Abstract
Bone represents a common site of metastasis from several solid tumours, including breast, prostate and lung malignancies. The onset of bone metastases (BM) is associated not only with serious skeletal complications, but also shortened overall survival, owing to the lack of curative treatment options for late-stage cancer.
Despite the diagnostic advances, BM detection often occurs in the symptomatic stage, underlining the need for novel strategies aimed at the early identification of high-risk patients. To this purpose, both bone turnover and tumour-derived markers are being investigated for their potential diagnostic, prognostic and predictive roles.
In this review, we summarize the pathogenesis of BM in breast, prostate and lung tumours, while exploring the current research focused on the identification and clinical validation of BM biomarkers.
Abbreviations: BM, bone metastases; SREs, skeletal related events; BTM, bone turnover markers; P1NP and P1CP, N and C terminal pro-peptides of type 1 collagen; BALP, bone specific alkaline phosphatase; TRACP-5b, tartrate-resistant acid phosphatase type 5b; NTX and CTX, N- and C- telopeptides of type 1 collagen; RANK, receptor activator of nuclear factor kB; RANK-L, RANK-ligand; OPG, osteoprotegerin; TNF, tumour necrosis factor; IL, interleukin; M-CSF, macrophage colony stimulating factor; PTH, parathyroid hormone; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor; PlGF, placental growth factor; BMDC, bone marrow derived cells; EMT, epithelial to mesenchymal transition; CXCR, C–X–C motif chemokine receptor; CXCL, C–X–C motif chemokine ligand; SDF-1, stromal cell-derived factor 1; CaSR, calcium sensing receptor; BC, breast cancer; DTC, disseminated tumour cells; PTH-rP, PTH related protein; IGF, insulin-like growth factor; PDGF, platelet-derived growth factor; PC, prostate cancer; BMPs, bone morphogenetic proteins; FGF, fibroblast growth factor; ER, estrogen receptor; Her2, human epidermal growth factor receptor 2; HR, hormone receptor; IL-1R, IL-1 receptor; ZNF217, zinc-finger protein 217; MAF, v-maf avian musculo-aponeurotic fibrosarcoma oncogene homolog; miRNA, micro RNA; TRAF3, TNF receptor associated factor 3; BSP, bone sialoprotein; CCL2, chemokine C-C ligand 2; CAPG, macrophage-capping protein; GIPC1, PDZ domain–containing protein member 1; PSA, prostate specific antigen; PDGFRα, PDGF receptor α; shRNA, short hairpin RNA; CTC, circulating tumour cells; LC, lung cancer; NSCLC, non-small cell LC; PYD, pyridinoline; DPD, deoxypyridinoline; uNTX, urinary NTX; β-CTX, CTX β isomer; 1CTP, cross-linked carboxy-terminal telopeptide of type 1 collagen; sBALP, serum BALP; BTA, bone-targeting agents
Keywords: Bone metastasis, Biomarkers, Bone turnover markers, Breast cancer, Prostate cancer, Lung cancer
1. Introduction
Bone metastases (BM) represent a frequent incurable complication of several malignancies, owing to specific interactions between cancer cells and the bone microenvironment that make it suitable for tumour cell implantation and growth [1]. Indeed, approximately 70% of patients suffering from advanced breast or prostate malignancies develop BM during the course of the disease [2], [3], with or without disease at other sites, while skeletal involvement characterizes approximately 30–40% of lung cancer (LC) patients [4].
Depending on the primary tumour, BM may exhibit a prevalent osteolytic or osteoblastic pattern, although in most cases a mixed radiological appearance is detected. Early-stage skeletal lesions are usually not detectable by current diagnostic tools, and their sensitivity and specificity are further limited when disease progression is slow and mimics non-malignant conditions. Moreover, the radiological appearance of BM may vary over time, both spontaneously and following anti-resorptive and anti-cancer treatments, and this ultimately complicates their monitoring [5].
As a consequence, BM are often not diagnosed until symptoms occur, leading to a significant impairment of patients’ quality of life; furthermore, late BM diagnosis increases the risk of skeletal related events (SREs) that include hypercalcemia, pathological fractures, spinal cord injury and unremitting pain requiring radiotherapy and/or surgery. Moreover, the occurrence of one SRE increases the risk of further SREs and significantly impairs overall survival [6].
Physiologically, a delicate balance exists between bone resorption and osteogenesis, with dysregulation evident during the evolution of BM [1]. Since bone turnover releases specific molecules in blood and urine [7], several attempts have been made to associate variations in those markers (bone turnover markers, BTM) with BM onset and progression. In particular, BTM have been extensively investigated for their potential as diagnostic tools and to provide prognostic information, as well as to monitor treatment response [7]. At present, the high inter- and intra-individual variability still represents a limitation to their routine use.
Additionally, novel tumour-derived markers are being explored that predict the risk of development of BM and potentially identify responders to adjuvant bone-targeted treatments [8], [9]. This may lead to intensified follow-up in selected patients as well as personalized adjuvant therapies, with the purpose to inhibit the onset of BM, which represent a non-curable condition.
Here, we will summarize the current view on BM pathogenesis in solid tumours, while exploring the recent advances in the BM biomarker field, focusing on breast, prostate and lung malignancies.
2. Methods
We first conducted an extensive research among previous international literature by using the PubMed database and key words such as “cancer osteotropism”, “bone metastasis biomarkers” and “bone turnover markers”, associated with “breast”, “prostate” or “lung cancer”. Then, we reviewed the references of relevant papers published in English between 1997 and 2017. Conference abstracts and papers were identified by reviewing the websites of relevant international oncology meetings.
2.1. Physiological bone turnover
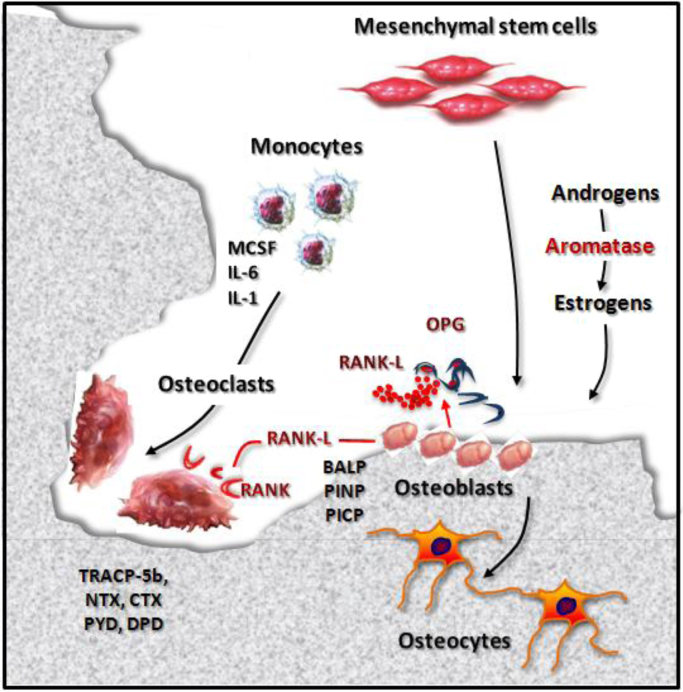
Bone turnover is the result of the opposing activities of osteoblasts and osteoclasts (Fig. 1). The former have a mesenchymal origin and are deputed to osteogenesis. They first synthesize pro-collagen, whose cleavage at N- and C-terminals produces type 1 collagen and P1NP/P1CP pro-peptides, respectively; these fragments are released into the bloodstream and undergo hepatic clearance [5], [10]. Then, osteoblasts secrete bone specific alkaline phosphatase (BALP) which hydrolyses pyrophosphate, a physiological inhibitor of bone matrix maturation, releasing inorganic phosphate [11]. Some osteoblasts are trapped in the newly formed matrix and become osteocytes, namely dendritic cells that commute mechanical stimuli into biochemical response, that in turn regulates bone turnover [12].
Fig. 1.
Physiological bone turnover. Bone turnover physiologically results from the opposite activities of osteoclasts and osteoblasts. The former derive from the monocyte/macrophage lineage and exert a bone resorptive function, through the secretion of H+ ions and enzymes, such as TRACP-5b. Osteoclastogenesis is enhanced by pro-osteoclastogenic cytokines (e.g. M-CSF, IL-6, IL-1). During bone erosion, type 1 collagen undergoes proteolytic cleavage which results in the release of degradation peptides (NTX, CTX, PYD, DPD), that are measurable in blood and urine. Conversely, osteoblasts have a mesenchymal origin and are deputed to osteogenesis. In particular, they synthesize pro-collagen whose cleavage at N- and C-terminals produces type 1 collagen, P1NP and P1CP peptides. Osteoblasts secrete also BALP which is necessary for the mineralization of bone matrix. Some osteoblasts become osteocytes, namely dendritic cells acting as mechano-transducers. Bone turnover is regulated by the RANK-L/RANK/OPG axis. Indeed, osteoblasts and stromal cells release RANK-L that, by binding its receptor RANK expressed by pre-osteoclasts, promotes their differentiation in osteoclasts. OPG partially inhibits this process, in order to prevent excessive bone resorption. Similarly, sex hormones exert a predominant anabolic effect. Adapted from D’Oronzo et al. 2015 [95]. Abbreviations: bone alkaline phosphatase (BALP), C-terminal fragment (CTX), deoxypyridinoline (DPD), interleukin-1 (IL-1), interleukin-6 (IL-6), macrophage colony stimulating factor (M-CSF), N-terminal fragment (NTX), osteoprotegerin (OPG). pro-collagen type 1 C-terminal propeptide (P1CP), pro-collagen type 1 N-terminal propeptide (P1NP), pyridinoline (PYD), receptor activator of nuclear factor kB (RANK), receptor activator of nuclear factor kB-ligand (RANK-L), tartrate-resistant acid phosphatase type 5b (TRACP-5b).
Osteoclasts are multinucleated bone-resorbing cells derived from the monocyte/macrophage lineage. Their erosive activity is based on the secretion of H+ ions and lytic enzymes, such as proteases and the tartrate-resistant acid phosphatase type 5b (TRACP-5b). Proteases degrade type 1 collagen, thereby releasing N- and C-terminal fragments (NTX and CTX, respectively) that are detectable in both blood and urine [13], [14].
Several factors contribute to the regulation of bone turnover, including the receptor activator of nuclear factor kB-ligand (RANK-L)/RANK/osteoprotegerin (OPG) axis. RANK-L belongs to the tumour necrosis factor (TNF) cytokine superfamily and is produced by osteoblasts and stromal cells. RANK-L stimulates osteoclast differentiation and maturation by interacting with its receptor RANK, expressed by pre-osteoclasts; excessive bone resorption is prevented by OPG, an osteoblast-derived soluble decoy receptor for RANK-L. A number of pro-osteoclastogenic (e.g. interleukin-1, IL-1; IL-6; macrophage colony stimulating factor, M-CSF) and anti-osteoclastogenic (e.g. IL-4, IL-18 and interferon-β) cytokines contribute to regulate the balance between bone resorption and osteogenesis [15], together with the hormones involved in calcium homeostasis. Indeed, vitamin D on one hand enhances bone resorption to increase calcium bioavailability, while on the other regulates the synthesis of bone matrix component such as osteocalcin, osteopontin and BALP. Parathyroid hormone (PTH) and calcitonin exert mutually opposite effects, with the former stimulating bone resorption and the latter enhancing osteogenesis [13].
Both estrogens and androgens have a predominant anabolic effect on the skeleton. Estrogens increase osteoblast number and activity, while inhibiting osteoclast maturation. Androgens exert not only direct effects on the growth plate, but also indirect regulatory activity, since they are converted to estradiol through the aromatization process [16].
2.2. The metastatic process and the bone microenvironment
The development of metastasis has been traditionally interpreted as the consequence of a late-stage detachment of cancer cells from the primary site, their subsequent intravasation into blood and lymphatic vessels and extravasation in distant organs. This process was considered stochastic and merely mechanical, with the first site reached by cancer cells regarded as the most likely metastatic site, due to tumour cell entrapment in small sized vessels [17]. In this context, the cells capable of escaping host immune response would first develop micrometastases and then macrometastases in months or even years, depending on the balance between tumour dormancy and proliferation [18].
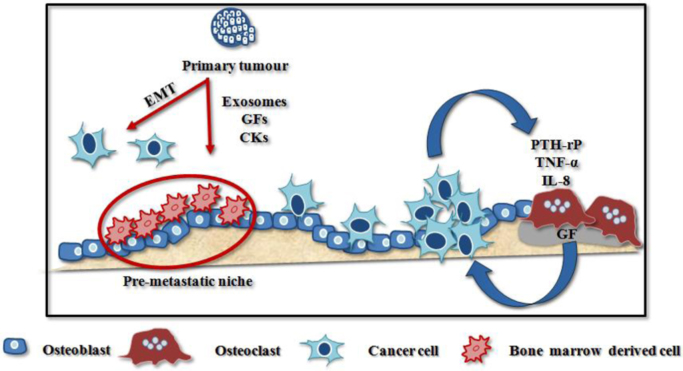
More recently, the primary tumour has turned out capable to release exosomes, growth factors (e.g. transforming growth factor-β, TGF-β; vascular endothelial growth factor, VEGF; placental growth factor, PlGF) and cytokines (e.g. TNF-α) that are able to recruit bone marrow derived cells (BMDC). BMDC increase vascular permeability, promote the extracellular matrix remodeling and modulate immune suppression to create the pre-metastatic niches, providing suitable microenvironments for cancer cell nesting and survival [19] (Fig. 2).
Fig. 2.
Establishment of osteolytic bone metastases. The onset of bone metastases is driven by the primary tumour through the recruitment of bone marrow derived cells. The latter promote the creation of a “pre-metastatic niche” by remodeling the extracellular matrix, exerting immune suppressive function and enhancing vascular permeability. Thanks to the up-regulation of specific chemokine receptors, some cancer cells migrate towards the bone niche, where they are able to survive for long periods in a quiescent state, until local and systemic conditions become suitable for the metastasis outgrowth. Thus, cancer cells alter the physiological bone turnover by releasing CKs that promote osteoclastogenesis and bone erosion. This phenomenon frees bone matrix-stored GFs which in turn promote cancer cell proliferation and perpetuate this vicious cycle. Adapted from D’Oronzo et al., 2017 [96]. Abbreviations: growth factors (GFs), cytokines (CKs).
Additionally, epithelial cancer cells undergo a morphological and functional remodeling (the so called epithelial-to-mesenchymal transition, EMT), to improve their migration capability and invasiveness. EMT is characterized by the acquisition of a spindle-like shape and the loss of intercellular junctions, accompanied by the down-regulation of epithelial markers (e.g. E-cadherin and cytokeratin) and the acquisition of mesenchymal ones (e.g. N-cadherin, fibronectin and vimentin) [20].
In order to explain the tropism of tumours for specific sites, Stephen Paget suggested that tumour cells (seeds) are more likely to metastasize towards a favorable environment (soil), able to support their growth [21]. According to this hypothesis, one would expect that tumours arising in paired organs (e.g. breast and lung) would easily generate metastases in the contralateral site, but this phenomenon is not frequently observed in clinical practice [22], [23].
This theory has been further developed, showing that cancer organotropism is driven by mutually attracting chemokine ligands and receptors, expressed by stromal and tumour cells, respectively. Indeed, bone-homing tumour cells overexpress the C–X–C motif chemokine receptor 4 (CXCR-4), whose ligand (C–X–C motif chemokine ligand 12, CXCL-12) is secreted by stromal cells, including those residing in bone. Other chemokine axes are involved in the bone-homing process (i.e. CXCR-3/CXCL-10 and CXCR-6/CXCL-16) [1], as well as the calcium sensing receptor (CaSR), expressed by advanced primary breast cancer (BC) [24] that is apparently responsible for the calcium-induced migration of BC cells in vitro [25].
Although a huge number of disseminated tumour cells (DTC) reaches the bone marrow, only a few of them successfully interact with the stroma, due to the expression of integrins [26]; in particular, αvβ3 and αvβ5 recognize and bind bone matrix proteins, while α4β1 interacts with the vascular cell adhesion molecule-1, expressed by bone marrow stromal cells [27], [28], [29]. Hence, DTC enter a state of dormancy and acquire the typical features of bone-residing cells (osteomimicry), in order to escape both cytotoxic treatments and immune response, until the microenvironment becomes suitable for their outgrowth months or, more usually, years later [1].
Once DTC start proliferating, osteolytic or osteoblastic BM may arise. The former, frequently associated with breast and lung tumours, derive from the establishment of a vicious cycle in which cancer cells secrete pro-osteoclastogenic factors (e.g. TNF-α, IL-8 and PTH related protein, PTH-rP). The enhanced bone resorption releases several matrix-stored growth factors (e.g. TGF-β; insulin-like growth factor, IGF; platelet-derived growth factor, PDGF) that further promote cancer cell proliferation [26]. On the other hand, prostate cancer (PC) BM are usually osteoblastic, due to the release of bone morphogenetic proteins (BMPs), TGF-β and fibroblast growth factor (FGF) that enhance the differentiation and activity of osteoblasts [15]. However, in most cases osteolytic and osteoblastic bone lesions coexist, suggesting the partial overlapping between those pathogenic mechanisms [30].
2.3. Application of bone turnover markers to the management of osteotropic tumours
Osteogenesis and bone resorption are both associated with the physiological release of phase-specific BTM, whose blood and/or urine levels reflect bone turnover and have been investigated as potential BM biomarkers (Table 1) [31].
Table 1.
Bone turnover biomarkers and potential clinical applications.
| Biomarker | Abbreviation | Potential clinical application | References |
|---|---|---|---|
| Bone formation markers | |||
| Bone alkaline phosphatase | BALP | Diagnosis of BM from solid tumours | [34], [42] |
| Prognostic role in the presence of BM from solid tumours | [97] | ||
| Prognostic role during BTA treatment | [44], [45], [48] | ||
| Prediction of response to atrasentan in PC | [50] | ||
| Pro-collagen type 1 N-terminal pro-peptide | P1NP | Diagnosis of BM from BC and PC | [32], [33], [34], [36], [37] |
| Pro-collagen type 1 C-terminal pro-peptide | P1CP | Diagnosis of BM from PC | [38] |
| Prediction of response to atrasentan in PC | [50] | ||
| Bone resorption markers | |||
| C-telopeptide of type 1 collagen | CTX | Diagnosis of BM from PC and LC | [38], [41] |
| N-telopeptide of type 1 collagen | NTX | Diagnosis of BM from solid tumours | [38], [41], [89] |
| Prognostic role in presence of BM from solid tumours | [97] | ||
| Prognostic role during BTA treatment | [44], [45], [46], [47], [48] | ||
| Prediction of response to atrasentan in PC; prediction of response to ZA | [49], [50] | ||
| Pyridinoline | PYD | Prediction of response to atrasentan in PC | [50] |
| Tartrate resistant acid phosphatase | TRACP | Diagnosis of BM from BC | [32], [34] |
| Cross-linked carboxy-terminal telopeptide of type 1 collagen | ICTP | Diagnosis of BM from LC | [41] |
| Receptor activator of nuclear factor KB-ligand/osteoprotegerin | RANK-L/OPG | Diagnosis of BM from BC | [35] |
“Bone formation” markers include P1NP and P1CP that are released in the circulation during pro-collagen cleavage, as well as BALP, produced by osteoblasts and critical for the maturation of bone matrix. On the other hand, “bone resorption” is accompanied by type 1 collagen degradation and the release of several collagen fragments, including not only CTX and NTX, but also pyridinoline (PYD) and deoxypyridinoline (DPD) that contribute to the mechanical stabilization of the protein. Other bone resorption markers are represented by the osteoblast-derived factors RANK-L and OPG, and the osteoclast-derived enzyme TRACP-5b. All the above-mentioned proteins are detectable in serum, while the peptides deriving from the degradation of type 1 collagen are also measurable in urine [10].
The next section will summarize the potential clinical applications of BTM in the management of bone-homing malignancies.
2.4. Diagnosis of bone metastases
Several studies have investigated the potential role of BTM in the diagnosis of BM from solid osteotropic tumours. In the BC setting, serum levels of TRACP and P1NP were reported to be significantly increased in the presence of BM, with the highest concentration of the latter observed in patients with more than seven skeletal lesions [32], [33]. In a recent prospective study, Lumachi and coworkers evaluated the accuracy of BTM in the detection of BC-related BM and achieved the highest value (82%, AUC = .889, 95% CI .798–.981) when combining BALP with P1NP and TRACP-5b [34]. On the other hand, another recent study focused on the RANK-L/OPG ratio that was found significantly increased in BC patients with BM. When considering a cut-off value ≥ .14, the sensitivity and specificity were 73% and 72%, respectively [35].
In PC patients, two studies showed a correlation between high serum P1NP levels and BM occurrence; interestingly, P1NP variations were detectable approximately eight months before the scintigraphic evidence of skeletal involvement [36], [37]. Other BTM were investigated in this clinical setting, including NTX, CTX, P1CP and BALP. In particular, BALP concentration significantly correlated with the extent of BM and was complementary to PSA during the staging of the disease; this observation suggested that the bone scan maybe unnecessary in a selected population [38], [39].
In the LC setting, urinary NTX (uNTX) levels > 62.5 pmol BCE/µmol creatinine strongly correlated with skeletal involvement [40], similarly to the increased serum concentration of CTX β isomer (β-CTX) and another peptide deriving from type 1 collagen degradation, namely cross-linked carboxy-terminal telopeptide of type 1 collagen (1CTP) (p < .001 in both instances) [41].
A meta-analysis involving 3268 patients with solid tumours showed that, in the presence of BM, serum BALP (sBALP) was significantly higher, as compared to patients without bone lesions (41.50 ± 26.61 µg/L vs 14.49 ± 5.52 µg/L, p < .05) [42]. Another meta-analysis that included 14 studies and 1279 patients, demonstrated the existence of a significant correlation between serum NTX and bone involvement [43].
Although promising, these results are characterized by sub-optimal specificity, sensitivity and diagnostic efficiency at the individual patient level that limit the clinical value of BTM for the diagnosis of BM in routine clinical practice.
2.5. Prognostic role of bone turnover markers
Several retrospective analyses investigated the association between BTM levels and clinical outcome, among patients with BM undergoing anti-resorptive therapies [7].
In particular, sBALP and uNTX levels were assessed in 1824 patients treated with zoledronic or pamidronic acid for BM from different malignancies; high sBALP significantly correlated with the occurrence of SREs, especially in PC patients, while moderate-high uNTX was associated with a two-fold increase in risk of skeletal complications (p < .001 in both instances) [44]. In parallel with these observations, Brown et al. described a significant correlation between the risk of SREs and the evidence of high sBALP and uNTX at baseline in bisphosphonate treated patients, when exploring data coming from the same placebo-controlled phase III trials of zoledronic acid in advanced cancers [45].
Conversely, normal NTX at baseline correlated with a significant reduction in the risk of death and pathological fractures (40% and 52%, respectively; p < .0001 in both instances), in patients with BM treated with zoledronic acid. Moreover, patients whose NTX values remained within the normal range throughout the trial duration (24 months) exhibited a 40–68% reduced risk of death or skeletal complications (p ≤ .0005), as compared to those with raised NTX levels [46]. Additionally, in patients with high baseline levels of NTX, the progressive normalization of the parameter during the first three months of bisphosphonate treatment (as compared to the failure to normalize) correlated with better survival and less skeletal complications [47].
In support of this, a recent retrospective study has analyzed data from 5543 patients treated with zoledronate or denosumab for BM and involved in three phase III trials. sBALP and/or uNTX ≥ median value (12.6 ng/ml and 10 nmol/mmol, respectively) after the first three months of treatment were associated with disease and BM progression, together with significantly reduced overall survival, as compared to lower BTM levels (p < .0001 in all instances) [48].
2.6. Role of bone turnover markers in predicting treatment efficacy
The potential role of BTM in predicting the efficacy of bone-targeting agents (BTA) was deduced from the databases belonging to three zoledronate randomized clinical trials, involving patients with BM from multiple myeloma or solid malignancies. In particular, patients with aggressive skeletal disease and baseline NTX ≥ 100 nmol/mmol creatinine, significantly benefited from zoledronate treatment, undergoing a 31% reduction of the risk of death (p = .0028) independently from the prevention of skeletal complications [49].
More recently, a prospective analysis has involved 778 PC patients recruited into a placebo-controlled phase III trial of docetaxel with or without the endothelin-A receptor antagonist atrasentan. The bone-targeting capability of this drug has been investigated owing to the emerging role of endothelin pathway in the progression of BM from PC. The authors evaluated two “bone formation” (BALP, P1CP) and two “bone resorption” (NTX, PYD) markers, both at baseline and at regular intervals, describing a correlation between worse survival and the evidence of high baseline levels and/or progressive increase of each marker (p < .001). The trial did not meet its primary endpoint, but the patients with the highest BTM concentration (upper 25th percentile) benefited from atrasentan in terms of median survival (13 months vs 5 months with placebo; p = .005) [50].
The role of BTM in predicting response to anti-cancer treatments other than BTA has also been explored. For instance, ALP levels have been monitored in PC patients with BM receiving abiraterone, over a median follow-up of 14 months. Some patients underwent an interesting phenomenon, called “ALP bouncing”, characterized by ALP rising within the first 2–4 weeks of abiraterone treatment, followed by its decline within 8 weeks. Interestingly, the lack of an ALP “bounce” has turned out to be the strongest predictor of poor overall survival, in association with the failure in PSA decrease (p < .001) [51].
2.7. Limitations of bone turnover markers
Several limitations still hamper the routine application of BTM assessment. These include patient's features (age, sex, food intake, natural diurnal variation, liver and/or kidney diseases) and concomitant treatments interfering with bone turnover, such as the hormone therapies currently administered to BC and PC patients [31]. Moreover, BTM levels physiologically undergo seasonal variations that need to be taken into consideration when interpreting the measurement [10], and will influence both the inter- and intra-assay variation coefficients. In addition to this, sBALP detection is even more difficult because of its cross-reactivity with the hepatic isoform of the protein [7].
2.8. Bone metastasis biomarkers in solid malignancies
Recent evidence suggests that the early identification of patients at high risk of BM could lead to the adoption of personalized adjuvant treatments, potentially able to modify the disease evolution, at least in BC patients [52], [53], [54]. Thus, the next sections will summarize the most recent advances in terms of BM biomarkers, focusing on breast, prostate and lung malignancies (Table 2).
Table 2.
Potential tumour-derived biomarkers for BM in breast, prostate and lung cancer.
| Bone metastasis biomarkers | References |
|---|---|
| Breast Cancer | |
| Genes | |
| Kang's signature (102 genes) | [57] |
| IL-1β | [59] |
| MAF | [62] |
| 15-gene signature | [58] |
| (APOPEC3B, ATL2, BBS1, C6orf61, C6orf167, MMS22L, CNS1, MFAP3L, NIP7, NUP155, PALM2, PH-4, PGD5, SFT2D2, STEAP3) | |
| ZNF217 | [61] |
| Proteins | |
| ER +, Her2-neu – | [55] |
| CAPG, GIPC1 | [8] |
| CXCR4, cadherin-11, osteopontin, BSP | [68] |
| HR+ | [56] |
| miRNAs | |
| Up-regulated: miR-10b, miR-373, miR-520c, miR-218, miR-214–3p | [63], [64], [65], [66] |
| Down-regulated: miR-126, miR-335, miR-206 | [67] |
| Prostate Cancer | |
| Genes | |
| PDGFRα | [72], [73] |
| IL-1β | [74] |
| Proteins | |
| IL-1β | [74] |
| CXCR-4 | [75] |
| miRNAs | |
| Up-regulated: miR−154, miR−379 | [76] |
| Down-regulated: miR-143, miR-145, miR-203 | [77], [78] |
| Lung Cancer | |
| Genes | |
| CD22, hepatocyte nuclear factor 1 α, adenomatous poliposis coli | [89] |
| Proteins | |
| BSP, CXCR-4, osteopontin, BMP4 | [90], [91] |
| Circulating PTH-rP | [92] |
| miRNAs | |
| Down-regulated: miR-33a | [93] |
2.9. Bone metastasis biomarkers in breast cancer
BC is the most frequent tumour in women, affecting annually more than 460,000 new patients in Europe, with a mortality incidence of 130,000/year [2]. Up to 70% of women with advanced BC develop BM; these are usually localized to the axial skeleton and demonstrate a lytic radiological appearance, although osteoblastic and mixed patterns are not unusual [6].
A retrospective study investigated the correlation between BC organotropism and the expression of estrogen receptor (ER), human epidermal growth factor receptor 2 (Her2) and proliferation rate as assessed by Ki67 staining, analyzed on 263 primary invasive breast tumours that subsequently resulted in metastases. The study showed that ER+ Her2- tumours with Ki67 score > 13% were associated with the highest incidence of BM (87.8%) [55]. Similar results have been recently described on a series of 490 BC patients, where hormone receptor positive (HR+) malignancies resulted in skeletal metastases in up to 72% of patients, while bone-only metastases were detected in 36% of women with luminal A BC [56].
Several attempts have been made to identify the BC “osteotropism gene signature”, including the one described by Kang and coworkers who identified a 102-gene expression profile in a bone metastatic subpopulation of the MDA-MB-231 BC cell line [57]. Another smaller signature, including 15 genes with high recurrence (82.4%) in bone-homing BC, was identified on a series of 157 primary breast tumours and included membrane molecules involved in protein binding [58].
Further investigation led to the evidence that IL-1β gene was significantly up-regulated in a bone-homing clone of MDA-MB-231 cells, obtained by seven passages in vivo. Subsequently, in 150 primary breast tumours screened for IL-1β expression, a significant correlation with the development of BM (p < .0001) was seen [59]. This evidence was followed by the pre-clinical evaluation of an IL-1 receptor (IL-1R) antagonist (Anakinra) for the prevention and treatment of BC-derived BM; 1 mg/kg/day treatment reduced the number of bone metastatic mice compared with placebo (40% vs 90%) and, interestingly, when the drug was administered before the tumour cell injection, only 10% of the animals developed BM [60].
Recently, another gene encoding the zinc-finger protein 217 (ZNF217) has been found to be significantly up-regulated in BC tumours developing BM (p = .005), particularly in those with an ER+ phenotype [61].
The application of genomics to BC research led to the evidence of a copy number variation associated with BM. In both osteotropic BC cell lines and primary breast tumours, amplification of the 16q23 region, with at least 1.5 copies of the region, normalized to the CEP16 centromeric probe per cell, was significantly correlated with BM occurrence (HR = 14.5, 95% I = 6.4 to 32.9, p < .001). This region (16q23) encodes a transcription factor (v-maf avian musculo-aponeurotic fibrosarcoma oncogene homolog, MAF) involved in the regulation of several genes participating to the establishment of BC BM, such as PTHrP [62].
As gene expression regulators, small non-coding micro RNAs (miRNAs) were also investigated as potential BM predictors. In particular, up-regulation of miR-10b, miR-373 and miR-520c was correlated with the enhanced migration and invasiveness of BC cells, both in vitro and in vivo [63], [64]. Moreover miR-218, that is physiologically involved in osteoblast differentiation, was found to be associated with BC cell osteomimicry [65]. Recently, miR-214-3p was shown to be significantly overexpressed in BC patients with osteolytic BM. Through a series of in vitro and in vivo studies, Liu and coworkers demonstrated that miR-214-3p can stimulate bone resorption by targeting the TNF receptor associated factor 3 (TRAF3) [66].
On the other hand, an array-based miRNA profiling was conducted on MDA-MB-231 cell subpopulations with different organotropism and revealed that some miRNAs were able to interfere with the metastatic process. In particular, miR-126 was shown to inhibit cancer cell proliferation, while miR-206 and miR-335 impaired cell migration and invasiveness. These miRNAs were down-regulated in bone-homing cells and, once re-expressed via retroviral transduction, significantly counteracted the development of BM in mice. Moreover, a qRT-PCR analysis performed on 20 primary breast tumours showed that low expression of any of the three miRNAs was associated with shorter median time to metastasis [67].
Several proteomic studies have been conducted to identify potential BM predictive biomarkers. A recent systematic review focused on 14 proteins that were shown to be significantly overexpressed in bone-homing BC cells (including CXCR-4, cadherin-11, osteopontin and bone sialoprotein, BSP), while the chemokine C-C ligand 2 (CCL2) has been inversely correlated with BM occurrence [68].
In a recent proteomic analysis, performed on MDA-MB-231 subpopulations with variable organotropism, macrophage-capping protein (CAPG) and PDZ domain–containing protein member 1 (GIPC1) were up-regulated in bone-homing cells, as compared to the parental population. To validate these proteins as biomarkers, breast tumour samples from 724 patients recruited in the AZURE trial, evaluating the role of adjuvant zoledronic acid in early BC [53], were analyzed. The authors showed that high expression of both proteins significantly correlated not only with the development of BM (p < .001) but also with the efficacy of adjuvant zoledronic acid in preventing BM (p = .008) [8]. This means that besides its role in the prognosis of BM, the combined CAPG/GIPC1 biomarker is predictive of treatment benefit and could potentially be applied to the selection of patients for adjuvant bisphosphonate treatment.
2.10. Bone metastasis biomarkers in prostate cancer
PC is the second most common tumour in European men and affects annually 420,000 new patients, with approximately 90,000 deaths/year. In most cases, it is diagnosed over the age of 70 [3], giving rise to BM in approximately 70–80% of patients with advanced disease, in the form of osteoblastic lesions [6], [69], [70].
Several attempts have been made to identify potential prognostic biomarkers for PC-related BM and a systematic review, analyzing data from 8644 patients, correlated skeletal involvement with a Gleason score ≥ 8, serum prostate specific antigen (PSA) ≥ 20 ng/ml and locally advanced disease at diagnosis [71]. However, subsequent studies partially contradicted these results giving rise to a significant debate about the parameters and cut-off levels to be employed [70].
Pre-clinical studies focused on two subpopulations of the PC3 cancer cell line (i.e. PC3-ML and PC3-N), characterized by similar chemokine receptors but differential osteotropism. In particular, PC3-ML cells were found to over-express platelet derived growth factor receptor α (PDGFRα) and this could partially explain their improved capability to survive in the bone marrow, under the proliferative stimulation provided by PDGF [72]. Indeed, once PC3-N cells were transduced to over-express PDGFRα, they underwent a significant improvement of the bone-homing potential; moreover, an anti-human PDGFRα monoclonal antibody was able to induce a 72% tumour burden reduction in murine femora and tibiae, as compared to placebo [73].
Interestingly, PDGFRα was found to up-regulate IL-1β; thus, a retrovirus-mediated over-expression of this gene in PC3-N cells was attempted, observing improved bone metastatic capability. On the other hand, the IL-1β knock down in PC3-ML cells, obtained through a short hairpin RNA (shRNA), impaired their osteotropism. Finally, 227 PC samples were screened for IL-1β and shown to be up-regulated in tumours with a Gleason score ≥ 7, as compared to less aggressive malignancies or healthy prostate samples [74].
In a meta-analysis involving 630 patients from 11 studies, CXCR-4 was identified as another potential biomarker and shown to be both more frequently expressed in PC samples, as compared to non-malignant tissue (p < .00001), and correlated with BM development (p = .003) [75].
miRNAs have also been investigated as potential BM biomarkers, through expression profiling performed on PC cell lines, primary tumours and BM tissues. In particular, miR-154 and miR-379 were found to be over-expressed, both in vitro and in vivo, in bone-homing PC cells with mesenchymal properties and enhanced invasiveness [76]. By contrast, miR-143, miR-145 and miR-203 were all down-regulated in osteotropic PC cells, compared to the corresponding primary tumours, as well as in BM samples. Once PC cells were transfected to over-express those miRNAs, they lost their “mesenchymal-like” features, and this led to impaired migration capability and invasiveness. miR-203 was also found to counteract the osteomimicry process through the down-regulation of osteoblast-derived genes, such as osteopontin, osteocalcin and Runx-2 [77], [78].
Among soluble biomarkers, circulating tumour cells (CTC) have been widely investigated in PC patients for prognostic purposes. In particular, several prospective trials established a correlation between CTC count and clinical outcome [79], [80], [81], [82], [83], [84], leading to the definition of a cut-off value, able to differentiate men with favorable outcome (with ≤ 4 CTC/7.5 ml) from those with unfavorable prognosis (with ≥ 5 CTC/7.5 ml). Moreover, de Bono et al. reported that a post-treatment 30% decline of the CTC count correlated with better overall survival, in patients treated with abiraterone or chemotherapy [85]. Interestingly, PC patients with both bone and visceral metastases exhibited the highest CTC count (median 26, range 0–207), as compared to those with soft tissue lesions (median 0, range 0–28). However, no correlations were found between CTC count and skeletal tumour burden, assessed by both bone lesion count (p = .54) and bone scan index (p = .81) [86]. Further CTC features, besides their number, have been investigated for prognostic purposes. Indeed, Okegawa et al. have recently described that the expression of epidermal growth factor receptor, in CTC isolated from castration-resistant PC patients treated with docetaxel, correlates with shorter overall survival (5.5 vs 20.0 months), as compared to its absence (p < .001) [87].
2.11. Bone metastasis biomarkers in lung cancer
LC is the third most common bone-homing tumour, with approximately 30–40% of patients developing BM during their lifetime [88].
A retrospective study of 661 patients with non-small cell LC (NSCLC) showed the presence of BM in 57.7% of cases at diagnosis, associated with a median overall survival of 9.5 months. In this series, osteolytic BM were detected in 74.3% of patients, while mixed (14.3%) and osteoblastic (11.4%) patterns were much less common [4].
Several markers have been investigated for the prediction of BM in early-stage patients and Zhang et al. have recently identified, by next generation sequencing, three genes (i.e. hepatocyte nuclear factor 1α, CD22 and adenomatous polyposis coli) with high mutation frequency (> 50%) in NSCLC patients with BM [89].
Among proteins, BSP was associated with BM occurrence by Scagliotti and coworkers. In particular, the authors screened primary NSCLC samples for the expression of 10 peptides and then matched the results coming from bone-metastatic tumours with a cohort associated with visceral metastasis and another one without secondary tumours. The immunohistochemical analysis showed a significant correlation between BSP expression and both worse disease outcomes (p = .02) and development of BM (p < .001) [90].
Later, another immunohistochemical study was conducted on 105 stage III NSCLC samples; four markers involved in BM pathogenesis (BSP, CXCR-4, osteopontin and BMP4) were included in a molecular model, established by multivariate logistic regression and then prospectively validated. The model exhibited a prediction sensitivity of 85.7% and a specificity of 66.7% [91].
Serum levels of cytokines involved in bone resorption were measured, at the time of diagnosis, in 1149 hypercalcemic LC patients in order to find out a potential circulating biomarker for BM prediction. Interestingly, PTH-rP concentration higher than 150 pmol/l was associated with the presence of hypercalcemia and significantly correlated with both BM incidence (71.4%) and decreased median survival (1.4 months) [92].
More recently, PTH-rP expression in LC cells has been inversely correlated with the intracellular levels of miR-33a, a physiological inhibitor of PTH-rP transcription. Thus, miR-33a could be considered not only an indirect biomarker of bone resorption but also a potential therapeutic target, for which further investigation is needed [93].
3. Conclusions
Bone represents a very common site of metastasis, especially from breast, prostate and lung cancer. The development of BM defines a non-curable condition associated with serious skeletal complications and worsened quality of life.
BM are often not identified until patients are symptomatic, since current diagnostic tools have a limited capability to detect early-stage lesions. This suggests the need for reliable and reproducible means for the timely identification of “high-risk” patients.
To this purpose, a number of pre-clinical and clinical studies have investigated tumour-derived markers for their potential BM predictive role, while BTM have been screened as diagnostic and prognostic markers, as well as for the development of anti-cancer drugs. However, several limitations currently hamper their routine use in the clinical practice, thus further investigation for clinical validation is needed before a change in guideline-based recommendations can be anticipated [94].
Disclosures
No relevant disclosures for bone biomarkers (SD and JB). Consultancy-Inbiomotion (RC).
Contributor Information
Stella D’Oronzo, Email: s.doronzo@sheffield.ac.uk.
Janet Brown, Email: j.e.brown@sheffield.ac.uk.
Robert Coleman, Email: r.e.coleman@sheffield.ac.uk.
References
- 1.Kan C., Vargas G., Pape F.L., Clézardin P. Cancer cell colonisation in the bone microenvironment. Int. J. Mol. Sci. 2016;17(10):1–16. doi: 10.3390/ijms17101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Research UK (CRUK) and National Cancer Intelligence Network (NCIN). CRUK BC statistics. 〈http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#KSe8ZK5GxJiqGX75.99〉. (Accessed February 11, 2017).
- 3.CRUK. Cancer statistics. 〈http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence#heading-One〉. Published 2013. (Accessed 11 February 2017).
- 4.Santini D., Barni S., Intagliata S., Falcone A., Ferraù F., Galetta D., Moscetti L., La Verde N., Ibrahim T., Petrelli F., Vasile E., Ginocchi L., Ottaviani D., Longo F., Ortega C., Russo A., Badalamenti G., Collovà E., Lanzetta G., Mansueto G., Adamo V., De Marinis F., Satolli M.A., Cantile F., Mancuso A., Tanca F.M., Addeo R., Russano M., Sterpi M., Pantano F., Vincenzi B., Tonini G. Natural history of non-small-cell lung cancer with bone metastases. Sci. Rep. 2015;5 doi: 10.1038/srep18670. (Article 18670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clamp A., Danson S S., Nguyen H., Cole D., Clemons M. Assessment of therapeutic response in patients with metastatic bone disease. Lancet Oncol. 2004;5(10):607–616. doi: 10.1016/S1470-2045(04)01596-7. [DOI] [PubMed] [Google Scholar]
- 6.Coleman R.E. Skeletal complications of malignancy. Cancer. 1997;80(Suppl. 8):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Coleman R.E., Brown J., Terpos E., Lipton A., Smith M.R., Cook R., Major P. Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions. Cancer Treat. Rev. 2008;34(7):629–639. doi: 10.1016/j.ctrv.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westbrook J.A., Cairns D.A., Peng J., Speirs V., Hanby A.M., Holen I., Wood S.L., Ottewell P.D., Marshall H., Banks R.E., Selby P.J., Coleman R.E., Brown J.E. CAPG and GIPC1: BC biomarkers for bone metastasis development and treatment. J. Natl. Cancer Inst. 2016;108(4):djv360. doi: 10.1093/jnci/djv360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.R. Coleman, A. Hall, R. Bell, D. Cameron, H. Marshall, J. Jean-Mairet, J. Tercero, F. Rojo, J. Albanell, R. Gomis, Impact of MAF gene amplification on disease recurrence and effects of adjuvant zoledronic acid in early breast cancer, in: Proceedings of San Antonio BC Symposium, Abstract P1-09-01, 2016.
- 10.Brown J.E., Sim S. Evolving role of bone biomarkers in castration-resistant prostate cancer. Neoplasia. 2010;12(9):685–696. doi: 10.1593/neo.10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halling Linder C., Ek-Rylander B., Krumpel M., Norgard M., Narisawa S., Millán J.L., Andersson G., Magnusson P. bone alkaline phosphatase and Tartrate-resistant acid phosphatase: potential co-regulators of bone mineralization. Calcif. Tissue Int. 2017;101(1):92–101. doi: 10.1007/s00223-017-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You L., Temiyasathit S., Lee P., Kim C.H., Tummala P., Yao W., Kingery W., Malone A.M., Kwon R.Y., Jacobs C.R. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42(1):172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raisz L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999;45(8):1353–1358. [PubMed] [Google Scholar]
- 14.Marotti G. Osteoncology Textbook. Poletto; 2010. Functional anatomy of bone; pp. 2–27. [Google Scholar]
- 15.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;50:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 16.Higano C.S. Androgen-deprivation-therapy-induced fractures in men with non metastatic prostate cancer: what do we really know? Nat. Clin. Pract. Urol. 2008;5(1):24–34. doi: 10.1038/ncpuro0995. [DOI] [PubMed] [Google Scholar]
- 17.Ewing J. 3rd edition. WB Saunders; Philadelphia: 1928. A Treatise on Tumours. [Google Scholar]
- 18.Klein C.A. Cancer. The metastasis cascade. Science. 2008;321(5897):1785–1787. doi: 10.1126/science.1164853. [DOI] [PubMed] [Google Scholar]
- 19.Sceneay J., Smyth M.J., Möller A. The pre-metastatic niche: finding common ground. Cancer Metastas. Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 20.Meng F., Wu G. The rejuvenated scenario of epithelial–mesenchymal transition (EMT) and cancer metastasis. Cancer Metastas. Rev. 2012;31:455–467. doi: 10.1007/s10555-012-9379-3. [DOI] [PubMed] [Google Scholar]
- 21.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 22.Chen M.T., Sun H.F., Zhao Y., Fu W.Y., Yang L.P., Gao S.P., Li L.D., Jiang H.I., Jin W. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population based analysis. Sci. Rep. 2016;7:9254. doi: 10.1038/s41598-017-10166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu F.Y., Zhou Q., Yang J.J., Zhong W.Z., Chen Z.H., Deng W., He Y.Y., Chen H.J., Zeng Z., Ke E., Zhao N., Zhang N., Sun H.W., Zhang Q.Y., Xie Z., Zhang X.C., Wu Y.L. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer. 2016;16:149. doi: 10.1186/s12885-016-2169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mihai R., Stevens J., McKinney C., Ibrahim N.B. Expression of the calcium receptor in human breast cancer-a potential new marker predicting the risk of bone metastases. Eur. J. Surg. Oncol. 2006;32:511–515. doi: 10.1016/j.ejso.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Saidak Z., Boudot C., Abdoune R., Petit L., Brazier M., Mentaverri R., Kamel S. Extracellular calcium promotes the migration of breast cancer cells through the activation of the calcium sensing receptor. Exp. Cell Res. 2009;315:2072–2080. doi: 10.1016/j.yexcr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Ren G., Esposito M., Kang Y. Bone metastasis and the metastatic niche. J. Mol. Med. 2015;93:1203–1212. doi: 10.1007/s00109-015-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clézardin P. Integrins in bone metastasis formation and potential therapeutic implications. Curr. Cancer Drug Targets. 2009;9:801–806. doi: 10.2174/156800909789760348. [DOI] [PubMed] [Google Scholar]
- 28.Sung V., Stubbs J.T., Fisher L., Aaron A.D., Thompson E.W. Bone sialoprotein supports breast cancer cell adhesion proliferation and migration through differential usage of the αvβ3 and αvβ5 integrins. J. Cell Physiol. 1998;176:482–494. doi: 10.1002/(SICI)1097-4652(199809)176:3<482::AID-JCP5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 29.Michigami T., Shimizu N., Williams P.J., Niewolna M., Dallas S.L., Mundy G.R., Yoneda T. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and α4β1-integrin enhances production of osteoclast-stimulating activity. Blood. 2000;96:1953–1960. [PubMed] [Google Scholar]
- 30.Lipton A., Uzzo R., Amato R.J., Ellis G.K., Hakimian B., Roodman G.D., Smith M.R. The Science and practice of bone health in oncology: managing bone loss and metastasis in patients with solid tumours. J. Natl. Compr. Cancer Netw. 2009;7(Suppl. 7):S1–S30. doi: 10.6004/jnccn.2009.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman R.E., Costa L., Saad F., Cook R., Hadji P., Terpos E., Garnero P., Brown J., Body J.J., Smith M., Lee K., Major P., Dimopoulos M., Lipton A. Consensus on the utility of bone markers in the malignant bone disease setting. Crit. Rev. Oncol. /Hematol. 2011;80:411–432. doi: 10.1016/j.critrevonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Wada N., Fujisaki M., Ishii S., Ikeda T., Kitajima M. Evaluation of bone metabolic markers in breast cancer with bone metastasis. Breast Cancer. 2001;8:131–137. doi: 10.1007/BF02967492. [DOI] [PubMed] [Google Scholar]
- 33.Oremek G., Sauer-Eppel H., Klepzig M. Total procollagen type 1 amino-terminal propeptide (total P1NP) as a bone metastasis marker in gynecological carcinomas. Anticancer Res. 2007;27:1961–1962. [PubMed] [Google Scholar]
- 34.Lumachi F., Basso S.M.M., Camozzi V., Tozzoli R., Spaziante R., Ermani M. Bone turnover markers in women with early stage breast cancer who developed bone metastases. A prospective study with multivariate logistic regression analysis of accuracy. Clin. Chim. Acta. 2016;460:227–230. doi: 10.1016/j.cca.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Elfar G.A., Ebrahim M.A., Elsherbiny N.M., Eissa L.A. Validity of osteoprotegerin and receptor activator of NF-κB ligand for detection of bone metastasis in breast cancer. Oncol. Res. 2016 doi: 10.3727/096504016X14768398678750. (Epub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koopmans N., de Jong I.J., Breeuwsma A.J., van der Veer E. Serum bone turnover markers (PINP and ICTP) for the early detection of bone metastases in patients with prostate cancer: a longitudinal approach. J. Urol. 2007;178:849–853. doi: 10.1016/j.juro.2007.05.029. (discussion follows) [DOI] [PubMed] [Google Scholar]
- 37.Klepzig M., Jonas D., Oremek G.M. Procollagen type-1 amino-terminal propeptide: a marker for bone metastases in prostate carcinoma. Anticancer Res. 2009;29(2):671–673. [PubMed] [Google Scholar]
- 38.Westerhuis L.W., Delaere K.P. Diagnostic value of some biochemical bone markers for the detection of bone metastases in prostate cancer. Eur. J. Clin. Chem. Clin. Biochem. 1997;35:89–94. doi: 10.1515/cclm.1997.35.2.89. [DOI] [PubMed] [Google Scholar]
- 39.Lorente D., Olmos D., Mateo J., Bianchini D., Seed G., Fleisher M., Danila D.C., Flohr P., Crespo M., Figueiredo I., Miranda S., Baeten K., Molina A., Kheoh T., Mc Cormack R., Terstappen L.W.M.M., Scher H.I., de Bono J.S. Decline in circulating tumor cell count and treatment outcome in advanced prostate cancer. Eur. Urol. 2016;70:985–992. doi: 10.1016/j.eururo.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izumi M., Nakanishi Y., Takayama K., Kimotsuki K., Inoue K., Wataya H., Minami T., Hara N. Diagnostic value of bone turnover metabolites in the diagnosis of bone metastases in patients with lung carcinoma. Cancer. 2001;91:1487–1493. doi: 10.1002/1097-0142(20010415)91:8<1487::aid-cncr1156>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Kong Q.Q., Sun T.W., Dou Q.Y., Tang Q., Pei F.X., Tu C.Q., Chen Z.Q. Beta-CTX and ICTP act as indicators of skeletal metastasis status in male patients with non-small cell lung cancer. Int. J. Biol. Markers. 2007;22:214–220. doi: 10.1177/172460080702200309. [DOI] [PubMed] [Google Scholar]
- 42.Du W.X., Duan S.F., Chen J.J., Huang J.F., Yin L.M., Tong P.J. Serum bone-specific alkaline phosphatase as a biomarker for osseous metastases in patients with malignant carcinomas: a systematic review and meta-analysis. J. Cancer Res. Ther. 2014;10(Special Issue):C140–C143. doi: 10.4103/0973-1482.145842. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y., Yi M., Cao J., Hou C., Zhou Y., Zhong Y. Serum cross-linked N-telopeptide of type I collagen for the diagnosis of bone metastases from solid tumours in the Chinese population: meta-analysis. J. Int. Med. Res. 2016;44(2):192–200. doi: 10.1177/0300060515600187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coleman R.E., Major P., Lipton A., Brown J.E., Lee K.A., Smith M., Saad F., Zheng M., Hei Y.J., Seaman J., Cook R. Predictive value of bone resorption and formation markers on cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J. Clin. Oncol. 2005;23(22):4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 45.Brown J.E., Cook R.J., Lipton A., Costa L., Coleman R.E. Prognostic factors for skeletal complications from metastatic disease in breast cancer. Breast Cancer Res. Treat. 2010;123:767–779. doi: 10.1007/s10549-010-0981-1. [DOI] [PubMed] [Google Scholar]
- 46.Lipton A., Cook R., Brown J., Body J.J., Smith M., Coleman R. Skeletal-related events and clinical outcomes in patients with bone metastases and normal levels of osteolysis: exploratory analyses. Clin. Oncol. 2013;25:217–226. doi: 10.1016/j.clon.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Lipton A., Cook R., Saad F., Major P., Garnero P., Terpos E., Brown J.E., Coleman R.E. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumours and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 48.Lipton A., Smith M.R., Fizazi K., Stopeck A.T., Henry D., Brown J.E., Shore N.D., Saad F., Spencer A., Zhu L., Warner D.J. Changes in bone turnover marker levels and clinical outcomes in patients with advanced cancer and bone metastases treated with bone antiresorptive agents. Clin. Cancer Res. 2016;22(23):5713–5721. doi: 10.1158/1078-0432.CCR-15-3086. [DOI] [PubMed] [Google Scholar]
- 49.Coleman R.E., Lipton A., Costa L., Cook R.J., Lee K., Saad F., Brown J.E., Terpos E., Major P.P., Kohno N., Smith M., Body J.J. Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumours and poor prognostic features – an exploratory analysis of placebo-controlled trials. J. Bone Oncol. 2013;2:70–76. doi: 10.1016/j.jbo.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lara P.N., Jr, Ely B., Quinn D.I., Mack P.C., Tangen C., Gertz E., Twardowski P.W., Goldkorn A., Hussain M., Vogelzang N.J., Thompson I.M., Van Loan M.D. Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. JNCI J. Natl. Cancer Inst. 2014;106(4):1–9. doi: 10.1093/jnci/dju013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mikah P., Krabbe L.M., Eminaga O., Herrmann E., Papavassilis P., Hinkelammert R., Semjonow A., Schrader A.J., Boegemann M. Dynamic changes of alkaline phosphatase are strongly associated with PSA-decline and predict best clinical benefit earlier than PSA-changes under therapy with abiraterone acetate in bone metastatic castration resistant prostate cancer. BMC Cancer. 2016;16:214. doi: 10.1186/s12885-016-2260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gnant M., Mlineritsch B., Stoeger H., Luschin-Ebengreuth G., Knauer M., Moik M., Jakesz R., Seifert M., Taucher S., Bjelic-Radisic V., Balic M., Eidtmann H., Eiermann W., Steger G., Kwasny W., Dubsky P., Selim U., Fitzal F., Hochreiner G., Wette V., Sevelda P., Ploner F., Bartsch R., Fesl C., Greil R. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozole plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol. 2015;26:313–320. doi: 10.1093/annonc/mdu544. [DOI] [PubMed] [Google Scholar]
- 53.Coleman R., Cameron D., Dodwell D., Bell R., Wilson C., Rathbone E., Keane M., Gil M., Burkinshaw R., Grieve R., Barrett-Lee P., Ritchie D., Liversedge V., Hinsley S., Marshall H. AZURE investigators, adjuvant zoledronic acid in patients with early BC: final efficacy analysis of the AZURE (big 01/04) randomised open- label phase 3 trial. Lancet Oncol. 2014;15:997–1006. doi: 10.1016/S1470-2045(14)70302-X. [DOI] [PubMed] [Google Scholar]
- 54.R. Coleman, M. Gnant, A. Paterson, T. Powles, G. von Minckwitz, K. Pritchard, J. Bergh, J. Bliss, J. Gralow, S. Anderson, V. Evans, H. Pan, R. Bradley, C. Davies, R. Gray, , Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer: a meta-analysis of individual patient data from randomized trials, in: Proceedings of San Antonio Breast Cancer Symposium, Abstract S4-07, 2013.
- 55.Savci-Heijink C.D., Halfwerk H., Hooijer G.K.J., Horlings H.M., Wesseling J., van de Vijver M.J. Retrospective analysis of metastatic behavior of breast cancer subtypes. BC Res. Treat. 2015;150:547–557. doi: 10.1007/s10549-015-3352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molnár I.A., Molnár B.A., Vízkeleti L., Fekete K., Tamás J., Deák P., Szundi C., Székely B., Moldvay J., Vári-Kakas S., Szász M.A., Ács B., Kulka J., Tőkés A.M. Breast carcinoma subtypes show different patterns of metastatic behavior. Virchows Arch. 2017;470:275–283. doi: 10.1007/s00428-017-2065-7. [DOI] [PubMed] [Google Scholar]
- 57.Kang Y., Siegel P.M., Shu W., Drobnjak M., Kakonen S.M., Cordòn-Cardo C., Guise T.A., Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 58.Savci-Heijink C.D., Halfwerk H., Koster J., van de Vijver M.J. A novel gene expression signature for bone metastasis in breast carcinomas. BC Res. Treat. 2016;156:249–259. doi: 10.1007/s10549-016-3741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nutter F., Holen I., Brown H.K., Cross S.S., Evans C.A., Walker M., Coleman R.E., Westbrook J.A., Selby P.J., Brown J.E., Ottewell P.D. Different molecular profiles are associated with breast cancer cell homing compared with colonization of bone: evidence using a novel bone-seeking cell line. Endocr. Relat. Cancer. 2014;21:327–341. doi: 10.1530/ERC-13-0158. [DOI] [PubMed] [Google Scholar]
- 60.Holen I., Lefley D.V., Francis S.E., Rennicks S., Bradbury S., Coleman R.E., Ottewell P. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. 2016;7(46):75571–75584. doi: 10.18632/oncotarget.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bellanger A., Donini C.F., Vendrell J.A., Lavaud J., Machuca-Gayet I., Ruel M., Vollaire J., Grisard E., Gyorffy B., Bièche I., Peyruchaud O., Coll J.L., Treilleux I., Maguer-Satta V., Josserand V., Cohen P.A. The critical role of the ZNF217 oncogene in promoting breast cancer metastasis to the bone. J. Pathol. 2017;242(1):73–89. doi: 10.1002/path.4882. [DOI] [PubMed] [Google Scholar]
- 62.Pavlovic M., Arnal-Estapé A., Rojo F., Bellmunt A., Tarragona M., Guiu M., Planet E., Garcia-Albéniz X., Morales M., Urosevic J., Gawrzak S., Rovira A., Prat A., Nonell L., Lluch A., Jean-Mairet J., Coleman R., Albanell J., Gomis R.R. Enhanced MAF Oncogene Expression and breast cancer Bone Metastasis. J. Natl. Cancer Inst. 2015;107(12):djv256. doi: 10.1093/jnci/djv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma L., Teruya-Feldstein J., Weinberg R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–689. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 64.Huang Q., Gumireddy K., Schrier M., le Sage C., Nagel R., Nair S., Egan D.A., Li A., Huang G., Klein-Szanto A.J., Gimotty P.A., Katsaros D., Coukos G., Zhang L., Puré E., Agami R. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat. Cell Biol. 2008;10(2):201–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 65.Hassan M.Q., Maeda Y., Taipaleenmaki H., Zhang W., Jafferji M., Gordon J.A., Li Z., Croce C.M., van Wijnen A.J., Stein J.L., Stein G.S., Lian J.B. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. Biol. Chem. 2012;287(50):42084–42092. doi: 10.1074/jbc.M112.377515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J., Li D., Dang L., Liang C., Guo B., Lu C., He X., Cheung H.Y.S., He B., Liu B., Li F., Lu J., Wang L., Shaikh A.B., Jiang F., Lu C., Peng S., Zhang Z., Zhang B.T., Pan X., Xiao L., Lu A., Zhang G. Osteoclastic miR-214 targets TRAF3 to contribute to osteolytic bone metastasis of breast cancer. Nature. 2017;7:40487. doi: 10.1038/srep40487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tavazoie S.F., Alarcón C., Oskarsson T., Padua D., Wang Q., Bos P.D., Gerald W.L., Massagué J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Awolaran O., Brooks S.A., Lavender V. Breast cancer osteomimicry and its role in bone specific metastasis; an integrative, systematic review of preclinical evidence. Breast. 2016;30:156–171. doi: 10.1016/j.breast.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 69.So A., Chin J., Ont O., Fleshner N., Saad F. Management of skeletal-related events in patients with advanced prostate cancer and bone metastases: incorporating new agents into clinical practice. J. Can. Urol. Assoc. 2012;6(6):465–470. doi: 10.5489/cuaj.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Briganti A., Suardi N., Gallina A., Abdollah F., Novara G., Ficarra V., Montorsi F. Predicting the risk of bone metastasis in prostate cancer. Cancer Treat. Rev. 2014;40(1):3–11. doi: 10.1016/j.ctrv.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Abuzallouf S., Dayes I., Lukka H. Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J. Urol. 2004;171(6 Pt 1):2122–2127. doi: 10.1097/01.ju.0000123981.03084.06. [DOI] [PubMed] [Google Scholar]
- 72.Dolloff N.G., Shulby S.S., Nelson A.V., Stearns M.E., Johannes G.J., Thomas J.D., Meucci O., Fatatis A. Bone-metastatic potential of human prostate cancer cells correlates with Akt/PKB activation by α platelet-derived Growth factor receptor. Oncogene. 2005;24(45):6848–6854. doi: 10.1038/sj.onc.1208815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Russell M.R., Jamieson W.L., Dolloff N.G., Fatatis A. The α-receptor for platelet-derived growth factor as a target for antibody-mediated inhibition of skeletal metastases from prostate cancer cells. Oncogene. 2009;28:412–421. doi: 10.1038/onc.2008.390. [DOI] [PubMed] [Google Scholar]
- 74.Liu Q., Russell M.R., Shahriari K., Jernigan D.L., Lioni M.I., Garcia F.U., Fatatis A. Interleukin-1β promotes skeletal colonization and progression of metastatic prostate cancer cells with neuroendocrine features. Cancer Res. 2013;73(11):3297–3305. doi: 10.1158/0008-5472.CAN-12-3970. [DOI] [PubMed] [Google Scholar]
- 75.Chen Q., Zhong T. The association of CXCR4 expression with clinic-pathological significance and potential drug target in prostate cancer: a meta-analysis and literature review. Drug Des. Dev. Ther. 2015;9:5115–5122. doi: 10.2147/DDDT.S82475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gururajan M., Josson S., Chu G.C., Lu C.L., Lu Y.T., Haga C.L., Zhau H.E., Liu C., Lichterman J., Duan P., Posadas E.M., Chung L.W.K. miR-154 and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate epithelial to mesenchymal transition and bone metastasis of prostate cancer. Clin. Cancer Res. 2014;20(24):6559–6569. doi: 10.1158/1078-0432.CCR-14-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng X., Guo W., Liu T., Wang X., Tu X., Xiong D., Chen S., Lai Y., Du H., Chen G., Liu G., Tang Y., Huang S., Zou X. Identification of miRs-143 and -145 that is associated with bone metastasis of prostate cancer and involved in the regulation of EMT. PLoS One. 2011;6(5):e20341. doi: 10.1371/journal.pone.0020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saini S., Majid S., Yamamura S., Tabatabai L., Suh S.O., Shahryari V., Chen Y., Deng G., Tanaka Y., Dahiya R. Regulatory role of mir-203 in prostate cancer progression and metastasis. Clin. Cancer Res. 2011;17(16):5287–5298. doi: 10.1158/1078-0432.CCR-10-2619. [DOI] [PubMed] [Google Scholar]
- 79.de Bono J.S., Scher H.I., Montgomery R.B., Parker C., Miller M.C., Tissing H., Doyle G.V., Terstappen L.W., Pienta K.J., Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008;14(19):6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 80.Danila D.C., Heller G., Gignac G.A., Espinoza R.G., Anand A., Tanaka E., Lilja H., Schwartz L., Larson S., Fleisher M., Scher H.I. Circulating tumour cell number and prognosis in progressive castration-resistant prostate cancer. Clin. Cancer Res. 2007;13(23):7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 81.Olmos D., Arkenau H.T., Ang J.E., Ledaki I., Attard G., Carden C.P., Reid A.H., A'Hern R., Fong P.C., Oomen N.B., Molife R., Dearnaley D., Parker C., Terstappen L.W., de Bono J.S. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann. Oncol. 2009;20(1):27–33. doi: 10.1093/annonc/mdn544. [DOI] [PubMed] [Google Scholar]
- 82.Scher H.I., Jia X., de Bono J.S., Fleisher M., Pienta K.J., Raghavan D., Heller G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10(3):233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goldkorn A., Ely B., Quinn D.I., Tangen C.M., Fink L.M., Xu T., Twardowski P., Van Veldhuizen P.J., Agarwal N., Carducci M.A., Monk J.P., Datar R.H., Garzotto M., Mack P.C., Lara P., Jr, Higano C.S., Hussain M., Thompson I.M., Jr, Cote R.J., Vogelzang N.J. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014;32(11):1136–1142. doi: 10.1200/JCO.2013.51.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fleisher M., Danila D.C., Fizazi K., Hirmand M., Selby B., Phung D., de Bono J.S., Scher H.I. Circulating tumor cell (CTC) enumeration in men with metastatic castration-resistant prostate cancer (mCRPC) treated with enzalutamide post-chemotherapy (phase 3 AFFIRM study) J. Clin. Oncol. 2015;33(Suppl. 15):5035. [Google Scholar]
- 85.Lorente J.A., Valenzuela H., Morote J., Gelabert A. Serum bone alkaline phosphatase levels enhance the clinical utility of prostate specific antigen in the staging of newly diagnosed prostate cancer patients. Eur. J. Nucl. Med. 1999;26:625–632. doi: 10.1007/s002590050430. [DOI] [PubMed] [Google Scholar]
- 86.Thalgott M., Rack B., Maurer T., Souvatzoglou M., Eiber M., KreB V., Heck M.M., Andergassen U., Nawroth R., Gschwend J.E., Retz M. Detection of circulating tumor cells in different stages of prostate cancer. J. Cancer Res. Clin. Oncol. 2013;139:755–763. doi: 10.1007/s00432-013-1377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okegawa T., Itaya N., Hara H., Tambo M., Nutahara K. Epidermal growth factor receptor status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with docetaxel chemotherapy. Int. J. Mol. Sci. 2016;17:2008. doi: 10.3390/ijms17122008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 89.Zhang K., Zhang M., Zhu J., Hong W. Screening of gene mutations associated with bone metastasis in non small cell lung cancer. J. Can. Res. Ther. 2016;12:186–190. doi: 10.4103/0973-1482.200597. [DOI] [PubMed] [Google Scholar]
- 90.Papotti M., Kalebic T., Volante M., Chiusa L., Bacillo E., Cappia S., Lausi P., Novello S., Borasio P., Scagliotti G.V. Bone sialoprotein is predictive of bone metastases in resectable non small cell lung cancer: a retrospective case-control study. J. Clin. Oncol. 2006;24:4818–4824. doi: 10.1200/JCO.2006.06.1952. [DOI] [PubMed] [Google Scholar]
- 91.Zhou Z., Chen Z.W., Yang X.H., Shen L., Ai X.H., Lu S., Luo Q.Q. Establishment of a biomarker model for predicting bone metastasis in resected stage III non small cell lung cancer. J. Exp. Clin. Cancer Res. 2012;31(34):1–6. doi: 10.1186/1756-9966-31-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hiraki A., Ueoka H., Bessho A., Segawa Y., Takigawa N., Kiura K., Eguchi K., Yoneda T., Tanimoto M., Harada M. Parathyroid hormone-related protein measured at the time of first visit is an indicator of bone metastases and survival in lung carcinoma patients with hypercalcemia. Cancer. 2002;95(8):1706–1713. doi: 10.1002/cncr.10828. [DOI] [PubMed] [Google Scholar]
- 93.Kuo P.L., Liao S.H., Hung J.Y., Huang M.S., Hsu Y.L. MicroRNA-33a functions as a bone metastasis suppressor in lung cancer by targeting parathyroid hormone related protein. Biochim. Biophys. Acta. 2013;1830:3756–3766. doi: 10.1016/j.bbagen.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 94.Van Poznak C.H., Temin S., Yee G.C., Janjan N.A., Barlow W.E., Biermann J.S., Bosserman L.D., Geoghegan C., Hillner B.E., Theriault R.L., Zuckerman D.S., Von Roenn J.H. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J. Clin. Oncol. 2011;29(9):1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 95.D’Oronzo S., Stucci S., Tucci M., Silvestris F. Cancer treatment-induced bone loss (CTIBL): pathogenesis and clinical implications. Cancer Treat. Rev. 2015;41:798–808. doi: 10.1016/j.ctrv.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 96.D’Oronzo S., Brown J., Coleman R. The value of biomarkers in bone metastasis. Eur. J. Cancer Care. 2017 doi: 10.1111/ecc.12725. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 97.Brown J.E., Cook R.J., Major P., Lipton A., Saad F., Smith M., Lee K., Zheng M., Hei Y., Coleman R.E. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumours. J. Natl. Cancer Inst. 2005;97(1):59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]