Abstract
Ceramides in the human stratum corneum (SC) are a mixture of diverse N-acylated fatty acids (FAs) with different chain lengths. C24 is the major class of FAs of ceramides. However, there are also other classes of ceramides with diverse chain lengths of FAs, and these lengths generally range from C16 to C26. This study aimed to prepare several types of phytoceramide containing diverse chain lengths of N-acylated FAs and compare them with C18-ceramide N-stearoyl phytosphingosine (NP) in terms of their effects on the physiological properties of the SC. We chose natural oils, such as horse fat oil, shea butter, sunflower oil, and a mixture of macadamia nut, shea butter, moringa, and meadowfoam seed oil, as sources of FAs and phytosphingosine as a sphingoid backbone to synthesize diverse phytoceramides. Each phytoceramide exhibited a distinctive formation of the lamellar structure, and their FA profiles were similar to those of their respective natural oil. The skin barrier properties, as analyzed in human skin, clearly demonstrated that all the phytoceramides improved the recovery rate of the damaged SC and enhanced hydration better than C18-ceramide NP did. In conclusion, natural oil-derived phytoceramides could represent a novel class of ceramides for cosmetic applications in the development of an ideal skin barrier moisturizer.
Keywords: fatty acid, chain length, phytoceramide, skin barrier, natural oil
Introduction
Permeability barrier function, which is the most important role of skin in maintaining skin moisture balance, is executed by lipid lamellar organization consisting of ceramides, cholesterol, and free fatty acids (FFAs) residing in the extracellular domain of stratum corneum (SC).1 Molecular species and the composition of epidermal ceramides are essential factors that determine the degree of permeability of the multilamellar structure.2,3 These lipid compositional changes affect lamellar packing of the lipids, which contributes to epidermal barrier function, and altered SC lipid composition has been typically observed from several skin diseases, such as atopic dermatitis (AD).4 According to recent studies, not only the composition and content of ceramides but also the chain length of N-acylated fatty acids (FAs) of ceramide has been identified as essential factors that affect the physical and chemical characteristics of lipid lamellar organization and skin barrier function.2,5 In the human skin barrier, various classes of ceramides with diverse chain lengths of FAs are present, mostly ranging from C16 to C26.6–8 Each skin lesion, such as those on the face or leg, has a unique profile of ceramides containing different FA chain lengths, which suggests that the skin permeability barrier function could be modulated by different ceramides with unique FA chain lengths.8 This notion was further emphasized by a report that demonstrated a relatively shorter chain length of FAs from atopic skin compared with that from normal skin.9 In the lesional skin of AD patients, short-chain ceramides were increased. In sodium lauryl sulfate-disrupted dry skin, the long-chain FA was reduced in length.10,11 Similarly, the distribution of hydrocarbon chain length in both ceramides and FAs are equally important in the formation of the correct lipid lamellar phases for the permeability barrier.2,4,5
Several research groups made efforts to ameliorate various abnormal skin conditions, such as atopic and dry skin, with altered lipid compositions upon the topical treatment of lipid mixtures that mimics SC lipid compositions.12,13 In 1995, Mao-Qiang et al13 initially reported that the topical treatment of an exogenous lipid mixture that consisted of a single ceramide class or a synthetic pseudoceramide together with cholesterol and FFA restored the abnormal barrier function of dry skin. Since then, numerous attempts to develop skin moisturizers containing ceramides from various sources, including ceramide N-stearoyl phytosphingosine (NP), ceramide N-(2-hydroxystearoyl)-4-hydroxysphinganine (AP), and some synthetic pseudoceramides, have been made to treat dry and atopic skin conditions.14–16 Although the aforementioned moisturizers improve abnormal skin conditions to some extent, they do not exactly mimic the skin lipid composition and structural organization observed in human SC.17
To our knowledge, attention has not been given to the existence of diverse profiles of the chain length of N-acylated FA in ceramides in terms of skin permeability barrier property. Most ceramide formulations aiming to ameliorate abnormal skin conditions, such as AD, contain either a single ceramide class or several ceramide classes that are natural or synthetic.12,17 To obtain diverse FA compositions and molecular species in terms of the carbon chain length, natural oils, including horse fat, shea butter, and sunflower oils, were selected. In addition, a plant oil mixture with macadamia nut, shea butter, moringa, and meadowfoam seed was synthesized as a diverse chain length containing complex.
For maintenance of intact permeability barrier, mechanical strength and integrity of the SC are required.18 Transepidermal water loss (TEWL) is a marker of damaged epidermal barrier function, and increased levels of TEWL after mechanical disruption, such as tape stripping, are relevant to lower hydration values.18 In addition, SC hydration influences skin barrier function, and an adequate supply of hydration contributes to the flexibility of the SC layer.19 The skin hydration levels are affected by several factors, including the thickness of the SC, the proper maturation of corneocytes, and the appropriate composition and organization of SC lipids.19 As a result, highly hygroscopic substances exist within the corneocytes.20 Among these factors, natural moisturizing factor (NMF) is considered a major contributor to skin hydration.19
In this study, we aimed to compare natural oil-derived ceramides based on phytosphingosine and a commercially available C18-ceramide NP in terms of their performance on maintaining the skin barrier function by conducting in vivo human studies.
Materials and methods
Synthesis of natural oil-derived phytoceramides and preparation of formulations for human study
Phytosphingosine-based ceramides derived from macadamia nut oil (phytocera-M), horse fat oil (phytocera-H), shea butter (phytocera-SB), sunflower oil (phytocera-SF), moringa oil (phytocera-MO), and meadowfoam seed oil (phytocera-MF) were prepared by conjugating pool of FAs derived from each natural oil to phytosphingosine. In addition, a plant oil mixture containing macadamia nut, shea butter, moringa, and meadowfoam seed oil was used and referred to as phytocera-Mix. Phytosphingosine manufactured from yeast fermentation was purchased (Doosan, Suwon-si, Korea). Each phytoceramide was produced by N-acylation of FAs to the amino group of phytosphingosine. Formulations were prepared using 3.5% of each phytoceramide, 1.5% phytosphingosine, 2.5% cholesterol, 2.5% medium-chain triglyceride (MCT; C8–C12,), 1.5% lecithin 95, 1.5% stearic acid, 1.5% oleic acid, 1% linoleic acid, and distilled water. The control formulation was prepared using C18-ceramide NP instead of phytoceramide.
Analysis of FA composition by gas chromatography (GC)
The FA composition of phytoceramides was analyzed after derivatization (saponification and methylation) with GC equipped with a Supelcowax 10 Capillary Column (30 m × 0.25 mm ID × 0.25 μm film thickness). The GC operation conditions were as follows: injector temperature = 305°C, detector temperature = 310°C flame ionization detector, temperature program = 175°C initial, 2.5°C/min rate, final 300°C for 20 min, and carrier gas = He.
Microscopic observations of Maltese cross appearance
Microscope images were observed according to previously described procedures with slight modifications.14 Emulsion drops of each phytoceramide were spread on the slide glass. The optical anisotropy was observed under the cross-polarized light (×200; Nikon Corporation, Tokyo, Japan).
Measurement of barrier recovery rate
Acute epidermal perturbation induced by tape stripping was performed according to previously described procedures with slight modifications.12 Ten female subjects (aged 24.5 ± 2.1 years) without any history of skin disorders volunteered for this study, who used phytocera-H. Ten female subjects (aged 24.0 ± 1.7 years) without any history of skin disorders volunteered for this study, who used phytocera-SB, phytocera-SF, and phytocera-Mix. Informed consent was obtained from all individual participants included in the study. In case of D-squame tape-induced damage, serial tape stripping was applied to a 2.5 cm2 area on the forearm skin until the TEWL value reached ~35–40 g/h/m2, and the topical application with 25 μL (10 μL/cm2) of each phytoceramide or C18-ceramide NP control followed for every 6 h. During a 24 h period, TEWL was measured using a MPA 580 (Courage & Khazaka, Cologne, Germany). The means of three individual measurements per tape-stripped site and time point were combined into one statistical value. The calculation of barrier recovery rate was converted to percentage of TEWL value compared with tape-stripped cases.
Measurement of SC cohesion
Ten female subjects (aged 24.5 ± 2.1 years) without any history of skin disorders volunteered for this study used phytocera-H. Informed consent was obtained from all individual participants included in the study. The method of SC cohesion measurement was performed according to previously described procedures with slight modifications.12
To measure SC cohesion against tape stripping, a 2.5 cm2 area of the forearms of all volunteers was pretreated with 25 μL (10 μL/cm2) of phytocera-H or C18-ceramide NP control for every 6 h. After 24 h, SC cohesion was evaluated by counting the number of D-squame tape stripping (CuDerm Corporation, Dallas, TX, USA) required to increase the value of TEWL >35 g/h/m2 from skins topically treated in an air-conditioned room at 25 ± 3°C and 50 ± 5% relative humidity.
Measurement of skin hydration
Eight female subjects (aged 25.4 ± 1.3 years) without any history of skin disorders volunteered for this study. Informed consent was obtained from all individual participants included in the study. The measurement of SC hydration was performed according to previously described procedures with slight modifications.9
To measure hydration, a 2.5 cm2 area of the forearms of all volunteers was treated twice a day with 25 μL (10 μL/cm2) of phytocera-H, phytocera-SB, phytocera-Mix, or C18-ceramide NP as a control. After 14 days of topical applications, skin hydration was evaluated using a Corneometer (Courage & Khazaka) from skins topically treated in an air-conditioned room at 20.4 ± 0.2°C and 40.5 ± 1.0% relative humidity. The changed value of arbitrary unit was calculated from each baseline and expressed as the mean ± standard error of the mean (SEM).
2-pyrrolidone-5-carboxylic acid (PCA) and urocanic acid (UCA) analysis
Quantitative analyses of NMF components, such as PCA and UCA, were conducted for eight female volunteers (aged 25.4 ± 1.3 years). The analytical method for PCA and UCA was performed according to previously described procedures with slight modifications.21 To quantify NMF components, a 2.5 cm2 area of the forearm of all volunteers was treated twice a day with phytocera-H, phytocera-SB, phytocera-Mix, or C18-ceramide NP as a control. After 14 days, the SC layer was harvested with D-squame tape (CuDerm Corporation) in an air-conditioned room at 20.4 ± 0.2°C and 40.5 ± 1.0% relative humidity. The first strip was discarded, and the second and third tape strips were pooled for the analysis. The total amount of SC protein on the tape was determined by bicinchoninic acid protein assay (Thermo Fisher Scientific, Waltham, MA, USA), and the levels of PCA and UCA were corrected for this protein value. Before high-performance liquid chromatography (HPLC) analysis, 750 μL of KOH solution was added to the tape strips, followed by 2 h of continuous shaking at 37°C. The alkaline extracts were neutralized with 3 μL of perchloric acid. A Synergi™ 4 μm Polar-RP-80A LC column (250 × 4.6 mm; Phenomenex, Macclesfield, UK) was attached to a Waters 600 HPLC System with a 486 Tunable Absorbance Detector (Waters Inc., Milford, MA, USA). The injection volume was 15 μL, and the flow rate was 0.4 mL/min. The mobile phase consisted of 4.3 mM hydrochloric acid, 0.1 mM sodium octane-1-sulfonate, and 2% acetonitrile at pH 2.4. PCA, trans-UCA, and cis-UCA were monitored at 210 nm.
Ethical approval of human study
All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards (approved by Dongguk University, DUIRB-20151127-010 and DUIRB-20161219-017).
Statistics
All data are presented as mean ± SEM. The data analysis between groups was performed using the Student’s paired t-test. Significance was defined as a p-value <0.05.
Results
Diverse composition of FAs
The FA compositions determined by GC are presented in Table 1. The FAs composition data of human SC were obtained from published data.8 The results clearly show that the FA composition of each natural oil-derived ceramide is well correlated with the profiles of that natural oil. FA chain compositions differed based on the type of oil. Although each natural oil provided a diversity of fatty acyl composition with respect to phytoceramide, the fatty acyl chain lengths were largely limited to long-chain FAs (C16–C18). To produce a phytoceramide containing more balanced and very long-chain fatty acyl moiety, such as C20, C22, and C24, a combination of several different plant oils such as macadamia nut, shea butter, moringa, and meadowfoam seed oil was designed to make phytocera-Mix. The fatty acyl chain compositions of phytocera-Mix, as shown in Table 1, seemed to more closely mimic those of human skin.
Table 1.
Diverse FA composition of phytoceramides analyzed using GC
| Sources of FA | FA chain length | 12:0 | 14:0 | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 | 20:0 | 20:1 | 20:4 | 22:0 | 22:1 | 22:2 | 24:0 | 24:1 | 26:0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human SC | FA in ceramide (face) | 0.1 | 4.3 | 9.8 | 4.3 | 6.1 | 3.8 | 0.3 | 7.0 | 2.0 | 43.9 | 10.8 | 7.7 | |||||
| FA in ceramide (leg) | 10.2 | 11.4 | 3.6 | 1.9 | 43.3 | 29.6 | ||||||||||||
| C18-ceramide NP | FA in ceramide | 100 | ||||||||||||||||
| Phytocera-H | FA in natural oil | 4.5 | 33.3 | 8.3 | 2.8 | 35.1 | 12.2 | 1.1 | ||||||||||
| FA in synthetic ceramide | 4.6 | 38.0 | 7.1 | 3.5 | 31.0 | 10.3 | 1.0 | |||||||||||
| Phytocera-M | FA in natural oil | 7.5 | 21.4 | 2.4 | 60.5 | 2.0 | 1.9 | 2.0 | ||||||||||
| FA in synthetic ceramide | 8.5 | 20.5 | 2.9 | 57.3 | 2.1 | 2.2 | 2.2 | |||||||||||
| Phytocera-SB | FA in natural oil | 0.29 | 0.20 | 6.12 | 28.41 | 60.49 | 1.65 | 1.15 | ||||||||||
| FA in synthetic ceramide | 0.30 | 0.20 | 6.13 | 27.93 | 59.67 | 1.77 | 1.01 | |||||||||||
| Phytocera-SF | FA in natural oil | 0.13 | 0.25 | 9.66 | 0.14 | 3.64 | 29.20 | 52.62 | 0.14 | 0.24 | 0.12 | 0.72 | 0.27 | |||||
| FA in synthetic ceramide | 0.12 | 0.24 | 7.80 | 0.13 | 2.99 | 28.14 | 58.11 | 0.17 | 0.18 | 0.10 | 0.56 | |||||||
| Phytocera-Mix | FA in natural oil | 8.04 | 3.14 | 6.31 | 52.25 | 2.90 | 2.35 | 15.86 | 3.20 | 3.04 | 3.11 | 0.70 | ||||||
| FA in synthetic ceramide | 7.88 | 2.67 | 4.24 | 51.01 | 3.49 | 2.23 | 18.79 | 3.45 | 3.94 | 3.82 | 0.63 |
Notes: The FA composition result of human SC was cited from previously published data.8 A distinct FA chain composition of each phytoceramide is shown. The FA composition of each natural oil-derived ceramide is well correlated with the profiles of that natural oil. Each analyzed data point was converted into percent.
Abbreviations: FA, fatty acid; GC, gas chromatography; SC, stratum corneum; NP, N-stearoyl phytosphingosine; phytocera-H, horse fat oil-derived ceramide; phytocera-M, macadamia nut oil-derived ceramide; phytocera-SB, shea butter-derived ceramide; phytocera-SF, sunflower oil-derived ceramide; phytocera-Mix, macadamia nut, shea butter, moringa, and meadowfoam seed oil-derived ceramide.
Microscopic observations of Maltese cross appearance
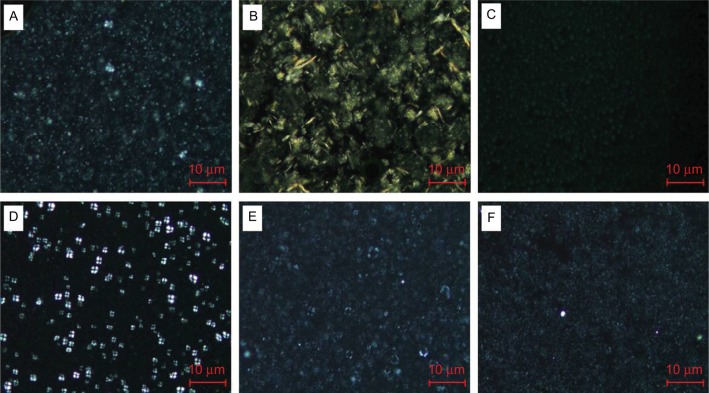
The cross polarization observation revealed a Maltese cross for the phytocera-M and phytocera-MO (Figure 1). The Maltese cross image is known for a distinct arrangement that appears in lamellar emulsion using cross-polarized microscopy.14 Owing to the hydrophobic interaction between alkyl chains, the molecular geometry prefers the formation of the strong hydrophobic forces between alkyl chains, resulting in macroscopic molecular crystallization in formulations. However, the Maltese cross appearance did not exhibit distinct optical anisotropy compared with other formulations. The result indicated that different FA profiles according to the types of phytoceramides affect physical behavior, such as lamellar structure formation.
Figure 1.
Polarized light photomicrographs of phytoceramides.
Notes: A Maltese cross appeared in the macadamia and moringa oil emulsion drops (D and E), whereas other formulations are not shown (×200; Nikon Corporation, Tokyo, Japan). Distinctive lamellar structure resulted from different FA profiles of each phytoceramides: (A) phytocera-H, (B) phytocera-SB, (C) phytocera-SF, (D) phytocera-M, (E) phytocera-MO, and (F) phytocera-MF.
Abbreviations: phytocera-H, horse fat oil-derived ceramide; phytocera-SB, shea butter-derived ceramide; phytocera-SF, sunflower oil-derived ceramide; phytocera-M, macadamia nut oil-derived ceramide; phytocera-MF, meadowfoam seed oil-derived ceramide.
Topical treatment of phytocera-H increased epidermal barrier recovery rate and SC cohesion
The result of topically applied phytocera-H on acute barrier-perturbed conditions is presented in Figure 2. Phytocera-H treatment of the damaged site exhibited a significantly accelerated recovery rate compared with the C18-ceramide NP control formulation (p < 0.05). Because the only difference between the phytocera-H and C18-ceramide NP was the composition of each ceramide, the result demonstrated that a mixture of ceramides composed of diverse FAs with different chain lengths had a stronger positive effect on skin barrier function than did a single C18-ceramide NP. As shown in Table 2, phytocera-H also markedly increased SC cohesion. In other words, it required a greater number of tape strippings to induce the same degree of barrier damage as the control group. This finding may be attributed to the increased distribution of the more tightly packed orthorhombic lipid lamellar organization upon application of phytocera-H.
Figure 2.
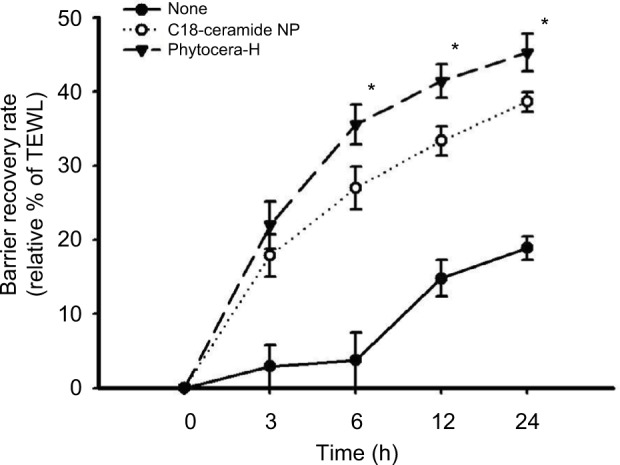
Increased epidermal barrier recovery rate by phytocera-H treatment of human forearm skin.
Notes: Topically treated phytocera-H affects rapid recovery than C18-ceramide NP control. The result indicates that phytocera-H accelerated recovery of mechanically damaged skin barrier compared to that of control. The recovery rate is presented as relative percent of the tape-perturbed value of each case and expressed as mean ± SEM. *p < 0.05. Tape stripping indicates tape-stripped control. C18-ceramide NP indicates tape-stripped conditions and C18-ceramide NP-treated control. Phytocera-H indicates tape-stripped conditions and phytocera-H-treated cases.
Abbreviations: phytocera-H, horse fat oil-derived ceramide; NP, N-stearoyl phytosphingosine; SEM, standard error of the mean; TEWL, transepidermal water loss.
Table 2.
SC cohesional activity of phytocera-H in human skin
| Treatment | TEWL after 24 h of treatment | TEWL after tape stripping | Number of tape strippings |
|---|---|---|---|
| None | 11.2 ± 1.9 | 35.3 ± 3.3 | 27.2 ± 6.9 |
| C18-ceramide NP | 11.0 ± 2.7 | 35.5 ± 2.9 | 29.8 ± 7.6 |
| Phytocera-H | 11.1 ± 1.9 | 35.3 ± 3.0 | 38.7 ± 12.0* |
Notes: Through topical application of phytocera-H, significantly increased SC cohesion resulted. From the result, it was suggested that the integrity of SC was significantly reinforced by the treatment of phytocera-H. Data are expressed as the mean ± SEM.
p < 0.05.
Abbreviations: SC, stratum corneum; phytocera-H, horse fat oil-derived ceramide; TEWL, transepidermal water loss; NP, N-stearoyl phytosphingosine; SEM, standard error of the mean.
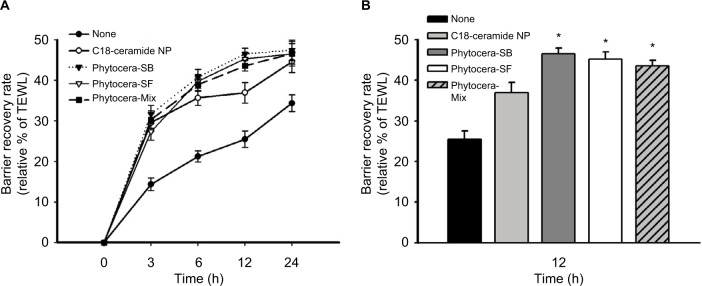
Acceleration of epidermal barrier recovery rate was also observed for other phytoceramides
Results obtained from phytocera-H prompted us to examine other phytoceramides derived from different oils. The effects of phytocera-SB and phytocera-SF on epidermal barrier recovery were similar to the effect of phytocera-H presented in Figure 3A. The recovery rate of each phytoceramide was significantly higher than that of the control C18-ceramide NP at 12 h after treatment (p < 0.05; Figure 3B). During epidermal barrier recovery, the initial recovery phase, which lasts until 12 h after the tape stripping process, is critical. This result indicated that phytocera-SB and phytocera-SF accelerated the recovery of the mechanically damaged skin barrier and effectively normalized the damaged lipid lamellar organization. However, the phytocera-Mix, which contained a very long-chain FA moiety, did not exhibit improved performance on the recovery of damaged epidermal barrier compared with phytocera-SB and phytocera-SF. Nonetheless, altogether, the results strongly support the notion that a mixture of phytoceramides mimicking the composition of ceramides found in human skin should be more effective than a single molecule of phytoceramide in providing a skin barrier protective effect.
Figure 3.
Increased epidermal barrier recovery rate by treatment of human forearm skin with phytocera-SB, phytocera-SF, and phytocera-Mix.
Notes: The recovery rate of phytocera-SB, phytocera-SF, and phytocera-Mix was significantly accelerated than that of the control C18-ceramide NP. (A) Time course change of recovery rate for 24h; (B) statistical significance was shown after 12h of topical treatment. Recovery rate is presented as relative percent of the tape-perturbed value of each case and expressed as mean ± SEM. *p < 0.05. Tape stripping indicates tape-stripped control, whereas C18-ceramide NP indicates tape-stripped conditions and C18-ceramide NP-treated control.
Abbreviations: phytocera-SB, shea butter-derived ceramide; phytocera-SF, sunflower oil-derived ceramide; phytocera-Mix, macadamia nut, shea butter, moringa, and meadowfoam seed oil-derived ceramide; NP, N-stearoyl phytosphingosine; SEM, standard error of the mean; TEWL, transepidermal water loss.
Enhancement of skin hydration by oil-derived phytoceramides
The results suggesting that all the oil-derived phytoceramides significantly improved the recovery rate after disruption of skin barrier by tape stripping in vivo human skin led us to further evaluate the effect of phytoceramides on skin hydration. Phytocera-H, phytocera-SB, and phytocera-Mix were topically applied for 14 days, and changes in skin hydration from baseline were measured using a Corneometer (Figure 4). All phytoceramides significantly increased skin hydration at 14 days after applications compared with the C18-ceramide NP control.
Figure 4.
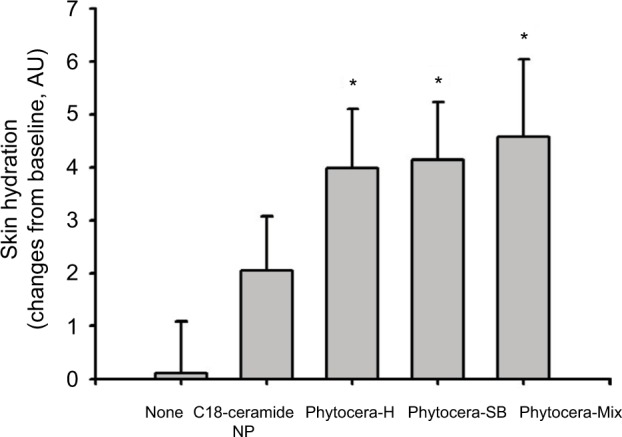
Improvement in skin hydration with topically applied phytoceramides.
Notes: Topical application of phytocera-H, phytocera-SB, and phytocera-Mix significantly increased skin hydration than C18-ceramide NP control. Changes in the value of AU was calculated from each baseline and expressed as mean ± SEM. *p < 0.05.
Abbreviations: phytocera-H, horse fat oil-derived ceramide; phytocera-SB, shea butter-derived ceramide; phytocera-Mix, macadamia nut, shea butter, moringa, and meadowfoam seed oil-derived ceramide; NP, N-stearoyl phytosphingosine; AU, arbitrary unit SEM, standard error of the mean.
PCA and UCA contents were increased by topically treated phytoceramides
The contents of PCA and UCA were measured to determine enhancement of skin hydration described in the previous section. Phytocera-H, phytocera-SB, and phytocera-Mix were topically applied for 14 days, and SC samples were collected by tape stripping. HPLC analysis was conducted to measure the amounts of PCA and UCA. Although the content of UCA isomers (trans-UCA and cis-UCA) was measured separately, the UCA content results indicated the total amount of UCA isomers as the sum of the concentration of both isomers. As shown in Table 3, phytocera-H, phytocera-SB, and phytocera- Mix treatment significantly increased PCA content at 14 days of applications compared with the C18-ceramide NP control. UCA content was also slightly increased by the topical treatment of all three phytoceramides compared with C18-ceramide NP, but statistical significance was not noted. These results suggest that the increase in NMF contents, such as PCA and UCA, contributed to the enhancement of SC hydration.
Table 3.
Changes in PCA and UCA contents by phytoceramide treatment of human forearm skin
| Treatment | None | C18-ceramide NP | Phytocera-H | Phytocera-SB | Phytocera-Mix |
|---|---|---|---|---|---|
| PCA content (μM/mL) | 164.7 ± 12.5 | 166.0 ± 13.1 | 176.1 ± 13.0* | 174.9 ± 11.5* | 179.5 ± 12.6* |
| UCA content (μM/mL) | 54.7 ± 6.0 | 56.4 ± 3.0 | 58.3 ± 8.2 | 59.0 ± 2.6 | 59.7 ± 10.7 |
Notes: An increase in the quantity of PCA and UCA contents was noted through topically treated phytocera-H, phytocera-SB, and phytocera-Mix. Data are expressed as mean ± SEM.
p < 0.05.
Abbreviations: PCA, 2-pyrrolidone-5-carboxylic acid; UCA, urocanic acid; NP, N-stearoyl phytosphingosine; phytocera-H, horse fat oil-derived ceramide; phytocera-SB, shea butter-derived ceramide; phytocera-Mix, macadamia nut, shea butter, moringa, and meadowfoam seed oil-derived ceramide; SEM, standard error of the mean.
Discussion
The primary purposes of this study were to synthesize a phytosphingosine-based ceramide pool containing N-acylated FAs with diverse chain lengths and to analyze its effect on the permeability barrier function of human skin. Initially, the study idea stemmed from previously published results. First, the skin barrier function, especially permeability barrier capability, depends on not only the level and composition of lipids, particularly the ceramide of SC, but also the chain length profiles of FAs in ceramides. Second, the N-acylated FA profiles of chain length analyzed from different parts of human skin, including face and leg, have a unique composition.2,5 A change in FA chain length is an important determinant for altered lipid lamellar organization in SC.5 Although C24 FA is the major constituent of ceramides from both human face and leg, the compositions of other FA species are distinctively different from each other, which suggests that a specific skin lesion exerts appropriate skin permeability barrier function by adopting a unique FA composition in ceramides.4,7,8,22
To obtain the diversity of FA chain length, horse fat oil, shea butter, sunflower oil, and a mixture of macadamia nut, shea butter, moringa, and meadowfoam seed oil were selected. These natural oils were chosen because they are currently being used in cosmetic formulations as key emollients and their FA profiles are distinctively different from each other. In addition, phytosphingosine was used as a sphingoid backbone in the synthetic process of ceramides. Phytosphingosine is one of the three major sphingoid backbones found in ceramide in human skin.23 Among these backbones, phytosphingosine is somewhat skin specific and is rarely found in other human organs. In addition, phytoceramides, ie, phytosphingosine-based ceramides (ceramide NP and AP), are the most abundant ceramide classes present in human SC.14–16 However, only limited classes of ceramides containing a specific FA, eg, ceramide NP and ceramide NS, have been used for the development of moisturizers.14–16
All formulations containing each phytoceramide were subjected to observations of Maltese cross appearance, and the results revealed that each phytoceramide exhibited a different lipid lamellar organization (Figure 1). In addition, markedly different physical properties in viscosity and texture were observed (data not shown). These results clearly demonstrated that a different FA composition in each phytoceramide is the only factor creating unique physicochemical properties.
The effects of each phytoceramide on the physiological properties of SC were evaluated based on two different aspects. The effect on the acute recovery of the damaged SC after tape stripping and the influence on skin hydration were assessed. First, formulated creams of phytocera-H or C18-ceramide NP mixed with cholesterol and FFAs were prepared and compared to assess their effect on the recovery rate in tape-stripped human skin. Once skin barrier was disrupted by tape stripping, ≥48 h is required for skin to fully restore the normal barrier function. Early stages in the recovery process after the disruption are critical for the entire recovery process. The application of phytocera-H accelerated recovery of permeability barrier much more rapidly than did the control cream. An increased enhancement of SC cohesion was also observed from a group treated with phytocera-H compared with that treated with C18-ceramide NP. Although the major determinant of SC cohesion is corneodesmosome, the integrity of intercellular lipid lamellar organization, which is interdigitated with the corneocyte lipid envelop, provides physical strength to support cohesion.24,25 Of note, the control cream that mimicked skin physiological lipid composition has been widely used in the development of moisturizers for dry and atopic skin. In fact, the control cream also markedly enhanced the recovery rate compared with the untreated control. Based on this preliminary result, we wanted to determine whether other natural oil-derived phytoceramides also exhibit similar performance. Thus, phytocera-SB, phytocera-SF, and phytocera-Mix were prepared. As shown in Figure 3, all the natural oil-derived phytoceramides exhibited outstanding performance in the recovery of permeability barrier and enhancement of SC hydration compared with C18-ceramide NP on human skin. The differences in each category of skin barrier assessment between C18-ceramide NP and the natural oil-derived phytoceramides were statistically significant, although only small number of volunteers were involved. However, no differences between oil-derived phytoceramides were observed, although each phytoceramide distinctively improved SC properties. In particular, the phytocera-Mix seemed slightly better than the other oil-derived phytoceramides, but no distinct differences were observed for other phytoceramides. If the number of participants is sufficiently large, the differences may be significant. In conclusion, the notion that a mixture of ceramides with diverse FA chain length, which more closely mimics the ceramide composition in human skin, should be more effective in restoration of damaged SC permeability barrier has been proven correct.
We also found that the augmentation of skin hydration by phytoceramides increased the amount of PCA, which is the most abundant molecule among NMF in corneocytes. Given that NMF, including PCA, is present inside corneocytes, the topical application of formulations of phytoceramides seemed to stimulate the generation of NMF in corneocytes. This finding may be due to the presence of phytosphingosine in each formulation. According to our recent study, phytosphingosine has a strong signaling effect on filaggrin synthesis and degradation to yield NMF (submitted separately).
Conclusion
In this study, we mainly conducted a series of in vivo human assessments to determine any differences between phytoceramides harboring diverse FAs and C18-ceramide NP regarding their effect on skin barrier function. We observed a striking contrast in their performance. The topical treatment of natural oil-derived ceramide, phytocera-H, phytocera-SB, phytocera-SF, and phytocera-Mix markedly improved epidermal homeostasis by strengthening the permeability barrier function in the intercellular space of SC (mortar) and corneocyte maturation by generation of NMF (brick). Further studies are required to elucidate the mechanism for the difference with respect to lipid lamellar organization at the SC.
Acknowledgments
This study was supported by the research program of Dongguk University, 2016.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580(23):5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Školová B, Janůšová B, Zbytovská J, et al. Ceramides in the skin lipid membranes: length matters. Langmuir. 2013;29(50):15624–15633. doi: 10.1021/la4037474. [DOI] [PubMed] [Google Scholar]
- 3.van Smeden J, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta. 2014;1841(3):295–313. doi: 10.1016/j.bbalip.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Uchiyama M, Oguri M, Mojumdar EH, Gooris GS, Bouwstra JA. Free fatty acids chain length distribution affects the permeability of skin lipid model membranes. Biochim Biophys Acta. 2016;1858(9):2050–2059. doi: 10.1016/j.bbamem.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Janůšová B, Zbytovská J, Lorenc P, et al. Effect of ceramide acyl chain length on skin permeability and thermotropic phase behavior of model stratum corneum lipid membranes. Biochim Biophys Acta. 2011;1811(3):129–137. doi: 10.1016/j.bbalip.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Kihara A, Mitsutake S, Mizutani Y, Igarashi Y. Metabolism and biological functions of two phosphorylated sphingolipids, sphingosine 1-phosphate and ceramide 1-phosphate. Prog Lipid Res. 2007;46(2):126–144. doi: 10.1016/j.plipres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Mizutani Y, Sun H, Ohno Y, et al. Cooperative synthesis of ultra long-chain fatty acid and ceramide during keratinocyte differentiation. PLoS One. 2013;8(6):e67317. doi: 10.1371/journal.pone.0067317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lampe MA, Burlingame AL, Whitney J, et al. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983;24(2):120–130. [PubMed] [Google Scholar]
- 9.Janssens M, van Smeden J, Gooris GS, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53(12):2755–2766. doi: 10.1194/jlr.P030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jungersted JM, Hellgren LI, Jemec GB, Agner T. Lipids and skin barrier function – a clinical perspective. Contact Dermatitis. 2008;58(5):255–262. doi: 10.1111/j.1600-0536.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 11.van Smeden J, Janssens M, Kaye EC, et al. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp Dermatol. 2014;23(1):45–52. doi: 10.1111/exd.12293. [DOI] [PubMed] [Google Scholar]
- 12.Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad. 2002;47(2):198–208. doi: 10.1067/mjd.2002.124617. [DOI] [PubMed] [Google Scholar]
- 13.Mao-Qiang M, Brown BE, Wu-Pong S, Feingold KR, Elias PM. Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131(7):809–816. doi: 10.1001/archderm.131.7.809. [DOI] [PubMed] [Google Scholar]
- 14.Park BD, Youm JK, Jeong SK, Choi EH, Ahn SK, Lee SH. The characterization of molecular organization of multilamellar emulsions containing pseudoceramide and type III synthetic ceramide. J Invest Dermatol. 2003;121(4):794–801. doi: 10.1046/j.1523-1747.2003.12470.x. [DOI] [PubMed] [Google Scholar]
- 15.Danby SG, Brown K, Higgs-Bayliss T, Chittock J, Albenali L, Cork MJ. The effect of an emollient containing urea, ceramide NP, and lactate on skin barrier structure and function in older people with dry skin. Skin Pharmacol Physiol. 2016;29(3):135–147. doi: 10.1159/000445955. [DOI] [PubMed] [Google Scholar]
- 16.Farwick M, Santonnat B, Lersch P, et al. An aquaporin-inspired lipid concentrate for mature skin. Cosmet Toiletries. 2008;123(4):69–74. [Google Scholar]
- 17.de Jager MW, Gooris GS, Ponec M, Bouwstra JA. Lipid mixtures prepared with well-defined synthetic ceramides closely mimic the unique stratum corneum lipid phase behavior. J Lipid Res. 2005;46(12):2649–2656. doi: 10.1194/jlr.M500221-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Berthaud F, Boncheva M. Correlation between the properties of the lipid matrix and the degrees of integrity and cohesion in healthy human stratum corneum. Exp Dermatol. 2011;20(3):255–262. doi: 10.1111/j.1600-0625.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- 19.Verdier-Sevrain S, Bonte F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6(2):75–82. doi: 10.1111/j.1473-2165.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 20.Rawlings AV. Sources and role of stratum corneum hydration. In: Elias PM, Feingold KR, editors. Skin Barrier. New York, NY: CRC Press; 2005. pp. 399–425. [Google Scholar]
- 21.Kezic S, Kammeyer A, Calkoen F, Fluhr JW, Bos JD. Natural moisturizing factor components in the stratum corneum as biomarkers of filaggrin genotype: evaluation of minimally invasive methods. Br J Dermatol. 2009;161(5):1098–1104. doi: 10.1111/j.1365-2133.2009.09342.x. [DOI] [PubMed] [Google Scholar]
- 22.Norlen L, Nicander I, Lundsjo A, Cronholm T, Forslind B. A new HPLC-based method for the quantitative analysis of inner stratum corneum lipids with special reference to the free fatty acid fraction. Arch Dermatol Res. 1998;290(9):508–516. doi: 10.1007/s004030050344. [DOI] [PubMed] [Google Scholar]
- 23.Coderch L, Lopez O, de la Maza A, Parra JL. Ceramides and skin function. Am J Clin Dermatol. 2003;4(2):107–129. doi: 10.2165/00128071-200304020-00004. [DOI] [PubMed] [Google Scholar]
- 24.Jonca N, Leclerc EA, Caubet C, Simon M, Guerrin M, Serre G. Corneodesmosomes and corneodesmosin: from the stratum corneum cohesion to the pathophysiology of genodermatoses. Eur J Dermatol. 2011;21(suppl 2):35–42. doi: 10.1684/ejd.2011.1264. [DOI] [PubMed] [Google Scholar]
- 25.Wertz PW, Swartzendruber DC, Kitko DJ, Madison KC, Downing DT. The role of the corneocyte lipid envelopes in cohesion of the stratum corneum. J Invest Dermatol. 1989;93(1):169–172. doi: 10.1111/1523-1747.ep12277394. [DOI] [PubMed] [Google Scholar]