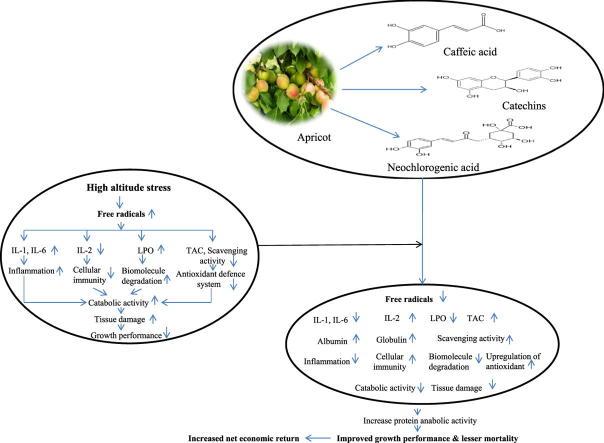
Graphical abstract
Keywords: Antioxidant, Broiler chickens, Growth performance, High altitude, Immune responses, Prunus armeniaca
Abstract
Extreme climatic conditions and hypobaric hypoxia at high altitude hinders the growth and productivity of chickens. The present study was carried out to examine the effect of aqueous extract of Prunus armeniaca seeds on health, survivability, antioxidants, plasma biochemical parameters, and immune status of broiler chickens at high altitude. Phytochemical analysis of extract revealed the presence of high phenolics, flavonoids, and carotenoids contents. Before the in vivo study, in vitro efficacy evaluation indicated a significant protective effect of the extract in chicken peripheral blood lymphocytes. For in vivo study, experimental groups include control (fed the basal diet), and treatment T1, T2, T3, T4, T5, and T6 which received an aqueous extract of P. armeniaca in drinking water at concentrations of 100, 150, 200, 300, 400, and 800 mg/kg body weight of chicken respectively, along with basal diet for 42 days. Body weight was significantly increased in all treatment groups as compared to control group and the highest body weight was recorded in T3 group. Higher profit was gained in treatment groups due to lesser mortality in chickens. Moreover, chicken in the treatment groups had significantly higher total antioxidant capacity, free radical scavenging activity, interleukin-2, total protein, albumin, globulin level and lower malondialdehyde, interleukin-6, glucose, cholesterol, triglyceride, ALT and AST level as compared to control group. Results suggest that, P. armeniaca extract at 200 mg/kg body weight of chicken, exhibited the beneficial effect on growth performance and survivability rate of broilers and therefore, could be useful as phytogenic feed additive for broiler chickens at high altitude cold desert.
Introduction
The growth performance of broiler poultry chickens that reared at cold arid high altitude Himalayas is very poor in spite of stunning progress that has been achieved in broiler poultry industry in India over the last two decades. The reasons for the poor growth performance could be attributed to stressful environmental conditions in this region, which are characterized with hypobaric hypoxia, extreme temperature variations (from +35 °C to −35 °C), high UV radiations, low humidity, and scarcity of fodders which affects livestock health. These all climatic adversities contribute to high altitude oxidative stress, which ultimately hinders the growth rate of poultry chickens by inducing their catabolic activities and thus, produces a low return of income for local poultry farmers [1], [2]. The major consequence of oxidative stress is the marked increase in cellular dysfunction and decline in the productiveness of antioxidant defense system due to increase generation of reactive oxygen species [3]. However, several fruits that found in Himalayan region are rich in phytomolecules such as polyphenols, flavonoids, vitamins, and carotenoids etc. and therefore, widely used as prophylactic and therapeutics agent in combating health problems associated with high altitude [4]. Moreover, supplementation of these fruit extracts to broiler chickens in the form of feed additive might have been beneficial, not beneficially affecting their nutritional and health status. These phytogenic feed additives would be less toxic and ideal to replace antibiotic growth promoters from broiler diet.
Prunus armeniaca is an edible fruit belong to family Rosaceae and is adapted to grow in climatic conditions with cool Winter and warm Summer [5]. In India P. armeniaca fruits are mainly cultivated in hilly regions of Himachal Pradesh, Jammu and Kashmir, and some North Eastern regions. Ladakh region in Jammu and Kashmir represented the major cultivated area for P. armeniaca [6]. Fresh P. armeniaca fruits exhibited pharmacological activity due to the presence of a large number of phytomolecules such as vitamins, polyphenols, flavonoids, carotenoids, and fatty acids [7], [8]. P. armeniaca seed (Kernel) is an important source of dietary protein along with a significant amount of oil and fibers [9] and exhibited higher antioxidative activity then flesh of the fruit [10]. Due to its pharmacological activity, it has been used in folk medicine as a remedy for various diseases [6]. A wide spectrum of pharmacological effect of P. armeniaca have been reported including antioxidant [11], antimicrobial [8], antitumor [11], immunomodulatory [12], anti-inflammatory [13], hepato-protective [14], radio-protective [15] and cardio-protective [16]. It has been reported by Jadhav et al. [17] that feeding of the P. armeniaca cake in lambs feed provides proper nutrition and does not create any adverse effect on lamb performance under high altitude climatic conditions of Ladakh. Improved growth performance in broilers was reported by Takeli [18] and Samli et al. [19] after supplementation of P. armeniaca kernel in broiler diet. However, to the best of authors knowledge, no research work has yet been conducted that investigates the effect of Prunus armeniaca seed extract on antioxidant, cytokines, blood biochemical level and health status of broiler chicken at high altitude. Therefore, the present study was undertaken to examine the effects of aqueous extract of Prunus armeniaca seeds on antioxidant, cytokines, blood biochemical level and growth performance of broiler chickens at high altitude cold desert.
Material and methods
Plant material and extraction
Dried P. armeniaca seeds (kernel) were collected commercially from Leh market (altitude = 3540 m above mean sea level). Upon arrival at the laboratory all the collected P. armeniaca seeds were ground in a stainless steel grinder to obtain fine homogeneous powder for the extraction. Powdered samples of P. armeniaca seeds were extracted with 100% distilled water in soxhlet apparatus (Borosil Glass Works Limited, Worli, Mumbai, India) for 24–48 h at 80 °C each batch. The extract was then filtered with a Buckner funnel and Whatman No 1 filter paper (Sigma-Aldrich, St. Louis, MO, USA). After that, the solvent was removed by the rotary evaporator (Rotavapor R-210, Buchi Labortechnik AG, Flawil, Switzerland) under reduced pressure (240 mili bars). The remaining extract material was then lyophilized at −88 to −90 °C in a lyophilizer (Lyochamber ALPHA 2–4 LD plus, Martin Christ GmbH, Osterodo am Harz, Germany) to obtain the dry extract which was then stored at −80 °C until use.
Characterisation of the extract
Aqueous extract of P. armeniaca seeds was evaluated for total antioxidant capacity and free radical scavenging activity and also phytochemically characterized for total phenolics, flavonoids, and carotenoid content.
Total antioxidant capacity (TAC)
TAC of the P. armeniaca seed extract was determined by ferric reducing antioxidant potential (FRAP) assay [20]. A similar method was also used for analysis of TAC of plasma samples. For this assay, 15 µL of sample (both extract and plasma) was allowed to react with 285 µL of FRAP Reagent (Prepared by mixing 10 volumes of 300 mmol/L acetate buffer, pH 3.6 with 1 vol of 20 mmol/L FeCL3 and 1 vol of 10 mmol/L 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) in 40 mmol/L HCl) and absorbance was measured at 593 nm. Aqueous solutions of FeSO4·7H2O were used for calibration and the result was expressed as FRAP value (µM Fe (II)/g of extract) or (µM Fe (II)/L of plasma).
DPPH radical scavenging capacity
The free radical scavenging capacity of the P. armeniaca seed extract was determined by 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay [21]. Similar assay was also used for determining scavenging activity of plasma samples.
ABTS radical scavenging capacity
2,2-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay was performed using the protocol described by Re et al. [22].
Total phenolic content
Total phenolic content in the P. armeniaca seed extract was measured by Folin-Ciocalteu calorimetric method [23] and phenolic content were expressed as mg of gallic acid equivalent (mg gallic acid/g of extract).
Total flavonoid content
Total flavonoid content in the P. armeniaca seed extract was measured by using the method described by Ordonez et al. [24] and total flavonoid content were expressed as mg of quercetin equivalent (mg quercetin/g of extract).
Determination of carotenoids
Total carotenoid content in the P. armeniaca seed extract was measured by using the method described by Ranjith et al. [25] and total carotenoids were expressed as mg of β-carotene equivalent (mg β-carotene/100 g of extract).
Evaluation of dose efficacy of P. armeniaca extract in-vitro
Efficacy of P. armeniaca seed extract was first evaluated in chicken peripheral blood lymphocytes (PBL) in vitro.
Blood collection and isolation of peripheral blood mononuclear cells (PBMC)
Fresh blood samples were collected from jugular/wing vein of healthy broiler chickens into an ethylenediaminetetraacetic acid (EDTA) containing tubes. Collected blood sample was diluted with phosphate buffered saline (PBS) in 1:1 ratio and was gently over layered on Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) in falcon tube followed by centrifugation (AllegraR X-15R centrifuge, Beckman Coulter) at 400g for 30 min. After centrifugation, PMBC were collected from gradient interface, and centrifuged twice at 200g for 10 min. For separation of adherent (Monocytes) and non-adherent (Lymphocytes) cells, PBMC were incubated at 41 °C in 5% CO2 incubator (CO-150, New Brunswick Scientific, USA) for 45 min by using plastic adherence technique described by Gupta et al. [26].
Cell culture
Thereafter, PBL suspension (100 µL/well) was cultured with 100 µL/well of different concentrations of P. armeniaca seed extract (100, 200, 400, and 800 ng/mL, and 1, 2, 4, 8, 50, 100, 200, and 400 μg/mL), 1 µg/mL of concanavalin A as positive control, and medium as negative control, in 96-well flat bottom microtiter plate for 24 h.
Proliferative activity
Proliferative activity of extract was determined with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay described by Mosmann [27]. After incubation of 24 h, 50 µL of MTT solution was added to each well and the plate was further incubated for 4 h. Thereafter, to solubilize the formazan product 100 µL dimethyl sulfoxide (DMSO) was added to each well. Absorbance was taken at 570 nm using the microplate reader (680 microplate reader, Bio-Rad Laboratories, California, USA). Percentage cell viability was calculated by employing following formula
Cytoprotective activity against H2O2-induced toxicity
To determine cytoprotective activity, PBL cells were treated simultaneously with different concentrations of P. armeniaca extract (100, 200, 400, 800 ng/mL, and 1, 2, 4, 8, 50, 100, 200, 400 μg/mL) and 100 µm H2O2 for 2 h. Cell viability was determined by using MTT assay.
In vivo experiment
The in vivo experiment was conducted after it was approved by the Institutional Animal Ethics Committee of DIHAR (Protocol no: DIHAR/IAEC/27/2015). For this experiment, 105 one day old RIR cross-bred broiler chicks were randomly assigned to seven groups in three replicates (5 chicks in each replicate) having 15 chickens in each group as per completely randomized design. Chickens in the control group were fed the basal diet, whereas the six treatment groups, T1, T2, T3, T4, T5, and T6 received an aqueous extract of P. armeniaca seeds in drinking water at concentration of 100, 150, 200, 300, 400, and 800 mg/kg body weight of chicken, respectively in addition of basal diet. The experimental period was of 42 days. The ingredients and chemical composition of basal diet are present in Supplementary file 1. The chickens were weighed individually at 0, 7, 14, 21, 28, 35, and 42 days. Feed and water were provided ad lib. Mortality was recorded daily and dead chickens were examined for coccidiosis and ascites on post-mortem examination. An economy was also calculated based on the rearing cost of chickens.
Blood collection
Eight chickens were randomly selected from each experimental group and blood samples were collected from jugular/wing vein of chickens at 0, 21, and 42 days. Collected blood samples were centrifuged at 3500 RPM for 10 min and isolated plasma samples were analysed for antioxidant, blood biochemical, and cytokines study.
Determination of blood biochemical parameters
All blood plasma biochemical parameters including cholesterol, triglyceride, high density lipoprotein (HDL), low density lipoprotein (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein, albumin, glucose, uric acid, and creatinine, were analysed with commercially available biochemical kits (Span Diagnostics, Surat, India) according to methodology suggested by manufacturer using biochemical semi-auto analyser (BIOTRON BTR-830, Biosystems, USA). For estimation of plasma globulin concentration, value of albumin was subtracted from the total protein value.
Determination of plasma antioxidant parameters
Plasma TAC and free radical scavenging capacity were determined as described earlier in the section.
Lipid peroxidation (LPO)
For estimation of LPO, level of malondialdehyde (MDA) in plasma samples was estimated by method of Buege and Aust [28] and plasma MDA concentration was expressed as nmol/mL.
Determination of inflammatory cytokines
The level of three inflammatory cytokines: IL-1, IL-2, and IL-6 in plasma samples were analysed using commercially available ELISA kits (Biolegend, San Diego, CA) according to methodology suggested in respective kit manuals.
Statistical analysis
Data were analysed by one way analysis of variance (ANOVA) using completely randomized design. Values were expressed as mean ± standard error. Duncan’s multiple range test was used to compare significant differences in the means of different experimental groups. P < 0.05 was assumed to be statistically significant. All statistical analysis was performed with SPSS statistical software package version 17.0 (SPSS, Chicago). For growth performance, number of chickens in experimental group [n = 15, (3 replicates with 5 chickens)] served as experimental unit for statistical analysis.
Results
Characterisation of aqueous extract of P. armeniaca seed
DPPH and ABTS radical scavenging capacity
The P. armeniaca extract scavenged the DPPH and ABTS radical in a dose-dependent manner at a concentration of 20–100 µg/mL and it was similar to positive control ascorbic acid (Table 1).
Table 1.
DPPH and ABTS radical scavenging activity of aqueous extract of Prunus armeniaca.
| Inhibition (%) |
||||
|---|---|---|---|---|
| DPPH radical scavenging capacity |
ABTS radical scavenging capacity |
|||
| Concentration (µg/mL) | P. armeniaca | Ascorbic acid | P. armeniaca | Ascorbic acid |
| 20 | 29.10 ± 0.62 | 39.57 ± 0.76 | 15.32 ± 0.28 | 21.36 ± 1.12 |
| 40 | 30.16 ± 0.66 | 45.40 ± 0.89 | 19.80 ± 0.31 | 29.37 ± 0.45 |
| 60 | 32.51 ± 0.79 | 49.80 ± 0.63 | 25.54 ± 0.41 | 35.86 ± 0.54 |
| 80 | 35.19 ± 0.73 | 53.98 ± 0.57 | 31.10 ± 0.57 | 41.18 ± 0.71 |
| 100 | 40.22 ± 0.85 | 60.59 ± 1.08 | 37.04 ± 0.69 | 55.94 ± 0.96 |
Value are given as mean ± S.E of four replicates.
Total antioxidant capacity (TAC)
TAC of P. armeniaca extract is presented in Table 2 and it was recorded to be 409.78 ± 16.61 µM Fe (II)/g of extract.
Table 2.
Total antioxidant capacity, total phenolic, flavonoids and carotenoid content in Prunus armeniaca seed extract.
| Sample | FRAP (µM Fe (II)/g of extract) | Total phenolic (mg GAE/g of extract | Flavonoids (mg QE/g of extract) | Carotenoids (mg/100 g extract) |
|---|---|---|---|---|
| P. armeniaca | 409.78 ± 16.61 | 0.68 ± 0.22 | 0.40 ± 0.14 | 0.68 ± 0.31 |
Value are given as mean ± S.E of four replicates.
Total phenolic, flavonoid, and carotenoid contents
The concentration of phenolic content in P. armeniaca extract is presented in Table 2 and was recorded to be 0.68 ± 0.22 mg/g of extract. The concentration of flavonoids and carotenoids in the P. armeniaca extract is also presented in Table 2 and was recorded to be 0.40 ± 0.14 mg/g of extract and 0.68 ± 0.31 mg/100 g of extract, respectively.
In vitro proliferative study in PBL
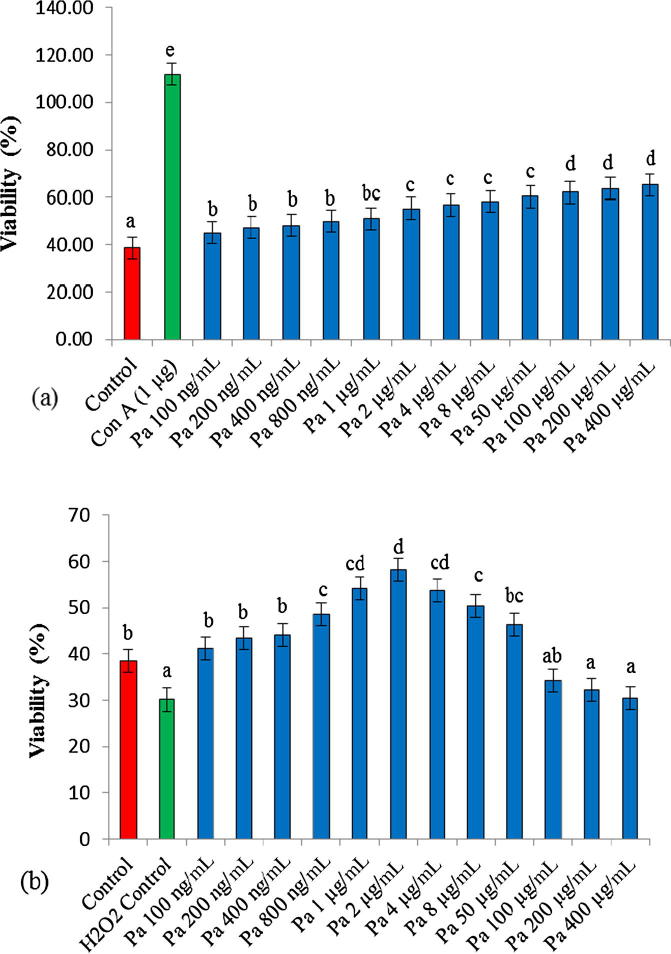
P. armeniaca extract stimulated the proliferation of chicken PBL at all tested concentrations in a dose dependent manner compared with the medium control (Fig. 1a). Highest proliferation was recorded at 400 µg/mL followed by 200 and 100 µg/mL and proliferation activity decreased with decrease in the extract concentration. In addition, P. armeniaca extract reduced the H2O2 induced cellular toxicity in a dose dependent manner and protects the lymphocytes against the toxic effects of H2O2 at all tested dose concentrations between 100 ng/mL to 50 µg/mL as compared with H2O2 treated control cells (Fig. 1b). P. armeniaca at concentration of 2 µg/mL exhibits maximum cytoprotective activity against toxic effects of H2O2.
Fig. 1.
In vitro efficacy of P. armeniaca extract. (a) Effect of aqueous extract of P. armeniaca on chicken PBL proliferation. Each bar represents the mean ± SE value obtained from four culture wells. Each value was compared with untreated control cells as well as with in different dose concentrations (b) Cytoprotective activity of extract against H2O2 induced toxicity in chicken PBL. Each value was compared with H2O2 stimulated cells as well as with in different dose concentrations. Bars having different superscripts (a, b, c, d, e) differ significantly (P < 0.05) according to Duncan’s multiple range test.
Growth performance
The effect of aqueous extract of P. armeniaca seeds on growth performance parameters of broiler chickens at high altitude is presented in Table 3. The mean values were compared with-in different experimental groups at different week intervals. At 42 day of age, live body weight of chicken was increased (P = 0.036) in all treatment groups as compared to control group and highest body weight was recorded in T3 group chicken (450.14 ± 8.59) (supplemented with P. armeniaca extract @ 200 mg/kg body weight of chicken) followed by T4, T2, T1, T5, and T6. Chickens in the control group revealed the lowest body weight (348.53 ± 10.41). Feed conversion ratio (FCR) value in T3 and T4 groups was significantly improved (P = 0.024) among the groups.
Table 3.
Effect of aqueous extract of Prunus armeniaca on growth performance of broiler chickens at high altitude.
| Treatments | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | Control | T1 | T2 | T3 | T4 | T5 | T6 |
| Initial live body weight (g/chick) | 37.20 ± 0.40 | 37.46 ± 0.55 | 38.00 ± 0.37 | 38.13 ± 0.63 | 37.13 ± 0.52 | 37.60 ± 0.66 | 38.06 ± 0.52 |
| Live weight at 21 day (g/chick) | 184.71 ± 7.69 | 195.46 ± 4.76 | 203.14 ± 8.04 | 209.20 ± 9.46 | 204.28 ± 8.32 | 198.00 ± 3.98 | 200.40 ± 10.78 |
| Live weight at 42 day (g/chick) | 348.53a ± 10.41 | 410.13b ± 8.30 | 417.57b,c ± 9.03 | 450.14d ± 8.59 | 440.28c,d ± 8.70 | 408.80b ± 10.41 | 398.15b ± 8.30 |
| Cumulative feed intake up to 42 day (g/chick) | 1511.21 ± 6.26 | 1514.12 ± 6.00 | 1524.41 ± 5.77 | 1519.33 ± 5.26 | 1519.40 ± 4.80 | 1525.46 ± 4.70 | 1518.48 ± 5.92 |
| Feed conversion ratio at 42 day | 4.86d ± 0.04 | 4.06b ± 0.05 | 4.02b ± 0.06 | 3.69a ± 0.05 | 3.77a ± 0.05 | 4.11b ± 0.04 | 4.22c ± 0.04 |
| Cumulative water intake up to 42 day (mL/chick) | 2245.60 ± 6.27 | 2250.52 ± 5.44 | 2240.84 ± 5.31 | 2255.56 ± 6.72 | 2250.35 ± 5.39 | 2250.19 ± 6.45 | 2240.15 ± 5.59 |
C, T1, T2, T3, T4, T5, and T6 represent groups of chickens received aqueous extract of Prunus armeniaca in drinking water at concentration level of 0, 100, 150, 200, 300, 400, and 800 mg/kg body weight of chicken respectively.
Results are presented as mean ± S.E, n = 15 (3 replicates with 5 chickens each).
Means bearing the different superscripts (a, b, c, d) in a row differ significantly (P < 0.05).
Economics and mortality in chickens during in vivo experiment
Highest mortality rate (20.00%, total 3 chicken out of 15) was recorded in the control group (Table 4) chickens followed by T1, T2, T4, and T6 (6.67%, total 1 out of 15). Post mortem examination revealed 13.30%, and 6.67% mortality in chickens induced from ascites in control and P. armeniaca seeds supplemented T2 group, respectively whereas 6.67% mortality induced from coccidiosis each in control and P. armeniaca seeds supplemented T1 group (Table 4).
Table 4.
Economics and mortality rate (%) in chicken supplemented with P. armeniaca.
| Description | Control | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|
| Total mortality (%) | 20.00 | 6.67 | 6.67 | 0.00 | 6.67 | 0.00 | 6.67 |
| Mortality by ascites (%) | 13.30 | 0.00 | 6.67 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mortality by coccidiosis (%) | 6.67 | 6.67 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mortality by other reasons (%) | 0.00 | 0.00 | 0.00 | 0.00 | 6.67 | 0.00 | 6.67 |
| Cost of extract/chicken (Rs.) | Nil | 0.88 | 1.48 | 1.89 | 2.80 | 3.54 | 6.98 |
| Cost of feed/chicken (@25/kg Rs.) | 37.78 | 37.85 | 38.11 | 37.98 | 37.99 | 38.14 | 37.96 |
| Total feed cost/chicken (Rs.) | 37.78 | 38.73 | 39.59 | 39.87 | 40.79 | 41.68 | 44.94 |
| Sale of chicken at 42 day (@Rs. 200/kg live weight)* | 69.70 | 82.02 | 83.51 | 90.02 | 88.05 | 81.76 | 79.63 |
| Loss due to mortality (Rs.)# | 209.10 | 82.02 | 83.51 | 0.00 | 88.05 | 0.00 | 79.63 |
| Total benefit per group (Rs.)## | – | 127.08 | 125.59 | 209.10 | 121.05 | 209.10 | 129.49 |
C, T1, T2, T3, T4, T5, and T6 represent groups of chickens received aqueous extract of P. armeniaca in drinking water at concentration level of 0, 100, 150, 200, 300, 400, and 800 mg/kg body weight of chicken, respectively.
Due to limited availability of fresh chickens at high altitude the rates are very high.
Loss due to mortality = Sale cost per chicken X total mortality.
Total benefit per group = Loss from mortality in control – loss from mortality in treatment.
Economics of the experiment were also calculated based on the rearing cost of 15 numbers of chickens. The additional cost of extract was included with feed cost whereas other expenditure remained constant. P. armeniaca extract reduced the mortality rate in treatment groups and which resulted in increased in the net return (Table 4).
Blood biochemical parameters
All the blood plasma biochemical parameters are presented in Table 5, Table 6, Table 7. P. armeniaca seed extract increased (P < 0.05) the level of plasma total protein, albumin, and globulin in the treatment groups as compared to control group (Table 5). Furthermore, the concentration of plasma glucose was higher (P = 0.037) in control group chicken as compared to treatment groups.
Table 5.
Plasma total protein, albumin, globulin, and glucose values of broilers supplemented with aqueous extract of Prunus armeniaca.
| Groups | 0 day | 21st day | 42nd day |
|---|---|---|---|
| Total protein (g/dL) | |||
| Control | 3.86 ± 0.09 | 4.11a ± 0.15 | 4.15a ± 0.21 |
| T1 | 3.87 ± 0.13 | 4.60bc ± 0.17 | 5.33c ± 0.19 |
| T2 | 3.80 ± 0.10 | 4.54b ± 0.14 | 5.22b ± 0.17 |
| T3 | 3.81 ± 0.13 | 4.76d ± 0.19 | 5.51d ± 0.20 |
| T4 | 3.84 ± 0.08 | 4.64c ± 0.16 | 5.40cd ± 0.23 |
| T5 | 3.80 ± 0.05 | 4.47b ± 0.14 | 5.16b ± 0.18 |
| T6 | 3.81 ± 0.07 | 4.51b ± 0.15 | 5.28bc ± 0.16 |
| Albumin (g/dL) | |||
| Control | 2.14 ± 0.06 | 2.17a ± 0.10 | 2.23a ± 0.15 |
| T1 | 2.15 ± 0.04 | 2.61c ± 0.13 | 3.16b ± 0.21 |
| T2 | 2.14 ± 0.06 | 2.53bc ± 0.09 | 3.14b ± 0.18 |
| T3 | 2.10 ± 0.08 | 2.68d ± 0.15 | 3.21b ± 0.20 |
| T4 | 2.11 ± 0.10 | 2.59c ± 0.14 | 3.20b ± 0.23 |
| T5 | 2.10 ± 0.08 | 2.41b ± 0.10 | 3.12b ± 0.18 |
| T6 | 2.11 ± 0.11 | 2.45b ± 0.12 | 3.17b ± 0.20 |
| Globulin (g/dL) | |||
| Control | 1.72 ± 0.08 | 1.94 ± 0.11 | 1.92a ± 0.09 |
| T1 | 1.72 ± 0.08 | 1.99 ± 0.16 | 2.17c ± 0.14 |
| T2 | 1.66 ± 0.06 | 2.01 ± 0.14 | 2.08b ± 0.17 |
| T3 | 1.71 ± 0.09 | 2.08 ± 0.19 | 2.30d ± 0.21 |
| T4 | 1.73 ± 0.10 | 2.05 ± 0.15 | 2.20c ± 0.16 |
| T5 | 1.70 ± 0.06 | 2.06 ± 0.21 | 2.04b ± 0.13 |
| T6 | 1.70 ± 0.06 | 2.06 ± 0.20 | 2.11bc ± 0.15 |
| Glucose (mg/dL) | |||
| Control | 316.25 ± 7.85 | 323.25c ± 5.37 | 308.25d ± 6.68 |
| T1 | 316.00 ± 6.67 | 282.25a ± 7.92 | 257.00a ± 5.95 |
| T2 | 316.75 ± 9.53 | 309.00b ± 6.45 | 293.25c ± 4.78 |
| T3 | 314.50 ± 7.92 | 285.25a ± 6.20 | 261.50a ± 4.29 |
| T4 | 314.25 ± 9.62 | 310.75b ± 5.97 | 289.00c ± 9.85 |
| T5 | 318.75 ± 5.54 | 313.00b ± 4.60 | 301.50cd ± 7.59 |
| T6 | 315.50 ± 7.59 | 289.40a ± 6.70 | 272.75b ± 4.87 |
C, T1, T2, T3, T4, T5, and T6 represent groups of chickens received aqueous extract of Prunus armeniaca in drinking water at concentration level of 0, 100, 150, 200, 300, 400, and 800 mg/kg body weight of chicken, respectively.
Results are presented as mean ± S.E, n = 8.
Means bearing the different superscripts (a, b, c, d) in a columns differ significantly (P < 0.05).
Table 6.
Plasma cholesterol, triglyceride, HDL, and LDL values of broilers supplemented with aqueous extract of Prunus armeniaca.
| Groups | 0 day | 21st day | 42nd day |
|---|---|---|---|
| Cholesterol (mg/dL) | |||
| Control | 184.50 ± 10.50 | 193.00c ± 06.09 | 189.75c ± 10.54 |
| T1 | 184.75 ± 09.19 | 175.25ab ± 05.55 | 159.25b ± 04.84 |
| T2 | 185.25 ± 11.03 | 174.75ab ± 13.13 | 153.25ab ± 04.58 |
| T3 | 186.50 ± 09.57 | 166.75a ± 05.46 | 147.00a ± 07.22 |
| T4 | 184.25 ± 11.50 | 175.25ab ± 15.63 | 166.25b ± 05.02 |
| T5 | 186.25 ± 10.75 | 173.00ab ± 15.86 | 168.75b ± 04.05 |
| T6 | 185.75 ± 10.15 | 179.75b ± 10.11 | 169.25b ± 04.78 |
| Triglyceride (mg/dL) | |||
| Control | 124.50 ± 2.50 | 126.50b ± 2.21 | 121.50b ± 1.25 |
| T1 | 125.75 ± 3.27 | 123.75ab ± 2.46 | 116.25ab ± 2.17 |
| T2 | 124.25 ± 3.27 | 124.50ab ± 3.12 | 120.75b ± 1.25 |
| T3 | 123.50 ± 2.59 | 118.25a ± 1.03 | 111.50a ± 1.84 |
| T4 | 124.75 ± 1.10 | 122.75ab ± 1.25 | 115.75ab ± 2.32 |
| T5 | 123.25 ± 1.25 | 122.25ab ± 1.43 | 120.00b ± 1.47 |
| T6 | 124.50 ± 2.50 | 126.50b ± 2.21 | 121.50b ± 1.25 |
| HDL (mg/dL) | |||
| Control | 18.26 ± 0.40 | 21.81 ± 0.44 | 23.45 ± 0.51 |
| T1 | 18.52 ± 0.51 | 22.27 ± 0.47 | 24.12 ± 0.49 |
| T2 | 17.84 ± 0.49 | 22.90 ± 0.55 | 23.81 ± 0.60 |
| T3 | 18.90 ± 0.46 | 23.04 ± 0.51 | 25.19 ± 0.58 |
| T4 | 17.89 ± 0.42 | 22.10 ± 0.55 | 24.33 ± 0.60 |
| T5 | 18.12 ± 0.50 | 22.65 ± 0.57 | 25.00 ± 0.62 |
| T6 | 18.55 ± 0.40 | 22.37 ± 0.38 | 24.26 ± 0.55 |
| LDL (mg/dL) | |||
| Control | 45.19 ± 0.79 | 41.32 ± 0.76 | 40.11 ± 0.84 |
| T1 | 46.11 ± 0.82 | 40.16 ± 0.90 | 39.85 ± 0.82 |
| T2 | 45.80 ± 0.85 | 41.03 ± 0.90 | 40.10 ± 0.78 |
| T3 | 46.31 ± 0.91 | 39.56 ± 0.95 | 38.83 ± 0.90 |
| T4 | 45.21 ± 0.90 | 40.76 ± 0.92 | 40.21 ± 0.89 |
| T5 | 46.07 ± 0.87 | 41.09 ± 0.90 | 39.74 ± 0.92 |
| T6 | 45.89 ± 0.91 | 40.70 ± 0.95 | 39.45 ± 0.93 |
C, T1, T2, T3, T4, T5, and T6 represent groups of chickens received aqueous extract of Prunus armeniaca in drinking water at concentration level of 0, 100, 150, 200, 300, 400, and 800 mg/kg body weight of chicken, respectively.
Results are presented as mean ± S.E, n = 8.
Means bearing the different superscripts (a, b, c) in a columns differ significantly (P < 0.05).
Table 7.
Plasma creatinine, uric acid, ALT, and AST values of broilers supplemented with aqueous extract of Prunus armeniaca.
| Groups | 0 day | 21 day | 42 day | |
|---|---|---|---|---|
| Creatinine (mg/dL) | ||||
| Control | 0.87 ± 0.13 | 1.25 ± 0.06 | 1.22 ± 0.12 | |
| T1 | 0.90 ± 0.11 | 1.27 ± 0.04 | 1.22 ± 0.06 | |
| T2 | 0.92 ± 0.12 | 1.22 ± 0.20 | 1.20 ± 0.05 | |
| T3 | 0.87 ± 0.12 | 1.22 ± 0.07 | 1.18 ± 0.07 | |
| T4 | 0.95 ± 0.09 | 1.22 ± 0.16 | 1.32 ± 0.12 | |
| T5 | 0.90 ± 0.09 | 1.20 ± 0.04 | 1.22 ± 0.04 | |
| T6 | 0.92 ± 0.13 | 1.21 ± 0.05 | 1.30 ± 0.10 | |
| Uric acid (mg/dL) | ||||
| Control | 5.02 ± 0.14 | 5.38 ± 0.16 | 6.18 ± 0.22 | |
| T1 | 5.01 ± 0.18 | 5.41 ± 0.24 | 6.18 ± 0.26 | |
| T2 | 5.01 ± 0.17 | 5.37 ± 0.21 | 6.15 ± 0.23 | |
| T3 | 5.06 ± 0.11 | 5.42 ± 0.26 | 6.17 ± 0.25 | |
| T4 | 5.02 ± 0.16 | 5.44 ± 0.19 | 6.10 ± 0.23 | |
| T5 | 5.02 ± 0.14 | 5.44 ± 0.21 | 6.12 ± 0.27 | |
| T6 | 5.01 ± 0.15 | 5.37 ± 0.22 | 6.15 ± 0.24 | |
| AST (IU/L) | ||||
| Control | 98.50 ± 2.10 | 89.75d ± 1.31 | 84.00c ± 3.24 | |
| T1 | 97.25 ± 3.19 | 68.50b ± 3.40 | 56.25a ± 2.32 | |
| T2 | 97.00 ± 3.89 | 64.00b ± 2.16 | 57.75a ± 2.56 | |
| T3 | 97.25 ± 1.65 | 57.00a ± 1.82 | 54.75a ± 1.49 | |
| T4 | 98.25 ± 0.85 | 72.75bc ± 2.92 | 57.25a ± 1.93 | |
| T5 | 98.50 ± 2.95 | 79.75c ± 1.49 | 68.75b ± 1.79 | |
| T6 | 98.00 ± 1.29 | 68.00b ± 3.58 | 61.00a ± 1.47 | |
| ALT (IU/L) | ||||
| Control | 23.50 ± 2.10 | 19.25b ± 1.49 | 18.50c ± 1.32 | |
| T1 | 22.50 ± 2.10 | 18.50b ± 1.19 | 14.50b ± 1.75 | |
| T2 | 23.75 ± 1.65 | 16.25ab ± 1.31 | 12.75b ± 1.31 | |
| T3 | 23.00 ± 2.79 | 13.75a ± 1.37 | 08.50a ± 0.64 | |
| T4 | 22.50 ± 1.32 | 17.25ab ± 1.10 | 14.75b ± 1.31 | |
| T5 | 22.75 ± 1.97 | 19.00b ± 1.29 | 13.75b ± 1.32 | |
| 23.00 ± 0.91 | 16.75ab ± 1.25 | 12.50b ± 0.95 | ||
C, T1, T2, T3, T4, T5, and T6 represent groups of chickens received aqueous extract of Prunus armeniaca in drinking water at concentration level of 0, 100, 150, 200, 300, 400, and 800 mg/kg body weight of chicken, respectively.
Results are presented as mean ± S.E, n = 8.
Means bearing the different superscripts (a, b, c, d) in a columns differ significantly (P < 0.05).
Plasma cholesterol was significantly reduced (P = 0.029) in the treatment groups and chicken in T3 group represented lowest cholesterol level among the groups (Table 6). Plasma triglyceride concentration was reduced significantly in T3 group chicken in comparison to control group.
The AST level was reduced in the treatment groups as compared to control group, whereas ALT level was recorded lowest in T3 group chicken among the groups (Table 7).
Plasma antioxidant level in chickens
Lipid peroxidation
MDA level was reduced in the treatment groups (P = 0.041) as compared to control group (Table 8). Among the treatment groups, the lowest MDA level was recorded in T3 group chicken.
Table 8.
Effect of Prunus armeniaca on MDA, TAC, and free radical-scavenging activity of broiler chickens.
| Groups | 0 day | 21st day | 42nd day | |
|---|---|---|---|---|
| MDA (nmol/mL) | ||||
| Control | 8.41 ± 0.29 | 8.06d ± 0.18 | 8.13e ± 0.19 | |
| T1 | 8.38 ± 0.31 | 6.47b ± 0.24 | 4.43b ± 0.17 | |
| T2 | 8.48 ± 0.36 | 6.60b ± 0.21 | 5.13c ± 0.16 | |
| T3 | 8.37 ± 0.27 | 6.07a ± 0.15 | 4.08a ± 0.18 | |
| T4 | 8.46 ± 0.40 | 6.84c ± 0.19 | 4.52b ± 0.08 | |
| T5 | 8.51 ± 0.40 | 7.02c ± 0.23 | 5.16c ± 0.09 | |
| T6 | 8.43 ± 0.33 | 6.90c ± 0.18 | 5.65d ± 0.15 | |
| FRAP value (µm/L) | ||||
| Control | 1098.26 ± 11.21 | 1189.04a ± 14.63 | 1167.57a ± 16.87 | |
| T1 | 1098.53 ± 10.06 | 1313.78c ± 17.48 | 1686.24c ± 17.61 | |
| T2 | 1097.33 ± 07.78 | 1392.45d ± 17.36 | 1487.58b ± 18.24 | |
| T3 | 1098.24 ± 07.92 | 1496.98e ± 15.47 | 1785.26e ± 18.08 | |
| T4 | 1096.34 ± 07.56 | 1380.01d ± 14.63 | 1741.18d ± 15.86 | |
| T5 | 1096.26 ± 07.60 | 1300.53c ± 19.58 | 1668.17c ± 17.29 | |
| T6 | 1097.31 ± 09.94 | 1224.30b ± 20.03 | 1474.65b ± 15.83 | |
| DPPH radical-scavenging activity (%) | ||||
| Control | 41.92 ± 0.21 | 43.14a ± 1.37 | 42.87a ± 2.11 | |
| T1 | 41.52 ± 0.40 | 55.94c ± 0.87 | 64.30c ± 1.87 | |
| T2 | 41.65 ± 0.47 | 49.26b ± 0.85 | 63.03c ± 1.41 | |
| T3 | 41.96 ± 0.27 | 59.61d ± 0.78 | 66.13d ± 0.63 | |
| T4 | 41.43 ± 0.48 | 56.67c ± 2.43 | 61.94bc ± 0.82 | |
| T5 | 41.91 ± 0.12 | 52.48bc ± 1.23 | 58.86b ± 2.93 | |
| T6 | 42.03 ± 0.16 | 48.13b ± 1.03 | 57.16b ± 1.82 | |
C, T1, T2, T3, T4, T5, and T6 represent groups of chickens received aqueous extract of Prunus armeniaca in drinking water at concentration level of 0, 100, 150, 200, 300, 400, and 800 mg/kg body weight of chicken, respectively.
Results are presented as mean ± S.E, n = 8.
Means bearing the different superscripts (a, b, c, d, e) in a columns differ significantly (P < 0.05).
Free radical scavenging capacity
Free radical scavenging capacity was higher in the treatment groups (P = 0.038) as compared to control group. Among the treatment groups, chicken in the T3 group represented highest scavenging activity (Table 8).
Total antioxidant capacity (TAC)
TAC was higher in all treatment groups as compared to control group (P = 0.020). In between treatment groups, chicken in T3 group represented highest TAC (Table 8).
Inflammatory cytokine level
The level of inflammatory cytokines: IL-1, IL-2, and IL-6 in plasma samples of chickens are presented in Table 9. The level of IL-2 was significantly (P < 0.05) increased in treatment groups as compared to control group. Furthermore, the level of proinflammatory cytokine IL-6 was reduced (P = 0.044) in the treatment groups as compared to control group chicken.
Table 9.
Plasma IL-1, IL-2, and IL-6 level in broilers supplemented with aqueous extract of Prunus armeniaca.
| Groups | 0 day | 21st day | 42nd day |
|---|---|---|---|
| IL-1 (pg/mL) | |||
| Control | 5.45 ± 0.33 | 5.48 ± 0.30 | 5.51 ± 0.34 |
| T1 | 5.43 ± 0.27 | 5.46 ± 0.41 | 5.49 ± 0.38 |
| T2 | 5.45 ± 0.21 | 5.48 ± 0.44 | 5.50 ± 0.49 |
| T3 | 5.46 ± 0.30 | 5.45 ± 0.35 | 5.47 ± 0.42 |
| T4 | 5.44 ± 0.36 | 5.43 ± 0.40 | 5.46 ± 0.50 |
| T5 | 5.45 ± 0.28 | 5.47 ± 0.43 | 5.49 ± 0.46 |
| T6 | 5.43 ± 0.33 | 5.45 ± 0.31 | 5.48 ± 0.40 |
| IL-2 (pg/mL) | |||
| Control | 8.56 ± 0.44 | 8.59a ± 0.51 | 8.60a ± 0.47 |
| T1 | 8.55 ± 0.49 | 8.80b ± 0.56 | 9.15b ± 0.56 |
| T2 | 8.54 ± 0.38 | 8.80b ± 0.50 | 9.12b ± 0.60 |
| T3 | 8.57 ± 0.45 | 8.93c ± 0.62 | 9.37c ± 0.65 |
| T4 | 8.56 ± 0.44 | 8.90c ± 0.56 | 9.36c ± 0.58 |
| T5 | 8.58 ± 0.33 | 8.82b ± 0.42 | 9.10b ± 0.60 |
| T6 | 8.55 ± 0.36 | 8.83b ± 0.50 | 9.15b ± 0.50 |
| IL-6 (pg/mL) | |||
| Control | 8.47 ± 0.24 | 8.56b ± 0.45 | 8.61b ± 0.40 |
| T1 | 8.49 ± 0.32 | 8.44a ± 0.26 | 8.39a ± 0.37 |
| T2 | 8.47 ± 0.26 | 8.45a ± 0.30 | 8.39a ± 0.26 |
| T3 | 8.45 ± 0.20 | 8.41a ± 0.28 | 8.35a ± 0.41 |
| T4 | 8.46 ± 0.36 | 8.44a ± 0.23 | 8.37a ± 0.30 |
| T5 | 8.50 ± 0.38 | 8.45a ± 0.37 | 8.37a ± 0.44 |
| T6 | 8.46 ± 0.38 | 8.43a ± 0.40 | 8.34a ± 0.30 |
C, T1, T2, T3, T4, T5, and T6 represent groups of chickens received aqueous extract of Prunus armeniaca in drinking water at concentration level of 0, 100, 150, 200, 300, 400, and 800 mg/kg body weight of chicken, respectively.
Results are presented as mean ± S.E, n = 8.
Means bearing the different superscripts (a, b, c) in a columns differ significantly (P < 0.05).
Discussion
Characterisation of extract
In this study, DPPH and ABTS radical scavenging capacity of the P. armeniaca extract was increased in a dose dependent manner, similar to positive control ascorbic acid. TAC represents the overall antioxidant capacity of plant extract in reducing oxidative stress and in this study, the P. armeniaca extract was found rich in TAC. This increase in the antioxidant defense system could be attributed to the higher content of carotenoids (β-carotene), polyphenolic compounds (catechins, neochlorogenic acid, caffeic acid) and flavonoids in P. armeniaca. It has been well reported that the antioxidant capacity of plant is associated with polyphenolic content [29] and in the present study P. armeniaca extract was found rich in total phenolics, flavonoids, and carotenoid contents.
In vitro dose efficacy of P. armeniaca extract
In the present study, P. armeniaca extract stimulated the proliferation of chicken PBL in a dose dependent manner and extract was not toxic to cells at higher dose concentrations. Furthermore, it also exhibited cytoprotective activity against H2O2 induced toxicity in chicken PBL. The ability of plant extract to stimulate lymphocyte proliferation and enhanced cytoprotection is mainly due to its higher antioxidant content [30]. As carotenoids along with polyphenolic compounds are the major bioactive components in P. armeniaca [7] and it is very likely that these antioxidants in P. armeniaca probably were responsible for enhanced cellular immunity in chickens.
In vivo growth performance
At high altitude, the growth performance of poultry chicken is adversely affected [31] and this is also clearly evident from this study where the final body weight of broilers recorded in the control group was 348.53 g after 42 days. The reason behind the low body weight could be attributed to hypobaric hypoxic conditions which affects the body metabolism due to disturbance in energy balance that leads to decrease in body mass with the increase in the catabolic activities [2], [31].
However, increase in the body weight in treatment groups might be due to the presence of bioactive molecules (carotenoids, catechins, neochlorogenic acid, caffeic acid) in P. armeniaca extract [7] which can stimulate increased digestion and metabolism of nutrients causing higher efficiency in the utilization of feed which results in enhanced growth in chickens. Net return also revealed an increase in the profit in the treatment groups as compared to control group due to a reduction in the mortality rate.
Blood biochemical parameters
The elevated total protein level in treatment groups might be due to higher protein and nutritional content of P. armeniaca [9] which causes greater absorption of amino acid in intestinal tissues, and increased protein synthesis. Albumin protein is a negative acute phase protein [32] and increased level of albumin in treatment groups possibly due to anti-inflammatory activity of P. armeniaca [13]. The increase in the globulin content may be due to the immune stimulating activity of bioactive molecules present inside P. armeniaca [12]. In the present study, P. armeniaca extract reduced the level of glucose in chickens and this may be due to reduced glucocorticoid secretion with P. armeniaca supplementation, which could limit protein and lipid catabolism due to reduced gluconeogenesis [33].
Reduced level of plasma cholesterol and triglyceride in treatment groups might be due to the reduced activity of HMG-CoA reductase enzyme by the P. armeniaca polyphenols [34]. Reduced level of ALT and AST in treatment groups indicates hepatoprotective activity [14], [35] of P. armeniaca extract in chicken liver cells.
Antioxidant parameters
In the present study, the P. armeniaca extract was supplemented to broiler chicken as antioxidant source and it enhanced antioxidant defense level while decreased the level of MDA in chickens. This might be due to the higher content of phytomolecules such as vitamins, carotenoids, polyphenols and flavonoids in the P. armeniaca extract. Previous reports of Ozturk et al. [35] and Yilmaz et al. [36] in laboratory animals indicated a remarkable reduction in MDA level and increased antioxidant defense level after administration of the P. armeniaca extract. Effective antioxidative properties of the P. armeniaca extract can also be implicated with an improved growth performance of broilers observed in this study. Therefore, under the high altitude stress condition P. armeniaca seed extract at dose concentration of 200 mg/kg body weight of chicken could be useful as a broiler feed additive for their better growth rate.
Inflammatory cytokines level
In the present study, the P. armeniaca extract reduced the level of proinflammatory cytokine IL-6 in treatment groups. This might be due to anti-inflammatory activity of polyphenolic compounds of P. armeniaca [13] through downregulating NF-kB signaling pathway by decreased phosphorylation of NF-kB [37]. Moreover, P. armeniaca extract stimulate the production of IL-2, which is produced by activating T helper cells 1 (Th1) and play a central role in cell mediated immunity [38]. This suggests that, P. armeniaca extract exerts immunomodulatory effects in broilers through mediating both cellular and humoral immunity.
Conclusions
Results revealed that, supplementation of aqueous extract of P. armeniaca seeds in broilers, had beneficial effects on their growth performance, survivability, antioxidant level, immune status and blood biochemical parameters. Higher profit was gained in P. armeniaca supplemented groups. In addition, P. armeniaca extract at dose concentration of 200 mg/kg body weight of chicken provides a better effect as compared to the other treatments level. Hence, it can be concluded that P. armeniaca seed extract has potential health benefits in broilers and it could be used as a source of phytogenic feed additive for improvement in their growth performance and to save the loss from high mortality at high altitude.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The present study was fully supported by Defence Research and Development Organisation (DRDO), Ministry of Defence, Government of India. The authors would particularly like to thank Dr R S Chauhan and Dr Malairaman Udayabanu for providing in vitro facility at Jaypee University of Information and Technology. Authors would like to thank Mr. Arun Sharma for helping in in vitro studies and all the staff of the DIHAR poultry division for the care of chicken and for their assistance during the blood sampling. Authors would also like to acknowledge Dr Vineeeth Ravindran T. for his technical assistance.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jare.2017.08.005.
Appendix A. Supplementary material
References
- 1.Biswas A., Bharti V.K., Deshmukh P.B., Venkatesan G., Srivastava R.B. Commercial poultry farming in cold arid region of Leh-Ladakh. In: Srivastava R.B., Selvamurthy W., editors. Innovatives in agro animal technologies. Satish Serial Publishing House; New Delhi: 2011. pp. 216–233. [Google Scholar]
- 2.Kalia S., Bharti V.K., Gogoi D., Giri D., Kumar B. Studies on the growth performance of different broiler strains at high altitude and evaluation of probiotic effect on their survivability. Sci Rep. 2017 doi: 10.1038/srep46074. Article ID 46074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller L.E., McGinnis G.R., Kliszczewicz B., Slivka D., Hailes W., Cuddy J. Blood oxidative-stress markers during a high-altitude trek. Int J Sport Nutr Exerc Metab. 2013;23:65–72. doi: 10.1123/ijsnem.23.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Kala C.P. Medicinal plants of the high altitude cold desert in India: diversity, distribution and traditional uses. Int J Biodivers Sci Manage. 2006;2:43–56. [Google Scholar]
- 5.Ahmadi H., Fathollahzadeh H., Mobli H. Some physical and mechanical properties of apricot fruits, pits and kernels (C.V. Tbarzeh) Am – Eurasian J Agric Environ Sci. 2008;3:703–707. [Google Scholar]
- 6.Wani S.M., Masoodi F.A., Wani T.A., Ahmad M., Gani A., Ganai S.A. Physical characteristics, mineral analysis and antioxidant properties of some apricot varieties grown in North India. Food Sci Technol. 2015;1:1–10. [Google Scholar]
- 7.Dragovic-Uzelac V., Levaj B., Mrkic V., Boras M. The content of polyphenol and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007;102:966–975. [Google Scholar]
- 8.Yigit D., Yigit N., Mavi A. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) kernels. Braz J Med Biol Res. 2009;42:346–352. doi: 10.1590/s0100-879x2009000400006. [DOI] [PubMed] [Google Scholar]
- 9.Nout M.J., Tuncel G., Brimer L. Microbial degradation of amygdalin of bitter apricot seeds (Prunus armeniaca) Int J Food Microbiol. 1995;24:407–412. doi: 10.1016/0168-1605(94)00115-m. [DOI] [PubMed] [Google Scholar]
- 10.Soong Y.Y., Barlow P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004;88:414–417. [Google Scholar]
- 11.Gomaa E.Z. In vitro antioxidant, antimicrobial and antitumor activities of bitter almond and sweet apricot (Prunus armeniaca L.) kernels. Food Sci Biotechnol. 2013;22:455–463. [Google Scholar]
- 12.Tian H., Yan H., Tan S., Zhan P., Mao X., Wang P. Apricot kernel oil ameliorates cyclophosphamide-associated immunosuppression in rats. Lipids. 2016;51(8):931–939. doi: 10.1007/s11745-016-4166-5. [DOI] [PubMed] [Google Scholar]
- 13.Minaiyan M., Ghannadi A., Asadi M., Etemad M., Mahzoun P. Anti-inflammatory effect of Prunus armeniaca L. (apricot) extracts ameliorates TNBS-induced ulcerative colitis in rats. Res Pharma Sci. 2014;9:225–231. [PMC free article] [PubMed] [Google Scholar]
- 14.Yilmaz I., Cetin A., Bilgic Y. Hepatoprotective effects of apricot against acetaminophen induced acute hepatotoxicity in rats. Am J Pharma Sci. 2015;3:44–48. [Google Scholar]
- 15.Kurus M., Ertan C., Celi M.R., Cetin A., Otlu A. Protective effect of apricot feeding in the pulmonary tissues of rats exposed to low dose X-Ray radiation. Indian J Appl Res. 2013;3:1–5. [Google Scholar]
- 16.Parlakpinar H., Olmez E., Acet A., Ozturk F., Tasdemir S., Ates B. Beneficial effects of apricot-feeding on myocardial ischemia-reperfusion injury in rats. Food Chem Toxicol. 2009;47:802–808. doi: 10.1016/j.fct.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Jadhav S.E., Guru Charan, Raj T., Bharti V.K., Singh S.B. Performance and blood biochemical profile of lambs fed local unconventional feed ingredients at cold and high altitude conditions of Ladakh. Indian J Anim Sci. 2011;81:730–734. [Google Scholar]
- 18.Tekeli A. Effect of apricot kernel on selected performance and blood parameters and meat fatty acid composition of broilers. J Anim Vet Adv. 2012;11:3697–3704. [Google Scholar]
- 19.Samli H.E., Terzioglu M., Okur A.A., Koc F., Senkoylu N. Effects of sweet apricot kernel meal on performance and intestinal microbiota in broiler chickens. J Taki Agric Faculty. 2014;11:38–43. [Google Scholar]
- 20.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”. The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 21.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 22.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice E.C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 23.Gao X., Ohlander M., Jeppsson N., Bjork L., Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of seabuckthorn (Hippophae rhamnoides) during maturation. J Agric Food Chem. 2000;48:1485–1490. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- 24.Ordonez E.A., Gomez J.D., Vattuone M.A., Isla M.I. Antioxidant activities of Sechium edule Swart extracts. Food Chem. 2006;97:452–458. [Google Scholar]
- 25.Ranjith A., Kumar K.S., Venogupalan V.V., Arumughan C., Shawney R.S., Singh V. Fatty acids, tocols, and carotenoids in pulp oil of three seabuckthorn species (Hippophae rhamnoides, H. salicifolia, and H. tibetana) grown in the Indian Himalayas. J Am Oil Chem Soc. 2006;83:359–364. [Google Scholar]
- 26.Gupta S., Aggarwal S., See D., Starr A. Cytokine production by adherent and non-adherent mononuclear cells in chronic fatigue syndrome. J Psychiatr Res. 1997;31:149–156. doi: 10.1016/s0022-3956(96)00063-5. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid calorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Buege J.A., Aust S.D. The thiobarbuturic acid assay. Methods Enzymol. 1978;52:306–307. [Google Scholar]
- 29.Skerget M., Kotnik P., Hadolin M., Hras A.R., Simonic M., Knez Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. [Google Scholar]
- 30.Geetha S., Sai Ram M., Singh V., Ilavazhagan G., Sawhney R.C. Antioxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides) – an in-vitro study. Ethnopharmacol. 2002;79:373–378. doi: 10.1016/s0378-8741(01)00406-8. [DOI] [PubMed] [Google Scholar]
- 31.Balog J.M., Anthony N.B., Cooper M.A., Kidd B.D., Huff G.R., Huff W.E. Ascites syndrome and related pathologies in feed restricted broilers raised in a hypobaric chamber. Poult Sci. 2000;79:318–323. doi: 10.1093/ps/79.3.318. [DOI] [PubMed] [Google Scholar]
- 32.Ritchie R.F., Palomaki G.E., Neveux L.M., Navolotskaia O., Ledue T.B., Craig W.Y. Reference distributions for the negative acute phase serum proteins, albumin, transferrin, and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 1999;13:273–279. doi: 10.1002/(SICI)1098-2825(1999)13:6<273::AID-JCLA4>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borges S.A., Fischer A.V., Silva D.A., Maiorka A. Acid base balance in broilers. World’s Poult Sci J. 2007;63:73–81. [Google Scholar]
- 34.Singh D.K., Banerjee S., Porter T.D. Green and black tea extract inhibit HMG-CoA reductase and activate amp-kinase to decrease cholesterol synthesis in hepatoma cells. J Nut Biochem. 2009;20:816–822. doi: 10.1016/j.jnutbio.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozturk F., Gul M., Ates B., Ozturk I.C., Cetin A., Vardi N. Protective effect of apricot (Prunus armeniaca L.) on hepatic steatosis and damage induced by carbon tetrachloride in wistar rat. Br J Nut. 2009;102:1767–1775. doi: 10.1017/S0007114509991322. [DOI] [PubMed] [Google Scholar]
- 36.Yilmaz I., Dogan Z., Erdemli E., Gursoyand S., Bag H.G. The effect of apricot on large intestine oxidative stress enzymes in rats. Cytol Histol. 2015 [Google Scholar]
- 37.Gonzales A.M., Orlando R.A. Curcumin and resveratrol inhibit nuclear factor kappaB-mediated cytokine expression in adipocytes. Nut Metabol. 2008;5:17. doi: 10.1186/1743-7075-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyman O., Sprent J. The role of interleukin-2 during homeostasis and activation of immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.