Abstract
Synaptic strength at excitatory synapses is determined by the presence of glutamate receptors (i.e. AMPA, NMDA, and kainate receptors) at the synapse. Synaptic strength is modulated by multiple factors including assembly of different receptor subunits, interaction with auxiliary subunits, and post-translational modifications of either the receptors or their auxiliary subunits. Using mass spectrometry, we found that the intracellular region of neuropilin and tolloid-like proteins (Neto) 1 and Neto2, the auxiliary subunits of kainate receptor (KARs), are phosphorylated by multiple kinases in vitro. Specifically, Neto2 was phosphorylated at serine 409 (Ser-409) by Ca2+/calmodulin-dependent protein kinase II (CaMKII) and protein kinase A (PKA) both in vitro and in heterologous cells. Interestingly, we observed a substantial increase in Neto2 Ser-409 phosphorylation in the presence of CaMKII, and this phosphorylation was reduced in the presence of the KAR subunit GluK1 or GluK2. We also found endogenous phosphorylation of Neto2 at Ser-409 in the brain. Moreover, Neto2 Ser-409 phosphorylation inhibited synaptic targeting of GluK1 because, unlike WT Neto2 and the phosphodeficient mutant Neto2 S409A, the Neto2 S409D phosphomimetic mutant impeded GluK1 trafficking to synapses. These results support a molecular mechanism by which Neto2 phosphorylation at Ser-409 helps restrict GluK1 targeting to the synapse.
Keywords: Ca2+/calmodulin-dependent protein kinase II (CaMKII), glutamate receptor, phosphorylation, synapse, trafficking, GluK1, Neto2
Introduction
KARs2 are a class of ionotropic glutamate-type receptors involved in synaptic transmission and neuromodulation. They have been implicated in neurodegenerative and brain disorders such as epilepsy, autism, and schizophrenia. These receptors modulate synaptic transmission at multiple levels: during development, neurotransmitter release, and postsynaptic depolarization. It is the precise localization of the KARs that governs their role in neuronal function. A number of factors such as post-translational modifications, alternative splicing, assembly of different KAR subunits, and interaction with other proteins target KAR to different sites (1–3). Of the known KAR-interacting partners, Neto1 and Neto2 are characterized as auxiliary subunits of KARs (4, 5). Neto1 and Neto2, although widely distributed in the brain, show specific enrichment in certain brain regions (6), interact with different KAR subunits, and modulate receptor synaptic localization as well as biophysical properties. More specifically, Neto1 and Neto2 affect the channel desensitization and rectification properties of KARs and the synaptic trafficking in a subunit-dependent manner (7–11). Various studies from knock-out mice suggest that both KARs and Neto proteins are important for stabilizing one another at the synapse (4–6, 12, 13). Although an interdependence of Neto1/2 and KARs on synaptic localization has been shown, underlying molecular mechanisms still need to be understood. Neto1 and Neto2 are single-pass transmembrane proteins containing an extracellular region with two CUB domains, an LDL domain, and an intracellular C terminus. There are a few reports that highlight the function of these specific domains: CUB domains mediate interactions with KAR subunits (4), the LDL domain affects channel kinetics (7), and the intracellular domain is important both for modifying channel properties and for regulating synaptic abundance of KARs (7, 12).
In this study, we found that the intracellular region of Neto1 and Neto2 can be phosphorylated by various kinases in vitro. We characterized phosphorylation at one of the residues on Neto2, Ser-409, by different biochemical methods and found that this residue is phosphorylated by CaMKII and PKA. We also demonstrated endogenous phosphorylation of this residue in the brain. Additionally, we found that Neto2 inhibits synaptic targeting of GluK1 when phosphorylated at Ser-409. This is the first report showing that Neto1 and Neto2 can be phosphorylated and how Neto phosphorylation regulates synaptic targeting of KARs. Future studies on the other identified phosphorylation residues will further our understanding of KAR trafficking by Netos.
Results
The C-terminal tails of Neto1 and Neto2 are phosphorylated
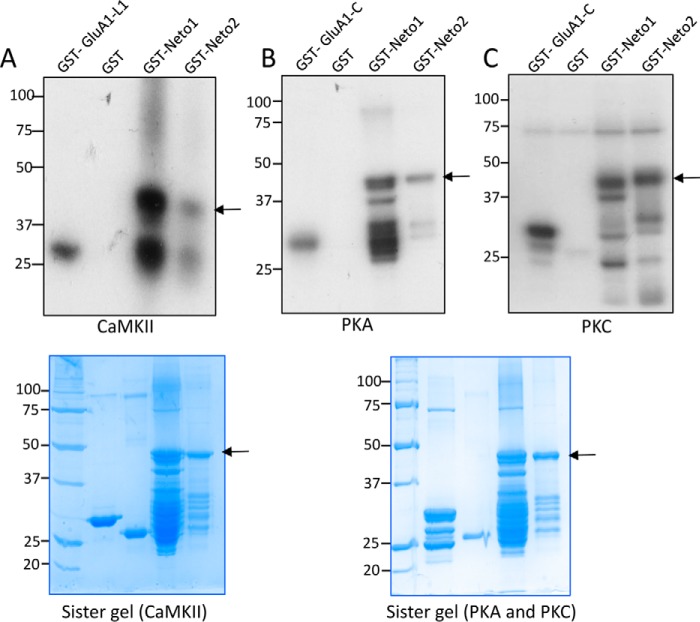
It is well established that the function and trafficking of synaptic proteins are modulated by different post-translational modifications such as phosphorylation. The intracellular C termini of Neto1 and Neto2 contain several serine and threonine residues that can be potentially phosphorylated. We began by testing whether Neto proteins can be phosphorylated in vitro. We expressed and purified the C termini as GST-Neto1 and GST-Neto2 fusion proteins and used these in an in vitro kinase assay. GST-Neto1 and GST-Neto2 were incubated with three different kinases, CaMKII, PKA, and PKC, along with [γ-32P]ATP in the kinase reaction buffer. The autoradiogram shows that the C termini of both Neto1 and Neto2 are phosphorylated by CaMKII, PKA, and PKC (Fig. 1, A–C).
Figure 1.
The intracellular C termini of Neto1 and Neto2 are phosphorylated. A–C, an in vitro kinase assay in which purified GST-Neto1 or GST-Neto2 C-terminal fusion protein was incubated with [γ-32P]ATP in the presence of the indicated kinase. The autoradiogram shows phosphorylated proteins. GST was used as a negative control; GST-GluA1 C-tail (C) and GST-GluA1 loop1 (L1) were used as positive controls for the indicated kinases. The lower panel shows the corresponding sister gels as visualized by Coomassie Brilliant Blue. The sister gel for the PKA and PKC reactions is the same. The arrow indicates the full-length GST-Neto C-terminal fusion proteins, and the lower bands are the degradation products generated during the purification process.
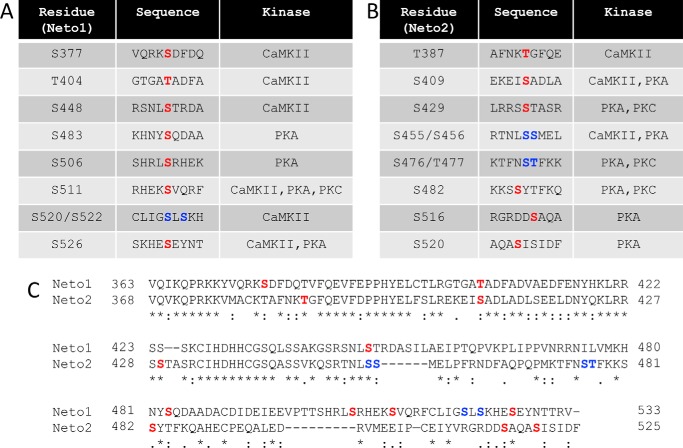
Subsequently, we identified the residues of Neto1 and Neto2 that can be phosphorylated by the specific kinases using mass spectrometry. We found that both Neto1 and Neto2 can be phosphorylated at multiple residues (highlighted in red) by different kinases (Fig. 2, A and B). In certain regions of the sequence, it was difficult to distinguish the specific residue due to the high abundance of Ser/Thr residues. For those cases, both residues are highlighted in blue. All three kinases, PKA, PKC, and CaMKII, phosphorylated multiple residues on both proteins. The C termini of Neto1 and Neto2 show a certain degree of sequence similarity, and we observed that some phosphorylated residues are common for both Neto1 and Neto2, and others are specific for each of the proteins (Fig. 2C).
Figure 2.
The C termini of Neto1 and Neto2 are phosphorylated at multiple residues. A and B, phosphorylated residues identified in the C termini of Neto1 (A) and Neto2 (B) by mass spectroscopy. Kinases phosphorylating the respective residues and residue numbers are indicated. Residues highlighted in red show specific phosphorylation at the particular residue (identified in at least two independent experiments); residues highlighted in blue are those residues at which one of the two residues is phosphorylated. C, sequence alignment of the C termini of Neto1 and Neto2 using Clustal Omega. Phosphorylated residues identified by mass spectroscopy are highlighted.
Neto2 is phosphorylated at Ser-409 by CaMKII and PKA
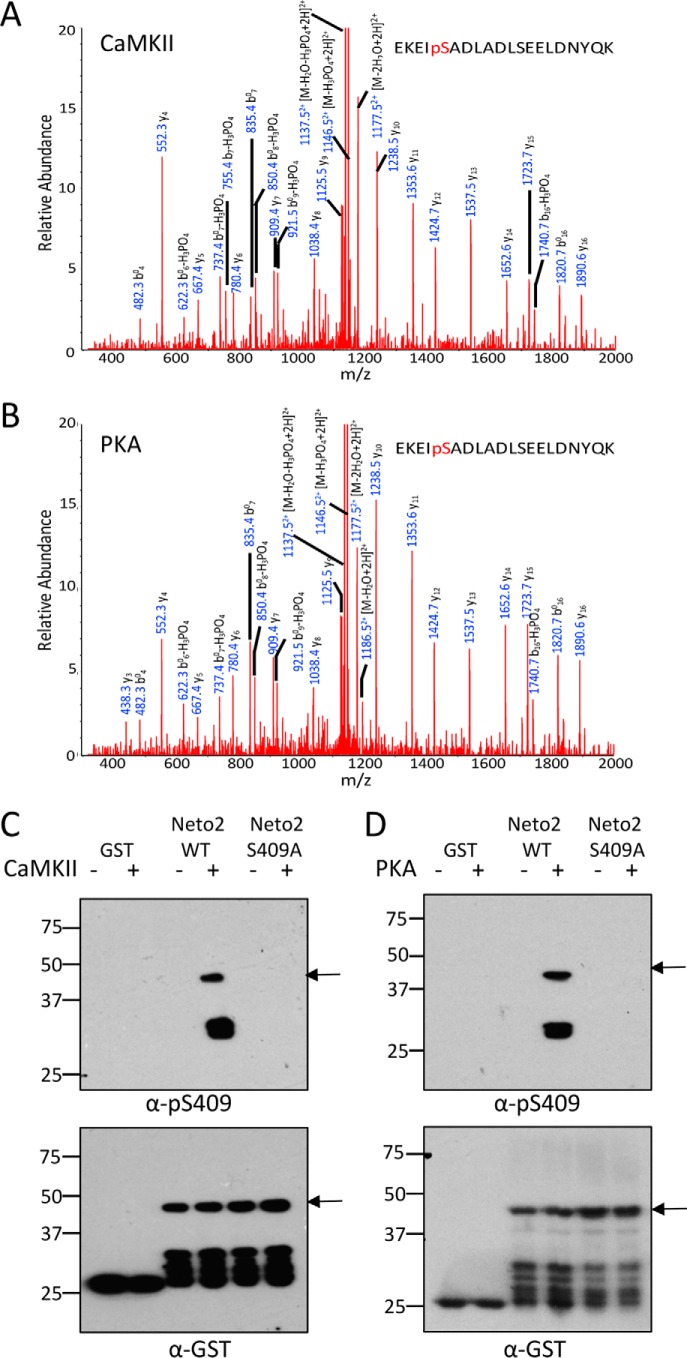
The mass spectrometry analysis shows that Neto2 is phosphorylated at Ser-409 by both CaMKII (Fig. 3A) and PKA (Fig. 3B). To evaluate the phosphorylation at Ser-409, we generated and characterized a phosphorylation state-specific antibody, Ser(P)-409. We mutated Ser-409 to Ala (S409A) and tested the specificity of the antibody in an in vitro kinase assay using purified GST-Neto2 (WT or S409A). Upon blotting with Ser(P)-409 antibody, a specific signal was observed only with GST-Neto2 WT when it was incubated with either CaMKII (Fig. 3C) or PKA (Fig. 3D). This experiment showed the specificity of the custom Ser(P)-409 antibody along with confirming that Neto2 is phosphorylated at Ser-409 directly by CaMKII and PKA. Subsequently, we tested the effect of CaMKII or PKA on full-length Neto2. We co-transfected COS-7 cells with Neto2 and a constitutively active mutant of CaMKII (T286D) or treated Neto2-transfected cells with forskolin to activate PKA. We observed a substantial increase in Neto2 Ser-409 phosphorylation both in the presence of CaMKII T286D and with forskolin treatment in heterologous cells using Ser(P)-409 antibody (Fig. 4, A–D), demonstrating the CaMKII- and PKA-mediated phosphorylation of Neto2 Ser-409 in situ. We subsequently immunoprecipitated Neto2 from brain lysates of young WT animals (age P1–P2), which show a high expression of the protein (14), and confirmed that Neto2 is endogenously phosphorylated at Ser-409 in the brain by immunoblotting with the custom Ser(P)-409 antibody (Fig. 4E). Phosphorylation at Ser-409 has also been previously reported in two independent proteomic studies using HeLa cells and the brain (15, 16). In the study using HeLa cells, multiple phosphorylated peptides of Neto2 were identified, whereas only Ser-409 was detected to be phosphorylated from the brain tissue (Table 1).
Figure 3.
Neto2 is phosphorylated at Ser-409 by CaMKII and PKA. A and B, MS/MS spectra of ions at m/z 1195. 54 that correspond to the doubly charged EKEIpSADLADLSEELDNYQK peptide detected upon phosphorylation of GST-Neto2 C terminus by CaMKII (A) or PKA (B). C and D, in vitro kinase assay using GST-Neto2 (WT or S409A) in the presence of CaMKII (C) or PKA (D) demonstrating phosphorylation of Neto2 at Ser-409 in vitro. The Western blot was probed with Ser(P)-409 antibody generated in rabbit using the epitope REKEIpSADLA. The lower panel shows the same blot probed with anti-GST to confirm the presence of proteins. GST was used as a negative control. Arrows indicate the full-length GST-Neto C termini fusion proteins.
Figure 4.
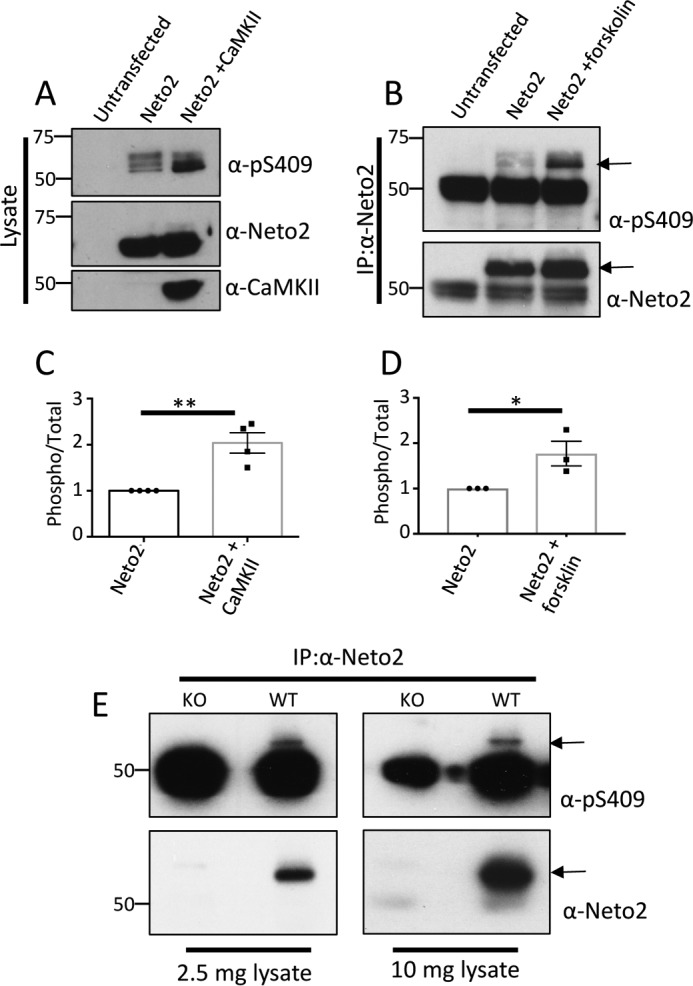
Neto2 is phosphorylated at Ser-409 in situ and in vivo. A, full-length Neto2 was expressed in COS-7 cells with or without CaMKII (T286D), and the lysate was probed for Ser-409 phosphorylation using Western blotting with the Ser(P)-409 antibody. B, full-length Neto2 was expressed in COS-7 cells, treated with 20 μm forskolin for 30 min, and probed for Ser-409 phosphorylation using the Ser(P)-409 antibody after immunoprecipitation with Neto2 antibody. Arrows indicate the corresponding bands for phosphorylated or total Neto2. The lower band observed is IgG heavy chain. C and D, mean ± S.E. of phosphorylated Neto2 in the presence of CaMKII (n = 4; **, p < 0.01) or forskolin (n = 3; *, p < 0.05). Quantitation was done by densitometry, and a ratio of phosphorylated/total Neto2 is plotted. Error bars represent S.E. E, detergent-solubilized brain lysate from WT pups (P1–P2) was immunoprecipitated (IP) with Neto2 antibody and immunoblotted with Ser(P)-409 antibody. Neto2 knock-out brain lysate was used as a control. Arrows indicate the corresponding band for phosphorylated/total Neto2. The lower band observed is IgG heavy chain.
Table 1.
Different phosphorylated (ph) peptides of Neto2 identified in the reported proteomic screens
Neto2 Ser-409 phosphorylation is affected by KAR expression
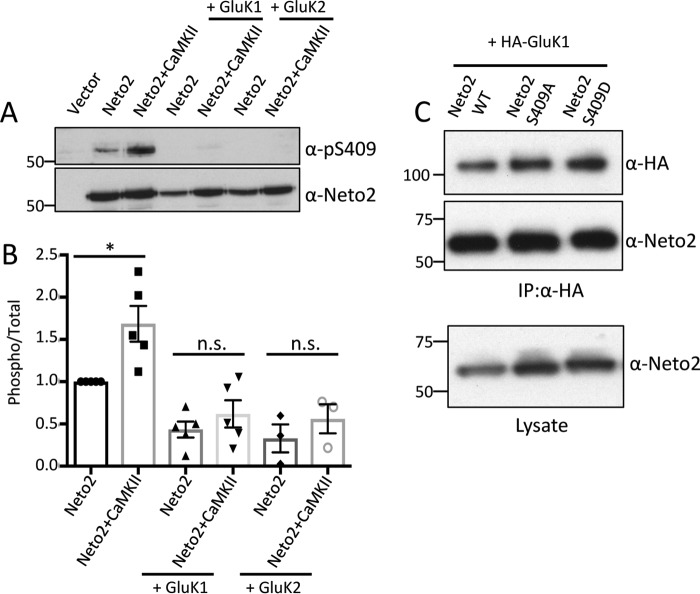
Neto2 is an important auxiliary subunit of KARs (5) and affects both the channel gating and synaptic targeting properties of different subunits of KARs. Therefore, we tested whether Neto2 Ser-409 phosphorylation is modulated in the presence of KARs. Although we found that Neto2 Ser-409 phosphorylation increased considerably in the presence of CaMKII T286D, no such increase was observed when either KAR subunit GluK1 or GluK2 was co-expressed (Fig. 5, A and B). We probed with the respective antibodies to confirm that both Neto2 and KAR subunits are being expressed and found no apparent difference in their expression under different conditions (supplemental Fig. S1). We subsequently tested whether binding between GluK1 and Neto2 is affected by this phosphorylation. However, we did not find any differences in the binding ability of Neto2 WT; Neto2 S409A, the phosphodeficient mutant; or Neto2 S409D, the phosphomimetic mutant (Fig. 5C).
Figure 5.
Neto2 Ser-409 phosphorylation is reduced upon co-expression with KARs. A, Neto2, CaMKII (T286D), and the indicated KAR subunit (HA-GluK1 or GluK2) were expressed in COS-7 cells and probed with Ser(P)-409 antibody. The blots were also probed with Neto2 antibody to determine total Neto2 amounts. B, mean ± S.E. (n = 5 for GluK1 and n = 3 for GluK2) of phosphorylated Neto2. Quantitation was done by densitometry, and a ratio of phosphorylated/total Neto2 is plotted (*, p < 0.05; n.s., non-significant). Error bars represent S.E. C, HA-GluK1 and Neto2 (WT or mutants) were co-expressed. The upper panel shows the immunoprecipitated (IP) GluK1 and the bound Neto2 using HA and Neto2 antibodies, respectively. Neto2 expression for different mutants was tested in the lysate alone (lower panel).
Neto2 Ser-409 phosphorylation inhibits synaptic targeting of KAR
We have previously shown that Neto2 induces synaptic targeting of GluK1 (9), and now we have demonstrated that Neto2 is phosphorylated at Ser-409 in the brain. So, we next tested whether phosphorylation of Neto2 Ser-409 affects KAR-mediated synaptic transmission. We co-expressed GluK1 and Neto2 (WT, S409A, or S409D) in CA1 neurons of rat hippocampal slices through biolistic transfection. Subsequently, we measured synaptic responses at −70 mV by dual whole-cell recordings. As previously reported, we found a substantial increase in GluK1 synaptic responses in the presence of Neto2 WT (Fig. 6A). Co-expression of GluK1 with the phosphodeficient mutant Neto2 S409A also showed a similar increase in synaptic responses (Fig. 6B). By contrast, there was significantly less enhancement of synaptic currents when co-expressed with Neto2 S409D, the phosphomimetic mutant (Fig. 6, C and D). We have previously shown that Neto2 itself does not regulate AMPAR-mediated synaptic transmission (9). Taken together, these results show that when Neto2 is phosphorylated at Ser-409 the synaptic targeting of GluK1 is inhibited.
Figure 6.
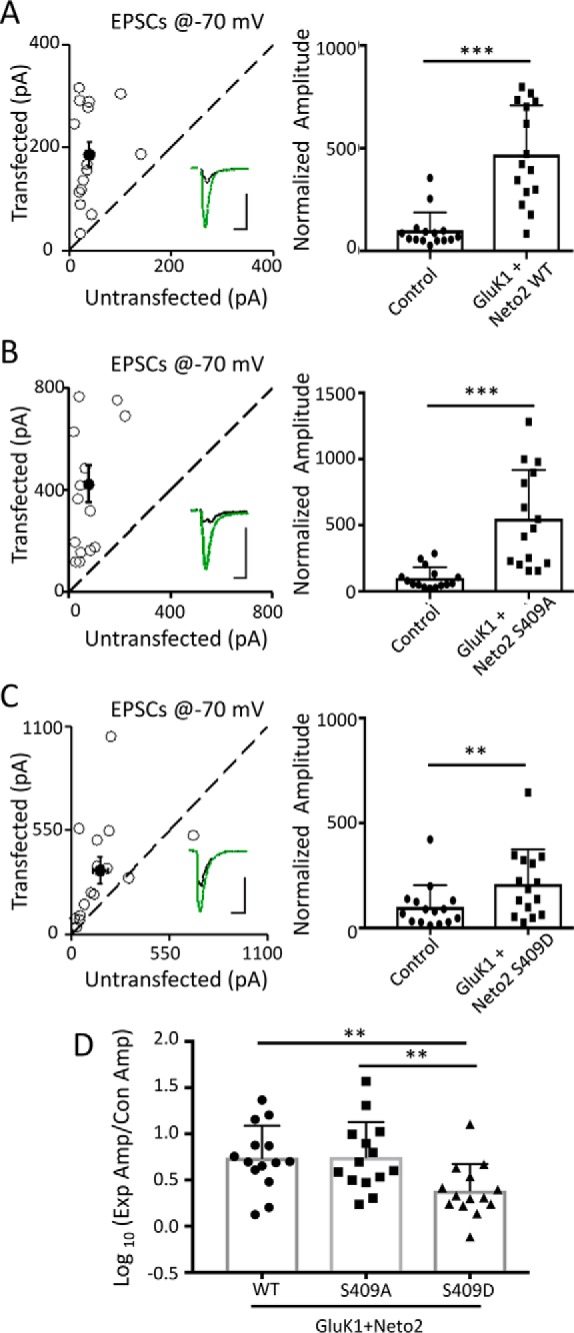
Neto2 Ser-409 phosphorylation inhibits synaptic targeting of GluK1 in CA1 pyramidal neurons. A–C, rat hippocampal slice cultures were biolistically transfected with GluK1/Neto2 (A), GluK1/Neto2 S409A (B), or GluK1/Neto2 S409D (C). Simultaneous dual whole-cell recordings from a transfected CA1 pyramidal neuron (green trace) and a neighboring wild-type neuron (black trace) were performed (n = 15 paired cells). The evoked EPSCs were measured at −70 mV. Open and filled circles (left panel) represent amplitudes for single pairs and mean ± S.E., respectively. Error bars represent S.E. Insets show sample current traces from control (black) and experimental (green) cells. The scale bars are 100 pA and 25 ms. Graphs show normalized evoked EPSC amplitudes (mean ± S.D.) of −70 mV pretreatment (A, 469.96 ± 61.41% control; ***, p < 0.0001; B, 549.91 ± 94.74% control; ***, p < 0.001; C, 209.38 ± 42.44% control; **, p < 0.01) presented in scatter plots. Error bars represent S.D. All the statistical analyses are compared with respective control neurons with two-tailed Wilcoxon signed rank sum test. D, logarithm summary of the evoked EPSC amplitude (Amp) ratio between the experimental (Exp) and respective control (Con) neurons (mean ± S.D.) for the above three transfections (GluK1/Neto2, 0.70 ± 0.09; GluK1/Neto2 S409A, 0.77 ± 0.09; GluK1/Neto2 S409D, 0.36 ± 0.08; GluK1/Neto2 versus GluK1/Neto2 S409A, p > 0.05; GluK1/Neto2 versus GluK1/Neto2 S409D; *, p < 0.05; GluK1/Neto2 S409A versus GluK1/Neto2 S409D; **, p < 0.005). Error bars represent S.D. The statistical analyses were done using a Mann-Whitney U test.
Discussion
Although there have been a number of studies showing that Neto1 and Neto2 interact with and regulate the trafficking properties of KARs, the precise mechanisms underlying this regulation are not fully understood. Our findings show a molecular mechanism by which phosphorylation of the Neto2 C terminus regulates GluK1 synaptic targeting. We have shown that the intracellular region of Neto1 and Neto2 can be phosphorylated by different kinases at specific residues in vitro. Furthermore, we have demonstrated that the Neto2 C terminus can be phosphorylated at Ser-409 by two different kinases, CaMKII and PKA, both of which are widely expressed in the brain and regulate synaptic plasticity by modulating multiple synaptic proteins including glutamate receptors (17). We further characterized Neto2 Ser-409 phosphorylation and found that it is significantly increased in the presence of constitutively active CaMKII or PKA activated by forskolin treatment. We were able to confirm endogenous phosphorylation of Neto2 Ser-409 in the brain, suggesting a potential role in vivo. Interestingly, we observed reduced phosphorylation of this residue in the presence of CaMKII when Neto2 was co-expressed with either GluK1 or GluK2. It is possible that the reduced Neto2 Ser-409 phosphorylation observed in the presence of KAR subunits is due to competition with KAR subunits, which are also substrates for different kinases (18–20). We tested using different mutants whether phosphorylation has any effect on binding with GluK1 but found no difference. This is possibly because the binding between the two proteins is spread through a large interface involving multiple domains of Neto2 (12) and thus may not be easy to alter. Importantly, we found that Neto2 Ser-409 phosphorylation affects synaptic targeting of GluK1. Upon co-expression of GluK1 with Neto2 S409D, a phosphomimetic mutation, we found reduced GluK1 at the synapses. The remaining enhancement seen with Neto2 S409D is presumably due to an incomplete blockade of synaptic trafficking, although we cannot completely exclude the possibility of some unrelated mechanism.
We, along with others, have previously shown that Neto2 is essential for synaptic targeting of GluK1 to synapses (9, 21). It has been reported that Neto2 interacts with the scaffolding protein glutamate receptor-interacting protein and regulates synaptic localization of KARs (12) and that deletion of the C terminus of either Neto1 or Neto2 abrogates synaptic targeting of GluK1 (9). It is also well established that Neto2 can directly interact with GluK1 (21), and Neto2 affects the levels of synaptic KARs and vice versa; however, no underlying mechanism has been described.
Analogous to the role Netos play in regulating KARs, TARPs are AMPAR auxiliary subunits whose role in modulating AMPAR trafficking and gating has been characterized extensively. It has been shown that CaMKII-mediated phosphorylation of the intracellular C termini of TARPs regulates AMPAR-mediated synaptic transmission (22, 23). Additionally, PKA-mediated phosphorylation of the C terminus of stargazin, one of the TARPs, is known to inhibit its binding to the scaffolding protein PSD-95, and the corresponding phosphomimetic mutant reduces synaptic AMPARs (24, 25). It has also been shown that the phosphomimetic mutant of stargazin alters the NMDAR-dependent long-term depression component of synaptic plasticity, whereas wild-type or phosphodeficient stargazin did not have any effect (26).
Our observation that Neto2 Ser-409 phosphorylation alters synaptic targeting of GluK1 explains such a molecular mechanism for KARs by which phosphorylation of auxiliary subunits affects receptor trafficking. Our observations of less GluK1 synaptic targeting in the presence of Neto2 S409D and reduced Ser-409 phosphorylation in the presence of KAR subunits suggest that Neto2 phosphorylated at Ser-409 helps restrict KAR from the synapses due to altered trafficking. Thus, phosphorylation of Neto2 Ser-409 is a possible regulatory mechanism adopted for restricting GluK1 from synapses. In conclusion, this is the first report showing a molecular mechanism by which Neto2 phosphorylation might control KAR synaptic content. Additionally, we identified multiple other phosphorylation sites in the C termini of Neto1 and Neto2, and deciphering their role will further our understanding of KAR synaptic regulation by Netos.
Experimental procedures
Constructs and antibodies
HA-GluK1 and full-length Neto2 were generated as described previously (9). GluK2 in pRK5 mammalian expression vector was obtained as a gift from Dr. P. Seeburg. GST-GluA1 loop1 and GST-GluA1 C-tail have been described previously (27). C-terminal tails of mouse Neto1 (amino acids 363–533) and rat Neto2 (amino acids 368–525) were cloned into pGEX4T1 in EcoRI and NotI sites to generate GST fusion proteins. Point mutations were generated using a QuikChange mutagenesis kit (Agilent Technologies), and the positive clone was confirmed by sequencing. Different antibodies used in the study were as follows: α-GST (A190-122A, Bethyl Laboratories; 1:25,000), α-CaMKII (C6974, Thermo Scientific; 1:1000), α-HA clone 3F10 (Roche Applied Science), α-GluK2 clone NL9 (Millipore), α-Neto2 (for immunoblotting, HPA013180, Sigma; 1:1000; for immunoprecipitation and experiments from the brain, ab109288, Abcam). The phosphorylation state-specific antibody was generated by New England Peptide. Rabbits were immunized with the synthetic phosphopeptide Ac-REKEIpSADLA-amide (where pS is phosphoserine). Serum was collected and affinity-purified using a column immobilized with the phosphopeptide as the antigen. For immunoblotting with this antibody, the membranes were blocked in 5% PhosphoBLOCKER (Cell Biolabs, Inc.) at room temperature for 1 h and incubated with the primary antibody at 4 °C overnight at a concentration of 1 μg/μl and secondary antibody at room temperature for 1 h, both diluted in 1% PhosphoBLOCKER.
Protein purification
The protein was expressed in Escherichia coli BL21 cells by induction with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside at 37 °C for 3 h. The induced bacterial pellet was resuspended in buffer (20 mm Tris-Cl, pH 8.0, 150 mm NaCl, 5 mm EDTA, 100 mm DTT, and protease inhibitors). To lyse the bacterial cells, they were sonicated followed by incubation with 1% Triton X-100 for 45 min at 4 °C. Subsequently, the lysate was centrifuged at 18,000 × g for 30 min at 4 °C to separate the soluble protein, which was then incubated with glutathione-Sepharose 4B beads (Amersham Biosciences) for 2 h at 4 °C. The nonspecific protein was removed by washes with PBS. The protein bound to beads was resuspended as a 50:50 slurry in PBS containing protease inhibitors.
In vitro kinase assay
The in vitro kinase assays were performed as described previously (28). The protein bound on the glutathione beads was incubated with the respective kinases in reaction buffer at 30 °C for 30 min. For PKA, reactions were incubated in 10 mm HEPES, pH 7.0, 20 mm MgCl2, 50 μm ATP, and 1 pmol of [γ-32P]ATP (3,000 Ci mmol−1) with 50 ng of purified PKA catalytic subunit (Promega). For PKC, reactions were performed in 20 mm HEPES, pH 7.4, 1.67 mm CaCl2, 1 mm DTT, 10 mm MgCl2, 50 μm ATP, and 1 pmol of [γ-32P]ATP (3,000 Ci mmol−1) with 10 ng of purified PKC (Promega). No lipid activators of PKC were included in the assay. For CaMKII, the assay was performed in 20 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 0.5 mm DTT, 0.1 mm EDTA, 2.4 μm calmodulin, 2 mm CaCl2, 100 μm ATP, and 1 pmol of [γ-32P]ATP (3,000 Ci mmol−1) with 25 ng of recombinant CaMKII-α (Calbiochem). The reaction was terminated by boiling samples in SDS sample buffer at 95 °C for 10 min. The proteins were resolved by SDS-PAGE and visualized by autoradiography. For samples analyzed by immunoblotting, the proteins were transferred onto PVDF membranes and probed by the indicated antibodies. For identifying phosphorylated residues by mass spectrometry, gels were stained with Coomassie Brilliant Blue, and the respective protein band was excised for further analysis.
Mass spectrometry
The in-gel samples were reduced with tris(2-carboxyethyl)phosphine, alkylated with N-ethylmaleimide, and digested with trypsin overnight at 37 °C. Tryptic peptides were desalted first and analyzed on an Orbitrap Elite mass spectrometer (Thermo Scientific) coupled to an Ultimate 3000 HPLC (Thermo-Dionex) via an Easy-Spray ion source (Thermo Scientific). Peptides were separated on an ES802 Easy-Spray column (Thermo Scientific; 75-μm inner diameter, 25-cm length, 3-μm C18 beads). A 40-min linear gradient of 2–27% mobile phase B (mobile phase A, 2% acetonitrile and 0.1% formic acid; mobile phase B, 98% acetonitrile and 0.1% formic acid) was used. The flow rate was set at 300 nl/min. The Thermo Scientific Orbitrap Elite mass spectrometer was operated in positive nanoelectrospray mode. The resolution of the survey scan was set at 60,000 at m/z 400 with a target value of 1 × 106 ions. The m/z range for MS scans was 300–1600. The collision-induced dissociation MS/MS data were acquired in data-dependent mode. The top 10 most abundant ions were selected for product ion analysis. The isolation window for MS/MS fragmentation was set to 2.0 Da. Dynamic exclusion was 12 s, and early expiration was enabled. Xcalibur RAW files were converted to peak list files in mgf format using Mascot Distiller (version 2.5.1). The database search was performed using Mascot Daemon (2.5.1) against a house-built database that contains the Neto1 and Neto 2 sequences and the NCBInr human database (10,962 sequences; 5,416,110 residues). The following database search parameters were used: enzyme, semitrypsin; variable modifications, N-ethylmaleimide (Cys), oxidation (Met), phospho (Ser/Thr), phospho (Tyr); peptide mass tolerance, ±10 ppm; fragment mass tolerance, ±0.3 Da. The MS/MS spectra of all phosphopeptides detected in the database search were manually checked. The phosphorylation sites listed were identified in at least two independent experiments. The sites identified only in one experiment have not been highlighted.
Phosphorylation in heterologous cells
COS-7 cells were transfected with the indicated plasmids using Lipofectamine 2000 reagent (Invitrogen). 40 h after transfection, cells were washed in PBS, lysed directly with SDS-PAGE sample buffer, sonicated, and boiled at 95 °C for 10 min. The proteins were resolved by SDS-PAGE and subjected to Western blotting using the indicated antibodies. For testing change in phosphorylation upon forskolin treatment (20 μm; 30 min; 37 °C), Neto2 was immunoprecipitated using Neto2 antibody to enrich the protein. The amount of phosphorylation in different conditions was quantitated by densitometry using ImageJ. Statistical significance was calculated using an unpaired t test.
Phosphorylation in brain
Pups from WT animals (P1–P2) were dissected, and the brains were homogenized in buffer containing protease and phosphatase inhibitors. The crude synaptosomal fraction was isolated and detergent-solubilized in 1% Triton X-100 as described previously (28). Different concentrations of the P2 solubilized fraction were immunoprecipitated with anti-Neto2 antibody, and the pulled down complexes were immunoblotted with the indicated antibodies.
Binding between GluK1 and Neto2
HA-GluK1 and Neto2 (WT or mutants) were co-expressed in HEK cells using PEI. The lysates were immunoprecipitated with HA-agarose (Sigma), and the bound Neto2 was detected by probing with Neto2 antibody. Immunoprecipitation of GluK1 was confirmed by probing with HA antibody.
Electrophysiology
Organotypic hippocampal slice cultures were made from P6–P8 rats. All experiments were performed in accordance with established protocols approved by the University of California San Francisco Institutional Animal Care and Use Committee. Transfections were carried out on day in vitro 2 after culturing using a Helios Gene Gun (Bio-Rad) with 1-μm DNA-coated gold particles. When biolistically expressing two plasmids, gold particles were coated with equal amounts of each plasmid, and plasmids always expressed different fluorescent markers. Observed frequency of co-expression was nearly 100%. Slices were maintained at 34 °C with changes of media every other day. On day in vitro 8, dual whole-cell recordings in the CA1 area were done by simultaneously recording responses from a fluorescent transfected neuron as well as a neighboring untransfected control neuron. Pyramidal neurons were identified by morphology and location. Series resistance was monitored online, and recordings in which series increased to >30 megaohms or varied by >50% between neurons were discarded. Dual whole-cell recordings measuring evoked EPSCs used artificial cerebrospinal fluid, bubbled with 95% O2 and 5% CO2, consisting of 119 mm NaCl, 2.5 mm KCl, 4 mm CaCl2, 4 mm MgSO4, 1 mm NaH2PO4, 26.2 mm NaHCO3, and 11 mm glucose. 100 μm picrotoxin was added to block inhibitory currents, and 4 μm 2-chloroadenosine was used to control epileptiform activity. The intracellular solution contained 135 mm CsMeSO4, 8 mm NaCl, 10 mm HEPES, 0.3 mm EGTA, 5 mm QX314-Cl, 4 mm MgATP, 0.3 mm Na3GTP, and 0.1 mm spermine. A bipolar stimulation electrode was placed in the stratum radiatum, and responses were evoked at 0.2 Hz. As the ratio of synaptic current amplitudes between experimental and control neurons is independent of the stimulation intensity (29), any evoking intensity was applied only when stable synaptic currents were observed. Peak AMPAR and KAR currents were recorded at −70 mV. All these data were analyzed offline with custom software (Igor Pro, Wavemetrics). Responses were collected with a Multiclamp 700A amplifier (Axon Instruments), filtered at 2 kHz, and digitized at 10 kHz. Significance of evoked dual whole-cell recordings compared with controls was determined using the two-tailed Wilcoxon signed rank sum test. For all experiments involving unpaired data, a Mann-Whitney U test with Bonferroni correction for multiple comparisons was used. Data analysis was carried out in Igor Pro, Excel (Microsoft), and GraphPad Prism (GraphPad Software).
Author contributions
R. M. L. performed all biochemistry experiments and data analysis. R. M. L. and K. W. R. wrote the manuscript. Y. L. performed and analyzed all mass spectrometry experiments. N. S. performed and analyzed all electrophysiology experiments. K. W. R. and R. A. N. helped design experiments and supervised the project.
Supplementary Material
Acknowledgments
We thank John D. Badger II for technical assistance. We thank members of the Roche laboratory for helpful discussions. We also acknowledge the laboratories of Roderick R. McInnes and Michael W. Salter for generating the Neto2 knock-out animals and the laboratory of Chris J. McBain for breeding the Neto2 knock-out animals.
This work was supported by the National Institutes of Health NINDS Intramural Research Program (to R. M. L., Y. L., and K. W. R.), National Institute of Mental Health Grants R01MH080379-09 and R01MH070957-11A1 (to N. S. and R. A. N.), and National Institutes of Health Grant ZIA-NS002994-15. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Fig. S1.
- KAR
- kainate receptor
- Neto
- neuropilin and tolloid-like protein
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- AMPAR
- AMPA receptor
- TARP
- transmembrane AMPA receptor regulatory protein
- P
- postnatal day
- EPSC
- excitatory postsynaptic current
- CUB domain
- complement C1r/C1s, Uegf, Bmp1.
References
- 1. Jaskolski F., Coussen F., Nagarajan N., Normand E., Rosenmund C., and Mulle C. (2004) Subunit composition and alternative splicing regulate membrane delivery of kainate receptors. J. Neurosci. 24, 2506–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hayes D. M., Braud S., Hurtado D. E., McCallum J., Standley S., Isaac J. T., and Roche K. W. (2003) Trafficking and surface expression of the glutamate receptor subunit, KA2. Biochem. Biophys. Res. Commun. 310, 8–13 [DOI] [PubMed] [Google Scholar]
- 3. Palacios-Filardo J., Aller M. I., and Lerma J. (2016) Synaptic targeting of kainate receptors. Cereb. Cortex 26, 1464–1472 [DOI] [PubMed] [Google Scholar]
- 4. Tang M., Pelkey K. A., Ng D., Ivakine E., McBain C. J., Salter M. W., and McInnes R. R. (2011) Neto1 is an auxiliary subunit of native synaptic kainate receptors. J. Neurosci. 31, 10009–10018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang W., St-Gelais F., Grabner C. P., Trinidad J. C., Sumioka A., Morimoto-Tomita M., Kim K. S., Straub C., Burlingame A. L., Howe J. R., and Tomita S. (2009) A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron 61, 385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Straub C., Hunt D. L., Yamasaki M., Kim K. S., Watanabe M., Castillo P. E., and Tomita S. (2011) Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat. Neurosci. 14, 866–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisher J. L., and Mott D. D. (2012) The auxiliary subunits Neto1 and Neto2 reduce voltage-dependent inhibition of recombinant kainate receptors. J. Neurosci. 32, 12928–12933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Straub C., Zhang W., and Howe J. R. (2011) Neto2 modulation of kainate receptors with different subunit compositions. J. Neurosci. 31, 8078–8082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheng N., Shi Y. S., Lomash R. M., Roche K. W., and Nicoll R. A. (2015) Neto auxiliary proteins control both the trafficking and biophysical properties of the kainate receptor GluK1. Elife 4, e11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang W., Devi S. P., Tomita S., and Howe J. R. (2014) Auxiliary proteins promote modal gating of AMPA- and kainate-type glutamate receptors. Eur. J. Neurosci. 39, 1138–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fisher J. L. (2015) The auxiliary subunits Neto1 and Neto2 have distinct, subunit-dependent effects at recombinant GluK1- and GluK2-containing kainate receptors. Neuropharmacology 99, 471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang M., Ivakine E., Mahadevan V., Salter M. W., and McInnes R. R. (2012) Neto2 interacts with the scaffolding protein GRIP and regulates synaptic abundance of kainate receptors. PLoS One 7, e51433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wyeth M. S., Pelkey K. A., Petralia R. S., Salter M. W., McInnes R. R., and McBain C. J. (2014) Neto auxiliary protein interactions regulate kainate and NMDA receptor subunit localization at mossy fiber-CA3 pyramidal cell synapses. J. Neurosci. 34, 622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vernon C. G., and Swanson G. T. (2017) Neto2 assembles with kainate receptors in DRG neurons during development and modulates neurite outgrowth in adult sensory neurons. J. Neurosci. 37, 3352–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huttlin E. L., Jedrychowski M. P., Elias J. E., Goswami T., Rad R., Beausoleil S. A., Villén J., Haas W., Sowa M. E., and Gygi S. P. (2010) A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mertins P., Qiao J. W., Patel J., Udeshi N. D., Clauser K. R., Mani D. R., Burgess M. W., Gillette M. A., Jaffe J. D., and Carr S. A. (2013) Integrated proteomic analysis of post-translational modifications by serial enrichment. Nat. Methods 10, 634–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lussier M. P., Sanz-Clemente A., and Roche K. W. (2015) Dynamic regulation of N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by posttranslational modifications. J. Biol. Chem. 290, 28596–28603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nasu-Nishimura Y., Jaffe H., Isaac J. T., and Roche K. W. (2010) Differential regulation of kainate receptor trafficking by phosphorylation of distinct sites on GluR6. J. Biol. Chem. 285, 2847–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pahl S., Tapken D., Haering S. C., and Hollmann M. (2014) Trafficking of kainate receptors. Membranes 4, 565–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu J., Liu Z. A., Pei D. S., and Xu T. J. (2010) Calcium/calmodulin-dependent kinase II facilitated GluR6 subunit serine phosphorylation through GluR6-PSD95-CaMKII signaling module assembly in cerebral ischemia injury. Brain Res. 1366, 197–203 [DOI] [PubMed] [Google Scholar]
- 21. Copits B. A., Robbins J. S., Frausto S., and Swanson G. T. (2011) Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J. Neurosci. 31, 7334–7340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sumioka A., Yan D., and Tomita S. (2010) TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron 66, 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park J., Chávez A. E., Mineur Y. S., Morimoto-Tomita M., Lutzu S., Kim K. S., Picciotto M. R., Castillo P. E., and Tomita S. (2016) CaMKII phosphorylation of TARPγ-8 is a mediator of LTP and learning and memory. Neuron 92, 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chetkovich D. M., Chen L., Stocker T. J., Nicoll R. A., and Bredt D. S. (2002) Phosphorylation of the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 binding site of stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J. Neurosci. 22, 5791–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi J., Ko J., Park E., Lee J. R., Yoon J., Lim S., and Kim E. (2002) Phosphorylation of stargazin by protein kinase A regulates its interaction with PSD-95. J. Biol. Chem. 277, 12359–12363 [DOI] [PubMed] [Google Scholar]
- 26. Tomita S., Stein V., Stocker T. J., Nicoll R. A., and Bredt D. S. (2005) Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron 45, 269–277 [DOI] [PubMed] [Google Scholar]
- 27. Lussier M. P., Gu X., Lu W., and Roche K. W. (2014) Casein kinase 2 phosphorylates GluA1 and regulates its surface expression. Eur. J. Neurosci. 39, 1148–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bemben M. A., Shipman S. L., Hirai T., Herring B. E., Li Y., Badger J. D. 2nd, Nicoll R. A., Diamond J. S., and Roche K. W. (2014) CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses. Nat. Neurosci. 17, 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herring B. E., and Nicoll R. A. (2016) Kalirin and Trio proteins serve critical roles in excitatory synaptic transmission and LTP. Proc. Natl. Acad. Sci. U.S.A. 113, 2264–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.