Abstract
Purpose
Microbial contamination of different cosmetic preparations, as a result of preservative failure, presents a major public health threat. Also, most of the known preservatives have serious consumer side effects. The antimicrobial activity of zinc oxide nanoparticles (ZnO NP) is well documented. Therefore, we aimed to determine the possible use of unirradiated and γ-irradiated ZnO NP as a cosmetic preservative.
Methods
The possible use of ZnO NP as a preservative was tested and compared to commonly used preservatives using a challenge test. Their activity was tested in six different types of preparations. The effect of γ radiation on the antimicrobial activity of ZnO NP was tested through determination of the obtained zone diameters against different microorganisms and the total aerobic microbial count in tested preparations. The antimicrobial activity, of unirradiated and γ-irradiated ZnO NP during storage was also determined.
Results
ZnO NP were superior to other commonly used preservatives in all tested cosmetic preparations. They pass the challenge test in all types of tested preparations. γ irradiation enhanced their antimicrobial activity in all tested preparations. The irradiation causes a reduction in NP sizes that is directly proportional to the applied radiation dose. Upon storage, ZnO NP were effective in maintaining the microbial count of the product within the acceptable range. Their activity in stored products was enhanced by γ irradiation.
Conclusion
Unirradiated and γ-irradiated ZnO NP can be used as effective preservatives. They are compatible with the components of all tested products. γ irradiation enhanced the antimicrobial activity of ZnO NP.
Keywords: antimicrobial activity, challenge test, γ irradiation, particle size, preservative, zinc oxide nanoparticles
Introduction
Microbial contamination of cosmetics is very crucial because of their daily use and direct contact with the skin. Their contamination arises from various sources such as environment, raw materials, and manufacturing process.1 Several studies have revealed that cosmetic products may be contaminated with pathogenic microorganisms to different levels.1–4 Escherichia coli, Pseudomonas species, Staphylococcus species, and Bacillus species were the most commonly recovered bacteria from cosmetics.5,6
Contamination with pathogenic organisms can adversely affect the product stability and cause hazards to consumer health.7 In addition, commonly used preservatives such as parabens, sodium benzoate, and phenoxyethanol have a well-known skin-sensitizing potential, and repeated exposure is responsible for the occurrence of contact allergy, especially when combined with other allergens and skin irritants.8 The cosmetic industry is also facing some restrictions regarding the use of some preservatives like parabens, which are accused of causing breast cancer.9 Therefore, there is a need for incorporation of more safe and effective antimicrobial agents in cosmetics.
Inorganic powders such as zinc oxide (ZnO) represent a promising alternative to these harmful organic preservatives. ZnO is listed as “generally recognized as safe” by the US Food and Drug Administration (21CFR 182.8991) and is widely used in topical pharmaceutical preparations.10 Several mechanisms have been proposed to account for the antimicrobial activity of ZnO: photochemical reactions coming from the semi-conductive properties of ZnO which generate reactive oxygen species capable of damaging the cell membranes of microorganisms, the partial dissolution of ZnO particles which releases cytotoxic Zn2+ ions in water, and the adsorption of ZnO particles onto the microbial cells that destabilizes the microbial cell walls.9 The compatibility of ZnO with different formulation ingredients in some topical preparations had been described by Pasquet et al.10
Nanotechnology represents a new research area of modern science. The antimicrobial activity of metal oxide nanomaterials has become of great concern. Their inhibitory effect on both Gram-positive and Gram-negative bacteria has been recently reported and zinc oxide nanoparticles (ZnO NP) exhibited the best activity compared to all tested metal oxide nanomaterials.11 Nanosized particles of ZnO have been claimed to possess pronounced antimicrobial activities than larger particles; considering the fact that the small size (less than 100 nm) and the high surface-to-volume ratio of NP allow for better interaction with bacteria.12 Pasquet et al9 revealed that the antimicrobial properties of ZnO particles are highly affected by its physicochemical properties. This phenomenon may not be prominent in the NP of ZnO due to their small diameter which helps in their dispersion in solutions.
Swaroop et al13 reported an increase in the antibacterial properties of ZnO NP with γ irradiation, on two tested Gram-negative bacteria (Klebsiella pneumoniae and P. aeruginosa). However, there are no studies considering the effect of γ irradiation on the antimicrobial properties of ZnO NP against other organisms. In this regard, ionizing radiation has been reported to modify the specific surface area of some solids.14
This study aimed to test the possible use of ZnO NP, either unirradiated or γ-irradiated, as a safe compatible effective preservative in different cosmetic preparations. The sustainability of this preservative effect upon storage was also tested.
Materials and methods
Samples
Six types of cosmetic products, without the addition of any preservatives, were used: sunblock cream, foundation cream, moisturizing cream, body lotion, face cream, and scrub cream. The constituents of the tested products are given in Table S1. They were supplied by Dr Joe Factory and Jolly for cosmetics, Cairo, Egypt. All the samples were stored at 4°C until use. Prior to use, the samples were inspected for any physical defects. Propylparabens and phenoxyethanol were supplied by Dr Joe Factory and Jolly for cosmetics, respectively. ZnO NP with diameter of 1–100 nm were purchased from Sigma-Aldrich (code: 544906, St Louis, MO, USA). They have a formula weight of 81.39 g mol−1 and a specific surface area of 15–25 m2g−1.
Microorganisms
Standard microorganisms were purchased from VACSERA (Giza, Egypt), and these were P. aeruginosa (ATCC 27856), E. coli (ATCC 25922), S. aureus (ATCC 25923), Candida albicans (ATCC 90028), and Aspergillus niger (ATCC 22343). In addition, P. aeruginosa, E. coli, S. aureus, and C. albicans isolates that were previously recovered from contaminated cosmetic preparations were also included in the study.
γ Irradiation facility
All irradiations were performed using the cobalt-60 source (Gamma cell 4000A, India) located at the National Center for Radiation Research and Technology, Cairo, Egypt. A dose rate of 1.77 kGy h−1 was used for the experiment.
Minimum inhibitory concentration and minimum bactericidal/fungicidal concentration of ZnO NP
The minimum inhibitory concentration (MIC) of ZnO NP was determined by broth macrodilution method according to the Clinical and Laboratory Standards Institute recommendations.15 ZnO NP were used in a concentration range of 0.01–2.5 µg mL−1. One milliLiter of each tested microbial strain, at 5×106 CFUmL−1 inoculum density, was added to different ZnO NP concentrations. They were incubated at 37°C for 24 h. The MIC values were taken as the lowest concentration of ZnO NP solution that inhibits the microbial growth. The minimum bactericidal/fungicidal concentration (MBC/MFC) was determined by subculturing 50 µL from each test tube showing no apparent growth on tryptic soy agar and Sabouraud dextrose agar plates, respectively, and incubating the plates at 37°C for 24 h. The least concentration of the test solution showing no visible growth (less than 10 CFU/plate) after subculturing was taken as MBC for bacteria and MFC for C. albicans. All measures were taken in duplicate.16
Efficiency of ZnO NP as a preservative compared to some commonly used preservatives
A challenge test was used to investigate the efficiency of ZnO NP as a compatible preservative in the tested cosmetic preparations.17 Freshly grown culture of the test organisms P. aeruginosa (ATCC 27856), E. coli (ATCC 25922), S. aureus (ATCC 25923), C. albicans (ATCC 90028), and A. niger (ATCC 22343) were harvested in sterile saline and adjusted to a density of 1×108 CFUmL−1. They were then used for inoculating 30 g of each cosmetic preparation, in aseptic state, to reach a final inoculum of 106 CFUg−1. Each product was divided into three equal parts and the test preservatives were added in the following concentrations: ZnO NP (0.62 µg g−1), propylparabens (0.3%), and phenoxyethanol (2%).18,19 All inoculated samples were shaken and incubated at 25°C for 28 days. Two grams of the inoculated samples were removed on days 0, 7, 14, and 28 and serially diluted in saline to determine their total aerobic microbial count. The efficiency of the dilution as a neutralizer of the preservative activity was confirmed through validation of the recovery of tested organisms.
According to United States Pharmacopeia (USP) 2014, the preservative in a tested sample will pass the test if it causes a reduction in the total aerobic microbial number of the challenging bacteria by >2 log reduction, from the initial count after 14 days, and no increase from the 14 days count after 28 days of challenge, and no increase from the initial count after 14 and 28 days of challenge with fungi.17
Antimicrobial activity of γ-irradiated ZnO NP in cosmetic preparations
The use of γ irradiation in microbial decontamination is advantageous for finished cosmetic products as well as raw materials. This method does not leave residues that can be harmful to workers or consumers.20 In addition, γ irradiation was found to enhance the antimicrobial activity of ZnO NP.13 Therefore, the antimicrobial activity of in situ γ-irradiated ZnO NP was determined through measurement of their inhibition zone diameter against different standard and isolated microbial strains as well as through determination of their effect on the total aerobic microbial count of the different tested preparations.
ZnO NP were added to each preparation at their MIC (0.62 µg g−1). The preparations were then divided into equal parts and exposed to different γ radiation doses (1, 3, 5, 7 kGy). γ-Irradiated cream portions without ZnO NP were used as a control.
Determination of inhibition zone diameters against different standard and isolated microbial strains
Overnight cultures of standard and isolated test organisms (S. aureus, P. aureginosa, E. coli, and C. albicans) were diluted to 5×106 CFU mL−1 in nutrient broth and streaked on the surface of solidified tryptic soy agar and Sabouraud dextrose agar plates for bacteria and C. albicans, respectively. Wells were then made in the inoculated agar plates and loaded with 50 µg of tested cream portions. The plates were incubated overnight at 37°C, and the diameters of the inhibition zones were then determined. The inhibition zone diameters were considered as a measure of the antimicrobial activity of irradiated ZnO NP in different tested preparations against standard and isolated microbial species.21
Determination of the total aerobic microbial count in tested preparations
One gm from each tested preparation was mixed with 9 mL sterile saline-tween and tenfold serial dilution was made in the same diluents. Aliquots of 0.1 mL were taken from each dilution and spread, in duplicate, on sterile plates containing tryptic soy agar. They were incubated at 35°C±2°C and examined daily for up to 72 h. The mean of the count of duplicate plates was reported in CFUmL−1.
Testing the possible effect of γ irradiation on the size of NP
Dry ZnO NP powder was suspended in deionized water at a concentration of 0.62 µg mL−1. This was sonicated at room temperature for 10 min to form a homogenous suspension. The resulting solution was divided into two portions and irradiated with either 3 or 7 kGy γ radiation doses, at room temperature. The average particle size of all samples was studied using Dynamic light scattering (DLS; Malvern, Malvern, UK) and transmission electron microscope (TEM; JEM-2100, Jeol USA, Inc., Peabody, MA, USA).22 The particle size of unirradiated solution was used as control.
Antimicrobial activity of unirradiated and γ-irradiated ZnO NP during product storage
The antimicrobial activity of ZnO NP during product storage was tested. Each product was divided into three equal parts in separate containers; ZnO NP (0.62 µg mL−1) were added to two of them, and one of the ZnO NP-containing preparations was exposed to γ irradiation dose of 7 kGy (the highest effective dose). The third container was used as a control with no added preservative. The containers were stored for 10 months, and samples were withdrawn every 2 months for the determination of total aerobic microbial count, as described in the “Determination of the total aerobic microbial count in tested preparations” section.
Results and discussion
MIC and MBC/MFC of ZnO NP
The MIC and MBC were the same for both the standard and isolated microorganisms: 0.31, 0.16, 0.62, and 0.31 µg mL−1 for E. coli, S. aureus, P. aeruginosa, and C. albicans, respectively. This indicated that ZnO NP are bactericidal in nature. The differences in the susceptibility of bacteria to ZnO NP was previously reported to be related to the differences in the cell wall structure, cell physiology, metabolism, or the degree of contact.23 The high MIC value recorded with P. aeruginosa indicated its higher resistance to ZnO NP, compared to other tested bacteria, which may be due to its intrinsic resistance caused by low intrinsic cell wall permeability and multiple efflux systems.24 Higher MIC values of ZnO NP against E. coli and S. aureus (6.25 µg mL−1 for both) were reported by Singh et al.12 Also, Yousef et al25 reported higher MIC values for ZnO NP against different organisms. This variation in the MIC values in different studies may be attributed to the difference in the method used for the preparation of NP, which can affect their particle size.25,26 Several mechanisms were proposed for the antimicrobial activity of ZnO NP including the generation of reactive oxygen species and the release of toxic zinc ions which can inhibit the active transport and disturb amino acid metabolism.27
The efficiency of ZnO NP as a preservative compared to some commonly used preservatives
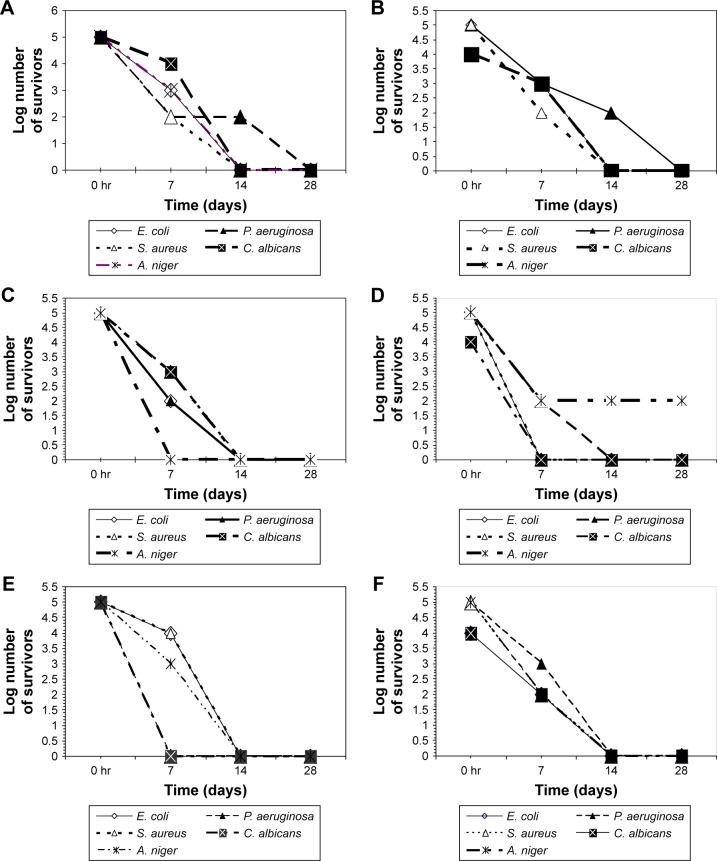
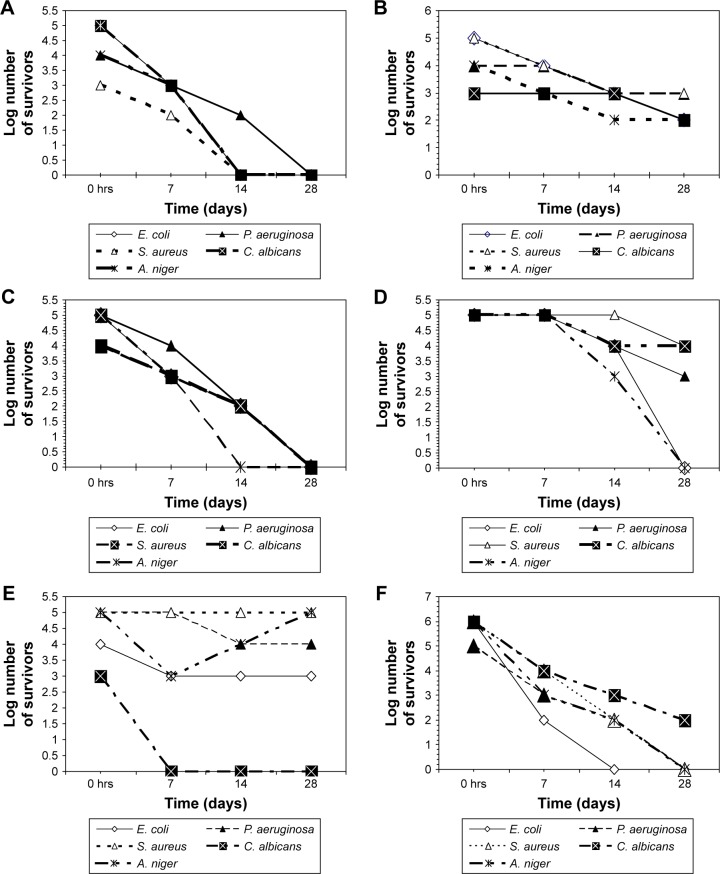
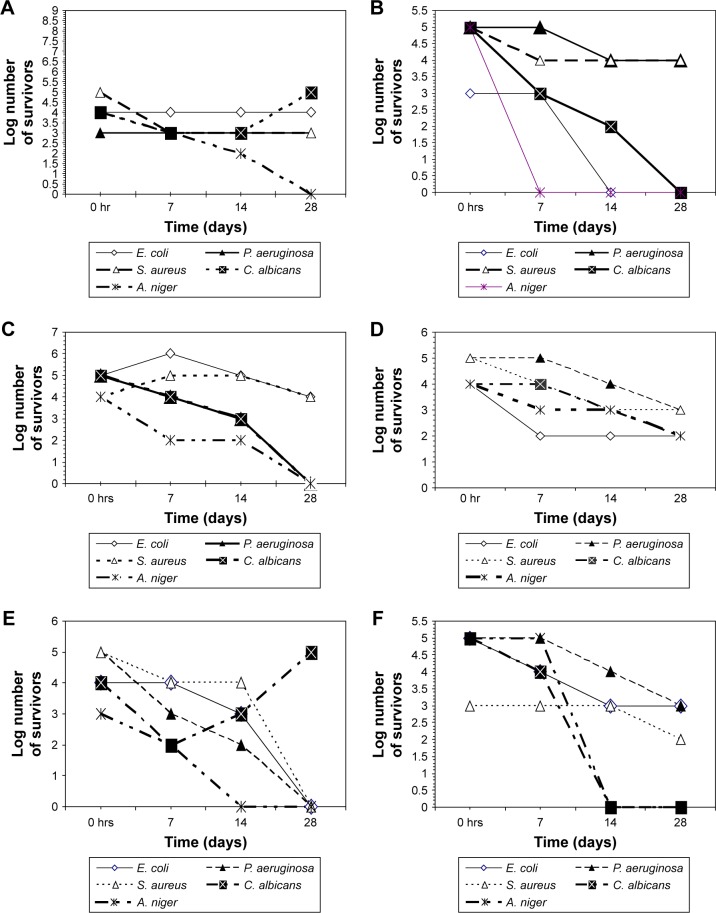
The challenge test revealed that ZnO NP were superior to propylparabens and phenoxyethanol (the most commonly used preservative). ZnO NP were capable of fulfilling the USP criteria for preservatives used in topical aqueous preparations in all tested cosmetic preparation types, indicating their compatibility with different preparation constituents (Figure 1). On the contrary, propylparaben was effective as a preservative and fulfilled the USP criteria for preservatives in only the sunblock, moisturizing cream, and the body scrub preparations. However, it failed USP criteria as a preservative in the foundation cream, body lotion, and the face cream (Figure 2). This may be caused by its incompatibility with various components of these preparations. Phenoxyethanol failed to fulfill the USP preservative criteria by the challenge test in any of the tested preparations (Figure 3).
Figure 1.
Number of survivors with time in tested preparations challenged with different microorganisms in the presence of ZnO NP as preservative.
Note: (A) Sunblock, (B) foundation cream, (C) moisturizing cream, (D) body lotion, (E) face cream, (F) body scrub.
Abbreviations: A. niger, Aspergillus niger; C. albicans, Candida albicans; E. coli, Escherichia coli; P. aeruginosa, Pseudomonas aeruginosa; S. aurerus, Staphylococcus aureus; ZnO NP, zinc oxide nanoparticles.
Figure 2.
Number of survivors with time in tested preparations challenged with different microorganisms in the presence of propylparaben as preservative.
Note: (A) Sunblock, (B) foundation cream, (C) moisturizing cream, (D) body lotion, (E) face cream, (F) body scrub.
Abbreviations: A. niger, Aspergillus niger; C. albicans, Candida albicans; E. coli, Escherichia coli; P. aeruginosa, Pseudomonas aeruginosa; S. aurerus, Staphylococcus aureus; ZnO NP, zinc oxide nanoparticles.
Figure 3.
Number of survivors with time in tested preparations challenged with different microorganisms in the presence of phenoxyethanol as preservative.
Note: (A) Sunblock, (B) foundation cream, (C) moisturizing cream, (D) body lotion, (E) face cream, (F) body scrub.
Abbreviations: A. niger, Aspergillus niger; C. albicans, Candida albicans; E. coli, Escherichia coli; P. aeruginosa, Pseudomonas aeruginosa; S. aurerus, Staphylococcus aureus; ZnO NP, zinc oxide nanoparticles.
Failure of nonsterile products preservative is well documented. Sutton and Jimenez28 reported that 15% of nonsterile product recalls in the years 2004–2011 were due to microbial contamination. Several cosmetic products recalls due to microbiological contamination still occur frequently.29 Therefore, this study highlights the efficiency of ZnO NP as a superior preservative to propylparaben and phenoxyethanol. Its safety is well documented. The Scientific Committee on Consumer Safety of the European Commission has documented the safety of using ZnO NP in topical preparations as an ultraviolet filter in concentrations up to 50% without any toxic in vivo effect, either acute or chronic. They reported that ZnO NP either coated or uncoated do not penetrate the skin. They have the advantage of being transparent compared to the larger ZnO particles.30 Several studies also documented the lack of acute dermal toxicity, sensitization, and irritation on using ZnO NP.31,32 The US Food and Drug Administration does not approve cosmetic preparations prior to market use. It issued a guidance on the safety of nanomaterials in cosmetic products, in June 2014.33 Since then, ZnO NP have been incorporated in a lot of sunblock preparations,34 and there has been no documented FDA product recall due to its use.35
Antimicrobial activity of γ-irradiated ZnO NP in cosmetic preparations
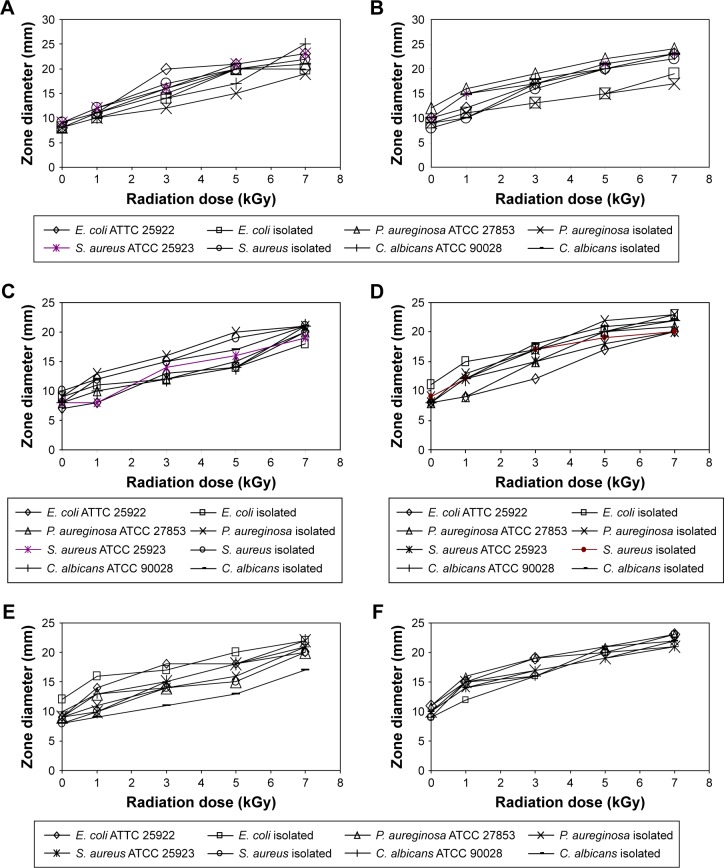
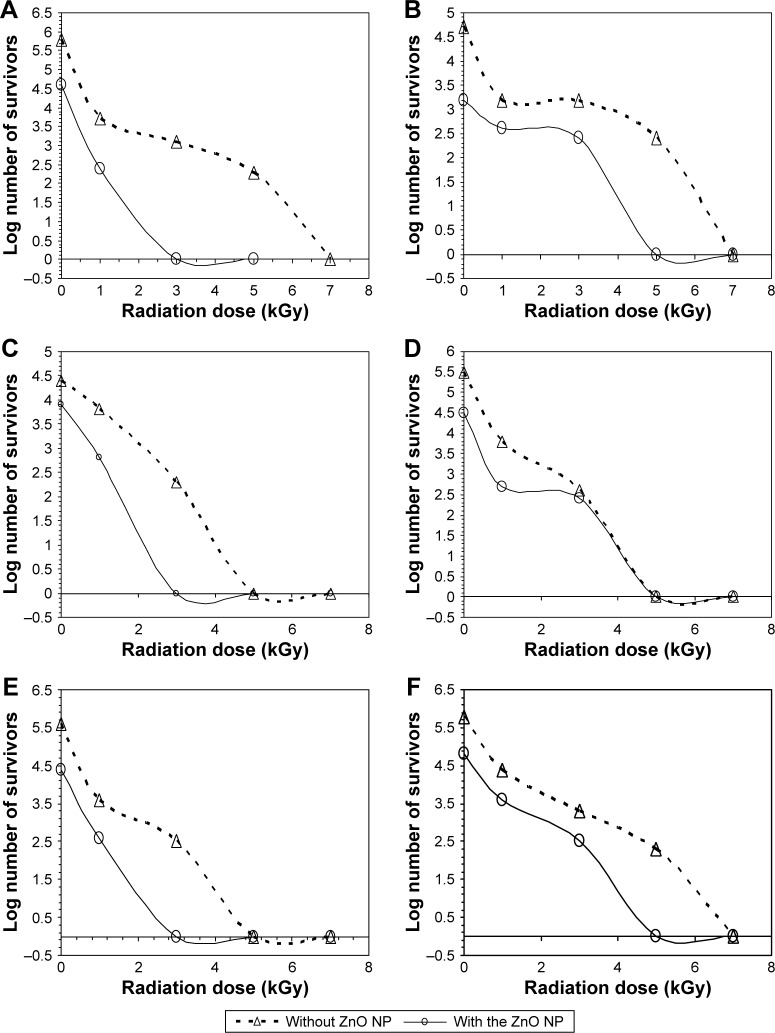
The antimicrobial activity of γ-irradiated ZnO NP in the tested cosmetic preparations was confirmed by the presence of inhibition zones as well as by the reduction in the total aerobic microbial count in all tested cosmetic preparations compared to the control (Figures 4 and 5). Cosmetics without ZnO NP did not show any inhibition zone against the tested microorganisms. The presence of ZnO NP reduced the initial aerobic microbial count in all of the tested preparations by at least one log. The antimicrobial activity of ZnO NP has been previously reported by Yousef et al,25 Wang et al,26 Liu et al,36 Rizwan et al,37 and Vani et al.38 However, in our study, we proved the compatibility of ZnO NP with the different tested cosmetics and its possible use in the preservation of these preparations. By applying different γ radiation doses to the preparations containing ZnO NP, the antimicrobial activity of ZnO NP was enhanced with the increase in the radiation dose. This was indicated by the increase in zone diameters against the tested microorganisms (Figure 4) and the decrease in the total aerobic microbial counts (Figure 5) until complete microbial decontamination at 3 or 5 kGy depending on the type of the preparation. The effect of γ radiation on the antimicrobial activity of ZnO NP has been reported previously on K. pneumoniae and P. aeruginosa.15 In this study, it was clear that applying γ radiation on ZnO NP resulted in enhancing its antimicrobial activity against S. aureus, E. coli, C. albicans, and P. aeruginosa. The γ-irradiated ZnO NP produced their antimicrobial activity in all tested cosmetic creams, indicating their compatibility with the tested constituents and the possible use of this combination in their preservation. The effect of γ irradiation may be due to its activation of ZnO NP and the production of H2O2 that can penetrate the microbial cell membrane and kill the bacteria.25,39,40 Also, it may be due to the reduction in the specific surface area of the particles by irradiation, as reported for some solids.14 This possible size reduction was further tested by using TEM and DLS analysis.
Figure 4.
The effect of different γ radiation doses on the zone diameters of ZnO NP-containing preparations against different microorganisms.
Note: (A) Sunblock, (B) foundation cream, (C) moisturizing cream, (D) body lotion, (E) face cream, (F) body scrub.
Abbreviations: C. albicans, Candida albicans; E. coli, Escherichia coli; P. aeruginosa, Pseudomonas aeruginosa; S. aurerus, Staphylococcus aureus; ZnO NP, zinc oxide nanoparticles.
Figure 5.
The effect of different γ radiation doses on the total aerobic microbial count in cosmetic preparations with and without 0.62 µg g−1 ZnO NP.
Note: (A) Sunblock, (B) foundation cream, (C) moisturizing cream, (D) body lotion, (E) face cream, (F) body scrub.
Abbreviation: ZnO NP, zinc oxide nanoparticles.
The effect of radiation on the size of ZnO NP
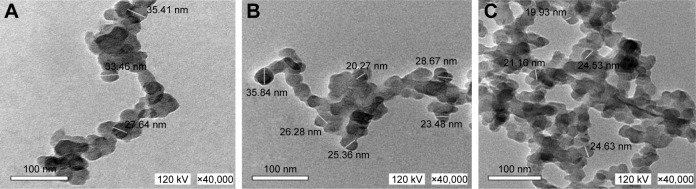
The results of the DLS measurement indicated a reduction in the size of ZnO NP with γ irradiation, with the particle size decreasing with the increase in the radiation dose. The predominant particle sizes were 127.5, 110.1, and 7.5 nm for unirradiated, 3, and 7 kGy-irradiated ZnO NP, respectively (Table 1). A similar reduction in ZnO NP size with γ irradiation has been also reported in the TEM micrographs (Figure 6). To the best of our knowledge, this is the first report on the reduction of ZnO NP diameter with γ irradiation, and this can account for the enhanced antimicrobial activity of ZnO NP with γ irradiation.
Table 1.
The average and predominant particle sizes of ZnO NP subjected to different γ radiation doses as determined by DLS
| γ irradiation dose (kGy) | Average particle size (nm) | Predominant particle size (nm) |
|---|---|---|
| 0 (unirradiated) | 70.9–307 | 127.5 |
| 3 | 61.2–265.6 | 110.1 |
| 7 | 4.8–18.17 | 7.5 |
Abbreviations: DLS, dynamic light scattering; ZnO NP, zinc oxide nanoparticles.
Figure 6.
TEM images of ZnO NP.
Note: (A) Unirradiated, (B) irradiated at 3 kGy dose of γ radiation, (C) irradiated at 7 kGy dose of γ radiation.
Abbreviations: TEM, transmission electron microscopy; ZnO NP, zinc oxide nanoparticles.
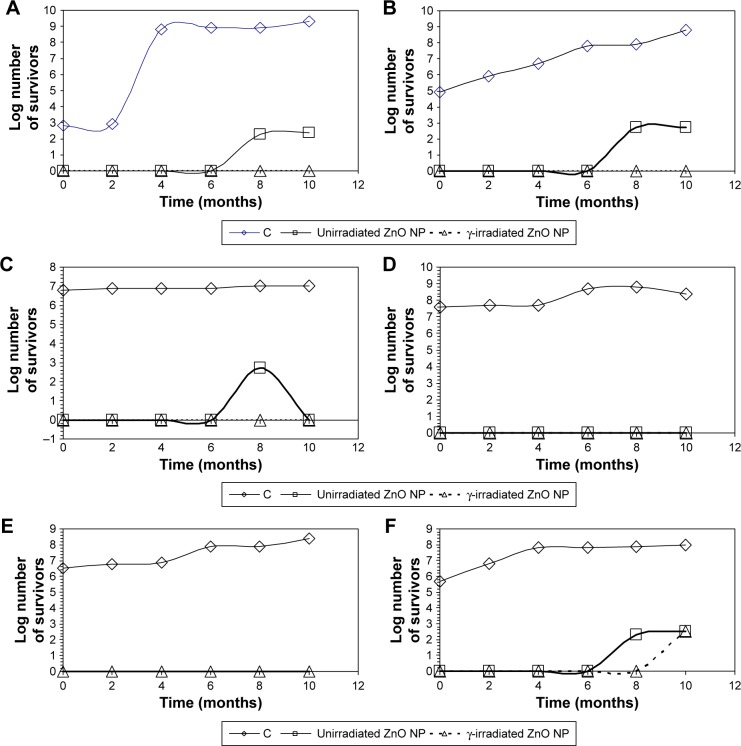
The antimicrobial activity of unirradiated and γ-irradiated ZnO NP during product storage
Preparations containing unirradiated ZnO NP (0.6 µg g−1) showed no detected growth (count <10 CFUg−1) up to 6 months. However, the count reached 102 CFUmL−1 after 8 months in most preparations, with no increase thereafter. γ-irradiated ZnO NP were superior to unirradiated nanoparticles and kept the microbial count below the detectable level (10 CFUmL−1) for up to 10 months except in body scrub preparation where the count reached the acceptable limit (102 CFUmL−1) after 10 months (Figure 7). This confirmed the efficiency of ZnO NP as a preservative in cosmetic preparations and the effect of γ radiation on enhancing their activity. In addition, unirradiated and γ-irradiated ZnO NP proved to be compatible with the components of the tested preparations during the product storage. The only limitation of this study is the short storage period, and so longer storage periods need to be tested.
Figure 7.
The number of survivors in cosmetic preparations, either without ZnO NP (C) or containing unirradiated and γ-irradiated ZnO NP, with time.
Note: (A) Sunblock, (B) foundation cream, (C) moisturizing cream, (D) body lotion, (E) face cream, (F) body scrub.
Abbreviation: ZnO NP, zinc oxide nanoparticles.
Conclusion
ZnO NP have a strong antimicrobial preservative activity against pathogenic organisms in topical preparations. This activity is enhanced by γ irradiation, mainly due to particle size reduction. ZnO NP provide a superior, safe, and more effective alternative to commonly known preservatives, and they also proved to be stable on storage.
Supplementary material
Table S1.
List of the 77 raw materials used in manufacture of the six tested products
| Sunblock | Foundation cream | Moisturizing cream | Body lotion | Face cream | Body scrub |
|---|---|---|---|---|---|
| Stearic acida | Stearic acida | Lanette 16 (cetyl alcohol)a | Stearic acidb | Stearic acidb | Stearic acidb |
| Sorbitan stearate | Lanet o (cetearyl alc)a | Lanet o (cetearyl alc)a | Lanet o (cetearyl alc)b | Lanet o (cetearyl alc)a | Citric acid |
| Caprylictriglyceride | Paraffin oila | Paraffin oila | Glyceryl monostearatea | Paraffin oilb | Propanediol |
| Lanette 16 (cetyl alcohol)a | Vaseline | Glycerina | Tapioca starch | Glycerinb | Sodium benzoate |
| Isopropyl myristatea | Vaseline | Linalool | Isopropyl myristateb | Lanet o (cetearyl alc)a | |
| Lanet o (cetearyl alc)a | Titanium dioxidea | Isopropyl myristatea | Paraffin oilb | Triethanolamineb | |
| Zinc oxidea | Vitamin E | Glycerinb | Mono propylene glycolb | Carbomer | |
| Glyceryl monostearateb | Octyl methoxy cinnamate | Triethanolaminea | Isopropyl myristateb | Tween 80 | Imidazolidinyl urea |
| Emulgin B2 | Triethanolamineb | Dimethicone silicone | Sodium hydroxide | ||
| Octocrylene | Jojoba oil | Panthenol | Mono propylene glycolb | Fragrancea | Sodium hydroxide |
| Cetyl palmitate | Emulgin B2 | Mono propylene glycola | Dimethicone silicone | Paraffin oilb | |
| Paraffin oila | Panthenol | Vitamin A | Butylene glycol | Glycerinb | |
| Laureth-23 | Silicone oil | Sesame oil | Lanolin alcohol | Isopropyl myristateb | |
| Glycerina | Zinc stearate | Fragrancea | Sodium carbomer | Triethanolamineb | |
| Simethicone | Talc powder | Fragranceb | Limonene | Titanium dioxideb | |
| Vaseline | Mono propylene glycola | Butylene glycol | Benzyl silicate | Zinc oxideb | |
| Allantoin | Lanolin alcohol | Myristyl alcohol | Mono propylene glycolb | ||
| Lactic acid | Sodium carbomer | Petroleum | |||
| Isopropyl myristatea | Hexyl cinnamal | Tween 80 | |||
| Tween 20 | Dimethicone silicone | ||||
| Vitamin E | Fragrancea | ||||
| Glycolic acid | Scrub (sand) | ||||
| Triethanolaminea | Calcium | ||||
| Tocopherylacetate | pantothenate | ||||
| Titanium dioxidea | Citrus grandis | ||||
| Zinc oxidea | Pyridoxine Hcl | ||||
| Benzophenone-3 | Tocopheryl acetate | ||||
| Siloxane | |||||
| Xanthan Gum | |||||
| Avobenzone | |||||
| Sucrose cocoate | |||||
| Octyl methoxy cinnamate | |||||
| Jojoba oil | |||||
| Iron oxide | |||||
| Alumina |
Notes:
Raw material from Dr Joe Factory;
Raw material from Jolly for cosmetics.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Haftbaradran B, Abedi D, Jalili M, Bagherinejad MR. Microbial quality survey of sunscreen products in Iranian market. Adv Biomed Res. 2014;3:180. doi: 10.4103/2277-9175.139534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hugbo PG, Onyekwelli OA, Igwe I. Microbial contamination and preservative capacity of some brands of cosmetic creams. Trop J Pharm Res. 2003;2:229–234. [Google Scholar]
- 3.Campana R, Scesa C, Patrone V, Vittoria E, Baffone W. Microbiological study of cosmetic products during their use by consumers: health risk and efficacy of preservative systems. Lett Appl Microbiol. 2006;43:301–306. doi: 10.1111/j.1472-765X.2006.01952.x. [DOI] [PubMed] [Google Scholar]
- 4.Neza E, Centini M. Microbiologically contaminated and over-preserved cosmetic products accccording rapex 2008–2014. Cosmetics. 2016;3(3):1–11. [Google Scholar]
- 5.Okeke IN, La Mikanara A. Bacteriological of skin moisturizing creams and lotions distributed in tropical developing country. J Appl Microbiol. 2001;91:922–928. doi: 10.1046/j.1365-2672.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 6.Tan ASB, Tuysuz M, Otuk G. Investigation of preservative efficacy and microbiological content of some cosmetics found on the market. Pak J Pharm Sci. 2013;26:153–157. [PubMed] [Google Scholar]
- 7.Roden K. Preservatives in personal care products. Official J Austr Socie Micro Inc. 2010;31:195–197. [Google Scholar]
- 8.Yazar K, Johnsson S, Lind M, Boman A, Liden C. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2010;64:265–272. doi: 10.1111/j.1600-0536.2010.01828.x. [DOI] [PubMed] [Google Scholar]
- 9.Pasquet J, Chevalier Y, Couval E, et al. Antimicrobial activity of zinc oxide particles on five micro-organisms of the challenge tests related to their physicochemical properties. Int J Pharm. 2014;460:92–100. doi: 10.1016/j.ijpharm.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Pasquet J, Chevalier Y, Couval E, Bouvier D, Bolzinger M. Zinc oxide as a new antimicrobial preservative of topical products: interactions with common formulation ingredients. Int J Pharm. 2015;479:88–95. doi: 10.1016/j.ijpharm.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomed. 2012;7:6003–6009. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh BN, Rawat AKS, Khan W, Naqvi AH, Singh BR. Biosynthesis of stable antioxidant ZnO nanoparticles by pseudomonas aeruginosa rhamnolipids. PLoS One. 2014;9:e106937. doi: 10.1371/journal.pone.0106937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swaroop K, Sheikh S, Chandrashekar KR, Somashekarappa H. Antibacterial studies of gamma irradiated zinc oxide nanoparticles on Klepsiella pneumonia and Pseudomonas aeruginosa. IOSR J Appl Phys. 2015;7:58–63. [Google Scholar]
- 14.El-molla SA, Ismail SA, Ibrahim MM. Effect of gamma irradiation and aging on surface and catalytic activity of nanosized CuO/MgO system. J Mex Chem Soc. 2011;55(3):154–163. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Disk Susceptibility Tests M02-A11. 11th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 16.Singh P, Nanda A. Antimicrobial and antifungal potential of zinc oxide nanoparticles in comparison to conventional zinc oxide particles. J Chem Pharm Res. 2013;5(11):457–463. [Google Scholar]
- 17.United States Pharmacopeia (USP) Chapter 51, Antimicrobial Effectiveness Testing. [Accessed January 2, 2017]. Available from: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c51.html.
- 18.Cashman AL, Warshaw EM. Parabens: a review of epidemiology, structure, allergenicity, and hormonal properties. Dermatitis. 2005;16(2):57–66. [PubMed] [Google Scholar]
- 19.Nicole V, Nguyen K, Kupiec TC. The essentials of United States pharmacopeia chapter <51> antimicrobial effectiveness testing. Int J Pharm Compd. 2014;18(2):123–130. [PubMed] [Google Scholar]
- 20.Ribeiro MH, Oliveira RS, Desantana MF. In vitro evaluation of gamma irradiation on a gel formation of Cratylia Mollis: rheological properties and microbiological control. J Cosmet Derm Sci Appl. 2012;(2):45–50. [Google Scholar]
- 21.Karvani ZE, Cherazi P. Antibacterial activity of ZnO nanoparticles on gram-positive and gram-negative bacteria. Afr J Microbiol Res. 2011;5(12):1368–1373. [Google Scholar]
- 22.Ghorbani HR, Mehr FP, Pazoki H, Rahmani BM. Sysnthesis of ZnO nanoparticles by precipitation method. Orient J Chem. 2015;31(2):1219–1221. [Google Scholar]
- 23.Slman A. Antibacterial activity of ZnO nanoparticles on some gram-positive and gram negative bacteria. Iraqi J Phys. 2012;18(10):5–10. [Google Scholar]
- 24.Bayroodi E, Razieh J. Modulation of antibiotic resistance in pseudomonas aeruginosa by ZnO nanoparticles. Iran J Microbiol. 2016;8:85–92. [PMC free article] [PubMed] [Google Scholar]
- 25.Yousef J, Danial E. In vitro antibacterial activity and minimum inhibitory concentration of zinc oxide and nano-particle zinc oxide against pathogenic strains. J Healt Sci. 2012;2(4):38–42. [Google Scholar]
- 26.Wang C, Liu L, Zhang A, Xie P, Lu J, Zou X. Antibacterial effects of zinc oxide nanoparticles on Escerichia coli K 88. Afr J Biotechnol. 2012;11(44):10248–10254. [Google Scholar]
- 27.Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7(3):219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton S, Jimenez LA. Review of reported recalls involving microbiological control 2004–2011 with emphasis on FDA considerations of “Objectionable Organisms”. Am Pharmaceut Rev. 2012;15:42–57. [Google Scholar]
- 29.US Food & Drug Administration Recalls & alerts. [Accessed August 1, 2017]. Available from: https://www.fda.gov/Cosmetics/ComplianceEnforcement/RecallsAlerts/default.htm.
- 30.Health and consumers . Zinc oxide (nano form) European Commission; [Accessed August 1, 2017]. Available from: https://ec.europa.eu/health/scientific_committees/opinions_layman/zinc-oxide/es/l-2/5.htm. [Google Scholar]
- 31.Kim S, Heo Y, Choi S, et al. Safety evaluation of zinc oxide nanoparticles in terms of acute dermal toxicity, dermal irritation and corrosion, and skin sensitization. Mol Cell Toxicol. 2016;12(1):93–99. [Google Scholar]
- 32.Nohynek GJ, Dufour EK, Roberts MS. Nanotechnology, cosmetics and the skin: is there a health risk? Skin Pharmacol Physiol. 2008;21:136–149. doi: 10.1159/000131078. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition Guidance for Industry Safety of Nanomaterials in Cosmetic Products. [Accessed June 29, 2017]. Available from: https://www.fda.gov/downloads/Cosmetics/GuidanceRegulation/GuidanceDocuments/UCM300932.pdf.
- 34.Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95–112. doi: 10.2147/NSA.S19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US Food & Drug Administration Enforcement Report. [Accessed August 1, 2017]. Available from: https://www.accessdata.fda.gov/scripts/ires/index.cfm.
- 36.Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O 157:H7. J Appl Microbiol. 2009;107:1193–1201. doi: 10.1111/j.1365-2672.2009.04303.x. [DOI] [PubMed] [Google Scholar]
- 37.Rizwan W, Young-Soon K, Amrita M, Soon-II Y, Hyung-Shik Sh. Formation of ZnO micro-flowers prepared via solution process and their antibacterial activity. J Nanoscale Res Lett. 2010;5(10):1675–1681. doi: 10.1007/s11671-010-9694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vani C, Sergin GK, Annamalia AA. Study on the effect of zinc oxide nanoparticles in Staphylococcus aureus. Int J Pharma Bio Sci. 2011;4(2):326–335. [Google Scholar]
- 39.Fang M, Chen JH, Xu XL, Yang PH, Hildebr HF. Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. Int JAntimicrob Agents. 2006;27:513–517. doi: 10.1016/j.ijantimicag.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Blake MD, Maness P, Huang Z, Wolfrum EJ, Huang J, Jacoby WA. Application of photo catalytic chemistry of titanium dioxide to disinfection and the killing of cancer cells. Sep Purif Methods. 1999;28(1):1–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
List of the 77 raw materials used in manufacture of the six tested products
| Sunblock | Foundation cream | Moisturizing cream | Body lotion | Face cream | Body scrub |
|---|---|---|---|---|---|
| Stearic acida | Stearic acida | Lanette 16 (cetyl alcohol)a | Stearic acidb | Stearic acidb | Stearic acidb |
| Sorbitan stearate | Lanet o (cetearyl alc)a | Lanet o (cetearyl alc)a | Lanet o (cetearyl alc)b | Lanet o (cetearyl alc)a | Citric acid |
| Caprylictriglyceride | Paraffin oila | Paraffin oila | Glyceryl monostearatea | Paraffin oilb | Propanediol |
| Lanette 16 (cetyl alcohol)a | Vaseline | Glycerina | Tapioca starch | Glycerinb | Sodium benzoate |
| Isopropyl myristatea | Vaseline | Linalool | Isopropyl myristateb | Lanet o (cetearyl alc)a | |
| Lanet o (cetearyl alc)a | Titanium dioxidea | Isopropyl myristatea | Paraffin oilb | Triethanolamineb | |
| Zinc oxidea | Vitamin E | Glycerinb | Mono propylene glycolb | Carbomer | |
| Glyceryl monostearateb | Octyl methoxy cinnamate | Triethanolaminea | Isopropyl myristateb | Tween 80 | Imidazolidinyl urea |
| Emulgin B2 | Triethanolamineb | Dimethicone silicone | Sodium hydroxide | ||
| Octocrylene | Jojoba oil | Panthenol | Mono propylene glycolb | Fragrancea | Sodium hydroxide |
| Cetyl palmitate | Emulgin B2 | Mono propylene glycola | Dimethicone silicone | Paraffin oilb | |
| Paraffin oila | Panthenol | Vitamin A | Butylene glycol | Glycerinb | |
| Laureth-23 | Silicone oil | Sesame oil | Lanolin alcohol | Isopropyl myristateb | |
| Glycerina | Zinc stearate | Fragrancea | Sodium carbomer | Triethanolamineb | |
| Simethicone | Talc powder | Fragranceb | Limonene | Titanium dioxideb | |
| Vaseline | Mono propylene glycola | Butylene glycol | Benzyl silicate | Zinc oxideb | |
| Allantoin | Lanolin alcohol | Myristyl alcohol | Mono propylene glycolb | ||
| Lactic acid | Sodium carbomer | Petroleum | |||
| Isopropyl myristatea | Hexyl cinnamal | Tween 80 | |||
| Tween 20 | Dimethicone silicone | ||||
| Vitamin E | Fragrancea | ||||
| Glycolic acid | Scrub (sand) | ||||
| Triethanolaminea | Calcium | ||||
| Tocopherylacetate | pantothenate | ||||
| Titanium dioxidea | Citrus grandis | ||||
| Zinc oxidea | Pyridoxine Hcl | ||||
| Benzophenone-3 | Tocopheryl acetate | ||||
| Siloxane | |||||
| Xanthan Gum | |||||
| Avobenzone | |||||
| Sucrose cocoate | |||||
| Octyl methoxy cinnamate | |||||
| Jojoba oil | |||||
| Iron oxide | |||||
| Alumina |
Notes:
Raw material from Dr Joe Factory;
Raw material from Jolly for cosmetics.