Abstract
Parkinson's disease is a neurodegenerative chronic condition with a declining trajectory and lack of a cure, making quality of life an important aspect of care. The purpose of this literature review was to analyze the state-of-the-science on the effects of non-pharmacological treatments on quality of life in person's with Parkinson's disease. Literature search was conducted using keywords in electronic databases up to September 1, 2016 and cross-searching the references of identified articles. Of the 259 articles generated, 26 met the eligibility criteria and were included in this review. The majority of studies (77%) were Level I evidence and 23% Level II evidence. The levels of study quality were: strong (50%), moderate (15%), and weak (35%). The interventions varied across studies with 15 studies evaluating a similar intervention. About 58% of the studies showed that the interventions improved quality of life. In conclusion, a variety of non-pharmacological interventions have been increasingly studied for their effects on quality of life in Parkinson's disease, showing initial promising results. However, most interventions were only examined by a limited number of studies and the minimal and optimal intervention doses needed for improving quality of life are yet unknown.
Keywords: Aging, Parkinson's disease, Quality of life, Therapy
Introduction
Parkinson's disease (PD) is a multifaceted, neurodegenerative chronic disease with a declining trajectory, afflicting approximately 0.4-1% of people 60 to 79 years of age and 1.9% of those 80 years old and older worldwide [1]. In the United States alone, PD affects over one million people with an approximate 60,000 new cases each year and an annual cost of $ 25 billion [2,3]. The symptoms of PD include motor (bradykinesia, postural instability, rigidity, and resting tremor) and non-motor (depression, anxiety, fatigue, sleep disturbances, and cognitive impairment) symptoms. While the etiologies of PD are still unknown, deficient dopamine in the substantia nigra of the brain is well-established as the cause for motor symptoms [4]. However, dopamine deficiency alone cannot explain the non-motor symptoms which can be more disabling and challenging to manage than motor symptoms [5].
Given the lack of a cure and the chronic nature of PD, improving quality of life (QOL) is paramount for persons with PD and their families. QOL is increasingly recognized as an essential outcome in PD intervention studies [6]. Medications such as levodopa/carbidopa and surgical interventions such as deep brain stimulation show strong efficacy on improving motor symptoms and QOL [7,8]. In contrast, the efficacy of drug treatments for non-motor symptoms is equivocal and drugs for non-motor symptoms could actually interfere with the effectiveness of levodopa/carbidopa and/or aggravate motor symptoms, leading to poor QOL [5,9]. On the other hand, non-pharmacological interventions, e.g., music and exercises, have been increasingly tested to improve motor and non-motor symptoms due to their low profile of adverse events [10]. However, it is unclear how these non-pharmacological interventions affect QOL in PD. Hence, the purpose of this review was to analyze the state-of-the-science on the effects of non-pharmacological interventions on QOL in persons with PD. Since the last review of QOL in persons with PD was conducted in 2012 [11], this review fills the gap with updated and new evidence.
Methods
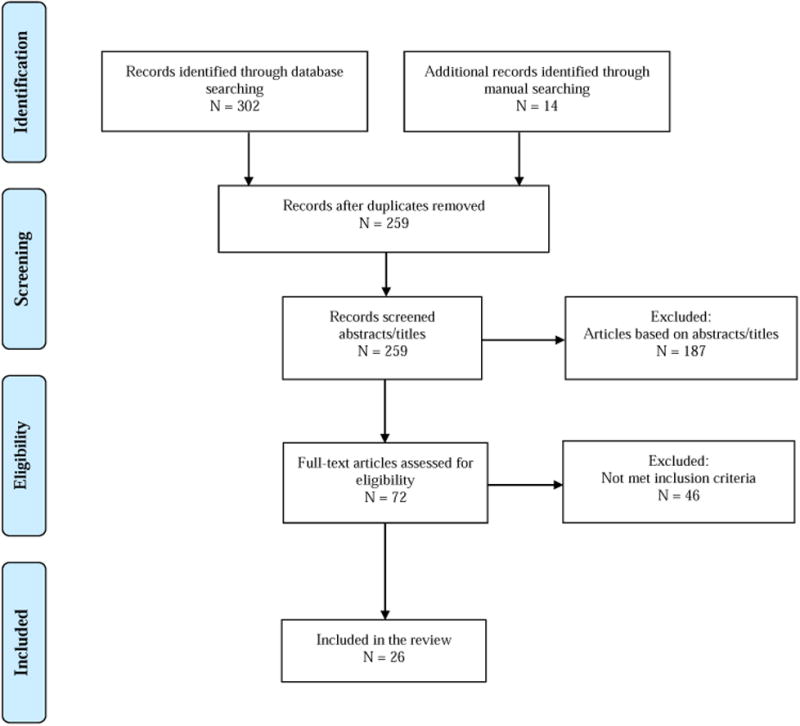
The literature search was conducted in 5 steps. First, electronic databases of PubMed, CINHAL, and PsycINFO were searched from the beginning dates of each database to September 1, 2016 by using keywords matched to medical subject headings: “Parkinson's disease/ therapy” and “QOL”. Second, articles were excluded using keywords matched to medical subject headings: “drug therapy”, “prescription drugs” and “deep brain stimulation”. Two hundred and forty five articles were generated. Third, the study titles and abstracts were reviewed using the following inclusion criteria: 1) publication in English; 2) QOL assessed as an outcome; and 3) randomized control trial (RCT), experimental, or quasi-experimental design. Forth, studies meeting the inclusion criteria were screened out if they met at least one of the exclusion criteria: 1) surgical intervention; and 2) case study. If the abstract of a study did not provide enough information to determine its eligibility, the full article was reviewed. Lastly, we cross-searched the references of the selected studies from database search and identified 14 additional studies. Of the total 259 studies identified, 26 met the eligibility criteria and were included in this review (Figure 1).
Figure 1. Flowchart of eligibility assessment.
The matrix system by Garrard was used to summarize the eligible studies (Tables 1 and 2) [12]. Each study was first rated for level of evidence: Level I (RCT or experimental) or Level II (quasi-experimental). Next, three authors (SA, YC, and TB) independently assessed the quality of each study using the Effective Public Health Practice Project (EPHPP) Quality Assessment Tool for Quantitative Studies. The EPHPP has 6 categories, including selection bias, study design, confounders, blinding, data collection method, and withdrawals. Each category is graded as ‘strong’, ‘moderate’ or ‘weak’ [13]. The category ratings were combined to generate an overall quality rating as: strong quality (no weak rating among the six categories), moderate quality (one weak rating), or weak quality (two or more weak ratings). The ratings agreed by at least two authors were assigned as the final overall quality rating for each study.
Table 1. Summary of the studies with QOL as the primary outcome (n=18).
| Citation, Level & Quality | Purpose | Study Design | N | Setting | Outcomes and Measures | Results/Conclusions |
|---|---|---|---|---|---|---|
| Exercise intervention | ||||||
| Argolo et al. 2013 Level: II Quality: Weak | Investigate the effect of 5-week motor swallowing exercise, twice a day, 5 days a week on swallowing and QOL | Pre-test post-test evaluation | 15 | University Medical Center | Swallowing: VSS and questionnaire QOL: SWAL-QOL | VSS showed improvement in bolus control*, piecemeal swallow*, residue on the tongue*, valleculae*, and pyriform sinuses*. SWAL-QOL improved on fear* and symptom frequency*. Reduction in swallowing events not related to QOL improvements. |
| Combs et al. 2011 Level: II Quality: Weak | Compare the effects of 12-week group boxing training, 3 times a week on balance, mobility, and QOL | Case series, pretest posttest design | 6 | Community | Balance: Functional Reach Test, BBS, Activities-specific Balance Confidence Scale Gait: TUG, 6MW, GaitRite Walkway System Disability: UPDRS QOL: PDQL | Improvements in balance, gait, disability, and PDQL at 12 weeks. Improvement continued at 24 and 36 weeks. Persons with mild PD show improvements earlier than those with moderate to severe PD. |
| Combs et al. 2013 Level: I Quality: Strong | Compare the effects of 12-week, 90-min non-traditional group boxing training, 3 times a week on function and QOL | A single blinded RCT to traditional group aerobic, resistance, balance exercise | 31 | Community | Balance: BBS Balance confidence: ABC Mobility: TUG Gait velocity: GaitRite Walk way System Gait endurance: 6MWT. QOL: PDQL | Boxing significantly improved gait velocity* and endurance*. Both groups improved balance*, mobility*, and QOL [PDQL score in boxing group: median 128.0 (range 61.0) in baseline vs median 132.0 (range 63.0) in follow up]*. Traditional exercise improved balance confidence*. |
| Cruise et al. 2011 Level: I Quality: Moderate | Evaluate the benefits of 12-week exercise, twice a week on cognition, mood, and QOL | Nonrandomized controlled trial with waitlist control | 28 | Community | Cognition: MMSE, WAIS verbal IQ Depression: GDS QOL: PDQ-39 | Exercise improved executive function*, but not QOL [PDQ-39 score in exercise group: 16.4 (7.68) in baseline vs 17.9 (7.38) in follow up]. |
| Dereli, Yaliman, 2010 Level: I Quality: Weak | Compare the effect of a 10-week physiotherapist-supervised exercise program, 3 times a week with unsupervised home exercise on QOL | Non-randomized, controlled trial | 32 | Outpatient exercise unit and home | QOL: PDQL and NHP. PD Severity: UPDRS Depression: BDI | Physiotherapy-supervised exercise improved more than home exercise on PDQL/NHP total score [for PDQL, median 11(Min-2 to Max23) vs median 4(Min-16 to Max38)]** / [for NHP, median-10.5 (Min-33 to Max 0) vs median-2 (Min-13 to Max 40)]**, PDQL Parkinson's symptoms [median 5 (Min 0 to Max10) vs median 2(Min-2 to Max 16)]**, and PDQL emotional function [median 3(Min 0 to Max 6) vs median 1 (Min-7 to Max 6)]**, as well as PD severity** and depression**. |
| Dibble et al. 2009 Level: I Quality: Weak | Examine the effect of 12-week high intensity resistance exercise, 3 times a week on bradykinesia and QOL | Exercise and control groups matched on age, gender, disease severity | 20 | Movement disorders clinic | Motor deficits: UPDRS motor subscale Muscle force: MVIC Bradykinesia: TMW and TUG QOL: PDQ-39 | The exercise group improved on all measures more than the control group, including PDQ-39 [within group effect size: 0.45 in exercise vs 0.08 in control]**, TMW**, TUG**, UPDRS motor subscale, and MVIC. |
| Kelly et al. 2014 Level: II Quality: Weak | Test the effects of16-week high-intensity exercise, 3 days a week, on muscle mass, mitochondrial function, and physical capacity | Pre-test post-test design with non-PA subjects as control | 15 | University clinical exercise facility | QOL: PDQ-39, PD Severity: UPDRS Fatigue: FSS Sleep: PSQI Depression: BDI Gait: FOGBalance: single leg balance test Walking: 6MWT Thigh muscle mass: DXA | Improvements in PDQ-39 ADLs [23.1 (3.3) vs 15.6 (2.2)]*, emotional well-being [25.8 (4.5) vs 17.8(4.5)]*, and cognition [31.3 (5.4) vs 25.0 (4.6)]*. Favorable changes in skeletal muscle at cellular and sub-cellular level. |
| Pedreira et al. 2013 Level: I Quality: Strong | Evaluate the effects of 4-week 40-min Nintendo Wii training, 3 days per week on QOL | Single blinded RCT with traditional physical therapy as control | 44 | Clinic | QOL: PDQ-39 | Nintendo subjects showed greater improvements in PDQ-39 total score than control [total score in intervention group: 34.3(18.61) in baseline vs 24.2 (16.00) in follow up]*. Nintendo subjects also showed significant improvement in ADL [35.2 (24.42) vs 26.4 (22.88)]*, stigma [29.4 (28.46) vs 15.3 (19.22)]*, social support [18.2 (22.90) vs 8.3 (16.31)]*, and communication [26.0 (19.14) vs 17.3 (14.88)]* from baseline to 4 weeks. |
| Van Eijkeren et al. 2008 Level: II Quality: Weak | Test the effect of6-week Nordic walking, 2 times per week on physical inactivity and QOL | Single-group repeated measures design | 19 | Community | Functional fitness: TMW, TUG, 6MWT QOL: PDQ-39 | Nordic walking significantly improved TMW *, TUG*, 6MWT * at 6 weeks. There was a trend towards improvement for QOL (p=0.08) at 6weeks. The improvements persisted to 5-month follow-up. |
| Villegas, Israel, 2014 Level: I Quality:Weak | Analyze the effects of 12-week Ai-Chi, twice a week on functional activities, QOL, and posture | Nonrandomized controlled trial | 15 | Community | Functional activity: UPDRS QOL: PDQ-39 Posture: SAPO (a postural assessment software) | Intervention subjects showed significant improvement in functional activities* and posture*, but not QOL [PDQ-39 score in intervention group: 65.6 (20.6) in baseline vs 52.3 (21.7) in follow up]. |
| Westheimer et al. 2015 Level: I Quality: Moderate | Examine the effect of a 8-week dance intervention, twice a week on motor symptoms and QOL | Pre-test post-test design | 14 | Community | Motor: UPDRS, BBS, Depression: BDI QOL: PDQ-39 | Dance improved motor symptoms, especially gait* and tremor. QOL didn’t show significant change [PDQ-39 score in intervention group: 25.3(20.3) in baseline vs 25.1(17.6) in follow up]. |
| Yousef et al. 2009 Level: I Quality: Moderate | Investigate the effect of 10-week exercise, 4 times a week on ADL and QOL | Non-randomized, controlled trial | 24 | Rehabilitation Clinic | ADL: SPES/SCOPA QOL: PDQL | For PDQL, except for emotional functioning [21.1 (3.4) vs 21.5 (3.2)], the exercise group showed significant better scores than control in PD symptoms [48.3 (9.8) vs 38.7 (8.4)]**, systemic symptoms [21.3 (4.3) vs 16.5 (3.0)]**, social functioning [21.0 (3.3) vs 17.9 (3.2)]**, and total score [115.4 (22.3) vs 95.6 (15.6)]**. The exercise group also showed a significant better score than control in SPES/SCOPA**. |
| Acupuncture | ||||||
| Eng et al. 2006 Level: II Quality: Weak | Evaluate the safety and efficacy of 6-month combined tuina massage, acupuncture, and qi qong, weekly on QOL | Pre-test post-test design | 25 | University outpatient PD Center | Treatment response: CGI. PD Severity: UPDRS, HY ADL: SEADL Depression: BDI QOL: PDQ-39 | No significant improvements in objective measures. Some subjective improvements as based on BDI* and PDQ-39 [PDQ-39 score: 23.2 (16.4) in baseline vs 19.6 (13.9) in follow up]*. |
| Neuromuscular electrical stimulation | ||||||
| Heijnen et al. 2012 Level: I Quality: Strong | Investigate the effects of 3-5 week adjuvant neuromuscular electronical stimulation (NMES) on QOL | Double-blind RCT | 88 | Community | QOL: SWAL-QOL and MDADI Dysphagia severity: DSS | All groups showed significant improvement in DSS* and restricted positive effects on QOL with minimal group differences [Effect data of MDADI total in NMES at motor group: median 7]*. Results remained unchanged at 3 months. |
| Patient education | ||||||
| A'Campo et al. 2010 Level: I Quality: Moderate | Evaluate the effects of 8-weekly 90-minute session of Patient Education Program Parkinson (PEPP) on QOL and well-being | Double-blind RCT | 64 | University outpatientneurological department | QOL: PDQ-39 SI Depression: SDS | None of the effects were significant. However, there was a trend towards improvement for QOL [PDQ-39 SI score change: 3.1 (7.81) in PEPP vs -1.8 (6.73) in control]. |
| Reflexology | ||||||
| Johns et al. 2010 Level: II Quality: Weak | Evaluate the effect of 8 reflexology treatments on well-being | Pre-test post-test design | 16 | Community | QOL: PDQ-39 | Based on PDQ-39's mean impact over 65 weeks [average mean 3.00], improvements in PDQ-39 ADLs [mean 3.21], emotional well-being [mean 3.07], and cognition [mean 3.15], but not mobility [mean 2.79], stigma [mean2.85], social support [mean 2.64], communication [mean 2.50], and discomfort |
| Self-management program | ||||||
| Tickle-Degnen et al. 2010 Level: I Quality: Strong | Determine the effect of 18- and 27-hour self-management rehabilitation on QOL | Double-blindRCT with 0-hourself-managementrehabilitation ascontrol | 117 | Academic Parkinson Center and community | QOL: PDQ-39 | At 6 weeks, significant effect on PDQ-39 beyond best medical therapy [31.0 (SE1.1) in control vs 27.6 (SE1.1) in 18-hour]** / [31.0 (SE1.1) in control vs 27.3 (SE1.1) in 27-hour]**. No differences between 18 and 27 hours of intervention. |
| Spa therapy | ||||||
| Brefel-Courbon et al. 2003 Level: I Quality: Strong | Assess the impact of 3-week spa therapy on QOL, motor and psychological functions and cost | Single-blind | 31 | Neurological University Hospital | QOL: PDQ-39, SF-36. Motor function: UPDRS Psychological function: GHQ-28 Direct medical costs | Spa group showed significant improvement, compared to control, over 4 weeks on PDQ-39: stigma [15 (SE3) in spa vs 4 (SE3) in control]** and communication [10 (SE2) in spa vs 1 (SE3) in control]**, and onSF-36: physical health [-30 (SE7) in spa vs -5 (SE8) in control]** and mental health [-9 (SE2) in spa vs 3 (SE2) in control]**. Similar results are found on motor function**, psychological function**. Spa cost less**. |
Note: All values are mean (standard deviation) unless otherwise noted; SE=Standard Error; Min=Minimum Value; Max=Maximum Value
Statistically significant when compared to baseline (p< 0.05)
Statistically significant when comparing between groups (p< 0.05)
ABC: Activities-specific Balance Confidence; ADL: Activities of Daily Living; BBS: Berg Balance Scale; BDI: Beck Depression Inventory; CGI: Clinical Global Impression scale; DSS: Dysphagia Severity Scale; DXA: Dual energy X-ray Absorptiometry; FOG: Freezing of Gait; FSS: Fatigue Severity Scale; GHQ-28: General Health Questionnaire-28; HY: Hoehn and Yahr staging; MDADI: MD Anderson Dysphagia Inventory; MMSE: Mini-Mental State Examination; MVIC: Maximal Voluntary Isometric Force; NHP: Nottingham Health Profile; PD: Parkinson's Disease; PDQ-39: Parkinson's Disease Questionnaire-39; PDQL: Parkinson's Disease Quality of Life; PDQ-39 SI: Parkinson's Disease Questionnaire-39 Summary Index; PSQI: Pittsburgh Sleep Quality Index; RCT: Randomized Controlled Trial; SDS: Self-rating Depression Scale; SEADL: Schwab and England Activities of Daily Living scale; SF-36: Medical Outcomes Study 36-item Short Form; SPES/SCOPA: Short Parkinson Evaluation Scale/Scales for Outcomes in Parkinson's disease; SWAL-QOL: Swallowing Quality of Life; TMW: 10-meter walking test; TU1G: Timed up and go; UPDRS: Unified Parkinson's Disease Rating Scale; VSS: Videofuoroscopy of Swallowing Score; 6MWT: 6-minute walk test.
Table 2. Summary of the studies with QOL as a secondary outcome (n=8).
| Citation, Level & Quality | Purpose | Study Design | N | Setting | Outcomes and Measures | Results/Conclusions |
|---|---|---|---|---|---|---|
| Exercise intervention | ||||||
| Lauhoff et al. 2013 Level: I Quality: Strong | Evaluate the effect of 6-week cycle ergometry training, once a week on exercise tolerance, balance and QOL | Interrupted time-series design | 23 | Community | Exercise tolerance†: 6MWT Gait efficiency: PCI Balance: BBS Functional ability: TUG PD-related disability: UPDRS ADL and motor subscales QOL: PDQ-39 | The intervention had a significant positive effect on balance**, functional ability**, and PD-related disability**. The intervention had a trend towards improvement in PDQ-39 for the sub-categories of cognition (p=0.799), emotions (p=0.591), and bodily discomfort (p=0.512). |
| Schenkman et al. 2012 Level: I Quality: Strong | Compare short-and long-term responses to 64-week supervised fexibility/balance/ function exercise and 64-week supervised aerobic exercise programs with 64-week home-based control exercise program | Double-blind RCT | 121 | Outpatient clinic | Physical function†: CS-PFP Balance: FRT Walking economy: Oxygen uptake. PD Severity: UPDRS ADL and motor subscales. QOL: PDQ-39 | Supervised fexibility/balance/function exercise resulted in significant improvement in physical function** than supervised aerobic exercise and control at 4-month follow up. Supervised flexibility/ balance/function exercise resulted in significant improvement in PD severity** than control at 4 and 16-month follow up. Walking economy** significantly improved in supervised aerobic exercise compared with supervised flexibility/balance/function exercise at 4, 10, and 16-month follow up. No group difference in balance and QOL measures at any follow up periods. |
| Volpe et al. 2013 Level: I Quality: Strong | Evaluate the feasibility of 24-week Irish set dancing, once a week, compared with routine physiotherapy | Single-blind pilot RCT | 24 | Rehabilitation facility | Motor disability†: UPDRS motor subscale Balance: BBS Gait: FOG QOL: PDQ-39 | The intervention group showed significantly improvement in motor disability** and gait**, which was more pronounced than the control group. There were trends towards improvements in balance and QOL [PDQ-39 score in intervention group: 30.6 (12.06) in baseline vs 22.2 (10.18) in follow up]. |
| Cognitive training | ||||||
| Paris et al. 2011 Level: I Quality: Strong | Analyze the efficacy of 4-week cognitive training, 3 times a week on cognition and QOL | Double-blinded multi-center RCT | 33 | Rehabilitation facility | Cognition†: WAIS III Digit Span Logical Memory, CVLT II, SDMT, TMT, Stroop, ROCFT (Recalls, Copy), RBANS-Line Orientation, FAS, TOL QOL: PDQ-39 Mood: GDS-15 | Cognitive training resulted in significant improvement in some cognitive performances than control. None of the effects were significant for QOL and mood in both groups [PDQ-39 score in intervention group: 47.9 (21.36) in baseline vs 50.3 (26.72) in follow up]. |
| Cueing training | ||||||
| Nieuwboer et al. 2007 Level: I Quality: Strong | Investigate the efficacy of 6-week home-based cueing program on gait, gait-related activity and QOL | Single-blind randomized crossover design | 153 | Community | Posture and gait†: UPDRS gait and balance items Balance: FOG, TUG Activity: NEADL, FES QOL: PDQ-39, CSI | Gait* and balance* improved significantly after the intervention. The intervention had a trend towards improvement in gait-related activity and QOL [In PDQ-39, intervention β estimate: -1.4 (SE1.14), p=.23]. |
| Music therapy | ||||||
| Pohl et al. 2013 Level: I Quality: Strong | Examine the feasibility and effects of 6-week music therapy (Ronnie Gardniner Rhythm and Music Method), twice a week on mobility, cognition, and QOL | Single-blinded RCT | 18 | Neurological Rehabilitation Center | Mobility†: PLM, TUG, and UPDRS motor-subscale. Cognition: text recall test, SDMT, Clox and Cube, Naming, Stroop QOL: PDQ-39 | No significant difference between groups. Suggestion of improvement in mobility, cognition and QOL [median PDQ-39 score difference between baseline and follow up in intervention group: -3.6]*. |
| Neuromuscular therapy | ||||||
| Craig et al. 2006 Level: I Quality: Strong | Determine the effects of 4-week neuromuscular therapy, twice a week on motor and non motor symptoms | Single-blinded RCT with control group receiving music relaxation therapy | 36 | University clinic facility | Motor symptoms†: UPDRS Part III (motor subscale) Treatment response: CGI QOL: PDQ-39 Depression: BDI Anxiety: BAI | Neuromuscular therapy resulted in significant improvement in motor symptoms** than control. No group difference in non-motor measures. Compared to baseline, intervention subjects showed significant improvement in QOL in 4-week [37.8 (22.20) vs 29.8 (18.84)]*, but not in 5-week follow up [37.8 (22.20) vs 32.2 (25.69)]. |
| Physiotherapy network | ||||||
| Munneke et al. 2010 Level: I Quality: Strong | Investigate the effects of 24-week ParkinsonNet on health-care costs and health outcomes | Double-blinded clusterRCT | 699 from 1 clusters | 6 Community clusters | Gait, balance, transfers, grasping, and physical capacity†: PSI-PD Functional mobility: M-PAS Mobility-related QOL: PDQ-39's mobility domain Costs: questionnaire based on micro costing | Primary outcome similar for cluster vs usual care participant. Secondary endpoint showed change in total health care costs** for cluster care participants, otherwise no change. There was no difference in QOL between the cluster and the usual care participants at all endpoints. |
indicating the primary outcome
Note: All values are mean (standard deviation) unless otherwise noted; SE=Standard Error
Statistically significant when compared to baseline (p<0.05)
Statistically significant when comparing between groups (p<0.05)
ADL: Activities of Daily Living; BAI: Beck Anxiety Inventory; BBS: Berg Balance Scale; BDI: Beck Depression Inventory; CGI: Clinical Global Impression scale; CS-PFP: Continuous Scale-Physical Functional Performance; CSI: Carer Strain Index; CVLT II: California Verbal Learning Test II; FES: Falls Efficacy Scale; FOG: Freezing of Gait; FRT: Functional Reach Test; GDS-15: Geriatric Depression Scale 15; M-PAS: Modified Parkinson Activity Scale; NEADL: Nottingham Extended Activities of Daily Living; PCI: Physiological Cost Index; PD: Parkinson's Disease; PDQ-39: Parkinson's Disease Questionnaire-39; PLM: Posturo-Locomotion-Manual; PSI-PD: Patient-Specifc Index for Parkinson's Disease; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; RCT: Randomized Controlled Trial; ROCFT: Rey-Osterrieth Complex Figure Test; SDMT: Symbol Digit Modalities Test; TOL: Tower of London; TUG: Timed up and go; UPDRS: Unifed Parkinson's Disease Rating Scale; WAIS III: Wechsler Adult Intelligence Scale III; 6MWT: 6-minute walk test.
Results
The studies were conducted in several countries, including France (n=1), Iran (n=1), Australia (n=1), Spain (n=1), Sweden (n=1), Turkey (n=1), Ireland (n=1), Belgium (n=1), Italy (n=1), United Kingdom (n=1), Brazil (n=3), Netherlands (n=4), and the United States (n=9). Twenty studies have Level I evidence and six Level II evidence. Thirteen of the 26 studies (50%) were rated with strong quality, 4 (15%) with moderate quality, and 9 (35%) with weak quality (Tables 1 and 2). In the following sections, we described the types of interventions tested, the QOL instruments used, and the effects of the interventions on QOL.
Types of the interventions tested
Fifteen studies (57.7%) tested an exercise intervention [14-28], and the remaining 11 studies each evaluated a different intervention including acupuncture [29], neuromuscular electrical stimulation [30], patient education [31], reflexology [32], self-management program [33], spa therapy [34], cognitive training [35], cueing training [36], music therapy [10], neuromuscular therapy [37], and physiotherapy network [38]. Eighteen of the 26 studies (69.2%) measured QOL asa primary outcome [14-20,22,24,25,27-34], while the remainders measured it as secondary outcomes [10,21,23,26,35-38].
QOL instruments used
Six different QOL instruments were used across the 26 studies. Twenty studies (77%) used the Parkinson's Disease Questionnaire 39 (PDQ-39) [10,17,19-27,29,31-38]. The PDQ-39 is a 39-item self-report questionnaire with a 5-point Likert-type scale, representing 8 domains (Mobility, Activities of Daily Living, Emotional Well-Being, Stigma, Social Support, Cognitions, Communication, and Bodily Discomfort). The PDQ-39 Summary Index (PDQ-39SI) is an overall score and expressed as a percentage ranging from 0 to 100. Lower scores indicate better QOL [39].
Four studies used the PD QOL scale (PDQL) [15,16,18,28]. The PDQL is a self-administered questionnaire on a 5-point Likert scale and contains 4 domains (Parkinson Symptoms, Systemic Symptoms, Social Functioning, and Emotional Functioning). Higher scores indicating better perceived QOL [40].
Two studies used the Swallowing QOL (SWAL-QOL) scale [14,30]. The SWAL-QOL measures swallowing-related QOL, including 44 items. It contains 11 domains, including burden, symptom frequency, food selection, eating duration, eating desire, communication, fear, mental health, social functioning, fatigue, and sleep. Higher scores indicates better perceived swalloing-related QOL [41].
Effects of the interventions on QOL
Fifteen of the 26 studies (58%) showed that the interventions significantly improved QOL [10,14-16,18-20,22,28-30,32-34,37], while the remaining 11 studies (42%) reported no effect [17,21,23-27,31,35,36,38]. Among the 12 different types of interventions tested, exercise intervention was examined by 15 studies and the other 11 interventions were only tested by one study.
Exercise intervention
Twelve of the 15 exercise studies treated QOL as a primary outcome (Table 1): 8 studies are level I evidence [16-19,22,25,27,28], and 4 are level II evidence [14,15,20,24]. Seven studies (58%) have weak quality [14,15,18-20,24,25] because of the lack of randomizationand blinding, 3 (25%) have moderate quality due to lack of randomization or blinding [17,27,28], and 2 (17%) have strong quality [16,22].
The modality of exercise included swallowing exercise [14], boxing [15,16], physiotherapist-supervised exercise [18], high intensity resistance exercise [19], combined strength, endurance, and/or balance exercises [17,20,28], Nintendo Wii [22], Nordic walking [24], Ai-Chi [25], and dance [27]. The duration of exercise interventions varied from 4 weeks [22], 5 weeks [14], 6 weeks [24], 8 weeks [27], 10 weeks [18,28] to 16 weeks [20] with 12 weeks as the most used duration [15-17,19,25]. The frequency of exercise was 2 [17,24,25,27], 3 [15,16,18-20,22], 4 [28], or 5 times a week [14]. Eight out of the 12 studies (67%) showed significant improvement in QOL [14-16,18-20,22,28].
The three exercise studies that treated QOL as a secondary outcome were all level I evidence with strong quality. The durations of the exercise interventions varied from 6 [21] to 24 [26] and 64 weeks [23]. However, none of these studies showed an effect on QOL (Table 2).
Acupuncture
One study investigated the effect of a weekly acupuncture therapy with massage for 6 months on QOL as a primary outcome (Table 1). This study is level II evidence and has weak quality due to the lack of randomization and blinding. QOL was significantly improved at 6 months compared to baseline [29].
Neuromuscular electrical stimulation
A study tested the effect of 3-5 weeks of adjuvant neuromuscular electrical stimulation, 5 times a week on QOL as a primary outcome [30]. This study is level I evidence and has strong quality. All groups improved their QOL over time with minimal group differences (Table 1).
Patient education
One study evaluated the effect of 8 weekly sessions of the Patient Education Program Parkinson (PEPP) on QOL [31]. This study is level I evidence and has moderate quality. Compared to the control group, PEPP showed a trend towards significance on QOL, a primary outcome (Table 1).
Reflexology
One study examined the effect of 8 reflexology treatments (acupressure) on QOL as a primary outcome [32]. This study is level II evidence and has weak quality due to the lack of randomization and blinding. The results showed within-group improvement in QOL, but not between-group differences (Table 1).
Self-management program
A self-management program was tested using 2 different doses of 18 hours and 27 hours over 6 weeks for its effect on QOL as a primary outcome [33]. This study is level I evidence and has strong quality. Compared to a control group, both doses improved QOL significantly (Table 1).
Spa therapy
One study evaluated the effect of a 3-week daily spa therapy (thermal baths, drinking mineral water, various types of showers, and underwater massage) on QOL as a primary outcome (Table 1). This study is level I evidence and has strong quality. Spa therapy improved QOL significantly compared to control [34].
Cognitive training
One study tested the effect of interactive multimedia and paper-and-pencil-based cognitive training program, 3 times a week for 4 weeks on QOL as a secondary outcome [35]. This study is level I evidence and has strong quality. The results showed that cognitive training had no significant effect on QOL (Table 2).
Cueing training
One study tested the effect of a 6-week cueing training that contains 3 rhythmical cueing modalities (auditory, visual, and somatosensory) on QOL as a secondary outcome [36]. This study is level I evidence and has strong quality. The results showed that cueing training had no significant effect on QOL (Table 2).
Music therapy
One study tested the effect of a 6-week, twice a week music therapy that contains stretching and some rhythmical movements on QOL as a secondary outcome [10]. This study is level I evidence and has strong quality. The results indicated that music therapy significantly improved QOL (Table 2).
Neuromuscular therapy
One study examined the effect of a 4-week, twice a week neuromuscular therapy on muscle pain and spasm (primary outcomes) as well as QOL (secondary outcome) [37]. This study is level I evidence and has strong quality. The results indicated that QOL improved over the 4-week period for the intervention group, but there was no significant difference between the 2 groups (Table 2).
Physiotherapy network
Munneke et al. used a physiotherapy network named Parkinson Net to train physiotherapists and facilitate communication between health professionals and persons with PD (Table 2). This study is level I evidence and has strong quality. Compared to the control group, Parkinson Net did not show effects on improving QOL, a secondary outcome [38].
Discussions
The main findings from our review are that 77% of the 26 included studies represented ‘Level I’ (RCT or experimental designs) and 23% ‘Level II’ (quasi-experimental designs) evidence. About 50% of the studies have ‘strong quality’, 15% ‘moderate quality’ and 35% ‘weak quality’. The main reasons for moderate or weak quality were the lack of randomization and blinding.
Eighteen of studies (69%) tested the intervention effects on QOL as a primary outcome and 8 (31%) of studies treated QOL as a secondary outcome. In addition, a variety of non-pharmacological interventions have been tested, including exercise, acupuncture, neuromuscular electrical stimulation, patient education, reflexology, self-management program, spa therapy, cognitive training, cueing training, music therapy, neuromuscular therapy, and physiotherapy network. Except for 15 studies that evaluated an exercise intervention, only one study tested each one of the other 11 interventions.
The findings from our review suggest that 8 (53%) exercise intervention studies indicated that exercise were effective at improving QOL in PD in contrast to findings from the other 7 (47%) including three studies measuring QOL as a secondary outcome. The discrepant findings seem to be influenced by several factors: 1) whether QOL was treated as a primary or secondary outcome: Studies that treated QOL as a primary outcome were more likely to have a suffcient large sample size and adequate power to detect a significant effect on QOL, whereas studies that evaluated QOL as a secondary outcome were not even if they were well designed; 2) the intervention mode and dose: There were substantial variations in the mode and dose of the exercise interventions across studies [14-28]. The dose-response relationships between exercise and QOL in PD have not been studied; 3) confounding factors: The intervention effects were further affected by sample characteristics, making the study findings less comparable. For example, the PD stages, duration, and drug regimen were quite heterogeneous across studies.
Furthermore, combined exercise may be more promising than a single mode exercise intervention. One study showed that 6-week music therapy combined with rhythmical dance of an hour, twice a week dose improved QOL [10]. Recently, dance programs like tango have been introduced [42,43], showing increased social contacts and cognition over time. These results suggest that music with physical movements can improve non-motor symptom (e.g., cognition) better than either component alone, which needs to be further tested in research with an aim to enhance QOL.
There are emerging but very limited evidence about the effects of other non-pharmacological interventions on QOL in persons with PD. Spa therapy [34], neuromuscular therapy [37], reflexology [32], and acupuncture [29] induced significant improvements of QOL in persons with PD. All these studies included a massage as an intervention element, but each study used a different PD sample: Spa therapy (n=31, mean age=67, mean PD duration=12 years), neuromuscular therapy (Intervention group: n=18, mean age=62, mean Hoehn & Yahr score=1.8), reflexology (n=16), and acupuncture (n=25, mean age=69, mean PD duration=6 years, mean UPDRS motor-subscale=24). Their findings are further limited because the reflexology [32] and acupuncture [29] used quasi-experimental designs without a control group. Furthermore, neuromuscular electrical stimulation [30], and self-management intervention improved QOL [33]. However, cognitive training [35], physiotherapy networks intervention [38], and patient education [31] did not show any benefits to PD symptoms and QOL. Similar to the findings of exercise studies, the results of these non-pharmacological interventions were likely affected by its design of considering QOL as a primary or secondary outcome.
Strengths and Limitations
This review has two notable strengths. First, it comprehensively examined the non-pharmacological interventions and their effects on QOL in persons with PD. Second, it used a strong method of conducting the literature search and review. This review is limited by the scope of literature search. We only searched three databases (PubMed, CINAHL and PsycINFO) where non-pharmacological interventions are most likely published. Although we did perform manual literature search via cross-referencing, it is possible that we have missed some relevant publications.
Implications for Research and Practice
Given the fact that only one study met the eligibility criteria for 11 of the 12 non-pharmacological interventions, there is a pressing need to increase the sheer volume of experimental studies to test out these interventions. Our findings suggest that randomization and blinding are the two major factors to consider in order improving the study quality. Future studies need to balance internal validity and generalizability. For example, limiting the study sample to early stage PD improves the sample homogeneity and internal validity, but reduces the generalizability of study findings to other Parkinson's stages. Design considerations are essential in future studies to help determine the minimally effective and optimal doses of the non-pharmacological interventions. Future research should build on this literature to further refine the interventions and test the minimally-effective and optimal doses as well as the dose-response relationships of each intervention for improving QOL across different stages of PD.
Despite the methodological concerns about existent studies, emerging evidence supports that non-pharmacological interventions likely play a role in improving QOL in persons with PD in clinical practice. Clinicians should become aware of the variety of non-pharmacological interventions and use a person-centered and tailored approach to select the non-pharmacological interventions and their doses most suitable to the patient at hand.
Conclusion
QOL is increasingly recognized as an essential outcome in PD intervention research. A variety of non-pharmacological interventions is potentially effective on improving quality of life and has been studied by at least one experimental study. Exercise is the most examined intervention. There is emerging evidence that other non-pharmacological interventions are potentially promising for improving QOL, but research is very limited currently. Little is known about the minimally effective and optimal doses of the non-pharmacological interventions.
Acknowledgments
The conception and initial development of this work was supported by the Edmond J. Safra Visiting Nursing Faculty Program award from the Parkinson's Disease Foundation that was awarded to TB and FY. This work was also supported by the National Institute on Aging of the National Institutes of Health Award Number 1R01AG043392-01A1 awarded to FY. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 2.American Parkinson Disease Association. Understanding the basics of PD 2015 [Google Scholar]
- 3.Parkinson's Disease Foundation. Statistics on Parkinson's 2016 [Google Scholar]
- 4.Beitz JM. Parkinson's disease: a review. Front Biosci (Schol Ed) 2014;6:65–74. doi: 10.2741/s415. [DOI] [PubMed] [Google Scholar]
- 5.Fritsch T, Smyth KA, Wallendal MS, Hyde T, Leo G, et al. Parkinson disease: research update and clinical management. South Med J. 2012;105:650–656. doi: 10.1097/SMJ.0b013e318273a60d. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Choi M, Jung D, Sohn YH, Hong J. A structural model of health-related quality of life in Parkinson's Disease patients. Western J Nurs Res. 2015;37:1062–1080. doi: 10.1177/0193945914528588. [DOI] [PubMed] [Google Scholar]
- 7.Foppa AA, Chemello C, Vargas-Pelaez CM, Farias MR. Medication therapy management service for patients with Parkinson's disease: A before-and-after study. Neurol Ther. 2016;5:85–99. doi: 10.1007/s40120-016-0046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salat D, Tolosa E. Levodopa in the treatment of Parkinson's disease: current status and new developments. J Parkinsons Dis. 2013;3:255–269. doi: 10.3233/JPD-130186. [DOI] [PubMed] [Google Scholar]
- 9.Stacy M. Medical treatment of Parkinson disease. Neurol Clin. 2009;27:605–631. doi: 10.1016/j.ncl.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Pohl P, Dizdar N, Hallert E. The Ronnie Gardiner Rhythm and Music Method - a feasibility study in Parkinson's disease. Disabil Rehabil. 2013;35:2197–2204. doi: 10.3109/09638288.2013.774060. [DOI] [PubMed] [Google Scholar]
- 11.Uitti RJ. Treatment of Parkinson's disease: focus on quality of life issues. Parkinsonism Relat Disord. 2012;18 Suppl 1:S34–S36. doi: 10.1016/S1353-8020(11)70013-X. [DOI] [PubMed] [Google Scholar]
- 12.Garrard J. Health sciences literature review made easy: The matrix method. 3rd. Jones & Bartlett; Publishers, Massachusetts, USA: 2010. [Google Scholar]
- 13.Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1:176–184. doi: 10.1111/j.1524-475X.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- 14.Argolo N, Sampaio M, Pinho P, Melo A, Nobrega AC. Do swallowing exercises improve swallowing dynamic and quality of life in Parkinson's disease? NeuroRehabilitation. 2013;32:949–955. doi: 10.3233/NRE-130918. [DOI] [PubMed] [Google Scholar]
- 15.Combs SA, Diehl MD, Staples WH, Conn L, Davis K, et al. Boxing training for patients with Parkinson disease: a case series. Phys Ther. 2011;91:132–142. doi: 10.2522/ptj.20100142. [DOI] [PubMed] [Google Scholar]
- 16.Combs SA, Diehl MD, Chrzastowski C, Didrick N, McCoin B, et al. Community-based group exercise for persons with Parkinson disease: a randomized controlled trial. NeuroRehabilitation. 2013;32:117–124. doi: 10.3233/NRE-130828. [DOI] [PubMed] [Google Scholar]
- 17.Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, et al. Exercise and Parkinson's: benefits for cognition and quality of life. Acta Neurol Scand. 2011;123:13–19. doi: 10.1111/j.1600-0404.2010.01338.x. [DOI] [PubMed] [Google Scholar]
- 18.Dereli EE, Yaliman A. Comparison of the effects of a physiotherapist-supervised exercise programme and a self-supervised exercise programme on quality of life in patients with Parkinson's disease. Clin Rehabil. 2010;24:352–362. doi: 10.1177/0269215509358933. [DOI] [PubMed] [Google Scholar]
- 19.Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves Quality Of Life in persons with Parkinson's disease: a preliminary study. Parkinsonism Relat Disord. 2009;15:752–757. doi: 10.1016/j.parkreldis.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Kelly NA, Ford MP, Standaert DG, Watts RL, Bickel CS, et al. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson's disease. J Appl Physiol (1985) 2014;116:582–592. doi: 10.1152/japplphysiol.01277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauhoff P, Murphy N, Doherty C, Horgan NF. A controlled clinical trial investigating the effects of cycle ergometry training on exercise tolerance, balance and quality of life in patients with Parkinson's disease. Disabil Rehabil. 2013;35:382–387. doi: 10.3109/09638288.2012.694962. [DOI] [PubMed] [Google Scholar]
- 22.Pedreira G, Prazeres A, Cruz D, Gomes I, Monteiro L, et al. Virtual games and quality of life in Parkinson's disease: A randomised controlled trial. Adv Parkinsons Dis. 2013;2:97–101. [Google Scholar]
- 23.Schenkman M, Hall DA, Baron AE, Schwartz RS, Mettler P, et al. Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther. 2012;92:1395–1410. doi: 10.2522/ptj.20110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Eijkeren FJ, Reijmers RS, Kleinveld MJ, Minten A, Bruggen JP, et al. Nordic walking improves mobility in Parkinson's disease. Mov Disord. 2008;23:2239–2243. doi: 10.1002/mds.22293. [DOI] [PubMed] [Google Scholar]
- 25.Villegas IL, Israel VL. Effect of the Ai-Chi method on functional activity, quality of life, and posture in patients with Parkinson Disease. Topics Geriatr Rehabil. 2014;30:282–289. [Google Scholar]
- 26.Volpe D, Signorini M, Marchetto A, Lynch T, Morris ME. A comparison of Irish set dancing and exercises for people with Parkinson's disease: a phase II feasibility study. BMC Geriatr. 2013;13:54. doi: 10.1186/1471-2318-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westheimer O, McRae C, Henchcliffe C, Fesharaki A, Glazman S, et al. Dance for PD: a preliminary investigation of effects on motor function and quality of life among persons with Parkinson's disease (PD) J Neural Transm (Vienna) 2015;122:1263–1270. doi: 10.1007/s00702-015-1380-x. [DOI] [PubMed] [Google Scholar]
- 28.Yousef B, Tadibi V, Khoei AF, Montazeri A. Exercise therapy, quality of life, and activities of daily living in patients with Parkinson disease: a small scale quasi-randomised trial. Trials. 2009;10:67. doi: 10.1186/1745-6215-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eng ML, Lyons KE, Greene MS, Pahwa R. Open-label trial regarding the use of acupuncture and yin tui na in Parkinson's disease outpatients: a pilot study on efficacy, tolerability, and quality of life. J Altern Complement Med. 2006;12:395–399. doi: 10.1089/acm.2006.12.395. [DOI] [PubMed] [Google Scholar]
- 30.Heijnen BJ, Speyer R, Baijens LW, Bogaardt HC. Neuromuscular electrical stimulation versus traditional therapy in patients with Parkinson's disease and oropharyngeal dysphagia: effects on quality of life. Dysphagia. 2012;27:336–345. doi: 10.1007/s00455-011-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.A'Campo LE, Wekking EM, Spliethoff-Kamminga NG, Le Cessie S, Roos RA. The benefits of a standardized patient education program for patients with Parkinson's disease and their caregivers. Parkinsonism Relat Disord. 2010;16:89–95. doi: 10.1016/j.parkreldis.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Johns C, Blake D, Sinclair A. Can reflexology maintain or improve the well-being of people with Parkinson's Disease? Complement Ther Clin Pract. 2010;16:96–100. doi: 10.1016/j.ctcp.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Tickle-Degnen L, Ellis T, Saint-Hilaire MH, Thomas CA, Wagenaar RC. Self-management rehabilitation and health-related quality of life in Parkinson's disease: a randomized controlled trial. Mov Disord. 2010;25:194–204. doi: 10.1002/mds.22940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brefel-Courbon C, Desboeuf K, Thalamas C, Galitzky M, Senard JM, et al. Clinical and economic analysis of spa therapy in Parkinson's disease. Mov Disord. 2003;18:578–584. doi: 10.1002/mds.10404. [DOI] [PubMed] [Google Scholar]
- 35.Paris AP, Saleta HG, de la Cruz Crespo Maraver M, Silvestre E, Freixa MG, et al. Blind randomized controlled study of the efficacy of cognitive training in Parkinson's disease. Mov Disord. 2011;26:1251–1258. doi: 10.1002/mds.23688. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–140. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craig LH, Svircev A, Haber M, Juncos JL. Controlled pilot study of the effects of neuromuscular therapy in patients with Parkinson's disease. Mov Disord. 2006;21:2127–2133. doi: 10.1002/mds.21132. [DOI] [PubMed] [Google Scholar]
- 38.Munneke M, Nijkrake MJ, Keus SH, Kwakkel G, Berendse HW, et al. Efficacy of community-based physiotherapy networks for patients with Parkinson's disease: a cluster-randomised trial. Lancet Neurol. 2010;9:46–54. doi: 10.1016/S1474-4422(09)70327-8. [DOI] [PubMed] [Google Scholar]
- 39.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res. 1995;4:241–248. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 40.de Boer AG, Wijker W, Speelman JD, de Haes JC. Quality of life in patients with Parkinson's disease: development of a questionnaire. J Neurol Neurosurg Psychiatry. 1996;61:70–74. doi: 10.1136/jnnp.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McHorney CA, Bricker DE, Kramer AE, Resenbek JC, Robbins J, et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: I. Conceptual foundation and item development. Dysphagia. 2000;15:115–121. doi: 10.1007/s004550010012. [DOI] [PubMed] [Google Scholar]
- 42.Foster ER, Golden L, Duncan RP, Earhart GM. Community-based Argentine tango dance program is associated with increased activity participation among individuals with Parkinson's disease. Arch Phys Med Rehabil. 2013;94:240–249. doi: 10.1016/j.apmr.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKee KE, Hackney ME. The effects of adapted tango on spatial cognition and disease severity in Parkinson's disease. J Mot Behav. 2013;45:519–529. doi: 10.1080/00222895.2013.834288. [DOI] [PMC free article] [PubMed] [Google Scholar]


