The human TP53 gene encodes not only full-length p53 protein (FLp53, also termed p53α) but also more than a dozen of p53 protein isoforms due to alternative pre-mRNA splicing, transcriptional initiation from alternative promoters, and alternative initiation of protein translation. Among those p53 protein isoforms, an amino-terminally truncated isoform lacking the first 132 amino acid residues but otherwise identical to FLp53 (Δ133p53α, hereafter simply called Δ133p53) physiologically originates from a transcriptional initiation from the alternative promoter within intron 4. We have previously shown that this natural p53 isoform functions to inhibit FLp53-induced cellular senescence in normal human cells including fibroblasts, CD8+ T lymphocytes and brain astrocytes.1-3 The unique features of Δ133p53 include its degradation via chaperone-assisted selective autophagy,1 unlike well-recognized, proteasome-mediated degradation of FLp53, and its human/primate-specific nature due to lack of an initiating methionine in any other organisms examined at the position corresponding to the human codon 133.3 Our new study uncovers a novel role of this p53 isoform in human pluripotent stem cells.4
The first striking finding is that all human pluripotent stem cells examined, including induced pluripotent stem cells (iPSC) and embryonic stem cells (ESC), consistently express abundant levels of endogenous Δ133p53 protein (at least 10-fold higher than human fibroblasts and attributed to both increased mRNA levels and reduced autophagic degradation) (Fig. 1), while FLp53 protein levels in iPSC and ESC widely vary from 0.3- to 2.3-fold of that in human fibroblasts.4 We have also found that human iPSC and ESC express reduced levels of some p53-inducible genes, i.e., those that primarily induce cellular senescence (such as p21WAF1 and microRNA-34a), but maintained or increased levels of others involved in apoptosis and DNA damage repair (such as BAX, PUMA and p53R2) (Fig. 1).4
Figure 1.
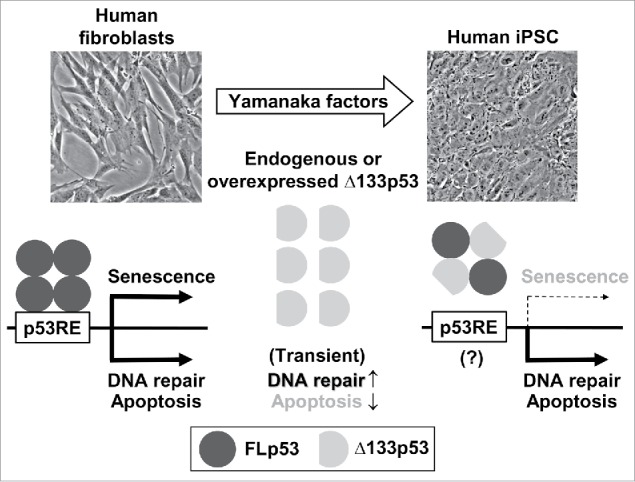
Δ133p53 enables iPSC reprogramming with genome stability in human cells. While human fibroblasts are committed to expressing p53-inducible genes involved in cellular senescence, apoptosis and DNA damage repair, human pluripotent stem cells are characterized by the preferential repression of those involved in cellular senescence, which is attributed to the activity of upregulated Δ133p53. Δ133p53 physically interacts with full-length p53 (FLp53) and dominant-negatively inhibits its binding to the p53 response element (p53RE) likely in a promoter context-dependent manner,4 although the exact stoichiometry of the Δ133p53-FLp53 interaction is still unknown (a heterotetramer consisting of 2 each is shown in this scheme). The molecular mechanisms by which Δ133p53 differentially regulates different subsets of p53-inducible genes are under investigation (indicated by a question mark). According to Gong et al.,5 Δ133p53 may also function independently of FLp53 to inhibit apoptosis and enhance DNA repair transiently during the reprogramming processes.
Our functional analysis suggests that Δ133p53 contributes to establishing this expression profile of different subsets of p53-inducible genes in iPSC and ESC, which is consistent with self-renewing capacity of these pluripotent stem cells (incompatible with p53-induced senescent proliferation arrest) and their ability to maintain genome stability (through p53-mediated repair of DNA damage and apoptotic elimination of severely damaged cells). Overexpression of exogenous Δ133p53 in human fibroblasts, while not repressing BAX, PUMA and p53R2, significantly represses p21WAF1 and microRNA-34a by dominant-negatively displacing FLp53 from the promoter regions of these genes,4 reproducing the expression profile of the p53-inducible genes in iPSC and ESC with upregulated endogenous Δ133p53 (Fig. 1). When induced to reprogram to iPSC by Yamanaka factors (Oct-4, Sox-2, Klf-4 and c-Myc), these Δ133p53-overexpressing human fibroblasts show 2- to 3-fold increased efficiency of iPSC generation compared with vector-transduced control fibroblasts,4 suggesting that increased levels of Δ133p53 plays a causative role in reprogramming human cells to pluripotent state (Fig. 1). Gong et al.5 also reported Δ133p53-mediated enhancement of iPSC generation through transient inhibition of apoptosis during reprogramming processes (Fig. 1).
Tumorigenicity is a possible safety concern associated with iPSC,6 especially when they are induced by inhibition of p53 activities.7 Nonetheless, iPSC clones we have established from Δ133p53-overexpressing fibroblasts, when injected into immuno-deficient mice, form well-differentiated benign teratomas with differentiation into all 3 germ layer-derived tissues and without malignant pathology.4 Both our study4 and Gong et al.5 suggest that Δ133p53 contributes to improved genome stability in iPSC. Their iPSC clones generated by a vector-based integrating method underwent chromosomal abnormalities, which were suppressed by overexpression of Δ133p53.5 Our iPSC clones generated by a synthetic mRNA-based, non-integrating method, whether or not their original fibroblasts overexpress Δ133p53, show normal karyotype without gross chromosomal abnormalities and have stable microsatellite repeats.4 Strikingly, these iPSC clones carry a fewer number of somatic mutations (such as single nucleotide substitutions and small insertions/deletions) than iPSC generated from p53-knocked-down fibroblasts.4 Overall, our findings support that p53 activities in human pluripotent stem cells are not simply inhibited, but rather are coordinately regulated by Δ133p53 to enable the establishment and maintenance of self-renewing capacity with secured genome stability (Fig. 1).
Δ133p53 functions to rescue aging- and tumor-associated functional decline in human CD8+ T lymphocytes, indicated by restored expression of central memory T cell markers CD62L and CD27 and loss of immune checkpoint proteins PD-1 and LAG-3.2 Δ133p53 also promotes human astrocytes to protect against neurodegeneration through inhibition of senescence-associated secretory phenotypes, including neurotoxic IL-6.3 Given the role of Δ133p53 in genetic and functional integrity of human pluripotent stem cells,4 we now propose that Δ133p53 contributes to multiple aspects of normal development and healthy lifespan in humans, and that it can be targeted for enhancement toward future clinical applications in T cell-mediated immunotherapy against cancer and chronic infection, astrocyte-mediated therapy for Alzheimer disease and other neurodegenerative diseases, and stem cell-based regenerative medicine. Considering that Δ133p53 is present only in humans and primates,3 we also speculate that the lack of a physiological counterpart of Δ133p53 in mice, which would require a substitutive, potentially tumorigenic mechanism, may be associated with higher incidences of teratocarcinoma from mouse iPSC and development of malignant tumors in their derived chimeras.6 Lastly, we are currently investigating the molecular details of the Δ133p53 regulation of different subsets of p53-inducible genes (Fig. 1).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
References
- [1].Horikawa I, Fujita K, Jenkins LM, Hiyoshi Y, Mondal AM, Vojtesek B, Lane DP, Appella E, Harris CC. Autophagic degradation of the inhibitory p53 isoform Δ133p53α as a regulatory mechanism for p53-mediated senescence. Nat Commun 2014; 5:4706; PMID:25144556; https://doi.org/ 10.1038/ncomms5706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mondal AM, Horikawa I, Pine SR, Fujita K, Morgan KM, Vera E, Mazur SJ, Appella E, Vojtesek B, Blasco MA, Lane DP, Harris CC.. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J Clin Invest 2013; 123:5247-57; PMID:24231352; https://doi.org/ 10.1172/jci70355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Turnquist C, Horikawa I, Foran E, Major EO, Vojtesek B, Lane DP, Lu X, Harris BT, Harris CC. p53 isoforms regulate astrocyte-mediated neuroprotection and neurodegeneration. Cell Death Differ 2016; 23:1515-28; PMID:27104929; https://doi.org/ 10.1038/cdd.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Horikawa I, Park KY, Isogaya K, Hiyoshi Y, Li H, Anami K, Robles AI, Mondal AM, Fujita K, Serrano M, Harris CC. Δ133p53 represses p53-inducible senescence genes and enhances the generation of human induced pluripotent stem cells. Cell Death Differ 2017; 24:1017-28; PMID:28362428; https://doi.org/ 10.1038/cdd.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gong L, Pan X, Chen H, Rao L, Zeng Y, Hang H, Peng J, Xiao L, Chen J. p53 isoform Δ133p53 promotes efficiency of induced pluripotent stem cells and ensures genomic integrity during reprogramming. Sci Rep 2016; 6:37281; PMID:27874035; https://doi.org/ 10.1038/srep37281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer 2011; 11:268-77; PMID:21390058; https://doi.org/ 10.1038/nrc3034 [DOI] [PubMed] [Google Scholar]
- [7].Krizhanovsky V, Lowe SW. Stem cells: The promises and perils of p53. Nature 2009; 460:1085-6; PMID:19713919; https://doi.org/ 10.1038/4601085a [DOI] [PMC free article] [PubMed] [Google Scholar]


