Abstract
PURPOSE
We aimed to evaluate limb salvage, defined as freedom from major amputation, and to identify predictors of major amputation in patients with infrapopliteal peripheral arterial disease (PAD) based on the updated 2015 TASC II anatomic classification treated by percutaneous transluminal angioplasty (PTA).
METHODS
This was a retrospective study of infrapopliteal PTA procedures performed for PAD over a 4-year period. Patient demographics, medical comorbidities, risk factors, angiographic imaging, technical details, and clinical follow-up were analyzed to determine limb salvage rates, technical success, and all-cause mortality. Predictors of major amputation following PTA were identified.
RESULTS
A total of 112 patients were treated by infrapopliteal PTA. Most lesions consisted of TASC C (44%) and D (34%) categories, were over 10 cm in length, and were occlusive and heavily calcified (89%). Overall technical success was 75%, with limb salvage rates of 77% at 1 year and 65% at 3 years following PTA. Smoking, previous stroke or cardiovascular events, and anticoagulation use were associated with an increased risk of major amputation following PTA.
CONCLUSION
PTA of complex infrapopliteal PAD is associated with good intermediate term limb salvage rates.
Infrapopliteal percutaneous transluminal angioplasty (PTA) is an established treatment for lower limb salvage in critical limb ischemia (CLI) and is highly attractive given its minimally invasive nature and comparable outcomes to tibial bypass surgery (1–5). Previous studies have reported outcomes of infrapopliteal PTA based on stratification of lesions using the Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) classification (4, 5). However, this was updated in 2015 to include a new classification for infrapopliteal lesions reflecting recent technical advancements (Table 1) (6). This new classification would have changed the stratification of lesions in previous studies towards lower categories; for example, a 3 cm occlusion previously classed as TASC D would now be considered a category B lesion and therefore outcomes pertaining to endovascular treatment of the contemporary and comparatively, more complex, TASC C and D lesions are not well established.
Table 1.
Updated 2015 TASC II classification for infrapopliteal disease (6)
| Classification | Description |
|---|---|
| TASC A | Single stenosis ≤5 cm length Similar stenosis or occlusion in other tibial vessels |
| TASC B | Multiple stenoses each ≤5cm or total ≤10 cm length or single occlusion <3 cm Similar stenosis or occlusion in other tibial vessels |
| TASC C | Multiple stenoses and/or single occlusion >10 cm length Similar stenosis or occlusion in other tibial vessels |
| TASC D | Multiple occlusions >10 cm length or dense calcification or poor collaterals Other tibial arteries occluded or dense calcification |
TASC, Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease.
The aims of this study are to evaluate limb salvage outcome following PTA based on the updated TASC II classification and to identify potential factors predicting limb salvage in a cohort of patients with complex infrapopliteal atherosclerotic disease.
Methods
A retrospective study was performed and included all infrapopliteal PTA procedures performed in a tertiary university teaching hospital over the study period (July 2010 to June 2014). As this was a retrospective review of current medical practice in our institution and as it did not influence individual patient management, it was categorized as a service evaluation and was exempted from Institutional Review Board approval. All patients gave written informed consent to angiography and angioplasty.
Study population
Patients were eligible for inclusion if clinical, imaging, and procedural details were available for review. Demographic information, clinical details and follow-up data were obtained from a combined review of electronic medical records and charts. Imaging and procedural details were retrieved from the radiology information and picture archiving and communication systems and reviewed in consensus on dedicated workstations.
Imaging studies and procedural details
All patients were assessed clinically and investigated with a combination of imaging (duplex ultrasound, computed tomography or magnetic resonance angiography) and physiologic measurements (ankle-brachial index and toe pressure) prior to treatment. Endovascular treatment was performed in a dedicated angiographic suite under conscious sedation, local anesthesia, and continuous monitoring. Following antegrade femoral arterial access, a 6 French introducer sheath (Avanti+, Cordis) was placed and diagnostic lower limb digital subtraction angiography was performed to plan treatment. Lesions were classified based on the TASC II anatomic classification system for infrapopliteal arterial disease (6).
In patients with multilevel tandem inflow lesions (iliac, femoral, or popliteal), the inflow segments were treated first with PTA with or without stenting. Infrapopliteal PTA was subsequently performed using a 0.014-inch platform to recanalize one or more vessels using a luminal or subintimal approach. The use of specific guidewires, support catheters, and angioplasty balloons were chosen at the operator’s discretion. PTA was performed following administration of 5000 IU of unfractionated heparin using low-profile noncompliant angioplasty balloons and mechanical inflation devices. Balloon sizes were selected to match the nondiseased luminal diameter of the treated vessel and were inflated to nominal pressure for 3 minutes. Drug-coated balloons, stents, or atherectomy devices were not routinely used in our cohort. Revascularization was targeted at the tibial artery supplying the area of tissue loss using the angiosome model. Technical success was defined as successful restoration of inline flow to the foot supplying the area of tissue loss. Partial success was defined as successful recanalization of the tibial vessel but with limited flow into the pedal arch or where target angiosome revascularization was not achieved.
Follow-up and outcomes
Patients were followed-up in a dedicated vascular outpatient clinic at regular intervals following endovascular intervention and included a clinical assessment of wound healing and peripheral pulses as well as repeat duplex imaging, ankle-brachial index or toe pressure measurements. All procedural and periprocedural complications occurring within 30-days were recorded. Any further treatment including surgical amputation procedures during the follow-up period were also recorded. The primary outcome was limb salvage, defined as freedom from major lower extremity amputation (below or above-knee amputation). Secondary outcomes included technical success of recanalization and all-cause mortality.
Statistical analysis
Statistical analyses were performed with SPSS version 23 (IBM Corporation). A standard linear model was designed using a forward-stepwise model selection method and information criterion (AICC) as the entry criteria. Using a combination of the standard linear model, binomial and multinomial logistic regression, potential predictors of the primary outcome were identified and a prediction model was formed and compared with the observed outcome for validation. A Cox proportional hazards regression model was also used to identify predictors and determine the associated hazard ratio (HR). Survival analysis was performed using the Kaplan-Meier method and survival curves were plotted for both limb salvage and all-cause mortality. A P value of <0.05 was taken to represent statistical significance. Continuous data were expressed as mean ± standard deviation unless specified.
Results
A total of 112 patients underwent infrapopliteal PTA and consisted of 83 male and 28 female patients with a mean age of 74 years (range, 46–95 years). The most common clinical indication was CLI with tissue loss (80%), followed by rest pain (13%) and severe claudication (7%).
Cardiovascular risk factors included hypertension (72%), diabetes mellitus (59%), previous stroke and cardiovascular disease (50%), hyperlipidemia (41%), smoking (34%), and chronic or end-stage renal disease (31%). Most patients were taking antiplatelet therapy with aspirin (68%) or clopidogrel (25%) and over one-half were taking statin therapy (53%). Approximately one-third (29%) received anticoagulation therapy for cardiovascular indications, most commonly atrial fibrillation.
Lesions were characterized based on pretreatment digital subtraction angiography using the updated 2015 TASC II classification for infrapopliteal lesions. As shown in Table 2, most lesions encountered in the cohort consisted of complex TASC C (44%) and D (34%) lesions over 10 cm in length with chronic total occlusion or heavy calcification (89%).
Table 2.
Summary of lesion characteristics and distribution of vessels treated by infrapopliteal angioplasty
| Lesion characteristics | n (%) |
|---|---|
| TASC II classification | |
| A | 2 (2) |
| B | 19 (18) |
| C | 49 (45) |
| D | 38 (35) |
|
| |
| Lesion length | |
| <3 cm | 2 (2) |
| 3–10 cm | 21 (21) |
| >10 cm | 79 (78) |
|
| |
| Lesion type | |
| Occlusion | 75 (89) |
| Stenosis | 9 (11) |
|
| |
| Vessels treated | |
| Anterior tibial artery | 73 |
| Posterior tibial artery | 31 |
| Peroneal artery | 38 |
| Tibioperoneal trunk | 16 |
TASC, TransAtlantic InterSociety Consensus for the Management of Peripheral Arterial Disease.
Approximately one-half had tandem inflow disease (48%) which was treated at the same or separate session by endovascular or surgical means. Most patients underwent revascularization of one crural vessel (62%), followed by two vessels (33%). Recanalization of three crural vessels was attempted in a small minority (5%). Technical success was achieved in 75%, with partial success in 6% and failure in 19%. When stratified by TASC category, technical success was achieved in 81% for A–C lesions and was 63% for D lesions. Seventeen patients required repeat intervention over the follow-up period (15% secondary reintervention rate).
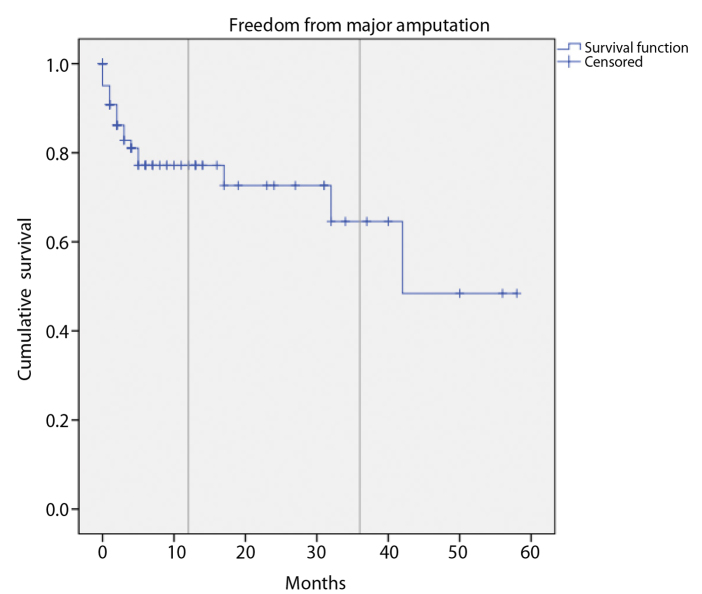
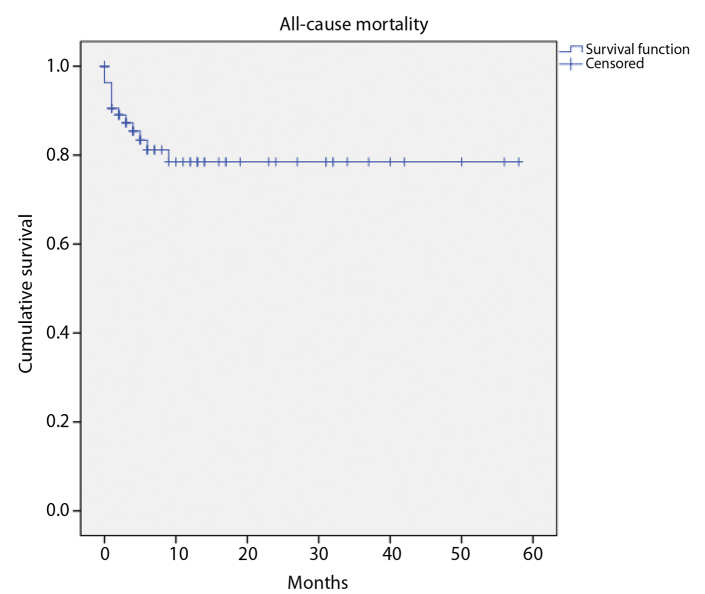
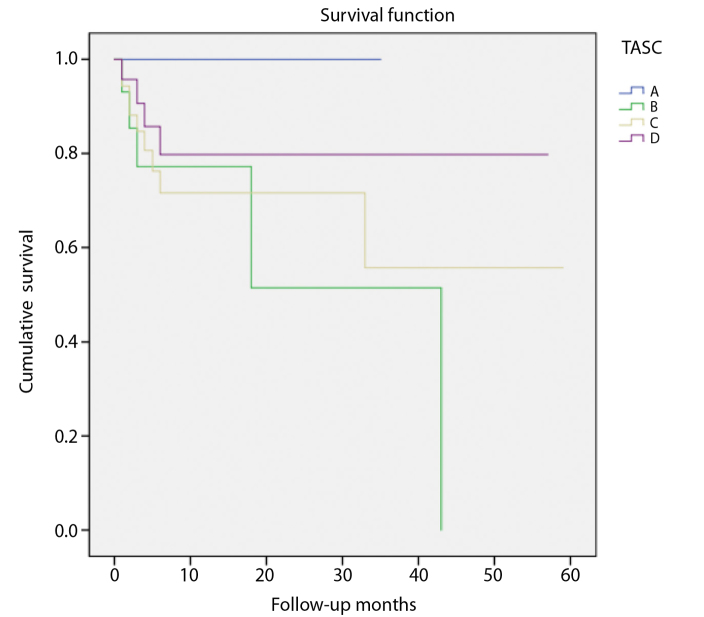
Follow-up was available in 93 patients (83%) over a mean duration of 11±14 months (range, 1–58 months). Eighteen patients (19%) underwent major amputation. The limb salvage rate was 77% at 1 year and 65% at 3 years following infrapopliteal PTA (Fig. 1). Sixteen mortalities occurred over the follow-up period with a 1-year cumulative survival of 79% (Fig. 2). The most common causes of death were from sepsis and vascular events (stroke and mesenteric ischemia). There was no significant difference in limb salvage when analyzed by TASC category (Fig. 3). However, there was a trend towards lower amputation risk at 3 years in TASC C (80%) compared with D (56%) lesions.
Figure 1.
Cumulative freedom from major amputation following infrapopliteal angioplasty over the study period.
Figure 2.
Cumulative survival from all-cause mortality following infrapopliteal angioplasty over the study period.
Figure 3.
Cumulative freedom from major amputation for each TASC category following infrapopliteal angioplasty shows no significant difference between groups. However, there was a trend for lower risk of major amputation in TASC A and C categories.
Significant predictors of major amputation were identified: smoking (HR, 1.3; 95% CI, 1.3–9.9), previous stroke or cardiovascular disease (HR, 1.1; 95% CI, 1.0–8.3) and anticoagulation use (HR, 1.1; 95% CI, 1.2–7.9). Other factors, including anatomic lesion characteristics and TASC classification were not found to be significantly predictive of major amputation.
Discussion
This is a study evaluating the clinical outcomes in patients with advanced infrapopliteal PAD as classified anatomically using the updated 2015 TASC II classification. The study population consisted predominantly of patients with complex TASC C and D lesions and multiple medical comorbidities, reflective of many patients with CLI. The majority of patients treated in our series had long (>10 cm) stenotic (TASC C) or calcified chronic total occlusive (TASC D) lesions which are very different from previously described TASC D lesions based on the older classification system. Multilevel arterial inflow disease coexisted in almost half of the study population. Despite the complexity of these lesions, endovascular treatment with PTA was technically successful in the majority of patients.
Technical success was lower in patients with TASC D lesions at 63%, compared with 75% as reported previously by Giles et al. (4) in 2008 when TASC D lesions were stratified based on the older classification. The updated TASC D classification (Table 1) is more conservative compared with the older definition of occlusions >2 cm in length and accounts for the observed differences as these lesions are now predominantly long (>10 cm) calcified chronic total occlusions.
Technical success for the remaining A–C lesions was higher at 81% but is still lower than success rates previously reported (4, 7). This is also consistent with the more conservative shift of categorizing longer stenotic lesions >10 cm as TASC C using the updated classification, which would have previously been regarded as TASC D. Therefore, the updated TASC categories represent a very different cohort of patients to those previously reported in the literature and the differences are particularly marked in the more advanced C and D categories. To our knowledge, our series represents the first reported technical success rates and clinical outcomes based on the updated TASC classification.
Despite the overall complexity of treated lesions in our series, the overall limb salvage at 1 and 3 years following conventional PTA is comparable to other published results and to outcomes from surgical bypass with a relatively low secondary reintervention rate of 15% (2–5, 7–15). Although newer technologies such as drug-coated angioplasty balloons (DCB) or drug-eluting stents (DES), and atherectomy devices were absent in our series, there has been recent interest in the use of DCB and DES in the treatment of infrapopliteal lesions (16–20). However, a meta-analysis has not shown any difference in amputation or mortality when compared with PTA or bare-metal stenting (21). Lesions treated with DCB or DES in published trials to date have tended to focus on shorter, less complex lesions and therefore, results are not directly comparable with those of many infrapopliteal PTA limb salvage trials where longer complex lesions predominate. Results from the IN.PACT DEEP trial have shown a trend towards more major amputations with DCB angioplasty when compared to PTA which led to a recall of the In. Pact Amphirion balloon by Medtronic in late 2013 (22). Robust evidence for infrapopliteal atherectomy is still lacking. A subgroup analysis from the DEFINITIVE LE study for directional atherectomy of infrapopliteal lesions showed high technical success and 1-year primary patency rates of 84%. However, the study cohort consisted of fewer CLI patients (less than half the cohort) and shorter nonocclusive lesions with absence of longer term data beyond 1 year (23). A Cochrane systematic review comparing atherectomy to PTA for treatment of peripheral vascular disease suggests that current evidence supporting atherectomy is poor and that further well-designed trials are needed (24). Therefore, it is likely that conventional infrapopliteal PTA will remain the mainstay of practice until further studies are available to support the use of these newer devices, particularly for long, occluded, or calcified infrapopliteal lesions.
A wide variety of factors influencing outcome following infrapopliteal PTA with varying impact have been proposed in the literature (3, 5, 10, 14, 15, 25). The accurate outcome prediction from endovascular therapy allows interventionists to tailor appropriate management strategies to individual patients with greater confidence and may minimize morbidity or mortality from under or overtreatment. Smoking, previous stroke or cardiovascular events and anticoagulation use were found to be significant predictors of major amputation in our cohort and emphasizes the need for aggressive cardiovascular risk factor reduction and concomitant optimal medical therapy in parallel to endovascular treatment in such patients.
Our study has a number of limitations. This was a retrospective and nonrandomized study and is therefore potentially subject to selection bias. A number of patients were referred from external institutions for treatment and returned to their referring hospital for local follow-up. Despite efforts to obtain as much follow-up data as possible, this was not possible in a small minority (17%) and final outcomes may be underestimated. As our patient cohort consisted of mostly complex TASC C and D lesions, the outcomes for TASC A and B lesions are unclear and the predictors for major amputation may differ.
In conclusion, the updated 2015 TASC II classification system for infrapopliteal disease represents a major paradigm shift in the classification of patients with infrapopliteal atherosclerotic disease with more accurate stratification of patients with complex disease into the later categories. Despite this, PTA of complex TASC C and D infrapopliteal lesions is associated with high technical success and good limb salvage rates in the intermediate term.
Main points.
The updated 2015 TASC II classification system for infrapopliteal disease represents a paradigm shift in the classification of infrapopliteal atherosclerotic lesions compared with the older TASC system.
The new TASC categories, particularly C and D lesions comparatively represent much more complex disease and includes long (>10 cm) stenotic or occlusive lesions with or without heavy calcification.
Despite this change, PTA of complex TASC C and D infrapopliteal lesions is associated with high technical success and good limb salvage rates in the intermediate term.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Rand T, Uberoi R. Current status of interventional radiology treatment of infrapopliteal arterial disease. Cardiovasc Intervent Radiol. 2013;36:588–598. doi: 10.1007/s00270-012-0524-8. https://doi.org/10.1007/s00270-012-0524-8. [DOI] [PubMed] [Google Scholar]
- 2.Soderstrom MI, Arvela EM, Korhonen M, et al. Infrapopliteal percutaneous transluminal angioplasty versus bypass surgery as first-line strategies in critical leg ischemia: a propensity score analysis. Ann Surg. 2010;252:765–773. doi: 10.1097/SLA.0b013e3181fc3c73. https://doi.org/10.1097/SLA.0b013e3181fc3c73. [DOI] [PubMed] [Google Scholar]
- 3.Conrad MF, Kang J, Cambria RP, et al. Infrapopliteal balloon angioplasty for the treatment of chronic occlusive disease. J Vasc Surg. 2009;50:799–805. doi: 10.1016/j.jvs.2009.05.026. https://doi.org/10.1016/j.jvs.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Giles KA, Pomposelli FB, Spence TL, et al. Infrapopliteal angioplasty for critical limb ischemia: relation of TransAtlantic InterSociety Consensus class to outcome in 176 limbs. J Vasc Surg. 2008;48:128–136. doi: 10.1016/j.jvs.2008.02.027. https://doi.org/10.1016/j.jvs.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Lo RC, Darling J, Bensley RP, et al. Outcomes following infrapopliteal angioplasty for critical limb ischemia. J Vasc Surg. 2013;57:1455–1463. doi: 10.1016/j.jvs.2012.10.109. https://doi.org/10.1016/j.jvs.2012.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Committee TS, Jaff MR, White CJ, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the inter-society consensus for the management of peripheral arterial disease (TASC II) J Endovasc Ther. 2015;22:663–677. doi: 10.1177/1526602815592206. https://doi.org/10.1177/1526602815592206. [DOI] [PubMed] [Google Scholar]
- 7.Tewksbury R, Pearch B, Redmond K, et al. Outcomes of infrapopliteal endoluminal intervention for transatlantic intersociety consensus C and D lesions in patients with critical limb ischaemia. ANZ J Surg. 2014;84:866–870. doi: 10.1111/ans.12460. https://doi.org/10.1111/ans.12460. [DOI] [PubMed] [Google Scholar]
- 8.Ferraresi R, Centola M, Ferlini M, et al. Longterm outcomes after angioplasty of isolated, below-the-knee arteries in diabetic patients with critical limb ischaemia. Eur J Vasc Endovasc Surg. 2009;37:336–342. doi: 10.1016/j.ejvs.2008.12.001. https://doi.org/10.1016/j.ejvs.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Haider SN, Kavanagh EG, Forlee M, et al. Twoyear outcome with preferential use of infrainguinal angioplasty for critical ischemia. J Vasc Surg. 2006;43:504–512. doi: 10.1016/j.jvs.2005.11.016. https://doi.org/10.1016/j.jvs.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Iida O, Soga Y, Yamauchi Y, et al. Clinical efficacy of endovascular therapy for patients with critical limb ischemia attributable to pure isolated infrapopliteal lesions. J Vasc Surg. 2013;57:974–981. doi: 10.1016/j.jvs.2012.10.096. https://doi.org/10.1016/j.jvs.2012.10.096. [DOI] [PubMed] [Google Scholar]
- 11.Nakama T, Watanabe N, Kimura T, et al. Clinical implications of additional pedal artery angioplasty in critical limb ischemia patients with infrapopliteal and pedal artery disease. J Endovasc Ther. 2016;23:83–91. doi: 10.1177/1526602815610119. https://doi.org/10.1177/1526602815610119. [DOI] [PubMed] [Google Scholar]
- 12.Nakano M, Hirano K, Iida O, et al. Prognosis of critical limb ischemia in hemodialysis patients after isolated infrapopliteal balloon angioplasty: results from the Japan below-the-knee artery treatment (J-BEAT) registry. J Endovasc Ther. 2013;20:113–124. doi: 10.1583/11-3782.1. https://doi.org/10.1583/11-3782.1. [DOI] [PubMed] [Google Scholar]
- 13.Park SW, Kim JS, Yun IJ, et al. Clinical outcomes of endovascular treatments for critical limb ischemia with chronic total occlusive lesions limited to below-the-knee arteries. Acta Radiol. 2013;54:785–789. doi: 10.1258/ar.2012.120217. https://doi.org/10.1177/0284185113479878. [DOI] [PubMed] [Google Scholar]
- 14.Sigala F, Kontis E, Hepp W, et al. Long-term outcomes following 282 consecutive cases of infrapopliteal PTA and association of risk factors with primary patency and limb salvage. Vasc Endovascular Surg. 2012;46:123–130. doi: 10.1177/1538574411432161. https://doi.org/10.1177/1538574411432161. [DOI] [PubMed] [Google Scholar]
- 15.Strom M, Konge L, Lonn L, Schroeder TV, Rordam P. Amputation-free survival after crural percutaneous transluminal angioplasty for critical limb ischemia. Scand J Surg. 2016;105:42–48. doi: 10.1177/1457496915571403. https://doi.org/10.1177/1457496915571403. [DOI] [PubMed] [Google Scholar]
- 16.Fanelli F, Cannavale A, Boatta E, et al. Lower limb multilevel treatment with drug-eluting balloons: 6-month results from the DEBELLUM randomized trial. J Endovasc Ther. 2012;19:571–580. doi: 10.1583/JEVT-12-3926MR.1. https://doi.org/10.1583/JEVT-12-3926MR.1. [DOI] [PubMed] [Google Scholar]
- 17.Liistro F, Porto I, Angioli P, et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation. 2013;128:615–621. doi: 10.1161/CIRCULATIONAHA.113.001811. https://doi.org/10.1161/CIRCULATIONAHA.113.001811. [DOI] [PubMed] [Google Scholar]
- 18.Spiliopoulos S, Theodosiadou V, Katsanos K, et al. Long-term clinical outcomes of infrapopliteal drug-eluting stent placement for critical limb ischemia in diabetic patients. J Vasc Interv Radiol. 2015;26:1423–1430. doi: 10.1016/j.jvir.2015.06.034. https://doi.org/10.1016/j.jvir.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Zeller T, Beschorner U, Pilger E, et al. Paclitaxel-coated balloon in infrapopliteal arteries: 12-month results from the BIOLUX P-II randomized trial (BIOTRONIK’S-first in man study of the Passeo-18 LUX drug releasing PTA balloon catheter vs. the uncoated Passeo-18 PTA balloon catheter in subjects requiring revascularization of infrapopliteal arteries) JACC Cardiovasc Interv. 2015;8:1614–1622. doi: 10.1016/j.jcin.2015.07.011. https://doi.org/10.1016/j.jcin.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Rastan A, Tepe G, Krankenberg H, et al. Sirolimus-eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J. 2011;32:2274–2281. doi: 10.1093/eurheartj/ehr144. https://doi.org/10.1093/eurheartj/ehr144. [DOI] [PubMed] [Google Scholar]
- 21.Baerlocher MO, Kennedy SA, Rajebi MR, et al. Meta-analysis of drug-eluting balloon angioplasty and drug-eluting stent placement for infrainguinal peripheral arterial disease. J Vasc Interv Radiol. 2015;26:459–473. doi: 10.1016/j.jvir.2014.12.013. https://doi.org/10.1016/j.jvir.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Zeller T, Baumgartner I, Scheinert D, et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. 2014;64:1568–1576. doi: 10.1016/j.jacc.2014.06.1198. https://doi.org/10.1016/j.jacc.2014.06.1198. [DOI] [PubMed] [Google Scholar]
- 23.Rastan A, McKinsey JF, Garcia LA, et al. One-year outcomes following directional atherectomy of infrapopliteal artery lesions: subgroup results of the prospective, multicenter DEFINITIVE LE trial. J Endovasc Ther. 2015;22:839–846. doi: 10.1177/1526602815608610. https://doi.org/10.1177/1526602815608610. [DOI] [PubMed] [Google Scholar]
- 24.Ambler GK, Radwan R, Hayes PD, Twine CP. Atherectomy for peripheral arterial disease. Cochrane Database Syst Rev. 2014;(3):CD006680. doi: 10.1002/14651858.CD006680.pub2. https://doi.org/10.1002/14651858.CD006680.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Keeling AN, Khalidi K, Leong S, et al. Below knee angioplasty in elderly patients: predictors of major adverse clinical outcomes. Eur J Radiol. 2011;77:483–489. doi: 10.1016/j.ejrad.2009.08.011. https://doi.org/10.1016/j.ejrad.2009.08.011. [DOI] [PubMed] [Google Scholar]