Abstract
The thyroid gland secretes primarily tetraiodothyronine (T4), and some triiodothyronine (T3). Under normal physiological circumstances, only one-fifth of circulating T3 is directly released by the thyroid, but in states of hyperactivation of thyroid-stimulating hormone receptors (TSHRs), patients develop a syndrome of relative T3 toxicosis. Thyroidal T4 production results from iodination of thyroglobulin (TG) at residues Tyr5 and Tyr130, whereas thyroidal T3 production may originate in several different ways. In this study, the data demonstrate that within the carboxyl-terminal portion of mouse TG, T3 is formed de novo independently of deiodination from T4. We found that upon iodination in vitro, de novo T3 formation in TG was decreased in mice lacking TSHRs. Conversely, de novo T3 that can be formed upon iodination of TG secreted from PCCL3 (rat thyrocyte) cells was augmented from cells previously exposed to increased TSH, a TSHR agonist, a cAMP analog, or a TSHR-stimulating antibody. We present data suggesting that TSH-stimulated TG phosphorylation contributes to enhanced de novo T3 formation. These effects were reversed within a few days after removal of the hyperstimulating conditions. Indeed, direct exposure of PCCL3 cells to human serum from two patients with Graves' disease, but not control sera, led to secretion of TG with an increased intrinsic ability to form T3 upon in vitro iodination. Furthermore, TG secreted from human thyrocyte cultures hyperstimulated with TSH also showed an increased intrinsic ability to form T3. Our data support the hypothesis that TG processing in the secretory pathway of TSHR-hyperstimulated thyrocytes alters the structure of the iodination substrate in a way that enhances de novo T3 formation, contributing to the relative T3 toxicosis of Graves' disease.
Keywords: post-translational modification (PTM), protein processing, protein secretion, thyroid, thyroid hormone, Graves' Disease, iodination, thyroglobulin
Introduction
In the body of vertebrate animals, thyroglobulin (TG)2 is the primary (if not exclusive) original source of thyroid hormones (1) that regulate central nervous system development and function, oxidative metabolism, thermogenesis, and body weight regulation, heart rate, cardiac output, LDL cholesterol levels, and other phenotypes (2, 3). The thyroid gland produces virtually 100% of the supply of l-thyroxine (T4) from the body. However, other than nongenomic actions (4), the main physiological effects of thyroid hormones are brought about by gene expression changes as a consequence of 3,3′,5-triiodo-l-thyronine (T3) interaction with nuclear thyroid hormone receptors (5).
Depending upon the species and conditions, there are somewhat differing views about the main sources of circulating T3. In otherwise normal thyroidectomized rats that are fully replaced with exogenous levothyroxine (i.e. normal serum T4), circulating T3 is decreased ∼55% (6) indicating a significant thyroidal contribution to circulating T3. In normal humans, classic studies have estimated that only ∼21% of daily T3 production is derived from thyroidal secretion (the rest coming from deiodination of T4 to T3 by deiodinases D1 and D2) (7). However, in patients with untreated Graves' disease (a disease of thyroidal hyperstimulation by TSH receptor-stimulating antibodies (8)), thyroid tissue is markedly enriched in T3 concurrent with increased T3 in the circulation (9, 10). Although some increased thyroidal T3 production in Graves' disease might be derived from intrathyroidal deiodination of T4 to T3 (11), the aforementioned study of untreated Graves' patients reported increased thyroid tissue T3 only after Pronase digestion (7). Moreover, mice with whole body D1/D2-double knock-out (DKO) nevertheless maintain normal circulating T3 levels (12). Taken together, these findings strongly imply that the thyroid gland has the capability to contribute importantly to circulating T3 via a mechanism involving de novo T3 formation, and this may be particularly important in Graves' disease.
The role of TG (a large homodimeric glycoprotein with a monomer molecular mass of 330 kDa and containing >2745 residues) in thyroid hormone synthesis is initiated upon its iodination (13, 14). Iodination is catalyzed by thyroid peroxidase, which provides the necessary oxidation to form diiodotyrosine (DIT) and monoiodotyrosine (MIT) within TG. Favored by these same oxidizing conditions, a coupling reaction involving a DIT acceptor residue and a corresponding DIT donor residue allows for the formation of T4 within the TG polypeptide; similarly, coupling of an MIT donor with a DIT acceptor allows for de novo T3 formation (15, 16). Classic studies report that thyroid peroxidase shows no marked specificity in its ability to catalyze TG iodination and coupling over that of lactoperoxidase or myeloperoxidase (16), whereas efficient T4 and T3 formation requires the TG substrate in its native conformation (17). Furthermore, despite ∼70 Tyr residues distributed broadly along the length of the protein, T4 and T3 formation are restricted to relatively few sites in TG, including an evolutionarily preferred DIT-DIT coupling of Tyr130–Tyr5 to yield T4 at position 5 (1) and a preferred T3 formation site at position 2746 of human TG (2744 of mouse TG, although the MIT coupling partner in either species remains unclear) (18, 19).
During its complex trafficking through the intracellular transport pathway of thyrocytes, TG undergoes considerable post-translational processing prior to its secretion and iodination (20). Many of these post-translational modifications are regulated indirectly by TSH-induced changes in the gene expression and activity of TG processing enzymes (21). Herein, we have examined de novo T3 formation within TG analyzed both from in vivo samples and after iodination in vitro. We directly demonstrate de novo T3 formation in TG and establish that this ability is directly related to the degree to which thyrocytes have been exposed to prior TSHR stimulation.
Results
De novo formation of T3 within TG
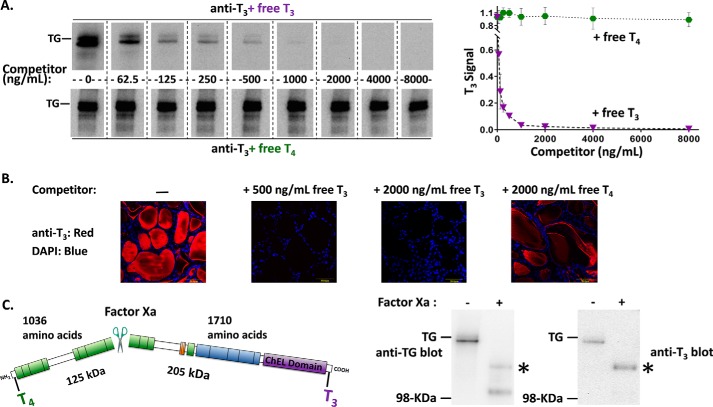
We developed a simple assay to detect the presence of T3 formation within thyroidal protein of euthyroid mice by immunoblotting using a mAb that recognizes T3 when contained within the TG protein backbone, in parallel with immunoblotting with a polyclonal antibody against TG. The addition of free T3 (half-maximal concentration ∼75 ng/ml) eliminated the immunoblotted mouse TG band with mAb anti-T3, whereas the addition of free T4 had little effect (Fig. 1A, blots at left; quantitation at right). Thyroidal immunofluorescence from euthyroid mice with mAb anti-T3 was distributed primarily in the follicle lumen where extracellular TG resides in “colloid”; this immunofluorescent signal was fully blocked by the addition of free T3 at 500 ng/ml but was only slightly diminished by free T4 even at 2000 ng/ml (Fig. 1B). Routinely, we added 500 ng/ml of free T4 in all immunoblotting experiments designed to detect T3 in TG, to ensure specificity. The data in Fig. 1 indicate that a basal level of T3 in TG is present under euthyroid (i.e. not TSH-hyperstimulated) conditions.
Figure 1.
T3 is enriched in the carboxyl-terminal region of TG. A, identical aliquots of normal mouse thyroid lysate (2 μg/lane) were loaded in every other lane, resolved by reducing SDS-PAGE and electrotransfer to nitrocellulose, and the membrane cut into strips. Each membrane strip was incubated with mAb anti-T3 (1:1000) plus an increasing concentration of either free T3 or free T4. The strips were then incubated simultaneously with identical HRP-conjugated goat anti-mouse antibody followed by enhanced chemiluminescence substrate, and then examined together in a single 20-s digital image exposure. Quantitation of the T3 signal within the TG band (gel shown at left; graph shown at right) represents the mean ± S.D. from 3 independent experiments. B, immunofluorescence with the same mAb anti-T3 (1:200) localizes T3-containing protein to the thyroid follicle lumen, and the immunofluorescence signal is specifically blocked upon addition of 500 ng/ml of free T3. All images are the same magnification; the yellow bar in each panel = 50 μm; nuclei are counterstained with DAPI. C, there is a single Factor Xa cleavage site in mouse TG that forms two predicted fragments. TG from normal mouse thyroid lysates ± digestion with Factor Xa was resolved by SDS-PAGE (4 μg of protein per lane), electrotransfer, and immunoblotting with anti-TG or anti-T3 antibodies. Two cleavage products of the expected size were detected with anti-TG; a polypeptide consistent with the C-terminal fragment (asterisk) was enriched in T3. Three independent experiments yielded results identical to those shown here. The position of a pre-stained molecular weight marker is indicated.
The primary sequence of mouse TG encodes a single predicted site for Factor Xa cleavage that excises the N-terminal one-third of the protein from the C-terminal two-thirds. As Dunn et al. (18) reported that the majority of T3 synthesized within TG is located in its C-terminal portion, we extracted mouse thyroid tissue and found by immunoblotting that a single band of intact Tg was cleaved to two fragments of the expected molecular mass after incubation with Factor Xa (Fig. 1C, left). Of these, the larger fragment corresponding to the C-terminal portion of TG was selectively enriched in T3 (Fig. 1C, right).
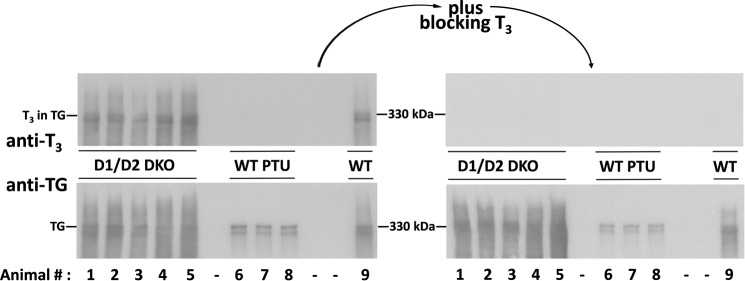
There are strong cell biological arguments to suggest that T3 contained within the TG protein does not result as a consequence of deiodination of T4 contained within TG. Specifically, almost all of the hormone-containing-TG is localized to the extracellular thyroid follicular lumen (Fig. 1B) (22), whereas the catalytic activities of the two enzymes responsible for T4 to T3 conversion (deiodinases D1 and D2) are topologically facing the cytosol (23). Indeed, we examined the T3 content of TG in the thyroids of animals devoid of D1 and D2 (12) and found that D1/D2-DKO mice had as much or more T3 contained within TG than in that from a wild-type reference animal (Fig. 2, left). All of the detected signal was derived from bona fide protein-bound T3 as it was completely blocked by the addition of free T3 added to the immunoblotting conditions (Fig. 2, right). As a negative control, TG obtained from PTU-treated mice contained no detectable T3 (Fig. 2 left). Together, these data provide strong support that de novo T3 formation within TG is independent of T4 deiodination.
Figure 2.
Evidence for de novo T3 formation within TG, independent of T4 deiodination. Gels at left: thyroid tissue homogenates from five different D1/D2-DKO mice, or wild-type C57BL/6 treated with PTU (WT PTU) to inhibit iodination in vivo, were analyzed by SDS-PAGE (4 μg of protein/lane), electrotransfer, and immunoblotting with anti-T3 (above) or anti-TG (below). Gels at right: the same samples immunoblotted in the presence of 500 ng/ml of free T3. Wild-type (WT) TG was run in lane 9 as a 330-kDa molecular mass marker.
TG secreted from thyrocytes after stimulation of TSHR is intrinsically more competent for de novo T3 formation
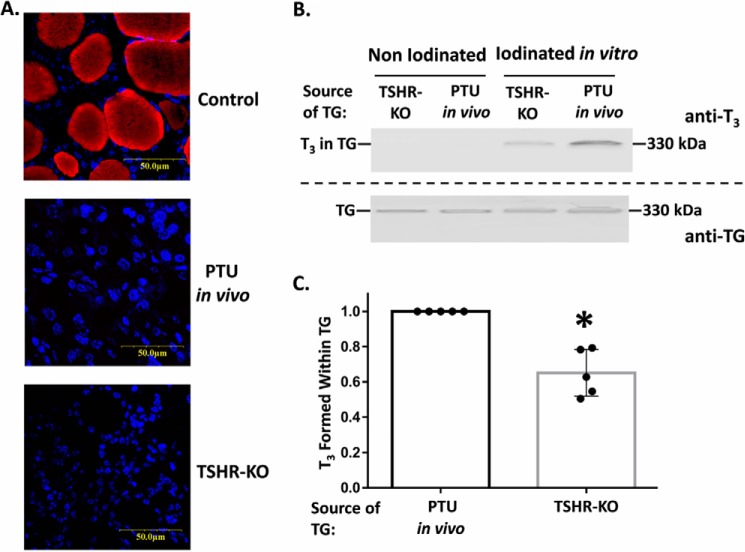
Iodination of TG is enhanced by TSH stimulation of the thyroid gland (21). However, independent of effects on sodium iodide symport activity, dual-function oxidase activity, or thyroid peroxidase activity, it has been hypothesized that the intrinsic ability of TG to form T3 may be modulated through prior thyroidal stimulation by TSH on its receptor (thyroid-stimulating hormone receptor (TSHR) (24)). To test this, we subjected the TG obtained from thyroids of TSHR-KO mice, and from PTU-treated WT mice (that develop hypothyroidism and a compensatory increase in TSH leading to stimulated TSHRs) to iodination under fixed conditions in vitro. In addition to eliminating any effects of TSHR stimulation on thyrocyte enzymes that promote TG iodination in vivo, the in vitro iodination method also normalizes for TG protein, and total protein, within each iodination reaction. Prior to the in vitro iodination reaction, neither thyroids from TSHR-KO nor PTU-treated mice exhibited detectable T3 within TG, as judged both by mAb anti-T3 immunofluorescence and immunoblotting (Fig. 3, A and B). This is precisely why, for this experiment, we did not examine TG from control mouse thyroid glands. After iodination in vitro, TG from TSHR-KO consistently exhibited less de novo T3 formation within TG (Fig. 3B, quantified in C).
Figure 3.
De novo T3 formation in TG from thyroid glands of mice with low and high TSHR activation states. A, thyroid tissue sections were examined by immunofluorescence (as in Fig. 1B) from WT mice (Control), or mice treated for 6 weeks with PTU in vivo to block thyroidal iodination and induce hypothyroidism with high TSH, or mice bearing genetic deletion of TSHR (TSHR-KO). Both of the latter sets of animals lacked immunofluorescently detectable T3, which is present in the thyroid gland of control mice. B, thyroid tissue extracts from TSHR-KO mice or mice rendered hypothyroid with PTU, both before and after iodination in vitro, were resolved by SDS-PAGE (3 μg of protein per lane), electrotransfer to nitrocellulose, and immunoblotting with mAb anti-T3 and anti-TG. Wild-type TG was run in an adjacent lane as a 330-kDa molecular mass marker. C, quantitation of the relative T3/TG band intensity ratio from five independent experiments; mean ± S.D.; *, p < 0.05 comparing thyroids from TSHR-KO to PTU-treated animals.
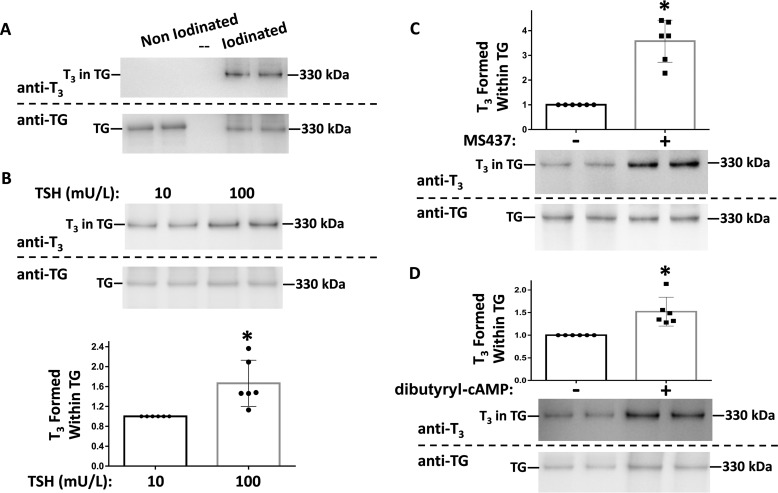
To directly test the effect of thyrocyte stimulation by TSH on the ability of TG to facilitate de novo T3 formation, we collected secretion from the rat thyrocyte cell line, PCCL3. Ordinarily, these cells are grown in the presence of TSH at a concentration of 1–10 milliunits/ml (25, 26). Under standard cell culture conditions, PCCL3 cells cannot iodinate their own secreted proteins; however, upon collection of the secretion followed by iodination in vitro, de novo T3 formation was readily detectable within TG (Fig. 4A). We cultured PCCL3 cells for several days in two concentrations of TSH that differed by an order of magnitude (10 and 100 milliunits/liter). TG secreted from PCCL3 cells after exposure to the higher TSH concentration consistently exhibited greater potential for T3 formation upon iodination in vitro (Fig. 4B, quantified in graph below the blot). Recently, a specific small molecule agonist of the TSHR, MS437, was described to activate Gαs, which mimics TSH in turning on downstream target genes (27). Upon growth of PCCL3 cells in the presence of MS437, in vitro iodination of secreted TG led to a marked increase in de novo T3 formation (Fig. 4C, quantified in graph above the blot). Stimulatory G proteins in thyrocytes activate adenylyl cyclase, which catalyzes cAMP synthesis (28). Upon incubation of PCCL3 cells in the presence of dibutyryl cAMP, in vitro iodination of secreted TG again demonstrated increased de novo T3 formation (Fig. 4D, quantified in the graph above the blot). These data strongly suggest that TSH, working through TSHR, stimulatory G proteins, and cAMP production, alters the intrinsic ability of TG to form T3 upon TG iodination.
Figure 4.
De novo T3 formation in TG secreted from the PCCL3 rat thyrocyte cell line. A, TG secreted into serum-free media bathing control PCCL3 was iodinated in vitro, or non-iodinated as a negative control. The samples were analyzed by SDS-PAGE (2 μg of protein/lane), electrotransfer, and immunoblotting with mAb anti-T3 and anti-TG, as indicated. Wild-type TG was run in an adjacent lane as a 330-kDa molecular mass marker. B, PCCL3 cells were grown at two different TSH concentrations: 10 or 100 milliunits/liter, as described under “Experimental procedures.” TG secreted into serum-free media was iodinated in vitro and analyzed by SDS-PAGE (2 μg of protein/lane), electrotransfer, and immunoblotting as in A. Quantitation of the relative T3/TG band intensity ratio from 6 independent samples is shown below; mean ± S.D.; *, p < 0.05 comparing higher to lower TSH. C, PCCL3 cells were incubated ± TSHR agonist MS437 (10 μm) as described under “Experimental procedures.” Secreted TG was iodinated in vitro followed by SDS-PAGE (3 μg of protein/lane), electrotransfer, and immunoblotting with mAb anti-T3 and anti-TG, as indicated. Quantitation of the relative T3/TG band intensity ratio from 6 independent samples is shown above; mean ± S.D.; * p < 0.05 compared with control. D, PCCL3 cells were incubated ± dibutyryl cAMP (0.1 mm) as described under “Experimental procedures,” and analyzed exactly as in panel C.
There are several significant post-translational modifications that may have structural consequences on TG synthesized in TSH-stimulated thyrocytes, which may impact its hormonogenic potential, including de novo T3 formation. These include the following: increased N-linked glycosylation (29) promoted in part by stimulation of oligosaccharyl transferase activity (30) and up-regulation of N-acetylglucosaminyltransferase 1 (31); increased complex sugars added in the thyrocyte Golgi complex including galactose (32); a marked decrease in the level of α2,6-bound sialic acid (33); down-regulation of tyrosine sulfation (34); increased formation of dityrosine cross-bridges (35) that can form non-disulfide-linked covalent TG dimers (36) and also increased TG phosphorylation (37), which can occur within TG carbohydrate, phosphotyrosine, and phosphoserine residues.
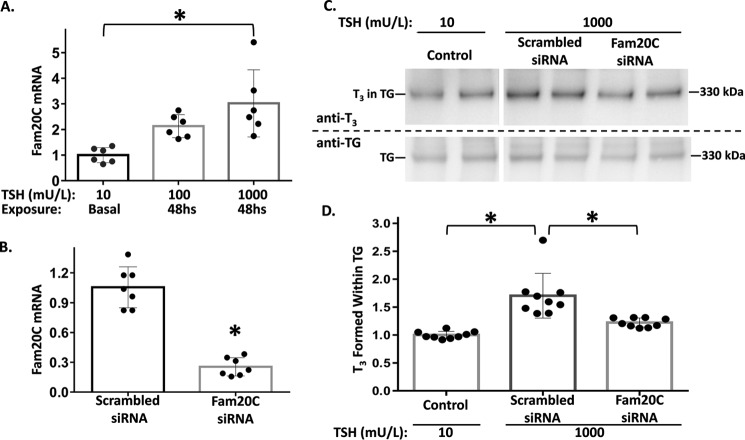
Recently, Fam20C was identified as a secretory pathway kinase that phosphorylates hundreds of secreted proteins, with a marked preference for Ser residues within the consensus sequence Ser-X-Glu/phospho-Ser (38). We observed a TSH dose-dependent 3-fold stimulation of Fam20C mRNA levels in PCCL3 cells (Fig. 5A). To examine the potential contribution of increased Fam20C mRNA on de novo T3 formation in TG, we eliminated this increase using siRNA knockdown of Fam20C in TSH-stimulated PCCL3 cells (Fig. 5B). Upon in vitro iodination of secreted TG, TSH-stimulated cells with knockdown of Fam20C showed significantly less de novo T3 formation (Fig. 5C, quantified in D). Moreover, treatment with calf intestinal phosphatase to dephosphorylate TG secreted from TSH-stimulated PCCL3 cells decreased de novo T3 formation in TG by 26% (data not shown). Taken together, these data suggest that TG phosphorylation (37) is one of the TSH-stimulated post-translational modifications that contribute to altering the structure of TG to increase de novo T3 formation within TG.
Figure 5.
Fam20C expression regulated by TSH stimulation in PCCL3 thyrocytes. A, PCCL3 cells were grown in complete media containing 10, 100, or 1000 milliunits/liter of TSH as described under “Experimental procedures.” At 48 h, cells were lysed, RNA was isolated and reverse transcribed, and Fam20C mRNA levels were quantified by qPCR normalized to that of HPRT1; the mean of 6 independent samples (each performed in duplicate) is shown; mean ± S.D.; *, p < 0.05. B, PCCL3 cells were lysed 48 h after siRNA-mediated knockdown of Fam20C or scrambled oligonucleotide control. The cells were grown in complete normal growth media (1000 milliunits/liter of TSH) prior to RNA isolation, and Fam20C mRNA was quantified by qPCR as in panel A (7 independent samples per condition). C, control secretion from PCCL3 cells (10 milliunits/liter of TSH) was collected as described in the legend to Fig. 4B; secretion from PCCL3 cells treated as in B was collected in serum-free cells in the presence of 1000 milliunits/liter of TSH. Secreted TG from all samples was iodinated in vitro followed by SDS-PAGE (3 μg of protein/lane), electrotransfer, and immunoblotting with mAb anti-T3 and anti-TG, as indicated. D, quantitation of the relative T3/TG band intensity ratio from 9 independent samples is shown; mean ± S.D.; *, p < 0.05 compared with scrambled oligonucleotide.
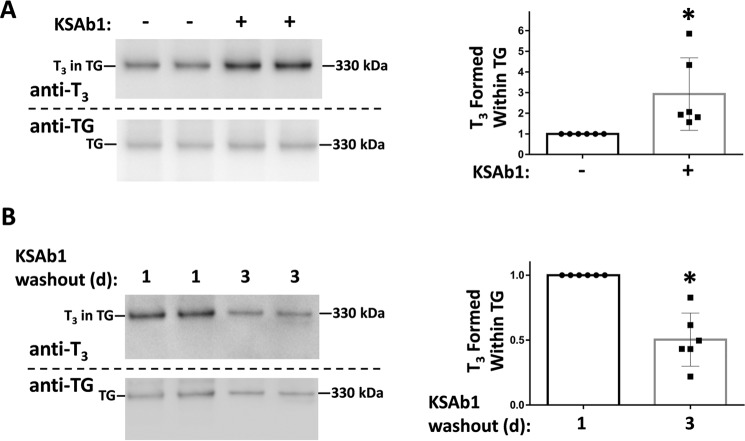
Graves' disease involves thyroidal overactivity leading to thyrotoxicosis, with a particular predilection to increased circulating T3, i.e. relative T3 toxicosis (9). To determine whether a TSHR-stimulating immunoglobulin could also promote enhanced de novo T3 formation, PCCL3 cells were cultured for 5 days in the presence or absence of KSAb1, a strong TSHR-stimulating immunoglobulin (39). After the culture period, serum-free secretion from PCCL3 cells was collected for the ensuing 24 h and the secreted proteins were iodinated in vitro. From the results shown in Fig. 6A, left (quantified at right), it was clear that TG secreted from PCCL3 cells that had been previously incubated with KSAb1 showed significantly increased de novo T3 formation.
Figure 6.
Effects of TSHR-stimulating immunoglobulin (KSAb1) on de novo T3 formation in TG. A, left: PCCL3 cells were preincubated ± mouse mAb KSAb1 TSHR-stimulating immunoglobulin (3 μg/ml). Secreted TG was iodinated in vitro followed by SDS-PAGE (3 μg of protein/lane), electrotransfer, and immunoblotting with mAb anti-T3 and anti-TG, as indicated. Wild-type TG was run in an adjacent lane as a 330-kDa molecular mass marker. Right, quantitation of the relative T3/TG band intensity ratio from 6 independent experiments is shown; mean ± S.D.; *, p < 0.05 compared with control lacking KSAb1 pretreatment. B, reversion of the stimulating effect of KSAb1. Left, the bathing media containing secreted TG was collected 1 day after removing KSAb1, and again for another 2 days after the first media collection. Secreted TG was iodinated in vitro followed by SDS-PAGE (2 μg of protein/lane), electrotransfer, and immunoblotting with mAb anti-T3 and anti-TG, as indicated. Right, quantitation of the relative T3/TG band intensity ratio from TG collected on days 2 + 3, compared with day 1 (normalized to 1.0) in 6 independent experiments is shown; mean ± S.D.; *, p < 0.05.
Thyrotoxicosis and complications of Graves' disease have been found to improve when the elevated concentrations of circulating TSHR-stimulating immunoglobulins are reversed, for example, by plasmapheresis (40). To examine reversibility of enhanced de novo T3 formation in TG, we washed out the KSAb1 and collected TG secreted over the next 24 h, or the subsequent 48 h thereafter. Upon iodination in vitro, it was apparent that beyond 24 h after washout of KSAb1, the subsequent secretion contained TG that had reverted back to a decreased ability for de novo T3 formation (Fig. 6B, left, quantified at right).
One clinical assay to screen for TSHR-stimulating immunoglobulins involves measuring responses to antibodies contained within the sera of human Graves' patients, in a cultured rat thyrocyte cell line (41). With this in mind, we cultured PCCL3 cells for 5 days in the presence of control sera from 5 individuals without Graves' disease or from 2 patients with Graves' disease. After exposure to the human sera, serum-free secretion from PCCL3 cells was collected for the ensuing 24 h and the secreted proteins were iodinated in vitro. From the results shown in Fig. 7, culture with each of the 5 control sera resulted in subsequent TG secretion with approximately the same potential for de novo T3 formation. By contrast, culture with unpurified sera from two Graves' disease patients both suggested an increase in the ability of the subsequently secreted TG to form T3 upon iodination.
Figure 7.
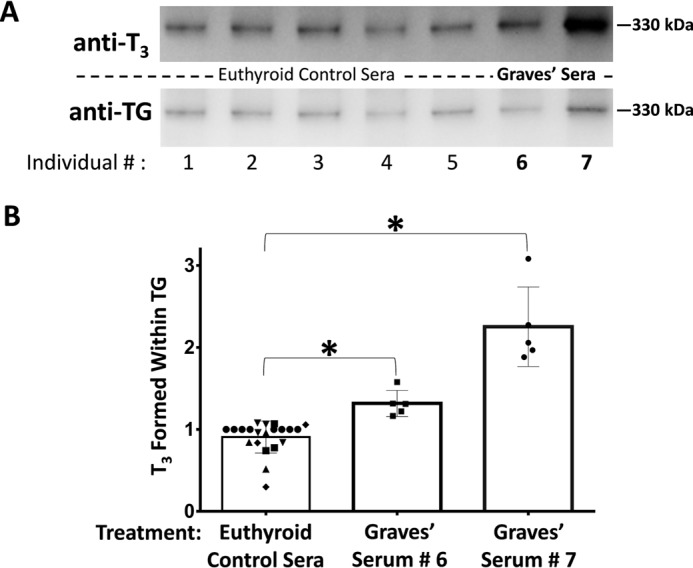
Stimulation of de novo T3 formation by serum from two human Graves' disease patients. A, PCCL3 cells were incubated for 5 days in media containing 40% serum from two patients with Graves' disease (#6 and #7), or 5 euthyroid controls (#1–#5). After washing, TG secreted thereafter into serum-free media bathing the cells was iodinated in vitro and analyzed by SDS-PAGE (2 μg of protein/lane), electrotransfer, and immunoblotting with anti-T3 and anti-TG, as indicated. Wild-type TG was run in an adjacent lane as a 330-kDa molecular mass marker. B, quantitation of the relative T3/TG band intensity with the ratio set to a value of 1.0 for control serum #1. For all sera tested, secreted TG was analyzed in at least three independent cultures and iodination in vitro; mean ± S.D.; *, p < 0.05 compared with controls.
Finally, we collected secretion from cultured primary human thyrocytes that were obtained from normal thyroid tissue of patients undergoing total thyroidectomy for localized thyroid cancer. In monolayer culture in TSH-containing medium to which additional iodide was not added, there was no detectable T3 found within TG secreted into the culture medium, regardless of the TSH dose (Fig. 8A). However, when the TSH concentration in the culture medium was increased by an order of magnitude, upon iodination in vitro, T3 formation in the subsequently secreted TG was clearly apparent (Fig. 8B). Taken together, these data indicate that both human TSHR-stimulating immunoglobulins, and human thyrocytes responding to stimulation of TSHR, increase de novo T3 formation in TG.
Figure 8.
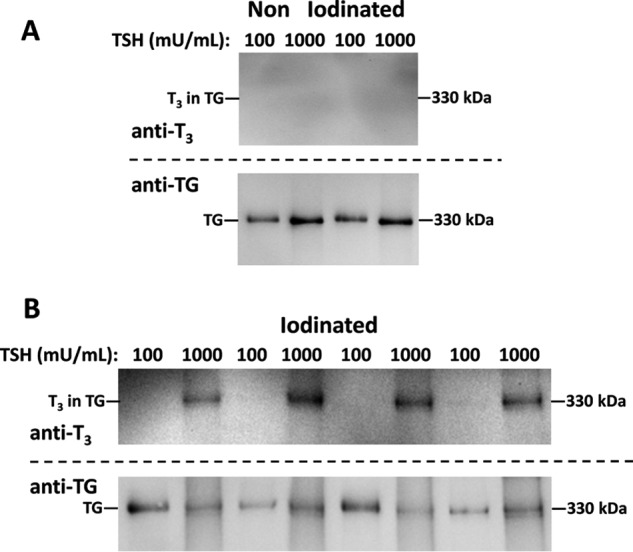
De novo T3 formation in TG secreted from primary culture of human thyrocytes. Human thyrocytes were grown at two different TSH concentrations: 100 or 1000 milliunits/ml, as described under “Experimental procedures.” A, TG secreted into serum-free media that was not iodinated in vitro served as a negative control; analyzed by SDS-PAGE (2 μg of protein/lane), electrotransfer, and immunoblotting with mAb anti-T3 and anti-TG, as indicated. B, TG secreted from human thyrocytes grown as in panel A was iodinated in vitro and analyzed as in panel A.
Discussion
Pioneering work of Dunn and others (15) helped lead to the identification of a few selected Tyr residues of the ∼70 on the TG protein that are favored for T4 and T3 formation. Remarkably, within the huge TG polypeptide, the most frequent site of T4 formation resides just 5 residues from the N terminus (42), whereas more than half of all T3 in TG is formed just 3 residues from the C terminus (24). It is known that DIT located at Tyr130 is the DIT “donor” to form T4 at Tyr5, but the precise mechanism of T3 formation at the C terminus has not been established (19), although it has been postulated to involve one of several potential upstream MIT donor residues in the TG polypeptide (43) including residue 2520 of human TG (44).
In this study, we have utilized a simple immunoblotting procedure to specifically identify T3 within TG (Fig. 1A). Essentially all of the immunodetectable T3 in TG from mouse thyroid tissue (Fig. 1B) resides within the C-terminal portion of the molecule (Fig. 1C) and this immunoreactivity is independent of T4 to T3 conversion (Fig. 2). However, from TG secreted both in mouse thyroid glands (Fig. 3) and a (rat-derived) thyrocyte cell line (Fig. 4), the degree of prior stimulation of TSHRs exerts a strong influence over the efficiency of de novo T3 formation upon TG iodination. Similar effects are observed either with high TSH itself (Fig. 4B), a TSHR agonist (Fig. 4C), a cAMP analog (Fig. 4D), or a TSHR-stimulating immunoglobulin (Fig. 6A). Moreover, these stimulating effects on de novo T3 formation are entirely reversible within a day after removing the TSHR-stimulating immunoglobulin (Fig. 6B). Our data are consistent with the work of Fassler et al. (24), who proposed that TSH alters the utilization of hormonogenic sites on TG through changes in TG structure, which is presumed to be a consequence of altered TG post-translational processing.
Of the many potential TSH-stimulated changes in gene expression of TG post-translational processing enzymes, we have examined Fam20C, a novel secretory pathway kinase often referred to as “casein kinase” (38). Of the potential sites that are both predicted casein kinase (45) and canonical Fam20C targets, multiple sites are specifically conserved between rat, mouse, and human TG; three of these fall within the ChEL domain; and one of these (at position 2721 of human TG) has been directly established to be a phospho-Ser residue by mass spectrometry of human TG (46) and is close to a primary site of T3 formation (24). Our current evidence suggests that TSHR stimulation can increase Fam20C mRNA levels within 48 h, and this increase contributes (along with other changes) to enhanced de novo T3 formation within TG (Fig. 5).
The observation that TSHR stimulation up-regulates the efficiency of de novo T3 formation upon TG iodination fits plausibly with the notion of a direct increase in de novo T3 formation within TG in Graves' disease, thereby contributing to increased intrathyroidal and secreted T3 in this condition (7, 11), which contributes to a state of relative T3 toxicosis (47, 48). Indeed, when collecting the secretion from PCCL3 cells incubated with serum from two Graves' disease patients, the secreted TG showed a clearly increased predisposition to form T3 upon iodination, in comparison to various control sera (Fig. 7). Moreover, TSHR activation also promotes enhancement of T3 formation in TG from human thyrocytes, as directly demonstrated in cultures of normal human thyroid tissue hyperstimulated with TSH (Fig. 8).
Interestingly, from repeated experiments, we were unable to detect any demonstrable evidence that TSHR stimulation alters TG in a way that enhances its efficiency in de novo T4 formation (data not shown), and this selectivity for de novo T3 formation is consistent with previous reports (49). Of course, in Graves' disease, there is also a general increase of T4 secretion that may be attributed to many TSHR-stimulated activities including increased iodide uptake, DUOX function, TPO function, TG synthesis, and endocytosis of colloid, to name but a few (50). More work is needed at both the TG structural level, and at the thyroid cell biological level, to understand how and why T3 is formed preferentially at residue 2746 of human TG (2744 of mouse TG), and how TSHR stimulation selectively increases de novo T3 formation. However, our data support the hypothesis that the TSHR-stimulated effects reflect alterations in TG post-translational processing that impact structurally on the carboxyl-terminal region of TG molecules to enhance de novo T3 formation.
Experimental procedures
Materials
NaI, lactoperoxidase, glucose oxidase, dextrose, fetal bovine serum, insulin, hydrocortisone, apo-transferrin, TSH, dibutyryl-cAMP, l-thyroxine (T4), and 3,3′,5-triiodo-l-thyronine (T3) were from Sigma. Protease inhibitor mixture was from Roche Applied Science. Penicillin/streptomycin, PBS, and DMEM/F-12 were from Gibco. Prolong gold anti-fade reagent with DAPI, lithium dodecyl sulfate gel loading buffer, 4–12% polyacrylamide BisTris “NuPAGE” gels, 10% polyacrylamide Tris glycine gels, SeeBlue Plus2 molecular weight markers, and the SuperScript III first-strand synthesis system were from Invitrogen. BCA protein assay and RNAiMAX reagent were from Thermo Scientific; Power SYBR Green PCR master mix was from Applied Biosystems; RNeasy RNA purification kit was from Qiagen; Factor Xa protease from New England Biolabs; and Citrisolv and Retrieve-All antigen retrieval reagent were from Fisher.
Antibodies
Mouse mAb anti-T3 clone 3A6 (≤0.1% cross-reactivity with T4 by ELISA), and a cross-adsorbed goat anti-mouse antibody conjugated to Alexa Fluor-555 were from Invitrogen; rabbit polyclonal anti-TG was as described (51). HRP-conjugated goat anti-mouse IgG was from Bio-Rad; HRP-conjugated goat anti-rabbit IgG was from Jackson ImmunoResearch.
Animal thyroid tissues used in this study
Mice were housed and fed as per an approved institutional protocol. C57BL/6 (WT) mice were 8–11 months old; D1/D2 double knock-out (DKO) mice (12) were 10 months old; TSHR-KO mice (52) were 3–5 months old. Where indicated, WT mice were fed low-iodide chow containing 0.15% PTU (Envigo) for 6–15 weeks. Thyroid glands were lysed by sonication in RIPA buffer ± SDS and containing a protease inhibitor mixture. Lysates were cleared at 12,000 × g for 10 min at 4 °C and total protein was determined by BCA or Bramhall assay (53).
Immunofluorescence with mAb anti-T3
Fresh thyroid tissues from wild-type, PTU-treated, or TSHR-KO mice were immersion fixed with 10% formaldehyde and paraffin-embedded. Six-μm tissue sections were de-paraffinized with Citrosolv, followed by antigen retrieval with Retrieve-All, and permeabilization with Triton X-100 (0.2%). First antibody incubation with mAb anti-T3 (1:200) in TBS plus 0.2% Tween 20 and 3% BSA ± free T3 or free T4 as described, was followed by a secondary antibody (noted above) diluted 1:5000 in the same buffer without hormone competitor. Images were captured using a ×40 oil objective in an Olympus FluoView 500 laser scanning confocal microscope.
Site-specific protease digestion of TG
Factor Xa protease has only one predicted cleavage site (Peptide Cutter, Expasy) in WT mouse TG, at amino acid 1036. Thyroid homogenate protein from C57BL/6 WT mice (140 μg) in RIPA buffer was adjusted to 1% SDS, 5 mm DTT and boiled for 1 min. The sample was then diluted to 0.01% SDS, 0.05 mm DTT in a total volume of 100 μl and digested with 8 μl of Factor Xa (New England Biolabs) for 15 min at 25 °C. Digestion was terminated by boiling in denaturing gel sample buffer containing 100 mm DTT for 5 min before SDS-PAGE.
PCCL3 cell culture and treatments
PCCL3 cells were cultured in DMEM/F-12 supplemented with 5% FBS plus penicillin/streptomycin and a four-hormone mixture containing 1 μg/ml of insulin, 1 nm hydrocortisone, 5 μg/ml of apo-transferrin, and 1 milliunit/ml of TSH.
For experiments with varying TSH concentrations, PCCL3 cells were seeded at 50,000 cells/well in 24-well plates. After 24 h, the cells were grown for 5 days in complete medium containing TSH at either 10 or 100 milliunits/liter. The cells were then washed in PBS and re-fed at the same TSH concentrations in serum-free media and cultured for 1 (100 milliunits/liter of TSH) or 3 days (10 milliunits/liter of TSH), leading to comparable amounts of secreted TG and total protein in the bathing media. For experiments studying the TSHR agonist MS437 (27), the PCCL3 cells were seeded as above and grown for 3 days in complete medium with 10 milliunits/liter of TSH plus 10 μm MS437 or vehicle (DMSO, 0.1%). The cells were then re-fed under the same conditions in serum-free bathing media, which were finally collected either at 2 (MS437) or 3 days (vehicle). For experiments studying the effects of dibutyryl-cAMP, PCCL3 cells were seeded as above. After 24 h, the cells were grown in complete medium with 10 milliunits/liter of TSH ± 0.1 mm dibutyryl cAMP. The media were changed on days 2 and 4, and the cells then re-fed under the same conditions in serum-free media, which were finally collected either at 2 (dibutyryl cAMP) or 3 days (negative control). For experiments studying the mouse mAb KSAb1 TSHR-stimulating immunoglobulin (39), the PCCL3 cells were seeded at 25,000 cells/well in 48-well plates. After 24 h, the cells were grown for 5 days in complete medium containing 10 milliunits/liter of TSH ± purified KSAb1. The cells were then re-fed under the same conditions in serum-free media, which were finally collected either at 1 day (KSAb1-stimulated) plus further days in fresh medium (this is referred to as “KSAb1 washout” for cells that had previously been KSAb1-stimulated) or 3 days (negative control).
Human sera were collected from patients with Graves' disease, or controls, with their consent under an approved Institutional Review Board (IRB) research protocol. For treatment of PCCL3 cells with human sera, the cells were initially plated as in the KSAb1 experiments described above. After 24 h, the cells were grown for 5 days in complete medium containing 10 milliunits/liter of TSH + 40% human serum. The cells were then re-fed with serum-free media containing 10 milliunits/liter of TSH and cultured for 24 h before collecting the media for TG analysis and iodination in vitro.
Fam20C effects in PCCL3 cells
PCCL3 cells were pretreated under basal conditions in complete media containing 10 milliunits/liter of TSH for 5 days and then plated at 100,000 cells/well in 12-well plates. For mRNA measurements, cells were then either continued in basal media or shifted to complete media containing either 100 or 1000 milliunits/liter of TSH for 48 h. RNA was purified, reverse-transcribed, and qPCR was performed in duplicate using a StepOnePlus thermal cycler (Applied Biosystems) using SYBR Green and the following primers: Fam20C, 5′-gaggcacaatgcggagatag-3′ and 5′-gaggcactctgcggaaatc-3′; and HPRT1, 5′-ctcatggactgattatggacaggac-3′ and 5′-gcaggtcagcaaagaacttatagcc-3′. Data were analyzed by comparative CT (ΔΔCT). For knockdown of Fam20C, PCCL3 cells grown in complete media containing 1,000 milliunits/liter of TSH were plated at 100,000 cells/well in 12-well plates. After 24 h 10 nm Fam20C siRNA (ID304334, Origene) or scrambled duplex oligonucleotide (SR30004, Origene) were transfected using RNAiMAX according to the manufacturer's instructions. After 6 h, media were replaced with complete media containing 1000 milliunits/liter of TSH for 18 h. At that time, the cells were re-fed under the same conditions in serum-free bathing media, which were finally collected after 48 h. The bathing media were used for in vitro iodination of TG and the lysed cells were used for RNA purification followed by qPCR.
Primary human thyrocyte culture and treatments
Normal human thyroid tissue was obtained from a patient undergoing total thyroidectomy for thyroid cancer at the National Institutes of Health Clinical Center. The patient provided informed consent on an approved IRB research protocol and materials were received anonymously with approval of research activity through the Office of Human Subjects Research, National Institutes of Health. Primary thyrocytes were prepared and propagated as described (54).
Human thyrocytes were plated in DMEM, 10% FBS plus penicillin/streptomycin at 100,000 cells/well in 12-well plates. After 24 h, the cells were cultured for 1 day in serum-free medium plus 0.1% BSA, and after one further day, the cells were re-fed and cultured for 6 days with DMEM plus penicillin/streptomycin and TSH at a concentration of 100 or 1000 milliunits/ml. These conditions were tested in two biological replicates, and results in the replicates were identical.
In vitro enzymatic iodination of TG
Iodination in vitro (55) included lactoperoxidase (30 ng/μl), glucose (2 μg/μl), glucose oxidase (0.352 ng/μl), 100 μm NaI, and 50–250 ng/μl of thyroidal protein. Incubations were initiated with the addition of the glucose oxidase, incubated for 2 h at 37 °C, and stopped by addition of gel sample buffer and boiling for 5 min. To roughly normalize the amount of TG protein in the samples being compared in each experiment, when required we diluted thyrocyte-secreted protein with a known quantity of protein from serum-free medium bathing 293T cells that do not express TG. However, in pilot experiments we found that normalizing the TG protein content may not be necessary as it did not affect the ratio of T3 formed per unit TG.
Western blotting
Samples (2–4 μg of total protein per lane) were subjected to SDS-PAGE under either reducing (Figs. 1–3) or nonreducing conditions (all other figures). Pre-stained molecular mass markers as well as WT TG (330 kDa) were run in lanes adjacent to the experimental samples. Electrotransfer to nitrocellulose was performed for 7 min at 20 V using the iBlot transfer apparatus (Invitrogen). Blocking was performed for 30 min at room temperature with 5% BSA in TBS plus 0.05% Tween 20 (TBS-T) and washed with TBS-T. Primary mouse mAb anti-T3 was diluted at 1:1000 containing (unless otherwise indicated) 500 ng/ml of free T4 (to eliminate any possibility of T4 cross-reactivity) and incubated overnight at 4 °C. Primary rabbit polyclonal anti-TG was diluted 1:5000 in 5% BSA/TBS-T and incubated for 1 h at room temperature. Species-specific HRP-conjugated secondary antibodies (1:5000 dilutions in blocking buffer) were incubated for 30 min at room temperature. Bands were visualized using the WesternBright Sirius kit as directed by the manufacturer (Advansta). Images were captured in a Fotodyne work station with a digital camera; exposure times averaged 20 s for anti-T3 blots and 5 s for anti-TG blots.
Quantitation of T3/TG band intensity ratio
Band intensities were quantified using ImageQuant 5.2 (Molecular Dynamics). The ratio of intensities of the bands corresponding to the T3 immunoreactivity within the TG band to the direct TG immunoreactivity from polyclonal anti-TG within the same band was calculated, with the control value set to 1.0. In Fig. 5D, the mean of control values was set to 1.0.
Data analysis
Statistical analyses were done using unpaired Student's t test with two-tailed p value (Figs. 3–5B and 6) or by one-way ANOVA followed by Dunnett's test (Figs. 5, A and D, and 7). The level of significance for all statistical tests was set to p < 0.05. Statistical values were calculated with GraphPad Prism version 6. Data are presented as mean ± S.D.
Author contributions
C. E. C., B. V., S. J. M., V. A. G., S. A., and Y. M. performed experiments, R. L., M. C. G., T. J. S., and P. A. designed experiments, and C. E. C. and P. A. interpreted data. All authors reviewed the results and approved the final submitted version of the manuscript.
Acknowledgment
We thank Dr. Terry Davies (Mt. Sinai School of Medicine) for helpful discussions during the course of this work.
Note added in proof
In the version of this article that was published as a Paper in Press on July 25, 2017, some incorrect panels were inadvertently used to assemble Fig. 1A. This error has now been corrected and does not affect the results or conclusions of this work.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK40344 (to P. A.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- TG
- thyroglobulin
- PTU
- propylthiouracil
- T3
- triiodothyronine
- T4
- thyroxine
- TSH
- thyroid stimulating hormone
- TSHR
- TSH receptor
- D1
- type 1 deiodinase
- D2
- type 2 deiodinase
- DKO
- D1/D2 double knockout
- DIT
- diiodotyrosine
- MIT
- mono-iodotyrosine
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- qPCR
- quantitative PCR.
References
- 1. Holzer G., Morishita Y., Fini J. B., Lorin T., Gillet B., Hughes S., Tohmé M., Deléage G., Demeneix B., Arvan P., and Laudet V. (2016) Thyroglobulin represents a novel molecular architecture of vertebrates. J. Biol. Chem. 291, 16553–16566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mullur R., Liu Y. Y., and Brent G. A. (2014) Thyroid hormone regulation of metabolism. Physiol. Rev. 94, 355–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calzà L., Fernández M., and Giardino L. (2015) Role of the thyroid system in myelination and neural connectivity. Compr. Physiol. 5, 1405–1421 [DOI] [PubMed] [Google Scholar]
- 4. Davis P. J., Goglia F., and Leonard J. L. (2016) Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 12, 111–121 [DOI] [PubMed] [Google Scholar]
- 5. Bernal J. (2017) Thyroid hormone regulated genes in cerebral cortex development. J. Endocrinol. 232, R83–R97 [DOI] [PubMed] [Google Scholar]
- 6. Chanoine J. P., Braverman L. E., Farwell A. P., Safran M., Alex S., Dubord S., and Leonard J. L. (1993) The thyroid gland is a major source of circulating T3 in the rat. J. Clin. Invest. 91, 2709–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Larsen P. R. (1975) Thyroidal triiodothyronine and thyroxine in Graves' disease: correlation with presurgical treatment, thyroid status, and iodine content. J. Clin. Endocrinol. Metab. 41, 1098–1104 [DOI] [PubMed] [Google Scholar]
- 8. Smith T. J., and Hegedüs L. (2016) Graves' disease. N. Engl. J. Med. 375, 1552–1565 [DOI] [PubMed] [Google Scholar]
- 9. Schimmel M., and Utiger R. D. (1977) Thyroidal and peripheral production of thyroid hormones: review of recent findings and their clinical implications. Ann. Intern. Med. 87, 760–768 [DOI] [PubMed] [Google Scholar]
- 10. Woeber K. A. (2006) Triiodothyronine production in Graves' hyperthyroidism. Thyroid 16, 687–690 [DOI] [PubMed] [Google Scholar]
- 11. Laurberg P. (1984) Mechanisms governing the relative proportions of thyroxine and 3,5,3′-triiodothyronine in thyroid secretion. Metabolism 33, 379–392 [DOI] [PubMed] [Google Scholar]
- 12. Galton V. A., Schneider M. J., Clark A. S., and St Germain D. L. (2009) Life without thyroxine to 3,5,3′-triiodothyronine conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology 150, 2957–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Targovnik H. M., Citterio C. E., and Rivolta C. M. (2011) Thyroglobulin gene mutations in congenital hypothyroidism. Horm. Res. Paediatr. 75, 311–321 [DOI] [PubMed] [Google Scholar]
- 14. Di Jeso B., and Arvan P. (2016) Thyroglobulin from molecular and cellular biology to clinical endocrinology. Endocr. Rev. 37, 2–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunn J. T., and Dunn A. D. (2001) Update on intrathyroidal iodine metabolism. Thyroid 11, 407–414 [DOI] [PubMed] [Google Scholar]
- 16. Taurog A. M. (2000) Hormone synthesis: thyroid iodine metabolism. in Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text (Braverman L.E., and Utiger R. D., eds) pp. 61–85, 8th Ed., Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 17. Lamas L., and Taurog A. (1977) The importance of thyroglobulin structure in thyroid peroxidase-catalyzed conversion of diiodotyrosine to thyroxine. Endocrinology 100, 1129–1136 [DOI] [PubMed] [Google Scholar]
- 18. Dunn J. T., Anderson P. C., Fox J. W., Fassler C. A., Dunn A. D., Hite L. A., and Moore R. C. (1987) The sites of thyroid hormone formation in rabbit thyroglobulin. J. Biol. Chem. 262, 16948–16952 [PubMed] [Google Scholar]
- 19. Dedieu A., Gaillard J. C., Pourcher T., Darrouzet E., and Armengaud J. (2011) Revisiting iodination sites in thyroglobulin with an organ-oriented shotgun strategy. J. Biol. Chem. 286, 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xavier A. C., Maciel R. M., Vieira J. G., Dias-da-Silva M. R., and Martins J. R. (2016) Insights into the posttranslational structural heterogeneity of thyroglobulin and its role in the development, diagnosis, and management of benign and malignant thyroid diseases. Arch. Endocrinol. Metab. 60, 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rousset B., Dupuy C., Miot F., and Dumont J. (2000) Chapter 2: Thyroid hormone synthesis and secretion, in Endotext (De Groot L. J., Chrousos G., Dungan K., Feingold K. R., Grossman A., Hershman J. M., Koch C., Korbonits M., McLachlan R., New M., Purnell J., Rebar R., Singer F., and Vinik A., eds) MDText.com, Inc., South Dartmouth, MA [Google Scholar]
- 22. Sellitti D. F., and Suzuki K. (2014) Intrinsic regulation of thyroid function by thyroglobulin. Thyroid 24, 625–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bianco A. C., and Larsen P. R. (2005) Cellular and structural biology of the deiodinases. Thyroid 15, 777–786 [DOI] [PubMed] [Google Scholar]
- 24. Fassler C. A., Dunn J. T., Anderson P. C., Fox J. W., Dunn A. D., Hite L. A., Moore R. C., and Kim P. S. (1988) Thyrotropin alters the utilization of thyroglobulin's hormonogenic sites. J. Biol. Chem. 263, 17366–17371 [PubMed] [Google Scholar]
- 25. Palmero C. Y., Miranda-Alves L., Sant'Ana Barroso M. M., Souza E. C., Machado D. E., Palumbo-Junior A., Santos C. A., Portilho D. M., Mermelstein C. S., Takiya C. M., Carvalho D. P., Hochman-Mendez C., Coelho-Sampaio T., and Nasciutti L. E. (2013) The follicular thyroid cell line PCCL3 responds differently to laminin and to polylaminin, a polymer of laminin assembled in acidic pH. Mol. Cell. Endocrinol. 376, 12–22 [DOI] [PubMed] [Google Scholar]
- 26. Ambesi-Impiombato F. S., Parks L. A., and Coon H. G. (1980) Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc. Natl. Acad. Sci. U.S.A. 77, 3455–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Latif R., Ali M. R., Ma R., David M., Morshed S. A., Ohlmeyer M., Felsenfeld D. P., Lau Z., Mezei M., and Davies T. F. (2015) New small molecule agonists to the thyrotropin receptor. Thyroid 25, 51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kleinau G., and Biebermann H. (2014) Constitutive activities in the thyrotropin receptor: regulation and significance. Adv. Pharmacol. 70, 81–119 [DOI] [PubMed] [Google Scholar]
- 29. Desruisseau S., Franc J. L., Gruffat D., and Chabaud O. (1994) Glycosylation of thyroglobulin secreted by porcine cells cultured in chamber system: thyrotropin controls the number of oligosaccharides and their anionic residues. Endocrinology 134, 1676–1684 [DOI] [PubMed] [Google Scholar]
- 30. Desruisseau S., Valette A., Franc J. L., and Chabaud O. (1996) Thyrotropin controls dolichol-linked sugar pools and oligosaccharyltransferase activity in thyroid cells. Mol. Cell. Endocrinol. 122, 223–228 [DOI] [PubMed] [Google Scholar]
- 31. Suban D., Zajc T., Renko M., Turk B., Turk V., and Dolenc I. (2012) Cathepsin C and plasma glutamate carboxypeptidase secreted from Fischer rat thyroid cells liberate thyroxin from the N-terminus of thyroglobulin. Biochimie 94, 719–726 [DOI] [PubMed] [Google Scholar]
- 32. Di Jeso B., Liguoro D., Ferranti P., Marinaccio M., Acquaviva R., Formisano S., and Consiglio C. (1992) Modulation of the carbohydrate moiety of thyroglobulin by thyrotropin and calcium in Fisher rat thyroid line-5 cells. J. Biol. Chem. 267, 1938–1944 [PubMed] [Google Scholar]
- 33. Grollman E. F., Saji M., Shimura Y., Lau J. T., and Ashwell G. (1993) Thyrotropin regulation of sialic acid expression in rat thyroid cells. J. Biol. Chem. 268, 3604–3609 [PubMed] [Google Scholar]
- 34. Nlend M. C., Cauvi D., Venot N., Desruisseau S., and Chabaud O. (1999) Thyrotropin regulates tyrosine sulfation of thyroglobulin. Eur. J. Endocrinol. 141, 61–69 [DOI] [PubMed] [Google Scholar]
- 35. Leonardi A., Acquaviva R., Marinaccio M., Liguoro D., Fogolari F., Di Jeso B., Formisano S., and Consiglio E. (1994) Presence of dityrosine bridges in thyroglobulin and their relationship with iodination. BBRC 202, 38–43 [DOI] [PubMed] [Google Scholar]
- 36. Haeberli A., Kneubuehl F., and Studer H. (1981) Changes in the polypeptide assembly of guinea pig thyroglobulin induced by thyrotropin-regulated thyroid activity. Endocrinology 109, 523–529 [DOI] [PubMed] [Google Scholar]
- 37. Consiglio E., Acquaviva A. M., Formisano S., Liguoro D., Gallo A., Vittorio T., Santisteban P., De Luca M., Shifrin S., and Yeh H. J. (1987) Characterization of phosphate residues on thyroglobulin. J. Biol. Chem. 262, 10304–10314 [PubMed] [Google Scholar]
- 38. Tagliabracci V. S., Wiley S. E., Guo X., Kinch L. N., Durrant E., Wen J., Xiao J., Cui J., Nguyen K. B., Engel J. L., Coon J. J., Grishin N., Pinna L. A., Pagliarini D. J., and Dixon J. E. (2015) A single kinase generates the majority of the secreted phosphoproteome. Cell 161, 1619–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gilbert J. A., Gianoukakis A. G., Salehi S., Moorhead J., Rao P. V., Khan M. Z., McGregor A. M., Smith T. J., and Banga J. P. (2006) Monoclonal pathogenic antibodies to the thyroid-stimulating hormone receptor in Graves' disease with potent thyroid-stimulating activity but differential blocking activity activate multiple signaling pathways. J. Immunol. 176, 5084–5092 [DOI] [PubMed] [Google Scholar]
- 40. Prummel M. F., and Wiersinga W. M. (1998) Immunomodulatory treatment of Graves' ophthalmopathy. Thyroid 8, 545–548 [DOI] [PubMed] [Google Scholar]
- 41. Kasagi K., Konishi J., Iida Y., Tokuda Y., Arai K., Endo K., and Torizuka K. (1987) A sensitive and practical assay for thyroid-stimulating antibodies using FRTL-5 thyroid cells. Acta Endocrinol. 115, 30–36 [DOI] [PubMed] [Google Scholar]
- 42. Marriq C., Lejeune P. J., Venot N., and Vinet L. (1991) Hormone formation in the isolated fragment 1–171 of human thyroglobulin involves the couple tyrosine 5 and tyrosine 130. Mol. Cell. Endocrinol. 81, 155–164 [DOI] [PubMed] [Google Scholar]
- 43. Cetrangolo G. P., Arcaro A., Lepore A., Graf M., Mamone G., Ferranti P., Palumbo G., and Gentile F. (2014) Hormonogenic donor Tyr2522 of bovine thyroglobulin. Insight into preferential T3 formation at thyroglobulin carboxyl terminus at low iodination level. Biochem. Biophys. Res. Commun. 450, 488–493 [DOI] [PubMed] [Google Scholar]
- 44. Xiao S., Dorris M. L., Rawitch A. B., and Taurog A. (1996) Selectivity in tyrosyl iodination sites in human thyroglobulin. Arch. Biochem. Biophys. 334, 284–294 [DOI] [PubMed] [Google Scholar]
- 45. Amanchy R., Periaswamy B., Mathivanan S., Reddy R., Tattikota S. G., and Pandey A. (2007) A curated compendium of phosphorylation motifs. Nat. Biotechnol. 25, 285–286 [DOI] [PubMed] [Google Scholar]
- 46. Mertins P., Mani D. R., Ruggles K. V., Gillette M. A., Clauser K. R., Wang P., Wang X., Qiao J. W., Cao S., Petralia F., Kawaler E., Mundt F., Krug K., Tu Z., Lei J. T., et al. (2016) Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 534, 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burch H. B., and Cooper D. S. (2015) Management of Graves disease: a review. JAMA 314, 2544–2554 [DOI] [PubMed] [Google Scholar]
- 48. Sterling K., Refetoff S., and Selenkow H. A. (1970) T3 thyrotoxicosis: thyrotoxicosis due to elevated serum triiodothyronine levels. JAMA 213, 571–575 [DOI] [PubMed] [Google Scholar]
- 49. Izumi M., and Larsen P. R. (1977) Triiodothyronine, thyroxine, and iodine in purified thyroglobulin from patients with Graves' disease. J. Clin. Invest. 59, 1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koibuchi N. (2012) Molecular mechanisms of thyroid hormone synthesis and secretion. Nihon Rinsho 70, 1844–1848 [PubMed] [Google Scholar]
- 51. Kim P. S., and Arvan P. (1991) Folding and assembly of newly synthesized thyroglobulin occurs in a pre-Golgi compartment. J. Biol. Chem. 266, 12412–12418 [PubMed] [Google Scholar]
- 52. Marians R. C., Ng L., Blair H. C., Unger P., Graves P. N., and Davies T. F. (2002) Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc. Natl. Acad. Sci. U.S.A. 99, 15776–15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bramhall S., Noack N., Wu M., and Loewenberg J. R. (1969) A simple colorimetric method for the determination of protein. Anal. Biochem. 31, 146–148 [DOI] [PubMed] [Google Scholar]
- 54. Morgan S. J., Neumann S., Marcus-Samuels B., and Gershengorn M. C. (2016) Thyrotropin stimulates differentiation not proliferation of normal human thyrocytes in culture. Front. Endocrinol. 7, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lamas L., Santisteban P., Turmo C., and Seguido A. M. (1986) Evidence, by in vitro enzymatic iodination of thyroglobulin, that the efficiency of coupling is determined by the initial iodide concentration. Endocrinology 118, 2131–2136 [DOI] [PubMed] [Google Scholar]