Abstract
PURPOSE
Wilms tumor (WT) and neuroblastoma (NB) are the most common pediatric abdominal malignant neoplasms of the kidney and adrenal gland. Differentiating them from each other is essential since their treatments are different. Here, we aimed to show the diffusion characteristics of WT and NB for differentiation.
METHODS
Diffusion-weighted imaging (DWI) of 17 histopathologically diagnosed lesions (10 NB and 7 WT in 8 female and 9 male patients) was evaluated retrospectively. The apparent diffusion coefficient (ADC) value for each tumor was calculated using region-of-interest (ROI) measurements by two observers. The mean ADC values were compared, and receiver operating characteristics (ROC) curve analysis was performed. Intraclass correlation was evaluated for the reliability of ADC measurement.
RESULTS
The mean ADC values measured by two observers were 0.787±0.09 ×10−3 mm2/s and 0.768±0.08 ×10−3 mm2/s for WT, and 0.524±0.16 ×10−3 mm2/s and 0.529±0.16 ×10−3 mm2/s for NB, respectively (P = 0.006 and P = 0.011). Intraclass correlation coefficient was 0.955. Utilizing ROC curve analysis, a cutoff ADC value of ≤0.645 ×10−3 mm2/s was obtained to differentiate NB from WT.
CONCLUSION
ADC values of NBs were significantly lower than WT with a perfect interobserver agreement. We suggest that DWI may have a role in differentiating the two tumors.
Wilms tumor (WT) and neuroblastoma (NB) are the most common pediatric abdominal malignant neoplasms of adjacent organs (1). Diagnostic imaging and laboratory findings are helpful for a precise diagnosis, which is necessary for management of these tumors, but on occasion making a correct diagnosis can be challenging (1–5). When a large-sized NB invades the kidney, it can be misdiagnosed as an exophytic WT (2). In addition, NB can display radiologic findings that are typical for WT, such as thrombosis in the renal vein, inferior vena cava, and/or right atrium, or rarely, lung metastases (3).
Diffusion-weighted imaging (DWI) is based on random movements of water molecules (Brownian motion) and has gained popularity in the determination of ischemia, infection, and fibrosis in radiology practice. Also, DWI can be used for the detection and characterization of abdominal tumors as well as monitoring the therapy response (6, 7). In the medical literature, several studies have assessed the utility of diffusion properties for differentiation of benign and malignant pediatric abdominal masses (8–12). However, data about the DWI characteristics of WT and NB are very rare (1, 3, 8–11). This study mainly focused on DWI properties of WT and NB and the ability to distinguish between them before a treatment decision.
Methods
The institutional review board approved the retrospective design of the study, and the need for informed consent was waived. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Patients
The hospital information system was searched for patients diagnosed as WT or NB by histopathologic evaluation between July 2011 and December 2015. Twenty patients who met this criterion were identified. The radiology database was searched for MRI examinations of identified patients before diagnostic tru-cut biopsy or surgery, and the MRIs of all twenty patients were found. MRIs were further evaluated for diagnostic quality and presence of DWI. Two patients’ MRI examination that did not include DWI and the third patient’s MRI examination that had a nondiagnostic DWI due to motion artifact were excluded from the study. Finally, seventeen patients with MRIs performed prior to any intervention were included in the survey. Patient’ age (months), gender, and interventions (biopsy or surgery) were noted.
Magnetic resonance imaging
MRI examinations were performed on a 1.5 Tesla MRI scanner (Avanto, Siemens Medical Solutions). Patients fasted for at least 4–6 hours before the MRI. MRIs were performed with the children sedated or under general anesthesia, in the supine position with the arms raised. Axial T1-weighted imaging single-shot fast spin-echo (SSFSE), respiratory triggered T2-weighted imaging SSFSE, fat-suppressed T1- and T2-weighted imaging, in- and out-of-phase T1-weighted imaging and dynamic contrast-enhanced axial 3D T1-weighted gradient echo fat-suppressed (VIBE) images were obtained in axial, coronal, and sagittal planes. The dynamic contrast-enhanced (DCE-MRI) sequence was performed after contrast administration (Gadoterate meglumine, Dotarem, Guerbet) of 0.2 mL/kg into the antecubital vein at a rate of 1 mL/s followed by a 6 mL saline flush. Respiratory triggered and fat suppressed single-shot echo planar DWI was obtained with a b value of 0, 50, 400, and 800 s/mm2 and tridirectional diffusion gradients before DCE-MRI on the axial plane. The parameters for DWI were TR, 6300 ms; TE, 79 ms; slice thickness, 4 mm; slice gap, 0.9 mm; matrix, 192×192; and FOV, 250 mm. Apparent diffusion coefficient (ADC) maps were automatically generated from DWI obtained at b values of 0, 50, 400, and 800 s/mm2 using standard postprocessing software.
Data collection and image analysis
The final diagnosis of WT or NB was made by histopathologic analysis. Two observers experienced in pediatric abdominal radiology and blinded to patient diagnosis evaluated the MRI in consensus. The T1- and T2-weighted imaging was used to delineate regions of necrosis and hemorrhage within the primary tumor if present. The signal intensity of WT and NB related to normal appearing renal parenchyma were noted as hypo, iso, or hyperintense. The contrast-enhanced fat-suppressed T1- weighted axial images were used for the measurement of tumor size since tumor borders were clearly outlined on this sequence. Maximum size, measured in any plane, was accepted as the tumor size. Both WT and NB were evaluated for vascular encasement (abdominal aorta and/or its branches), aortic displacement, and midline crossing. In addition, the presence of claw sign in WT and patient’s diagnosis regarding conventional MRI findings were noted.
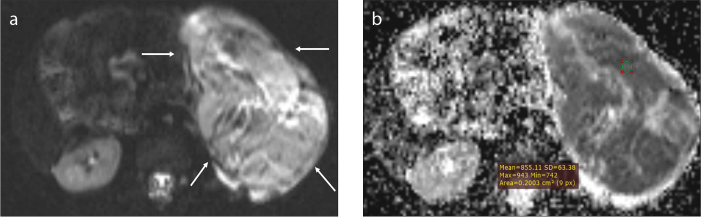
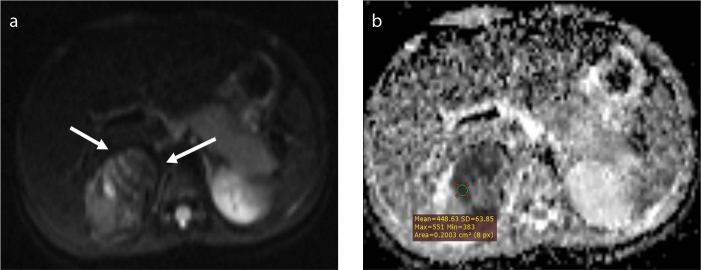
The circle region-of-interest (ROI) of 0.2 cm2 was placed on ADC maps that displayed maximum contrast enhancement on contrast-enhanced T1-weighted imaging. Two observers measured ADC values independently on a workstation. The mean of at least four ADC measurements from different tumor sites was used for statistical analysis (Figs. 1 and 2). The T2-weighted images were used to avoid measurements from cystic necrotic areas. Also, maximum care was given to prevent measurements from regions of hemorrhagic or necrotic/cystic areas.
Figure 1. a, b.
A 60-month-old girl with a 10.5 cm sized Wilms tumor. The tumor on the left kidney (a, arrows) doses not cross the midline and is hyperintense relative to paraspinal muscles on DWI. The mean ADC value of the selected ROI is 0.855 ×10−3 mm2/s (b).
Figure 2. a, b.
A 24-month-old girl with a 4.5 cm sized neuroblastoma. The tumor (a, arrows) is confined to the right adrenal region and is hyperintense on DWI. The mean ADC value of the selected ROI is 0.448 ×10−3 mm2/s (b).
Statistical analysis
All lesions were classified as WT or NB by histopathologic findings, and ADC values were compared by utilizing the MedCalc Statistical Software version 12.7.7 (MedCalc Software bvba). Mann-Whitney U test was performed to analyze continuous variables due to a low number of observations causing normality test to fail. Pearson chi-square and Fisher-Freeman-Halton tests were used to compare the nonparametric variables. Receiver operating characteristics (ROC) curve analysis was performed to determine optimal cutoff values of numeric data that showed a significant difference. The Youden index was chosen for estimating the optimal cutoff values. The intraclass correlation coefficient (ICC) was evaluated to determine the reliability of ADC measurements by ROI. Spearman correlation analysis performed to display the correlation between histopathologic diagnosis and morphologic diagnosis regarding conventional MRI. Correlation was accepted as poor if <0.4, fair if between 0.4 and 0.59, good if between 0.6 and 0.74, and excellent if between 0.75 and 1.00. A significant difference was determined with bidirectional P < 0.05.
Results
All 17 patients underwent surgery and were diagnosed as WT or NB histopathologically. There were 8 females and 9 males: 3 females and 4 males had WT; 5 females and 5 males had NB. The mean age was 46.36±21.55 months (range, 4.5–72 months) for patients with WT and 21.40±16.64 months (range, 2–50 months) for patients with NB (P = 0.025). The mean lesion size was 11.9±4.94 cm (range, 4.5–20 cm) for WT and 7.53±3.49 cm (range, 3.8–12 cm) for NB (P = 0.070). Signal intensity on T1- and T2-weighted imaging, presence of necrosis, hemorrhage, and aortic displacement between WT and NB were not significantly different, while vascular encasement and midline crossing were nearly significant for neuroblastoma (P = 0.050 for both parameters; Table 1). The claw sign was only present in 5 patients with WT (71.4%). Observers diagnosed all NB and 6 WT correctly based on MRI features (Spearman’s rho rs=0.883, P ≤ 0.001).
Table 1.
MRI findings of Wilms tumor and neuroblastoma
| Neuroblastoma | Wilms tumor | P | ||
|---|---|---|---|---|
| T1-WI SIa | Hypointense | 4 (5.3) | 5 (3.7) | 0.503b |
| Isointense | 4 (3.5) | 2 (2.5) | ||
| Hyperintense | 2 (1.2) | 0 (0.8) | ||
|
| ||||
| T2-WI SIa | Hypointense | 2 (1.2) | 0 (0.8) | 0.485c |
| Hyperintense | 8 (8.8) | 7 (6.2) | ||
|
| ||||
| Necrosis | Present | 6 (5.3) | 3 (3.7) | 0.637c |
| Absent | 4 (4.7) | 4 (3.3) | ||
|
| ||||
| Hemorrhage | Present | 1 (1.2) | 1 (0.8) | 1.000c |
| Absent | 9 (8.8) | 6 (6.2) | ||
|
| ||||
| Vascular encasement | Present | 7 (4.7) | 1 (3.3) | 0.050c |
| Absent | 3 (5.3) | 6 (3.7) | ||
|
| ||||
| Aortic displacement | Present | 1 (2.4) | 3 (1.6) | 0.250c |
| Absent | 9 (7.6) | 4 (5.4) | ||
|
| ||||
| Crossing midline | Present | 7 (4.7) | 1 (3.3) | 0.050c |
| Absent | 3 (5.3) | 6 (3.7) | ||
Cell values are observed (expected) values.
T1-WI SI, T1-weighted imaging signal intensity; T2-WI SI, T2-weighted imaging signal intensity.
Signal intensity patterns were relative to kidney parenchyma;
Pearson chi-square;
Fisher’s exact test.
The mean ADC values measured by two observers were 0.787±0.09 ×10−3 mm2/s and 0.768±0.08 ×10−3 mm2/s for WT and 0.524±0.16 ×10−3 mm2/s and 0.529±0.16 ×10−3 mm2/s for NB, respectively (P = 0.006 and P = 0.011) (Table 2). ICC for the reliability of ADC measurements was excellent (ICC=0.955). Using the Youden J index on ROC curve analysis, threshold values of 0.645 ×10−3 mm2/s and 0.646 ×10−3 mm2/s were obtained for ADC values in differentiating NB from WT, respectively. Sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios, and accuracy values are presented in Table 2.
Table 2.
ROC curve analysis of ADC values to discriminate neuroblastoma from Wilms tumor
| Observer 1 | Observer 2 | |
|---|---|---|
| AUC | 0.90 (0.658–0.991) | 0.871 (0.622–0.982) |
| P | <0.001 | <0.001 |
| Cutoff value (x10−3 mm2/s) | ≤0.645 | ≤0.646 |
| Youden index J | 0.8 (0.5–1.0) | 0.8 (0.5–1.0) |
| Sensitivity (%) | 80 (44.4–97.5) | 80 (44.4–97.5) |
| Specificity (%) | 100 (59–100) | 100 (59–100) |
| PPV (%) | 100 (95.4–100) | 100 (95.4–100) |
| NPV (%) | 83.3 (76.6–88.9) | 83.3 (76.6–88.9) |
| (+) likelihood ratio | ∞ | ∞ |
| (−) likelihood ratio | 0.2 (0.06–0.7) | 0.2 (0.06–0.7) |
| Accuracy (%) | 90 | 90 |
Numbers are given with 95% confidence intervals in parentheses.
ROC, receiver operating characteristics; ADC, apparent diffusion coefficient; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value.
Discussion
A precise diagnosis of WT or NB is essential before treatment since management and treatment of these tumors are quite different. Nephrectomy is the first step for WT treatment, while neoadjuvant chemotherapy is the mainstay for patients with highrisk abdominal NB, to preserve the kidney and its functions before surgery (4, 5). WTs are generally spherical intrarenal tumors with a pseudocapsule and contain calcification in 9% of cases, whereas NBs contain calcifications in approximately 90% of patients. On T1-weighted imaging, WT is isointense, while NB is generally hypointense. But, on T2-weighted imaging, both WT and NB are hyperintense relative to the kidney parenchyma. WT and NB may contain cystic components due to intratumoral hemorrhage, necrosis, and the obstruction of the renal pelvis and collecting system. Thus, heterogeneous contrast enhancement can be observed on MRI or CT. WT can invade the inferior vena cava and extend into the right atrium, while NB can encase aorta and its branches. However, these findings are often not helpful for the discrimination of WT and NB particularly when big masses are present. Therefore, discriminating WT from NB can be challenging.
We found that NB has significantly lower ADC value than WT in our study. This difference could be due to higher cellular density and less necrosis in NB relative to WT. There are a few studies concerning the role of DWI for the pediatric abdomen, which are generally for the differentiation of malignant pediatric abdominal masses from benign masses. The results of these studies are conflicting. Humphries et al. (8) found insignificant ADC values, while Kocaoğlu et al. (9) and Gawande et al. (11) found significantly lower ADC values in malignant masses than in benign masses. Platzer et al. (12) compared the DWI findings in pediatric renal masses and obtained insignificant ADC values. Gahr et al. (10) revealed significant differences concerning ADC values in subtypes of NB, namely ganglioneuroma, ganglioneuroblastoma, and NB. The mean ADC value of patients with WT in our study was similar to the studies of Kocaoğlu et al. (9) and Gawande et al. (11), but lower than the value obtained by Humphries et al. (8) which included three patients with WT and measured the ADC values with various sized ROIs (between 1 and 82 cm2). This measurement strategy might be responsible for the high ADC values in their study since they presumably included both solid and cystic parts of the tumors (8). We placed smaller ROIs (0.2 cm2) that included only the most enhancing areas of lesions on DCE-MRI, as did Kocaoğlu et al. (9) and Gawande et al. (11). We also looked for the interobserver agreement to display the repeatability of our ROI measurements since we used small ROIs. We found an ICC of 0.955, indicating excellent agreement.
Our significantly lower ADC values of NB were also lower than the mean ADC values of NBs in previous studies (9–11). In our study, one patient had a differentiated NB while the rest of the tumors were undifferentiated or poorly differentiated, which could explain the difference. The diffusion of poorly differentiated and undifferentiated NB was much more restricted than the differentiating NB due to the excessively disorganized cells and reduced extracellular space, which causes highly restricted diffusion on DWI (13).
In this study, the signal intensity on T1-and T2–weighted imaging, the presence of necrosis and hemorrhagia, and aorta displacement were not statistically significant, but the vascular encasement and the midline crossing were suggestive for NB (4, 5). We morphologically diagnosed all NBs and six cases of WT definitively using MRI features. The one WT that we could not definitively diagnose encased the left renal artery and crossed the midline morphologically. However, it had high ADC value of 0.906 ×10−3 mm2/s compatible with our findings. When evaluating the ADC values, two patients with NB had ADC values higher than 0.645 ×10−3 mm2/s. Among them, one lesion was confined to the adrenal gland, and the other one displayed vascular encasement, midline crossing and had liver and bone metastasis, which were typical for NB.
In this study, the small number of patients could be considered as a limitation. However, both NB and WT have low prevalences, and we eliminated patients with low-quality images and artifacts.
In conclusion, DWI should be a part of pediatric abdominal MRI, which may be helpful in characterizing and differentiating NB from WT, particularly if they have equivocal features.
Main points.
Precise diagnosis is essential since the treatment of Wilms tumor and neuroblastoma are different.
Diffusion-weighted imaging can be helpful for differentiating between challenging cases of neuroblastoma and Wilms tumor.
Diffusion-weighted imaging should be a part of abdominal magnetic resonance imaging.
Footnotes
This research was presented as a poster at the 2016 International Pediatric Radiology Conjoint Meeting & Exhibition with a small number of cases (May 15–20, 2016. Chicago, Illinois, USA).
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Kim S, Chung DH. Pediatric solid malignancies: neuroblastoma and Wilms tumor. Surg Clin North Am. 2006;86:469–487. doi: 10.1016/j.suc.2005.12.008. https://doi.org/10.1016/j.suc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Brisse HJ, McCarville MB, Granata C, et al. Guidelines for imaging and staging of neuroblastic tumors: consensus report from the International Neuroblastoma Risk Group Project. Radiology. 2011;261:243–257. doi: 10.1148/radiol.11101352. [DOI] [PubMed] [Google Scholar]
- 3.Gaetan G, Ouimet A, Lapierre C, Teira P, Sartelet H. Neuroblastoma presenting like a Wilms’ tumor with thrombus in inferior vena cava and pulmonary metastases: a case series. Springerplus. 2014;3:351. doi: 10.1186/2193-1801-3-351. https://doi.org/10.1186/2193-1801-3-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosfeld JL. Neuroblastoma. In: Grosfeld JL, O’Neill JA, Fonkalsrud EW, Coran AG, editors. Pediatric Surgery. Elsevier Science; Philadelphia: 2006. pp. 467–494. https://doi.org/10.1016/B978-0-323-02842-4.50031-0. [Google Scholar]
- 5.Henry MC, Tashjian DB, Breuer CK. Neuroblastoma update. Curr Opin Oncol. 2005;17:19–23. doi: 10.1097/01.cco.0000147901.12325.90. https://doi.org/10.1097/01.cco.0000147901.12325.90. [DOI] [PubMed] [Google Scholar]
- 6.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–1635. doi: 10.2214/AJR.06.1403. https://doi.org/10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 7.Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797–1810. doi: 10.1148/rg.296095521. https://doi.org/10.1148/rg.296095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries PD, Sebire NJ, Siegel MJ, Olsen ØE. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology. 2007;245:848–854. doi: 10.1148/radiol.2452061535. https://doi.org/10.1148/radiol.2452061535. [DOI] [PubMed] [Google Scholar]
- 9.Kocaoglu M, Bulakbasi N, Sanal HT, et al. Pediatric abdominal masses: diagnostic accuracy of diffusion weighted MRI. Magn Reson Imaging. 2010;28:629–636. doi: 10.1016/j.mri.2010.02.010. https://doi.org/10.1016/j.mri.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Gahr N, Darge K, Hahn G, Kreher BW, von Buiren M, Uhl M. Diffusion-weighted MRI for differentiation of Neuroblastoma and ganglioneuroblastoma /ganglioneuroma. Eur J Radiol. 2011;79:443–446. doi: 10.1016/j.ejrad.2010.04.005. https://doi.org/10.1016/j.ejrad.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Gawande RS, Gonzalez G, Messing S, Khurana A, Daldrup-Link HE. Role of diffusion-weighted imaging in differentiating benign and malignant pediatric abdominal tumors. Pediatr Radiol. 2013;43:836–845. doi: 10.1007/s00247-013-2626-0. https://doi.org/10.1007/s00247-013-2626-0. [DOI] [PubMed] [Google Scholar]
- 12.Platzer I, Li M, Winkler B, et al. Detection and differentiation of paediatric renal tumours using diffusion-weighted imaging: an explorative retrospective study. Cancer Res Front. 2015;1:178–190. https://doi.org/10.17980/2015.178. [Google Scholar]
- 13.Vilanova JC, Baleato S, Balliu E. Musculoskeletal applications of DWI. In: Luna A, Ribes R, Soto JA, editors. Diffusion MRI outside the brain: a case-based review and clinical applications. Springer Science & Business Media; Berlin Heidelberg New York: 2011. pp. 339–364. [Google Scholar]