Abstract
Cell-cell communication is critical to the development, maintenance, and function of multicellular organisms. Classical mechanisms for intercellular communication include secretion of molecules into the extracellular space and transport of small molecules through gap junctions. Recent reports suggest that cells also can communicate over long distances via a network of transient intercellular nanotubes. Such nanotubes have been shown to mediate intercellular transfer of organelles as well as membrane components and cytoplasmic molecules. Moreover, intercellular nanotubes have been observed in vivo and have been shown to enhance the transmission of pathogens such as human immunodeficiency virus (HIV)-1 and prions in vitro. These studies indicate that intercellular nanotubes may play a role both in normal physiology and in disease.
Classical mechanisms of intercellular communication include those that are relatively well characterized such as the secretion of molecules (e.g., neurotransmitters or chemokines) into the extracellular space, and the transport of small molecules through gap junctions formed between neighboring cells. In addition, it has been suggested that cells outside the nervous system can communicate via long cellular extensions. For example, long and dynamic filopodia have been observed to extend from developing sea urchin embryos1 and Kornberg et al. observed filopodia-like cytoplasmic extensions of cells, which they termed cytonemes, in Drosophila wing imaginal discs2 (Figure 1.1). These studies suggest that some of the signaling previously thought to be mediated by diffusible signals may instead be the result of direct interactions mediated by cellular extensions.3
Figure 1.
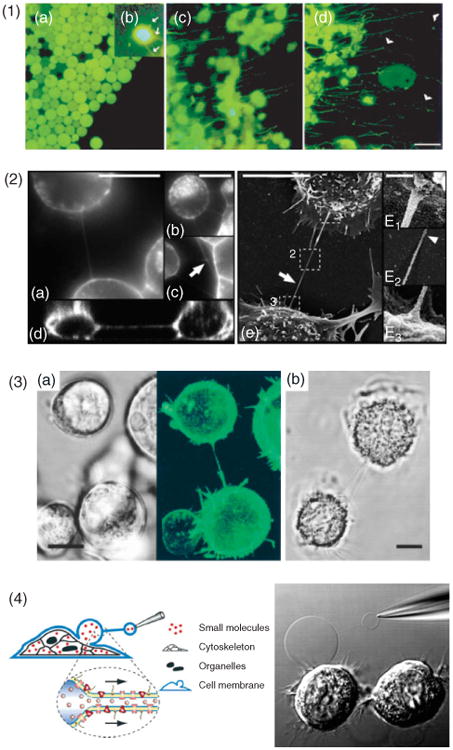
Intercellular nanotubes. (1) Growing cellular nanotubes (cytonemes) in culture of fragments cut from Drosophila wing discs. Fluorescence microscopy initially revealed round green fluorescent protein (GFP)-containing cells, (a), but after approximately 40 min in culture, multiple small processes containing GFP emerged (green). (b), These processes extended and retracted rapidly. After approximately 60 min, the cells extended long, GFP-containing processes, (c, d). The cytonemes were found to grow unidirectionally toward C-fragment cells (nonfluorescent) (d, arrowheads). Scale bar, 10 μm. (Reprinted with permission from Ref Ref 2. Copyright 1999 Elsevier). (2) The architecture of tunneling nanotubes (TNTs) between cultured rat pheochromocytoma (PC12) cells was analyzed by 3D live-cell microscopy. Cells were connected with surrounding cells via one, (a), or several TNTs, (b). Rarely, branched TNTs were observed (c, arrow). A selected (x–z) section obtained from a confocal 3D reconstruction illustrates how TNTs do not adhere to the substrate, (d), scale bar, 15 μm. (e), The ultrastructure of TNTs in PC12 cells was analyzed by scanning electron microscopy, scale bar, 10 μm, E1-F3: 200 nm. (Reprinted with permission from Ref 4. Copyright 2004 AAAS). (3) Membrane nanotubes connect immune cells. A nanotube connecting Epstein–Barr virus-transformed human B-cells (a) where the fluorescence image (right) shows emission from glycosylphosphatidylinositol anchored GFP (green). (b) Membrane nanotube between two primary human macrophages. Scale bar, 10 μm. (Reprinted with permission from Ref 5. Copyright 2004 AAI). (4) An artificial cellular nanotube with schematic representation showing the formation of a daughter vesicle from a cell membrane bleb. Membrane blebs were induced in NG108-15 cells by a combination of DTT and formaldehyde; subsequently, one bleb was electroinjected with a buffer-filled micropipette. Following translation of the micropipette, a nanotube connection was formed and the injected buffer led to the formation of a daughter vesicle at the micropipette tip. Organelles and cytoskeletal structures remained within the cell, while the bleb most likely enclosed low molecular weight cytosolic molecules. Both the bleb and the daughter vesicle have membrane proteins embedded in the membrane. (Adapted with permission from Ref 6. Copyright 2007 ACS).
Long-range cell–cell communication between mammalian cells has recently been complemented with a newly discovered class of intercellular structures. Rustom et al. reported that cultured neuronal rat pheochromocytoma cells (PC12) cells could be connected by tubular structures containing membrane and filamentous (F) actin4 (Figure 1.2). These structures were suspended in the medium between connected cells and, thus, did not rest on the substratum. Furthermore, they were shown to mediate the transfer of subcellular organelles between cells by actin-dependent mechanisms and membrane-bound proteins were seen diffusing between cells, suggesting a seamless transition of the plasma membranes of the two connected cells. Because of the unexpected properties displayed by these tubular structures, they were initially called tunneling nanotubes.
Since the first report, it has become clear that similar structures, here collectively called intercellular nanotubes (ICNs), readily form between a variety of cell types, including natural killer (NK) cells and Epstein–Barr virus (EBV)-transformed B-cells5 (Figure 1.3), cultured DU154 prostate cancer cells,7 THP-1 monocytes,8 endothelial progenitor cells, rat cardiac myocytes,9 human and murine macrophages,10,11 and astrocytes.12 Studies have shown that ICNs can support transfer of organelles,4,9,13 membrane-bound components, or cytoplasmic molecules.8,14 Furthermore, the growing number of reports implicating ICNs as pathways for pathogens, such as bacteria,11 virus,15–17 and prions18 indicates that ICNs may also play a role in disease. The possibility that ICNs are only a consequence of in vitro cell culture conditions has been ruled out by recent observations of ICNs in vivo.19
Prior to the description of ICNs, artificial nanotube–vesicle networks (NVN) had been reported in liposome systems20 and it was demonstrated that NVNs could be functionalized with membrane proteins.21 More recently, it was shown that NVNs could be constructed directly from the plasma membrane of cultured cells6 (Figure 1.4). The similarities between cellular and artificial nanotubes suggest that NVNs could act as model systems to study some aspects of the formation and transport mechanisms of ICNs.22
The mechanisms for cell–cell communication supported by nanotubes are still poorly understood. Studies have revealed a substantial heterogeneity in structure, formation process, and functional properties.17,23–25 There is also some confusion regarding the terminology and definitions of ICNs, illustrating the immaturity of this research field. In this review, we discuss the literature of these cellular structures, nanotubular support for long-range intercellular communication, and other functional roles of intercellular nanotubes in biology. We also cover what can be learned from artificial lipid model systems and describe new ultrasensitive analytical techniques that could provide insight into their compositions and functions. We start by reviewing the recent literature suggesting a biological function of ICNs.
Biological Function
Signaling Across ICN
Watkins and Salter recently demonstrated that ICNs could facilitate calcium signaling between myeloid cells8 (Figure 9.1). Calcium fluxes influence many cellular events and are known to be transmitted between adjacent cells through gap junctions or through autocrine activity of secreted adenosine triphosphate. Coordinated intercellular calcium oscillations offer a rapid mechanism of local intercellular communication and are thought to be critical in synchronizing cellular activities. Watkins and Salter8 observed that dendritic cells (DCs) at the receiving end of signals delivered by nanotubes rapidly underwent morphological changes, demonstrating how important messages might be delivered through ICNs. A physical disruption of ICNs between the cells prevented cell-to-cell transmission of calcium fluxes, indicating that, at least in some situations, ICNs can function as an alternative to established intercellular calcium signaling pathways. Based on the speed by which the calcium flux propagates, action potential was ruled out. Instead, as is the case for gap junction-dependent intercellular calcium transmission,26 ICNs may allow cell-to-cell transport of the second messenger inositol trisphosphate (IP3), which induces calcium release from internal stores. Here, heterogeneity among different cell types is apparent as, for example, ICNs between T-cell do not facilitate intercellular calcium signaling.17
Figure 9.
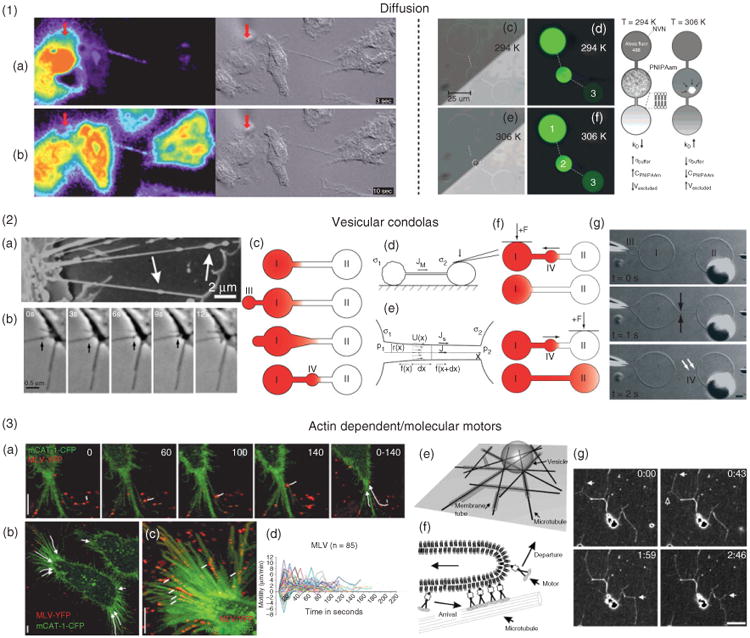
Transport of cargo in intercellular nanotubes (ICNs). (1) Diffusional translocation of molecules is effective on the cellular scale. (a) and (b), Time sequence of the spread of calcium signal (pseudocolored) on the left and the corresponding differential interference contrast image on the right. (Reprinted with permission from Ref 8. Copyright 2005 Elsevier). (c–g), Model systems for studies of diffusional effects in a nanotube–vesicle network (NVN). Diffusive transport rates were controlled with a thermoactuated hydrogel valve (in container 2). (Adapted with permission from Ref 95. Copyright 2008 ACS). (2) Constitutive membrane flow and Marangoni transport are tightly linked to actin polymerization and membrane tension. (a), Vesicular gondolas formed as an integral part of ICNs in the human urothelial cell line. (b), Fusion of a gondola (arrows) with a cell body, showing directional movement of the gondola along a nanotube. (Adapted with permission from Ref 51. Copyright 2008 Elsevier). Gondola formation in NVN systems. (c), Schematic for the formation of nanotube-integrated mobile vesicles in NVNs. (d), A difference of membrane tension induces Marangoni transport between vesicles: by pressing a vesicle with a microfiber, a flow toward the tense vesicle is created (σ2 > σ1). (e), Stationary state of a lipid tube connecting tense and floppy vesicles. (f), By selective manipulation of a node in NVNs, introduced gondolas can be delivered to a vesicle of choice. (g), Time-sequences of the formation of nanotube-integrated mobile vesicles. (3) Transportation along and elongation of nanotubes are regulated by actin polymerization and molecular motors. (a–d), Virus cell surfing is actin and myosin II dependent. (Reprinted with permission from Ref 35. Copyright 2005 originally published in JCB). (e–g), An example where microtubule connected membrane tubes are formed from giant vesicles by dynamic association of motor proteins (Reprinted with permission from Ref 96. Copyright 2006 National Academy of Sciences).
ICN-mediated Protein Transfer
Another potential functional role of ICNs could be to mediate the exchange of proteins between cells.27 It has also been shown by Niu et al.28 that intercellular transfer of proteins, lipid transfer, and cytoplasmic component transfer can occur simultaneously and that a direct cell–cell contact is required. Several reports have shown that immune cells can swap proteins during transient encounters.29,30 For example, T-cells can acquire antigen from target cells, making them susceptible to being killed by other cytotoxic T lymphocytes (CTL), in a mechanism that has been termed fratricide killing.31 Similarly, it has recently been shown that NK cells that have acquired activating ligands during encounters with target cells can trigger activation in other NK cells.101 The mechanisms for membrane transfer are largely unknown, as discussed in recent reviews.24,32 Potential routes of transfer include membrane bridges that have been observed in the immune synapse between CTL and target cells,33 membrane protrusions within and surrounding the immune synapse in NK cells,34 and ICNs.23 Flux of membrane proteins over membrane nanotubes has been used to demonstrate that NVNs can be reconstituted with plasma membrane lipids and proteins of cultured cells.6
ICN-Mediated Pathogen Transfer
Virus
In recent years, HIV has been found to transfer between cells directly via ICNs by mechanisms that may facilitate the evasion of the immune defense. Lehman et al. showed that viruses can undergo inward trafficking along filopodia similar to the myosin II-dependent retrograde flow observed in several other systems.35 As the virus reached the cell body, it could be endocytosed, thus infecting the cell. In a following study, it was shown that uninfected cells could extend filopodia toward infected cells, establishing an ICN, or viral cytoneme.16 It was shown that viruses could undergo retrograde flow along this newly formed ICN, leading to infection of the previously uninfected cell [see labels a–c in Figure 2.1], and that the stability of the ICN was dependent on a receptor–ligand interaction.
Figure 2.
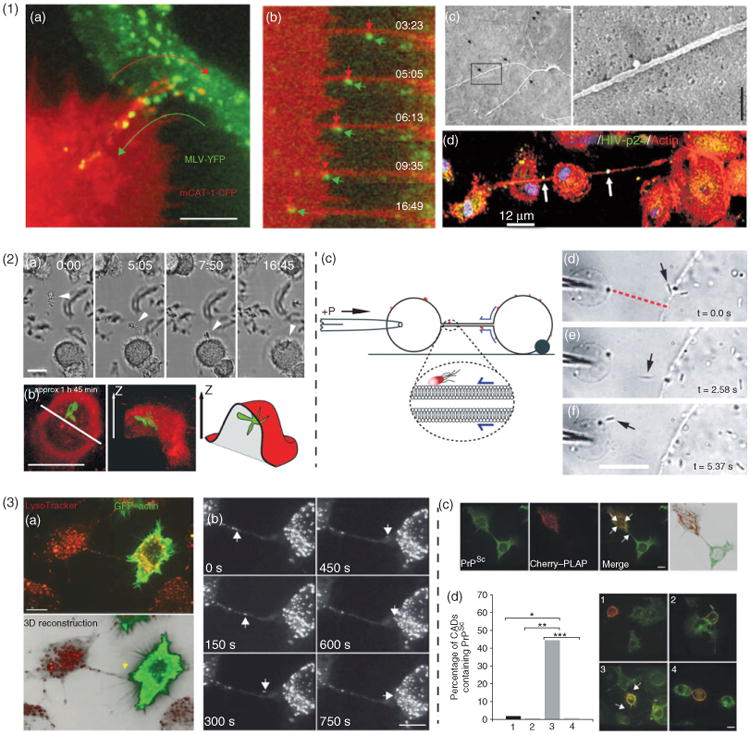
Pathogens exploit intercellular nanotubes (ICNs). (1) Viral particles. ICNs mediate cell-to-cell transmission of retroviruses. (a), Superimposed video frames illustrate the opposing movements of a receptor-expressing filopodium (red) and the movement of viral particles (green). Scale bar, 5 μm. (b), Time-lapse sequence of viral particles moving from the infected cell toward the noninfected target cells. The moving viral particle (green) was colocalized with mCAT1 receptor (red). (c), The viruses move along the outer surface of filopodial bridges toward target cells and correlated to single approximately 100-nm particles observed by SEM (black arrows). Scale bar, 500 nm. (a-c, Adapted with permission from Ref 16. Copyright 2007 Macmillan Publishers Ltd.). (d), Cellular nanotubes can be induced by human immunodeficiency virus (HIV)-infection of macrophages. Distinct HIV vesicles localized in nanotubular processes: actin (red), HIV-p24 (green), and DAPI (blue). Arrows denote HIV-p24 positive vesicles being transported across long nanotubes. (Adapted with permission from Ref 15. Copyright 2009 Elsevier). (2) Bacteria. Bacteria can surf along membrane nanotubes, aided by constitutive flow of nanotube surface. (a), Brightfield time-lapse sequence showing Mycobacterium bovisBCG expressing soluble green fluorescent protein (GFP) incubated with human macrophages. A cluster of bacteria (arrowhead) is shown trapped on an ICN connecting two macrophages and transported along the nanotube to the cell body where they were phagocytosed. (b), To confirm that the bacteria were indeed internalized, the cell membrane was labeled by the addition of membrane dye directly to the imaging chamber, schematic view to the left. (Adapted with permission from Ref 11. Copyright 2004 AAI). (c), Schematic illustrating the principle of extratubular Marangoni transport of bacteria over nanotubes in nanotube–vesicle networks (NVNs). Bacteria attached to the outer leaflet of the vesicle bilayer were transported over the nanotube by membrane flow. (d–f), Micrograph sequence demonstrating the surfing of bacteria between neighboring vesicles in NVNs. Scale bar, 20 μm. (Reproduced with permission from Ref 36. Copyright 2008 RSC). (3) Prions. Prions transfer through ICNs. (a), A three-dimensional reconstruction showing an ICN (yellow arrow) connecting a Cath.a-differentiated central nervous system cell (CAD cell) transfected to express actin-GFP (green) and labeled with LysoTracker (red) and an untransfected CAD cell. (b), A video sequence captures a vesicle moving inside a nanotube [termed tunneling nanotube (TNT)] and entering the cytoplasm of the recipient cell. (c), Diseased prions (PrPSc) were found in vesicular structures (white arrows) inside TNTs, as well as in the cytoplasm of the transfected cell, showing that PrPSc can transfer through TNTs. (d), Quantification of endogenous PrPSc transfer from prion infected CAD cells (ScCADs) to CAD cells through TNTs. CAD cells (red) cocultured with ScCAD (1–3) or with ScCAD in the presence of latrunculin to block TNT formation (4). Efficient transfer of PrPSc is detected only in cells connected through TNTs: (1), CAD cells not touching ScCAD cells; (2), CAD cells in direct cell contact with ScCAD cells; (3), CAD cells in contact with ScCAD cells through TNTs; (4), latrunculin-treated cocultures. Scale bar, 10 μm. (Adapted with permission from Ref 18. Copyright 2009 Macmillan Publishers Ltd.).
In another study, Sowinski et al. proposed17 that HIV-1 spread using ICNs formed by short-term intercellular unions between T-cells. Here, transfer was also observed to be receptor dependent, as T-cells lacking CD4 were not infected in cocultures with infected T-cells. The ICNs between T-cells appear to be distinct from those observed by Mothes and coworkers35 as a dynamic junction was observed along T-cell nanotubes or at their contact with cell bodies. Unlike the report from Sherer et al.16, images of T-cell ICNs indicate that these could extend from the infected cells allowing ‘forward’ transfer of virus particles along the tube toward the uninfected cell.
Recently, it was reported that an HIV infection of primary human macrophages induces the formation of ICNs with a time course that correlates with that of viral replication15 [see label d in Figure 2.1]. The authors reported that several classes of ICNs and viruses seemed to be localized both inside and on the surface of these nanotubes. It has also been shown that HIV infection modifies cell-cell interaction, enhancing the number of viral synapses, filopodial bridges and ICNs in a manner that is dependent on lymphocyte function associated antigen 1 (LFA-1), an integrin previously known to also facilitate HIV replication.37 Taken together, these reports have shown that ICNs are accessible to viruses and that there are mechanisms for transmission between cells and possibly even upregulation of ICNs during infection. However, the details of these processes are largely unknown.
Bacteria
Mycobacterium bovis bacillus calmette-Guerin (BCG) has been observed to bind and surf along ICNs, connecting human macrophages. This surfing was mediated by a constitutive flow of nanotube membrane that appeared to be similar to the retrograde flow suggested for viral transport.16 Önfelt et al. demonstrated that bacteria could be transported along the nanotube to the cell body where they were phagocytosed11 [see labels a and b in Figure 2.2], but it is unclear if this process was dependent on specific receptors mediating binding to the bacteria and anchoring to F-actin inside the ICNs. However, for human neutrophils Galkina et al.38 proposed a mechanism by which nitric oxide-induced ICNs enable neutrophils to bind and aggregate bacteria at a distance from the neutrophil cell body. Experiments in NVN systems have also shown similar surfing of bacteria, both on the outer and inner leaflet of the bilayer membrane, with the aid of tension induced membrane flow [see label c in Figure 2.2].36 Thus, these studies suggest that ICNs can capture bacteria in the extracellular space and transport them to cell bodies for phagocytosis. One of the most intriguing processes relating to ICNs is cell-to-cell transfer of the bacteria Listeria monocytogenes.39 The bacterium takes over of the host cell's cytoskeleton machinery and polymerizes a so-called comet tail, using host-produced actin filaments and is pushed through the host cell's membrane to invade neighboring cells, creating a trailing nanotube-like structure between the cells in the process.
Prions
How prions spread to and through the central nervous system has been a long-standing question. Reports have indicated that cell–cell contact enhances the infection process; for example, it has been shown that scrapie prions transfer by cell–cell contact40 and observed that prion proteins incorporated in vesicles are capable of intercellular transfer through neurites.41 Gousset et al.18 have now demonstrated that prion protein can travel through ICNs between neuronal cells, or connecting primary neurons with bone marrow-derived DCs, suggesting a potential route for prion spread from the peripheral site of entry to the nervous system via ICNs (Figure 2.3).
Wild-type prion protein traveling through ICN suggests that prion can spread via these structures.18 Similarly, the misfolded, diseased form of the prion protein (PrPSc) could be transported through ICNs and the speed and pattern of migration indicated a vesicular transport mechanism. It was shown that diseased prions only spread when cells were connected by ICNs as other types of cell–cell contact did not allow spreading of PrPSc.
In Vivo
In vitro model systems play a crucial role in the investigation of the molecular mechanisms for the formation and function of ICNs. To rule out the possibility that ICNs are merely an artifact of in vitro culture conditions, the community has also started to address the issue of in vivo observation of ICNs. Cellular extensions that resemble ICNs were observed in tissue before the report by Rustom et al. in 2004. Among them is the report of cytonemes in the fruit fly imaginal discs2 (Figure 1.1) and interconnecting filopodia in mouse blastocyst.42 The first example of ICNs formed in animals was demonstrated in mouse corneas19 (Figure 3). These were generally short and straight and corresponded closely to previous descriptions of membrane nanotubes in vitro. In this study, the frequency of ICNs was significantly increased in corneas subjected to trauma and lipopolysaccharides, which suggests that nanotubes have an important role in cell–cell communication between widely spaced DCs during inflammation. The authors speculated that ICNs between DCs in vivo could represent a significant means of transmitting antigen, thus amplifying local immune surveillance in an environment like the mammalian cornea where antigen-presenting cells are scarce.
Figure 3. In vivo.
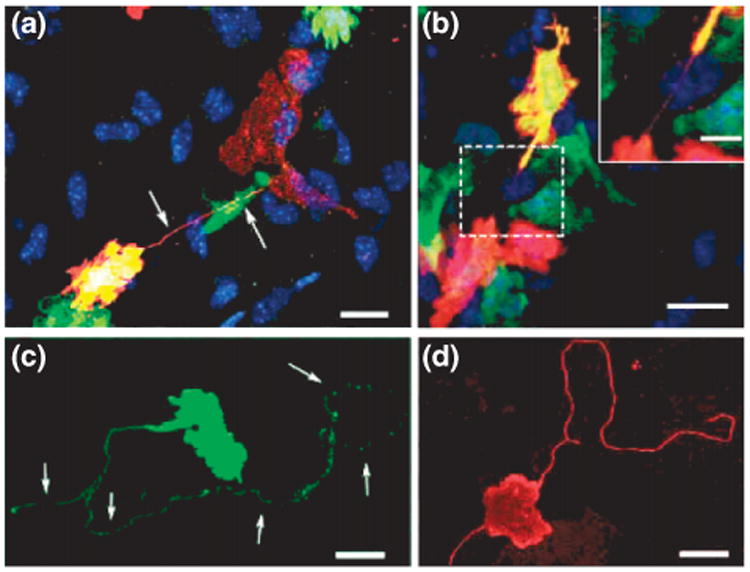
intercellular nanotubes (ICNs) observed between cells in the corneal stroma tissue of mice. (a), Chimeric mouse corneal whole mount reveals a donor-derived (green fluorescent protein (GFP)+ /green) major histocompatibility complex (MHC) class II+ (red) and double positive (yellow) cell connected via a fine ICN (arrows) to a resident MHC class II+ GFP- cell (red only). (b), Two donor-derived MHC class II+ cells expressing varying amounts of GFP joined by a fine, straight ICN. (c) and (d), Long, nonbridging membrane nanotubes on MHC class II+ cells in the naive, (c), and inflamed, (d), mouse corneal stroma. Scale bar, 20 μm; inset scale bar, 10 μm. (Reprinted with permission from Ref 19. Copyright 2008 AAI).
Structure and Dynamics of Icns
Within the lamellipodia of migrating cells are actin structures, which, when they spread beyond the lamellipodium frontier, are called filopodia. As a cell migrates along a surface, it extends filopodia at the leading edge, attaching the cell to the substratum at focal adhesion spots further down the migratory pathway.43 Connected with the ability to generate mobility, the filopodia participate in fundamental physiological processes like wound healing, developmental processes such as neurite outgrowth, serving as precursors for dendritic spines in neurons,44 and in cell signaling.45
Mechanisms for the up- and downregulation of the actin filaments46 [see label b in Figure 4.1] perform a wide range of important functions in cell motility, as well as in locating and transporting protein complexes in the cell. The dynamic assembly and disassembly of actin structures, such as lamellipodia and filopodia, are controlled by protein binding to existing actin filaments to form a branching filament network.47,48 This F-actin regulatory pathway is likely to also affect formation of ICN.49
Figure 4.
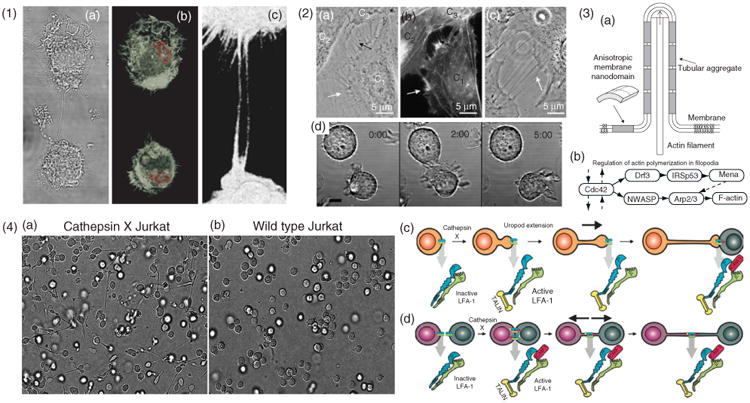
Cytoskeletal structure and formation of ICNs (1) Actin structure of ICNs illustrated with macrophages, (a), differential interference contrast micrograph, (b), micrograph showing a 3D rendered confocal stack. F-actin stained by phalloidin (white) and the nucleus stained with Hoescht (red). (c), A zoomed-in region showing the phalloidin stained nanotubes. (2) Formation of ICNs. (a–c), Bridging nanotubes. (a), Phase contrast image of live T24 cells, (b), fluorescence micrograph with actin labeling of the same cells as in A. The white arrows in (a) and (b) indicate short and dynamic membrane protrusions with which the approaching cell explores its surroundings. The black arrow in, A, points at protrusions that have already connected to the target cell. Bridging nanotubes can be more than 20 μm in length and bifurcations are occasionally observed (arrow in C). (Reprinted with permission from Ref 51. Copyright 2008 Elsevier). (d), Time-lapse imaging of 721.221 cells forming a transient contact demonstrates that a connecting nanotube can form as cells move apart after contact. Scale bar, 10 μm. (Reprinted with permission from Ref 5. Copyright 2004 AAI). (3) Schematic illustration, (a), of stabilization of nanotubular membrane protrusions by accumulation of anisotropic membrane nanodomains in the tubular region. The growing actin filaments push the membrane outward. The protrusion could additionally be stabilized by accumulated anisotropic nanodomains that favor an anisotropic cylindrical geometry of the membrane. The cylindrical-shaped anisotropic membrane domains, once assembled in the membrane region of a nanotubular membrane protrusion, keep the protrusion mechanically stable even if the cytoskeletal components (actin filaments) are disintegrated. (Reprinted with permission from Ref 51. Copyright 2008 Elsevier). (b), The general regulatory pathway of actin polymerization in filopodia46 (4) LFA-1 activation is responsible for two distinct pathways of nanotube formation in Jurkat T-cells. (a), Example of the extension and nanotube outgrowth by Cathepsin X-up regulated Jurkat T-cells in a three-dimensional environment, leading to increased ICN-mediated cell-to-cell contact. (b), Wt cells remained in a spherical shape. Nanotubes form upon uropod elongation in the absence of prior intercellular contact. (c), The proposed mechanism of nanotube outgrowth via persistent LFA-1 activation (mediated by cathepsin X). Talin binds to the cytoplasmic tail of LFA-1, followed by binding of ICAM-1 to the extracellular domain, enabling inside-out driven actin reorganization, uropod formation, elongation, and ICN formation. (d), The proposed mechanism of nanotube formation following intercellular contact between T-cells. LFA-1/ICAM-1 interactions arising in T-cell aggregation are enhanced in cathepsin X-upregulated Jurkat cells. Prolonged LFA-1 activation enables cytoskeletal reorganization, similar to that associated with uropod outgrowth, with talin-binding to the cytoplasmic tail of LFA-1 and subsequent membrane nanotube formation as cells depart. (Modified with permission from Ref 52. Copyright 2009 Springer Science+Business Media).
Cytoskeletal Structure of ICNs
Several reports have shown that actin is a main cytoskeletal content of ICNs5,13 (Figure 4.1), and studies on fixed samples have revealed F-actin organization with an implantation pattern into the cell resembling structures seen in filopodia.50 Interrupting actin polymerization reduces transfer efficacy via ICNs.18,49 There are also examples of ICNs that contain microtubuli, such as in macophages,11 urothelial cell lines,51 and in human prostate cancer cells.7
Formation of ICNs
Researchers have observed different mechanisms for ICN formation. For example, it has been shown that ICNs can form de novo, growing from filopodial structures.4,16 Membrane tube bundles emanate from a single cell and dynamically protrude out into the surrounding media seeking contact with neighboring cells, [see labels a and b in Figure 4.2], and occasionally three-way junctions (bifurcation) can be observed, [see label c in Figure 4.2].
ICN can also form during separation after tight cell–cell contacts [see label d in Figure 4.2]. Immune synapses between NK cells and EBV-transformed B-cells were observed to result in nanotube formation as cells separated. Similarly, ICNs have been observed after transient contacts between macrophages5 and T-cells.17,52 When the membranes of the two cells detach, extensions containing both actin and cytokeratin filaments form as the cells move apart. Cytokeratins provide these nanotubes with stronger mechanical properties, preventing rupture because of cell migration or environmental stresses.53
Recently, putative molecular mechanisms have been proposed to explain the formation of ICNs. The formation of ICNs between T-cells, for example, was proposed to occur through LFA-1, and integrin activation by the cysteine protease cathepsin X was suggested to cause the elongation of nanotubes toward target cells as well as to support ICN formation when cells move apart52(Figure 4.4).
Membrane Continuity
An unresolved question is which mechanism regulates whether the ICNs are open-ended, with a continuous membrane between the connected cells4,8 (Figure 5(a)), or contain an intercellular junction17 (Figure 5(b)). Rustom reported evidence that green fluorescent protein (GFP) bound to the plasma membrane could transfer between cells connected by ICNs4 and Önfelt et al. reported the colocalization of membrane components at the base of an ICN between two EBV-transformed B-cells, possibly indicating fused plasma membranes.5 In T24 cells, actin–GFP have been observed to spread into nontransfected neighboring cells51 connected by ICNs. However, in that case, lipid material was not observed to pass through the junction, which would be expected if the tube had a continuous membrane. Several other observations suggest that ICNs are not open-ended tunnels, but instead contain a distinct junction between the two connected cells. For instance, two reports show that T-cell nanotubes contain such a junction17,52 (Figure 5(b)). Thus, there is compelling evidence that ICNs are heterogeneous with regard to the structure of the interface between connected cells. One important future challenge is to resolve under what circumstances and by which mechanisms membranes can fuse, leading to open-ended ICNs.
Figure 5.
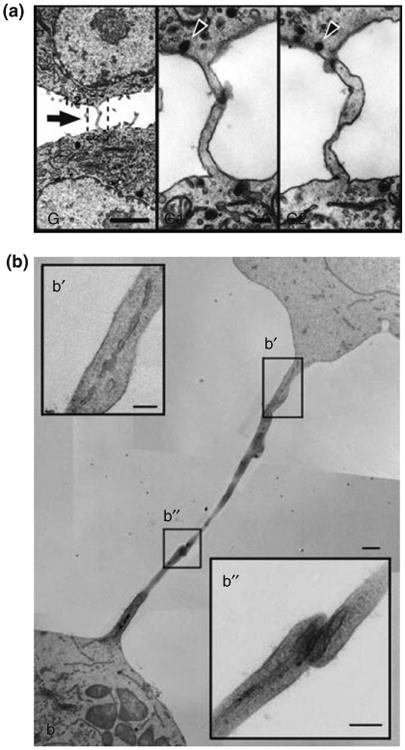
Membrane continuity of intercellular nanotubes. (a) Transmission electron microscope (TEM) ultrastructure of a tunneling nanotube (TNT) between rat pheochromocytoma cells where a serial sectioning showed that, at any given point along TNTs, the membrane appeared to be continuous. From Rustom et al.4 reprinted with permission from AAAS. (b) TEM ultrastructure of an ICN between T-cells reveals a closed border i.e., no direct cytoplasmic contact or membrane mixing. (b′, b″) Insets show higher magnifications of the nanotube and the junction. Scale bar, 500 nm. (Reprinted with permission from Ref 17. Copyright 2008 Nature Publishing Group).
Model Nanotubes and Formation of NVNs
Methods based on pipette aspiration can be used to probe the physical and chemical properties in single lipid nanotube extensions and nanotube three-way junctions in model systems like NVNs. Formation of model nanotubes from vesicles can be performed by extracting membrane material with methods that apply a point force to the lipid membrane, for instance by micromanipulation54 (Figure 6), optical tweezers55 (Figure 7.1), or by using motor proteins56 [see label (e-g) in Figure 9.3].
Figure 6.
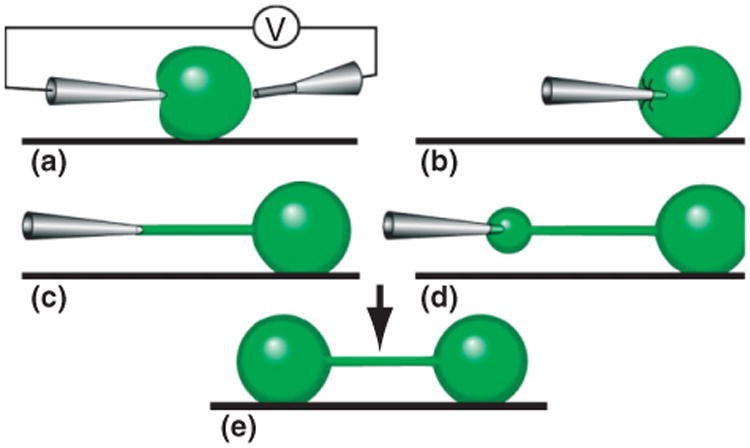
Schematic illustration of the nanotube-vesicle network fabrication principle. (a), By a combination of mechanical deformation and electric pulses across the liposome, a microinjection needle is inserted into a unilamellar liposome connected to a multilamellar protrusion (not shown). (b), After the lipid has adhered to the injection needle, the micropipette is pulled away from the liposome. (c), A lipid nanotube is created between the tip of the micropipette and the liposome. (d), By applying a low pressure in the microinjection needle, ejected liquid expands the nanotube into a liposome at the microinjection tip, transferring additional lipid material from the multilamellar liposome (not shown). (e), After the liposome has reached a desired size, it is allowed to adhere to the surface. Thereafter, the micropipette is removed by applying electric pulses and simultaneously pulling it out of the liposome.
Figure 7.
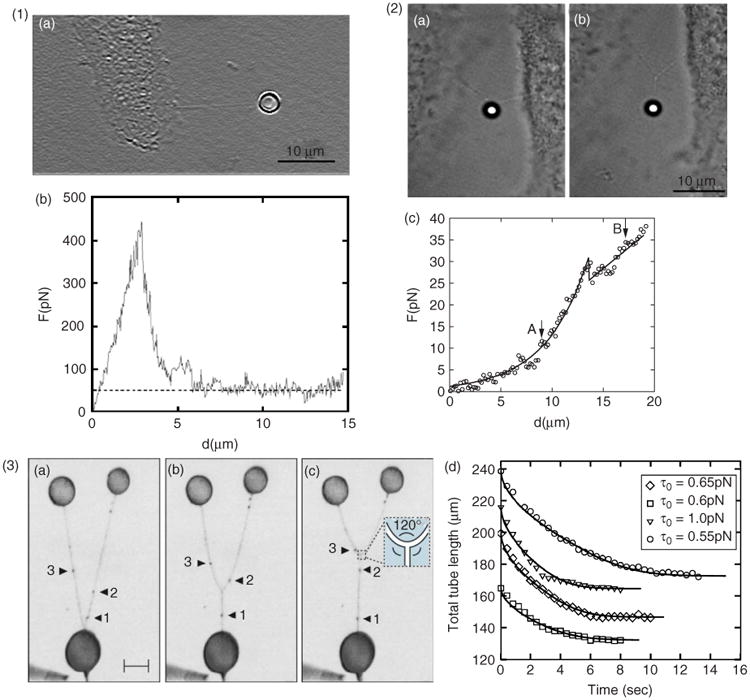
Membrane dynamics and three-way junction formation. (1) Direct nanotube extraction from cell by laser trap manipulation. (a), As a membrane nanotube is extracted from the cell, force versus microscope stage displacement, (b), is recorded, where the dashed line indicates the plateau average of the force. (2) Transition from a V to a Y shape of cellular nanotubes. (a), Before bifurcation, (b), after bifurcation, and, (c), the corresponding force versus microscope stage displacement. Points corresponding to images (a) and (b) are marked by arrows; the bifurcation position coincides with the dip in the curve seen in image (c). (Reprinted with permission from Ref 50. Copyright 2008 Springer Science+Business Media). (3) Model systems such as nanotube–vesicle networks can provide insight into membrane properties. (a–c), Inverted fluorescence images showing merging of nanotubes. Based on observations of the surfactant flow (black arrows, 1–3) on the nanotubes in model membrane systems, it is shown that a Y-junction propagates with a zipper-like mechanism. The surfactants from two nanotube branches undergo 1:1 mixing at the junction, and spontaneously form the extension of the third nanotube branch. Scale bar, 30 μm. (d), Experimental data for the total nanotube length of four different Y junctions, and fits obtained by numerical integration of the fluid-string model. The value in the inset is the nanotube string tension estimated from the fits. (Adapted with permission from Ref 57. Copyright 2006 American Physical Society).
With a method based on the principle of electroporation,58 a microinjection technique can be utilized to create biomimetic NVNs22 (Figure 6). The networks are composed of surface-immobilized phospholipid bilayer vesicles, interconnected with vesicles 1–50 μm in diameter and 10–15 – 10–12 L in volume. The width of these synthetic lipid nanotubes is approximately 25–300 nm in diameter, similar to the dimension observed for ICNs. By electromechanical insertion of a pipette tip into a unilamellar vesicle, followed by lateral pulling of the micropipette away from the vesicle, a nanotube is formed. Buffer solution in the pipette is injected into the nanotube orifice, forming a vesicle of controlled size that can be immobilized on the surface. The networks have controlled connectivity and precise topography with regard to the container size, the angle between nanotube extensions, and nanotube length.59 The internal fluid composition of individual vesicles is defined during the formation of the network by the selection of the solution within the micropipette.60 The protocols allow for the formation of NVNs of high geometrical complexity,61 where each node within a network can have a unique chemistry62 and material can actively be transported in the nanotubes.63 In addition, the NVNs can also be created from live cells based on these techniques,6 (Figure 1.4) to study transport phenomena such as protein transfer onto nanotubes.
Membrane Dynamics of Nanotubes
Upon the formation of ICNs, the plasma membrane must undergo substantial adaptive changes. The plasma membrane is a fluid lipid bilayer that responds elastically to applied mechanical forces, and these forces will become distributed throughout the entire surface area. To buffer against changes in membrane tension cells maintain a plasma membrane reservoir in the form of ruffles or by addition of lipids from internal stores.64 This reservoir of excess cell membrane makes the extraction of nanotubes feasible.
To understand the behavior and geometry of ICNs, a physical description of membrane mechanics is beneficial. One approach suggested by Helfrich in the 1970s is to use the thin elastic shell theory.65 The contribution to the elastic free energy of the lipid membrane is described by a set of independent shape deformations of the membrane surface. Any such membrane deformation must increase the total energy compared to that at equilibrium. With the development of the theories of lipid membrane mechanics, the principles of vesicle bilayer and NVN formation, based on properties such as thermal transitions, elasticity, rigidity, cohesion, and colloidal interactions, have become better understood.66,67 It is now generally accepted that the shape of a lipid vesicle is determined primarily by the bending elasticity and curvature of the vesicle.
Once formed, artificial membrane nanotubes can remain stable.56 NVNs are also stable in a local energy minima as long as the network is anchored to a substrate.20 It has been suggested that the stability of the tubular membrane protrusions without the inner supporting rod-like cytoskeleton is a consequence of the accumulation of anisotropic membrane components, or nanodomains, [see label (a) in Figure 4.3], in the bilayer membrane of nanotubular protrusions.68 These properties of lipid bilayers and plasma membranes provide insight into the stability of ICNs once formed, even if actin filaments are disassembled.51
Membrane dynamics of nanotubes formed from cells, for example, the nanotubes observed in human peripheral blood69 and tubulovesicular extensions in human neutrophils70 acquired with measurements of nanotube extraction forces, provide information on their structure and elastic properties. Overall, despite their actin content50 (Figure 7.1), the elastic dynamics of ICN do not appear to be fundamentally different from those observed for hollow tubes in endoplasmatic reticulum, Golgi apparatus71 or artificial systems.57 Considering these similarities, understanding the dynamics of pure lipid membrane networks, the principles of their reorganization and the transport inside them will facilitate understanding the organization and trafficking inside ICNs.
Three-way Junctions
In coalescence experiments in model systems, in which two nanotubes are pulled from the same vesicle and brought closer to each other until they merge,72 the force required to extract a tether and the angle between tethers at coalescence directly yield the bending rigidity and the membrane tension of the membrane nanotubes, and can therefore provide information on the physical dimensions of nanotubes. For instance, such measurements with soybean lipid composition have yielded nanotube radii of 110 ± 26 nm.73 As the tubes coalesce during micromanipulation, they rearrange into three-way junctions (Y-junction) and a similar mechanism can induce self-organization and restructuring of the NVN.74,75 Similarly, three-way junctions51 and V–Y transitions10,50 have been observed in ICNs (Figure 7.2). Surface free energy considerations show that three-way junctions appear spontaneously in NVN systems when two adjacent nanotubes overcome the critical coalescence distance.76 In relaxed NVN systems, three-way junctions are positioned so that connected tubes form 120° relative to each other, thus minimizing the distance required to connect the vesicles.
Interestingly, the three-way junctions observed for ICNs5,50 also often display 120° angles between connected nanotubes (Figure 7.2). In model systems, the path minimization mechanism is well understood, since the lipid networks are fluid and numerical models can be used to study the dynamics of self-organization57 (Figure 7.3). However, considering that ICNs often contain F-actin, the mechanism behind dynamically moving three-way junctions is less clear for cellular systems. Furthermore, three-way junctions in NVNs are fully open, allowing molecules to diffuse in all directions. Whether this can also be the case for similar junctions in ICNs is unknown. Judging from the observed heterogeneity of ICNs, it is unlikely that the answer is straightforward.
Materials and Methods of Nanotubular Studies
Techniques employed for the study of ICNs have mostly been based on microscopy. Fluorescence microscopy77 (Figure 8.1) offers spatiotemporal information of protein trafficking and signal propagation, while electron microscopy78,79 (Figure 8.2) gives high resolution information about cellular structures. Most of our understanding of ICNs has been provided by fluorescence or electron microscopy studies, both on live cells using various fluorescent markers and on fixed cells using immunofluorescence or electron microscopy. Unfortunately, microscopy techniques offer little biochemical information that bulk biochemical analysis methods (e.g., mass spectrometry80) can provide.
Figure 8.
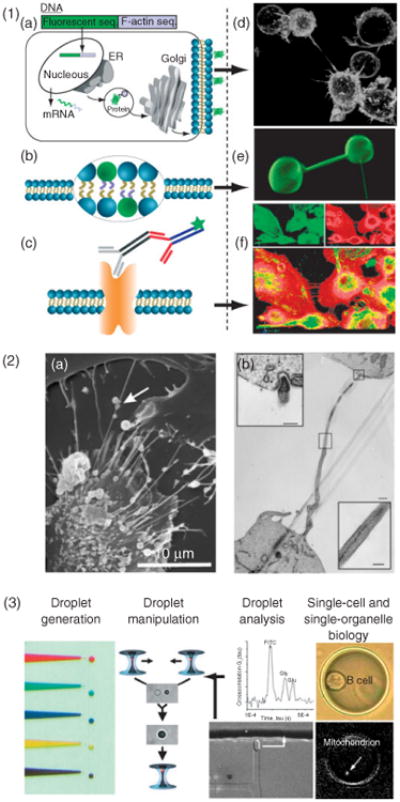
Materials and methods of nanotubular studies. (1) Fluorescent imaging techniques such as confocal imaging provide spatiotemporal information based on fluorescent staining. Labeling of cells is based on, (a), genetically modified cell lines expressing fluorescent proteins, (b), membrane stains, or, (c), antibodies. The panels to the right give specific examples of nanotubes tagged using these methods. (d), B-cells expressing glycosylphosphatidylinositol anchored GFP. (e), nanotube-vesicle network stained with the membrane dye DiO. (f), Primary macrophages stained with phalloidin for F-actin (red) and with monoclonal antibody against α-tubulin (green). (2) Electron microscopy provides structural details on a nanometer resolution scale. (a), Scanning electron microscopy can provide external structural information. Adapted from Veranic et al.51 © 2008, with permission from Elsevier. (b), Transmission electron microscopy illuminates the internal structure, such as membrane junctions. (Adapted with permission from Ref 51. Copyright 2008 Nature Publishing Group). (3) Femtoliter- and picoliter-sized droplets as nanolaboratories for manipulating single cells and subcellular compartments is a potential route to analyze cellular nanotubes. A potential route to analyze intercellular nanotubes could be the use of droplet-nanolaboratories as has been demonstrated for single cells and subcellular compartments. (Reprinted with permission from Ref 81. Copyright 2009 ACS).
Development of ultrasensitive bioanalytical methodologies for the analysis of single cells and organelles could help to address this issue.81 One particular suite of developed tools, for example, should be able to extract detailed chemical information from subcellular structures, organelles, and ICNs from live cells while preserving the spatiotemporal information offered by high-resolution fluorescence microscopy81 For instance, single-cell nanosurgery82,83 could be used to isolate single or small groups of ICNs. Once isolated, ICNs could be encapsulated in aqueous droplets and used as nanolabs for the biochemical manipulation84 and analysis of the composition.84–86 A schematic work flow involved in using a droplet nanolab approach for the analysis of single cells, organelles, and ICNs is depicted in Figure 8.3.
Besides the development and application of new sensitive analytical techniques to the study of ICNs, improvements of existing techniques will also enhance our ability to study ICNs. For example, microscopy techniques, such as stimulated emission depletion,87,88 can improve resolution beyond the diffraction limit; computer-based methods, such as the automated detection of nanotubes,89 facilitate data gathering and analysis. As a complement, model systems such as NVNs21,22 may provide fundamental understanding of mechanistic principles, for instance, of transport phenomena36,90,91 and the regulation of reactions.92,93
Transfer and Transport Mechanisms
Recent studies have shown that ICNs can transport a range of cargo, including proteins and organelles, by several different mechanisms. Clearly, in ICNs containing cytoskeletal structures and associated motor proteins, there are potential mechanisms for active transport that are lacking in artificial systems. However, studies of NVN systems have shown that more passive means of transport, for example, diffusion and Marangoni flow, can have functional properties.
Diffusion Inside Nanotubes
There are some indications that diffusion could be a functional mechanism for communication via ICNs. For instance, the calcium signals transmitted between macrophages8 could be mediated by the diffusion of IP3. This mode of transport would require an open-ended tubular structure. Mixing and transport by diffusion could be efficient considering the small length scales of cells networked by ICNs as well as NVNs. Theoretical studies modeling nanotube–vesicle topologies show that network geometry influences the result and can be used to describe how content concentrations in involved vesicles evolve over time.94 The effects of compartmentalization on diffusional modification have been investigated in NVNs95 (Figure 9.1), illustrating how diffusion can be harnessed.
Passive Membrane Transport Along the Nanotube Surface
Cell-surface proteins and/or patches of membrane might transfer via nanotubular connections.4,5 In addition to biological ATP-dependent mechanisms such as the previously mentioned retrograde flow, it has been demonstrated both theoretically and experimentally that a gradient or difference in membrane tension across a lipid membrane surface can drive the lipids to flow from regions of low tension to regions of high tensions, in order to eliminate the tension difference97 (Figure 9.2). In the case of bilayer membranes, nonuniform lipid distributions can be a result of, for example, fluid convection, temperature gradients, electric fields, laser light, and mechanical means like cell movement or the extension of membrane protrusions through actin polymerization or through mechanical force in model systems. The spatial variation in interfacial tension created by such protrusions generates tension gradients over membrane regions that produce a membrane flow directed toward regions of higher surface tension.90 In NVNs, it has been shown that this tension driven surface flow of the lipid membrane induces a Marangoni plug flow of the solution inside the nanotube21,98 which in turn transports contents from one vesicular compartment to another.36
Formation of Bulges or Gondolas
Related to the transport of membrane material over nanotubes, several separate reports have identified moving vesicles, bulges, or gondolas integrated into ICNs.4,5,7 Such expansions of intercellular nanotubes (Figure 9.2) have in some studies been interpreted as cargo too large to fit the inner diameter of the tube.69 Formation of bulges in ICNs is reminiscent of observations in model systems.91 Where a sudden tension difference has been shown to be a mechanism for gondola formation or pearling. In cellular systems, such a change in tension could be caused, for example, by diverging cells. The anchoring of a structural polymer to the membrane99 has also been linked to pearling behavior in model systems. It has been suggested that a similar mechanism may also take place in cells.51 Rearrangement of local constituents, such as lipids and proteins along the nanotubes, enable and favor the formation of the dilatations in cellular nanotubes. Pearling behavior in cells to create gondolas69 might hence be caused by a gradual disruption of the actin cytoskeleton.100
Molecular Motor Dependent Transport
Organelle transport along actin inside nanotubes is mediated by, for example, the translation of myosin V along F-actin. For instance, unidirectional vesicular traffic for PC12 cells4 has been reported, while bidirectional trafficking11 along microtubules was shown for human primary macrophages. In normal rat kidney (NRK) cells and PC12 cells, nanotubes mediate the transfer of various cellular components, including endocytotic organelles.4 The observation of unidirectional transport along ICNs has been suggested to be linked to active transport by myosin motors along actin filaments for organelle transfer13 and viral spread.35The observed reduction of viral infection and organelle transfer in the presence of F-actin depolymerizing agents suggests that transport along nanotubes and filopodia is mediated by the underlying actin cytoskeleton and is controlled by myosin. Because myosin II is a plus-end motor that mediates minus end motility toward the cell body, it must regulate the movement of entire actin filaments, a process called retrograde F-actin flow.35 In addition, a reduction of organelle transfer was also observed after microtubules were depolymerized with nocodazole, although less pronounced as compared to F-actin depolymerizing conditions. This observation might suggest that NRK cell microtubules do not participate in the intercellular translocation itself; instead they convey a function of targeting endosomal organelles to nanotubular entry sites in the cell periphery.
Actin-driven Surfing
Surfing, where cargo is transported along the outside surface of nanotubes (Figure 9(c)) has been demonstrated by the intercellular trafficking of bacteria11 or viral particles.16,17 Surfing is caused by retrograde flow that may be dependent on myosin II. The mechanism is a two-step process, in which the cargo first attaches to the membrane through anchors, such as transmembrane receptors that bind to a virus,16 bacteria,11 or similar object on the outer leaflet of the cell membrane. The binding event is followed by a flow of membrane material toward the target cell transporting the attached cargo.
Conclusion
The discovery of intercellular nanotubes as a novel form of intercellular communication has triggered a lot of attention and new research. However, further research is needed to understand the fundamental properties of ICNs, establish if they are as common in vivo as they are in vitro and, thus, could play a role in intercellular communication and disease on the systemic level.
This review has focused on a variety of topics connected to ICNs including their structure and biological function, which transport processes they support. We also suggest that an interdisciplinary approach, involving studies in artificial systems such as NVNs and the use of novel ultrasensitive bio-analytical methodologies could be a route toward understanding more about ICNs. However, analysis of the biochemical compositions and biomechanical properties of ICNs involves technical challenges. Such analysis includes implementation of novel capabilities offered by, for example, droplet microfluidics and nanosurgery. Application of such new methodologies suggests exciting new possibilities for ICN research.
Acknowledgments
This work was supported by a fellowship from the Knut & Alice Wallenberg Foundation (to J. Hurtig), by the National Institutes of Health and the National Science Foundation (to D. T. Chiu), and from Swedish Foundation for Strategic Research and Swedish Research Council (to B. Önfelt).
References
- 1.Wolpert L, Gustafson T. Studies on the cellular basis of morphogenesis of the sea urchin embryo. The formation of the blastula. Exp Cell Res. 1961;25:374–382. doi: 10.1016/0014-4827(61)90287-7. [DOI] [PubMed] [Google Scholar]
- 2.Ramirez-Weber F, Kornberg T. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 3.Miller J, Fraser S, McClay D. Dynamics of thin filopodia during sea urchin gastrulation. Development. 1995;121:2501–2511. doi: 10.1242/dev.121.8.2501. [DOI] [PubMed] [Google Scholar]
- 4.Rustom A, Saffrich R, Markovic I, Walther P, GerdesH Nanotubular highways for intercellular organelletransport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 5.Önfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 6.Bauer B, Davidson M, Orwar O. Direct reconstitution of plasma membrane lipids and proteins in nanotubevesicle networks. Langmuir. 2006;22:9329–9332. doi: 10.1021/la060828k. [DOI] [PubMed] [Google Scholar]
- 7.Vidulescu C, Clejan S, O'Connor K. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med. 2004;8:388–396. doi: 10.1111/j.1582-4934.2004.tb00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 10.Önfelt B, Davis D. Can membrane nanotubes facilitate communication between immune cells? BiochemSoc Trans. 2004;32:676–678. doi: 10.1042/BST0320676. [DOI] [PubMed] [Google Scholar]
- 11.Önfelt B, Nedvetzki S, Benninger RKP, Purbhoo MA, Sowinski S, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 12.Gimsa U. Actin is not required for nanotubular protrusions of primary astrocytes grown on metal nano-lawn. Mol Membr Biol. 2007;24:243–255. doi: 10.1080/09687860601141730. [DOI] [PubMed] [Google Scholar]
- 13.Gurke S, Barroso JFV, Hodneland E, Bukoreshtliev NV, Schlicker O, et al. Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp Cell Res. 2008;314:3669–3683. doi: 10.1016/j.yexcr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Belting M, Wittrup A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J Cell Biol. 2008;183:1187–1191. doi: 10.1083/jcb.200810038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254:142–148. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherer N, Lehmann M, Jimenez-Soto L, Horensavitz C, Pypaert M, et al. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 18.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 19.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson A, Karlsson R, Karlsson M, Cans AS, Strömberg A, et al. Networks of nanotubes and containers. Nature. 2001;409:150–152. doi: 10.1038/35051656. [DOI] [PubMed] [Google Scholar]
- 21.Davidson M, Karlsson M, Sinclair J, Sott K, Orwar O. Nanotube-vesicle networks with functionalized membranes and interiors. J Am Chem Soc. 2003;125:374–378. doi: 10.1021/ja027699o. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson M, Davidson M, Karlsson R, Karlsson A, Bergenholtz J, et al. Biomimetic nanoscale reactors and networks. Annu Rev Phys Chem. 2004;55:613–649. doi: 10.1146/annurev.physchem.55.091602.094319. [DOI] [PubMed] [Google Scholar]
- 23.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 24.Gerdes HH, Carvalho RN. Intercellular transfermediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20:470–475. doi: 10.1016/j.ceb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 2008;18:414–420. doi: 10.1016/j.tcb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boitano S, Dirksen E, Sanderson M. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 27.Rechavi O, Goldstein I, Kloog Y. Intercellular exchange of proteins: The immune cell habit of sharing. FEBS Lett. 2009;583:1792–1799. doi: 10.1016/j.febslet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Niu XL, Gupta K, Yang JT, Shamblott MJ, Levchenko A. Physical transfer of membrane and cytoplasmic components as a general mechanism of cell-cell communication. J Cell Sci. 2009;122:600–610. doi: 10.1242/jcs.031427. [DOI] [PubMed] [Google Scholar]
- 29.Carlin LM, Eleme K, McCann FE, Davis DM. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med. 2001;194:1507–1517. doi: 10.1084/jem.194.10.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanherberghen B, Andersson K, Carlin LM, Nolte-t Hoen ENM, Williams GS, et al. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc Natl Acad Sci U S A. 2004;101:16873–16878. doi: 10.1073/pnas.0406240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorak-Stolinska P, Kemeny DM, Noble A. Activation-induced cell death in human T cells is a suicidal process regulated by cell density but superantigen induces T cell fratricide. Cell Immunol. 2002;219:98–107. doi: 10.1016/s0008-8749(02)00598-1. [DOI] [PubMed] [Google Scholar]
- 32.Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 33.Stinchcombe J, Bossi G, Booth S, Griffiths G. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 34.Williams GS, Collinson LM, Brzostek J, Eissmann P, Almeida CR, et al. Membranous structures transfer cell surface proteins across NK cell immune synapses. Traffic. 2007;8:1190–1204. doi: 10.1111/j.1600-0854.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann M, Sherer N, Marks C, Pypaert M, Mothes W. Actin-and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170:317. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurtig J, Orwar O. Injection and transport of bacteria in nanotube-vesicle network. Soft Matter. 2008;4:1515–1520. doi: 10.1039/b800333e. [DOI] [PubMed] [Google Scholar]
- 37.Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, et al. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol. 2009;83:6234–6246. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galkina SI, Romanova JM, Stadnichuk VI, Molotkovsky JG, Sud'ina GF, et al. Nitric oxide-induced membrane tubulovesicular extensions (cytonemes) of human neutrophils catch and hold Salmonella enterica serovar Typhimurium at a distance from the cell surface. FEMS Immunol Med Microbiol. 2009;56:162–171. doi: 10.1111/j.1574-695X.2009.00560.x. [DOI] [PubMed] [Google Scholar]
- 39.Tilney L, Portnoy D. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanu N, Imokawa Y, Drechsel D, Williamson R, Birkett C, et al. Transfer of scrapie prion infectivity by cell contact in culture. Curr Biol. 2002;12:523–530. doi: 10.1016/s0960-9822(02)00722-4. [DOI] [PubMed] [Google Scholar]
- 41.Magalhaes AC, Baron GS, Lee KS, Steele-Mortimer O, Dorward D, et al. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J Neurosci. 2005;25:5207–5216. doi: 10.1523/JNEUROSCI.0653-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salas-Vidal E, LomelI H. Imaging filopodia dynamics in the mouse blastocyst. Dev Biol. 2004;265:75–89. doi: 10.1016/j.ydbio.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 43.McClay DR. The role of thin filopodia in motility and morphogenesis. Exp Cell Res. 1999;253:296–301. doi: 10.1006/excr.1999.4723. [DOI] [PubMed] [Google Scholar]
- 44.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 45.Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007;2007:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- 46.KEGG—Kyoto Encyclopedia of Genes and Genomes. Regulation of Actin Cytoskeleton Available at http://www.genome.jp/kegg-bin/show_pathway?hsa04810.
- 47.Wood W, Martin P. Structures in focus—filopodia. Int J Biochem Cell Biol. 2002;34:726–730. doi: 10.1016/s1357-2725(01)00172-8. [DOI] [PubMed] [Google Scholar]
- 48.Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- 49.Bukoreshtliev NV, Wang X, Hodneland E, Gurke S, Barroso JF, et al. Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 2009;583:1481–1488. doi: 10.1016/j.febslet.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 50.Pontes B, Viana NB, Campanati L, Farina M, Neto VM, et al. Structure and elastic properties of tunneling nanotubes. Eur Biophys J. 2008;37:121–129. doi: 10.1007/s00249-007-0184-9. [DOI] [PubMed] [Google Scholar]
- 51.Veranic P, Lokar M, Schutz GJ, Weghuber J, Wieser S, et al. Different types of cell-to-cell connections mediated by nanotubular structures. Biophys J. 2008;95:4416–4425. doi: 10.1529/biophysj.108.131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obermajer N, Jevnikar Z, Doljak B, Sadaghiani AM, Bogyo M, et al. Cathepsin X-mediated beta(2) integrin activation results in nanotube outgrowth. Cell Mol Life Sci. 2009;66:1126–1134. doi: 10.1007/s00018-009-8829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp Cell Res. 2007;313:2021–2032. doi: 10.1016/j.yexcr.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Evans E, Yeung A. Hidden dynamics in rapid changes of bilayer shape. Chem Phys Lipids. 1994;73:39–56. [Google Scholar]
- 55.Cuvelier D, Chiaruttini N, Bassereau P, Nassoy P. Pulling long tubes from firmly adhered vesicles. Europhys Lett. 2005;71:1015–1021. [Google Scholar]
- 56.Roux A, Cappello G, Cartaud J, Prost J, Goud B, et al. A minimal system allowing tubulation with molecular motors pulling on giant liposomes. Proc Natl Acad Sci U S A. 2002;99:5394–5399. doi: 10.1073/pnas.082107299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lobovkina T, Dommersnes P, Joanny JF, Hurtig J, Orwar O. Zipper dynamics of surfactant nanotube Y junctions. Phys Rev Lett. 2006;97:188105. doi: 10.1103/PhysRevLett.97.188105. [DOI] [PubMed] [Google Scholar]
- 58.Chiu DT, Davidson M, Stromberg A, Ryttsen F, Orwar O. Electrical and optical methods for the manipulation and analyses of single cells. Proc SPIE. 2001;4255:1–8. [Google Scholar]
- 59.Sott K, Karlsson M, Pihl J, Hurtig J, Lobovkina T, et al. Micropipet writing technique for production of two-dimensional lipid bilayer nanotube-vesicle networks on functionalized and patterned surfaces. Langmuir. 2003;19:3904–3910. [Google Scholar]
- 60.Karlsson M, Sott K, Cans AS, Karlsson A, Karlsson R, et al. Micropipet-assisted formation of microscopic networks of unilamellar lipid bilayer nanotubes and containers. Langmuir. 2001;17:6754–6758. [Google Scholar]
- 61.Hurtig J, Karlsson M, Orwar O. Topographic SU 8 substrates for immobilization of three-dimensional nanotube-vesicle networks. Langmuir. 2004;20:5637–5641. doi: 10.1021/la0498051. [DOI] [PubMed] [Google Scholar]
- 62.Lizana L, Bauer B, Orwar O. Controlling the rates of biochemical reactions and signaling networks by shape and volume changes. Proc Natl Acad Sci U S A. 2008;105:4099–4104. doi: 10.1073/pnas.0709932105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hurtig J, Gustafsson B, Tokarz M, Orwar O. Electrophoretic transport insurfactant nanotube networks wired on microfabricated substrates. Anal Chem. 2006;78:5281–5288. doi: 10.1021/ac060229i. [DOI] [PubMed] [Google Scholar]
- 64.Raucher D, Sheetz M. Characteristics of a membrane reservoir buffering membrane tension. Biophys J. 1999;77:1992–2002. doi: 10.1016/S0006-3495(99)77040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch. 1973;28C:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 66.Evans E, Needham D. Physical properties of surfactant bilayer membranes: thermal transitions, elasticity, rigidity, cohesion and colloidal interactions. J Phys Chem. 1987;91:4219–4228. [Google Scholar]
- 67.Lipowsky R. The conformation of membranes. Nature. 1991;349:475–481. doi: 10.1038/349475a0. [DOI] [PubMed] [Google Scholar]
- 68.Iglic A, Hägerstrand H, Veranic P, Plemenitas A, Kralj-Iglic V. Curvature-induced accumulation of anisotropic membrane components and raft formation in cylindrical membrane protrusions. J Theor Biol. 2006;240:368–373. doi: 10.1016/j.jtbi.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 69.Iglic A, Hägerstrand H, Bobrowska-Ḧ{a}gerstrand M, Arrigler V, Kralj-Iglic V. Possible role of phospholipid nanotubes in directed transport of membrane vesicles. Phys Lett A. 2003;310:493–497. [Google Scholar]
- 70.Galkina SI, Sud'ina GF, Ullrich V. Inhibition of neutrophil spreading during adhesion to fibronectin reveals formation of long tubulovesicular cell extensions (cytonemes) Exp Cell Res. 2001;266:222–228. doi: 10.1006/excr.2001.5227. [DOI] [PubMed] [Google Scholar]
- 71.Upadhyaya A, Sheetz MP. Tension in tubulovesicular networks of Golgi and endoplasmic reticulum membranes. Biophys J. 2004;86:2923–2928. doi: 10.1016/S0006-3495(04)74343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cuvelier D, Derényi I, Bassereau P, Nassoy P. Coalescence of membrane tethers: experiments, theory, and applications. Biophys J. 2005;88:2714–2727. doi: 10.1529/biophysj.104.056473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tokarz M, Hakonen B, Dommersnes P, Orwar O, Akerman B. Electrophoretic transport of latex particles in lipid nanotubes. Langmuir. 2007;23:7651–7658. doi: 10.1021/la700336u. [DOI] [PubMed] [Google Scholar]
- 74.Karlsson M, Sott K, Davidson M, Cans AS, Linderholm P, et al. Formation of geometrically complex lipid nanotube- vesicle networks of higher-order topologies. Proc Natl Acad Sci U S A. 2002;99:11573–11578. doi: 10.1073/pnas.172183699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lobovkina T, Dommersnes P, Joanny JF, Orwar O. Formation and release of circular lipid nanotubes. Soft Matter. 2008;4:467–470. doi: 10.1039/b715771a. [DOI] [PubMed] [Google Scholar]
- 76.Derenyi I, Julicher F, Prost J. Formation and interaction of membrane tubes. Phys Rev Lett. 2002;88 doi: 10.1103/PhysRevLett.88.238101. Art. No. 238101. [DOI] [PubMed] [Google Scholar]
- 77.Lichtman JW, Conchello JA. Fluorescence microscopy. Nat Methods. 2005;2:910–919. doi: 10.1038/nmeth817. [DOI] [PubMed] [Google Scholar]
- 78.Dalby MJ, Riehle MO, Johnstone H, Affrossman S, Curtis ASG. Investigating the limits of filopodial sensing: a brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia. Cell Biol Int. 2004;28:229–236. doi: 10.1016/j.cellbi.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Galkina SI, Molotkovsky JG, Ullrich V, Sud'ina GF. Scanning electron microscopy study of neutrophil membrane tubulovesicular extensions (cytonemes) and their role in anchoring, aggregation and phagocytosis. The effect of nitric oxide Exp Cell Res. 2005;304:620–629. doi: 10.1016/j.yexcr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 80.Dass C. Fundamentals of Contemporary Mass Spectroscopy. New York: John Wiley & Sons; 2007. [Google Scholar]
- 81.Chiu DT, Lorenz RM. Chemistry and Biology in Femtoliter and Picoliter Volume Droplets. Acc Chem Res. 2009;42:649–658. doi: 10.1021/ar8002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeffries GDM, Edgar JS, Zhao YQ, Shelby JP, Fong C, et al. Using polarization-shaped optical vortex traps for single-cell nanosurgery. Nano Lett. 2007;7:415–420. doi: 10.1021/nl0626784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shelby JP, Edgar JS, Chiu DT. Monitoring cell survival after extraction of a single subcellular organelle using optical trapping and pulsed-nitrogen laser ablation. Photochem Photobiol. 2005;81:994–1001. doi: 10.1562/2005-02-02-RA-431. [DOI] [PubMed] [Google Scholar]
- 84.Lorenz RM, Edgar JS, Jeffries GDM, Zhao YQ, McGloin D, et al. Vortex-trap-induced fusion of femtoliter-volume aqueous droplets. Anal Chem. 2007;79:224–228. doi: 10.1021/ac061586w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edgar JS, Pabbati CP, Lorenz RM, He MY, Fiorini GS, et al. Capillary electrophoresis separation in the presence of an immiscible boundary for droplet analysis. Anal Chem. 2006;78:6948–6954. doi: 10.1021/ac0613131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schiro PG, Kuyper CL, Chiu DT. Continuous-flow single-molecule CE with high detection efficiency. Electrophoresis. 2007;28:2430–2438. doi: 10.1002/elps.200600730. [DOI] [PubMed] [Google Scholar]
- 87.Klar TA, Jakobs S, Dyba M, Egner A, Hell SW. Fluorescence microscopy with diffraction resolution barrier broken by stimulated emission. Proc Natl Acad Sci U S A. 2000;97:8206–8210. doi: 10.1073/pnas.97.15.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Westphal V, Hell SW. Nanoscale Resolution in the Focal Plane of an Optical Microscope. Phys Rev Lett. 2005;94:143903. doi: 10.1103/PhysRevLett.94.143903. [DOI] [PubMed] [Google Scholar]
- 89.Hodneland E, Lundervold A, Gurke S, Tai XC, Rustom A, et al. Automated detection of tunneling nanotubes in 3D images. Cytometry A. 2006;69:961–972. doi: 10.1002/cyto.a.20302. [DOI] [PubMed] [Google Scholar]
- 90.Dommersnes PG, Orwar O, Brochard-Wyart F, Joanny JF. Marangoni transport in lipid nanotubes. Europhys Lett. 2005;70:271–277. [Google Scholar]
- 91.Karlsson R, Karlsson A, Orwar O. Formation and Transport of Nanotube-Integrated Vesicles in a Lipid Bilayer Network. J Phys Chem B. 2003;107:11201–11207. [Google Scholar]
- 92.Markström M, Gunnarsson A, Orwar O, Jesorka A. Dynamic microcompartmentalization of giant unilamellar vesicles by sol-gel transition and temperature induced shrinking/swelling of poly(N-isopropyl acrylamide) Soft Matter. 2007;3:587–595. doi: 10.1039/b610351k. [DOI] [PubMed] [Google Scholar]
- 93.Sott K, Lobovkina T, Lizana L, Tokarz M, Bauer B, et al. Controlling enzymatic reactions by geometry in a biomimetic nanoscale network. Nano Lett. 2006;6:209–214. doi: 10.1021/nl052078p. [DOI] [PubMed] [Google Scholar]
- 94.Lizana L, Konkoli Z, Orwar O. Tunable filtering of chemical signals in a simple nanoscale reaction-diffusion network. J Phys Chem B. 2007;111:6214–6219. doi: 10.1021/jp068313p. [DOI] [PubMed] [Google Scholar]
- 95.Markström M, Lizana L, Orwar O, Jesorka A. Thermoactuated diffusion control in soft matter nanofluidic devices. Langmuir. 2008;24:5166–5171. doi: 10.1021/la7035967. [DOI] [PubMed] [Google Scholar]
- 96.Koster G, VanDuijn M, Hofs B, Dogterom M. Membrane tube formation from giant vesicles by dynamic association of motor proteins. Proc Natl Acad Sci U S A. 2003;100:15583–15588. doi: 10.1073/pnas.2531786100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chizmadzhev Y, Kumenko D, Kuzmin P, Chernomordik L, Zimmerberg J, et al. Lipid flow through fusion pores connecting membranes of different tensions. Biophys J. 1999;76:2951–2965. doi: 10.1016/S0006-3495(99)77450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karlsson R, Karlsson M, Karlsson A, Cans AS, Bergenholtz J, et al. Moving-wall-driven flows in nanofluidic systems. Langmuir. 2002;18:4186–4190. [Google Scholar]
- 99.Tsafrir I, Sagi D, Arzi T, Guedeau-Boudeville MA, Frette V, et al. Pearling instabilities of membrane tubes with anchored polymers. Phys Rev Lett. 2001;86:1138. doi: 10.1103/PhysRevLett.86.1138. [DOI] [PubMed] [Google Scholar]
- 100.Bar-Ziv R, Moses E. Instability and “pearling” states produced in tubular membranes by competition of curvature and tension. Phys Rev Lett. 1994;73:1392. doi: 10.1103/PhysRevLett.73.1392. [DOI] [PubMed] [Google Scholar]
- 101.McCann FE, Eissmann P, Önfelt B, Leung R, Davis DM. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J Immunol. 2007;178:3418–3426. doi: 10.4049/jimmunol.178.6.3418. [DOI] [PubMed] [Google Scholar]
Further Reading
On the following topics we recommend these reviews as further reading.
Intercellular nanotubes
- Gurke S, Barroso JFV, Gerdes HH. The art of cellular communication: tunneling nanotubes bridge the divide. Histochemistry and Cell Biology. 2008;129(5):539–550. doi: 10.1007/s00418-008-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Köhler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Önfelt B, Sattentau Q, Davis DM. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nature Cell Biology. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nature Reviews Immunology. 2007;7(3):238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- Gerdes HH, Carvalho RN. Intercellular transfer mediated by tunneling nanotubes. Current Opinion in Cell Biology. 2008 doi: 10.1016/j.ceb.2008.03.005. [DOI] [PubMed] [Google Scholar]
Nanotube-Vesicle Networks
- Karlsson M, Davidson M, Karlsson R, Karlsson A, Bergenholtz J, Konkoli Z, Jesorka A, Lobovkina T, Hurtig J, Voinova M, Orwar O. Biomimetic Nanoscale Reactors and Networks. Annual Review of Physical Chemistry. 2004;55:613–649. doi: 10.1146/annurev.physchem.55.091602.094319. [DOI] [PubMed] [Google Scholar]
- Jesorka A, Orwar O. Liposomes: Technologies and Analytical Applications. Annual Review of Analytical Chemistry. 2008;1:801–832. doi: 10.1146/annurev.anchem.1.031207.112747. [DOI] [PubMed] [Google Scholar]
HIV and ICNs
- Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends in Cell Biology. 2008;18(9):414–420. doi: 10.1016/j.tcb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nature Reviews Immunology. 2008;8(6):447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. Cell Biology: Tube travel for HIV? Nature Reviews Microbiology. 2008;6(3):175. doi: 10.1038/nrmicro1866. [DOI] [Google Scholar]
- Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nature Reviews Microbiology. 2008;6(11):815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]


