Abstract
Bone morphogenetic proteins (BMPs) regulate diverse cellular responses during embryogenesis and in adulthood including cell differentiation, proliferation, and death in various tissues. In the adult pituitary, BMPs participate in the control of hormone secretion and cell proliferation, suggesting a potential endocrine/paracrine role for BMPs, but some of the mechanisms are unclear. Here, using a bioactivity test based on embryonic cells (C3H10T1/2) transfected with a BMP-responsive element, we sought to determine whether pituitary cells secrete BMPs or BMP antagonists. Interestingly, we found that pituitary-conditioned medium contains a factor that inhibits action of BMP-2 and -4. Combining surface plasmon resonance and high-resolution mass spectrometry helped pinpoint this factor as thrombospondin-1 (TSP-1). Surface plasmon resonance and co-immunoprecipitation confirmed that recombinant human TSP-1 can bind BMP-2 and -4 and antagonize their effects on C3H10T1/2 cells. Moreover, TSP-1 inhibited the action of serum BMPs. We also report that the von Willebrand type C domain of TSP-1 is likely responsible for this BMP-2/4-binding activity, an assertion based on sequence similarity that TSP-1 shares with the von Willebrand type C domain of Crossveinless 2 (CV-2), a BMP antagonist and member of the chordin family. In summary, we identified for the first time TSP-1 as a BMP-2/-4 antagonist and presented a structural basis for the physical interaction between TSP-1 and BMP-4. We propose that TSP-1 could regulate bioavailability of BMPs, either produced locally or reaching the pituitary via blood circulation. In conclusion, our findings provide new insights into the involvement of TSP-1 in the BMP-2/-4 mechanisms of action.
Keywords: bone morphogenetic protein (BMP), pituitary gland, structural model, surface plasmon resonance (SPR), thrombospondin, C3H10T1/2 cells, bone morphogenetic protein antagonist
Introduction
Bone morphogenetic proteins (BMPs),3 members of the transforming growth factor β (TGFβ) superfamily, were originally identified by their ability to induce endochondral bone formation (1, 2). They are now known to regulate diverse cellular responses during embryogenesis and in adulthood including cell differentiation, proliferation, and death in various tissues. The BMP system appears as a critical component of the local regulation in several endocrine tissues including the ovary, pituitary, hypothalamus, and adrenal (3, 4). At the pituitary level, BMPs not only govern organogenesis but also participate to the control of hormone secretion and/or cell proliferation in different differentiated cell types like lactotropes (5, 6), corticotropes (7, 8), and gonadotropes (9, 10). In these latter cells BMPs as well as activins, other members of the TGFβ superfamily, participate to the regulation of follicle-stimulating hormone (FSH) synthesis and release in addition to the gonadotropin-releasing hormone (GnRH) and the gonadal steroids. Whereas activins are potent stimulators of FSH secretion (11–13), BMPs modulate FSH secretion with a species-dependent effect. In rat pituitary cells and murine LβT2 gonadotrope cell lines, recombinant human (rh) BMP-6 and BMP-7 at high concentrations or rhBMP-2 and rhBMP15 at lower concentrations stimulate basal FSH secretion and FSHβ promoter activity (9, 14, 15). Moreover, rhBMP-4 increased the release of FSH in response to activin and activin plus GnRH (16). In contrast, in ovine pituitary cells, rhBMP-4 inhibits FSH secretion and antagonizes the effects of activin (10, 17). Concerning lactotropes and corticotropes, previous studies reported that BMP-4 induces prolactin secretion in lactotropes (6, 18) meanwhile inhibiting ACTH secretion in corticotropes (7, 8). Moreover, recent studies also revealed a role of BMP-4 in the pituitary pathogenesis. It promotes pituitary prolactinoma while it inhibits corticotrope pathogenesis in Cushing's disease (5, 8).
BMP signaling occurs through heteromeric receptor complexes composed of type 1 and type 2 transmembrane serine/threonine kinase receptors. Three type 1 receptors are known to bind BMPs, which include the activin-receptor-like kinase 2 (ALK2), ALK3 (also known as BMPR-IA), and ALK6 (BMPR-IB) (19, 20). Similarly, three type 2 receptors possess binding affinity for BMPs, including BMPR-II, activin type IIA receptor (ActR-IIA), and ActR-IIB. The ligand binding induces the trans-phosphorylation of the type 1 receptor by the type 2 receptor. Consequently, the activated BMPR-I phosphorylates intracellular receptor-activated SMADs, SMAD1, SMAD5, and SMAD8, which then interacts with SMAD4 (Co-SMAD). The receptor-activated SMAD–Co-SMAD complex translocates to the nucleus and acts as transcription factors, either activating or repressing gene expression (21). BMP signaling is modulated by several extra- and intracellular modulators acting at multiple levels. A large number of extracellular antagonists, such as noggin, the chordin family, gremlin, twisted gastrulation protein, and the Dan family (22, 23) bind BMPs and block their interaction with the receptors, thus inhibiting BMP signaling (24). Moreover, non-signaling membrane pseudoreceptors or intracellular inhibitory SMADs are also able to block the BMP signaling (25).
Several BMP mRNAs are present in pituitary. For example, BMP-2, BMP-4, BMP-7, and GDF9 (growth and differentiation factor 9) mRNAs were detected in ewe pituitary (10, 26). Furthermore, ALK3, ALK6, and BMPR-II are found on different cell types including gonadotropes and corticotropes (10). These data suggest that pituitary BMPs can exert paracrine/autocrine actions on hormone synthesis and release. Alternatively, BMPs can act as endocrine factors supplied by the blood. Indeed, BMP-4, BMP-6, and BMP-9 were found in bovine serum (27), suggesting a potential endocrine role for BMPs at pituitary level.
In this context the first aim of the present study was to investigate whether pituitary cells produced BMPs and/or BMP inhibitors as well as whether the serum conveys BMPs. In the absence of available antibodies and protein assays for ovine BMPs, a sensitive in vitro bioassay based on mouse C3H-B12 cells was used. These cells are mesenchymal embryonic C3H10T1/2 cells stably transfected with an expression construct (BRE-Luc) containing a BMP-responsive element fused to firefly luciferase reporter gene (28). This assay presents the advantage to monitor the bioactivity of the protein and is not isoform-specific. It can also allow to detect factors that inhibit BMP action.
Interestingly, we found that pituitary cell-conditioned media exhibited an inhibitory activity for BMP-induced luciferase activity. We then conducted the identification of the putative inhibitory factor combining surface plasmon resonance and high resolution tandem mass spectrometry. Last, based on sequence and structure analysis, we provide insights into the molecular basis of interaction between BMP-4 and this inhibitor.
Results
Conditioned media (CM) from pituitary cells did not exhibit BMP activity
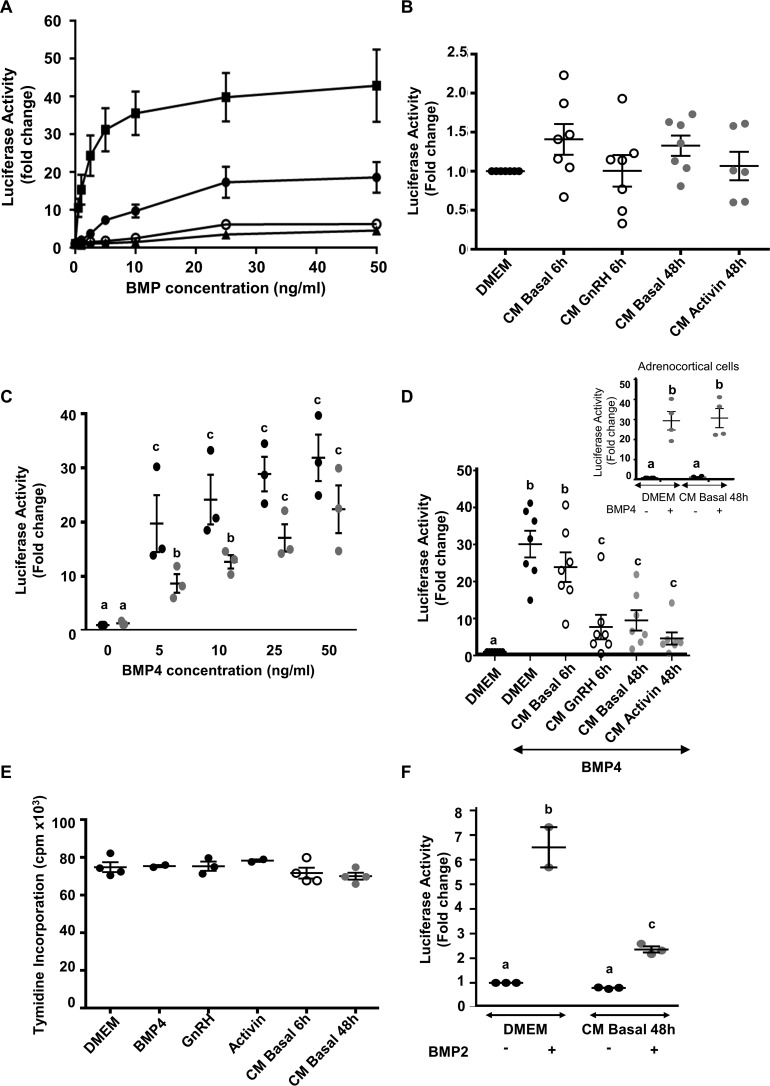
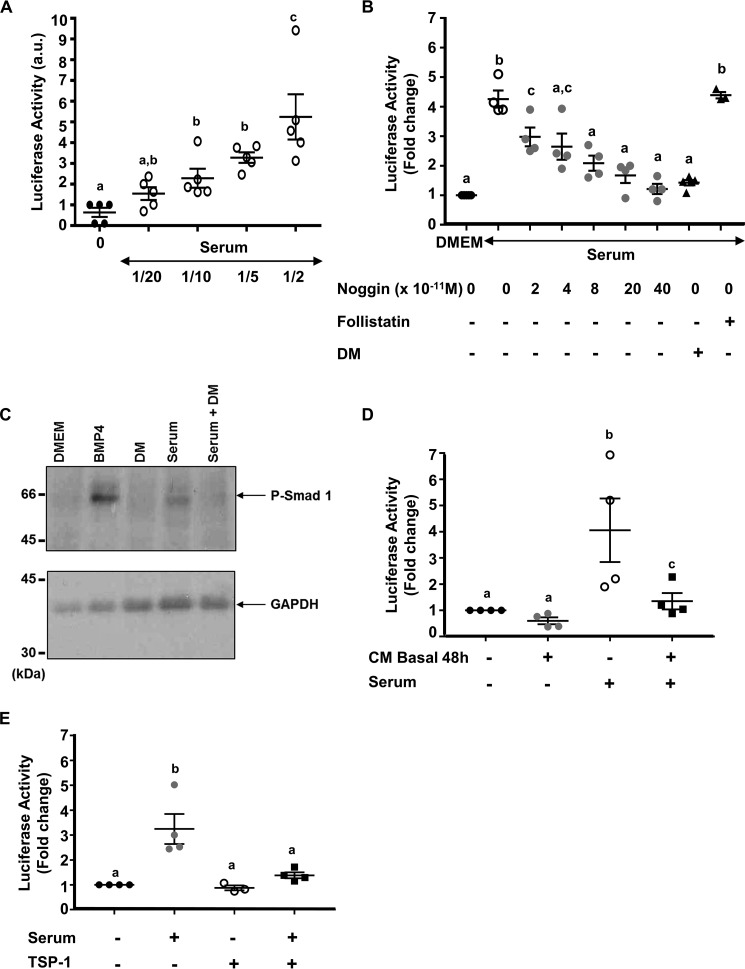
First, the BMP effect on the BRE-Luc construct was determined by treating C3H-B12 cells with increasing concentrations of BMP-2, BMP-4, BMP-6, or BMP-7 (0–50 ng/ml) overnight and monitoring changes in the luciferase activity. BMPs stimulated luciferase activity in a dose-dependent manner (Fig. 1A). BMP-4 was the most potent inducer of luciferase activity with an ED50 of 1.2 ng/ml and detection threshold of 0.25 ng/ml, whereas BMP-7 was the least potent inducer with a detection threshold of 25 ng/ml.
Figure 1.
Effect of BMPs or pituitary CM on luciferase activity from C3H-B12 cells. A, dose-dependent induction of the BRE-Luc construct from C3H-B12 cells by BMPs. C3H-B12 cells were treated overnight with increasing concentrations of either BMP-2 (●), BMP-4 (■), BMP-6 (▴), or BMP-7 (ο) before assaying for luciferase activity as described under “Experimental Procedures.” Note that BMP-6 and BMP-7 curves are superimposed. Results are expressed as arbitrary units. Each point represents the mean ± S.E. of three experiments. B–D, effect of pituitary CM on luciferase activity from C3H-B12 cells. C3H-B12 cells were exposed overnight to DMEM or CM before assaying for luciferase activity. B, CM were from ovine pituitary cells treated or not with 10−8 m GnRH for 6 h (CM GnRH 6 h) or with 10−9 m activin for 48 h (CM activin 48 h). Values are the mean ± S.E. from seven experiments. C, pituitary media conditioned for 48 h (CM basal 48 h) were supplemented with increasing concentrations of BMP-4 before exposition to C3H-B12 cells. Values are the mean ± S.E. from three experiments. D, comparison of pituitary media conditioned for 6 h with 10−8 m GnRH (CM GnRH 6 h) or without GnRH (CM basal 6 h) or for 48 h with 10−9 m activin (CM activin 48 h) and supplemented with rh-BMP-4 (10 ng/ml) before exposition to C3H-B12 cells. Values are the mean ± S.E. from seven experiments. Inset, CM were from ovine adrenocortical cell cultures conditioned for 48 h (CM Basal 48) supplemented with BMP-4 (10 ng/ml) before exposition to C3H-B12 cells. Values are the mean ± S.E. from three experiments. E, effect of pituitary CM on C3H-B12 cell proliferation. C3H-B12 cells were exposed overnight to DMEM supplemented or not with BMP-4 (10 ng/ml), GnRH (10−8 m), or activin (10−9 m) or to pituitary CM (CM basal 6 h or CM basal 48 h) before assaying for cell proliferation. F, effect of pituitary media conditioned for 48 h (CM basal 48 h) and supplemented with BMP-2 (10 ng/ml) on luciferase activity from C3H-B12 cells. Values are the mean ± S.E. from three experiments. Bars with different letters indicate that group means are significantly different at p < 0.05.
To determine whether CM from ovine pituitary cells exhibited BMP activity, C3H-B12 cells were exposed to CM from cultured pituitary cells, which were treated or not with 10−8 m GnRH for 6 h (CM GnRH 6 h) or with either 10−9 m activin for 48 h (CM activin 48 h). Luciferase activity from C3H-B12 was not modified compared with C3H-B12 cells exposed to Dulbecco's modified Eagle's medium (DMEM-0.1% bovine serum albumin (BSA) i.e. non-conditioned media; Fig. 1B). This result suggests that conditioned media exhibits no or low amounts of bioactive BMP whatever the treatment and the incubation period of the pituitary cells.
Conditioned media from pituitary cells exhibited an inhibitory activity on BMP action
To evaluate the potential presence of BMP inhibitors in CM, we supplemented pituitary cell media conditioned for 48 h with increasing doses of BMP-4 and incubated them with C3H-B12 cells. A dose-dependent increase in the luciferase activity was observed (Fig. 1C, gray spots). However, this increase was impaired relative to that obtained with DMEM, 0.1% BSA supplemented with similar doses of BMP-4 (Fig. 1C, black spots). A dose of 50 ng/ml BMP-4 in CM was necessary to recover full activity of BMP-4 on C3H-B12 cells compared with 5 ng/ml in DMEM, 0.1% BSA (Fig. 1C). These results suggest that an inhibitory activity of BMP action is present in CM. Moreover, the inhibitory activity of BMP action was more elevated in the medium conditioned for 48 h (CM basal 48 h) than for 6 h (CM basal 6 h) (72% of inhibition and 17%, respectively, versus DMEM + BMP-4) (Fig. 1D). As comparison, we analyzed CM basal 48 h from adrenocortical cells cultured in the same conditions as the pituitary cells. The C3H-B12 cell luciferase activity induced by BMP-4 (10 ng/ml) supplemented in adrenal cell CM was similar to that of BMP-4 diluted in DMEM, 0.1% BSA indicating that the CM from adrenal cells did not exhibit a detectable inhibitory activity of BMP action (Fig. 1D, inset).
Treatment of pituitary cells with GnRH for 6 h increased the CM inhibitory activity on BMP action
To determine whether FSH regulatory factors, such as GnRH and activin, were capable of modulating the production of the inhibitory activity, CM from pituitary cells treated with these factors in dose and time conditions known to affect FSH secretion were supplemented with 10 ng/ml rhBMP-4 and incubated with C3H-B12 cells overnight.
In the presence of BMP-4, CM from pituitary cells treated with GnRH for 6 h (CM GnRH 6 h) impaired the increase in luciferase activity of C3H-B12 cells versus DMEM + BMP-4 (77% inhibition) (p < 0.01) more than did CM from non-treated cells (CM basal 6 h) (17% inhibition) (p > 0.05) (Fig. 1D). This suggests that GnRH increased the CM inhibitory activity on BMP action.
When the CM from pituitary cells treated with activin for 48 h (CM activin 48 h) were added with BMP-4, they tended to impair the increase in luciferase activity versus DMEM + BMP-4 more than did CM from non treated cells (CM basal 48 h) (83% of inhibition versus 72%), although the difference was not statistically different (Fig. 1D). The treatment of C3H-B12 cells with pituitary CM, BMP-4, GnRH, or activin did not affect cell proliferation compared with DMEM, 0.1% BSA (Fig. 1E).
Moreover, we tested the ability of the pituitary CM to decrease the effect of another BMP, BMP-2. Fig. 1F shows that luciferase activity was impaired when BMP-2 was added to CM conditioned for 48 h compared with the addition in DMEM, similarly to the effect observed with BMP-4.
Conditioned media from pituitary cells exhibited BMP-4-binding protein
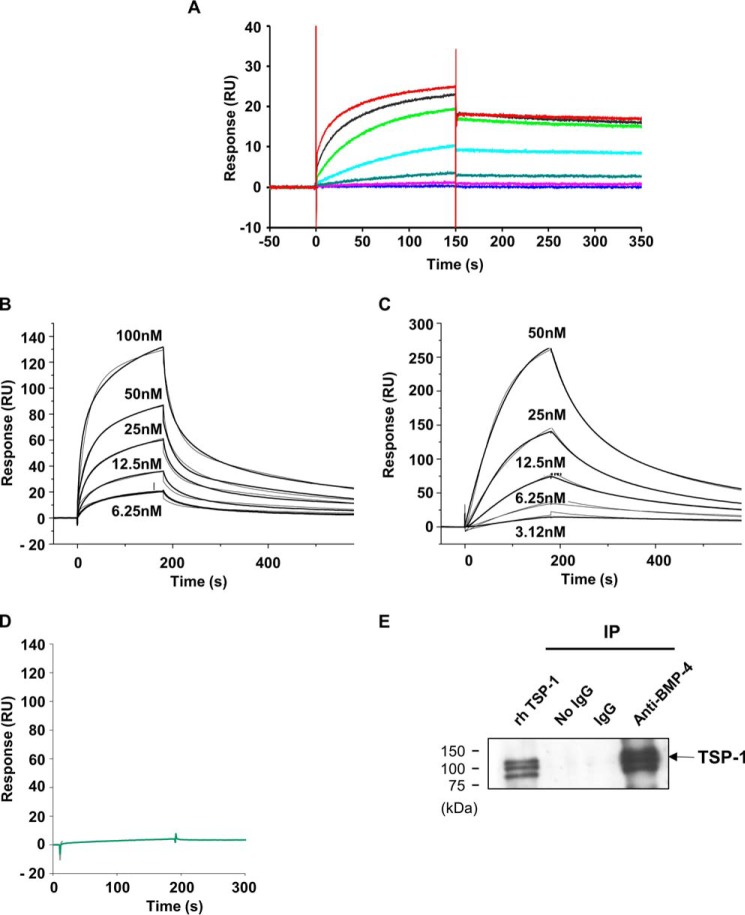
To explore the hypothesis that the CM factor(s) responsible for the inhibition of BMP action can be the BMP-4-binding protein(s), interaction between conditioned media and BMP-4 was analyzed using surface plasmon resonance (Biacore). The injection of CM (1/10 diluted) resulted in binding to high density immobilized rhBMP-4, whereas the injection of DMEM, 0.1% BSA led to a low nonspecific binding signal (Fig. 2). Moreover, the interaction signal was more elevated with media conditioned for 48 h compared with media conditioned for 6 h. To concentrate the binding factor and eliminate small molecules, the CM volumes were 10-fold reduced using high molecular mass polyethylene glycol (PEG) dialysis. The concentrated media exhibited an increased interaction signal compared with crude CM (Fig. 2). Collectively, these results demonstrated that an interaction occurs between pituitary CM and BMP-4. Note that the differences in interaction signal observed between media conditioned for 6 h and 48 h are consistent with the changes observed in the biological effect of the corresponding CM on CH3-B12 cells (Fig. 1D).
Figure 2.
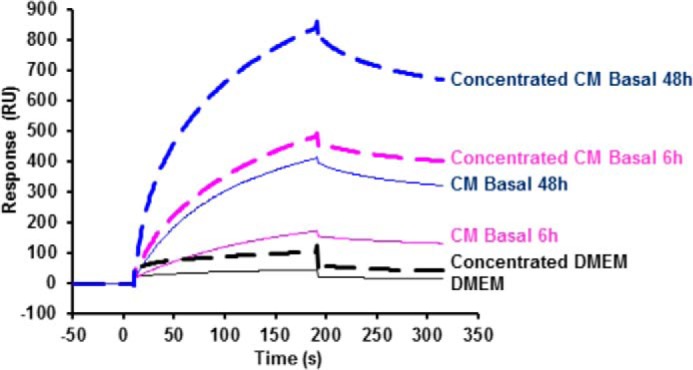
Interaction between pituitary conditioned media and BMP-4 analyzed by surface plasmon resonance. BMP-4 was immobilized at intermediate density (4600 RU) on a flow cell of a CM5 sensorchip, and DMEM-BSA or -diluted DMEM-BSA or medium conditioned for 6 h (CM basal 6 h) or for 48 h (CM basal 48 h) diluted were then injected for 180 s over the chip at 30 μl/min, and dissociation was studied for 120 s (plain lines). The sensorgrams are subtracted with the nonspecific interaction values obtained on an activated-deactivated control flow cell. Spotted lines represent aliquots of media concentrated over PEG as described under “Results” and -diluted before injection. The figure shows one representative experiment. Similar results were obtained with CM provided by six independent pituitary cultures.
BMP-4-binding protein identified as thrombospondin-1 by tandem mass spectrometry
The CM fraction bound to BMP-4 on CM5 sensorchip was eluted and analyzed by on-line nanoflow liquid chromatography tandem mass spectrometry after tryptic digestion. The only three detectable peptides allowed the identification of the predicted thrombospondin-1 isoform 1 (TSP-1) (Table 1), a 450-kDa secreted homotrimeric protein that regulates a wide range of functions (29). These peptides were not detected when elution was performed after injection of DMEM, 0.1% BSA on CM5 sensorchip instead of conditioned media. These results demonstrated that BMP-4 chip acts as a specific and efficient affinity separation method.
Table 1.
Protein and peptides identified by tandem mass spectrometry in the fraction from CM bound to BMP-4 coated CM5 sensorchip
| Identified peptides | Protein description | Accession number (nrNCBI) | Taxonomy | Gene | Theoretical molecular weight |
|---|---|---|---|---|---|
| TGDEWTVDSCTECR | Predicted thrombospondin-1 | gi 426232958 | Ovis aries | TSP-1 | 129 kDa |
| CENTDPGYNCLPCPPR | |||||
| QVTQSYWDTNPTR |
Recombinant thrombospondin-1 interacted with BMP-4 as well as BMP-2 and antagonized its action
To confirm the interaction between TSP-1 and BMP-4, surface plasmon resonance analysis was conducted. The injection of rhTSP-1 on low density BMP-4-immobilized CM5 sensorchip led to dose-dependent binding (Fig. 3A). To determine affinity data, we used the method of steady-state analysis. The equilibrium dissociation constant (KD) for the interaction was calculated at 10−8 m in two independent experiments.
Figure 3.
Interaction between rhTSP-1 and BMP-4. A, interaction between rhTSP-1 and BMP-4 analyzed by surface plasmon resonance using BMP-4 immobilized sensorchip. To reduce TSP avidity effects, BMP-4 was immobilized at low (60 RU) density on a flow cell of a CM5 sensorchip. Increasing concentrations of rhTSP-1 (from bottom to top: 1, 2, 4, 8, 16, 32, 64 nm) were then injected for 180 s over the chip at 30 μl·min−1. Dissociation was studied for 600 s (cut here after 200 s to better see the first phase of association). The sensorgrams are double-subtracted for the nonspecific interaction and for the buffer effect. B, interaction between rhTSP-1 and BMP-4 analyzed by surface plasmon resonance using TSP-1 immobilized sensorchip. TSP-1 was immobilized at 1500 RU on a flow cell of a CM5 sensorchip. Increasing concentrations of BMP-4 from 6.25 to 100 nm were then injected for 180 s at 10 μl·min−1. C, interaction between rhTSP-1 and BMP-2 analyzed by surface plasmon resonance using TSP-1-immobilized sensorchip. TSP-1 was immobilized at 1500 RU on a flow cell of a CM5 sensorchip. Increasing concentrations of BMP-2 from 3.12 to 50 nm were then injected for 180 s at 10 μl·min−1. D, interaction between rhTSP-1 and rh-activin A analyzed by surface plasmon resonance. rhTSP-1 was immobilized on a CM5 sensorchip, and 100 nm activin A (a) or BMP-4 (b) were then injected over the chip at 10 μl/min. E, interaction between rhTSP-1 and BMP-4 analyzed by co-immunoprecipitation (IP). BMP-4 and TSP-1 were incubated together, immunoprecipitated with anti-BMP-4 antibody, and immunoblotted with anti-TSP-1 antibody (fourth lane). The second and third lanes show the result after omission of the antibody or its replacement by nonspecific IgG, respectively. Lane 1 shows the detection of rhTSP-1 (100 ng). These experiments were performed three times with similar results.
BMP-4 binding to TSP-1 was further assayed using TSP-1-immobilized CM5 sensorchip. As expected, the injection of BMP-4 resulted in a dose-dependent binding signal (Fig. 3B). BMP-2 binding to TSP-1 was also studied on TSP-1-immobilized CM5 sensorchip. Dose-dependent binding signal was observed (Fig. 3C) with a kinetics slightly different from that of BMP-4. The dissociation constants (KD) were: 5.95 × 10−7 m for BMP-4 and 1.26 × 10−7 for BMP-2. Evaluation of the kinetic parameters showed faster association and dissociation rates for BMP-2 (Table 2).
Table 2.
Kinetic parameters of the interaction between BMP-4 and TSP-1 analysed by surface plasmon resonance
| Interacting proteins | ka | kd | KD | Rmax | χ2 |
|---|---|---|---|---|---|
| m−1 s−1 | s−1 | m | RU2 | ||
| BMP4-TSP1 | 6.9 × 104 | 0.037 | 5.95 × 10−7 | 421.76 | 2.58 |
| BMP2-TSP1 | 1.23 × 106 | 0.154 | 1.26 × 10−7 | 609.8 | 4.52 |
In contrast, no interaction between TSP-1 and activin, chosen as control, was observed (Fig. 3D).
To add more experimental data showing the interaction between BMP-4 and TSP-1, co-immunoprecipitation experiments were performed. When BMP-4 and TSP-1 are incubated together, precipitation with anti-BMP-4 antibody co-precipitates TSP-1 as detected by Western blot analysis (Fig. 3E, fourth lane). In contrast, when the anti-BMP-4 antibody was omitted or replaced by a nonspecific IgG, TSP-1 was not detected (Fig. 3E, second and third lanes, respectively). These observations confirm the interaction between BMP-4 and TSP-1.
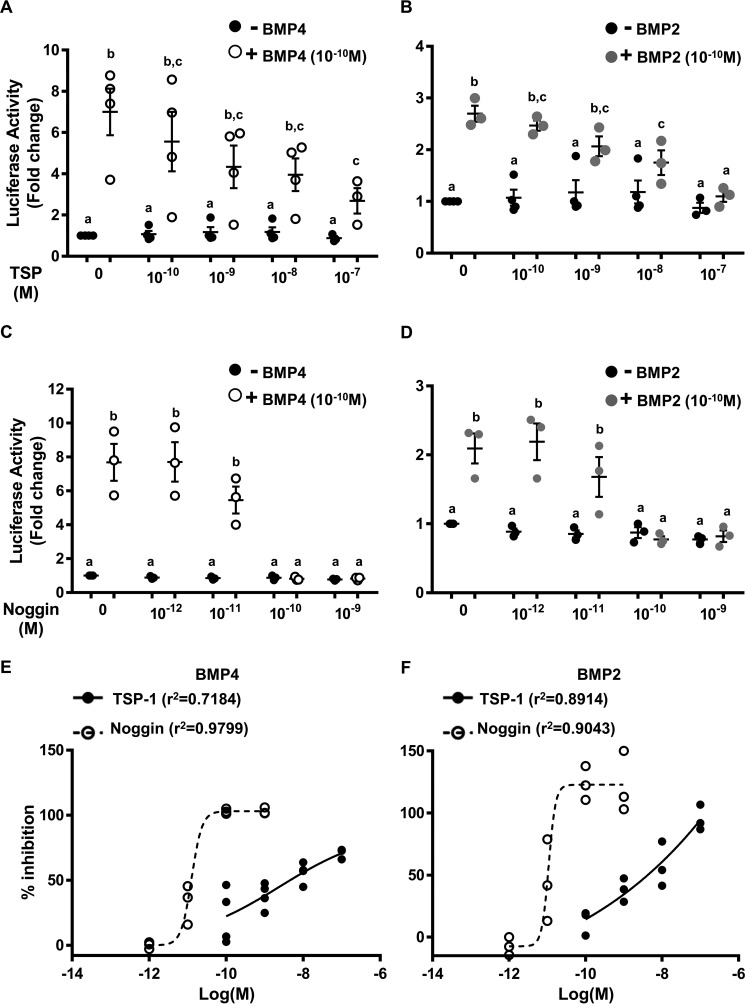
Furthermore, to assess the biological relevance of the BMP-4 and TSP-1 interaction, we studied the ability of TSP-1 to block BMP-4 or BMP-2 action. As shown in Fig. 4, A and B, incubation of TSP-1 with BMP-4 or BMP-2 led to antagonize BMP action as demonstrated in CH3-B12 cell bioassay where rhTSP-1 inhibited the activity of BMP-4 or BMP-2 (2.5 ng/ml, i.e. 10−10 m) on luciferase transcription in a dose-dependent manner. Dose-response curves were fitted using the Hill equation (Fig. 4, E and F). Half-maximal inhibitory concentration (IC50) values were estimated at 11.4 ± 6.3 and 8.5 ± 3.9 nm, respectively, with Hill slope values of 0.36 ± 0.1 and 0.61 ± 0.13. These values are in agreement with a negative cooperative interaction. TSP-1 alone did not affect luciferase transcription (Fig. 4A). As a comparison, noggin, a well-known BMP-2/4-binding protein, inhibited the activity of BMP-4 as well as BMP-2 (2.5 ng/ml, i.e. 10−10 m) (Fig. 4, C and D). When the data were fitted with the Hill equation, estimated IC50 values were 0.023 ± 0.001 and 0.021 ± 0.003 nm, respectively, with Hill slope values of 7.4 ± 0.6 and 6.9 ± 0.8 (Fig. 4, E and F). These values suggest a positive cooperative interaction.
Figure 4.
Antagonization of BMP-4 or BMP-2 action on luciferase activity from C3H-B12 cells. A–D, antagonization of BMP-4 or BMP-2 action by TSP-1 (A and B) or noggin (C and D). C3H-B12 cells were exposed to BMP-4 or BMP-2 (2.5 ng/ml, i.e. 10−10 m) in the presence of increasing concentrations (10−10–10−7 m) of rhTSP-1 or (10−12–10−9 m) of noggin overnight before assaying for luciferase activity. E and F, the data points, expressed as percentages of inhibition, were fitted using nonlinear regression to the Hill equation giving IC50 values of 11.4 ± 6.3, 8.5 ± 3.9, 0.02 ± 0.001, 0.02 ± 0.003 nm (A, B, C, and D, respectively) and Hill slope factors are 0.36 ± 0.1, 0.61 ± 0.13, 7.4 ± 0.6, and 6.9 ± 0.8 (A, B, C, and D, respectively). Values are the mean ± S.E. from at least three independent experiments with duplicate determinations. Bars with different letters indicate that group means are significantly different at p < 0.05.
Thrombospondin-1 mRNA and protein are expressed in pituitary cells
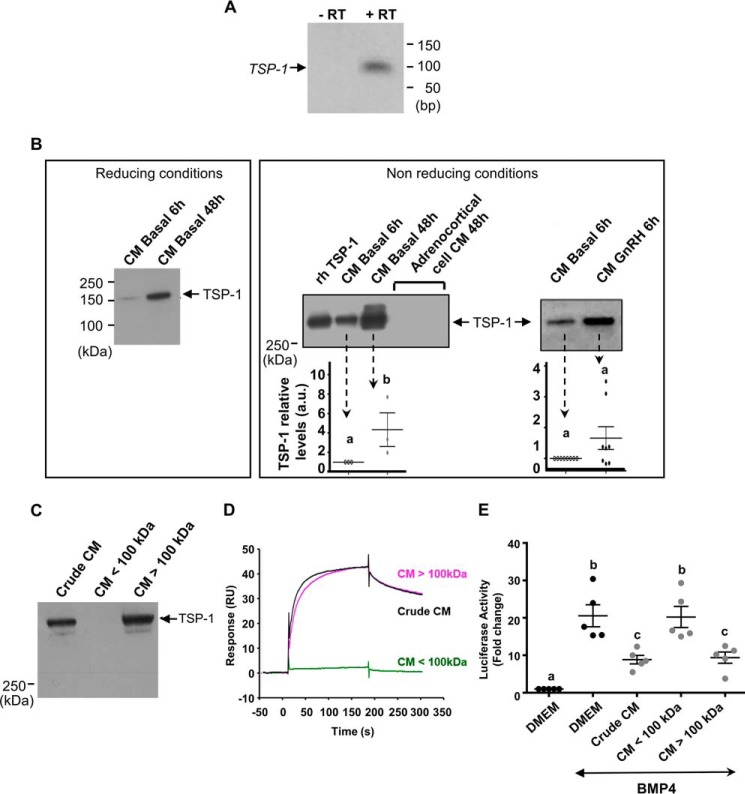
To confirm our findings from mass spectrometry identifying TSP-1 as a factor present in pituitary cell CM, the expression of its mRNA was analyzed by RT-PCR in cells collected at the time of CM recovery (48 h). Fig. 5A shows the presence of a single PCR product at the expected size for TSP-1 mRNA, i.e. 98 bp, whereas no product was detected when reverse transcriptase was omitted.
Figure 5.
Expression of pituitary TSP-1 and interaction between TSP-1-enriched CM fractions and BMP-4. A, TSP-1 mRNA expression in ovine pituitary cells. Cells were cultured for 48 h before RNA extraction. TSP-1 mRNA were analyzed by RT-PCR after 30 cycles (lane 2). PCR was performed with omission of reverse transcriptase (lane 1). B, TSP-1 protein expression in CM from pituitary cells. Cells were cultured for 6 h (CM basal 6 h) or 48 h (CM basal 48 h) or 6 h with GnRH (CM GnRH 6 h) before CM recovery and Western blot analysis in reducing or non-reducing conditions. CM from corticoadrenal cells were prepared under the same conditions. As a control, rhTSP-1 (50 ng) was loaded. Protein loading was checked by membrane staining with Ponceau S. The histograms represent the mean ± S.E. of densitometric analysis obtained with CM from six independent pituitary cultures. C, the presence of TSP-1 in high molecular mass fraction from ovine pituitary CM. Media from pituitary cells conditioned for 48 h were fractionated using 100-kDa cut-off membranes. The presence of TSP-1 in the crude CM, the filtrate (CM <100 kDa), and the retentate (CM >100 kDa) was analyzed by Western blotting. Protein loading was checked by membrane staining with Ponceau S. The figure shows a representative experiment. These experiments were performed with CM from six independent pituitary cultures. D, SPR interaction between high molecular mass fraction from pituitary CM and BMP-4. The filtrate (CM <100 kDa) -diluted and the retentate (CM >100 kDa) -diluted obtained as described above were injected for 180 s at 10 μl/min on a 60 RU immobilized BMP-4 flow cell of a CM5 sensor chip. The crude CM diluted 1/10 was injected under the same conditions. The sensorgrams are subtracted with the nonspecific interaction values obtained on the activated-deactivated control flow cell. The figure shows a representative experiment. Independent experiments were performed with CM from three different pituitary cultures. E, antagonization of BMP-4 action by the high molecular mass fraction from pituitary CM on luciferase activity from C3H-B12 cells. C3H-B12 cells were exposed to the crude CM ½-diluted, the filtrate (CM <100 kDa) ½-diluted, and the retentate (CM >100 kDa) -diluted in the presence of rhBMP-4 (2,5 ng/ml) overnight before assaying for luciferase activity. Values are the mean ± S.E. from four independent experiments with duplicate determinations. Bars with different letters indicate that group means are significantly different at p < 0.05.
Western blot analysis confirmed the presence of the protein in CM with an apparent Mr of 150 or 450 in reducing or non-reducing conditions, respectively, corresponding to TSP-1 (Fig. 5B). As expected, the level of TSP-1 was increased in CM 48 h compared with CM 6 h. This result is consistent with the increase of the inhibition of BMP-4 action on luciferase activity from B12 cells observed with media from pituitary cells conditioned for 48 h compared with 6 h (Fig. 1D). TSP-1 level also tended to increase in the presence of CM from pituitary cells treated with GnRH for 6 h compared with CM basal 6 h, although the difference was not significant (p < 0.1, Fig. 5B).
High molecular mass factor-enriched pituitary CM interacted with BMP-4 and antagonized its action
To validate that a high molecular mass factor like ovine TSP-1 present in pituitary CM is able to bind BMP-4 and inhibit its action, conditioned media were fractionated using 100-kDa cut-off membranes. The presence of TSP-1 in the retentate (CM >100 kDa) but not in the filtrate (CM <100 kDa) was confirmed by Western blotting (Fig. 5C). Fig. 5D shows that retentate (CM >100 kDa), but not filtrate (CM <100 kDa), bound to BMP-4-immobilized sensorchip. Consistent with this result is the inhibition of BMP-4 (10 ng/ml) action observed with the retentate but not with the filtrate on luciferase transcription in CH3-B12 cells (Fig. 5E).
Blood serum exhibited BMP activity
The above data showed that pituitary cells produced TSP-1 and that TSP-1 was able to bind BMPs. We made the hypothesis that pituitary TSP-1 could bind BMPs reaching the pituitary through the blood. We first asked whether ovine serum contains BMPs. To address this question, C3H-B12 cells were exposed to dilutions of serum from adult ewes. Fig. 6A showed that serum induced a dose-dependent increase of luciferase activity in C3H-B12 cells, suggesting that BMP-like activity was present. Serum did not affect cell proliferation or morphology in our assay conditions when diluted ½ or more (data not shown).
Figure 6.
BMP activity in ovine serum. A, C3H-B12 cells were exposed overnight to different dilutions of ovine serum before assaying for luciferase activity. Values are the mean ± S.E. from one representative experiment with duplicate determinations. These experiments were performed three times with similar results. C3H-B12 cells were exposed overnight to ovine serum ½-diluted supplemented with increasing doses of noggin from 0 to 4 × 10−10 m, 2.5 × 10−6 m dorsomorphin (DM) or 10−8 m follistatin (B) or pituitary CM (CM basal 48 h) diluted ½ (D) or 10−7 m rhTSP-1 (E) before assaying for luciferase activity. Values are the mean ± S.E. from four experiments with duplicate determinations. C, activation of SMAD1 in C3H-B12 cells by ovine serum. C3H-B12 cells were exposed for 1 h to BMP-4 (10 ng/ml) or serum ½-diluted or serum ½-diluted supplemented with dorsomorphin (2.5 × 10−6 m). Total proteins were isolated from the cells, and Western blotting was performed with phospho-SMAD1 antibody. Equal loading of the proteins was confirmed by using GAPDH antibody. Bars with different letters indicate that group means are significantly different at p < 0.05. a.u., absorbance units.
To test whether the increase in C3H-B12 luciferase activity was specific of a BMP action, ovine sera were supplemented with increasing doses of noggin (0–4 × 10−10 m), a BMP-2/4-binding protein. The serum-induced luciferase activity was decreased in a dose-dependent manner by noggin demonstrating the BMP specificity (Fig. 6B). When serum was supplemented with dorsomorphin (2.5 × 10−6 m), a small-molecule inhibitor of BMP type I receptors that selectively blocks ALK2, ALK3, and ALK6 activity (30), serum-induced luciferase activity in C3H-B12 cells was not detected (Fig. 6B). In contrast, follistatin, known as a preferential activin-binding protein, did not affect serum-induced luciferase activity at the dose of 10−8 m as illustrated in Fig. 6B.
To determine whether serum is able to induce BMP signaling in a short delay, the SMAD1 pathway in C3H-B12 cells was analyzed. Western blotting was performed after cell treatment with serum (½-diluted) for 60 min. As shown in Fig. 6C, treatment with BMP-4 or with serum induced SMAD-1 phosphorylation. When serum was supplemented with dorsomorphin (2.5 μm), phospho SMAD-1 immunoreactivity in C3H-B12 cells was not detected. These results suggest that serum rapidly recruits BMP signaling to stimulate luciferase activity in C3H-B12 cells.
Conditioned media from pituitary cells as well as rhTSP-1 inhibit BMP activity of the serum
Treatment of C3H-B12 cells with both CM and serum (1/1) showed that pituitary CM inhibited the BMP effect of serum on luciferase activity from C3H-B12 cells (Fig. 6C). This BMP counteraction was mimicked when rhTSP-1 (10−7 m) was added to serum ½-diluted (Fig. 6D). Note that TSP-1 alone had no effect on luciferase activity (Fig. 6D).
TSP-1 shared sequence similarity with proteins of the chordin family
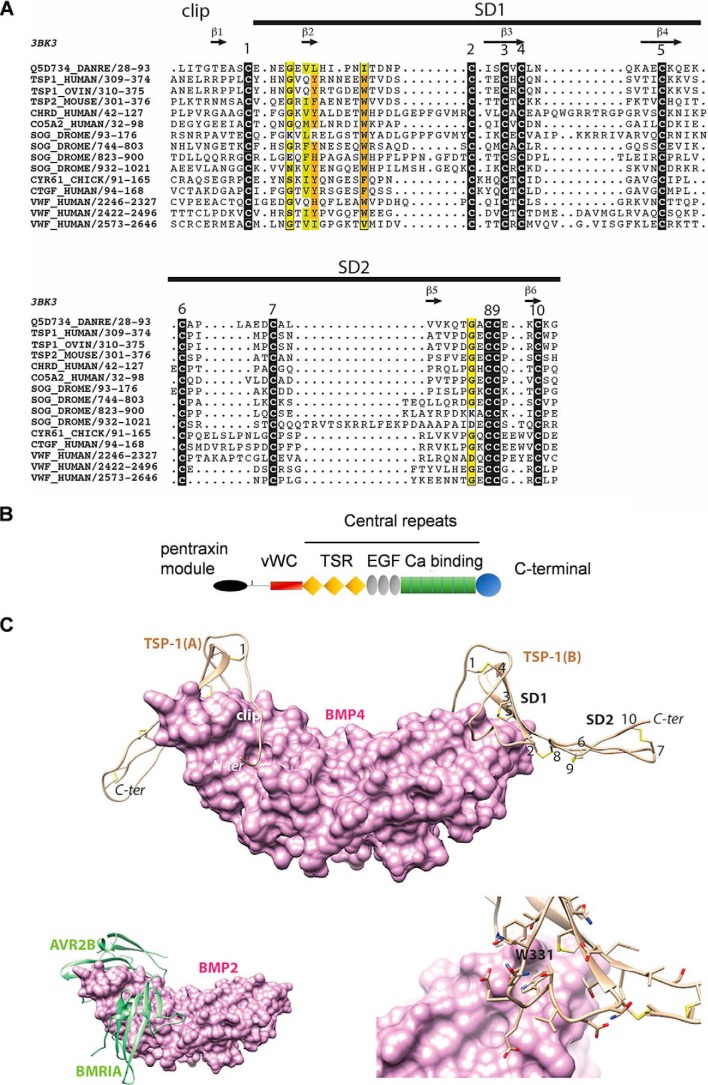
As defined by searching domain databases (HH-PRED Proba 98.85 with Pfam0093), ovine TSP-1 contains a von Willebrand type C domain (VWC) between amino acids 319 and 373. VWC domains have originally been identified in the von Willebrand factor, a large multimeric plasma protein involved in homeostasis, and are present in a wide variety of extracellular proteins. Among these are members of the Chordin modulator family, including Chordin and Crossveinless 2 (CV-2), also known as BMP-binding endothelial regulator (BMPER), which binds BMP-2 with nanomolar affinities and competes for binding BMP type I and type II receptors (31). As observed in the whole VWC family, sequence identities are low; only the 10 cysteine residues and spacing are conserved together with a very limited number of other positions (Fig. 7A). However, these conserved cysteine residues form the cysteine knot that constitutes a signature of these BMP antagonists (23, 32). Fig. 7B represents the domain architecture of TSP-1 including the VWC domain as described in Webb and Sali (33).
Figure 7.
A, multiple sequence alignment of the VWC domain of TSP-1 with other VWC domains from the chordin family, including that of CV-2 (Q5D734_DANRE) whose 3D structure is known (PDB ID 3bk3). Black boxes indicate highly conserved cysteine residues. Observed secondary structures and disulfide bonds are reported above and below the alignment, respectively. Sequences are designated with their UniProt identifiers. B, schematic diagram of domain architecture of TSP-1 (modified from Ref. 43) vWC = vWC domain; TSR = thrombospondin type 1 domain repeats; EGF = EGF like domain repeats; Ca binding = calcium-binding type 3 repeats. C, top, model of the 3D structure of the TSP-1 VWC domain (ribbon representation) in complex with BMP-4 (surface representation). The three subdomains (clip, SD1, and SD2) are shown together with the five disulfide bridges (numbering is as depicted in Fig. 1). The model was built based on the 3D structure of CV-2 in complex with BMP-2 (PDB ID 3BK3) (34) and on the alignment shown in Fig. 1. Bottom left, experimental structure of BMP-2 bound to its high-affinity type I receptor BMPR-IA and its low-affinity type II receptor ActR-IIB (PDB ID 2H62) (34) in order to show the overlap with the VWC domain-binding sites. Bottom right, focus on the Trp-331 amino acid, fitting with a groove at the surface of BMP-4.
Model of the 3D structure of the TSP-1 VWC domain in complex with BMP-4
We built a model of the 3D structure of the TPS1 VWC domain in complex with BMP-4 (ovine sequences) based on the experimental 3D structure of Danio rerio CV-2 in complex with human BMP-2 (HH-Pred Proba 98.67 with PDB ID 3bk3) (34) (Fig. 7C). The TSP-1 VWC domain (amino acids 317–374) shares the global architecture of the CV-2 VWC domain, made of a tripartite organization with an N-terminal clip segment and two small subdomains, called SD1 and SD2. Based on the similarity we observed with the CV-2: BMP-2 interaction, we suggest that the VWC domain of TSP-1 behaves as a paperclip relative to the BMP-4 3D structure, with on the one hand the N-terminal clip folding into the BMP-4 wrist epitope (blocking interaction with type I receptor) and on another hand the SD1 interacting with the BMP-4 knuckle epitope (blocking interaction with type II receptor) (Fig. 7C, top and bottom left).
BMP-2 and BMP-4 share high sequence identity (62%), whereas there is little sequence similarity between CV-2 and TSP-1 (26% identity), a general trend observed in the whole VWC family. However, some positions are highly conserved, such as two generally occupied by aromatic amino acids (in orange in Fig. 7C, bottom right, Tyr-325 and Trp-331 in TSP-1). Interestingly, the side chain Trp-331 is located inside a groove at the surface of BMP-4.
Discussion
BMPs regulate a large array of cellular processes in diverse tissues. In the anterior pituitary, they play various roles both for the initiation of organogenesis during the development and for the regulation of a number of pituitary functions in adult. Some of their actions regard the regulation of prolactin, ACTH, or FSH synthesis and release (6–10). Previous results reported that several BMPs, in particular rhBMP-4 and rhBMP-6, are able to modulate FSHβ mRNA expression and FSH release from pituitary cells (10, 17). Together with the presence of some BMP mRNAs as well as BMPR-IA (ALK3), BMPR-IB (ALK6), and BMPR-II receptors in the pituitary (10), these data suggest that BMPs can be produced locally and exert paracrine action on hormone synthesis. To explore the presence of BMPs in biological samples, the bioassay based on C3H-B12 cells represents a meaningful approach and exhibits several advantages because it is rapid, sensitive, and detects bioactive BMPs (28).
In this context our results showed that conditioned media from ovine pituitary cells did not exhibit BMP activity whatever the treatment (GnRH, activin) and the incubation period (6 h or 48 h) of the pituitary cells, suggesting that, at least in vitro, pituitary cells do not release BMPs or do so in very low amounts and that GnRH or activin does not modify their levels. Alternatively, pituitary cells can release BMP isoforms that are not detected in the bioassay. For example, the detection threshold for rhBMP-7 is ∼25 ng/ml, and the bioassay could fail to detect small concentrations of BMP-7. However, even when concentrated pituitary media were used, we did not observe any increase in luciferase activity (data not shown). One also must consider that BMPs can function in a highly locally manner limiting their diffusion in the medium. Furthermore, our bioassay only detects BMPs biologically active; thus, the BMPs present in the conditioned medium can be biologically inactive. Indeed, their activities depend on the ability of cell environment to convert the protein from an inactive to an active form (35). This process relies on proprotein convertase whose expression pattern is cell-specific. No data concerning pituitary cells are available. Another point we considered is that the biological activity of BMPs can be blocked by binding proteins produced locally.
In agreement with this last point, our results support the assumption that pituitary cells produce BMP antagonist(s). Indeed, when pituitary CMs were supplemented with BMP-4 or BMP-2, the increase in the C3H-B12 cells' luciferase activity was impaired relative to that obtained with DMEM supplemented with BMP-4 or BMP-2. Additional preliminary data indicate that pituitary CM also reduced BMP-6 and BMP-7 action. This suggests that an inhibitor(s) of BMP action is present in CM. Moreover, the inhibitory activity was more elevated when pituitary cell media were conditioned for 48 h compared with 6 h indicating that the inhibitor(s) of BMP action accumulated during the culture. In contrast, pituitary CM did not affect C3H-B12 cell proliferation or morphology under our assay conditions. Our data also underlined a selective presence of inhibitor(s) in pituitary cells as CM from adrenocortical cells did not inhibit BMP-4 activity. Treatment of pituitary cells with GnRH for 6 h induced an increase of the inhibitory activity of BMP action compared with untreated pituitary cells (basal 6 h). This effect suggests an action of this inhibitor on gonadotrope function. It can be postulated that GnRH could block, through such a BMP inhibitor, BMP signaling induced by low amounts of local BMPs or more probably by circulating BMPs. Such an action could promote FSH synthesis and release as BMP-4 inhibits FSH secretion in ewe pituitary (10, 17).
The following focus of the study was aimed at identifying the pituitary factor(s) responsible for the inhibition of BMP action. In agreement with the hypothesis that the inhibitory action can be attributed to extracellular BMP antagonist(s), i.e. binding protein(s) able to prevent BMP signaling, real time surface plasmon resonance (SPR) analysis revealed an interaction between BMP-4 and pituitary-conditioned media highlighting the presence of a BMP-binding protein. Notably, the interaction signal intensity was correlated to the inhibitory action of the CM on C3H-B12 cell luciferase activity, with a lower interaction with media conditioned for 6 h compared with 48 h. Step-combining SPR and tandem mass spectrometry allowed for the first time the identification of TSP-1 as a BMP-2/-4-binding protein.
TSP-1 is a 450-kDa multifunctional homotrimeric glycoprotein that belongs to a family of extracellular matrix-binding proteins. It is produced by different cell types like platelets, endothelial cells, and macrophages, and is involved in a variety of biological functions including angiogenesis, inflammation, cell proliferation, differentiation, apoptosis, and cell-cell interactions (36, 37). The multifaceted action of TSP-1 depends on its ability to physically interact with different ligands like components of the extracellular matrix, cell receptors, growth factors, or cytokines (36, 37). Among the TGFβ family, the only known example is the interaction between TSP-1 and TGFβ. Indeed, TSP-1 binding to the latent TGFβ1 complex induces activation by stimulating a conformation change (38, 39).
Our present data contribute to expand the field of TSP-1 interactome by demonstrating for the first time that TSP-1 physically interacts with BMP-4 and BMP-2. This interaction is revealed when either BMP-4 or TSP-1 are immobilized on sensor chips. Recombinant TSP-1 binds BMP-4 or BMP-2 with relatively high affinity, with a KD value calculated from SPR experiments of ∼10−7–10−8 m. The association and dissociation rates were higher for BMP-2 compared with BMP-4. In contrast, TSP-1 does not bind activin A. The interaction between TSP-1 and BMP-4 was further confirmed by the co-immunoprecipitation assays. Furthermore, we show that the binding between TSP-1 and BMP-4 or BMP-2 antagonizes BMP activity. Indeed, rhTSP-1 as well as high molecular mass-enriched pituitary CM containing TSP-1 prevents BMP-4 bioactivity on C3H-B12 cells. Even if the affinities of TSP-1 for BMP-4 or BMP-2 ∼10−8 m are weaker than the affinities of known BMP-binding proteins like noggin (10−11 m) or chordin, which range ∼10−10 m (40) and 10−13 m (41), the abundance of TSP-1 in CM as well as its high molecular mass may explain the relevance of the antagonism. Alternatively, TSP-1 may need other factors present in CM for optimal function. In the bioassay, 10−7 m rhTSP-1 did not inhibit BMP-4 action to a similar extent as pituitary media conditioned for 48 h. This result suggests either that rhTSP-1 has a less potent biological effect than pituitary TSP-1 or that TSP-1 concentrations in the CM are higher than 10−7 m. Also, CM may contain additional inhibitory factors. Interestingly, we did not identify another BMP-binding protein(s) than TSP-1 after combining SPR and MS, suggesting that if they are present they are in low amounts. Moreover, we noticed that our data argue in favor of a negative cooperative interaction between TSP-1 and BMPs in contrast to the positive cooperative binding shown between noggin and BMPs.
The bioinformatics analysis we made here reinforce evidence for binding between TSP-1 and BMP-4 by suggesting that the N-terminal VWC domain of TSP-1 mediates this interaction, as observed for BMP antagonists of the chordin family (34). We indeed show that the VWC domain of TSP-1 shares significant sequence similarities with the VWC domains of the chordin family, supporting the fact that the TSP-1 VWC domain may also adopt a modular tripartite architecture, with an N-terminal clip segment and two subdomains termed SD1 and SD2. Crystal structure analysis revealed how the CV-2 VWC domain blocks BMP-2 receptor binding by acting on two distinct epitopes specific for BMP type I and type II receptors, respectively (34, 42). According to the model of the 3D structure we built of the TSP-1 VWC domain in complex with BMP-4, structurally close to BMP-2, we thus suggest that TSP-1 could adopt a similar mechanism to antagonize BMP-4.
This mode of interaction between TSP-1 and BMP-4 is different from the binding between TSP-1 with TGFβ that involves the WSHWSPW sequence located in the second TSR domain of TSP-1 adjacent to the N-terminal (33, 38, 43). The interaction between this motif and TGFβ is critical for TSP-1-mediated activation of the TGFβ latent complex (44).
The study presented here identifies a novel role for TSP-1 as a BMP-2/-4-binding protein. Our results underline that TSP-1 is synthesized by pituitary cells and released in the extracellular compartment. The cell type responsible for TSP-1 synthesis is not known, although in the rat, TSP-1 immunoreactivity is partly distributed in pituitary endothelial cells surrounding the vessels (45). One possible role of TSP-1 at the pituitary level is to block the action of BMPs produced locally or to prevent the action of BMPs reaching pituitary by blood. Indeed, using the C3H-B12 cells, we detected BMP activity in ovine serum both at SMAD1 signaling and luciferase activity levels extending the results of Herrera and Inman (27). This BMP activity was inhibited in a dose-dependent manner by noggin, a BMP-2/4 antagonist, or by dorsomorphin, an ALK2/3/6 inhibitor, but not by follistatin, an activin-binding protein. Serum factors are able to recruit BMP signaling in a short delay as they stimulated SMAD-1 phosphorylation after 1 h of treatment as did BMP-4. The SMAD-1 phosphorylation was prevented by dorsomorphin. These data demonstrate the BMP specificity of the response observed with sera. Furthermore, the conditioned media from pituitary cell cultures as well as rhTSP-1 antagonized the effect of ovine serum on the luciferase activity from C3H-B12 cells.
The ability of TSP-1 to antagonize BMP-4 action suggests that TSP-1 might be important in pituitary processes where BMP-4 is involved. Our previous work demonstrated that the treatment of ewe pituitary cells with BMP-4 inhibits FSH secretion (10). Given the presence of BMPs in serum, if not in pituitary, we postulate that TSP-1 can modulate BMP action on FSH secretion through their interaction. The demonstration of such a biological relevance will require additional investigation under physiological conditions. In TSP-1-deficient mice, the homozygous animals are viable and fertile but they produced fewer litters than the wild-type animals (46). Whether FSH is altered was not studied. Further exploration of the pituitary function of these animals will help to gain insight into the role of TSP-1.
Beyond the pituitary, the TSP-1 antagonistic role of BMP-2/-4 may be important in other tissues where they exhibit pleiotropic activities (4, 20, 42), and it is tempting to speculate that their antagonism may be important for cell proliferation, differentiation, apoptosis, and/or migration. As example, TSP-1 as well as BMP-4 are also known to play crucial roles in the pathogenesis of tumors, including the pituitary (5, 47, 48). Whereas TSP-1 has been shown to control tumor growth by inhibiting angiogenesis in various tissues (33, 49, 50), BMP-4 is able to promote angiogenesis (47, 51). All together, these results raise the possibility that molecular interaction between TSP-1 and BMP-4 may be crucial to maintain an appropriate balance between proliferation and differentiation and/or regulate tissue homeostasis.
In conclusion, we show that ewe pituitary cells release a BMP-2/-4 antagonist, identified as TSP-1, and we provide structural evidence for physical interaction between TSP-1 and BMP-2/-4. This suggests that TSP-1 could regulate the bioavailability of BMPs either produced locally or reaching the pituitary by blood. Further study is necessary to elucidate the role of the couple TSP-1/BMP-2/4. Collectively, these data provide new insights into the mechanisms of BMP action.
Experimental procedures
Reagents
Cell culture media used were DMEM and F-12 (nutrient mixture F-12 Ham) from Sigma. Gentamicin, nystatin, l-ascorbic acid, apotransferrin, fetal calf serum (FCS), BSA, luciferin substrate, 17β-estradiol, and dorsomorphin were purchased from Sigma. Collagenase A and DNase 1 were from Roche Diagnostics. Geneticin sulfate (G418 sulfate) was from InvivoGen (San Diego, CA). Recombinant human activin A, BMP-2, BMP-4, BMP-6, BMP-7, and follistatin were obtained from R&D Systems (Lille, France). Recombinant human TSP-1 was from R&D systems (carrier-free TSP-1) for surface plasmon resonance studies and from EMP Genetech (Ingolstadt, Germany) for biological assays. Human noggin was from ProSci Inc. (Poway, CA). GnRH (Relefact® LH-RH) was from Aventis (Frankfurt, Germany). Monoclonal mouse TSP-1 antibody (A6.1) was purchased from Santa Cruz Biotechnology, Inc. (Heidelberg, Germany). Rabbit antibodies against phosphorylated SMAD1 were kindly provided by C. H. Helding (Ludwig Institute for Cancer Research, Uppsala, Sweden).
Ewe pituitary cell cultures
Pituitaries from adult Ile-de-France ewes were collected throughout the year and dissociated for cell cultures as previously described (8). Briefly, pituitaries were finely sliced and placed in F-12 containing 5% FCS, 0.4 mg/ml collagenase A, and 0.025 mg/ml DNase 1 and incubated for 90 min in a shaking water bath at 37 °C followed by manual dispersion through different needle sizes. Cells were then centrifuged at 100 × g for 10 min and washed 3 times. The pellet was resuspended in DMEM supplemented with 5% FCS (culture medium). Cells were seeded in 6-well plates at 3 × 106 cells/well in culture medium and allowed to attach for 2 days in a humidified atmosphere with 5% CO2 in air at 37 °C.
Adrenals were also collected, and cortical cells were dissociated according the same protocol as pituitaries
Preparation of conditioned media from pituitary cell cultures
At day 2 of culture, media were replaced with serum-free DMEM containing 0.1% BSA. One hour later the medium was changed and replaced by serum-free DMEM, 0.1% BSA supplemented or not with 10−8 m GnRH for 6 h or with 10−9 m activin A for 48 h. The choice of concentrations was based on our previous studies determining 10−8 m and 10−9 m as optimal doses for GnRH and activin, respectively, to regulate in vitro FSH secretion. CM were then collected, and non-adherent cells and debris were removed by centrifugation at 2000 × g for 5 min at 4 °C before assaying for BMP bioactivity on C3H-B12 cells. In another set of experiments aimed to concentrate the BMP inhibitory factor and eliminate small molecules, CM were put in a dialysis bag with a cut-off of 3 kDa placed over a bed of PEG (Mr 35 kDa) until the volumes were 10-fold reduced.
Moreover, an ovine BMP inhibitory factor enrichment strategy for high molecular mass factors from CM was performed in some assays using filtration on molecular mass cut-off membranes (Vivaspin® 500, Sartorius, Germany). One ml of CM was applied on a 100-kDa cut-off membrane previously rinsed with deionized water and centrifuged at 15,000 × g for 10 min. Filtrates and retentates (∼20 μl) were recovered for subsequent analysis assaying BMP activity.
Ewe blood samples
Blood samples were collected from the jugular vein from six adult ewes throughout the year. They were stored at 4 °C for 24 h and then centrifuged at 4000 × g for 30 min. Blood sera were collected, filtered through 0.2 μm, and treated at 56 °C for 1 h. They were stored at −20 °C until the BMP bioassay was performed.
C3H-B12 cell cultures
The mouse C3H-B12 cells were maintained in DMEM containing 10% FCS, 3 μg/ml gentamicin, 2 μg/ml nystatin, and 200 μg/ml Geneticin sulfate.
BMP bioassay
C3H-B12 cells were plated at 2 × 105 cells per well in 48-well plates with DMEM containing 10% FCS and allowed to attach for 5 h in a humidified atmosphere with 5% CO2 in air at 37 °C. The cells were rinsed twice with PBS and treated with serum-free DMEM, 0.1% BSA containing or not BMPs or with conditioned media from ovine pituitary cells or with ovine serum at 37 °C overnight. Cell extracts were then prepared for the luciferase activity assay. The cells were rinsed twice in ice-cold PBS and then lysed with 75 μl of passive lysis buffer (Promega). Sample were centrifuged at 12,000 × g for 1 min to pellet the cell debris. The supernatants were collected before luciferase assay (Luciferase Assay System, Promega). Luciferase activity was measured on a Luminoskan Ascent (Thermo Labsystems, Issy Les Moulineaux, France). The reported luciferase activities represent the emitted luminescence normalized over respective controls that are obtained in the absence of stimulating factor. All experiments were performed at least three times with two or three independent wells per condition.
Proliferation measurement
C3H-B12 cells were plated at 2 × 105 cells per well in 48-well plates with DMEM containing 10% FCS and allowed to attach for 5 h in a humidified atmosphere with 5% CO2 in air at 37 °C. The cells were rinsed twice with PBS, and conditioned medium from ovine pituitary cells or other reagents was added with 0.1 μCi/well [methyl-3H]thymidine and incubated at 37 °C overnight. Cells were then washed with PBS solubilized in 0.5 n NaOH. Thymidine incorporation was assessed using a liquid scintillation analyzer (PerkinElmer Life Sciences).
SPR analysis
SPR measurements were performed on a BIACORE T100 (GE Healthcare) instrument. Carrier free BMP-4 was immobilized on the flow cell(s) of CM5 sensor chips (GE Healthcare) using standard amine coupling, according to the manufacturer's instructions. The analyses were carried out at 25 °C with 10 mm Hepes (pH 7.4), 150 mm NaCl, 0.05% Tween 20 (HBS-P) as running buffer. Conditioned media diluted in HBS-P and 0.2-μm filtered were injected for 180 s at a flow rate of 30 μl/min. Dissociation was studied for 120 s. The surfaces were regenerated for 60 s with 3 m guanidine followed by a wash of the injection needle and a 300-s running buffer wash. The sensorgrams were subtracted with the nonspecific interaction results obtained on an activated-deactivated control flow cell.
The interaction of BMP-4 with purified rhTSP-1 was studied in near conditions on 60 resonance unit (RU) low density immobilized BMP-4. TSP-1 was injected at concentrations of 1–67 nm for 150 s at a flow rate of 30 μl/min, and dissociation was studied for 600 s. Regeneration was achieved by injection of 5 mm EDTA for 60 s followed by a 120-s running buffer wash. The sensorgrams were corrected by a double subtraction for the nonspecific interaction in the control activated/deactivated flow cell and for the buffer contribution in the same flow cell (52). Affinity was calculated using the steady-state 1:1 binding model (GE Healthcare Biacore T100 evaluation software, version 2.0.4). This model calculates the equilibrium dissociation KD for a stoichiometric 1:1 model of interaction from a plot of steady-state binding levels (Req) against analyte concentration (C), here TSP-1. Req = (CRmax/KD + C) + RI, where RI is bulk refractive index contribution, which is assumed to be the same for all sample concentrations, and Rmax is the analyte (TSP-1) binding capacity of the surface in RU.
The interaction of BMP-4 as well as BMP-2 with TSP-1 was further studied on carrier-free TSP-1 immobilized on the flow cells of CM5 sensor chip on a Biacore T200 (GE Healthcare). BMP-4 or BMP-2 were injected at concentrations of 6.25–100 nm or 3.12–50 nm, respectively, for 180 s at a flow rate of 10 μl/min, and dissociation was studied for 600 s. Regeneration was achieved by injection of 5 mm EDTA for BMP-4 or with 0.01% SDS for BMP-2. Binding affinity and kinetic parameters were evaluated using the bivalent fitting model (Biacore T200 software). This model describes the binding of a bivalent analyte, here BMP, to immobilized ligand. In some assays using TSP-1-immobilized sensorchip, activin A was injected at a concentration of 100 nm for 180 s at a flow rate of 10 μl/min.
Identification of the BMP-4-binding protein combining SPR and high resolution mass spectrometry
BMP-4 was immobilized on the 4 flow cells of a CM5 sensor chip at a level ∼4000 RU. A pool of 48 h CM filtrated on 0.2 μm and ¼-diluted was injected on these flow cells at a 5 μl/min flow rate for 180 s 6-fold, and this cycle was 4-fold repeated. As the control, DMEM-BSA was injected under the same conditions. The CM fraction bound to BMP-4 was then recovered using 3 m guanidine for 60 s. A volume of 80 μl was acidified at 0.1% formic acid and concentrated with a solid phase extraction technique using Zip Tip C4 (Millipore Corp., Billerica, MA). Cysteine reduction and alkylation were performed by the addition of 10 mm dithiothreitol in 50 mm ammonium bicarbonate for 30 min at 56 °C and 55 mm iodoacetamide in 50 mm ammonium bicarbonate for 20 min at room temperature in the dark, respectively. Proteins were digested overnight with 6.25 ng/μl trypsin (Sequencing Grade, Roche Diagnostics) and acidified with 0.1% formic acid.
The in-solution-digested peptides were analyzed by on-line nanoflow liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS) using a linear ion trap Fourier Transform Mass Spectrometer (FT-MS) LTQ Orbitrap Velos (Thermo Fisher Scientific, Bremen, Germany) coupled to an Ultimate® 3000 RSLC Chromatographer (Dionex, Amsterdam, The Netherlands). Mobile phases consisted of 0.1% formic acid, 97.9% water, 2% acetonitrile (v/v/v) (A) and 0.1% formic acid, 15.9% water, 84% acetonitrile (v/v/v) (B). Samples were desalted for 10 min at 5 ml/min with 4% solvent B using an LCPackings trap column (Acclaim PepMap 100 C18, 100-μm diameter, 6.2 cm long, 3-μm particles) and separated using a LCPackings nano-column (Acclaim PepMap C18, 75-μm diameter, 6.5-cm-long, 3-μm particles). Separation was performed at a flow rate of 300 nl/min by applying a gradient of 4–55% B for 60 min. The mass spectrometer was operated in positive mode in data-dependent mode with high-resolution full scan MS spectra (R = 60,000) and low-resolution MS/MS spectra (R = 7,500). In the scan range of m/z 300–1800, the 20 most intense peptide ions with charge states ≥2 were sequentially isolated and fragmented by collision-induced dissociation mode. Dynamic exclusion was activated for 30 s with a repeat count of 1. Polydimethylcyclosiloxane (m/z, 445.1200025) ions were used as lock mass for internal calibration.
All raw data files were converted to Mascot Generic Format (MGF) with Proteome Discoverer 1.3 software (Thermo Fisher Scientific). All MS/MS data were analyzed using MASCOT 2.3 search engine (Matrix Science) against the mammalia section of a locally maintained copy of nrNCBI (1426555 sequences, download 05/14/2013). Enzyme specificity was set to trypsin with two missed cleavages using carbamidomethylcysteine, oxidation of methionine, and N-terminal protein acetylation as variable modifications. The mass tolerance was set at 5 ppm for parent and 0.8 Da for fragment ions. Identification mascot results were subjected to Scaffold 3 software (v 3.6, Proteome Software, Portland, OR) for validation using Peptide and Protein Prophet algorithms (53, 54). Peptide identifications were accepted if they could be established at >95.0% probability. Proteins were accepted with at least two distinct peptides.
Co-immunoprecipitation
Co-immunoprecipitation experiments were performed to further confirm that TSP-1 binds to BMP-4. Recombinant TSP-1 (4 μg/ml, i.e. 10−8 m) and BMP-4 (0.2 μg/ml, i.e. 10−8 m) were placed alone or in combination in 1 ml of binding buffer (PBS with 0.5% BSA) for 1 h at room temperature with rocking. The pulldown anti-BMP-4 antibody (R&D Systems #MAB757, 500 ng/tube) or nonspecific IgG (500 ng/tube) were added to each reaction tube and incubated at 4 °C overnight with rocking. In another control tube, IgG were omitted. The following day 20 μl of protein A/G-agarose beads (Santa Cruz Biotechnology) were added to each tube and incubated for 1 h with rocking. Then beads were rinsed 5 times in PBS by centrifugation for 5 min at 1000 × g. Beads were then resuspended in 40 μl of SDS sample buffer for Western blotting and boiled to remove proteins from beads. Co-precipitated TSP-1 was detected by immunoblotting as described below.
Reverse transcription and PCR
Total RNA from pituitary cells were extracted using TRI Reagent (Sigma) according to the manufacturer's instructions. Remaining DNA was removed by RNase-free DNase (Qiagen) treatment. RT-PCR was performed as previously described (26). Complementary DNA was synthesized from 1 μg of RNA in a volume of 20 μl containing 150 ng of oligo(dT) (Promega), 1 mm deozynucleotide triphosphates, 20 units of RNasin, 1× RT buffer, and 12 units of Moloney murine leukemia virus reverse transcriptase (Promega). For the negative control, the reverse transcriptase was omitted. The RNA denaturation was performed at 70 °C for 10 min, and the reverse transcription was performed at 37 °C for 1 h. PCR was run on an iCycler from Bio-Rad using IQ SYBR Green Supermix (Bio-Rad) (1×) and primers 5′-GACTGGCTTCTGGACTTC-3′ (forward) and 5′-GCACTGGCTTATGATGGT-3′ (reverse) for ovine TSP-1 amplification (amplicon size = 98 bp). The equivalent of 10 ng of starting RNA was used in each reaction.
Western blotting
For TSP-1 detection, media from pituitary cells conditioned for 6 or 48 h (volume equivalent to 10 μg protein) were diluted in reducing or non-reducing Laemmli buffer, run in 8% SDS-PAGE, and electroblotted onto PVDF membranes. Membranes were blocked for 1 h at room temperature in Tris-buffered saline containing 0.1% Tween, 1% BSA and incubated with the TSP-1 antibody diluted at 1:1000 for 1 h at room temperature. After washing, the membranes were incubated with the secondary antibody, a peroxidase-conjugated anti-mouse IgG (Bio-Rad) diluted 1:10,000 for 1 h. Immunoreactive proteins were detected using enhanced chemiluminescence reagents (Amersham Biosciences). Membranes were exposed to Hyperfilm ECL for 1 or 5 min.
To investigate activation of SMAD 1 after BMP-4 or serum treatment, C3H-B12 cells were plated at 2 × 105 cells per well in 48-well plates with DMEM containing 10% FCS and allowed to attach for 5 h in a humidified atmosphere with 5% CO2 in air at 37 °C. The cells were rinsed twice with PBS and treated with serum-free DMEM, 0.1% BSA containing either rhBMP4 (10 ng/ml) or with ovine serum at 37 °C for 60 min. The cells were then washed with ice-cold PBS and lysed in lysis buffer (150 mm NaCl, 10 mm Tris, pH 7.4, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 0.5% Igepal CA-630, 100 mm sodium fluoride, 10 mm sodium pyrophosphate, 10 mm sodium orthovanadate, proteases inhibitor mixture). The concentrations of the proteins were determined with BC Assay Protein Quantification kit (Interchim, France). After 4 min at 95 °C in the reducing SDS-PAGE buffer containing β-mercaptoethanol, 10 μg of proteins were separated in 10% SDS-PAGE gels and electroblotted onto Immobilon-P membranes (Millipore). Membranes were blocked for 1 h at room temperature in Tris-buffered saline-0.1% Tween containing 1% BSA and incubated with the anti-phospho SMAD1 or the anti-GAPDH protein diluted at 1:20,000 and 1:1,000, respectively, overnight at 4 °C. After washing, the membranes were incubated with the secondary antibody, a peroxidase-conjugated anti-rabbit IgG (Bio-Rad) diluted 1:15,000 for 1 h. Immunoreactive proteins were detected using enhanced chemiluminescence reagents.
Sequence and structure analysis
Searches within the domain and structure databases were performed using HH-PRED (55). Modeling was made using Modeler v9.15 (56). Manipulation and visualization of 3D structures were made using Chimera (57).
Data analysis and statistics
All experiments were performed in duplicate in at least three independent cell cultures. For each figure, the number of cultures is indicated in the legend. All data are presented as the mean ± S.E. Statistical significance was determined by analysis of variance with Bonferroni post hoc analysis for multiple group comparison. Differences were considered significant when p < 0.05. The statistical analyses were performed using GraphPad Prism version 6.00 (GraphPad Software, San Diego, CA).
To calculate the IC50 values and Hill slopes corresponding to the inhibition of BMP-4 or BMP-2 action by TSP-1 or noggin, concentration-response data were fitted using a nonlinear regression analysis to the Hill equation (GraphPad Prism version 6.00).
Author contributions
C. S. conducted most of the experiments, analyzed the results, and wrote the paper with C. T. I. C. performed the bioinformatics analysis and modeling experiments. C. S. and I. B. performed the cell cultures, BMP bioassay, and Western blotting. J. F. participated to cell cultures. D. L-.A. provided the C3H10T1/2 cells for BMP bioassay. X. C. contributed to TSP-1 enrichment from conditioned media. P. M. participated to the bioinformatic analysis. G. H. and V. L. performed the mass spectrometry analysis. S. C., M. P., and C. H. designed and performed SPR experiments. C. T. conceived, designed, and supervised the study and wrote the paper with C. S. and with comments of other authors. All authors approved the final paper.
Acknowledgments
We thank the Experimental Unit “Unité Expérimentale de Physiologie Animale de l'Orfrasière,” Jean-Philippe Dubois, and the staff of the INRA (Institut National de la Recherche Agronomique) slaughterhouse of Nouzilly for animal management. We also thank Stephane Fabre and Alan McNeilly for helpful discussions. We acknowledge Yves Combarnous for helpful suggestions, Maxime Capelle and Marie Champion for technical assistance, and Elodie Chaillou for critical reading of the manuscript.
This work was supported by Institut National de Recherche Agronomique and the French “Région Centre.” The authors declare that they have no conflicts of interest with the contents of this article.
- BMP
- bone morphogenetic protein
- TGFβ
- transforming growth factor β
- GnRH
- gonadotropin releasing hormone
- CM
- conditioned media
- ALK
- activin receptor-like kinase
- BMPR-I
- BMP receptor type I
- BMPR-II
- BMP receptor type II
- ActR-II
- activin receptor type II
- BRE
- BMP-responsive element
- Luc
- luciferase reporter
- SPR
- surface plasmon resonance
- TSP-1
- thrombospondin 1
- rh
- recombinant human
- VWC
- von Willebrand type C domain
- CV-2
- Crossveinless 2
- RU
- resonance unit.
References
- 1. Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., and Wang E. A. (1988) Novel regulators of bone formation: molecular clones and activities. Science 242, 1528–1534 [DOI] [PubMed] [Google Scholar]
- 2. Chen D., Zhao M., and Mundy G. R. (2004) Bone morphogenetic proteins. Growth Factors. 22, 233–241 [DOI] [PubMed] [Google Scholar]
- 3. Otsuka F. (2010) Multiple endocrine regulation by bone morphogenetic protein system. Endocr. J. 57, 3–14 [DOI] [PubMed] [Google Scholar]
- 4. Otsuka F. (2013) Multifunctional bone morphogenetic protein system in endocrinology. Acta Med. Okayama. 67, 75–86 [DOI] [PubMed] [Google Scholar]
- 5. Paez-Pereda M., Giacomini D., Refojo D., Nagashima A. C., Hopfner U., Grubler Y., Chervin A., Goldberg V., Goya R., Hentges S. T., Low M. J., Holsboer F., Stalla G. K., and Arzt E. (2003) Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a SMAD/estrogen receptor crosstalk. Proc. Natl. Acad. Sci. U.S.A. 100, 1034–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giacomini D., Páez-Pereda M., Stalla J., Stalla G. K., and Arzt E. (2009) Molecular interaction of BMP-4, TGF-β, and estrogens in lactotrophs: impact on the PRL promoter. Mol. Endocrinol. 23, 1102–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nudi M., Ouimette J. F., and Drouin J. (2005) Bone morphogenic protein (SMAD)-mediated repression of proopiomelanocortin transcription by interference with Pitx/Tpit activity. Mol. Endocrinol. 19, 1329–1342 [DOI] [PubMed] [Google Scholar]
- 8. Giacomini D., Páez-Pereda M., Theodoropoulou M., Labeur M., Refojo D., Gerez J., Chervin A., Berner S., Losa M., Buchfelder M., Renner U., Stalla G. K., and Arzt E. (2006) Bone morphogenetic protein-4 inhibits corticotroph tumor cells: involvement in the retinoic acid inhibitory action. Endocrinology 147, 247–256 [DOI] [PubMed] [Google Scholar]
- 9. Huang H. J., Wu J. C., Su P., Zhirnov O., and Miller W. L. (2001) A novel role for bone morphogenetic proteins in the synthesis of follicle-stimulating hormone. Endocrinology 142, 2275–2283 [DOI] [PubMed] [Google Scholar]
- 10. Faure M. O., Nicol L., Fabre S., Fontaine J., Mohoric N., McNeilly A., and Taragnat C. (2005) BMP-4 inhibits follicle-stimulating hormone secretion in ewe pituitary. J Endocrinol. 186, 109–121 [DOI] [PubMed] [Google Scholar]
- 11. Carroll R. S., Kowash P. M., Lofgren J. A., Schwall R. H., and Chin W. W. (1991) In vivo regulation of FSH synthesis by inhibin and activin. Endocrinology 129, 3299–3304 [DOI] [PubMed] [Google Scholar]
- 12. Weiss J., Harris P. E., Halvorson L. M., Crowley W. F. Jr., Jameson J. L. (1992) Dynamic regulation of follicle-stimulating hormone-β messenger ribonucleic acid levels by activin and gonadotropin-releasing hormone in perifused rat pituitary cells. Endocrinology 131, 1403–1408 [DOI] [PubMed] [Google Scholar]
- 13. Weiss J., Guendner M. J., Halvorson L. M., and Jameson J. L. (1995) Transcriptional activation of the follicle-stimulating hormone β-subunit gene by activin. Endocrinology 136, 1885–1891 [DOI] [PubMed] [Google Scholar]
- 14. Lee K. B., Khivansara V., Santos M. M., Lamba P., Yuen T., Sealfon S. C., and Bernard D. J. (2007) Bone morphogenetic protein 2 and activin A synergistically stimulate follicle-stimulating hormone β subunit transcription. J Mol Endocrinol. 38, 315–330 [DOI] [PubMed] [Google Scholar]
- 15. Otsuka F., and Shimasaki S. (2002) A novel function of bone morphogenetic protein-15 in the pituitary: selective synthesis and secretion of FSH by gonadotropes. Endocrinology 143, 4938–4941 [DOI] [PubMed] [Google Scholar]
- 16. Nicol L., Faure M. O., McNeilly J. R., Fontaine J., Taragnat C., and McNeilly A. S. (2008) Bone morphogenetic protein-4 interacts with activin and GnRH to modulate gonadotrophin secretion in LβT2 gonadotrophs. J. Endocrinol. 196, 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young J. M., Juengel J. L., Dodds K. G., Laird M., Dearden P. K., McNeilly A. S., McNatty K. P., and Wilson T. (2008) The activin receptor-like kinase 6 Booroola mutation enhances suppressive effects of bone morphogenetic protein 2 (BMP-2), BMP-4, BMP-6 and growth and differentiation factor-9 on FSH release from ovine primary pituitary cell cultures. J. Endocrinol. 196, 251–261 [DOI] [PubMed] [Google Scholar]
- 18. Tsukamoto N., Otsuka F., Miyoshi T., Inagaki K., Nakamura E., Suzuki J., Ogura T., Iwasaki Y., and Makino H. (2011) Activities of bone morphogenetic proteins in prolactin regulation by somatostatin analogs in rat pituitary GH3 cells. Mol. Cell. Endocrinol. 332, 163–169 [DOI] [PubMed] [Google Scholar]
- 19. Kawabata M., Imamura T., and Miyazono K. (1998) Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 9, 49–61 [DOI] [PubMed] [Google Scholar]
- 20. Miyazono K., Kusanagi K., and Inoue H. (2001) Divergence and convergence of TGF-β/BMP signaling. J. Cell. Physiol. 187, 265–276 [DOI] [PubMed] [Google Scholar]
- 21. Zwijsen A., Verschueren K., and Huylebroeck D. (2003) New intracellular components of bone morphogenetic protein/SMAD signaling cascades. FEBS Lett. 546, 133–139 [DOI] [PubMed] [Google Scholar]
- 22. Vitt U. A., Hsu S. Y., and Hsueh A. J. (2001) Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol. Endocrinol. 15, 681–694 [DOI] [PubMed] [Google Scholar]
- 23. Avsian-Kretchmer O., and Hsueh A. J. (2004) Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol. Endocrinol. 18, 1–12 [DOI] [PubMed] [Google Scholar]
- 24. Yanagita M. (2005) BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev. 16, 309–317 [DOI] [PubMed] [Google Scholar]
- 25. Gazzerro E., and Canalis E. (2006) Bone morphogenetic proteins and their antagonists. Rev. Endocr. Metab. Disord. 7, 51–65 [DOI] [PubMed] [Google Scholar]
- 26. Sallon C., Faure M. O., Fontaine J., and Taragnat C. (2010) Dynamic regulation of pituitary mRNAs for BMP-4, BMP receptors and activin/inhibin subunits in the ewe during the oestrous cycle and in cultured pituitary cells. J. Endocrinol. 207, 55–65 [DOI] [PubMed] [Google Scholar]
- 27. Herrera B., and Inman G. J. (2009) A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP-4, BMP-6 and BMP9 in bovine and human serum. BMC Cell Biol. 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Logeart-Avramoglou D., Bourguignon M., Oudina K., Ten Dijke P., and Petite H. (2006) An assay for the determination of biologically active bone morphogenetic proteins using cells transfected with an inhibitor of differentiation promoter-luciferase construct. Anal. Biochem. 349, 78–86 [DOI] [PubMed] [Google Scholar]
- 29. Myszka D. G. (1999) Improving biosensor analysis. J. Mol. Recognit. 12, 279–284 [DOI] [PubMed] [Google Scholar]
- 30. Keller A., Nesvizhskii A. I., Kolker E., and Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 [DOI] [PubMed] [Google Scholar]
- 31. Nesvizhskii A. I., Keller A., Kolker E., and Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 [DOI] [PubMed] [Google Scholar]
- 32. Söding J., Biegert A., and Lupas A. N. (2005) The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244–W248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Webb B., and Sali A. (2014) Protein structure modeling with MODELLER. Methods Mol. Biol. 1137, 1–15 [DOI] [PubMed] [Google Scholar]
- 34. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., and Ferrin T. E. (2004) UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 35. Adams J. C. (1997) Thrombospondin-1. Int. J. Biochem. Cell Biol. 29, 861–865 [DOI] [PubMed] [Google Scholar]
- 36. Yu P. B., Hong C. C., Sachidanandan C., Babitt J. L., Deng D. Y., Hoyng S. A., Lin H. Y., Bloch K. D., and Peterson R. T. (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 4, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang J. L., Huang Y., Qiu L. Y., Nickel J., and Sebald W. (2007) von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J. Biol. Chem. 282, 20002–20014 [DOI] [PubMed] [Google Scholar]
- 38. Brazil D. P., Church R. H., Surae S., Godson C., and Martin F. (2015) BMP signalling: agony and antagony in the family. Trends Cell Biol. 25, 249–264 [DOI] [PubMed] [Google Scholar]
- 39. Zhang J. L., Qiu L. Y., Kotzsch A., Weidauer S., Patterson L., Hammerschmidt M., Sebald W., and Mueller T. D. (2008) Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev. Cell. 14, 739–750 [DOI] [PubMed] [Google Scholar]
- 40. Constam D. B., and Robertson E. J. (1999) Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J. Cell Biol. 144, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bornstein P. (1995) Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J. Cell Biol. 130, 503–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Resovi A., Pinessi D., Chiorino G., and Taraboletti G. (2014) Current understanding of the thrombospondin-1 interactome. Matrix Biol. 37, 83–91 [DOI] [PubMed] [Google Scholar]
- 43. Schultz-Cherry S., Chen H., Mosher D. F., Misenheimer T. M., Krutzsch H. C., Roberts D. D., and Murphy-Ullrich J. E. (1995) Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin 1. J. Biol. Chem. 270, 7304–7310 [DOI] [PubMed] [Google Scholar]
- 44. Sweetwyne M. T., and Murphy-Ullrich J. E. (2012) Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biol. 31, 178–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zimmerman L. B., De Jesús-Escobar J. M., and Harland R. M. (1996) The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86, 599–606 [DOI] [PubMed] [Google Scholar]
- 46. Song K., Krause C., Shi S., Patterson M., Suto R., Grgurevic L., Vukicevic S., van Dinther M., Falb D., Ten Dijke P., and Alaoui-Ismaili M. H. (2010) Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J. Biol. Chem. 285, 12169–12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weber D., Kotzsch A., Nickel J., Harth S., Seher A., Mueller U., Sebald W., and Mueller T. D. (2007) A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor. BMC Struct. Biol. 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adams J. C., and Lawler J. (2004) The thrombospondins. Int. J. Biochem. Cell Biol. 36, 961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adams J. C., and Lawler J. (2011) The thrombospondins. Cold Spring Harb. Perspect. Biol. 3, a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young G. D., and Murphy-Ullrich J. E. (2004) The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-β complex. J. Biol. Chem. 279, 47633–47642 [DOI] [PubMed] [Google Scholar]
- 51. Sarkar A. J., Chaturvedi K., Chen C. P., and Sarkar D. K. (2007) Changes in thrombospondin-1 levels in the endothelial cells of the anterior pituitary during estrogen-induced prolactin-secreting pituitary tumors. J. Endocrinol. 192, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lawler J., Sunday M., Thibert V., Duquette M., George E. L., Rayburn H., and Hynes R. O. (1998) Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J. Clin. Invest. 101, 982–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ye L., and Jiang W. G. (2016) Bone morphogenetic proteins in tumour associated angiogenesis and implication in cancer therapies. Cancer Lett. 380, 586–597 [DOI] [PubMed] [Google Scholar]
- 54. Recouvreux M. V., Camilletti M. A., Rifkin D. B., Becu-Villalobos D., and Díaz-Torga G. (2012) Thrombospondin-1 (TSP-1) analogs ABT-510 and ABT-898 inhibit prolactinoma growth and recover active pituitary transforming growth factor-β1 (TGF-β1). Endocrinology 153, 3861–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sid B., Sartelet H., Bellon G., El Btaouri H., Rath G., Delorme N., Haye B., and Martiny L. (2004) Thrombospondin 1: a multifunctional protein implicated in the regulation of tumor growth. Crit. Rev. Oncol. Hematol. 49, 245–258 [DOI] [PubMed] [Google Scholar]
- 56. Vailhé B., and Feige J. J. (2003) Thrombospondins as anti-angiogenic therapeutic agents. Curr. Pharm. Des. 9, 583–588 [DOI] [PubMed] [Google Scholar]
- 57. Pardali E., and Ten Dijke P. (2012) TGFβ signaling and cardiovascular diseases. Int. J. Biol. Sci. 8, 195–213 [DOI] [PMC free article] [PubMed] [Google Scholar]