Biochemistry. In the article “The 30-kDa C-terminal domain of the RecB protein is critical for the nuclease activity, but not the helicase activity, of the RecBCD enzyme from Escherichia coli” by Misook Yu, Jehanne Souaya, and Douglas A. Julin, which appeared in number 3, February 3, 1998, of Proc. Natl. Acad. Sci. USA (95, 981–986), the following correction should be noted. The symbols in the graph (Fig. 3C) were identified incorrectly in the manuscript. The corrected legend and graph with accompanying symbols are printed below.
Figure 3.
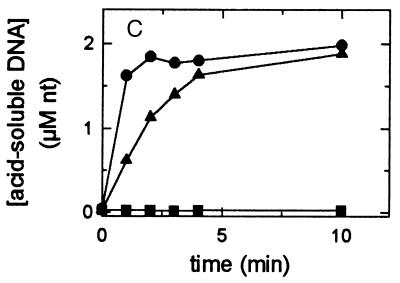
Nuclease assays. All reaction mixtures contained buffer A and 10 mM MgCl2. (A) ssDNA exonuclease assay with 100 nM single-stranded linear 5′-32P-labeled 25-mer as the substrate. (B) ssDNA endonuclease assay using 6 nM single-stranded circular M13 phage DNA as the substrate. (C) dsDNA exonuclease assay using AvaI-digested [3H]pTZ19R. Each reaction contained 2.3 μM (nucleotides) double-stranded [3H]pTZ19R, 250 μM ATP, and 9 nM RecB1–929CD (▪), 0.2 nM RecBCD (▴), or 9 nM RecBChD (•).