Abstract
Working memory is involved in the maintenance and manipulation of information essential for complex cognition. While the neural substrates underlying working memory capacity have been studied in humans, considerably less is known about the circuitry mediating working memory capacity in rodents. Therefore, the present experiments tested the involvement of medial prefrontal cortex (mPFC) and dorsal striatum (STR) in the odor span task (OST), a task proposed to assay working memory capacity in rodents. Initially, Long Evans rats were trained to dig in scented sand for food following a serial delayed nonmatching-to-sample rule. Temporary inactivation of dorsomedial (dm) STR significantly reduced span in well trained rats. Inactivation of mPFC or contralateral disconnection of the mPFC and dmSTR also reduced span. Infusing the GluN2B-containing NMDA receptor antagonist Ro 25-6981 into mPFC did not affect span; however, span was significantly reduced following bilateral Ro 25-6981 infusions into dmSTR or contralateral disconnection of mPFC (inactivation) and dmSTR (Ro 25-6981). These results suggest that span capacity in rats depends on GluN2B-containing NMDA receptor-dependent interactions between the mPFC and the dmSTR. Therefore, interventions targeting this circuit may improve the working memory capacity impairments in patients with schizophrenia, Alzheimer's disease, and Parkinson's disease.
Working memory is a type of short-term memory necessary for storage, maintenance, and manipulation of information for higher order cognition (Goldman-Rakic 1996; Baddeley 2003; D'Esposito 2007) that is impaired in individuals with brain disorders including schizophrenia (Barch and Smith 2008), Alzheimer's disease (Huntley and Howard 2010), and Parkinson's disease (Owen et al. 1992; Gabrieli et al. 1996; Bublak et al. 2002). Experiments using working memory tasks in rodents with strong translational potential to humans may provide insight into the neural circuitry underlying working memory and the development of novel therapeutics for treating working memory impairments (Barch et al. 2012; Dudchenko et al. 2013). Working memory is often divided into a number of constructs including goal maintenance, interference control, and capacity (Barch and Smith 2008; Moore et al. 2013). Most working memory tasks used with rodents do not include a component related to capacity (Dudchenko 2004; Dudchenko et al. 2013), although the odor span task (OST) first developed by Dudchenko et al. (2000) has received attention in this regard (Dudchenko et al. 2013; Moore et al. 2013). The OST is a serial delayed nonmatching-to-sample task (Fig. 1A) in which rodents receive food reward for choosing a bowl of sand scented with a novel odor, either by digging in the sand (Dudchenko et al. 2000; Young et al. 2007; Rushforth et al. 2010, 2011; Davies et al. 2013a,b; Murray et al. 2017) or by flipping a lid covering the sand (MacQueen et al. 2011; April et al. 2013; Galizio et al. 2013). Since limited information exists regarding the neural circuitry involved in odor span, the goal of the present experiments was to assess the role of a corticostriatal circuit in performance of the OST by rats.
Figure 1.
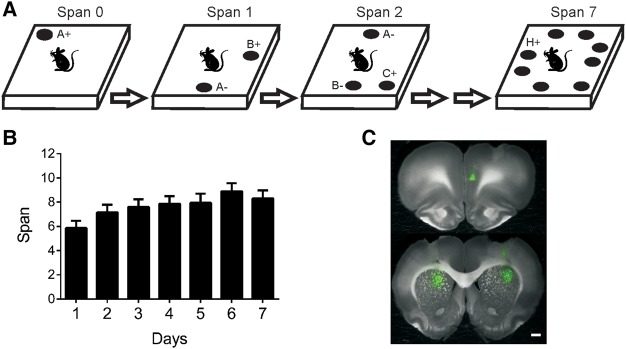
(A) Illustration of the OST. See text for details. Odors are indicated with letters. On subsequent trials for a given span, the bowl (black circle) that contains the novel odor is rewarded (+) while previously encountered bowl(s) are not (−). Bowls are added one at a time to the platform until an error occurs. Span is calculated as the number of bowls on the platform for the last error free trial minus 1. Note that all bowls are moved around the platform before each new trial. (B) Mean odor spans during the 7 d of training prior to the first treatment for all rats in the three experiments (n = 24). Rats had significantly higher spans on days 4–7 than day 1 (see Results for details). (C) Infusions sites of florescent muscimol in the right hemisphere of the mPFC (top), left hemisphere of the dmSTR (bottom left), and right hemisphere of the dlSTR (bottom right).
In humans, working memory involves the frontal cortex and striatum (Frank et al. 2001; McNab and Klingberg 2008; Baier et al. 2010) and working memory capacity correlates with frontostriatal connectivity and its modulation by dopamine during task performance (Wallace et al. 2011). We have recently shown that the medial prefrontal cortex (mPFC) is required for performance of the OST in rats using temporary lesions (Davies et al. 2013b); however, it is unknown which brain areas interact with the mPFC to support span capacity. In rodents, the mPFC projects strongly to the dorsal striatum (STR) (Sesack et al. 1989; Voorn et al. 2004; Mailly et al. 2013) and lesions of the dorsomedial (dm) STR impair performance in working memory tasks without a capacity component (White 2009). Given these studies, we tested whether odor span capacity in rats depends on the dorsal STR. The rodent dorsal STR can be functionally divided into dmSTR, which receives substantial projections from the prelimbic area of mPFC, and the dorsolateral striatum (dlSTR), which receives substantial projections from the sensory-motor cortical areas (McGeorge and Faull 1989; Voorn et al. 2004). Therefore, we performed bilateral inactivations of the mPFC, dmSTR, and dlSTR separately and also used a disconnection design (Floresco et al. 1997; Hannesson et al. 2004; Baker and Ragozzino 2014) to test whether functional interactions between areas are necessary for span.
Research examining the neurochemical modulation of working memory capacity in humans has focused on dopamine (Cools et al. 2008; Landau et al. 2009). We and others have shown that ionotropic glutamate receptors are involved in performance of the OST in rats. In particular, reduced and increased span capacity have been noted following either blockade of GluN2B-containing NMDA receptors with the antagonist Ro 25-6981 (Davies et al. 2013a) or genetically overexpressing GluN2B-containing NMDA receptors in the forebrain (Cui et al. 2011), respectively. Therefore, we also tested whether GluN2B-containing NMDA receptors in the mPFC and dorsal STR were involved in the OST.
Results
Training
Rats from all three experiments were initially trained to dig in a bowl of sand for a buried Froot Loop (dig training phase). This phase of training took an average of 5.13 d to complete (range = 3–9 d). After dig training, rats were trained for an average of 7.13 d (range = 3 to 12 d) in the nonmatching-to-sample task until they got 5/6 trials correct for three sessions. Rats were then trained on the OST for an average of 14.87 d (range, 11–23 d). Figure 1B displays the average spans obtained during the 7 training days before the first infusion for rats in all experiments. Analysis of these data with a repeated-measures ANOVA showed a significant effect of day (F(6,144) = 4.23, P < 0.001). Post hoc analyses demonstrated that spans were significantly higher for the 4 d immediately before infusions were initiated than the seventh day before. The improvement in performance during OST training has been observed previously (Davies et al. 2013a,b).
Inactivation of dmSTR impairs odor span
In this experiment, we inactivated the dmSTR or dlSTR as these regions receive distinct projections from the frontal cortex (Voorn et al. 2004), which has been implicated in the OST (Davies et al. 2013a,b). Following bilateral inactivation of dmSTR with Mus/Bac (Fig. 2A), rats had significantly lower spans (1.65 ± 0.66 odors) than when they were treated with vehicle (7.20 ± 1.31 odors; t(8) = 5.82, P < 0.001). Latency to initiate digging did not differ between the treatments (Mus/Bac = 11.92 ± 4.59 sec; vehicle 3.59 sec ± 0.66 sec; t(8) = 1.70, P = 0.13). Following bilateral inactivation of dlSTR (Fig. 2B), rats had an average span of 2.80 ± 1.15 odors, lower than the average span following vehicle infusions (6.15 ± 0.83 odors). However, this effect failed to reach significance (t(8) = 2.19, P = 0.060). Latency to begin digging did not differ between treatments (Mus/Bac = 10.89 ± 3.23 sec; vehicle = 5.10 sec ± 2.73 sec; t(8) = −1.70, P = 0.13). Figure 2C displays representative infusion sites for the dmSTR and dlSTR and Figure 1C displays images of the spread of fluorescent muscimol following infusion into either site.
Figure 2.
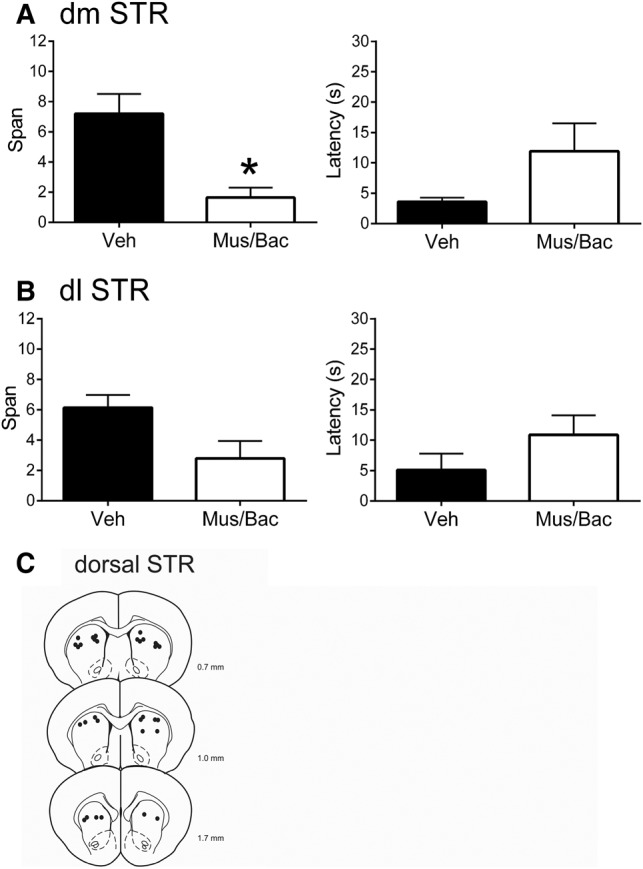
Effects of dmSTR or dlSTR inactivations on performance of the OST (n = 9). (A) Mean spans of rats following vehicle (Veh) or muscimol and baclofen (Mus/Bac) infusions into dmSTR (left). Mean latency of the rats to begin digging in a bowl for the dmSTR treatments (right). (B) Mean spans of rats following Veh or Mus/Bac infusions into dlSTR (left). Mean latency of the rats to start digging in a bowl for the dlSTR treatments (right). (C) Representative infusions sites in the dmSTR and dlSTR for the experiments that occurred in A and B. Numbers refer to the anterior–posterior location of the plates relative to bregma. * Refers to a significant difference between treatments (P < 0.05).
Projections from mPFC to dmSTR are necessary for odor span capacity
Given that the mPFC (Davies et al. 2013a,b) and dmSTR (Fig. 2) are involved in the OST, we next sought to test whether direct interactions between these areas are necessary using a disconnection procedure (Christakou et al. 2001; Baker and Ragozzino 2014). Bilateral inactivation of mPFC significantly impaired span (Fig. 3A; Mus/Bac = 2.67 ± 0.94 odors; vehicle = 7.76 ± 1.28 odors; t(6) = 4.21, P = 0.006). Latency to dig did not differ between the treatments (t(6) = 1.06, P = 0.33). In the second part of this experiment (Fig. 3B), we confirmed the role of the dmSTR in the OST as bilateral inactivation of dmSTR with Mus/Bac significantly reduced span (5.64 ± 1.40 odors) compared with vehicle infusions (10.95 ± 1.51 odors; t(6) = 2.58, P = 0.042). Latency to dig did not differ between treatments (t(6) = 0.23, P = 0.82).
Figure 3.
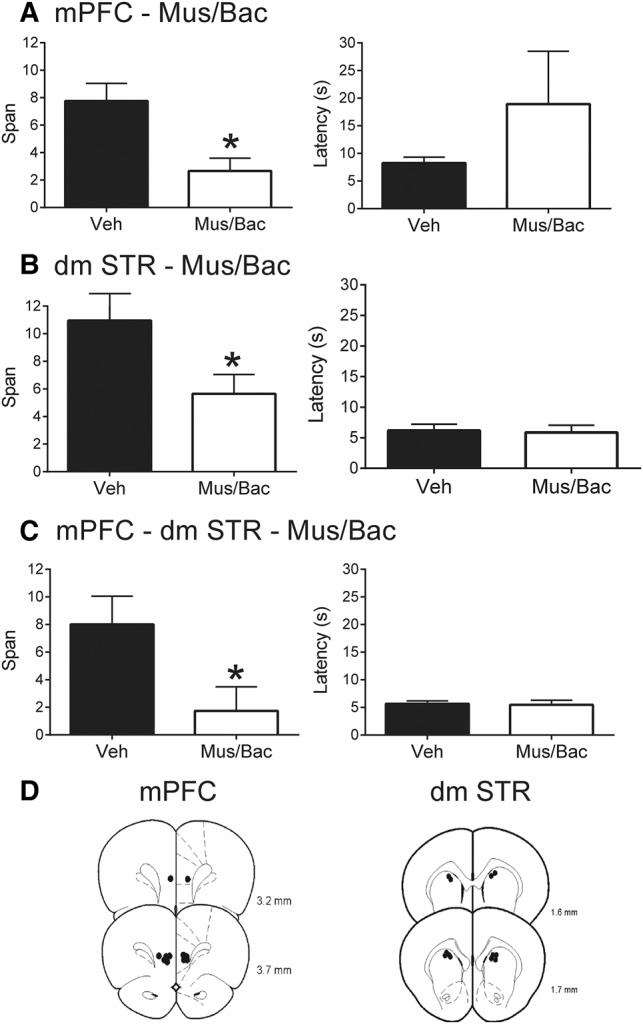
Effects of mPFC or dmSTR inactivations on performance of the OST (n = 7). (A) Mean spans of rats following vehicle (Veh) or muscimol and baclofen (Mus/Bac) infusions into the mPFC (left). Mean latency of the rats to begin digging in a bowl for the mPFC treatments (right). (B) Mean spans of rats following Veh or Mus/Bac infusions into the dmSTR (left). Mean latency of rats to start digging in a bowl for the dmSTR treatments. (C) Mean spans of rats following Veh or Mus/Bac for contralateral disconnection of mPFC and dmSTR (left; see text for details). Mean latency of rats to start digging in a bowl for the contralateral disconnection treatments (right). (D) Representative infusions sites in the mPFC (left) for experiments A and C, and dmSTR (right) for experiments B and C. Numbers refer to the anterior–posterior location of the plates relative to bregma. * Refers to a significant difference between treatments (P < 0.05).
When we tested whether temporary disconnection of mPFC and dmSTR would impair performance of the OST, unilateral infusions of Mus/Bac into the mPFC of one hemisphere and the dmSTR of the opposite hemisphere significantly impaired span without affecting latency to dig during the task (Fig. 3C). Following the disconnection with Mus/Bac, rats had a span of 1.74 ± 0.34 odors which was significantly lower than vehicle-treated rats (8.00 ± 2.05 odors; t(6) = 3.45, P = 0.013). Latency to dig did not differ between treatments (t(6) = 0.22, P = 0.83). Figure 3D displays the representative infusion sites for the mPFC and dmSTR for this experiment.
Odor span capacity depends on activation of GluN2B-containing NMDA receptors in the mPFC–dmSTR circuit
NMDA receptors containing GluN2B subunits have been implicated in the OST in two studies. Cui et al. (2011) showed increased odor span capacity in mice that overexpressed GluN2B-containing NMDA receptors in the forebrain. Previous research from our laboratory (Davies et al. 2013a) showed that blocking GluN2B-containing NMDA receptors with systemic injections of Ro25-6981 impaired span. Direct infusion of Ro 25-6981 into mPFC impaired odor span in the majority (6/7) of rats tested, although this effect failed to reach significance in that previous study (Davies et al. 2013a). Therefore, we assessed whether Ro 25-6981 infusions into mPFC or dmSTR affected odor span in a new sample of eight rats. Following Ro 25-6981 infusions in mPFC, span did not differ significantly in this sample with a mean span of 8.19 ± 1.42 odors while vehicle treatment resulted in a mean span of 9.23 ± 1.65 (Fig. 4A, left side; t(7) = 0.50, P = 0.63). Latency to respond did not differ between treatments (Fig. 4A, right side; t(7) = 0.57, P = 0.59).
Figure 4.
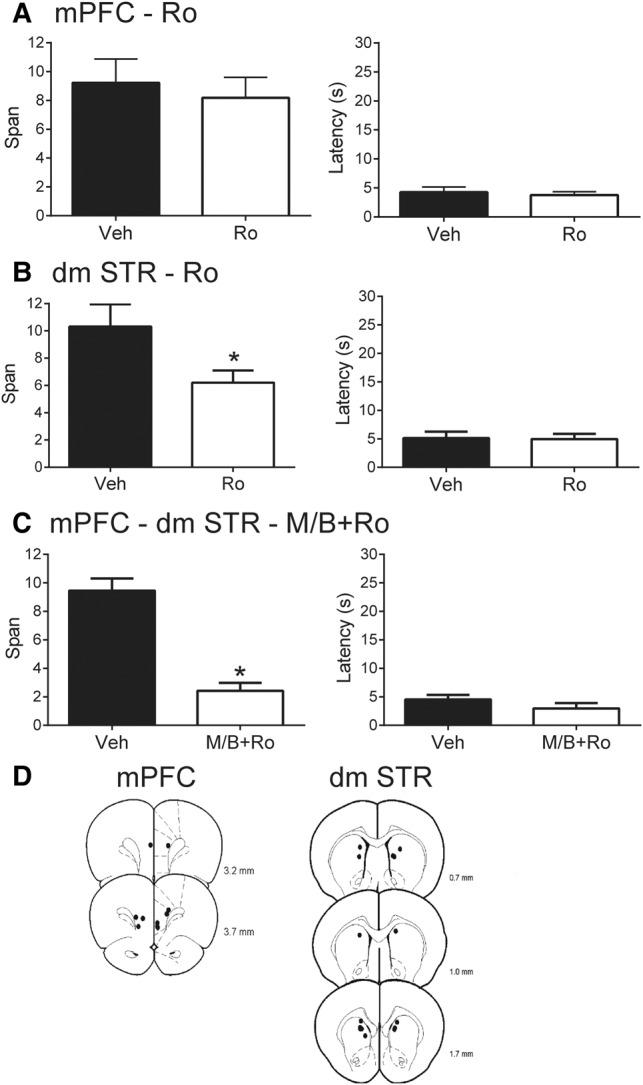
The effects of blocking GluN2B-containing NMDA receptors on performance on the OST (n = 8). (A) Mean spans of rats following vehicle (Veh) or Ro 25-6981 (Ro) infusions into the mPFC (left). Mean spans of rats in the same mPFC treatments with two poorly performing vehicle rats removed (middle). Mean latency of the rats to begin digging in a bowl for the mPFC treatments (right). (B) Mean spans of rats following Veh or Ro infusions into the dmSTR (left). Mean latency of the rats to begin digging in a bowl for the dmSTR treatments (right). (C) Mean spans of rats following Veh or muscimol and baclofen+Ro (M/B+Ro) for contralateral disconnection in mPFC (M/B) and dmSTR (Ro) (left; see text for details). Mean latency of rats to start digging in a bowl for the contralateral disconnection treatments (right). (D) Representative infusions sites in the mPFC (left) for experiments A and C, and dmSTR (right) for experiments B and C. Numbers refer to the anterior–posterior location of the plates relative to bregma. * Refers to a significant difference between treatments (P < 0.05).
In contrast, bilateral infusions of Ro 25-6981 into dmSTR significantly impaired span without affecting response latency (Fig. 4B). Following blockade of GluN2B-containing NMDA receptors with Ro 25-6981, rats had an average span of 6.21 ± 0.89 odors while vehicle treated rats had an average span of 10.31 ± 1.64 odors (t(7) = 2.87, P = 0.024). Latency to respond did not differ between treatments (Ro 25-6981 = 4.95 ± 0.93 sec, vehicle = 5.15 ± 1.11 sec; t(7) = 0.24, P = 0.82).
To assess the role of glutamatergic mPFC inputs in activating postsynaptic GluN2B-containing NMDA receptors in dmSTR, we performed disconnections of the mPFC and dmSTR by unilaterally infusing the mPFC with Mus/Bac and the dmSTR of the opposite hemisphere with Ro 25-6981 as reported previously (Baker and Ragozzino 2014). Disconnection of mPFC (Mus/Bac) and dmSTR (Ro 25-6981) reduced span to 2.43 ± 0.56 odors, which was significantly lower than the vehicle treatment of 9.45 ± 0.85 (Fig. 4C; t(6) = 7.44, P < 0.001). Latency to dig did not differ between treatments; contralateral disconnection 2.97 sec ± 0.94 compared with vehicle 4.55 ± 0.80 (t(6) = 1.56, P = 0.17). A unilateral infusion into mPFC (Mus/Bac) and dmSTR (Ro 25-6981) was also performed to confirm the specificity of the disconnection procedure. Following unilateral infusions ipsilaterally into both sites, rats displayed a mean span of 6.88 ± 1.56, which was not significantly different from their vehicle treatment (t(6) = 1.80, P = 0.12). Figure 4D displays the representative infusion sites for the mPFC and dmSTR.
Discussion
To the best of our knowledge, the present experiments are the first to assess the role of a corticostriatal circuit in the OST in rats. Temporary inactivation of dmSTR and dlSTR reduced span in the OST (Figs 2, 3B), although the reduction was only significant following dmSTR inactivation. Previous studies have assessed the role of dorsal striatum in working memory using tasks in rodents without a capacity component (White 2009; Kang et al. 2013). Lesions of the medial aspect of the dorsal striatum impair performance of delayed alternation (Dunnett et al. 1999; Moussa et al. 2011) while small lesions centered in the middle of the dorsal striatum impair reference but not working memory in a radial arm maze task (Colombo et al. 1989; Packard and White 1990). Packard et al. (1989) failed to show a working memory impairment following dorsal striatum lesions. Upon further investigation, impairments following lesions were shown when rats were given food on the maze during adaptation, but no impairment was observed when adaptation was performed without food (Packard et al. 1992). These results suggest that the appetitive component of the OST may be necessary for the observed reduction in working memory capacity following the dmSTR and dlSTR inactivations. In our second experiment, blocking GluN2B-containing NMDA receptors in the dmSTR reduced span in the OST (Fig. 4B). This impairment is consistent with previous studies showing that blocking NMDA receptors in the dmSTR impairs working memory (Smith-Roe et al. 1999) and types of behavioral flexibility including reversal learning (Watson and Stanton 2009) and behavioral switching (Baker and Ragozzino 2014).
The involvement of mPFC in a variety of working memory tasks including delayed alternation (Kolb 1990; Baeg et al. 2003) and the delayed win-shift task on the radial arm maze (Floresco et al. 1997; Lapish et al. 2008) is well known (Holmes and Wellman 2009). Importantly, these tasks do not explicitly measure working memory capacity (Dudchenko et al. 2013). Thus, the disruptive effects of mPFC inactivation on the OST (Fig. 3A) are noteworthy. Previous research with the OST has confirmed reduced spans in rats following infusions of Mus/Bac (Davies et al. 2013b) or the AMPA receptor antagonist CNQX (Davies et al. 2013a) into the mPFC; however, significant changes in response latencies complicated interpretation of these results (Davies et al. 2013a,b). In the present study, no significant changes in response latency following mPFC infusions of Mus/Bac were found. One possible reason for this discrepancy in the latency results is that Davies et al. (2013a,b) infused a higher volume (0.5 µL) of drug than in the present study (0.3 µL). Importantly, mPFC inactivation does not impair performance of an olfactory sensitivity test without a memory component (Davies et al. 2013b). Taken together, these results support the involvement of the rodent mPFC in working memory, and the OST in particular.
In our previous study (Davies et al. 2013a), blocking GluN2B-containing NMDA receptors in mPFC did not significantly alter OST performance, consistent with the present results (Fig. 4A). Interestingly, systemic injections of Ro25-6981 (10 mg/kg) impair span (Davies et al. 2013a) and overexpression of GluN2B-containing NMDA receptors in the forebrain and striatum (Cui et al. 2011) increase the spans of mice tested on the OST. Given the present results with targeted infusions, we propose that GluN2B-containing NMDA receptors in dmSTR, but not mPFC, are critical for the OST. However, caution should be taken when generalizing this interpretation to other working memory tasks as GluN2B-containing NMDA receptors in dorsolateral PFC are involved in the persistent firing exhibited by delay neurons during the delayed oculomotor response task in monkeys (Wang et al. 2013). We and others (Wang et al. 2013) have used the compound Ro 25-6981 which is 5000 times more selective for GluN2B subunits compared with GluN2A subunits. While we cannot eliminate the possibility that some GluN2A-containing NMDA receptors were also affected, it is highly likely that the effects we observed on odor span were due to effects of Ro 25-6981 on GluN2B-containing NMDA receptors. In other studies, systemic administration of broad spectrum NMDA receptor antagonists including CPP (Davies et al. 2013b), MK-801 (acute treatment) (MacQueen et al. 2011; Galizio et al. 2013), and ketamine (repeated injections) (Rushforth et al. 2011) impairs performance of the OST. Whether these impairments are the result of reduced GluN2B-containing NMDA receptor activity in dmSTR will require further investigation.
The mPFC sends dense glutamatergic projections to the dmSTR (McGeorge and Faull 1989; Voorn et al. 2004) that are positioned to activate GluN2B-containing NMDA receptors on medium spiny projection neurons and interneurons (Standaert et al. 1994; Smeal et al. 2008). To test whether direct communication between these areas is critical for performance of the OST, we performed contralateral disconnections of these regions. Unilateral inactivation of the mPFC combined with either inactivation (Fig. 3C) or Ro 25-6981 (Fig. 4C) into the contralateral dmSTR reduced span in the OST. Importantly, unilateral, ipsilateral infusions into both regions did not affect span. Taken together, these findings suggest that activity of glutamatergic projection neurons from mPFC to dmSTR enable performance of the OST by activating GluN2B-containing NMDA receptors in dmSTR.
It should be noted that following our manipulations, rats still performed the task, albeit at a significantly reduced level (i.e., their spans were not 0). If memories for the odors were maintained in these areas, span should have been completely eliminated by the infusions. However, it is unknown where memory for the odors is maintained during the OST. In addition, partially preserved performance of the rats may be the result of the relatively small volume (0.3 µL) used for our infusions. Thus, some portion of the mPFC or dmSTR were likely unaffected by our infusions and could have supported a partial memory load. The infusions also increased latency to initiate digging in some cases, although these effects did not reach significance. Anecdotal observations made by the experimenters suggest that some rats took somewhat longer to initiate any movement on the testing platform following infusions while other rats sampled more bowls than controls before digging. In addition, as latencies were never decreased following manipulations, it is unlikely that the rats were impulsively digging in the first bowl they sampled on the platform. Future experiments will systematically assess the rats’ behavior following brain manipulations. Group spans also varied from 6 to 10 following vehicle infusions. Variability in performance is characteristic of the OST as noted previously for individual testing days (Dudchenko et al. 2000) and following prolonged training (Davies et al. 2013a). While this variability has the potential to complicate interpretation of the results, we note that in the present data set, Mus/Bac infusions into dmSTR were tested in two separate groups. A similar degree of impairment was noted in each case (Fig. 2A, span from 7 to 2; Fig. 3B, span from 11 to 5). Impairments in span following inactivation of mPFC have also been observed in the present data set and our previous work (Davies et al. 2013b). Thus, we are confident that the disruptive effects of mPFC and dmSTR inactivation on span are robust.
Presently, the specific contributions of the mPFC and dmSTR to performance of the OST are not well understood. Corticostriatal interactions are implicated in working memory in a number of tasks (Frank et al. 2001; McNab and Klingberg 2008; Baier et al. 2010; Wallace et al. 2011) and theories suggest that information is maintained in cortical circuits during the delay while the striatum may serve to “gate” or control the maintenance and updating of this information at the start of the delay via cortico–striato–thalamic loops (Alexander et al. 1986; Frank et al. 2001; Gruber et al. 2006). Electrophysiological recordings during the delayed oculomotor response task in primates support these ideas as persistent neuronal activity in the dorsolateral PFC and caudate nucleus have been found during shorter delays of <4 sec (Schultz and Romo 1988; Hikosaka et al. 1989; Goldman-Rakic 1996; Kawagoe et al. 1998, Wang et al. 2013 but see also Lundqvist et al. 2016). Neurons recorded from rat dmSTR in a delayed nonmatching-to-position task with longer delays (up to 10 sec) show sequential patterns of activation over the course of the delay following initial responses to the sample stimulus early in the delay suggesting that the dmSTR may be involved in maintaining memory for the stimulus as well (Akhlaghpou et al. 2016). However, the OST has characteristics which distinguish it from simpler delayed response tasks. These include the longer retention interval (20–30 min for rats that reach spans greater than 10) and repeated exposure to the previously sampled stimuli in each “trial” of a given span. Thus, other maze based tasks such as the win-shift radial arm maze task, which puts trial-unique demands on memory over typical delays of 30 min, may engage corticostriatal circuits similarly to the OST. Recordings of neural ensembles from the mPFC of rats support its role in monitoring behavior and attending to actions (Lapish et al. 2008). Thus, during the OST, neuronal activity in mPFC may enable rats to organize actions through glutamatergic afferents to the dmSTR which activate GluN2B-containing NMDA receptors. Notably, corticostriatal projections have a greater GluN2B-containing NMDA receptor component than thalamostriatal projections (Smeal et al. 2008), blocking striatal NMDA receptors reduces spontaneous firing of medium spiny neurons in rats (Sandstrom and Rebec 2003; Pomata et al. 2008), and stimulation of medial prefrontal cortex induces short- and long-term patterns of synaptic plasticity in dorsal striatum (Galiñanes et al. 2011; Höhn et al. 2011). Thus, disruption of these processes by impeding the influence of mPFC inputs on dmSTR neurons may underlie the impairment of span we observed.
The present results confirm roles for the dmSTR and mPFC in the OST. Results of the disconnection experiments support the hypothesis that glutamatergic projections from mPFC to dmSTR activate GluN2B-containing NMDA receptors in the dmSTR to enable odor span. As the OST is one of the few tasks thought to involve the capacity component of working memory in rodents, these results may inform the development of therapies to improve the working memory capacity impairments in disorders such as schizophrenia, Alzheimer's disease, and Parkinson's disease (Barch and Smith 2008; Dudchenko et al. 2013). Assessments of potential therapies in rodent models of brain disorders that involve impaired odor span may prove particularly fruitful in this regard (Murray et al. 2017).
Materials and Methods
Animals
Three groups (total n = 24) of adult male Long Evans rats (265–415 g; Charles River, Quebec, Canada) were tested in the experiments using a within subjects design. The rats were individually housed in clear plastic cages in a colony room on a 12 h light–dark cycle (lights on at 07:00) with ad libitum access to water. Except for several days after arrival and surgery, rats were food restricted to maintain 85% of their free feeding weight. Experiments were conducted in accordance with the standards of the Canadian Council on Animal Care and were approved by the University of Saskatchewan Animal Research Ethics Board.
Apparatus
Training and testing occurred on a 91.5 cm2 platform covered with black corrugated plastic with a 2.5 cm tall border on the outer edge. The platform was fastened to a metal frame with casters attached and stood 95 cm above the floor. It was surrounded by a beige curtain to block visual cues in the room. Velcro was used to fasten white porcelain bowls (4.5 cm high, 9 cm in diameter) to the platform and stop the rats from spilling the sand. Pieces of Velcro were equally spaced along the edge of the platform (one piece in each corner and five additional pieces on each side) and the bowls for a given trial were placed randomly on the pieces of Velcro.
Odors
Premium Play Sand (Quikrete Cement and Concrete Products) was sifted to remove rocks and then odors (0.5 g of a single dried spice) were mixed into the sand (100 g per bowl). The odor and sand mixtures were stored in separate Ziploc bags when not in use and new batches of sand and odors were freshly mixed every 7 d. Twenty-four different scents were used in the experiments: allspice, anise seed, basil, caraway, celery seed, cinnamon, cloves (0.1 g), cocoa, coffee, cumin, dill, fennel seed, garlic, ginger, lemon and herb, marjoram, mustard powder, nutmeg, onion powder, orange, oregano, paprika, sage, and thyme. Spices were purchased from a local grocery store. The odors used each day were selected using a random list generator and rats were regularly exposed to all odors. Bowls containing scented sand were placed on the platform as needed for each trial.
Training on the odor span task
Previously published protocols were followed closely (Davies et al. 2013a,b; Murray et al. 2017). Behavioral training was conducted during the light phase with the experimenter blind to the treatments administered. Rats were handled three times for 3 min before dig training. Dig Training/Shaping. First, rats were trained to dig for a food reward (Kellogg's Froot Loops) in a bowl filled with 100 g of unscented sand. Rats were placed opposite to a bowl on the platform for three separate phases. In the first phase, the food reward was positioned on top of the sand, in the second phase the food reward was incompletely buried, and in the third phase, the food reward was fully buried. Rats were trained until they would reliably dig for the food reward regardless of the bowl's location on the platform. Odor nonmatching-to-sample. Once the rats consistently dug in unscented bowls, they moved onto the nonmatching-to-sample task. In the sample phase of a trial, the rat was presented with a scented bowl of sand randomly positioned on the platform. After the rat dug and consumed the food reward, it was removed from the platform and placed behind a curtain to obscure its view of the platform. The experimenter then positioned the bowl at a random location on the opposite end of the platform and added a second bowl with a different odor to the platform. In the choice phase of the trial, the rat was placed on the platform opposite to both bowls and then allowed access to both bowls. A food reward was only in the bowl with the novel odor for that trial. A choice was scored if the rat dug or placed its paws or nose on the sand and an error was scored if the rats chose the previously rewarded bowl. The rats performed six nonmatching-to-sample trials each day until they chose the novel odor on five of the six trials for 3 d.
Odor span task (Fig. 1A)
After reaching criterion on the nonmatching-to-sample task, rats were introduced to the OST. Trials were run as described for the nonmatching-to-sample task except that bowls with novel odors (for that trial) were added to the platform and previous bowls remained until the rat made an error (i.e., dug in any of the bowls except the novel one) which resulted in the trial ending. Previously presented bowls were randomly repositioned before each new bowl was added to the platform. Thus, rats could not use spatial cues to guide their choice. The span for a given trial was scored as the number of odor bowls correctly chosen minus one. The latency to dig on each trial was recorded by the experimenter with a stopwatch and averaged for all trials run on a given day. Each rat performed 1–3 “spans” per day (rats with high spans performed 1 span while rats with medium spans performed 2 spans, and rats with low spans performed 3 spans) with a break between spans occurring while other rats were tested. Averaging the spans reduced variance in our sample, as reported with other memory tests (Winters and Reid 2010). The mean of all spans for a given day is reported in the figures. Once a mean span of 7 or higher was reached for two training days, surgery was performed on the rats.
Probe sessions
To determine whether the rats were using the sample odors to solve the task, two types of probe sessions were conducted. The first probe session was implemented to test if the odor of the food reward guided behavior. In this probe session (three bowls on the platform: span of 2), rats were presented with bowls as described in the OST with the exception that the food reward was absent from all bowls. If the rat made a correct choice, the experimenter placed a food reward on top of the sand in the correct bowl. The second probe was implemented to test if the rats marked the bowls when they examined them. During this probe session (two bowls on the platform: span 1), all of the bowls and sand were replaced with new bowls and new sand that contained the same odors. If rats were marking the bowls, performance would be impaired on these trials. The rats’ performance was 100% accurate during the first probe with food reward absent, and 96% accurate (since one rat made an error) during the second probe of switching the bowls and sand (data not shown). The rat that made an error in the second probe was retested the next day on the same probe twice and got both trials correct.
Surgery and infusions
Rats were anesthetized with isoflurane and prepared for surgery using previously reported procedures (Davies et al. 2013a,b). Rats from each squad were implanted with guide cannulae (23 Ga) bilaterally to target two of the following three brain areas: mPFC (AP + 3.00 mm; ML ± 0.70 mm; DV −3.20 mm from bregma), dmSTR (AP + 0.80 mm; ML ± 2.20 mm; DV −3.40 mm), or dlSTR (AP + 0.80 mm; ML ± 3.60 mm DV −3.40 mm). Obdurators (0.033 cm diameter stainless steel wire) were placed into the cannula to prevent obstruction. Following surgery, rats were allowed to recover for at least a week before training resumed. Rats were habituated to the infusion procedure on three separate days during the week before infusions were administered. Bilateral infusions were performed by inserting custom made needles (30 Ga stainless steel tubing) linked via PE-50 tubing to an infusion pump (PHD 2000, Harvard Apparatus) 1 mm past the end of the cannula. Drugs were infused over 1 min and the infusion needles remained in place for an additional minute after the infusion to allow diffusion of the drug. Rats were tested on the OST 15 min following brain infusions. On treatment days, rats were tested for ∼30 min (1–3 spans) without a break between spans.
Experiment 1: Bilateral inactivation of dmSTR or dlSTR. Rats (n = 9) for this experiment had cannulae implanted over the dmSTR and dlSTR. Infusion needles were inserted into one area bilaterally and either the GABA receptor agonists muscimol (Abcam) and baclofen (Mus/Bac; Abcam) or vehicle (PBS) was delivered to the dmSTR or dlSTR. The agonists were dissolved separately in PBS at a concentration of 500 ng/µL and then mixed together before infusion (McFarland and Kalivas 2001; St Onge and Floresco 2010; Davies et al. 2013b; Sangha et al. 2014).
Experiment 2: Bilateral inactivation of mPFC, bilateral inactivation of dmSTR, or disconnection of mPFC and dmSTR. The same infusion method was used as for experiment 1. Seven rats were tested following three treatments: bilateral mPFC inactivation, bilateral dmSTR inactivation, and contralateral disconnection (unilateral infusions in mPFC and dmSTR of opposite hemispheres). The disconnection procedure was used to block transmission of information within the mPFC–dmSTR pathway in each hemisphere. This procedure has been used to define the route of serial information transfer between different brain regions in a number of tasks (Floresco et al. 1997; Hannesson et al. 2004; Baker and Ragozzino 2014).
Experiment 3: Role of GluN2B-containing NMDA receptors in mPFC and dmSTR in the OST. GluN2B-containing NMDA receptors were selectively targeted with Ro 25-6981. Eight rats were tested in a counterbalanced order following either vehicle (12% DMSO; 88% PBS) or Ro 25-6981 (2.5 µg/0.5 µL) (Zhang et al. 2008; Brigman et al. 2013; Davies et al. 2013a), delivered bilaterally to mPFC or dmSTR. In the contralateral disconnection treatment, Mus/Bac was infused into the mPFC and Ro 25-6981 was infused into the dmSTR. One rat was not tested in the disconnection experiment (bilateral or ipsilateral) as it failed to perform the task reliably on training days. Postmortem examination of its brain revealed evidence of an infection in the mPFC.
Histology
After testing on the OST was complete, rats were sacrificed with isoflurane and perfused with saline. Brains were removed and post-fixed in a 10% formalin–10% sucrose solution. Brains were sectioned on a sliding microtome and infusion sites were determined using standard protocols with reference to a rat brain atlas (Paxinos and Watson 1997).
Fluorescent muscimol infusions
In order to assess the spread of muscimol in the mPFC and dorsal STR following infusions, two rats were anesthetized and fluorescent muscimol (BODIPY TMR-X Conjugate, Life Technologies) (Allen et al. 2008) was infused using the stereotaxic coordinates described above. One rat was infused unilaterally into the mPFC while the other had unilateral infusions into the dmSTR and dlSTR of different hemispheres. Sixty minutes following the infusions, the rats were anesthetized with isoflurane, perfused with saline, and brain slices (200 µm) were cut with a vibratome. Images (Fig. 1C) of the fluorescent muscimol conjugate were captured using a Zeiss Discovery V8 stereoscope equipped with a 16-bit, 1344 × 1024 ORCA-R2 CCD camera (C10600-10B, Hamamatsu) cooled to −35°C. BODIPY TMR-X muscimol conjugate was excited using a filtered (HQ 535/50) Schott KL 1600 wide spectrum LED light source and epifluorescence filtered with an ET 605/70 installed just prior to the camera.
Data analysis
Odor spans and latencies to choose the bowls were manually recorded during testing and entered into Microsoft Excel (2010) and Statistical Package for the Social Sciences (SPSS version 19) for analysis. All descriptive values are reported as means ± standard error of the mean. Comparisons were performed using paired t-tests and repeated measures analysis of variance with Tukey's post hoc tests where appropriate. Statistical tests were considered significant if P values were <0.05.
Acknowledgments
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) and Operating Grant from the Canadian Institutes for Health Research to J.G.H. J.G.H. is a Canadian Institutes of Health Research New Investigator. D.A.D. received funding from a Saskatchewan Innovation & Opportunity Scholarship and an NSERC Postgraduate Scholarship. J.K.C. received funding from the Branch Out Neurological Foundation.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.045419.117.
References
- Akhlaghpou H, Wiskerke J, Choi JY, Taliaferro JP, Au J, Witten IB. 2016. Dissociated sequential activity and stimulus encoding in the dorsomedial striatum during spatial working memory. Elife 5: e19507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9: 357–381. [DOI] [PubMed] [Google Scholar]
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. 2008. Imaging the spread of reversible brain inactivations using fluorescent muscimol. J Neurosci Methods 171: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April LB, Bruce K, Galizio M. 2013. The magic number 70 (plus or minus 20): variables determining performance in the rodent odor span task. Learn Motiv 44: 143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. 2003. Working memory: looking back and looking forward. Nat Rev Neurosci 4: 829–839. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. 2003. Dynamics of population code for working memory in the prefrontal cortex. Neuron 40: 177–188. [DOI] [PubMed] [Google Scholar]
- Baier B, Karnath H-O, Dieterich M, Birklein F, Heinze C, Müller NG. 2010. Keeping memory clear and stable – the contribution of human basal ganglia and prefrontal cortex to working memory. J Neurosci 30: 9788–9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Ragozzino ME. 2014. Contralateral disconnection of the rat prelimbic cortex and dorsomedial striatum impairs cue-guided behavioral switching. Learn Mem 21: 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Smith E. 2008. The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biol Psychiatry 64: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Moore H, Nee DE, Manoach DS, Luck SJ. 2012. CNTRICS imaging biomarkers selection: working memory. Schizophr Bull 38: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, et al. 2013. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci 16: 1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublak P, Müller U, Grön G, Reuter M, von Cramon DY. 2002. Manipulation of working memory information is impaired in Parkinson's disease and related to working memory capacity. Neuropsychology 16: 577–590. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. 2001. Functional disconnection of a prefrontal cortical–dorsal striatal system disrupts choice reaction time performance: implications for attentional function. Behav Neurosci 115: 812–825. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Davis HP, Volpe BT. 1989. Allocentric spatial and tactile memory impairments in rats with dorsal caudate lesions are affected by preoperative behavioral training. Behav Neurosci 103: 1242–1250. [DOI] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. 2008. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci 28: 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Jin J, Zhang X, Xu H, Yang L, Du D, Zeng Q, Tsien JZ, Yu H, Cao X. 2011. Forebrain NR2B overexpression facilitating the prefrontal cortex long-term potentiation and enhancing working memory function in mice. PLoS One 6: e20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DA, Greba Q, Howland JG. 2013a. GluN2B-containing NMDA receptors and AMPA receptors in medial prefrontal cortex are necessary for odor span in rats. Front Behav Neurosci 7: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DA, Molder JJ, Greba Q, Howland JG. 2013b. Inactivation of medial prefrontal cortex or acute stress impairs odor span in rats. Learn Mem 20: 665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M. 2007. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci 362: 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA. 2004. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev 28: 699–709. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. 2000. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci 20: 2964–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. 2013. Animal models of working memory: a review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci Biobehav Rev 37: 2111–2124. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Nathwani F, Brasted PJ. 1999. Medial prefrontal and neostriatal lesions disrupt performance in an operant delayed alternation task in rats. Behav Brain Res 106: 13–28. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. 1997. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci 17: 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O'Reilly RC. 2001. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci 1: 137–160. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Singh J, Stebbins GT, Goetz CG. 1996. Reduced working memory span in Parkinson's disease: Evidence for the role of a frontostriatal system in working and strategic memory. Neuropsychology 10: 322–332. [Google Scholar]
- Galiñanes GL, Braz BY, Murer MG. 2011. Origin and properties of striatal local field potential responses to cortical stimulion: temporal regulation by fast inhibitory connections. PLoS One 6: e28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, Deal M, Hawkey A, April B. 2013. Working memory in the odor span task: effects of chlordiazepoxide, dizocilpine (MK801), morphine, and scopolamine. Psychopharmacology (Berl) 225: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. 1996. Regional and cellular fractionation of working memory. Proc Natl Acad Sci 93: 13473–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Dayan P, Gutkin BS, Solla SA. 2006. Dopamine modulation in the basal ganglia locks the gate to working memory. J Comput Neurosci 20: 153–166. [DOI] [PubMed] [Google Scholar]
- Hannesson DK, Howland JG, Phillips AG. 2004. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci 24: 4596–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. 1989. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiol 61: 814–832. [DOI] [PubMed] [Google Scholar]
- Höhn S, Dallérac G, Faure A, Urbach YK, Nguyen HP, Riess O, von Hörsten S, Le Blanc P, Desvignes N, El Masioui N, et al. 2011. Behavioral and in vivo electrophysiological evidence for presymptomatic alteration of prefrontalstriatal processing in the transgenic rat model for Huntington disease. J Neurosci 31: 8986–8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. 2009. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev 33: 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley JD, Howard RJ. 2010. Working memory in early Alzheimer's disease: a neuropsychological review. Int J Geriatr Psychiatry 25: 121–132. [DOI] [PubMed] [Google Scholar]
- Kang S, Ling Q-I, Liu W-T, Lu B, Liu Y, He L, Liu J. 2013. Down-regulation of dorsal striatal RhoA activity and impairment of working memory in middle-aged rats. Neurobiol Learn Mem 103: 3–10. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. 1998. Expectation of reward modulates cognitive signals in the basal gangliz. Nat Neurosci 1: 411–416. [DOI] [PubMed] [Google Scholar]
- Kolb B. 1990. Animal models for human PFC-related disorders. Prog Brain Res 85: 501–519. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. 2009. Striatal dopamine and working memory. Cereb Cortex 19: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Durstewitz D, Chandler LJ, Seamans JK. 2008. Successful choice behavior is associated with distinct and coherent network states in anterior cingulate cortex. Proc Natl Acad Sci 105: 11963–11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist M, Rose J, Herman P, Brincat SL, Buschman TJ, Miller EK. 2016. γ and β bursts underlie working memory. Neuron 90: 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen DA, Bullard L, Galizio M. 2011. Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiol Learn Mem 95: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailly P, Aliane V, Groenewegen HJ, Haber SN, Deniau JM. 2013. The rats prefrontalstriatal system analyzed in 3D: evidence for multiple interacting functional units. J Neurosci 33: 5718–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. 2001. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21: 8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. 1989. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29: 503–537. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. 2008. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci 11: 103–107. [DOI] [PubMed] [Google Scholar]
- Moore H, Geyer MA, Carter CS, Barch DM. 2013. Harnessing cognitive neuroscience to develop new treatments for improving cognition in schizophrenia: CNTRICS selected cognitive paradigms for animal models. Neurosci Biobehav Rev 37: 2087–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa R, Poucet B, Amalric M, Sargolini F. 2011. Contributions of dorsal striatal subregions to spatial alternation behavior. Learn Mem 18: 444–451. [DOI] [PubMed] [Google Scholar]
- Murray BG, Davies DA, Molder JJ, Howland JG. 2017. Maternal immune activation during pregnancy in rats impairs working memory capacity of the offspring. Neurobiol Learn Mem 141: 150–156. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Lange KW, Robbins TW. 1992. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain 115: 1727–1751. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. 1990. Lesions of the caudate nucleus selectively impair ‘reference memory’ acquisition in the radial maze. Behav Neural Biol 53: 39–50. [DOI] [PubMed] [Google Scholar]
- Packard MG, Hirsh R, White NM. 1989. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci 9: 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Winocur G, White NM. 1992. The caudate nucleus and acquisition of win-shift radial-maze behavior: effect of exposure to the reinforcer during maze adaptation. Psychobiology 20: 127–132. [Google Scholar]
- Paxinos G, Watson C. 1997. The rat brain in stereotaxic coordinates. Elsevier Academic Press, Amsterdam. [Google Scholar]
- Pomata PE, Belluscio MA, Riquelme LA, Murer MG. 2008. NMDA receptor gating of information flow through the striatum in vivo. J Neurosci 28: 13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacott S, Shoaib M. 2010. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neurosci Lett 471: 114–118. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Steckler T, Shoaib M. 2011. Nicotine improves working memory span capacity in rats following sub-chronic ketamine exposure. Neuropsychopharmacology 36: 2774–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom MI, Rebec GV. 2003. Characterization of striatal activity in conscious rats: contribution of NMDA and AMPA/kainate receptors to both spontaneous and glutamate-driven firing. Synapse 47: 91–100. [DOI] [PubMed] [Google Scholar]
- Sangha S, Robinson PD, Greba Q, Davies DA, Howland JG. 2014. Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology 39: 2405–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Romo R. 1988. Neuronal activity in the monkey striatum during the initiation of movements. Exp Brain Res 71: 431–436. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. 1989. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290: 213–242. [DOI] [PubMed] [Google Scholar]
- Smeal RM, Keefe KA, Wilcox KS. 2008. Differences in excitatory transmission between thalamic and cortical afferent to single spiny efferent neurons of rat dorsal striatum. Eur J Neurosci 28: 2041–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Roe SL, Sadeghian K, Kelley AE. 1999. Spatial learning and performance in the radial arm maze is impaired after N-methyl-D-aspartate (NMDA) receptor blockade in striatal subregions. Behav Neurosci 113: 703–717. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Testa CM, Young AB, Penney JB Jr. 1994. Organization of N-methyl-D-aspartate gluatmate receptor gene expression in basal ganglia of the rat. J Comp Neurol 343: 1–16. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. 2010. Prefrontal cortical contribution to risk-based decision making. Cereb Cortex 20: 1816–1828. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. 2004. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27: 468–474. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Vytlacil JJ, Nomura EM, Gibbs SE, D'Esposito M. 2011. The dopamine agonist bromocriptine differentially affects fronto-striatal functional connectivity during working memory. Front Hum Neurosci 5: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. 2013. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77: 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Stanton ME. 2009. Spatial discrimination reversal learning in weanling rats is impaired by striatal administration of an NMDA-receptor antagonist. Learn Mem 16: 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM. 2009. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behav Brain Res 199: 3–23. [DOI] [PubMed] [Google Scholar]
- Winters BD, Reid JM. 2010. A distributed cortical representation underlies crossmodal object recognition in rats. J Neurosci 30: 6253–6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kerr LE, Kelly JS, Marston HM, Spratt C, Finlayson K, Sharkey J. 2007. The odour span task: a novel paradigm for assessing working memory in mice. Neuropharmacology 52: 634–645. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Wu LJ, Gong B, Ren M, Li BM, Zhuo M. 2008. Induction- and conditioning-protocol dependent involvement of NR2B-containing NMDA receptors in synaptic potentiation and contextual fear memory in the hippocampal CA1 region of rats. Mol Brain 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]