Abstract
PURPOSE
We aimed to identify modifiable and nonmodifiable risk factors for hemoptysis complicating computed tomography (CT)-guided transthoracic needle biopsy.
METHODS
All procedures performed in our institution from November 2013 to May 2015 were reviewed. Hemoptysis was classified as mild if limited to hemoptoic sputum and abundant otherwise. Presence of intra-alveolar hemorrhage on postbiopsy CT images was also evaluated. Patient- and lesion-related variables were considered nonmodifiable, while procedure-related variables were considered modifiable.
RESULTS
A total of 249 procedures were evaluated. Hemoptysis and alveolar hemorrhage occurred in 18% and 58% of procedures, respectively, and were abundant or significant in 8% and 17% of procedures, respectively. Concordance between the occurrence of significant alveolar hemorrhage (grade ≥2) and hemoptysis was poor (κ=0.28; 95% CI [0.16–0.40]). In multivariate analysis, female gender (P = 0.008), a longer transpulmonary needle path (P = 0.014), and smaller lesion size (P = 0.044) were independent risk factors for hemoptysis. Transpulmonary needle-path length was the only risk factor for abundant hemoptysis with borderline statistical significance (P = 0.049).
CONCLUSION
The transpulmonary needle path should be as short as possible to reduce the risk of abundant hemoptysis during CT-guided transthoracic needle biopsy.
Computed tomography (CT)-guided transthoracic needle biopsy (TTNB) is a well-established method for investigating pulmonary nodules and masses. It offers a better diagnostic yield than bronchoscopy for the assessment of lung nodules and peripheral lesions (1, 2). Referral for CT-guided TTNB is expected to increase in the coming years. One reason is the higher proportion of adenocarcinomas among current lung cancers, which are more likely to be peripheral and not reachable by bronchoscopy (3, 4). Another reason is the recommendation of re-biopsy for molecular analysis of non-small cell lung cancers that progress or recur (5–7). Although TTNB is considered a safe and efficient method for obtaining a definite pathologic diagnosis, complications are reported in up to two-thirds of procedures (8, 9). The three main complications of TTNB are pneumothorax, alveolar hemorrhage, and hemoptysis. These complications are rarely life threatening, and pulmonary bleeding is usually self-resolving. However, severe hemoptysis can necessitate endobronchial tamponade, arterial embolization, or surgery (8, 10).
It is important for the operator to be aware of potentially modifiable risk factors. Several modifiable risk factors for pneumothorax have been reported in the literature, including angle between needle and pleural surface (11), transpulmonary needle-path length (12, 13), patient position during the procedure (11), and operator’s level of experience (12). A large pleural angle, a long needle path, the prone position, and limited expertise are all reported to increase the risk of pneumothorax. By contrast, data on risk factors for hemoptysis in this setting are scarce, and most published studies of transthoracic biopsy have combined alveolar hemorrhage and hemoptysis under the term “pulmonary bleeding.” Chakrabarti et al. (14) have shown that clinical judgment can reliably assess the risk of pneumothorax but not that of pulmonary bleeding.
The purpose of this study was to identify modifiable and nonmodifiable risk factors for hemoptysis during CT-guided transthoracic needle biopsy.
Methods
The study was approved by the local ethics committee. The need for patients’ written informed consent was waived because of the retrospective design.
Procedures
We reviewed all TTNB procedures targeting pulmonary lesions performed in our tertiary referral hospital from November 2013 to May 2015. The following laboratory parameters were required before performing biopsy: platelet count >100 000/mm3, prothrombin time >60%, and partial thromboplastin time ≤ 1.5 times the normal limit. Aspirin and clopidogrel were withheld for 5 and 10 days, respectively, and heparin was withheld for 1 day. Pulmonary hypertension is a contraindication to TTNB at our institution.
The procedures were performed under CT guidance using multislice CT devices (Brillance 16, Philips Healthcare; Somatom Sensation 16 or Somatom Definition DS, Siemens Healthcare). The biopsies were performed or supervised by two different radiologists with 15- and 2-year experience in interventional radiology. Coaxial 17-gauge needles with 18-gauge automated cutting needles (Bard Monopty, Bard) were used for all procedures. The automated cutting needle penetration depth was 22 mm.
Patients were positioned in the supine, prone, or lateral position depending on the location of the target lesion. Unenhanced 3 mm thick CT images were used to plan an optimal puncture site and needle trajectory. Local anesthesia was administered by sub-cutaneous injection of 2% lidocaine. The needle position was checked several times until the coaxial tip reached the proper position for biopsy. The number of samples was indicated on the radiologic report. After needle removal, postbiopsy CT images were systematically acquired to detect complications. Patients were observed for 30 minutes in the radiology department. They were discharged the day after the procedure if the chest X-ray showed no signs of pneumothorax.
Study variables
CT images of the procedures, radiologic reports, and patient charts were reviewed in consensus by two radiologists (G.C. and M.A.A.) with respectively 3 years and 1 year of experience in chest imaging.
The study variables are shown in Table 1 and in Figs. 1, 2, and 3. Patient- and lesion-related variables were considered nonmodifiable, while procedure-related variables were considered modifiable. Patient-related variables included age and sex. Lesion-related variables included lesion size, defined as the longest diameter in the axial plane (Fig. 1), the lobar location (lower lobes or elsewhere), peripheral (contact between the lesion and the peripheral pleura) or nonperipheral location (Fig. 2), attenuation characteristics (distinguishing solid, subsolid, cavitated, and necrotic lesions), and the final pathologic diagnosis. Pathologic findings were divided into 5 categories: adenocarcinoma, squamous cell carcinoma, metastasis of extrathoracic malignancies, other tumors, and benign lesions.
Table 1.
Patients, lesions, and procedures
| No hemoptysis n=205 | Any hemoptysis n=44 | Mild hemoptysis n=24 | Abundant hemoptysis n=20 | |
|---|---|---|---|---|
| Nonmodifiable parameters | ||||
|
| ||||
| Female gender | 64 (31) | 24 (54) | 14 (58) | 10 (50) |
|
| ||||
| Age (years) | 66.8±10.4 | 68.3±10.2 | 66.9±8.4 | 70.1±12.0 |
|
| ||||
| Lesion size (cm) | 4.4±4.1 | 2.7±1.7 | 2.5±1.2 | 3.0±2.2 |
|
| ||||
| Nonperipheral location | 96 (47) | 26 (59) | 15 (63) | 11 (55) |
|
| ||||
| Lower lobe location | 67 (33) | 20 (45) | 12 (50) | 8 (40) |
|
| ||||
| Lesion morphology | ||||
| Cavitated | 12 (6) | 3 (7) | 3 (12) | 0 |
| Solid | 177 (87) | 39 (89) | 21 (88) | 18 (90) |
| Subsolid | 5 (2) | 1 (2) | 0 | 1 (5) |
| Necrotic | 11 (5) | 1 (2) | 0 | 1 (5) |
|
| ||||
| Lesion attenuation (Hounsfield units) | 37.2±19.9 | 39.3±25.5 | 32.5±19.6 | 49.4 (30) |
|
| ||||
| Histologic type | ||||
| Adenocarcinoma | 76 (37) | 22 (50) | 11 (46) | 11 (55) |
| Squamous cell carcinoma | 29 (14) | 4 (9) | 2 (8) | 2 (10) |
| Metastasis | 23 (11) | 5 (11) | 4 (17) | 1 (5) |
| Other tumor | 39 (19) | 7 (16) | 3 (12) | 4 (20) |
| Nontumoral | 38 (19) | 6 (14) | 4 (17) | 2 (10) |
|
| ||||
| Modifiable parameters | ||||
| Patient position | ||||
| Prone | 78 (38) | 16 (36) | 9 (37) | 7 (35) |
| Supine | 86 (42) | 19 (43) | 10 (42) | 9 (45) |
| Contralateral decubitus | 41 (20) | 9 (20) | 5 (21) | 4 (20) |
|
| ||||
| Ipsilateral decubitus | 23 (11) | 6 (14) | 4 (17) | 2 (10) |
|
| ||||
| Transpulmonary needle-path length (cm) | 1.5±1.6 | 2.5±1.9 | 2.4±1.9 | 2.5±1.9 |
|
| ||||
| Coaxial tip outside the target lesion | 31 (15) | 10 (23) | 2 (8) | 8 (40) |
|
| ||||
| Lesion diameter along the needle path (cm) | 2.3±1.7 | 1.7 ±1.3 | 1.6±0.8 | 1.9 ±1.7 |
|
| ||||
| <2.2 cm | 119 (58) | 34 (77) | 18 (75) | 16 (80) |
|
| ||||
| Operator: | ||||
| Operator 1 | 141 (69) | 29 (66) | 16 (67) | 13 (66) |
| Operator 2 | 64 (31) | 15 (34) | 8 (33) | 7 (34) |
|
| ||||
| Number of per-procedure CT acquisitions until biopsy | 2.0±1.3 | 2.7±1.8 | 2.5±1.7 | 3.0±1.9 |
|
| ||||
| Number of tissue sampling | 2.9±1.0 | 2.5±1.1 | 2.6±1.6 | 2.3±1.0 |
Data are presented as n (%) or mean ± standard deviation.
Figure 1.
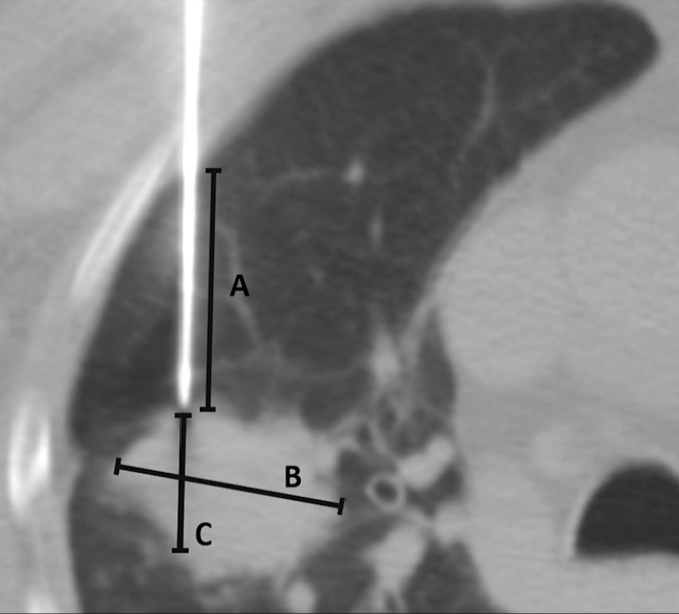
Computed tomography (CT) image obtained during CT-guided transthoracic needle biopsy (TTNB) illustrating measurements of transpulmonary needle-path length (measurement A), lesion size (measurement B), and lesion diameter along the needle path (measurement C).
Figure 2. a, b.
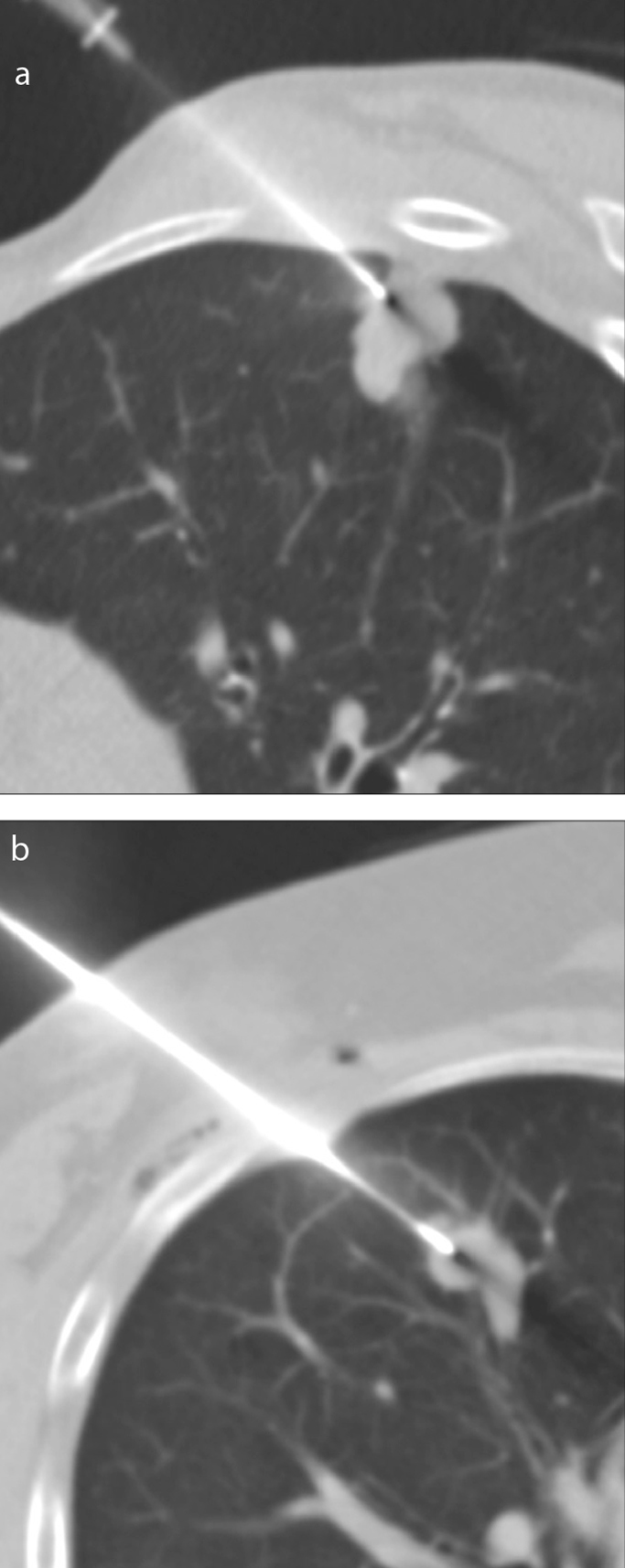
Axial CT images obtained during CT-guided TTNB illustrating the classification of nodule situation. Panel (a) shows a peripheral lung nodule abutting the peripheral pleura. Panel (b) shows a nonperipheral lung nodule distant from the peripheral pleura.
Figure 3. a–c.
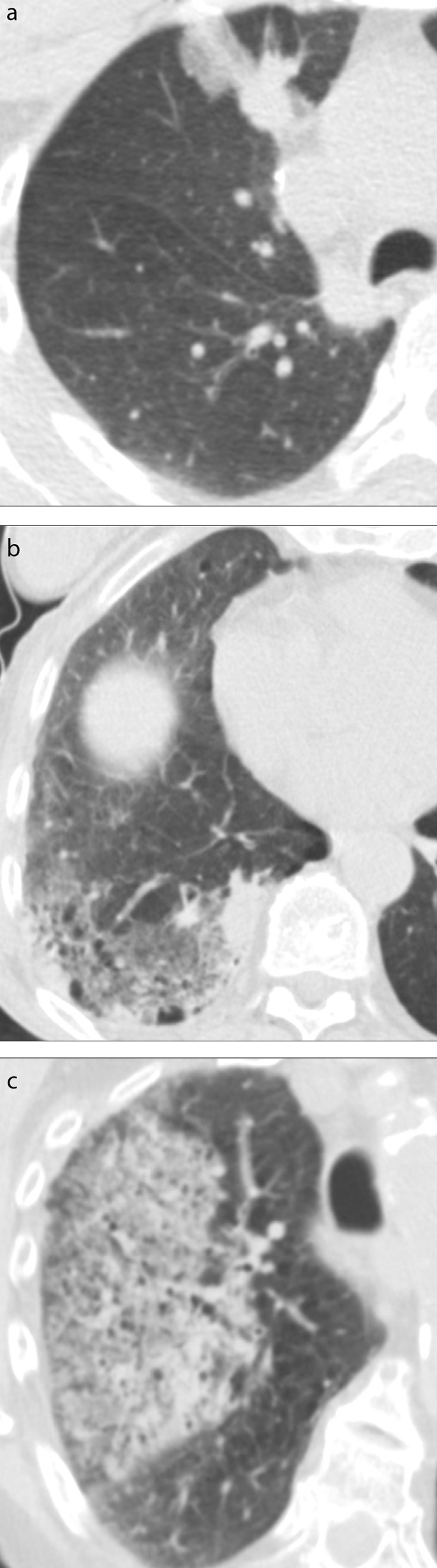
Post-biopsy axial CT images illustrating the alveolar hemorrhage grading scheme. Panel (a) shows a limited subsegmental ground-glass opacity <2 cm in width along the needle tract in the anterior segment of the right upper lobe, corresponding to a grade 1 hemorrhage. Panel (b) shows a >2 cm ground-glass opacity extending in the posterior basal segments of the right lower lobe, corresponding to a grade 2 hemorrhage. Panel (c) shows a ground class opacity extending to the right upper lobe, corresponding to grade 3 hemorrhage.
Procedure-related variables included the radiologist’s experience level, the patient’s position, the number of consecutive CT acquisitions after pleural crossing performed until the needle tip reached the proper position for biopsy, the coaxial tip position (inside or outside the target lesion), the length of the transpulmonary needle path, and the lesion diameter along the needle track, the latter two dimensions being measured on the last CT images acquired prior to the first sampling (Fig. 1). The number of tissue sampling was also analyzed.
Postbiopsy CT images were analyzed to detect new ground-glass opacities indicating alveolar hemorrhage. The structured reports used in our institution, which mention hemoptysis when present, were also reviewed for hemoptysis and its abundance. Hemoptysis was categorized as mild when limited to hemoptoic sputum, and otherwise as abundant. Alveolar hemorrhage defined as any new ground-glass opacity was considered grade 1 in case of needle tract hemorrhage 2 cm or less in width, grade 2 if measuring more than 2 cm in width, and grade 3 when lobar or greater, as derived from the classification used by Tai et al. (15) (Fig. 3). Each patient’s hospital charts were reviewed to determine if specific treatment for hemoptysis had been necessary.
We considered that a definitive diagnosis had been obtained when the pathologic report indicated a precise tumoral or non-tumoral diagnosis.
Statistical analysis
Standard descriptive analyses (proportions, means, and standard deviations [SD]) and univariate analyses were performed first, using the Chi-square test for categorical variables and the Wilcoxon test for continuous variables. A logistic regression model was then used to identify predictors of hemoptysis, and a multinomial regression model was used to identify predictors of hemoptysis abundance. For multivariate analysis, predictive models were constructed in successive steps, as follows: we first modeled patient characteristics, followed by lesion characteristics, and then procedure-related variables. Odds ratios (OR) and 95% confidence intervals (CI) are reported as appropriate. P values below 0.05 were considered to indicate statistical significance. All analyses were done with the R statistical software package (version 3.1.3, R Foundation for Statistical Computing).
Results
Characteristics of patients and procedures are summarized in Table 1. We analyzed a total of 249 biopsies in 240 patients (86 women and 154 men). Seven patients had 2 biopsies and one patient had 3 biopsies. Mean age was 67±10.5 years (range, 31–89 years).
The mean size of the lesions was 41±38 mm (median, 31 mm). Of the lesions, 35% were located in the lower lobes (n=87) and 49% in a nonperipheral location (n=122). The mean transpulmonary needle-path length was 17±17 mm and the mean lesion diameter along the needle path was 22±16 mm. Diameter of the lesion along the needle path was smaller than the 22 mm needle-throw of the cutting needle in 61% of the lesions (153/249). The coaxial needle tip remained outside the target lesion in only 41 procedures (17%).
The mean number of tissue samples per procedure was 3±1, and 243 procedures (98%) yielded a definite diagnosis. Among the 243 lesions with a definite diagnosis, 201 were malignant (adenocarcinomas, n=98; squamous cell carcinoma, n=33; metastasis, n=28; other malignant tumors, n=42) and 42 were nonmalignant (benign tumors, n=4; others, n=38).
Pulmonary bleeding, including alveolar hemorrhage and hemoptysis, complicated 60% of the procedures (150/249). Alveolar hemorrhage occurred in 145 procedures (58%). There were 59 cases of grade 1 alveolar hemorrhage (24%), 85 cases of grade 2 alveolar hemorrhage (34%) and one case of grade 3 alveolar hemorrhage (0.4%). Hemoptysis occurred in 44 cases (18%). It was mild in 24 cases (10%) and abundant in 20 cases (8%). Concordance between the occurrence of significant alveolar hemorrhage (grade ≥ 2) and hemoptysis was poor (κ=0.28; 95% CI [0.16–0.40]). All pulmonary bleeds resolved spontaneously; none necessitated embolization or surgery.
In univariate analysis, female gender (P = 0.002) and smaller lesion size (P < 0.001) were significant nonmodifiable predictors of hemoptysis (any abundance) and mild hemoptysis. Among the modifiable variables, transpulmonary needle-path length (P < 0.001, P = 0.006) and lesion diameter along the needle axis (P = 0.015, P = 0.004) were significant risk factors for hemoptysis (any abundance), and for mild hemoptysis, respectively. Lower-lobe lesion location (P = 0.046) was a significant predictor of mild hemoptysis only. Univariate analysis also identified smaller lesion size (P = 0.026), higher lesion attenuation (P = 0.002) and coaxial needle tip outside the target lesion (P < 0.001) as significant predictors of abundant hemoptysis (Table 2).
Table 2.
Univariate analysis of nonmodifiable and modifiable parameters as predictors of hemoptysis
| Any hemoptysis | Mild hemoptysis | Abundant hemoptysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Nonmodifiable parameters | ||||||
|
| ||||||
| Female gender | 2.64 (1.37–5.17) | 0.002 | 2.86 (1.22–6.92) | 0.002 | 2.94 (0.76–4.91) | 0.061 |
|
| ||||||
| Age (years) | 1.01 (0.98–1.05) | 0.35 | 1.00 (0.96–1.04) | 0.94 | 1.03 (0.99–1.08) | 0.067 |
|
| ||||||
| Lesion size (cm) | 0.75 (0.60–0.89) | <0.001 | 0.68 (0.49–0.88) | <0.001 | 0.84 (0.65–1.02) | 0.026 |
|
| ||||||
| Nonperipheral location | 1.64 (0.83–3.20) | 0.13 | 1.84 (0.78–4.54) | 0.074 | 1.30 (0.52–3.30) | 0.46 |
|
| ||||||
| Lower lobe location | 1.72 (0.88–3.32) | 0.10 | 2.00 (0.88–4.71) | 0.046 | 1.27 (0.48–3.20) | 0.50 |
|
| ||||||
| Lesion morphology | 0.65 | 0.59 | 1 | |||
| Cavitated | 2.75 (0.30–60.31) | ∞ (0.45–∞) | 0 (0–19.00) | |||
| Solid | 2.42 (0.45–44.95) | ∞ (0.28–∞) | 1.12 (0.17–25.03) | |||
| Subsolid | 2.20 (0.07–63.85) | 0 (0–∞) | 2.10 (0.05–92.11) | |||
| Necrotic | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
|
| ||||||
| Lesion attenuation (Hounsfield units) | 1.00 (0.99–1.02) | 0.56 | 0.99 (0.96–1.01) | 0.11 | 1.02 (1.00–1.05) | 0.002 |
|
| ||||||
| Histologic type | 0.54 | 0.53 | 0.22 | |||
| Adenocarcinoma | 2.10 (0.73–7.63) | 1.96 (0.49–13.12) | 3.41 (0.62–63.81) | |||
| Squamous cell carcinoma | 1.00 (reference) | 1.00 (reference) | 1.74 (0.16–38.71) | |||
| Metastasis | 1.58 (0.38–7.01) | 2.58 (0.46–19.78) | 1.00 (reference) | |||
| Other tumor | 1.30 (0.36–5.36) | 1.08 (0.17–8.58) | 2.57 (0.36–51.75) | |||
| Nontumoral | 1.14 (0.30–4.83) | 1.55 (0.28–11.70) | 1.29 (0.12–28.46) | |||
|
| ||||||
| Modifiable parameters | ||||||
|
| ||||||
| Patient position | 0.98 | 0.99 | 0.93 | |||
| Prone | 1.00 (reference) | 1.07 (0.38–2.61) | 1.00 (reference) | |||
| Supine | 1.08 (0.52–2.26) | 1.00 (reference) | 1.17 (0.42–3.38) | |||
| Lateral | 1.07 (0.42–2.59) | 1.06 (0.31–3.16) | 1.08 (0.27–3.77) | |||
|
| ||||||
| Ipsilateral decubitus | 1.25 (0.44–3.11) | 0.65 | 1.6 (0.44–4.65) | 0.34 | 0.83 (0.13–3.10) | 0.75 |
|
| ||||||
| Transpulmonary needle-path length (cm) | 1.36 (1.14–1.65) | <0.001 | 1.29 (1.03–1.62) | 0.006 | 1.34 (1.04–1.71) | 0.002 |
|
| ||||||
| Coaxial tip outside the target lesion | 1.65 (0.71–3.59) | 0.22 | 2.31 (0.64–14.77) | 0.13 | 4.0 (1.45–10.33) | <0.001 |
|
| ||||||
| Lesion diameter along the needle axis (cm) | 0.75 (0.56–0.96) | 0.015 | 0.66 (0.41–0.95) | 0.004 | 0.87 (0.60–1.17) | 0.25 |
|
| ||||||
| <2.2 cm | 2.45 (1.19–5.49) | 0.011 | 2.00 (0.80–5.69) | 0.064 | 2.69 (1.01–9.60) | 0.013 |
|
| ||||||
| Operator | 0.70 | 0.82 | 0.66 | |||
| Operator 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Operator 2 | 1.14 (0.56–2.44) | 1.08 (0.42–2.59) | 1.17 (0.43–2.99) | |||
|
| ||||||
| Number of per-procedure CT acquisitions until biopsy | 1.33 (1.08–1.62) | 0.005 | 1.17 (0.89–1.49) | 0.15 | 1.38 (1.07–1.76) | 0.001 |
|
| ||||||
| Number of tissue sampling | 0.65 (0.44–0.92) | 0.011 | 0.79 (0.50–1.21) | 0.19 | 0.55 (0.31–0.91) | 0.001 |
When the lesion diameter along the needle axis was smaller than the 22 mm needle-throw, the odds ratio for abundant hemoptysis was 2.69 (1.01–9.60; P = 0.013), but this was not a risk factor for mild hemoptysis. Lesion size correlated with lesion diameter along the needle axis (P < 0.001). The number of consecutive CT acquisitions was significantly higher in patients with hemoptysis (any abundance) (P = 0.001) or abundant hemoptysis (P = 0.005). This was not the case for mild hemoptysis (P = 0.15). A smaller number of biopsy sampling was paradoxically associated to an increased occurrence of hemoptysis (any abundance) (P = 0.011) and abundant hemoptysis (P = 0.001). The rate of hemoptysis was not significantly different between the two radiologists (29/170 [17%] vs. 15/79 [19%], P = 0.70) (Table 2).
In multivariate analysis (Table 3), female gender (P = 0.008), a longer transpulmonary needle path (P = 0.014) and smaller lesion size (P = 0.044) were the only independent risk factors for hemoptysis of any abundance and for mild hemoptysis. The needle-path length was the only independent risk factor for abundant hemoptysis, with P value at the limit of significance (P = 0.05). As lesion size and patient gender interacted (P < 0.001, smaller lesions being found in women), we performed multivariate analyses within gender strata. These analyses identified no other predictors apart from transpulmonary needle-path length (OR=1.26 [1.04; 1.54]; P = 0.019) and lesion size (OR=0.78 [0.63; 0.95]; P = 0.022).
Table 3.
Multivariate analysis of risk factors for hemoptysis
| Any hemoptysis | Mild hemoptysis | Abundant hemoptysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Female gender | 2.47 (1.24–5.00) | 0.008 | 2.77 (1.13–6.83) | 0.013 | 2.19 (0.83–5.78) | 0.083 |
|
| ||||||
| Transpulmonary needle-path length | 1.29 (1.06–1.58) | 0.014 | 1.29 (1.01–1.66) | 0.041 | 1.30 (0.99–1.72) | 0.049 |
|
| ||||||
| Lesion size | 0.82 (0.66–0.97) | 0.044 | 0.69 (0.50–0.95) | 0.022 | 0.94 (0.75–1.17) | 0.57 |
|
| ||||||
| Coaxial tip outside the lesion | 1.33 (0.81–2.33) | 0.28 | 0.27 (0.06–1.30) | 0.10 | 2.67 (0.94–7.54) | 0.062 |
Discussion
We found that female gender, smaller lesion size, and longer transpulmonary needle path were independent risk predictors for hemoptysis during transthoracic needle biopsy. Few studies have specifically examined predictors of hemoptysis in this setting (13, 16), and most grouped hemoptysis together with alveolar hemorrhage under the term “pulmonary bleeding” (12, 14, 17–21).
Alveolar hemorrhage is a frequent complication of lung TTNB but is usually limited to the needle path. It is reported to occur in up to 66% of procedures (9), while hemoptysis is reported to occur in up to 20% of procedures (22). Interestingly, we found that the occurrence of hemoptysis and alveolar hemorrhage were independent. Hemoptysis and alveolar hemorrhage occurred in respectively 18% and 58% of our procedures, values on the high side of previously reported rates. However, as suggested by Tai et al. (15), hemoptysis limited to hemorrhagic sputum may have been underreported. The higher rate of hemoptysis in our study may also be related to the use of cutting needles and a coaxial technique, which were found to increase the risk of bleeding in some series (15, 16, 23) but not in others (17, 23–25).
An association between female gender and pulmonary bleeding has already been reported, based on both univariate and multivariate analysis (15, 16, 18). Tai et al. (15) have suggested that females may have confounding morbidities, without any further explanation. We also found a significant interaction between female gender and smaller lesion size. Because the smaller lesion size in women might have been a confounding factor, we performed a second multivariate analysis that excluded gender, but this did not unmask other risk factors.
Small lesion size is one of the most frequently reported risk factors for pulmonary bleeding (12, 13, 15, 17–19, 24, 26). A higher bleeding rate has been reported for lesions with diameters smaller than 20 mm (12, 19, 26), a value corresponding to the throw-length of most cutting needles. Some authors have speculated that the higher bleeding rate observed in patients with lesions smaller than 20 mm may be due to extension of the cutting needle into the normal surrounding lung parenchyma (12, 19, 26). We distinguished between the target lesion size and its diameter along the needle axis. A diameter along the needle axis smaller than the 22 mm needle throw was not a significant risk factor in multivariate analysis, and neither was “coaxial needle tip outside the target lesion”, despite the significance of both parameters in univariate analysis. The observed interaction between lesion size and lesion diameter along the needle axis might have masked a relation between the latter parameter and the risk of hemoptysis. It is noteworthy in this respect that a study of tumor perfusion based on dynamic contrast-enhanced CT showed higher perfusion of smaller lesions, which might contribute to a higher risk of hemoptysis (27).
The association observed here between transpulmonary needle-path length and hemoptysis is in keeping with previous reports (12, 13, 15, 17–20, 24, 28). Transpulmonary needle-path length is a modifiable parameter that depends on patient positioning. Increasing the length of lung parenchyma crossed by the biopsy needle increases the risk of damaging pulmonary vessels, a known risk factor for pulmonary bleeding (17). In a comparison of two techniques for TTNB of small subpleural lesions, the long needle-path technique was associated with a higher risk of bleeding than the short needle-path technique (28). Longer transpulmonary needle paths also correspond to more centrally located lesions that are difficult to reach and sometimes require multiple needle reorientations (15, 17). However, in our study, bleeding never occurred during needle positioning but only after obtaining at least one sample. It has been suggested that the larger size of pulmonary vessels around centrally located lesions may explain the increased risk of major bleeding (17). This is supported by our finding that transpulmonary needle-path length was the only independent risk factor for abundant hemoptysis.
None of the other factors we evaluated was independently predictive of hemoptysis. In particular, we found no association between patient position (including the ipsilateral decubitus) and hemoptysis. This is in keeping with previous studies showing no influence of patient position on the risk of pulmonary bleeding or pneumothorax (20, 29). Some authors found age, emphysema, subsolid type, nonperipheral, middle, and basal location, and dual antiplatelet therapy to be significantly associated with pulmonary hemorrhage after TTNB, but most of these studies used only univariate analysis (15–17, 21, 29). Although pulmonary hypertension has also been identified as a potential risk factor, no significant association between pulmonary hypertension and high-grade pulmonary bleeding was found in recent studies (15, 30). Similar to the report of Yeow et al. (12), the level of experience was not found to influence the hemoptysis risk in our study. The influence of the radiologist experience level may have been masked by the fact that difficult cases, especially biopsies of small nodules, were preferably referred to the more experienced radiologist.
All factors predictive of hemoptysis identified here are also known predictors of pneumothorax. However, transpulmonary needle-path length is the only modifiable parameter, and also the only predictor of abundant hemoptysis. Choosing a patient position that shortens the transpulmonary needle path could reduce the risk of both hemoptysis and abundant hemoptysis, and has also been reported to reduce the risk of pneumothorax (12–14, 20, 29, 31). However, in patients with small subpleural lesions, a better diagnostic yield has been reported with an indirect approach that involves a longer intrapulmonary needle path (28, 32).
We found that a smaller number of biopsy sampling was paradoxically associated to an increased occurrence of hemoptysis. This is due to the fact that hemoptysis was often observed after the first sampling, leading to interruption of the procedure.
This study has several limitations resulting from its retrospective design. Data on hemoptysis were collected from the radiology reports, with a risk of inconsistent estimation of its abundance. However, the use of a structured report form that included hemoptysis occurrence probably limited this risk, as did the simple distinction we made between mild (limited to sputum) and abundant hemoptysis. Another limitation is the small number of cases of abundant hemoptysis, together with interactions between some of the study variables, which may have masked other predictors in multivariate analysis. None of the hemoptysis cases required specific therapy. We had data on hospitalization duration for all patients but we could not distinguish between hospitalization due to hemoptysis or other reasons. Thus, we could not use the stratification of complication severity proposed in the Quality Improvement Guidelines for Percutaneous Biopsy of the Society of Interventional Radiology (33) in which hemoptysis is considered as a major complication if it requires hospitalization or specific therapy. The proportion of biopsies leading to a definite diagnosis was in the upper range of values reported in these guidelines (98% vs. 77%–96%) (33).
In conclusion, we found that female gender, smaller lesion size, and a longer transpulmonary needle path were predictive of hemoptysis after lung TTNB. Needle-path length was the only predictor of abundant hemoptysis. The risk of hemoptysis can thus be reduced by optimizing the needle trajectory and patient position, thereby shortening the transpulmonary needle path.
Main points.
Concordance between the abundance of hemoptysis and that of pulmonary hemorrhage is poor.
The risk of hemoptysis is higher in small lesions and with longer transpulmonary needle paths.
The transpulmonary needle path should be as short as possible to reduce the risk of abundant hemoptysis.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142:385–393. doi: 10.1378/chest.11-1764. https://doi.org/10.1378/chest.11-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labbé C, Beaudoin S, Martel S, et al. Diagnostic yield of non-guided flexible bronchoscopy for peripheral pulmonary neoplasia. Thorac Cancer. 2015;6:517–523. doi: 10.1111/1759-7714.12223. https://doi.org/10.1111/1759-7714.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. https://doi.org/10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 4.Brooks DR, Austin JHM, Heelan RT, et al. Influence of type of cigarette on peripheral versus central lung cancer. Cancer Epidemiol Biomark Prev. 2005;14:576–581. doi: 10.1158/1055-9965.EPI-04-0468. https://doi.org/10.1158/1055-9965.EPI-04-0468. [DOI] [PubMed] [Google Scholar]
- 5.Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17:1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. https://doi.org/10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chouaid C, Dujon C, Do P, et al. Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01) Lung Cancer. 2014;86:170–173. doi: 10.1016/j.lungcan.2014.08.016. https://doi.org/10.1016/j.lungcan.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Wagnetz U, Menezes RJ, Boerner S, et al. CT screening for lung cancer: implication of lung biopsy recommendations. AJR Am J Roentgenol. 2012;198:351–358. doi: 10.2214/AJR.11.6726. https://doi.org/10.2214/AJR.11.6726. [DOI] [PubMed] [Google Scholar]
- 8.Laurent F, Montaudon M, Latrabe V, Bégueret H. Percutaneous biopsy in lung cancer. Eur J Radiol. 2003;45:60–68. doi: 10.1016/s0720-048x(02)00286-3. https://doi.org/10.1016/S0720-048X(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 9.Freund MC, Petersen J, Goder KC, Bunse T, Wiedermann F, Glodny B. Systemic air embolism during percutaneous core needle biopsy of the lung: frequency and risk factors. BMC Pulm Med. 2012;12:2. doi: 10.1186/1471-2466-12-2. https://doi.org/10.1186/1471-2466-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore EH. Technical aspects of needle aspiration lung biopsy: a personal perspective. Radiology. 1998;208:303–318. doi: 10.1148/radiology.208.2.9680552. https://doi.org/10.1148/radiology.208.2.9680552. [DOI] [PubMed] [Google Scholar]
- 11.Ko JP, Shepard JO, Drucker EA, et al. Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology. 2001;218:491–496. doi: 10.1148/radiology.218.2.r01fe33491. https://doi.org/10.1148/radiology.218.2.r01fe33491. [DOI] [PubMed] [Google Scholar]
- 12.Yeow K-M, Su I-H, Pan K-T, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126:748–754. doi: 10.1378/chest.126.3.748. https://doi.org/10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 13.Loh SE, Wu DD, Venkatesh SK, et al. CT-guided thoracic biopsy: evaluating diagnostic yield and complications. Ann Acad Med Singap. 2013;42:285–290. [PubMed] [Google Scholar]
- 14.Chakrabarti B, Earis JE, Pandey R, et al. Risk assessment of pneumothorax and pulmonary haemorrhage complicating percutaneous co-axial cutting needle lung biopsy. Respir Med. 2009;103:449–455. doi: 10.1016/j.rmed.2008.09.010. https://doi.org/10.1016/j.rmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Tai R, Dunne RM, Trotman-Dickenson B, et al. Frequency and severity of pulmonary hemorrhage in patients undergoing percutaneous CT-guided transthoracic lung biopsy: single-institution experience of 1175 cases. Radiology. 2016;279:287–296. doi: 10.1148/radiol.2015150381. https://doi.org/10.1148/radiol.2015150381. [DOI] [PubMed] [Google Scholar]
- 16.Song YS, Park CM, Park KW, et al. Does antiplatelet therapy increase the risk of hemoptysis during percutaneous transthoracic needle biopsy of a pulmonary lesion? AJR Am J Roentgenol. 2013;200:1014–1019. doi: 10.2214/AJR.12.8931. https://doi.org/10.2214/AJR.12.8931. [DOI] [PubMed] [Google Scholar]
- 17.Nour-Eldin N-EA, Alsubhi M, Naguib NN, et al. Risk factor analysis of pulmonary hemorrhage complicating CT-guided lung biopsy in coaxial and non-coaxial core biopsy techniques in 650 patients. Eur J Radiol. 2014;83:1945–1952. doi: 10.1016/j.ejrad.2014.06.023. https://doi.org/10.1016/j.ejrad.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Yildirim E, Kirbas I, Harman A, et al. CT-guided cutting needle lung biopsy using modified coaxial technique: factors effecting risk of complications. Eur J Radiol. 2009;70:57–60. doi: 10.1016/j.ejrad.2008.01.006. https://doi.org/10.1016/j.ejrad.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Yeow KM, See LC, Lui KW, et al. Risk factors for pneumothorax and bleeding after CT-guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol. 2001;12:1305–1312. doi: 10.1016/s1051-0443(07)61556-5. https://doi.org/10.1016/S1051-0443(07)61556-5. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Du Y, Yang HF, Yu JH, Xu XX. CT-guided percutaneous core needle biopsy for small (≤20 mm) pulmonary lesions. Clin Radiol. 2013;68:e43–e48. doi: 10.1016/j.crad.2012.09.008. https://doi.org/10.1016/j.crad.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155:137–144. doi: 10.1059/0003-4819-155-3-201108020-00003. https://doi.org/10.7326/0003-4819-155-11-201112060-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu C-H, Hsiao C-H, Chang Y-C, et al. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol. 2012;7:143–150. doi: 10.1097/JTO.0b013e318233d7dd. https://doi.org/10.1097/JTO.0b013e318233d7dd. [DOI] [PubMed] [Google Scholar]
- 23.Guimaraes M, Marchiori E, Hochhegger B, Chojniak R, Gross J. CT-guided biopsy of lung lesions: defining the best needle option for a specific diagnosis. Clinics. 2014;69:335–340. doi: 10.6061/clinics/2014(05)07. https://doi.org/10.6061/clinics/2014(05)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aktas AR, Gozlek E, Yazkan R, et al. Transthoracic biopsy of lung masses: Non technical factors affecting complication occurrence. Thorac Cancer. 2015;6:151–158. doi: 10.1111/1759-7714.12156. https://doi.org/10.1111/1759-7714.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurent F, Latrabe V, Vergier B, Michel P. Percutaneous CT-guided biopsy of the lung: comparison between aspiration and automated cutting needles using a coaxial technique. Cardiovasc Intervent Radiol. 2000;23:266–272. doi: 10.1007/s002700010067. https://doi.org/10.1007/s002700010067. [DOI] [PubMed] [Google Scholar]
- 26.Laurent F, Latrabe V, Vergier B, Montaudon M, Vernejoux J-M, Dubrez J. CT-guided transthoracic needle biopsy of pulmonary nodules smaller than 20 mm: results with an automated 20-gauge coaxial cutting needle. Clin Radiol. 2000;55:281–287. doi: 10.1053/crad.1999.0368. https://doi.org/10.1053/crad.1999.0368. [DOI] [PubMed] [Google Scholar]
- 27.Kiessling F, Boese J, Corvinus C, et al. Perfusion CT in patients with advanced bronchial carcinomas: a novel chance for characterization and treatment monitoring? Eur Radiol. 2004;14:1226–1233. doi: 10.1007/s00330-004-2288-2. https://doi.org/10.1007/s00330-004-2288-2. [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Krishnamurthy S, Broemeling LD, et al. Small (</=2-cm) subpleural pulmonary lesions: short-versus long-needle-path CT-guided Biopsy--comparison of diagnostic yields and complications. Radiology. 2005;234:631–637. doi: 10.1148/radiol.2342031423. https://doi.org/10.1148/radiol.2342031423. [DOI] [PubMed] [Google Scholar]
- 29.Heyer CM, Reichelt S, Peters SA, Walther JW, Müller K-M, Nicolas V. Computed tomography–navigated transthoracic core biopsy of pulmonary lesions. Acad Radiol. 2008;15:1017–1026. doi: 10.1016/j.acra.2008.02.018. https://doi.org/10.1016/j.acra.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Manhire A, Charig M, Clelland C, et al. Guidelines for radiologically guided lung biopsy. Thorax. 2003;58:920–936. doi: 10.1136/thorax.58.11.920. https://doi.org/10.1136/thorax.58.11.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson J, Murchison J, Patel D. CT-guided lung biopsy: factors influencing diagnostic yield and complication rate. Clin Radiol. 2003;58:791–797. doi: 10.1016/s0009-9260(03)00221-6. https://doi.org/10.1016/S0009-9260(03)00221-6. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka J, Sonomura T, Shioyama Y, et al. “Oblique path”--the optimal needle path for computed tomography-guided biopsy of small subpleural lesions”. Cardiovasc Intervent Radiol. 1996;19:332–334. https://doi.org/10.1007/BF02570185. [PubMed] [Google Scholar]
- 33.Gupta S, Wallace MJ, Cardella JF, Kundu S, Miller DL, Rose SC. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol. 2010;21:969–975. doi: 10.1016/j.jvir.2010.01.011. https://doi.org/10.1016/j.jvir.2010.01.011. [DOI] [PubMed] [Google Scholar]


