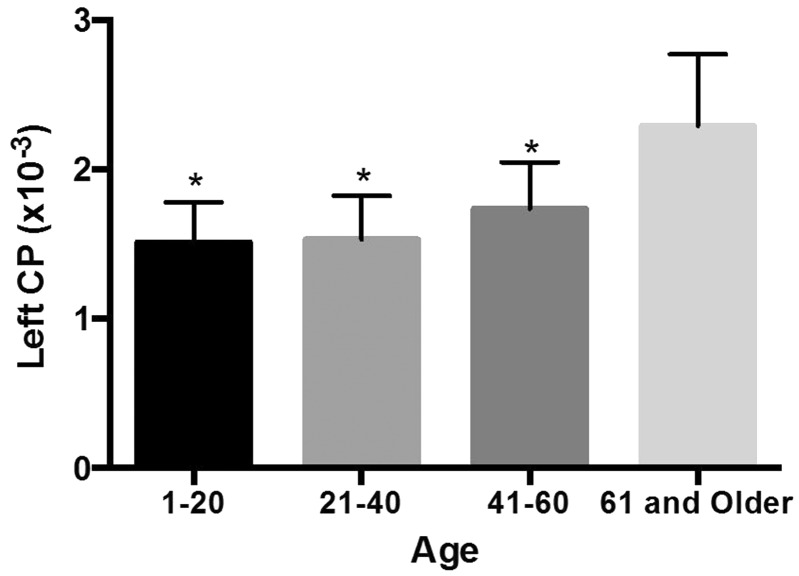
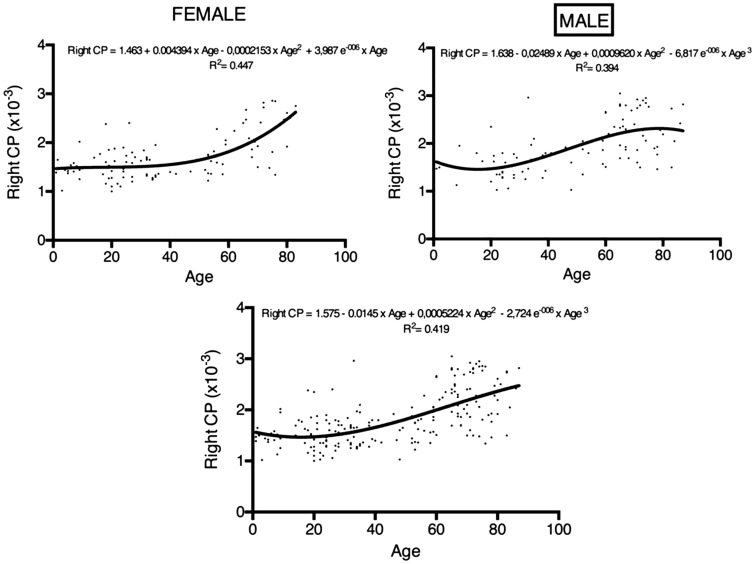
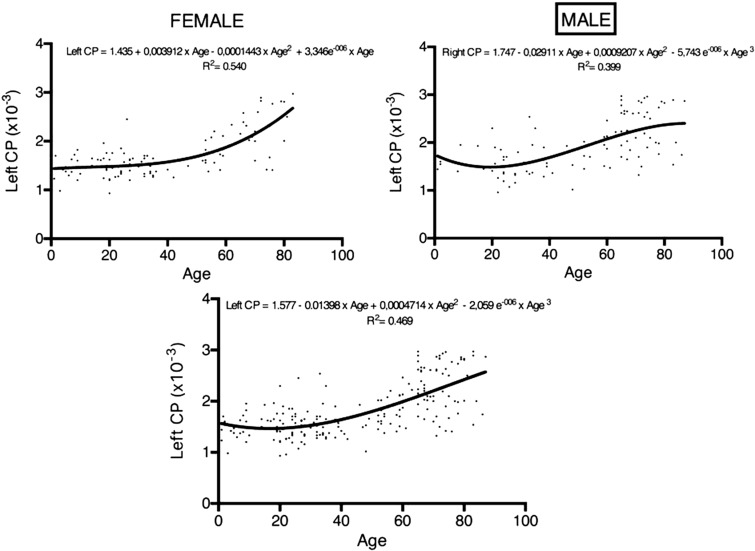
The patients were divided into four groups based on age: 1 to 20 years old, 21 to 40 years old, 41 to 60 years old, and 60 years and older (Table 1). The ADCs of the CP of the age groups are shown in Figures 1 and 2. For the right and left ADCs, the age groups significantly differed (p < 0.05): The pairwise group comparisons showed that the ADCs of the 60 years and older age group was significantly higher compared to the three other groups. However, the ADCs were not significantly different between the 1- to 20-year-old, 21- to 40-year-old, and 41- to 60-year-old groups (Table 1; Figures 1 and 2). There is a constant slope of ADC value of the CPs until the fourth decade, hereafter it slightly increases. The increase is steeper in women compared to men (Figures 3 and 4).
Table 1.
ADC values of both CPs in various age groups.
| Age groups | n | Mean ± SD (×10−3) | |
|---|---|---|---|
| Right CP | 1–20 | 39 | 1.51 ± 0.31a |
| 21–40 | 63 | 1.54 ± 0.32a | |
| 41–60 | 32 | 1.72 ± 0.37a | |
| 61 and older | 68 | 2.28 ± 0.46 | |
| Left CP | 1–20 | 39 | 1.51 ± 0.27a |
| 21–40 | 63 | 1.53 ± 0.29a | |
| 41–60 | 32 | 1.74 ± 0.31a | |
| 61 and older | 68 | 2.29 ± 0.48 |
Difference from ≥61-year-old age group.
ADC: apparent diffusion coefficient; CP: choroid plexus.
Figure 1.
Mean ADC values in the age groups in the right LV’s CPs.
(* Difference from ≥61-year-old age group).
Figure 2.
Mean ADC values in the age groups in the left LV’s CPs.
(* Difference from ≥61-year-old age group).
ADC: apparent diffusion coefficient; LV: lateral ventricle; CPs: choroid plexuses.
Figure 3.
Regression analysis displaying the trends of age-related ADC values of CPs (right, left and overall) separately in women and in men. Vertical axis is ADC value of right, left and all CP. Horizontal axis is age. Regression equation is given on each graph.
ADC: apparent diffusion coefficient; CPs: choroid plexuses.
Figure 4.
Regression analysis displaying the trends of age-related ADC values of CPs (right, left and overall) separately in women and in men. Vertical axis is ADC value of right, left and all CP. Horizontal axis is age. Regression equation is given on each graph.
ADC: apparent diffusion coefficient; CPs: choroid plexuses.
Discussion
The present study showed a significant increase in diffusion occurred in the CP and dilatation of the LV with aging. The ADC value is constant until the age of 60, but it increases significantly at >61 years; interestingly, women had a more significant increase of ADC value compared to men after the sixth decade. This cannot be explained by presence of any systemic disease, alcohol or drug consumption, neuropathologic and cognitive status, because of lack of data, although we have focused on healthy individuals. There is increasing interest on gender effects on brain aging. Neuroimaging studies indicate that age-related changes begins earlier in males, the brain varies in different subregions of the brain, and brain atrophy is greater in males.13,14 The clinical explanation of the findings needs studying of the neurocognitive, behavioral and neurophysiologic domains.
Increase in CSF volume is one of the first basic changes in the CSF and the ventricular system; the increase is mainly attributed to brain atrophy. In healthy aging, the ventricular volume increases by 25%–30% in the fifth to eighth decade compared to the second to fourth decades. An increase in diffusion of the CPs that likely reflects the histopathological and functional changes in CPs related to aging was presented in previous human and animal studies.1,5,6,8,15 DWI is a functional MRI sequence that employs the post-processing model of the mono-exponential algorithm model to derive the semi-quantitative parameters of ADC. However, the actual diffusion of water molecules in living tissues is not free. It is heterogeneous and limited by many kinds of barriers, such as the cell membrane, organelles, and microvasculars. In a CP voxel, several compartments are present, including the CSF compartment, especially because of partial volume effects. The increase in ADC could first be attributed to an increase of the CSF fractional volume in the voxel. Diffusion occurs as a result of the random microscopic motion of water molecules because of their intrinsic thermal energy, reflecting the molecular motion of water, probing its mobility on the cellular scale.16,17 The diffusion coefficient, measured in mm2/sec, relates the average motion, in a mean-square sense, to the observation time; higher values of this coefficient indicate more mobile water molecules. Thus, higher water mobility with higher ADCs results in lower observed signal intensity in the diffusion-weighted sequence. Conversely, decreased water mobility with reduced ADCs results in a higher observed signal. The degree of tissue cellularity and the presence of intact cell membranes help determine the resistance of water molecule diffusion. Low cellular tissues or tissues consisting of cells with disrupted membranes permit greater movement of water molecules.11,18 Brain maturation and aging may also be quantified by ADC changes. As a result of the high water content of the pediatric brain, the ADCs of the gray and white matter of healthy neonates and infant brains are higher than adult brains. The ADCs decrease over time as the brain matures. The ADCs for all parts of the brain that are more apparent in the white matter and the lentiform nucleus may increase minimally with aging.1,19–21 We hypothesize that increase of the CP ADC value in the elderly is related to decreased functional integrity of the BCSFB. Histologically, CPs are composed of tightly packed villous folds consisting of a single layer of cuboidal epithelial cells overlying a central core of highly vascularized stroma. The choroidal epithelial cells are joined together by encircling tight intercellular junctions.3 These epithelial tight junctions are concentrated in short lengths of several complex strands close to the apical side of CSF; the epithelial tight junctions together with the arachnoid membrane form the BCSFB. Under normal conditions, the BCSFB prevents free passage of molecules between the blood and the CSF via the paracellular route between cells.1 The extraction of the proteins from the vascular space is thought to be by simple diffusion via the paracellular spaces. Simple diffusion across the cellular layer could be limited by the paracellular diffusion rates and by the size of the cellular pores or gaps. The diffusion rate is inversely related to molecular weight. Animal models have shown a twofold increase in the extraction of higher molecular weight molecules via paracellular routes in older animals compared to younger animals, which suggests the functional integrity of the BCSFB had decreased. The increased protein content of the CSF according to age was also attributed to the contribution of the decreased functional integrity of the BCSFB with reduced CSF flow.1 A significant elevation in CSF protein was found in healthy humans older than 40 years old22 and in patients with neurological diseases. The increase in the protein content may result from the circulation across a leaky BCSFB, as well as the contribution of proteins from neighboring nervous tissue.23,24
Previously, age-related changes in the CPs were determined according to the occurrence of macroscopic calcifications that can be measured on computed tomography scans.8,25 MR perfusion study conclusions were also concomitant with those of experimental studies.1,8 A significant decrease in capillary permeability and an increase in the mean transit time in older individuals were found in dynamic susceptibility MRI examinations. A rapid hyperbolic-like decrease in the factor of choroidal capillary permeability was described as a decrease in the global exchange area or an increase in the thickness of the interface that reduces the mass transfer between the compartments. Reduced blood mean transit time was found to be correlated with rat studies.6,15 Functional MRI studies enable direct in vivo examination of the changes at the CP’s capillary level.
The present study has several limitations. Firstly, histopathological correlation was not possible. Secondly, the accuracy of the ADC measurements could be debated as the CPs are embedded in the LVs; they occasionally show hyperintensity on DWI with a mild elevation of ADCs that are usually higher than white matter. However, in spite of the potential risk of CSF contamination during ROI replacement, a clear positive correlation of the ADCs of CP and age was found. The similar ADCs of the right and left LVs’ CPs suggested that the measurements were accurate.
There are some similarities between age-related changes in the CP ventricular system and Alzheimer disease and normal-pressure hydrocephalus.3 In Alzheimer disease, epithelial atrophy, thickening of the basement membrane, and stroma fibrosis are more prominent compared to normal senescence. Synthesis, secretory, and transportation functions are significantly altered resulting in decreased CSF turnover, reduced beta-amyloid clearance, and increased glycation phenomena as well as oxidative stress.25 This study showed that there was a clear progressive increase in water diffusivity in the CPs during aging. The increased diffusion of the CPs is suggested to reflect the increased diffusion via the paracellular spaces and reduced functional integrity of the BCSFB during aging. Increased mean age in the modern population causes age-related brain disorders such as Alzheimer disease that have overwhelming economic, physical and emotional effects both on patients and their families. Early diagnosis and personalized therapeutic strategies should alter the prognosis of this devastating process. ADC values of the CP should be used as a quantitative neuroimaging biomarker of aging. It may help to diagnose as well as follow up the aging process in the geriatric population. Future studies should investigate the differences between normal aging and Alzheimer disease.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Chen RL, Kassem NA, Redzic ZB, et al. Age-related changes in choroid plexus and blood-cerebrospinal fluid barrier function in the sheep. Exp Gerontol 2009; 44: 289–296. [DOI] [PubMed] [Google Scholar]
- 2.May C, Kaye JA, Atack JR, et al. Cerebrospinal fluid production is reduced in healthy aging. Neurology 1990; 40: 500–503. [DOI] [PubMed] [Google Scholar]
- 3.Redzic ZB, Preston JE, Duncan JA, et al. The choroid plexus-cerebrospinal fluid system: From development to aging. Curr Top Dev Biol 2005; 71: 1–52. [DOI] [PubMed] [Google Scholar]
- 4.Emerich DF, Skinner SJM, Borlogan CV, et al. The choroid plexus in the rise, fall and repair of the brain. Bioessays 2005; 27: 262–274. [DOI] [PubMed] [Google Scholar]
- 5.Serot JM, Béné MC, Foliguet B, et al. Altered choroid plexus basement membrane and epithelium in late onset Alzheimer’s disease: An ultrastructural study. Ann N Y Acad Sci 1997; 826: 507–509. [DOI] [PubMed] [Google Scholar]
- 6.Serot JM, Foliguet B, Béné MC, et al. Choroid plexus and ageing in rats: A morphometric and ultrastructural study. Eur J Neurosci 2001; 14: 794–798. [DOI] [PubMed] [Google Scholar]
- 7.Preston JE. Ageing of choroid plexus-cerebrospinal fluid system. Microsc Res Tech 2001; 52: 31–37. [DOI] [PubMed] [Google Scholar]
- 8.Bouzerar R, Chaarani B, Gondry-Jouet C, et al. Measurement of choroid plexus perfusion using dynamic susceptibility MR imaging: Capillary permeability and age-related changes. Neuroradiol 2013; 55: 1447–1454. [DOI] [PubMed] [Google Scholar]
- 9.Eastwood JD, Lev MH, Wintermark M, et al. Correlation of early dynamic CT perfusion imaging with whole-brain MR diffusion and perfusion imaging in acute hemispheric stroke. Am J Neuroradiol 2013; 24: 1869–1875. [PMC free article] [PubMed] [Google Scholar]
- 10.Rajeshkannan R, Moorthy S, Sreekumar KP, et al. Clinical applications of diffusion weighted MR imaging: A review. Neuroradiology 2006; 16: 705–710. [Google Scholar]
- 11.Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: Concepts and applications. Radiographics 2009; 29: 1797–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita T, Moritani T, Hiwatashi A, et al. Clinically silent choroid plexus cyst: Evaluation by diffusion-weighted MRI. Neuroradiology 2005; 47: 251–255. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Kobayashi S, Yamaguchi S, et al. Gender effects on age-related changes in brain structure. AJNR Am J Neuroradiol 2000; 21: 112–118. [PMC free article] [PubMed] [Google Scholar]
- 14.Gur RE, Gur RC. Gender differences in aging: Cognition, emotions, and neuroimaging studies. Dialogues Clin Neurosci 2002; 4: 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masseguin C, LePanse S, Corman B, et al. Aging affects choroidal proteins involved in CSF production in Sprague-Dawley rats. Neurobiol Aging 2005; 26: 917–927. [DOI] [PubMed] [Google Scholar]
- 16.Engelter ST, Provenzale JM, Petrella JR, et al. The effect of aging on the apparent diffusion coefficient of normal-appearing white matter. AJR Am J Roentgenol 2000; 175: 425–430. [DOI] [PubMed] [Google Scholar]
- 17.Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol 2003; 45: 169–184. [DOI] [PubMed] [Google Scholar]
- 18.Maas LC. Diffusion tensor imaging: Basic principles and emerging clinical applications. Applied Radiology Online 2003; 32(Suppl): S49–S57. [Google Scholar]
- 19.Gideon P, Thomsen C, Henriksen O. Increased self-diffusion of brain water in normal aging. J Magn Reson Imaging 1994; 4: 185–188. [DOI] [PubMed] [Google Scholar]
- 20.Helenius J, Soinne L, Perkiö J. Diffusion-weighted MR imaging in normal human brains in various age groups. AJNR Am J Neuroradiol 2002; 23: 194–199. [PMC free article] [PubMed] [Google Scholar]
- 21.Chun T, Filippi CG, Zimmerman RD, et al. Diffusion changes in the aging human brain. AJNR Am J Neuroradiol 2000; 21: 1078–1083. [PMC free article] [PubMed] [Google Scholar]
- 22.Garton MJ, Keir G, Lakshmi MV, et al. Age-related changes in cerebrospinal fluid protein concentrations. J Neurol Sci 1991; 104: 74–80. [DOI] [PubMed] [Google Scholar]
- 23.Reiber H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci 2003; 21: 79–96. [PubMed] [Google Scholar]
- 24.Serot JM, Béné MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci 2003; 8: 515–821. [DOI] [PubMed] [Google Scholar]
- 25.Jovanović I, Ugrenović S, Antić S, et al. Morphometric and some immunohistochemical characteristics of human choroids plexus stroma and psammoma bodies. Microsc Res Tech 2007; 70: 617–627. [DOI] [PubMed] [Google Scholar]