Abstract
Obesity is a chronic, relapsing, multi-factorial disease characterized by abnormal or excessive adipose tissue accumulation that may impair health and increase disease risks. Despite the ever-increasing prevalence and economic and societal burden, the current approaches to treat obesity are not standardized or generally effective. In this manuscript, we describe a current working paradigm developed by a consensus approach for the multidisciplinary treatment of obesity in the GI practice. Obesity should be managed as a continuum of care focusing on weight loss, weight loss maintenance and prevention of weight regain. This approach needs to be disseminated throughout the health care system, coordinated by a multidisciplinary team and include gastroenterologists who are in a unique position to address obesity. Gastroenterologists are in the front line of managing the morbidity resulting from obesity, and have expertise in use of the essential tools to manage obesity: nutrition, pharmacology, endoscopy and surgery.
Keywords: obesity, weight loss, weight loss management
Introduction
Obesity is a multi-factorial disorder based on genetics, biological, microbial, and environmental factors that promote a positive energy balance mainly driven by an increased food intake and decreased energy expenditure (1,2) that lead to excess weight gain, adiposity and increased risk of diseases (3), including cardiovascular disease (4), type-2 diabetes mellitus (5), sleep apnea (6-9), cancer (10), reproductive disorders (11), endocrine disorders (12), psychological disorders (13-16), bone, joint and connective-tissue disorders (17,18), and gastrointestinal disorders (19). In fact, obesity is associated with the top ten causes of death and with co-morbidities before death (20).Treating obesity decreases mortality and improves the associated co-morbidities.
In this manuscript, we describe a recently proposed working paradigm for the treatment of obesity in the GI practice. Obesity should be managed as a continuum of care focusing on weight loss, weight loss maintenance and prevention of weight regain. This continuum of care should be coordinated by a multidisciplinary team that includes gastroenterologists because of their expertise in nutrition, pharmacology, endoscopy and surgery.
Step 1: Define the Treatment Goal
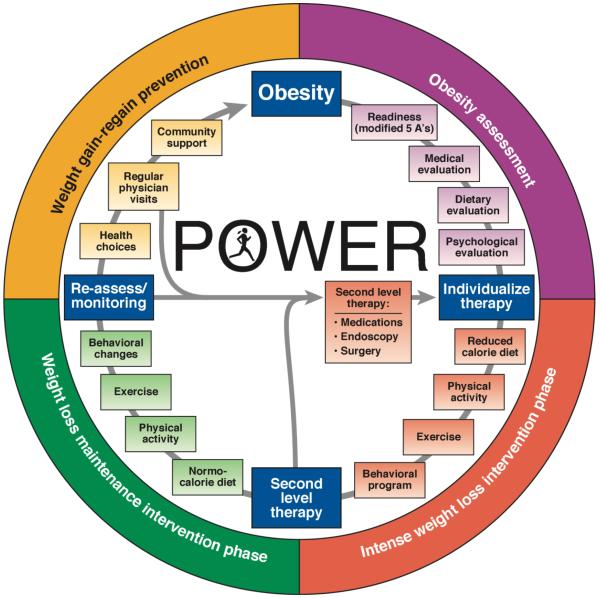
Once the patient is ready to be treated for obesity, a management program should focus on three main goals: 1) weight loss, 2) weight loss maintenance and 3) prevention of weight loss regain. Each stage of obesity management needs to be addressed separately and all the stages are equally important. Thus, maintenance of weight loss, and prevention of weight re-gain after initial successful weight loss (avoiding the yo-yo effect) are essential aspects of ongoing care (21). In the weight loss phase, the goals should be realistic and based on the evidence on the associated health benefits associated with the degree of weight loss, rather than focusing on cosmetic improvements. An initial and realistic weight loss goal should be moderate; the usual recommendation is 5-10% reduction in total body weight. Losing 5-10% is sufficient to have a significant improvement on insulin resistance, hypertension, fatty liver, weight-bearing joint arthritis, obstructive sleep apnea and all cardiovascular risks factors with exception of serum LDL levels (22,23). In addition, losing more that 15% total body weight is associated with a reduction of cardiovascular mortality and morbidity (24). Thus, multidisciplinary obesity management programs should aim for higher initial weight loss. Subsequently, similar emphasis should be given to maintain the weight loss achieved and to avoid rapid weight regain. Finally, when patients have successfully lost weight and maintained the new weight level for over a year, follow up and education should be maintained consistently to avoid weight regain (Figure 1) (21).
Figure 1.
POWER: Practice Guide on Obesity and Weight Management, Education and Resources (with permision from from ref. 21)
Selection of Therapy in Patients with Obesity
The selection of therapy for the obese patient should be accomplished by a multidisciplinary team, in which physicians’ partner with other professionals to provide a comprehensive assessment and intervention. The physician typically has training in obesity medicine or is a gastroenterologist with expertise in nutrition, and the team includes a bariatric surgeon, a mid-level provider (physician assistant, nurse practitioner or nurse), a registered dietitian nutritionist, a behavioral therapist (e.g. psychiatric social worker, psychiatrist or psychologist), a physical therapist and medical assistants. From the initial contact with the patient and through the continuum of care, the team, including ancillary staff, should embrace obesity as a chronic medical problem, deal respectfully and foster motivation and inspiration to achieve the proposed goals.
There are typically two different scenarios when approaching a patient with obesity: the patient may specifically be referred or seek care for obesity; alternatively, the patient with obesity presents with another medical condition. In the latter scenario, the physician needs to decide whether it is appropriate to embark on discussion of obesity during that visit and assess patient’s readiness to embark on a weight management program. If the patient is not ready for such a commitment, the subject should be not forced to do so.
The recommendation not to embark on a weight management program is predicated on the practical notion that it is essential for gastroenterologists who see many patients with obesity-related comorbidities, such as nonalcoholic fatty liver disease, reflux esophagitis, gallbladder disease, pancreatitis and colon cancer, and cannot pursue obesity management in patients who are not committed to do so.
If the patient is ready to discuss their obesity, the 2013 American Heart Association (AHA)/American College of Cardiology (ACC)/The Obesity Society (TOS) Guideline for the Management of Overweight and Obesity in Adults recommends that the clinician partner with the patient to assess whether the patient is ready to undertake the measures necessary to achieve weight loss before beginning comprehensive counseling efforts (25). The 5 As (Ask, Advise, Assess, Assist and Arrange), originally developed for smoking cessation, serve as an effective tool for obesity counseling (26). Motivational interviewing using open-ended questions, affirmation, reflections, and summaries (OARS) is another useful tool (27,28).
The next essential step is to introduce the phases and expectations of the weight management program (see goals above). The physician or other members of the team evaluate several personal factors for the individual patient: dietary patterns, physical activity, abnormal behaviors and psychosocial concerns, medical comorbidities, secondary causes of weight gain, potential barriers to weight loss, prior attempts at weight loss and weight gain, current medications, family history and comorbidities such as cardiovascular disease, diabetes (e.g. by urinalysis or finger stick blood glucose) and obstructive sleep apnea [using validated sleep apnea questionnaires or the sleep apnea clinical score (29)]. With the assessment completed, the multidisciplinary team can suggest the appropriate intervention based on the health needs, expected weight loss, the patient’s wishes and expectations according to the patient’s BMI (Table 1 or NIH recommendations) or individualized to the patient’s phenotype, such as behavioral or psychological issues associated with documentation of accelerated gastric emptying of solids (30).).
Table 1.
Treatment Recommendations for Obesity Based on American Heart Association (AHA)/American College of Cardiology (ACC)/The Obesity Society (TOS) Obesity Guideline (57)
| BMI category (kg/m2) | |||||
|---|---|---|---|---|---|
| Treatment | 25–26.9, overweight |
27–29.9, overweight |
30-34.9, class I obesity |
35–39.9, class II obesity |
>40, class III obesity |
| Lifestyle: diet, physical activity, behavior therapy |
With comorbidities |
With comorbidities |
+ | + | + |
| Pharmacotherapy | With comorbidities |
+ | + | + | |
| Endoscopy* | + | + | As bridge therapy |
||
| Surgery | With comorbidities |
+ | |||
modified in ref. 21).
Obesity Interventions
The comprehensive obesity intervention should be tailored to the individual and to the phase in the weight management program, low calorie during the weight loss phase, and normo-caloric diet and increased physical activity during the weight loss maintenance phase. In both phases of weight loss intervention and maintenance of weight loss,, the diet, physical activity and behavioral program are the cornerstone of treatment (Figure 1); the team can consider adding tools (medications, endoscopy or surgery) to facilitate adherence to the program and counteract the metabolic slow-down seen with weight loss. It is important to emphasize to the patient that these tools will only assist and support the lifestyle changes.
Diet
The 2013 Obesity Guideline suggests that is essential to create an energy deficit below that required for energy balance: 1200-1500kcal/day for women, and 1,500-1800kcal/day for men; or an energy deficit of 500kcal/day for women or 750kcal/day for men below the estimated daily energy requirement (25), or more aggressive under the supervision of the multidisciplinary team. A very low calorie diet may be considered if there is adequate supervision, avoiding its use in pregnant or breast-feeding women or in adolescents, and prevention of micronutrient or vitamin deficiencies or monitoring for development of symptomatic gallstones.
In the phases of weight maintenance and prevention of weight regain, patients should consume a normo-calorie diet and avoid resuming the “previous” high-calorie” diet. These recommendations can be given by the team’s registered dietitian nutritionist or a commercial weight loss program. A recent review of 45 studies involving commercial weight loss programs based on diet and behavioral modification (which included 39 randomized, controlled trials) showed that, at 12 months, the commercial diets achieved greater weight loss than control/education and counseling: Weight Watchers® by >2.6%, Jenny Craig® >4.9%, and very-low-calorie programs (e.g. Medifast® and OPTIFAST®) >4.0%. These commercial programs incorporate group sessions (e.g. Weight Watchers®) or more expensive 1-on-1 counselling (e.g. Jenny Craig®) (31). Partnering with a commercial program is an acceptable strategy, especially if behavioral or group sessions are not easily available in the GI practice.
Physical Activity
A comprehensive lifestyle intervention program should include increased aerobic physical activity (such as brisk walking) for ≥150 min/week (equal to ≥30 min/day most days of the week), and a goal of >10,000 steps per day. Higher levels of physical activity, approximately 200 to 300 min/week, are recommended to maintain the weight lost or minimize (and hopefully prevent) weight regain in the long term (>1 year) (32).
Behavioral Therapy
The diet and physical activity can be integrated with an established health center, work place or commercial behavior program to facilitate adherence to diet and activity recommendations including regular self-monitoring of food intake, body weight, physical activity, food cravings, emotional or binge eating. These same behaviors are recommended to maintain lost weight, with the addition of frequent (i.e., weekly or more frequent) monitoring of body weight (25).
Pharmacology
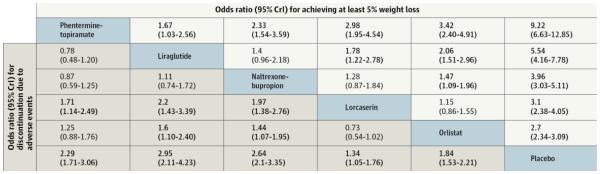
Medications approved for management of obesity should be used as a second approach, always in combination with a comprehensive lifestyle program for patients with BMI ≥30kg/m2 or BMI ≥27kg/m2 with weight-related co-morbidities such as hypertension, type 2 diabetes, dyslipidemia and obstructive sleep apnea (25). Table 2 provides an overview of the medications approved for long-term use including expected outcomes, contraindications and side effects. A recent network meta-analysis of twenty-eight randomized clinical trials with 29,018 patients (median age, 46 years; 74% women; median baseline body weight, 100.5kg; median baseline body mass index, 36.1kg/m2) reported efficacy of the different drugs compared to the median 23% of placebo participants who had at least 5% weight loss. High attrition rates (30%-45% in all trials) were associated with lower confidence in estimates (Figure 2 and Table 3) (33). We include below brief summaries of FDA-approved pharmacotherapy options. Prescribers should refer to each product label for additional detail. One approach is to prescribe medication and monitor its efficacy or adverse effects over 3-6 months before replacing with another approved medication. An alternative approach is to select the first medication based on overall appraisal of phenotype. For example, in patients with extremely strong appetite or hyperphagia, a centrally acting medication (lorcaserin, bupropion-naltrexone, or phentermine-topiramate) might be the drug of first choice. In contrast, patients with documented acceleration of gastric emptying would be excellent candidates for a long acting GLP-1 receptor agonist.
Table 2.
Pharmacological Actions and Efficacy of Currently Approved Medications for Long-Term Treatment of Obesity [summarized from Yanovski and Yanovski. JAMA 2014;311:74-86 (58); Astrup et al. Lancet 2009;374:1606–16 (59); and Pi-Sunyer et al. NEJM 2015:373:11-22 (60)]
| DRUG | Mechanism of action | Dose | Weight loss (kg) vs. placebo |
Adverse effects |
|---|---|---|---|---|
| Orlistat 60 mg (Alli®) or 120 mg (Xenical®; 3× within 1 hour of a fat- containing meal) |
Lipase inhibitor causing excretion of ~30% of ingested triglycerides in stool |
60mg or 120mg, with 3 meals |
60mg dose: −2.5 (−1.5 to −3.5) 120mg dose: −3.4 (−3.2 to −3.6) |
Oily spotting, flatus, fecal urgency, fatty oily stool, increased defecation, fecal incontinence |
| Phentermine plus topiramate- ER (Qsymia®; 3.75mg/ 23mg for 2 weeks, increased to 7.5mg/46mg |
Noradrenergic + GABA- receptor activator, kainite /AMPA glutamate receptor inhibitor causing appetite suppression |
3.75mg/23mg to 7.5mg/46mg; once daily |
7.5mg/46mg: −6.7 (−5.9 to −7.5) 15mg/92mg: −8.9(−8.3 to −9.4) |
Paresthesia, dizziness, taste alterations, insomnia, constipation, dry mouth, elevation in heart rate, memory or cognitive changes |
| Lorcaserin (Belviq®; 10mg, 2×) |
Highly selective serotonergic 5-HT2C receptor agonist causing appetite suppression |
10mg twice daily |
−3.2 (−2.7 to −3.8) |
Headache, dizziness, fatigue, nausea, dry mouth, cough, and constipation; and in T2DM back pain, cough, and hypoglycemia |
| Bupropion SR/ Naltrexone SR (Contrave®) |
Dopamine/norepinephrine reuptake inhibitor and opioid receptor antagonist |
360/32mg p.o. daily |
5-6 % | Headache, nausea, insomnia, constipation tremor |
| Liraglutide, 1.2mg escalating over 4 weeks to 3.0mg SQ (Saxenda ®) |
Glucagon-like peptide 1 agonist causing appetite suppression and possibly delaying gastric emptying |
1.2mg escalating to 3.0mg SQ once daily |
1.2mg dose: −2·1 (0·6– 3·6) 1.8mg dose: −2·8 (−4·3 to −1·3) 2.4mg dose: −3·5‡ (−5·0 to −2·0) 3mg dose: −4·4 (2·9– 60) |
Nausea and vomiting |
Figure 2.
Comparison of Weight Loss and Adverse Events with Pharmacological Weight Loss Agents in Network Meta-analysis (33)
Table 3.
Summary of Direct Meta-analysis for All Weight Loss and Adverse Event Outcomes (33)
| Pharmacological Intervention |
Number of Studies |
Active Intervention | Control (placebo, unless noted otherwise) |
OR or Weighted Mean Difference, kg (95% CI) |
||
|---|---|---|---|---|---|---|
| No. with event |
Total no. |
No. with event |
Total no. |
|||
| ≥5% weight loss | ||||||
| Orlistat | 16 | 3140 | 5315 | 1694 | 4694 | 2.69 (2.36-3.07) |
| Lorcaserin | 3 | 1562 | 3350 | 729 | 3288 | 3.09 (2.49-3.83) |
| Naltrexone- bupropion |
4 | 1081 | 2044 | 274 | 1319 | 3.90 (2.91-5.22) |
| Phentermine- topiramate |
2 | 1019 | 1479 | 290 | 1477 | 9.10 (7.68- 10.78) |
| Liraglutide | 3 | vs placebo: 1798 vs orlistat: 53 |
2921 72 |
Placebo: 380 Orlistat: 29 |
1503 67 |
5.09 (4.07-6.37) 3.66 (1.79-7.46) |
| ≥10% weight loss | ||||||
| Orlistat | 14 | 1520 | 4859 | 684 | 4249 | 2.41 (2.08-2.78) |
| Lorcaserin | 3 | 742 | 3350 | 276 | 3288 | 3.17 (2.53-3.97) |
| Naltrexone- bupropion |
4 | 599 | 2044 | 112 | 1319 | 4.11 (2.80-6.05) |
| Phentermine- topiramate |
2 | 702 | 1479 | 109 | 1477 | 11.34 (9.10- 14.13) |
| Liraglutide | 3 | vs placebo: 930 vs orlistat: 27 |
2921 72 |
Placebo: 146 Orlistat: 9 |
1503 67 |
4.36 (3.61-5.26) 3.87 (1.65-9.04) |
| Mean weight loss in excess of placeboa | ||||||
| Orlistat | 14 | - | 3391 | - | 2777 | −2.63 (−2.94 to − 2.32)b |
| Lorcaserin | 3 | - | 3350 | - | 3288 | −3.25 (−3.55 to − 2.95)b |
| Naltrexone- bupropion |
2 | - | 1297 | - | 967 | −4.95 (−5.54 to − 4.36)b |
| Phentermine- topiramate |
1 | - | 981 | - | 979 | −8.80 (−9.62 to − 7.98)b |
| Liraglutide | 3 | vs placebo: - vs orlistat: - |
2921 72 |
vs. placebo: - vs. orlistat: - |
1503 67 |
−5.24 (−5.60 to − 4.87)b −3.90 (−5.18 to − 2.62)b |
| Discontinuation of therapy due to adverse events | ||||||
| Orlistat | 16 | 439 | 5323 | 224 | 4704 | 1.84 (1.55-2.18) |
| Lorcaserin | 3 | 250 | 3350 | 190 | 3288 | 1.40 (0.96-2.03) |
| Naltrexone- bupropion |
4 | 501 | 2044 | 175 | 1319 | 2.60 (2.15-3.14) |
| Phentermine- topiramate |
2 | 274 | 1479 | 132 | 1477 | 2.32 (1.86-2.89) |
| Liraglutide | 3 | vs placebo: 292 vs orlistat: 7 |
2921 72 |
Placebo: 57 Orlistat: 2 |
1503 67 |
2.82 (2.10-3.77) 3.50 (0.70- 17.49) |
continuous outcome: event rate not applicable;
weighted mean difference for excess weight loss vs. placebo;
OR, odds ratio
Phentermine (Adipex ®)
Phentermine was approved by the FDA in 1959 (34). It is approved only for short-term use (three months). Phentermine is an adrenergic agonist, which promotes weight loss by activating the sympathetic nervous system, causing an increased resting energy expenditure and appetite suppression (35). The recommended dosage of phentermine is 15 to 37.5mg orally once daily, but dosage should be individualized to achieve adequate response with the lowest effective dose.
Orlistat (Xenical®)
Orlistat was approved by the FDA in 1999 for chronic weight management (34). It is also available as an over-the-counter medication (Alli®) (36). Orlistat reduces fat absorption from the gastrointestinal tract by inhibiting pancreatic and gastric lipases (37). The recommended dosage of orlistat is one 120-mg capsule (Xenical®) or one 60-mg capsule (Alli®) three times a day with each main meal containing fat. Additionally, patients should take a multivitamin to ensure adequate intake of fat soluble vitamins.
Phentermine/topiramate ER (Qsymia®)
Phentermine and topiramate extended release (ER) was approved by the FDA in 2012 (34). Topiramate, a drug approved for treatment of epilepsy and migraine, reduces caloric intake by modulation of gamma-aminobutyric acid receptors, inhibition of carbonic anhydrase, and antagonism of glutamate, which reduce food intake by decreasing appetite and increasing satiation (38). Phentermine/topiramate ER is available in four doses, which should be taken once daily in the morning. Gradual dose escalation, which helps minimize risks and adverse events, should proceed as follows: initially 3.75/23mg daily for 14 days; followed by 7.5/46mg daily; and at 12 weeks, option to increase to 11.25/69mg daily and then 15/96mg daily.
Lorcaserin (Belviq®)
Lorcaserin was approved by the FDA in 2012 (34). It is a serotonin 5-HT2c receptor agonist which is thought to reduce food intake and increase satiety by selectively activating receptors on anorexigenic pro-opiomelanocortin (POMC) neurons in the hypothalamus. The recommended dose of lorcaserin is 10mg twice daily.
Bupropion SR/naltrexone SR (Contrave®)
Bupropion/naltrexone sustained release (SR) was approved by the FDA in 2014 (34). Bupropion is a dopamine/norepinephrine reuptake inhibitor approved for depression in the 1980s and smoking cessation in 1997. Naltrexone is an opioid receptor agonist approved for opiate dependency in 1984 and alcohol addiction in 1994. The two medications have a synergistic effect (39). The combination reduces appetite and food cravings. Naltrexone SR/bupropion SR tablets contain 8mg naltrexone and 90mg bupropion. Initial prescription should be for one tablet daily with instructions to increase by one tablet a week to a maximum dose of two tablets in the morning, two tablets in the evening (32/360mg).
Liraglutide (Saxenda®)
Liraglutide 3mg dose was approved by the FDA in 2014. Liraglutide is a glucagon-like peptide-1 (GLP-1) analogue (34). Weight loss is mediated by reduced energy intake by reducing appetite, increasing satiety and delaying gastric emptying (40,41). Liraglutide is administered as a subcutaneous injection once daily. It is initiated at a dose of 0.6mg daily for one week with instructions to increase by 0.6mg weekly up to a maximum dose of 3.0mg.
Bariatric Endoscopy
Bariatric endoscopy or endoscopic bariatric therapy (EBT) should be used in combination with a lifestyle program, typically when diet, lifestyle and pharmacological interventions have not achieved therapeutic objectives. In recent years, the FDA has approved two intragastric balloons, a gastric aspiration device and an endoscopic suturing device that can be used for weight loss therapy (Table 4). There are a number of other EBTs currently being used in several countries or that are under development and in the pipeline for presentation to FDA for approval and marketing. Those devices are described in detail elsewhere (42).
Table 4.
Overview of FDA-Approved Bariatric Endoscopic Procedures (21)
| Name | Mechanism | Total body weight loss % |
Adverse effects* |
|---|---|---|---|
| Orbera intragastric balloon |
Gastric occupying space; delays gastric emptying |
11.3%a,(43) | Nausea, fullness, 1% migration |
| Re-shape intragastric balloon |
7.9%b,(42) | Nausea, fullness | |
| AspireAssist aspiration therapy |
Facilitates partial removal of gastric contents |
12.1(44) | Less than 0.5% risk of peritonitis, ulceration and abdominal pain. |
| Endoscopic sleeve gastroplasty |
Restrictive procedure; delays gastric emptying |
20%c,(45) | Nausea, pain, reflux, risk of bleeding |
Intragastric balloon removed at 6 months and reported: TBWL at 12 months;
Intragastric balloon removed at 6 months and reported: TBWL at 9 months;
Intragastric balloon removed at 6 months and reported: TBWL at 18 months
Intra-gastric Balloons
The ReShape Duo® was approved in 2015 for patients with BMI 30-40kg/m2 and with at least one medical comorbidity of obesity. The ReShape Duo consists of two spherical balloons connected by a flexible silicone rod. The device is placed endoscopically and filled with 900ml methylene blue-tinted saline. The Reshape Duo is removed endoscopically at six months. The REDUCE trial compared Reshape DUO to diet and lifestyle intervention alone. The mean percentage weight loss of the balloon group was 8.4% at 6 months and 7.5% at 9 months compared to the control group which was 5.4% at 6 months and 4.6% at 9 months. Adverse events were: balloon deflation without migration (6%) and early removal for intolerance (9%) (43).
The Orbera® is a spherical, large capacity silicone polymer device approved for treatment of patients with BMI 30-40 kg/m2, with or without comorbidities. The balloon is placed under direct endoscopic visualization and filled with 400-700ml of normal saline. The Orbera® balloon is currently deployed for a maximum duration of up to 6 months, and then deflated and extracted endoscopically. Based on a meta-analysis of 17 studies including 1683 patients, the pooled percent total body weight loss (TBWL) after Orbera balloon was 12.3% (95% CI, 7.9%-16.73%) at 3 months, 13.16% (95% CI, 12.37%-13.95%) at 6 months, and 11.27% (95% CI, 8.17%-14.36%) at 12 months after insertion (44). Adverse events included esophagitis (1.27%), gastric perforation (0.1%), gastric outlet obstruction (0.76%), gastric ulcer (0.2%), balloon rupture (0.36%), and death (0.07%) (44).
Aspiration Therapy
The AspireAssist Aspiration Therapy System was approved by the FDA in 2016 for patients with a BMI of 35.0-55.0kg/m2. The device system consists of an endoscopically-placed percutaneous gastrostomy device (A-tube) and a skin port with a counter that deactivates the device after a standard number of uses. To reactivate the aspiration capability, the patient has to make return visits for dietary counseling at regular intervals. The system includes an attachable aspiration accessory with a water-filled reservoir that permits instillation of fluid into the stomach to facilitate partial removal of gastric contents (44). In the randomized, controlled, pivotal study, subjects in the device arm lost an average of 31.2 pounds (12.1% of TBWL), and the control group lost an average of 9.0 pounds (3.5% TBWL) (45). The severe adverse events reported were peritonitis requiring antibiotics, skin ulceration requiring device repositioning, and abdominal pain requiring medical management. The study reported positive changes in eating behavior in the active treatment arm.
Endoscopic Sleeve Gastroplasty
Endoscopic sleeve gastroplasty (ESG) is a procedure which uses an FDA–approved endoscopic suturing device (OverStitchTM; Apollo Endosurgery, Austin, TX) to deploy a series of full-thickness sutures to produce gastric volume reduction and delay in gastric emptying. The sutures are endoluminally placed to reduce the greater curvature of the stomach (42). In a multicenter clinical trial of 242 patients, the average TBWL was 16.8%±6.4 at six months, 18.2% ±10 at 12 months, and 19.8% ±11.6 at 18 months. The ESG was associated with 2% serious adverse events: two perigastric inflammatory fluid collections (adjacent to the gastric fundus) that resolved with percutaneous drainage and antibiotics, one self-limited hemorrhage from splenic laceration, one pulmonary embolism 72 hours after the procedure, and one pneumoperitoneum and pneumothorax requiring chest tube placement. All 5 patients recovered fully (46).
Trans-Oral Outlet Reduction (TORe)
The Trans-Oral Outlet Reduction (TORe) is a procedure that uses an FDA–approved endoscopic suturing device (OverStitchTM) to reduce the gastrojejunal anastomotic aperture in patients with a Roux-en-Y gastric bypass who have regained significant weight. The procedure was evaluated in a multicenter, double-blind, randomized, sham-controlled trial (RESTORe) (47) using a previous device, and showed mean weight loss of 3.5kg in the TORe arm vs. 0.4kg in the sham arm (p=0.021). More recently, in a larger, uncontrolled series, the mean weight loss was 8.4kg at three years. The adverse events of transient pharyngeal pain, epigastric pain, nausea and vomiting were frequently reported.
Bariatric Surgery
Bariatric surgery in combination with lifestyle changes continues to be the more efficacious and durable management for severe obesity (48-50). The number of bariatric procedures performed in the United States continues to increase, with 190,000 operations in 2015. The three most commonly performed bariatric operations in the United States currently are: laparoscopic sleeve gastrostomy (SG), laparoscopic Roux en-Y gastric bypass (RYGB), and laparoscopic adjustable gastric banding (AGB). Overall, perioperative mortality for bariatric surgery ranges from 0.1-0.3% (51). Perioperative and nutritional deficiencies are overall low, but vary widely according to the procedure and, particularly, according to the length of the by-passed small intestine with RYGB.
Long-term studies assessing the weight loss outcomes of bariatric surgery have demonstrated improvement in all-cause survival as expressed in improved mortality when compared to cohorts with severe obesity and weight-related diseases that did not undergo surgical intervention (24,49). In addition to weight loss, diseases associated with metabolic syndrome including diabetes, hypertension, and hypertriglyceridemia, as well as obstructive sleep apnea have been shown to improve or resolve in many patients who underwent bariatric surgery (52). Additionally, other obesity-related diseases improved with bariatric surgery, including NAFLD, GERD, polycystic ovary syndrome (PCOS), degenerative joint disease, pseudotumor cerebri, and cardiovascular disease.
Laparoscopic Sleeve Gastrectomy
Laparoscopic sleeve gastrectomy (SG) consists of a surgical procedure to remove two-thirds to three-quarters of the stomach, leaving a tubular gastric conduit based on the lesser curvature. The outcome is consistent with a restrictive procedure, inducing early satiation and decreasing appetite. The expected TBWL is 25%, with concomitant improvement in weight-related co-morbid conditions (53). Contra-indications to performing SG include established Barrett’s esophagus and refractory GERD. Complication rates are low (<1 to 2.7%) including stenosis and staple-line dehiscence.
Laparoscopic Roux en-Y Gastric Bypass
Laparoscopic Roux en-Y gastric bypass (RYGB) is now the second most common bariatric surgical procedure in the U.S. The operation results in creation of a small gastric pouch in the cardia of the stomach anastomosed to a Roux limb connecting the pouch to the mid-jejunum. The remaining stomach is left in place, and the duodenal loop and proximal jejunum anastomosed to the Roux limb (surgical jejuno-jejunostomy anastomosis). The result is not only bypass of food from the remnant stomach, but also the duodenum and proximal jejunum. The Roux limb constitutes the alimentary channel, and the biliopancreatic limb transports bile and pancreatic enzymes distally to the entry of food into the small intestine via the gastric pouch. Mixing of food with digestive enzymes occurs distal to the convergence of these two limbs, in the area termed the common channel. The expected TBWL is from 25 to 45%, with improvements specifically in metabolic diseases, especially diabetes and obesity-related diseases (50,52,54). Contra-indications include history of inflammatory bowel disease and disease states that potentially could be affected by altered absorption (e.g. post-organ transplantation requiring immunosuppression medications).
Adjustable Gastric Banding
Adjustable gastric banding (AGB) involves placement of a soft, silicone ring just distal to the esophagogastric junction. The ring includes a balloon that can be filled intermittently to induce fullness and satiety, without complete obstruction. Access to the balloon is by means of a needle percutaneously through a subcutaneously placed port. The expected TBWL is approximate 15-20% (55), however, there is wide variability in the outcome and, in general, this operation is falling out of favor, especially relative to the SG procedure, due to insufficient weight loss, short duration of benefit or adverse events. Though perioperative complication rates are low, long-term complications of varying degrees are higher, with up to 20% of patients needing repeat bariatric surgery. Contra-indications include large hiatal hernia, severe GERD, and esophageal motility disorders.
Expert Opinion and Guidelines in the Management of Obesity
The current guidelines for weight loss suggest that selection of the appropriate intervention plus lifestyle changes should be based on BMI and comorbidities (Table 1). However, physicians should discuss all the appropriate options along with expected weight loss, potential side effects and, most importantly, the patient’s wishes. Furthermore, physicians should recognize special comorbidities that may favor one intervention over another. One such example is the presence of poorly controlled diabetes which may benefit from the effects of a GLP-1 agonist, such as liraglutide, over and above the metabolic enhancement resulting from the weight loss.
Consensus among medical and surgical societies involved in care of patients with diabetes has strongly recommended a role of bariatric “metabolic” surgery with specific guidance and indications (56).
While, there are no clear guidelines or society recommendations, it has been proposed that certain patient characteristics such as gastrointestinal and psychological traits may predict better response to treatment based on an individualized approach that matches the pharmacological or physiological effects of a drug or device and the patient phenotype (discussed elsewhere in detail ref. 30).
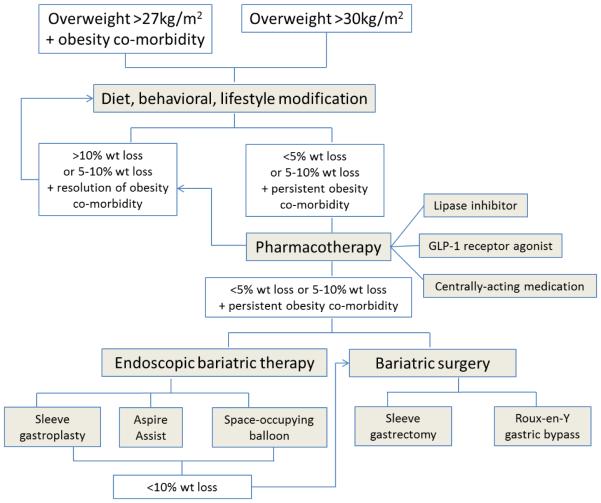
In the meantime, we recommend considering a multidisciplinary approach to manage obesity, based on a strong foundation of diet, physical activity and behavioral program (Figure 1), and enhanced by FDA-approved medications and devices (21). One approach that has been recommended is to commence a reduce calorie diet in combination with a bariatric endoscopy therapy (e.g. intragastric balloon) to produce rapid and high degree of weight loss within the first three months to kick start weight management. This is followed by initiation of medications and/or different diets to produce greater weight loss and start supporting long-term maintenance of weight loss. An overall summary algorithm is presented in Figure 3.
Figure 3.
Algorithm for management of obesity
Conclusion
Obesity should be managed through a continuum of care focusing on initiating weight loss, weight loss maintenance, and prevention of weight regain. This approach needs to be disseminated throughout the healthcare system and coordinated by a multidisciplinary team. The team should include gastroenterologists who are in a unique position to address obesity, as they are in the front line managing the gastrointestinal morbidity resulting from obesity and have access to and expertise in use of the essential tools to manage obesity, such as nutrition, pharmacology, endoscopy and surgery.
Acknowledgments
Funding
Dr. M Camilleri’s work in obesity is supported by grant R56-DK67071 from National Institutes of Health.
Abbreviations used
- ACC
American College of Cardiology
- AGB
adjustable gastric banding
- AHA
American Heart Association
- AND
Academy of Nutrition and Dietetics
- BMI
body mass index
- CVD
cardiovascular disease
- EBT
endoscopic bariatric therapy
- GERD
gastroesophageal reflux disease
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NASPGHAN
North American Society for Pediatric Gastroenterology, Hepatology and Nutrition
- NIH
National Institutes of Health
- PCOS
polycystic ovary syndrome
- POMC
pro-opiomelanocortin
- SG
sleeve gastrectomy
- SAGES
Society of American Gastrointestinal and Endoscopic Surgeons
- T2DM
type 2 diabetes mellitus
- TOS
The Obesity Society
- TBWL
total body weight loss
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions: Andres Acosta: drafting, writing and critical revision of the manuscript Michael Camilleri: drafting, writing and critical revision of the manuscript
Conflict of Interest Statement
Andres Acosta: Dr. Acosta is a stockholder of Gila Therapeutics, Inc and serves on the scientific advisory boards of Gila Therapeutics, Inversago, and General Mills.
Dr. Camilleri has served as an advisor to Enteromedics (St. Paul, MN) and ReShape Medical (San Clemente, CA), and has received medications from Vivus and NovoNordisk for research studies on obesity.
REFERENCES
- 1.Bray GA, Bouchard C. Handbook of obesity : epidemiology, etiology, and physiopathology. 3rd CRC Press; Boca Raton: 2014. [Google Scholar]
- 2.Acosta A, Abu Dayyeh BK, Port JD, Camilleri M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut. 2014 Jan 8;63(4):687–95. doi: 10.1136/gutjnl-2013-306235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nature medicine. 2006 Jan;12(1):62–6. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 4.Guize L, Pannier B, Thomas F, Bean K, Jego B, Benetos A. Recent advances in metabolic syndrome and cardiovascular disease. Arch Cardiovasc Dis. 2008 Sep;101(9):577–83. doi: 10.1016/j.acvd.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Hill MJ, Metcalfe D, McTernan PG. Obesity and diabetes: lipids, 'nowhere to run to'. Clin Sci (Lond) 2009 Jan;116(2):113–23. doi: 10.1042/CS20080050. [DOI] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Bixler EO, Chrousos GP, Pejovic S. Obesity and sleep disturbances: meaningful sub-typing of obesity. Arch Physiol Biochem. 2008 Oct;114(4):224–36. doi: 10.1080/13813450802521507. [DOI] [PubMed] [Google Scholar]
- 7.Vgontzas AN. Does obesity play a major role in the pathogenesis of sleep apnoea and its associated manifestations via inflammation, visceral adiposity, and insulin resistance? Arch Physiol Biochem. 2008 Oct;114(4):211–23. doi: 10.1080/13813450802364627. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Lin HM, Papaliaga M, Calhoun S, Vela-Bueno A, Chrousos GP, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond) 2008 May;32(5):801–9. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 9.de Sousa AG, Cercato C, Mancini MC, Halpern A. Obesity and obstructive sleep apnea-hypopnea syndrome. Obes Rev. 2008 Jul;9(4):340–54. doi: 10.1111/j.1467-789X.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- 10.Garfinkel L. Overweight and cancer. Ann Intern Med. 1985 Dec;103(6):1034–6. doi: 10.7326/0003-4819-103-6-1034. Pt 2. [DOI] [PubMed] [Google Scholar]
- 11.Deitel M, To TB, Stone E, Sutherland DJ, Wilk EJ. Sex hormonal changes accompanying loss of massive excess weight. Gastroenterol Clin North Am. 1987 Sep;16(3):511–5. [PubMed] [Google Scholar]
- 12.Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Int J Androl. 2008 Apr;31(2):201–8. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 13.Bean MK, Stewart K, Olbrisch ME. Obesity in America: implications for clinical and health psychologists. J Clin Psychol Med Settings. 2008 Sep;15(3):214–24. doi: 10.1007/s10880-008-9124-9. [DOI] [PubMed] [Google Scholar]
- 14.Stunkard AJ, Wadden TA. Psychological aspects of severe obesity. Am J Clin Nutr. 1992 Feb;55(2 Suppl):524S–32S. doi: 10.1093/ajcn/55.2.524s. [DOI] [PubMed] [Google Scholar]
- 15.Rapaka R, Schnur P, Shurtleff D. Obesity and addiction: common neurological mechanisms and drug development. Physiol Behav. 2008 Sep 3;95(1-2):2–9. doi: 10.1016/j.physbeh.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Warren MW, Gold MS. The relationship between obesity and drug use. Am J Psychiatry. 2007 Aug;164(8):1268. doi: 10.1176/appi.ajp.2007.07030388. author reply -9. [DOI] [PubMed] [Google Scholar]
- 17.Anandacoomarasamy A, Fransen M, March L. Obesity and the musculoskeletal system. Curr Opin Rheumatol. 2009 Jan;21(1):71–7. doi: 10.1097/bor.0b013e32831bc0d7. [DOI] [PubMed] [Google Scholar]
- 18.Magliano M. Obesity and arthritis. Menopause Int. 2008 Dec;14(4):149–54. doi: 10.1258/mi.2008.008018. [DOI] [PubMed] [Google Scholar]
- 19.Acosta A, Camilleri M. Gastrointestinal morbidity in obesity. Annals of the New York Academy of Sciences. 2014 Apr;1311:42–56. doi: 10.1111/nyas.12385. [Research Support, N.I.H., Extramural Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007 May;132(6):2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Acosta A, Streett S, Kroh MD, Cheskin LJ, Saunders KH, Kurian M, et al. POWER: Practice Guide on Obesity and Weight Management, Education and Resources. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.10.023. Submitted. [DOI] [PubMed] [Google Scholar]
- 22.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009 Nov 14;374(9702):1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013 Jul 11;369(2):145–54. doi: 10.1056/NEJMoa1212914. [Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. The New England journal of medicine. 2007 Aug 23;357(8):741–52. doi: 10.1056/NEJMoa066254. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 25.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American College of Cardiology. 2014 Jul 1;63(25):2985–3023. doi: 10.1016/j.jacc.2013.11.004. Pt B. [DOI] [PubMed] [Google Scholar]
- 26.Kushner R. Models of Obesity Care: Implications for Practice. Obesity Consults. 2015;3(1):17–29. [Google Scholar]
- 27.Kisely S, Ligate L, Roy MA, Lavery T. Applying Motivational Interviewing to the initiation of long-acting injectable atypical antipsychotics. Australas Psychiatry. 2012 Apr;20(2):138–42. doi: 10.1177/1039856212437257. [DOI] [PubMed] [Google Scholar]
- 28.Searight R. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009 Feb 15;79(4):277–84. [Review] [PubMed] [Google Scholar]
- 29.Gay PC. Sleep and sleep-disordered breathing in the hospitalized patient. Respir Care. 2010 Sep;55(9):1240–54. [PubMed] [Google Scholar]
- 30.Camilleri M, Acosta A. Gastrointestinal traits: individualizing therapy for obesity with drugs and devices. Gastrointestinal endoscopy. 2016 Jan;83(1):48–56. doi: 10.1016/j.gie.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudzune KA, Bleich SN, Clark JM. Efficacy of Commercial Weight-Loss Programs. Annals of internal medicine. 2015 Sep 1;163(5):399. doi: 10.7326/L15-5130-3. [Comment Letter] [DOI] [PubMed] [Google Scholar]
- 32.Riebe D, Franklin BA, Thompson PD, Garber CE, Whitfield GP, Magal M, et al. Updating ACSM's Recommendations for Exercise Preparticipation Health Screening. Med Sci Sports Exerc. 2015 Nov;47(11):2473–9. doi: 10.1249/MSS.0000000000000664. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 33.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA. 2016 Jun 14;315(22):2424–34. doi: 10.1001/jama.2016.7602. [Meta-Analysis Research Support, N.I.H., Extramural Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saunders K, Igel L, Kumar R, Shukla A, Aronne L. Pharmacotherapy for Obesity. Endocrinol Metab Clin North Am. 2016 doi: 10.1016/j.ecl.2016.04.005. (In press) [DOI] [PubMed] [Google Scholar]
- 35.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001 Jan;39(1):32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 36.GlaxoSmithKline Consumer Healthcare L, editor. Alli. Alli (orlistat) Moon Township, PA: 2015. [Google Scholar]
- 37.Zhi J, Melia AT, Guerciolini R, Chung J, Kinberg J, Hauptman JB, et al. Retrospective population-based analysis of the dose-response (fecal fat excretion) relationship of orlistat in normal and obese volunteers. Clinical pharmacology and therapeutics. 1994 Jul;56(1):82–5. doi: 10.1038/clpt.1994.104. [Clinical Trial Clinical Trial, Phase I Randomized Controlled Trial] [DOI] [PubMed] [Google Scholar]
- 38.Acosta A, Camilleri M, Shin A, Vazquez-Roque MI, Iturrino J, Burton D, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015 Mar;148(3):537–46. doi: 10.1053/j.gastro.2014.11.020. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, et al. Rational design of a combination medication for the treatment of obesity. Obesity. 2009 Jan;17(1):30–9. doi: 10.1038/oby.2008.461. [Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 40.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. International journal of obesity. 2014 Jun;38(6):784–93. doi: 10.1038/ijo.2013.162. [Randomized Controlled Trial Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acosta A, Camilleri M, Burton D, O'Neill J, Eckert D, Carlson P, et al. Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiol Rep. 2015 Nov;3(11) doi: 10.14814/phy2.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu Dayyeh BK, Edmundowicz SA, Jonnalagadda S, Kumar N, Larsen M, Sullivan S, et al. Endoscopic bariatric therapies. Gastrointestinal Endoscopy. 2015 May;81(5):1073–86. doi: 10.1016/j.gie.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2013 Mar-Apr;9(2):290–5. doi: 10.1016/j.soard.2012.07.007. [Clinical Trial, Phase I Comparative Study Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 44.Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, Sullivan S, et al. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointestinal endoscopy. 2015 Sep;82(3):425–38. doi: 10.1016/j.gie.2015.03.1964. e5. [DOI] [PubMed] [Google Scholar]
- 45.Thompson CC, Dayyeh BKA, Kushner R, Sullivan S, Schorr AB, Amaro A, et al. The AspireAssist Is an Effective Tool in the Treatment of Class II and Class III Obesity: Results of a One-Year Clinical Trial. Gastroenterology. 150(4):S86. [Google Scholar]
- 46.Lopez-Nava G, Sharaiha RZ, Neto MG, Kumta NA, Topazian M, Shukla A, et al. Endoscopic Sleeve Gastroplasty for Obesity: A Multicenter Study of 242 Patients With 18 Months Follow-Up. Gastroenterology. 150(4):S26. [Google Scholar]
- 47.Thompson CC, Chand B, Chen YK, Demarco DC, Miller L, Schweitzer M, et al. Endoscopic suturing for transoral outlet reduction increases weight loss after Roux-en-Y gastric bypass surgery. Gastroenterology. 2013 Jul;145(1):129–37. doi: 10.1053/j.gastro.2013.04.002. [Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov't] e3. [DOI] [PubMed] [Google Scholar]
- 48.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. The American journal of medicine. 2009 Mar;122(3):248–56. doi: 10.1016/j.amjmed.2008.09.041. [Meta-Analysis Research Support, Non-U.S. Gov't Review] e5. [DOI] [PubMed] [Google Scholar]
- 49.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. The New England journal of medicine. 2007 Aug 23;357(8):753–61. doi: 10.1056/NEJMoa066603. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 50.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014 Mar;149(3):275–87. doi: 10.1001/jamasurg.2013.3654. [Meta-Analysis Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. The New England journal of medicine. 2009 Jul 30;361(5):445–54. doi: 10.1056/NEJMoa0901836. [Multicenter Study Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2004 Oct 13;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [Meta-Analysis Research Support, Non-U.S. Gov't Review] [DOI] [PubMed] [Google Scholar]
- 53.Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2009 Jul-Aug;5(4):469–75. doi: 10.1016/j.soard.2009.05.011. [Review] [DOI] [PubMed] [Google Scholar]
- 54.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. The New England journal of medicine. 2014 May 22;370(21):2002–13. doi: 10.1056/NEJMoa1401329. [Comparative Study Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garb J, Welch G, Zagarins S, Kuhn J, Romanelli J. Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obesity surgery. 2009 Oct;19(10):1447–55. doi: 10.1007/s11695-009-9927-2. [Comparative Study Meta-Analysis Review] [DOI] [PubMed] [Google Scholar]
- 56.Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care. 2016 Jun;39(6):861–77. doi: 10.2337/dc16-0236. [Review] [DOI] [PubMed] [Google Scholar]
- 57.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013 Nov 12; [Google Scholar]
- 58.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014 Jan 1;311(1):74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–16. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 60.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. The New England journal of medicine. 2015 Jul 2;373(1):11–22. doi: 10.1056/NEJMoa1411892. [Comparative Study Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]