Abstract
One of the key concerns with the development of radical-generating reactive therapeutics is the ability to control the activation event within a biological environment. To that end, a series of quinoline-metal-loenediynes of the form M(QuiED)·2Cl (M = Cu(II), Fe(II), Mg(II), or Zn(II)) and their independently synthesized cyclized analogs have been prepared in an effort to elucidate Bergman cyclization (BC) reactivity differences in solution. HRMS(ESI) establishes a solution stoichiometry of 1:1 metal to ligand with coordination of one chloride counter ion to the metal center. EPR spectroscopy of Cu(QuiED)·2Cl and Cu (QuiBD)·2Cl denotes an axially-elongated tetragonal octahedron (g║ > g⊥ > 2.0023) with a dx2–y2 ground state, while the electronic absorption spectrum reveals a pπ Cl→Cu(II) LMCT feature at 19,000 cm −1, indicating a solution structure with three nitrogens and a chloride in the equatorial plane with the remaining quinoline nitrogen and solvent in the axial positions. Investigations into the BC activity reveal formation of the cyclized product from the Cu(II) and Fe(II) complexes after 12 h at 45 °C in solution, while no product is observed for the Mg(II) or Zn(II) complexes under identical conditions. The basis of this reactivity difference has been found to be a steric effect leading to metal–ligand bond elongation and thus, a retardation of solution reactivity. These results demonstrate how careful consideration of ligand and complex structure may allow for a degree of control and selective activation of these reactive agents.
Keywords: Bergman cyclization, Metalloenediynes, Diradicals, Plaque dissolution, Alzheimer’s disease
1. Introduction
Alzheimer’s disease (AD) is the leading cause of dementia and affects over 24 million people worldwide, primarily those in an older demographic [1]. AD is pathologically characterized by the accumulation of misfolded amyloid-β (Aβ) peptides that can self-assemble to form fibrils and eventually, insoluble plaques [2–4]. In addition to this self-assembly, AD-afflicted brains also demonstrate dyshomeostasis and miscompartmentalization of metal ions [2,3,5–7]. In particular, these Aβ plaques contain elevated levels of divalent Cu, Fe, and Zn. Cu(II) and Zn(II) can bind to Ab peptides and facilitate peptide aggregation, while redox-active metals such as Fe(II) and Cu(II) can generate reactive oxygen species and lead to oxidative stress via Fenton-like chemistry [2,3,6,8–13].
Due to the dyshomeostasis of divalent Cu, Fe, and Zn, there has been considerable interest in developing of small molecule frameworks capable of regulating metal ion distribution and pathology of the fibrils through chelation. Examples of such small molecule metal chelators include the hydroxyl-quinoline based antifungal drug clioquinol (CQ) [14,15] and the second-generation drug candidate PBT2 [16,17], both of which chelate Cu(II) or Zn(II) bound to the fibrils and aid in the disaggregation process. The vast majority of these metal chelating compounds act on a timescale of 12–24 h, thus allowing for the determination of a metal-bound structure and ligand binding kinetics. While the ability to study these properties is beneficial for gaining an understanding of how these ligands act, the disaggregation timescale can be drastically decreased by partnering in situ metal sequestration with a reactive moiety as part of a dual-threat approach to fibril disassembly.
One of the key issues to overcome within the paradigm of reactive therapeutics is the ability to control the activation event in a biological environment. Our latest approach to such bifunctional chelators involves a pyridine-containing enediyne ligand (PyED) that upon metal chelation, produces a potent 1,4-diradical species capable of H-atom abstraction. In the presence of Mg(II) ions in solution, PyED reveals significant H-atom abstraction activity within 2.5 h at room temperature [18]. Additionally, this ligand acts on a timescale of 2 h at medically-relevant hyperthermal temperatures (43 °C) to demonstrate significant radical-induced disaggregation of preformed Aβ fibrils in the presence of Cu(II) or Zn(II) ions [19]. Comparison to a non-radical generating cyclized analog validates that the radicals produced during the Bergman cyclization process are involved in the successful disaggregation observed. These results demonstrate that PyED can be activated by a variety of divalent metals and performs H-atom abstraction on short timescales under biologically relevant conditions. Unfortunately, the speed of the metal-chelation and radical generation events make it difficult to study the solution structure of these metal complexes, or to differentiate the reaction kinetics of the various activating metal ions. The ease with which multiple metals can be used to induce the radical generation process also makes it more challenging to control within a biological system and less kinetically informative in a chemical environment.
Our experience with enediyne activation via metal coordination and photochemical diradical formation has taught us that slight changes in the enediyne molecular framework can have a large impact on the activation parameters [20–24]. The ligand (Z)-N,N′-bis[quinolin-2-yl-meth-(E)-ylidene]oct-4-ene-2,6-diyne-1,8-diamine (QuiED) [25] seemingly engenders only a small change to the structure of the PyED framework through addition of a fused aromatic ring on each pyridine functionality. By leaving the binding moiety intact and altering only the steric consequences of the ligand, metal-mediated initiation of the reaction is hindered, permitting the activation potential for specific metal ions to be elucidated. This may allow metal selectivity to govern ligand reactivity based on environmental access to specific ions. Thus, herein we report the synthesis and Bergman cyclization parameters of novel QuiED–metalloenediyne complexes containing Fe(II), Cu(II), Zn(II), and Mg(II). The radical-generation event has been investigated relative to the non-radical generating control compounds using the independently cyclized ligand quinoline-2-ylmethyl-(2-{[quino-line-2-ylmethylene-amino]-methyl}-benzyl)-amine (QuiBD).
2. Experimental
2.1. Materials and instrumentation
All complex syntheses and reactivity studies were performed under a nitrogen atmosphere using standard Schlenk and drybox techniques unless otherwise specified. All chemicals used in the syntheses of the metalloenediynes and cyclized analogs were of the highest purity available and purchased from Sigma Aldrich. Solvents were dried and degassed according to standard procedure unless otherwise noted. The ligand (Z)-N,N′-bis[quinolin-2-yl-meth-(E)-ylidene]oct-4-ene-2,6-diyne-1,8-diamine (QuiED) was synthesized according to previously published procedure [25].
NMR spectra were obtained on a Varion Inova 400 or 500 MHz NMR spectrometer using the residual proton resonance of the solvent as an internal reference. Mass spectrometry data were obtained using an Agilent 1200 HPLC-6130 MSD mass spectrometer, a Waters Q-TOF Ultima ESI mass spectrometer (Mass Spectrometry Facility, Indiana University, Bloomington), or a Waters Q-TOF Ultima ESI mass spectrometer (Mass Spectrometry Laboratory, University of Illinois at Urbana-Champaign). Differential scanning calorimetry (DSC) measurements were obtained using a TGA Q10 DSC system with a TA Instruments thermal analyzer at a heating rate of 10 °C min−1. Optical spectra were recorded on a Perki-nElmer LAMBDA 650 UV/Visible (UV–Vis) spectrophotometer or an Agilent diode array 8453 UV–Vis spectrophotometer, while infrared spectra were measured on a Thermo Electron Corporation Nicolet 6700 FT-IR spectrometer. Electron paramagnetic resonance signals were obtained using a MeOH or a 1:1 DMSO:MeOH glass at 77 K on a Bruker EMX X-band EPR spectrometer operating under the following conditions: microwave frequency, 9.4 GHz; microwave power, 10 mW; modulation amplitude, 2.0 G; modulation frequency, 100.0 kHz; receiver gain, 3.56 × 104. The EPR spectra were simulated and fitted and parameters obtained using the WINEPR SimFonia software (Version 1.25). The room temperature magnetic moment of each complex was measured according to Evans method [26]. Elemental analyses were performed by Robertson Microlit Laboratories (Madison, NJ). Elemental analyses of metalloenediyne complexes routinely return variable results for repeated runs of the same sample with consistently lower than the expected wt% (difference up to 0.8%). This is caused by radical polymerization of metalloenediynes in the solid state during the high temperature combustion and consequently incomplete combustion of the products during analysis. Raman spectroscopy was performed using a Renishaw 1000B micro-Raman instrument with λex = 785 nm and spectra analyzed using the GramsAI software package.
2.1.1. Raman spectroscopy microscope slide preparation
Ag colloid was prepared according to a previously published synthesis [27]. A solution of ascorbic acid (176 mg, 1 mmol) in H2O (25 mL) was prepared under sonication. AgNO3 (5 mL, 100 mM) was injected into this solution, causing a brown precipitate to form. After an additional 4 min of sonication, the mixture was centrifuged (5000 rpm × 5 min, 1677×g) and sequentially washed with H2O and EtOH before final reconstitution in 1 mL EtOH. The metallic suspension (5 µL) was dried onto an Al foil-covered microscope prior to sample preparation.
2.2. Ligand synthesis
2.2.1. Characterization of (Z)-N,N′-bis[1-quinolin-2-yl-meth-(E)-ylidene]oct-4-ene-2,6-dyine-1,8-diamine (QuiED)
The ligand was prepared using a previously published synthesis [25] and additional characterization information has been included here. NMR (400 MHz, 298 K, CD2Cl2): δH 8.92 (s, 2H), 8.17–8.07 (m, 6H), 7.85 (d, J = 8.4 Hz, 2H), 7.24 (t, J = 7.6 Hz, 2H), 7.58 (t, J = 6.8 Hz, 2H), 6.06 (s, 2H), 5.00 (s, 4H); (400 MHz, 298 K, CD2Cl2): δC 163.88, 155.24, 148.29, 136.82, 130.12, 130.12, 129.21, 128.15, 127.87, 120.09, 118.68, 92.62, 85.99, 48.44; IR (KBr, cm−1): 3268, 3054, 2963, 2923, 2213, 1646, 1618, 1596, 1562, 1503, 1463, 1427, 1376, 1336, 1308, 1262, 1207, 1142, 1113, 1096, 1018, 953, 895, 872, 831, 801, 753, 701, 621; DSC: 103 °C (onset), 117 °C (maximum); HRMS(ESI): calcd for 413.1766, found 413.1772.
2.2.2. Synthesis of N,N′-bis-quinolin-2-ylmethyl-oct-4-ene-2,6-diyne-1,8-diamine (QuiEDH)
NaBH4 (12 equiv) was added to a solution of QuiED in methanol at 0 °C and stirred for 4 h. H2O (50 mL) was added to quench excess NaBH4 and the aqueous layer extracted with CH2Cl2 (3 × 100 mL). Organic fractions were combined, dried with Na2SO4, and taken to dryness in vacuo to yield the product as a deep yellow oil. Yield: 88%; NMR (500 MHz, 298 K, CD2Cl2): δH 8.02 (d, J = 6.8 Hz, 2H), 7.96 (d, J = 6.4 Hz, 2H), 7.76 (d, J = 6.0 Hz, 2H), 7.64 (t, J = 6.4 Hz, 2H), 7.48 (t, J = 6.0 Hz, 2H), 7.40 (d, J =7.2 Hz, 2H), 5.84 (s, 2H), 4.14 (s, 4H), 3.72 (s, 4H); (500 MHz, 298 K, CD2Cl2): δC 160.53, 148.11, 136.59, 129.73, 129.32, 128.04, 127.72, 126.40, 120.96, 119.71, 96.16, 81.52, 54.30, 39.31; IR (KBr, cm−1): 3058, 2959, 2924, 2854, 2206, 1675, 1618, 1599, 1565, 1504, 1456, 1426, 1376, 1262, 1211, 1115, 1017, 954, 871, 827, 751, 621; HRMS (ESI): calcd for 417.2079, found 417.2092.
2.2.3. Synthesis of quinoline-2-ylmethyl-(2-{[quinoline-2-ylmethylene-amino]-methyl}-benzyl)-amine (QuiBD)
A solution of 1,2-phenylenedimethanamine and 2-quinolinecar-boxaldehyde in CH2Cl2 was stirred over Na2SO4 at room temperature. After 4 h, charcoal (1 g) was added and the reaction stirred for an addition 30 min. The reaction mixture was filtered and solvent removed in vacuo to yield the product as an orange-yellow oil. Yield: 92%; NMR (400 MHz, 298 K, CD2Cl2): δH 8.61 (s, 2H), 8.14–8.03 (m, 6H), 7.80 (d, J = 7.6 Hz, 2H), 7.73–7.69 (m, 2H), 7.56 (t, J = 8.0 Hz, 2H), 7.44−7.31 (m, 4H), 5.12 (s, 4H); (400 MHz, 298 K, CD2Cl2): δC 163.85, 155.42, 148.28, 137.85, 136.73, 130.09, 130.09, 130.02, 129.99, 129.20, 128.16, 127.80, 118.73, 62.84; IR (KBr, cm−1): 3060, 2964, 2869, 1642, 1617, 1596, 1561, 1502, 1462, 1428, 1374, 1341, 1307, 1263, 1206, 1145, 1113, 1042, 1017, 953, 936, 891, 872, 834, 805, 780, 751, 621; HRMS(ESI): calcd for 415.1923, found 415.1943.
2.2.4. Synthesis of quinoline-2-ylmethyl-(2-{[(quinoline-2-yl-methyl)-amino]-methyl}-benzyl)-amine (QuiBDH)
NaBH4 (12 equiv) was added to a solution of QuiBD in methanol at 0 °C and stirred for 4 h. H2O (50 mL) was added to quench excess NaBH4 and the aqueous layer extracted with CH2Cl2 (3 × 100 mL). Organic fractions were combined, dried with Na2SO4, and taken to dryness in vacuo to yield the product as a bright yellow oil. Yield: 89%; NMR (400 MHz, 298 K, CD2Cl2): δH 8.12 (d, J = 8.0 MHz, 2H), 8.01 (d, J = 8.0 MHz, 2H), 7.93 (t, J =7.0 MHz, 2H), 7.81 (d, J = 7.5 Hz, 2H), 7.73 (d, J = 8.0 MHz, 2H), 7,68 (t, J = 8.0 MHz, 2H), 7.63 (t, J =8.0 MHz, 2H), 7.34–7.22 (m, 2H), 4.09 (s, 4H), 3.89 (s, 4H); (500 MHz, 298 K, CD2Cl2): δC 160.85, 148.12, 138.67, 136.55, 130.70, 129.70, 129.32, 128.01, 127.79, 126.45, 126.34, 120.98, 54.30, 52.18; IR (KBr, cm−1): 3047, 2923, 2852, 1618, 1600, 1565, 1504, 1454, 1426, 1375, 1308, 1243, 1140, 1048, 953, 910, 832, 748, 621; HRMS(ESI): calcd for 419.2236, found 419.2222.
2.3. General synthesis of M(QuiED)·2Cl and M(QuiBD)·2Cl
A solution of QuiED or QuiBD (1 equiv) in methanol was added to a stirring solution of MCl2xH2O (1 equiv) in methanol at 0 °C and the reaction was stirred for 4 h. Solvent was evaporated under vacuum at 0 °C and the crude product was then stirred in diethyl ether for 2 h at the same temperature. The suspension was filtered to yield the solid metal complex.
2.3.1. ((Z)-N,N’ -Bis[1-quinolin-2-yl-meth-(E)-ylidene]oct-4-ene-2,6-dyine-1,8-diamine)copper(II) chloride, Cu(QuiED)·2Cl
The product was isolated as a deep red-brown solid. Yield: 92%; IR (KBr, cm−1) 3381, 3142, 3054, 2175, 1701, 1617, 1598, 1563, 1511, 1464, 1434, 1408, 1379, 1347, 1304, 1262, 1216, 1145, 1052, 1031, 989, 959, 936, 873, 836, 774, 757, 639, 619; µeff = 1.5 (0)µB (298 K); DSC: 73 °C (onset), 98 °C (maximum); HRMS(ESI): calcd for 475.0984, found 475.0967.
2.3.2. (Quinolin-2-ylmethyl-(2-{[(quinoline-2-ylmethylene)-amino]-methyl}-benzyl)-amine)copper(II) chloride, Cu(QuiBD)·2Cl
The product was isolated as a deep purple-red solid. Yield: 94%; IR (KBr, cm−1) 3390, 3224, 3049, 2925, 2617, 1720, 1643, 1616, 1589, 1563, 1509, 1463, 1435, 1380, 1339, 1302, 1227, 1209, 1144, 1099, 1039, 981, 959, 934, 874, 835, 784, 752, 640, 619; µeff = 1.3(9)µB (298 K); Elemental Anal. Calc. for C28H22N4CuCl2 0.5H2O; C, 60.27; H, 4.15; N, 10.04, found: C, 60.18; H, 4.25; N, 10.04%; HRMS(ESI): calcd for 477.1140, found 477.1119.
2.3.3. ((Z)-N,N’ -Bis[1-quinolin-2-yl-meth-(E)-ylidene]oct-4-ene-2,6-dyine-1,8-diamine)iron(II) chloride, Fe(QuiED)·2Cl
The product was isolated as a shiny brown solid. Yield; 89%; IR (KBr, cm−1) 3388, 3060, 2971, 2931, 2181, 1641,1613, 1598, 1566, 1505, 1465, 1429, 1378, 1343, 1304, 1217, 1145, 1117, 870, 833, 755, 620; µeff= 5.1(8)µB (298 K); DSC: 83 °C (onset), 118 °C (maximum); HRMS(ESI): calcd for FeC28H20N4Cl+ 503.0726, found 503.0742.
2.3.4. (Quinolin-2-ylmethyl-(2-{[(quinoline-2-ylmethylene)-amino]-methyl}-benzyl)-amine)iron(II) chloride, Fe(QuiBD)·2Cl
The product was isolated as a deep brown-black solid. Yield: 97%; IR (KBr, cm−1): 3381, 3009, 2924, 1707, 1643, 1616, 1590, 1563, 1508, 1463, 1433, 1378, 1337, 1299, 1224, 1207, 1143, 1126, 1058, 1036, 977, 956, 931, 872, 833, 780, 752, 634, 623; µeff = 5.1(9)µB (298 K); Elemental Anal. Calc. for C28H22N4FeCl2 CH2Cl2H2O; C, 54.07; H, 4.07; N, 8.70, found: C, 53.85; H, 3.75; N, 8.67%. Residual methylene chloride is present from the ligand addition step; HRMS(ESI): calcd for FeC28H22N4Cl+ 505.0882, found 505.0859.
2.3.5. ((Z)-N,N’-Bis[1-quinolin-2-yl-meth-(E)-ylidene]oct-4-ene-2,6-dyine-1,8-diamine)magnesium(II) chloride, Mg(QuiED)·2Cl
The product was isolated as a dark tan solid. Yield: 52%; NMR (400 MHz, 298 K, (CD3)2SO): δH 8.84 (s, 2H), 8.71 (d, J = 8 Hz, 2H), 8.60 (d, J = 8 Hz, 2H), 8.25 (d, J = 8 Hz, 2H), 8.08 (d, J = 8 Hz, 2H), 7.90 (t, J= 8 Hz, 2H), 7.71 (t, J= 8 Hz, 2H), 6.26 (s, 2H), 5.07 (s, 4H); IR (KBr, cm−1): 3226, 2357, 2251, 2177, 1851, 1631, 1598, 1566, 1504, 1465, 1428, 1377, 1336, 1309, 1210, 1143, 1118, 1071, 1017, 980, 957, 894, 835, 755, 617; DSC 115 °C (onset), 186 °C (maximum); HRMS(ESI): calcd for MgC28H20N4Cl+ 471.1227, found 471.1243.
2.3.6. (Quinolin-2-ylmethyl-(2-{[(quinoline-2-ylmethylene)-amino]-methyl}-benzyl)-amine)magnesium(II) chloride, Mg(QuiBD)]·2Cl
The product was isolated as a light tan solid. Yield: 63%; NMR (400 MHz, 298 K, (CD3)2SO): δH 8.72 (s, 2H), 8.70 (d, J = 8.0 Hz, 2H), 8.60 (d, J =8.4 Hz, 2H), 8.24 (d, J = 8.8 Hz, 2H), 8.08 (d, J = 8.0 Hz, 2H), 7.89 (t, J = 8.0 Hz, 2H), 7.70 (t, J = 8.0 Hz, 2H), 7.25 (s, 4H), 5.12 (s, 4H); (500 MHz, 298 K, (CD3)2SO) δC 163.02, 154.42, 147.16, 137.29, 136.72, 130.06, 129.39, 129.09, 128.30, 127.99, 127.62, 127.56, 117.87, 61.21; IR (KBr, cm−1): 3310, 3208, 1630, 1595, 1564, 1522, 1498, 1466, 1429, 1374, 1320, 1223, 1207, 1191, 1144, 1114, 1082, 1038, 1015, 966, 953, 892, 874, 837, 808, 771, 751, 620; Elemental Anal. Calc. for C28H22N4MgCl2·4H2O; C, 57.81; H, 5.20; N, 9.63; found: C, 57.58; H, 4.89; N, 9.30%; HRMS(ESI): calcd for MgC28H22N4Cl+ 473.1383, found 473.1404.
2.3.7. ((Z)-N,N’-Bis[1-pyridin-2-yl-meth-(E)-ylidene]oct-4-ene-2,6-dyine-1,8-diamine)zinc(II) chloride, Zn(QuiED)·2Cl
The product was isolated as a dark tan solid. Yield: 58%; NMR (400 MHz, 298 K, (CD3)2SO): δH 8.86 (s, 2H), 8.37 (d, J = 8.0 Hz, 2H), 8.08 (d, J = 8.0 Hz, 2H), 8.02–7.99 (m, 4H), 7.79 (t, J = 8.0 Hz, 2H), 7.66 (t, J = 8.0 Hz, 2H), 6.27 (s, 2H), 5.08 (s, 4H); IR (KBr, cm−1): 3449, 3245, 3060, 2927, 2179, 1647, 1617, 1599, 1566, 1511, 1466, 1437, 1411, 1381, 1350, 1307, 1209, 1145, 1134, 1059, 1024, 1014, 942, 873, 836, 805, 783, 755, 640, 619; DSC: 123 °C (onset), 205 °C (maximum); HRMS(ESI): calcd for ZnC28H20N4Cl+ 511.0668, found 511.0692.
2.3.8. (Quinolin-2-ylmethyl-(2-{[(quinoline-2-ylmethylene)-amino]-methyl}-benzyl)-amine)zinc(II) chloride, Zn(QuiBD)·2Cl
The product was isolated as a bright yellow solid. Yield: 73%; NMR (400 MHz, 298 K, (CD3)2SO): δH 8.64 (s, 2H), 8.42 (d, J =8.4 Hz, 2H), 8.11 (d, J = 8.8 Hz, 2H), 8.08 (d, J =8.4 Hz, 2H), 8.02 (d, J = 8.8 Hz, 2H), 7.81 (t, J = 3.6 Hz, 2H), 7.66 (t, J = 3.7 Hz, 2H), 7.40 (s, 4H), 4.92 (s, 4H); (500 MHz, 298 K, (CD3)2SO)δC 160.92, 154.81, 148.21, 136.79, 136.62, 130.78, 130.55, 129.90, 129.79, 129.40, 128.13, 127.80, 121.65, 59.55; IR (KBr, cm−1) 3314, 3210, 3061, 1651, 1617, 1600, 1592, 1567, 1510, 1469, 1433, 1385, 1350, 1310, 1279, 1252, 1215, 1150, 1127, 1052, 1039, 993, 967, 940, 870, 836, 806, 783, 777, 755, 746, 709, 640, 617; Elemental Anal. Calc. for C28H22N4ZnCl2·CH2Cl2; C, 54.79; H, 3.81; N, 8.81, found: C, 54.71; H, 3.88; N, 9.08%. Residual methylene chloride is present from the ligand addition step; HRMS(ESI): calcd for ZnC28H22N4Cl+ 513.0824, found 513.0805.
2.4. Cyclization reactivity assays
1,4-Cyclohexadiene (25 equiv) was added to a solution of M (QuiED)·2Cl or M(QuiBD)·2Cl in methanol at 45 °C. After 12 h, the reaction was cooled to 0 °C and NaBH4 (12 equiv) was added. Solvent was removed in vacuo after 2 h and the residue was dissolved in minimal DMF. A saturated EDTA solution (pH 10.6) was added and the reaction stirred for 2 h at room temperature. The reaction was extracted with dichloromethane (3 × 50 mL); organic fractions were combined, and the solution volume reduced to 100 mL in vacuo. The organic solution was washed with H2O (3 × 150 mL) to remove residual DMF. The organic layer was dried with Na2SO4, filtered, and taken to dryness in vacuo to yield the reaction products. In situ formation of the cyclized product was characterized by HRMS(ESI) of the crude reaction product mixture.
3. Results and discussion
3.1. Synthesis of quinoline–metalloenediynes and cyclized controls
The tetradentate ligand QuiED was prepared as previously described in the literature [25] and characterized by its 1H and 13C NMR spectra and mass signatures. QuiED is readily soluble in dichloromethane and dimethyl sulfoxide (DMSO) and sparingly soluble in MeOH. In an effort to characterize the Bergman cyclization product generated by thermal activation of QuiED and to assist with structural elucidation of metal complexes, the independently cyclized ligand QuiBD, was synthesized. Schiff base condensation of (Z)-octa-4-en-2,6-diyne-1,8-diamine with two equivalents of 2-quinolinecarboxaldehyde (Scheme S1) results in the desired product, which was characterizated using NMR, IR spectroscopy, and high resolution mass spectrometry (HRMS). The cyclized ligand is readily soluble in dichloromethane and DMSO, as well as MeOH.
A series of air-stable quinoline-metalloenediynes and their independently cyclized analogs were prepared by addition of QuiED or QuiBD to a solution of MCl2 XH2O (M = Cu(II), Fe(II), Mg(II), or Zn(II)) in MeOH at 0 °C. After stirring for 4 h, the solvent was evaporated and the solid stirred in Et2O for 2 h at the same temperature. The solid was collected by filtration and dried under vacuum (Scheme 1). All complexes were characterized in both solution and the solid state. They demonstrate limited solubility in MeOH; however they are readily soluble in DMSO. The integrity of the enediyne moiety in the final products is demonstrated by the presence of a diagnostic alkyne stretch in the IR spectrum at 2175 (Cu(QuiED)·2Cl), 2181 (Fe(QuiED)·2Cl), 2179 (Zn(QuiED)·2Cl), and 2177 (Mg(QuiED)·2Cl) cm−1. Additionally, although the Raman spectrum of Fe(QuiED)·2Cl is not clearly resolved, a weak alkyne vibration is observable at ∼2180 cm−1 that is not detected in the cyclized analog (Fig. 1). Raman spectroscopy of Fe(QuiBD)·2Cl also reveals vibrations between 1472 and 1311 cmr−1 that are consistent with aromatic and quinoline-containing complexes [28]. Moreover, the signal at 1225 cm−1 arises from a CH deformation mode while the feature at 567 cm−1 corresponds to in-plane ring deformations. The C=N stretch from the imine [29,30] is also observed at 1637 cm−1.
Scheme 1.
Synthesis of metalloenediyne M(QuiED)·2Cl and cyclized control M(QuiBD)·2Cl constructs. M = Cu(II), Fe(II), Zn(II), or Mg(II).
Fig. 1.
Raman spectra of Fe(QuiED)·2Cl (red) and Fe(QuiBD)·2Cl (blue). The spectrum of Fe(QuiED)·2Cl exhibits a diagnostic alkyne vibration (2180 cm−1) that is not present in the cyclized control complex Fe(QuiBD)·2Cl. (Color online.)
HRMS of the metal complexes using electrospray ionization reveals that all of the metalloenediyne complexes and the cyclized analogs are comprised of a 1:1 metal:ligand stoichiometry in solution. The complexes containing the divalent metal centers Fe, Mg, and Zn possess a solution composition of [MLCl]+ (L = QuiED or QuiBD), indicating that one of the chloride anions is coordinated to the metal center. However, the Cu(II) complexes exhibit a parent ion peak corresponding to [CuL]+, signifying that the weakly bound chloride (vide infra) dissociates during ionization, and the Cu(II) metal center is reduced to Cu(I) in vacuo to yield a complex with the overall charge of +1. The Evans method measurements [26] of the Cu(QuiED)·2Cl and Cu(QuiBD)·2Cl complexes confirm that Cu(II) is present in the complex and the reduction is a result of the ionization process. In addition, EPR measurements corroborate the Cu(II) metal centers in these complexes (vide infra). This phenomenon is not observed in the other metal complexes as Cu(II) is the only divalent metal in the series to have a favorable one-electron reduction potential.
3.2. Electronic spectroscopy
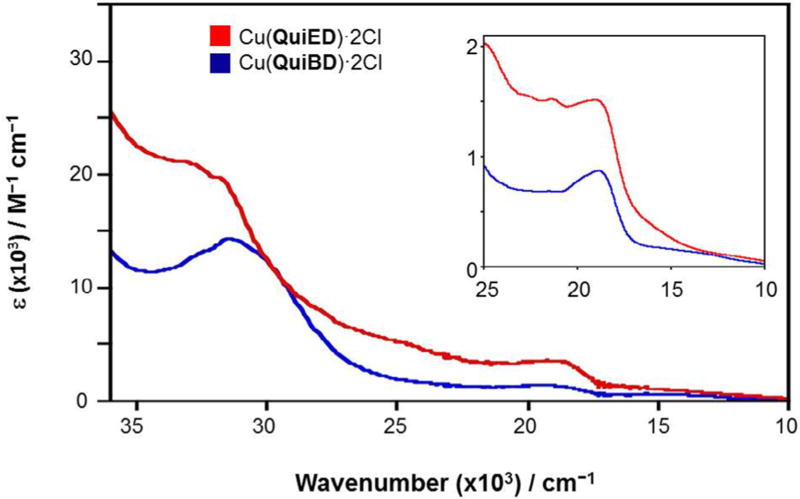
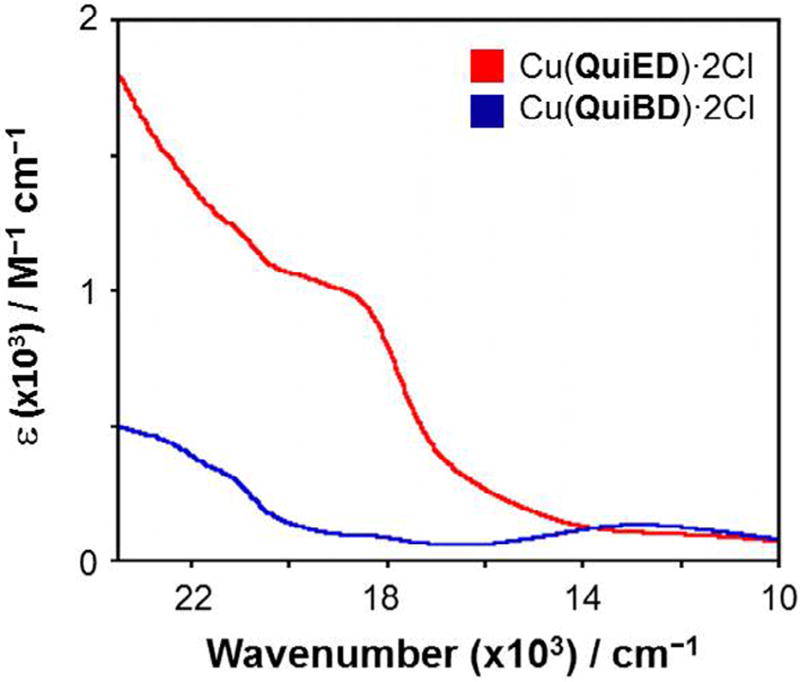
The abundance of information available on the electronic absorption spectra of Cu(II) complexes containing N4 coordination allows for a spectroscopic assessment of the structure of the Cu(II) complexes via electronic spectroscopy. Cu(II) complexes with N4 coordination are able to adopt coordination numbers ranging from 4 to 6 [31] and geometries spanning from tetrahedral to square planar and distorted octahedral. The square planar Cu(II) complexes in the literature exhibit ligand field absorption features in the range of 17,000–20,000 cm−1 deriving from transitions from the filled dxy, dz2, and dxz,yz orbitals to the one-electron hole in the dx2–y2 orbital. As the geometry and coordination number shifts from 4-coordinate square planar to 6-coordinate octahedral to 4-coordinate pseudotetrahedral, these d–d bands shift to lower energies as a result of the reduced ligand field splitting of the d-orbitals [32–34]. The electronic absorption profiles for both Cu(QuiED)·2Cl and Cu(QuiBD)·2Cl acquired in MeOH are shown in Fig. 2. These complexes demonstrate ligand field transitions between 11,000 and 14,000 cm−1, indicating the corresponding structures are 6-coordinate, distorted octahedral [35,36], however, the lower values for the ligand field transitions suggest significant distortion from planarity of the equatorial ligands. From the ligand field regions of the spectra alone, it is impossible to determine whether all four nitrogen donors of the ligand lie within the xy plane. A second feature at slightly higher energy is observed at 18,975 cm−1 (ε = 873 M−1 cm−1) (QuiBD) and 19,011 cm−1 (ε = 1514 M−1 cm−1) (QuiED) (Fig. 2, inset). These values correlate well with those for π-donation to the copper center from a chloride ligand in the equatorial plane (19,000–24,500 cm−1, ε = 1000–3500 M−1 cm−1) [34,37–41]; however, the molar absorption value for Cu(QuiBD)·2Cl is lower than commonly observed, indicating that chloride ligand π-overlap with the metal d-orbital is weak compared to Cu(QuiED) and may be subject to dissociation in the presence of a coordinating solvent. To this end, spectra obtained in DMSO demonstrate loss of the pπ Cl→Cu(II) LMCT band in the cyclized complex, demonstrating substitution of the weakly bound chloride by the oxygen of DMSO (Fig. 3). This analysis is in good agreement with an energy minimized structure for the Mg(II)–pyridine metalloenediyne complex that exhibits three nitrogens in the equatorial plane with the fourth coordinated in an axial position [18].
Fig. 2.
Electronic spectra of Cu(QuiED)·2Cl (red) and Cu(QuiBD)·2Cl (blue) in MeOH. Inset displays spectra in the visible region for Cu(QuiED)·2Cl (red) and Cu (QuiBD)·2Cl (blue). (Color online.)
Fig. 3.
Electronic spectra of Cu(QuiED)·2Cl (red) and Cu(QuiBD)·2Cl (blue) acquired in DMSO in the visible region. (Color online.)
In the higher energy region, there are four sets of transitions that occur in overlapping sections; π–π* from the quinoline ligand (40,500–36,000 cm−1, ε= 20,000–30,000 M−1 cm−1) [42–44], pσ(imine)→dx2–y2(Cu(II)) LMCT (45,000–37,000 cm−1, ε = 3000–4500 M−1 cm−1) [34,45,46], pσ(Cl)→dx2–y2(Cu(II)) LMCT (37,000–29,000 cm−1, ε = 3000–6000 M−1 cm−1) [38–41], and pπ(imine)→dx2–y2(Cu(II)) LMCT (40,000–30,000 cm−1, ε = 1000– 3000 M−1 cm−1) [34,45,47]. The broad absorption bands at 31,646 cm−1 (ε = 14,273 M−1 cm−1) (QuiBD) and 33,223 cm−1 (ε = 21,066 M−1 cm−1) (QuiED) correspond to the expected pσ(Cl)→dx2–y2(Cu(II)) and pπ(imine)→dx2–y2(Cu(II)) LMCT transitions superimposed upon the tail end of the intense quinoline π–π* and p r (imine)→dx2–y2(Cu(II)) LMCT features that lie to higher energy (Fig. 2).
The electronic absorption spectra for both Fe(QuiBD)·2Cl and Fe (QuiED)·2Cl exhibit characteristic absorption features centered at 33,898 cm−1 (ε = 13,427 M−1 cm−1) (QuiBD) and 33,003 cm−1 (ε = 8098 M−1 cm−1) (QuiED), corresponding to the π→π* internal transitions of the diimine ligand in high spin (HS) Fe(II) complexes (Fig. 4) [48–52]. The intensities of these transitions occur on the lower range of those observed for many HS Fe(II) compounds containing diimine or pyridine-type ligands [53,54]. Multiple MLCT absorption features arising from the t2g donor orbitals on the Fe (II) to the π* acceptor orbitals of the ligands [48,55,56] are observed in the Fe(QuiBD)·2Cl and Fe(QuiED)·2Cl spectra. More intense MLCT bands centered at 27,932 cm−1 (ε = 3926 M−1 cm−1) (QuiBD) and 27,855 cm−1 (ε = 3460 M−1 cm−1) are superimposed upon the tail-end of the intense ligand π→π* transitions described above, while the features at 22,624 cm−1 (ε = 781 M−1 cm−1) (QuiBD) and 21,459 cm−1 (ε = 462 M−1 cm−1) are often observed as shoulders with low molar extinction values (22,000– 24,000 cm−1, ε = 300–1000 M−1 cm−1) [51,52]. While two distinct bands are not clearly resolved for the imine and quinoline charge transfer transitions in these spectra, this phenomenon is common for d6 compounds when the functionalities are similar to one another, such as pyridine and quinoline nitrogens [34] and, in general, high spin (HS) Fe(II) octahedral structures demonstrate a broad absorption band due to weak Jahn Teller distortion [34]. Additionally, the intensity of the t2g→π* transitions observed for the cyclized complex is slightly greater than that for the uncyclized analog, indicating better overlap between the metal t2g donor orbitals and the π* acceptor orbitals on the ligands (Fig. 4, inset) [54].
Fig. 4.
Electronic spectra of Fe(QuiED)·2Cl (red) and Fe(QuiBD)·2Cl (blue) acquired in MeOH. Inset shows visible region for Fe(QuiED)·2Cl (red) and Fe(QuiBD)·2Cl (blue). (Color online.)
3.3. EPR spectroscopy
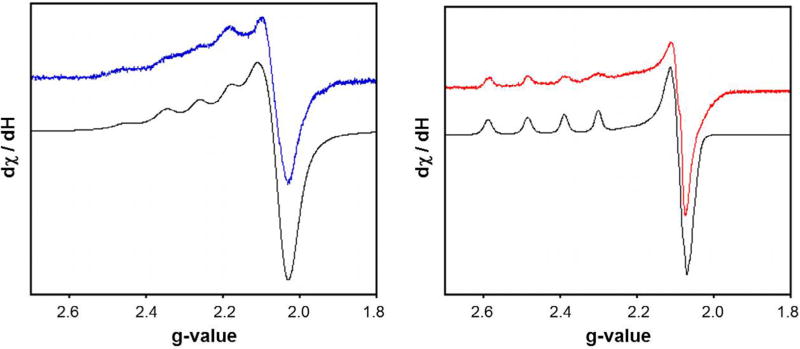
In order to gain further insight into the solution structure of the copper metalloenediynes, EPR signatures of Cu(QuiED)·2Cl and Cu (QuiBD)·2Cl were obtained in frozen solutions of MeOH at 77 K (Fig. 5). The spectra of both complexes reveal that the Cu(II) centers are axial (g║ > g⊥> 2.0023) with a dx2–y2 ground state [57–59] and the spin Hamiltonian parameters of g║ and g⊥ are indicative of six-coordinate, tetragonally-elongated Cu(II) centers (Table 1) [60–63]. The spectrum of Cu(QuiED)·2Cl reveals a major species with g⊥= 2.09 and g║ = 2.44, while the cyclized analog displays g⊥ = 2.06 and g║ = 2.22. These larger g-values correlate with data for other Cu(II) complexes containing an electron-withdrawing chloride ligand bound in the xy plane [64], which increases the anisotropy of the ligand set in this plane. Additionally, increased g║ values are also observed for aza-aromatic nitrogens such as pyridine and quinoline [65].
Fig. 5.
X-band EPR spectra of Cu(QuiBD)·2Cl (600 µM, blue trace) and Cu(QuiED)·2Cl (600 µM, red trace) at 77 K in MeOH. Simulated spectra are shown in black. (Color online.)
Table 1.
X-band EPR spectral parameters of Cu(QuiBD)·2Cl and Cu(QuiED)·2Cl at 77 K in 1:1 MeOH.
| Complex | Species | g║ | g⊥ | AIICu (×10−4) (cm−1) | G | g║/A║ | Percentage |
|---|---|---|---|---|---|---|---|
| Cu(QuiED)·2Cl | 1 | 2.44 | 2.09 | 123 | 4.89 | 198 | 85 |
| 2 | 2.35 | 2.09 | 114 | 3.76 | 206 | 15 | |
| Cu(QuiBD).2Cl | 1 | 2.22 | 2.06 | 114 | 3.84 | 195 | 75 |
| 2 | 2.31 | 2.05 | 125 | 6.25 | 185 | 25 |
While the g⊥ values are similar between the two complexes, there is a significant difference in the g║ values. This difference is likely due to a combination of a decrease in ligand covalency [60,66] and increased oxygen coordination. Deviation of g║ from the free electron value (ge = 2.0023) derives from spin–orbit coupling of the dxy orbital into the dx2–y2 ground state. Since the amount of orbital angular momentum localized on copper is directly related to the degree of metal–ligand covalency [60,66], a g║ > 2.3 is indicative of more ionic character in the overall ligand coordination for the metalloenediyne. The smaller value for the cyclized ligand (g║ = 2.22) suggests a more covalent and tighter Cu(II) binding interaction compared to the uncyclized enediyne (g║ = 2.44) [58,59]. This is consistent with the cyclized analog having a more constricted binding pocket and thus, a higher degree of covalency between the ligand and metal center. The more relaxed binding of the larger enediyne chelate may also allow for increased dissociation of the axial quinoline nitrogen and binding of solvent, increasing the oxygen coordination and ultimately g║.
Both Cu(QuiBD)·2Cl and Cu(QuiED)·2Cl in MeOH reveal the presence of two solution species in their EPR spectra. In the Cu (QuiBD)·2Cl system, the smaller g║ value of 2.22 observed for the major species (∼75%) suggests the second quinoline functionality is bound in the axial position (Fig. 6A). Although not clearly resolved, a second species is observed with g║ = 2.31 (∼25%) possessing a more oxygen-rich environment surrounding the metal center. This second species may be attributed to the substitution of the more weakly bound axial quinoline nitrogen by the solvent containing an oxygen-donor (Fig. 6B). A similar observable also occurs in the EPR spectrum of Cu(QuiED)·2Cl, however, the major and minor species are reversed relative to the cyclized analog. The major species with g║ = 2.44 is represented by the structure containing solvent displacement of the axial quinoline nitrogen due to reduced metal-ligand covalency, while the second species (∼15%) with a lower g║ value (g║ = 2.35) corresponds to the axial quinoline nitrogen remaining bound to the Cu(II) center.
Fig. 6.
Proposed structures of the multiple solution species observed for Cu(QuiED)·2Cl and Cu(QuiED)·2Cl. N represents the imine nitrogens and Q represents the nitrogens of the quinoline rings.
As noted by Hathway et al. [60], G values, (G = (g║ – 2)/(g⊥ – 2)), greater than 4 correlate with only slight misalignment of the equatorial axes, while values less than 4 suggest that the complex geometry is significantly distorted [60]. The G values for the chloro-complexes containing the axial nitrogen in both the QuiED (G = 3.76) and QuiBD (G = 3.84) complexes (Fig. 6A) are close to 4, implying minor equatorial asymmetry, while the species with increased oxygen coordination from solvent (G = 4.89, QuiED; G = 6.25, QuiBD) (Fig. 6B) reveal a smaller distortion. Further structural data regarding geometric distortion can be elucidated from the ratio g║/A║, which is an empirical factor that evaluates the distortion of the dihedral angle for an axially-elongated tetragonal complex. Values between 105 and 135 cm are assigned to a square planar arrangement of the equatorial ligands, while a progressive rise up to 250 cm indicates increasing distortion of the dihedral angle (∼40°) [65,67–69]. The calculated g║/A║ values reveal significant distortion from planarity in both the uncyclized (198) and cyclized (195) Cu(II) complexes.
As both complexes demonstrate limited solubility in MeOH at 77 K, spectra were also acquired in 1:1 MeOH:DMSO solution. Under these conditions there are slight changes observed in the spectra of both the cyclized and uncyclized complexes (Table 2, Fig. 7). The Cu(QuiED)·2Cl spectrum maintains only a small percentage of the minor species in the new solvent mixture (∼15%), indicating that the solution structure remains largely unchanged. The spectrum for Cu(QuiBD)·2Cl however, displays a decrease in the major species observed (∼50%, Fig. 6A) and the presence of a third species in solution with a g║ representative of increased oxygen coordination. Taken in consideration with the loss of the Cl→ Cu(II) LMCT band in the electronic spectrum in pure DMSO, one of these oxygen-rich species likely contains solvent in the axial position (Fig. 6B), while the second correlates with a coordinated DMSO in place of the equatorial chloride (Fig. 6C). The more tightly bound QuiBD ligand contains a stronger imine–Cu(II) bond, subsequently weakening the Cu(II)–Cl interaction compared to that in QuiED, and allowing for substitution by effectively coordinating solvents such as DMSO.
Table 2.
X-band EPR spectral parameters of Cu(QuiBD)·2Cl and Cu(QuiED)·2Cl at 77 K in 1:1 DMSO/MeOH.
| Complex | Species | g║ | g⊥ | AIICu (×10−4) (cm−1) | G | g║/A║ | Percentage |
|---|---|---|---|---|---|---|---|
| Cu(QuiED).2Cl | 1 | 2.40 | 2.08 | 129 | 5.06 | 186 | 85 |
| 2 | 2.32 | 2.08 | 117 | 4.05 | 198 | 15 | |
| Cu(QuiBD).2Cl | 1 | 2.23 | 2.06 | 104 | 3.83 | 214 | 50 |
| 2 | 2.32 | 2.05 | 119 | 6.48 | 195 | 25 | |
| 3 | 2.32 | 2.05 | 100 | 6.55 | 232 | 25 |
Fig. 7.
X-band EPR spectra acquired in 1:1 DMSO/MeOH of Cu(QuiBD)·2Cl (600 µM, blue trace) and Cu(QuiED)·2Cl (600 µM, red trace) at 77 K. Simulated spectra are shown in black. (Color online.)
3.4. Bergman cyclization reactivity assays
The closely related pyridine–Mg(II) enediyne complex demonstrates Bergman cyclization in MeOH within 2.5 h at ambient temperature [18]. The additional fused aromatic ring on each side of the quinoline ligand, however, leads to significant retardation of the solution reactivity relative to the pyridine analog PyED. Heating of the Mg(II)–quinoline complex for 12 h at 45 °C in MeOH results in no Bergman cyclized product by HRMS(ESI) analysis (Table 3). The absence of observable product is maintained even after 24 h at the elevated temperature. As Mg(II) has no ligand field electronic preference for a specific structure, ligand sterics therefore dictate the geometry of a complex. Thus, the drastic difference in reactivity between the Mg(II)–PyED and Mg(II)–QuiED metalloenediynes must arise from a steric crowding of the additional aromatic rings. Even with the quinoline moieties positioned in orthogonal axes, steric hindrance of the aromatic rings must lead to relaxation of the Mg–N heterocycle and Mg–N imine bonds relative to the corresponding PyED structure. The bond elongation generates a greater interalkynyl distance and consequently, the absence of reactivity for the Mg(QuiED)·2Cl complex as compared to the facile cyclization turnover of Mg(PyED).
Table 3.
Detection of cyclized product QuiBDH via HRMS (ESI) upon heating of M(QuiED)·2Cl or M(QuiBD)·Cl control for 12 h at 45 °C.
| Metal | +QuiBD (m/z) | +QuiED (m/z) |
|---|---|---|
| None | 419.2243 | – |
| Cu(II) | 419.2245 | 419.2245 |
| Fe(II) | 419.2248 | 419.2255 |
| Zn(II) | 419.2236 | Not observed |
| Mg(II) | 419.2223 | Not observed |
In contrast to Mg(II), Zn(II) centers have a slight preference for N- versus O-ligation and are able to adopt ligand-dependent coordination numbers ranging from 4 to 6 due to their d10 electronic configuration. Within this theme, the Bergman cyclization reactivity of Zn(QuiED)·2Cl mirrors that demonstrated by Mg(QuiED)·2Cl (Table 3), even though the structural origin of this observation may or may not be the same. The Zn(QuiED)·2Cl can maintain a 6-coordinate structure similar to the Mg(II) –quinoline metalloenediyne, or possibly adopt a 4-coordinate tetrahedral structure that would be expected to exhibit similarly high Bergman cyclization temperatures [23,24,70–72]. However, since addition of free pyridine–enediyne to preformed Zn(II) –Aβ fibrils at 43 °C leads to significant radical-induced fibril disaggregation within 2 h [19], and we would predict the binding motifs of the pyridine and quinoline ligands to be parallel, this indicates that the solution structure preference for Zn–metalloenediynes cannot be 4-coordinate. Thus, the geometries of the Zn(II) and Mg(II) structures must be analogous and it is the lengthening of the ligand–metal bonds that is responsible for the decreased reactivity observed compared to the pyridine metalloenediynes.
In contrast, Cu(QuiED)·2Cl and Fe(QuiED)·2Cl demonstrate Bergman cyclization reactivity within 12 h at 45 °C (Table 3). Isolation of the organic reaction products is achieved via reduction of the imine bonds with NaBH4 and extraction of the metal center using EDTA (Scheme 2). Formation of the benzannulated product was confirmed by comparison to the control complexes Cu (QuiBD)·2Cl and Fe(QuiBD)·2Cl under analogous conditions (Scheme S2). The HRMS(ESI) signatures of the Berman cyclized product were obtained via the independently synthesized amine ligand QuiBDH (Scheme S3). Qualitative analysis by mass spectrometry reveals approximately 20% turnover to form cyclized products. While appreciable yield of the specific Bergman cyclization product has been achieved in some systems containing an intramolecular radical trap [20,73,74], the bimolecular radical quenching here precludes the formation of a single product in high yields.
Scheme 2.
Thermal activation of M(QuiED)·2Cl at 45 °C to yield QuiBDH via Bergman cyclization. M = Cu(II) or Fe(II).
Thus, while the electronic and EPR data reveal full coordination spheres for both the Cu(II) and Fe(II) systems, comparable to that of Zn(II) and Mg(II), there is a stark difference in the reactivity between these two sets of quinoline–metalloenediynes. It has previously been demonstrated that the pyridine enediyne ligand has a higher binding affinity for Cu(II) over Zn(II) [19] and in general, chelating ligands containing these imine, pyridine, or quinoline functionalities demonstrate a higher affinity for Cu(II) and Fe(II) compared to Zn(II) and Mg(II) [75–78]. This greater degree of covalency exhibited by the Cu(II)– and Fe(II)–QuiED complexes allows for tighter bond strengths between the enediyne and metal center, leading to a lowered activation energy for these metal centers relative to the Mg(II)- and Zn(II)–QuiED complexes above. Additionally, although the Bergman cyclized product is detected after 12 h at 45 °C for the Cu(QuiED)·2Cl, this activity is severely diminished compared to the reactivity exhibited by the Cu(II)–PyED analog. Contrary to the long reaction times and high temperatures required for Cu(QuiED), the addition of PyED to preformed Cu(II)–Aβ fibrils results in significant radical-induced disaggregation within only 2 h at 37 °C [19]. The steric crowding of the addition fused aromatic rings of QuiED forces a relaxation of the M–N bond lengths in the Cu(II) system relative to the PyED equivalent and subsequently, a retardation in reactivity.
4. Conclusions
In an effort to gain insight into enediyne activation via metal chelation, a series of quinoline–metalloenediyne complexes and their cyclized analogs have been successfully synthesized and demonstrate substantial differences in solution Bergman cyclization reactivity between activating metal ions. HRMS(ESI) reveals a 1:1 stoichiometry of metal to ligand and coordination of one of the chloride counter ions to the metal center in solution. Electronic and EPR spectroscopic analysis of Cu(QuiED)·2Cl and Cu(QuiBD)·2Cl denotes a tetragonal solution structure that includes three nitrogens and a chloride in the equatorial plane with the remaining quinoline nitrogen and solvent in the axial positions. Solution reactivity assays show formation of the cyclized product from the Cu (II) of Fe(II) complexes after 12 h at 45 °C, however no product is observed for the Mg(II) or Zn(II) complexes under identical conditions. The source of the reactivity differences of these quinoline analogs has been traced to a steric effect leading to metal–ligand bond elongation and subsequent retardation of solution reactivity. Thus, QuiED offers a degree of control via reaction with divalent metals and a window into how simple modifications to enediyne ligands may allow for the selective activation of these reactive agents within biological systems.
Supplementary Material
Acknowledgments
The generous support of the National Science Foundation (CHE-1265703) is gratefully acknowledged for the funding of this research. The authors thank Joan Walker for assistance in acquisition of Raman spectra.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.poly.2015.10.041.
References
- 1.Alzheimer’s Association. Alzheimers Dement. 2012;8:131. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Kepp KP. Chem. Rev. 2012;112:5193. doi: 10.1021/cr300009x. [DOI] [PubMed] [Google Scholar]
- 3.Savelieff MG, DeToma AS, Derrick JS, Lim MH. Acc. Chem. Res. 2014;47:2475. doi: 10.1021/ar500152x. [DOI] [PubMed] [Google Scholar]
- 4.Miller Y, Ma B, Nussinov R. Chem. Rev. 2010;110:4820. doi: 10.1021/cr900377t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakob-Roetne R, Jacobsen H. Chem. Angew. Int. Ed. 2009;48:3030. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 6.Duce JA, Bush AI. Prog. Neurobiol. 2010;92:1. doi: 10.1016/j.pneurobio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Barnham KJ, Bush AI. Curr. Opin. Chem. Biol. 2008;12:222. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Scott LE, Orvig C. Chem. Rev. 2009;109:4885. doi: 10.1021/cr9000176. [DOI] [PubMed] [Google Scholar]
- 9.Faller P. ChemBioChem. 2009;10:2837. doi: 10.1002/cbic.200900321. [DOI] [PubMed] [Google Scholar]
- 10.DeToma AS, Salamekh S, Ramamoorthy A, Lim MH. Chem. Soc. Rev. 2012;41:608. doi: 10.1039/c1cs15112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zatta P, Drago D, Bolognin S, Sensi SL. Trends Pharmacol. Sci. 2009;30:346. doi: 10.1016/j.tips.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Faller P, Hureau C. Dalton Trans. 2009:1080. doi: 10.1039/b813398k. [DOI] [PubMed] [Google Scholar]
- 13.Telpoukhovskaia MA, Orvig C. Chem. Soc. Rev. 2013;42:1836. doi: 10.1039/c2cs35236b. [DOI] [PubMed] [Google Scholar]
- 14.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y-S, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Neuron. 2001;30:665. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 15.Crouch PJ, Tew DJ, Du T, Nguyen DN, Caragounis A, Filiz G, Blake RE, Trounce IA, Soon CPW, Laughton K, Perez KA, Li Q-X, Cherny RA, Masters CL, Barnham KJ, White AR. J. Neurochem. 2009;108:1198. doi: 10.1111/j.1471-4159.2009.05870.x. [DOI] [PubMed] [Google Scholar]
- 16.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW. Lancet Neurol. 2008;7:779. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 17.Faux NG, Ritchie CW, Gunn A, Rembach A, Tsatsanis A, Bedo J, Harrison J, Lannfelt L, Blennow K, Zetterberg H, Ingelsson M, Masters C, Tanzi R, Cummings L, Herd CM, Bush A. J. Alzheimer’s Dis. 2010;20:509. doi: 10.3233/JAD-2010-1390. [DOI] [PubMed] [Google Scholar]
- 18.Rawat DS, Zaleski JM. J. Am. Chem. Soc. 2001;123:9675. doi: 10.1021/ja011215r. [DOI] [PubMed] [Google Scholar]
- 19.Porter MR, Kochi A, Karty JA, Lim MH, Zaleski JM. Chem. Sci. 2015;6:1018. doi: 10.1039/c4sc01979b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nath M, Pink M, Zaleski JM. J. Am. Chem. Soc. 2005;127:478. doi: 10.1021/ja045979t. [DOI] [PubMed] [Google Scholar]
- 21.Rawat DS, Zaleski JM. Synlett. 2004:393. [Google Scholar]
- 22.Chandra T, Allred RA, Kraft BJ, Berreau LM, Zaleski JM. Inorg. Chem. 2004;43:411. doi: 10.1021/ic030218x. [DOI] [PubMed] [Google Scholar]
- 23.Rawat DS, Benites PJ, Incarvito CD, Rheingold AL, Zaleski JM. Inorg. Chem. 2001;40:1846. doi: 10.1021/ic010014l. [DOI] [PubMed] [Google Scholar]
- 24.Chandra T, Pink M, Zaleski JM. Inorg. Chem. 2001;40:5878. doi: 10.1021/ic010424+. [DOI] [PubMed] [Google Scholar]
- 25.Routt SM, Zhu J, Zaleski JM, Dynlacht JR. Int. J. Hypertherm. 2011;27:435. doi: 10.3109/02656736.2011.578607. [DOI] [PubMed] [Google Scholar]
- 26.Baker MV, Field LD, Hambley TW. Inorg. Chem. 1988;27:2872. [Google Scholar]
- 27.Zhang M, Zhao A, Sun H, Guo H, Wang D, Li D, Gan Z, Tao W. J. Mater. Chem. 2011;21:18817. [Google Scholar]
- 28.Socrates G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts. 3. Wiley, West Sussex; England: 2004. p. 366. [Google Scholar]
- 29.Alex S, Turcotte P, Fournier R, Vocelle D. Can. J. Chem. 1991;69:239. [Google Scholar]
- 30.Kazitsyna LA. Dokl. Akad. Nauk. SSSR. 1963;151:573. [Google Scholar]
- 31.Addison AW, Carpenter M, Lau LKM, Wicholas M. Inorg. Chem. 1978;17:1545. [Google Scholar]
- 32.Hathaway BJ. J. Chem. Soc., Dalton Trans. 1972:1196. [Google Scholar]
- 33.Solomon EI. Comments Inorg. Chem. 1984;3:238. [Google Scholar]
- 34.Lever ABP. Inorganic Electronic Spectroscopy. 2. Elsevier, Amsterdam: 1984. [Google Scholar]
- 35.Batra G, Mathur P. Inorg. Chem. 1992;31:1575. [Google Scholar]
- 36.Pradilla SJ, Chen HW, Koknat FW, Fackler JP. Inorg. Chem. 1979;18:3519. [Google Scholar]
- 37.Harris CM, Patil HRH, Sinn E. Inorg. Chem. 1967;6:1102. [Google Scholar]
- 38.Willett RD, Liles OL, Michelson C. Inorg. Chem. 1967;6:1885. [Google Scholar]
- 39.Allen GC, Hush NS. Inorg. Chem. 1967;6:4. [Google Scholar]
- 40.DeKock CW, Gruen DM. J. Chem. Phys. 1966;44:4387. [Google Scholar]
- 41.Desjardins SR, Penfield KW, Cohen SL, Musselman RL, Solomon EI. J. Am. Chem. Soc. 1983;105:4590. [Google Scholar]
- 42.Xue Y, Liu Y, An L, Zhang L, Yuan Y, Mou J, Liu L, Zheng Y. Comput. Theor. Chem. 2011;965:146. [Google Scholar]
- 43.Kumru M, Küçük V, Kocademir M, Alfanda HM, Altun A, Sarı L. Spectrochim. Acta. 2015;134:81. doi: 10.1016/j.saa.2014.06.094. Part A. [DOI] [PubMed] [Google Scholar]
- 44.Deng X, Chai X, Wei C, Fu L. Anal. Sci. 2011;27:493. doi: 10.2116/analsci.27.493. [DOI] [PubMed] [Google Scholar]
- 45.Fawcett TG, Bernarducci EE, Krogh-Jespersen K, Schugar HJ. J. Am. Chem. Soc. 1980;102:2598. [Google Scholar]
- 46.Miskowski VM, Thich JA, Solomon R, Schugar HJ. J. Am. Chem. Soc. 1976;98:8344. [Google Scholar]
- 47.Bernarducci E, Bharadwaj PK, Krogh-Jespersen K, Potenza JA, Schugar HJ. J. Am. Chem. Soc. 1983;105:3860. [Google Scholar]
- 48.Williams RJP. J. Chem. Soc. 1955:137. [Google Scholar]
- 49.Mori K, Kagohara K, Yamashita H. J. Phys. Chem. C. 2008;112:2593. [Google Scholar]
- 50.Renz F, Hasegaw M, Hoshi T, El-ayaan U, Linert W, Fukuda Y. Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A. 1999;335:531. [Google Scholar]
- 51.Krumholz P. J. Am. Chem. Soc. 1953;75:2163. [Google Scholar]
- 52.Krumholz P, Serra OA, De Paoli MA. Inorg. Chim. Acta. 1975;15:25. [Google Scholar]
- 53.Linert W, Konecny M, Renz F. J. Chem. Soc. Dalton Trans. 1994:1523. [Google Scholar]
- 54.Loeb KE, Zaleski JM, Westre TE, Guajardo RJ, Mascharak PK, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 1995;117:4545. [Google Scholar]
- 55.Byers W, Lever ABP, Parish RV. Inorg. Chem. 1968;7:1835. [Google Scholar]
- 56.Bryant G, Fergusson J, Powell H. Aust. J. Chem. 1971;24:257. [Google Scholar]
- 57.Manzur J, Mora H, Vega A, Spodine E, Venegas-Yazigi D, Garland MT, El Fallah MS, Escuer A. Inorg. Chem. 2007;46:6924. doi: 10.1021/ic700544b. [DOI] [PubMed] [Google Scholar]
- 58.Balasubramanian S, Krishnan CN. Polyhedron. 1986;5:669. [Google Scholar]
- 59.Kivelson D, Neiman R. J. Chem. Phys. 1961;35:149. [Google Scholar]
- 60.Hathaway BJ, Billing DE. Coord. Chem. Rev. 1970;5:143. [Google Scholar]
- 61.Searl JW, Smith RC, Wyard SJ. Proc. Phys. Soc. 1959;74:491. [Google Scholar]
- 62.Singh B, Yadav BP, Aggarwal RC. Ind. J. Chem. 1984;23A:441. [Google Scholar]
- 63.Peisach J, Blumberg WE. Arch. Biochem. Biophys. 1974;165:691. doi: 10.1016/0003-9861(74)90298-7. [DOI] [PubMed] [Google Scholar]
- 64.Ahmadi RA, Hasanvand F, Bruno G, Rudbari HA, Amani S. ISRN Inorg. Chem. 2013;2013:1. [Google Scholar]
- 65.Linss M, Weser U. Inorg. Chim. Acta. 1986;125:117. [Google Scholar]
- 66.Hathaway BJ, Copper . In: Comprehensive Coordination Chemistry: Theory and Background. Wilkinson G, editor. Vol. 5. Pergamon Press; Oxford: 1987. pp. 533–774. [Google Scholar]
- 67.Sakaguchi U, Addison AW. J. Chem. Soc. Dalton Trans. 1979:600. [Google Scholar]
- 68.Yokoi H, Addison AW. Inorg. Chem. 1977;16:1341. [Google Scholar]
- 69.Murali M, Palaniandavar M, Pandiyan T. Inorg. Chim. Acta. 1994;224:19. [Google Scholar]
- 70.Warner BP, Millar SP, Broene RD, Buchwald SL. Science. 1995;269:814. doi: 10.1126/science.269.5225.814. [DOI] [PubMed] [Google Scholar]
- 71.Lindahl SE, Park H, Pink M, Zaleski JM. J. Am. Chem. Soc. 2013;135:3826. doi: 10.1021/ja308190q. [DOI] [PubMed] [Google Scholar]
- 72.Bhattacharyya S, Clark AE, Pink M, Zaleski JM. Inorg. Chem. 2009;48:3916. doi: 10.1021/ic801116q. [DOI] [PubMed] [Google Scholar]
- 73.Nath M, Huffman JC, Zaleski JM. Chem. Commun. 2003:858. [PubMed] [Google Scholar]
- 74.Boerner LJK, Pink M, Park H, LeSueur A, Zaleski JM. Chem. Commun. 2013;49:2145. doi: 10.1039/c3cc34471a. [DOI] [PubMed] [Google Scholar]
- 75.Puranik DB, Singh A, Chang EL. J. Coord. Chem. 1996;39:321. [Google Scholar]
- 76.Chen C, Men G, Bu W, Liang C, Sun H, Jiang S. Sens. Actuators, B. 2015;220:463. [Google Scholar]
- 77.Obali AY, Ucan HI. J. Mol. Struct. 2015;1081:74. [Google Scholar]
- 78.Cheng J, Ma X, Zhang Y, Liu J, Zhou X, Xiang H. Inorg. Chem. 2014;53:3210. doi: 10.1021/ic5000815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.