Abstract
BACKGROUND
Reproductive factors reflective of endogenous sex hormone exposure might have an effect on cardiac remodeling and the development of heart failure (HF).
OBJECTIVES
This study examined the association between key reproductive factors and the incidence of HF.
METHODS
Women from a cohort of the Women’s Health Initiative were systematically evaluated for the incidence of HF hospitalization from study enrollment through 2014. Reproductive factors (number of live births, age at first pregnancy, and total reproductive duration [time from menarche to menopause]) were self-reported at study baseline in 1993 to 1998. We employed Cox proportional hazards regression analysis in age- and multivariable-adjusted models.
RESULTS
Among 28,516 women, with an average age of 62.7 ± 7.1 years at baseline, 1,494 (5.2%) had an adjudicated incident HF hospitalization during an average follow-up of 13.1 years. After adjusting for covariates, total reproductive duration in years was inversely associated with incident HF: hazard ratios (HRs) of 0.99 per year (95% confidence interval [CI]: 0.98 to 0.99 per year) and 0.95 per 5 years (95% CI: 0.91 to 0.99 per 5 years). Conversely, early age at first pregnancy and nulliparity were significantly associated with incident HF in age-adjusted models, but not after multivariable adjustment. Notably, nulliparity was associated with incident HF with preserved ejection fraction in the fully adjusted model (HR: 2.75; 95% CI: 1.16 to 6.52).
CONCLUSIONS
In postmenopausal women, shorter total reproductive duration was associated with higher risk of incident HF, and nulliparity was associated with higher risk for incident HF with preserved ejection fraction. Whether exposure to endogenous sex hormones underlies this relationship should be investigated in future studies.
Keywords: cardiovascular disease, menarche, menopause, pregnancy, women
Endogenous sex hormones present during a woman’s reproductive period prior to menopause may have unique effects on her lifetime risk of atherosclerosis, hypertension, cardiac remodeling, and heart failure (HF) (1,2). Two physiological phenomena affecting endogenous sex hormone levels during the reproductive period include menstrual cycling and pregnancy. The protective effects of menstrual cycles are frequently hypothesized to explain why premenopausal women have a lower risk of cardiovascular disease (CVD) compared with postmenopausal women of similar age, although this is still a controversial hypothesis (2,3). Moreover, data suggest that women with early menopause have an elevated risk of coronary heart disease (CHD) and stroke, and there are conflicting data as to whether early menopause also predicts HF (4–7).
In addition to menstrual cycling, pregnancy involves alterations in cardiovascular hemodynamics, fluid balance, inflammatory and thrombotic pathways, and exposure to endogenous sex hormones, all of which impart a variety of peripartum and postpartum risks to women (8–11). Previous analyses have suggested a relationship between reproductive factors, such as total number of pregnancies and number of live births, with the subsequent development of CVD, including HF (12–21). A variety of mechanisms have been proposed, but the relationship remains incompletely understood and is likely multifactorial. Notably, a higher number of pregnancies leading to live births has been associated with increases in left ventricular (LV) mass and end-diastolic volume, as well as with a decrease in LV ejection fraction, which may lead to HF development (18).
By comparison, infertility has also been associated with increased maternal cardiovascular risk: women who reported at least 5 years of infertility before having a successful pregnancy demonstrated a 19% higher incidence of CVD compared with women not reporting infertility (17). Consistent with these findings, an analysis of the Swedish population registry found a J-shaped relationship between a woman’s number of live births and her subsequent risk of CVD, finding that both nulliparous women and women with at least 5 live births had increased risk compared with women with 2 live births (16). A higher number of pregnancies correlates with an earlier age at first birth, which also has been demonstrated to be independently associated with higher risk for CHD (22). It is unclear to what extent socioeconomic factors may affect this risk (23).
To better characterize the relationship between reproductive factors and subsequent HF risk, we examined postmenopausal women in the WHI (Women’s Health Initiative) to test associations among total number of live births, age at first pregnancy lasting at least 6 months, and total reproductive duration (time from menarche to menopause) with incident HF. Our hypotheses were that nulliparity and multiparity (>2 pregnancies) would be associated with an increased risk of HF, as would a younger age at first pregnancy and a shorter total reproductive duration.
METHODS
The design of WHI has been described previously (24,25). Briefly, a total of 161,808 women were enrolled in the observational study and clinical trial components from 1993 to 1998 in the United States. We studied the subset of 44,174 WHI participants included in the University of North Carolina (UNC) HF cohort, for whom HF outcomes were centrally adjudicated from enrollment through September 2014. This cohort includes all women randomized to the hormone trial component of WHI (n = 27,347) and all black participants (n = 11,880) and Hispanic participants (n = 4,947) from the other clinical trials and the observational study.
Figure 1 depicts the creation of our analytic sample. All women with pre-existing CVD (n = 467), including CHD, HF, stroke, or myocardial infarction, were excluded from the analysis, as were women with missing data (n = 15,191). Of the remaining participants, 28,516 women had completed menopause at enrollment and were included in the current analyses.
Figure 1. Creation of the Study Sample: WHI.
After excluding patients with cardiovascular disease (CVD) and missing reproductive data, the study included 28,516 participants from the University of North Carolina (UNC) heart failure (HF) cohort of the WHI (Women’s Health Initiative).
Baseline characteristics, including a detailed reproductive history, were obtained by interviews and questionnaires (24,25). Reproductive factors were defined as the age at first pregnancy lasting at least 6 months, total number of live births, and total reproductive duration (defined as the age at menarche subtracted from age at menopause). Selected covariates included age at screening, household income, education level, ethnicity, U.S. region, body mass index, hypertension (defined by self-report, systolic blood pressure $ 140 mm Hg or diastolic blood pressure >90 mm Hg at screening, or use of antihypertensive medication), diabetes (defined by fasting glucose >126 mg/dl or use of diabetes medication), hyperlipidemia requiring the use of medication, smoking status, history of breast-feeding for at least 1 month, history of pregnancy loss, prior hysterectomy, and usage of oral contraception or menopausal hormone therapy.
Within the cohort, all confirmed cases of HF hospitalization and patient-reported development of HF, angina, or CVD during hospitalization were sent to trained physicians at UNC for adjudication. These physicians reviewed the HF hospitalization medical records and classified cases following the algorithm used in the ARIC (Atherosclerosis Risk in Communities) study (26). Initial and subsequent hospitalizations were included, and information such as ejection fraction and diastolic dysfunction were included, when available, from the medical record. Cases were classified into 1 of the following 5 categories: definite acute decompensated heart failure (ADHF), possible ADHF, chronic stable HF, unclassifiable, or HF unlikely.
The primary outcome for this analysis was the time to development of a first hospitalization for definite or probable ADHF or newly diagnosed stable HF. Additional outcomes included the time to development of heart failure with reduced ejection fraction (HFrEF) (defined as EF <50%) and time to development of heart failure with preserved ejection fraction (HFpEF) (defined as EF $ 50%), analyzed individually. In the 90 cases where a participant developed both HFpEF and HFrEF during the analytic period, the 2 classes of HF outcomes were not considered mutually competing events.
STATISTICAL ANALYSIS
Cox proportional hazards regression models, both age- and multivariable-adjusted, estimated associations among the number of live births, age at first pregnancy, and total reproductive duration with incident hospitalized HF, and with HFrEF and HFpEF individually. We tested the assumption of proportionality of hazards with Kaplan-Meier survival analysis, and we performed sensitivity analyses to test the competing influence of death during follow up. A p value <0.05 was considered statistically significant. All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
In secondary analyses, we separately evaluated the individual determinants of reproductive duration-age at menarche and age at menopause-with incident HF hospitalization. We also investigated effect modification by race/ethnicity by including an interaction term crossing race/ethnicity with reproductive duration in the regression model.
Recognizing that 42% of women in our study sample reported a history of hysterectomy prior to enrollment (Table 1), we separately evaluated the incidence of our primary outcome among women with natural menopause (without history of hysterectomy or oophorectomy) and among women with surgical menopause (with history of hysterectomy and/or oophorectomy). In secondary sensitivity analyses, we evaluated the incidence of our primary outcome among women without oral contraceptive use or menopausal hormone therapy, and after adjusting for infertility.
Table 1.
Baseline Characteristics (N = 28,516)
| Age at screening, yrs | 62.7 ± 7.1 |
|
| |
| Age at menopause, yrs | 47.1 ± 7.3 |
|
| |
| Age at menarche, yrs | 12.6 ±1.5 |
|
| |
| Reproductive duration, yrs | 34.4 ± 7.4 |
|
| |
| Age at first pregnancy, yrs | 20–24* (N/A) |
|
| |
| Total number of Live births | 3.3 ±1.7 |
|
| |
| History of pregnancy Loss | 12,135 (42.7) |
|
| |
| History of breastfeeding for >1 month | 16,618 (58.8) |
|
| |
| Hysterectomy prior to screening | 12,098 (42.4) |
|
| |
| Oral contraceptive use (any) | 12,393 (43.5) |
|
| |
| Menopausal hormone therapy (any) | 111,012 (38.6) |
|
| |
| Body mass index at screening, kg/m2 | 29.6 ± 6.2 |
|
| |
| Systolic blood pressure, mm Hg | 129.1 ± 17.6 |
|
| |
| Diabetes | 2,516 (8.8) |
|
| |
| High cholesterol requiring medications | 3,904 (13.9) |
|
| |
| Smoking status | |
| Current smoker | 2,857 (10.1) |
| Former smoker | 10,964 (38.9) |
| Never smoked | 14,374 (51.0) |
|
| |
| Household income | |
| <$35,000 | 13,669 (51.0) |
| $35,000 to $74,999 | 9,955 (37.0) |
| $ $75,000 | 3,179 (11.9) |
|
| |
| Education | |
| High school and below | 11,405 (40.3) |
| Some college and above | 16,870 (60.7) |
|
| |
| U.S. region | |
| Northeast | 5,764 (20.2) |
| South | 8,916 (31.3) |
| Midwest | 6,710 (23.5) |
| West | 7,126 (25.0) |
|
| |
| Ethnicity | |
| American Indian | 89 (0.3) |
| Asian or Pacific Islander | 355 (1.3) |
| Black or African American | 8,848 (31.1) |
| Hispanic/Latino | 3,564 (12.5) |
| White (non-Hispanic) | 15,408 (54.1) |
| Other | 221 (0.8) |
Values are mean ± SD or n (%).
Age at first pregnancy lasting at least 6 months was recorded as a categorical variable (age <20, 20–24, 25–29, 30–34, 35–39, 40–44, or >45 years), and the mean of the rank order was the category age 20–24 years.
N/A = not applicable.
RESULTS
Of the 44,174 women included in the UNC HF adjudication cohort, 28,516 women had no missing reproductive data, were free of prevalent CVD, and were included in the analysis (Figure 1). Baseline characteristics of this sample are presented in Table 1. The mean age at screening was 62.7 years, and the mean ages at menarche and menopause were 12.6 and 47.1 years, respectively, with a mean total reproductive duration of 34.4 years. The average number of live births was 3.3.
The mean and median follow-up were 13.1 and 15.2 years, respectively. Minimum follow-up was 9 days and maximum follow-up was 19.6 years. There were 1,494 cases of incident adjudicated HF hospitalization, of which 505 were cases with HFrEF and 586 were cases with HFpEF. In the remaining 403 cases, ejection fraction was not available or was undetermined. There were 5,600 deaths during follow up: 827 deaths (15%) were preceded by HF events, including 271 with HFpEF, 204 with HFrEF, and 352 with undetermined ejection fraction.
PRIMARY ANALYSES
Shorter total reproductive duration was associated with increased risk of HF, with an age-adjusted hazard ratio (HR) of 0.98 per year (95% confidence interval [CI]: 0.97 to 0.98 per year; p <0.0001) and a multivariable-adjusted HR of 0.99 per year (95% CI: 0.98 to 0.99 per year; p = 0.02). This equates to a 5-year adjusted HR of 0.95 (95% CI: 0.91 to 0.99) (Table 2, Central Illustration).
Table 2.
Reproductive Factors and Incident Total HF
| Number of Women (n = 28,516) | Incident HF Events | Incident HF Events Per 100 Person-Yrs | Age-Adjusted
|
Multivariable-Adjusted*
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p Value Across Group | Hazard Ratio | 95% CI | p Value Across Group | ||||
| Number of Live births | 0.21 | 0.48 | |||||||
| 0 | 188 | 16 | 7.5 | 1.80 | 1.07–3.03 | 1.70 | 0.95–3.03 | ||
| 1 | 3,237 | 138 | 3.4 | Reference | N/A | Reference | N/A | ||
| 2 | 7,113 | 332 | 3.6 | 1.05 | 0.86–1.28 | 1.13 | 0.91–1.41 | ||
| 3 | 7,041 | 358 | 3.9 | 1.03 | 0.84–1.25 | 1.17 | 0.94–1.45 | ||
| 4 | 5,051 | 287 | 4.4 | 1.12 | 0.91–1.38 | 1.19 | 0.95–1.50 | ||
| $ 5 | 5,886 | 363 | 5.0 | 1.13 | 0.92–1.38 | 1.15 | 0.92–1.45 | ||
|
| |||||||||
| Age at first pregnancy, yrs | <0.0001 | 0.26 | |||||||
| <20 | 6,967 | 377 | 4.4 | 1.42 | 1.16–1.75 | 1.14 | 0.91–1.43 | ||
| 20–24 | 12,822 | 645 | 3.9 | 1.07 | 0.88–1.29 | 0.99 | 0.81–1.22 | ||
| 25–29 | 6,202 | 336 | 4.1 | 1.01 | 0.82–1.23 | 1.04 | 0.83–1.29 | ||
| $ 30 | 2,525 | 136 | 4.2 | Reference | N/A | Reference | N/A | ||
|
| |||||||||
| Reproductive duration (per additional yr) | 28,516 | 1,494 | 4.1 | 0.98 | 0.97–0.98 | <0.0001 | 0.99 | 0.98–0.99 | 0.02 |
Multivariable models were adjusted for: age at screening, household income, education level, ethnicity, U.S. region, body mass index, hypertension, diabetes, hyperlipidemia, smoking status, breastfeeding, history of pregnancy loss, prior hysterectomy, and usage of oral contraception or menopausal hormone therapy.
CI = confidence interval; HF = heart failure.
CENTRAL ILLUSTRATION. Reproductive Factors and Incidence of HF.
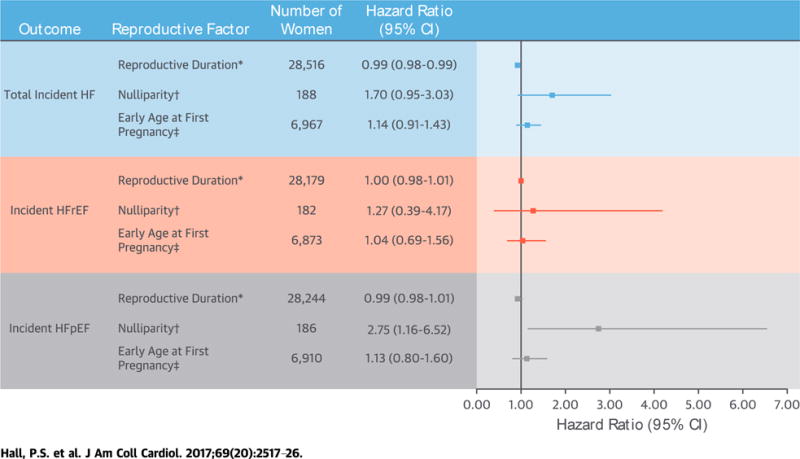
We examined the association between key reproductive factors and incidence of heart failure (HF) in a cohort of the WHI (Women’s Health Initiative). Hazard ratios and 95% confidence intervals (CIs) were adjusted for age at screening, household income, education level, ethnicity, U.S. region, body mass index, hypertension, diabetes, hyperlipidemia, smoking status, breastfeeding, history of pregnancy Loss, prior hysterectomy, and usage of oral contraception or menopausal hormone therapy. In postmenopausaL women, shorter total reproductive duration was associated with a higher risk of incident HF, and nulliparity was associated with a higher risk for incident heart failure with preserved ejection fraction (HFpEF). *Per year. †Compared with women with 1 Live birth. ‡Age of first pregnancy Lasting at Least 6 months at <20 years of age compared with referent $ 30 years of age. HFrEF = heart failure with reduced ejection fraction.
Younger age at first pregnancy (age <20 years) and nulliparity were associated with increased risk of incident HF in age-adjusted models (HR: 1.42; 95% CI: 1.16 to 1.75; and HR: 1.80; 95% CI: 1.07 to 3.03, respectively). However, these associations were not statistically significant after multivariable adjustment (younger age at first pregnancy HR: 1.14; 95% CI: 0.91 to 1.43; nulliparity HR: 1.70; 95% CI: 0.95 to 3.03) (Table 2).
Shorter total reproductive duration was associated with increased risk of HFrEF in age-adjusted models (HR: 0.98; 95% CI: 0.97 to 0.99), but not after multivariable adjustment (Table 3). Nulliparity was associated with a statistically significant increased risk of HFpEF in both age-adjusted (HR: 2.57; 95% CI: 1.22 to 5.44) and multivariable-adjusted models (HR: 2.75; 95% CI: 1.16 to 6.52) (Table 4). This association remained significant in sensitivity analyses after additional adjustment for infertility (HR: 2.62; 95% CI: 1.24 to 5.55). Early age at first pregnancy and shorter total reproductive duration were associated with increased risk of HFpEF in age-adjusted but not multivariable-adjusted models (Table 4).
Table 3.
Reproductive Factors and Incident HF With Reduced Ejection Fraction
| Number of Women (n = 28,179) | Incident HF Events | Incident HF Events Per 100 Person-Yrs | Age-Adjusted
|
Multivariable-Adjusted*
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p Value Across Group | Hazard Ratio | 95% CI | p Value Across Group | ||||
| Number of Live births | 0.51 | 0.68 | |||||||
| 0 | 182 | 4 | 1.9 | 1.43 | 0.51–3.97 | 1.27 | 0.39–4.17 | ||
| 1 | 3,207 | 45 | 1.1 | Reference | N/A | Reference | N/A | ||
| 2 | 7,044 | 109 | 1.2 | 1.05 | 0.74–1.50 | 1.18 | 0.80–1.76 | ||
| 3 | 6,959 | 118 | 1.3 | 1.05 | 0.74–1.48 | 1.28 | 0.86–1.90 | ||
| 4 | 4,977 | 95 | 1.5 | 1.15 | 0.80–1.65 | 1.30 | 0.86–1.97 | ||
| $ 5 | 5,810 | 134 | 1.9 | 1.29 | 0.91–1.83 | 1.40 | 0.94–2.10 | ||
|
| |||||||||
| Age at first pregnancy, yrs | 0.12 | 0.99 | |||||||
| <20 | 6,873 | 124 | 1.5 | 1.31 | 0.92–1.88 | 1.04 | 0.69–1.56 | ||
| 20–24 | 12,677 | 230 | 1.4 | 1.10 | 0.79–1.54 | 1.04 | 0.72–1.50 | ||
| 25–29 | 6,128 | 106 | 1.3 | 0.95 | 0.67–1.36 | 1.00 | 0.68–1.47 | ||
| $ 30 | 2,501 | 45 | 1.4 | Reference | N/A | Reference | N/A | ||
|
| |||||||||
| Reproductive duration (per additional yr) | 28,179 | 505 | 1.4 | 0.98 | 0.97–0.99 | <0.0001 | 1.00 | 0.98–1.01 | 0.54 |
Table 4.
Reproductive Factors and Incident HF With Preserved Ejection Fraction
| Number of Women (n = 28,244) | Incident HF Events | Incident HF Events Per 100 Person-Yrs | Age-Adjusted
|
Multivariable-Adjusted*
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | p Value | 95% CI Across Group | Hazard Ratio | p Value | 95% CI Across Group | ||||
| Number of Live births | 0.11 | 0.22 | |||||||
| 0 | 186 | 8 | 3.8 | 2.57 | 1.22–5.44 | 2.75 | 1.16–6.52 | ||
| 1 | 3,211 | 49 | 1.2 | Reference | N/A | Reference | N/A | ||
| 2 | 7,063 | 122 | 1.3 | 1.08 | 0.78–1.51 | 1.20 | 0.84–1.73 | ||
| 3 | 6,971 | 146 | 1.6 | 1.16 | 0.84–1.62 | 1.30 | 0.91–1.87 | ||
| 4 | 4,999 | 124 | 1.9 | 1.34 | 0.96–1.88 | 1.37 | 0.94–1.99 | ||
| $ 5 | 5,814 | 137 | 1.9 | 1.17 | 0.84–1.63 | 1.20 | 0.83–1.74 | ||
|
| |||||||||
| Age at first pregnancy, yrs | <0.001 | 0.07 | |||||||
| <20 | 6,910 | 152 | 1.8 | 1.51 | 1.09–2.10 | 1.13 | 0.80–1.60 | ||
| 20–24 | 12,697 | 252 | 1.5 | 1.06 | 0.78–1.43 | 0.84 | 0.61–1.15 | ||
| 25–29 | 6,133 | 128 | 1.6 | 0.96 | 0.69–1.32 | 0.89 | 0.63–1.23 | ||
| $ 30 | 2,504 | 54 | 1.7 | Reference | N/A | Reference | N/A | ||
|
| |||||||||
| Reproductive duration (per additional yr) | 28,244 | 586 | 1.6 | 0.98 | 0.97–0.99 | <0.001 | 0.99 | 0.98–1.01 | 0.23 |
Sensitivity analyses considering death as a competing event were not materially different from the reported results (data not shown).
SECONDARY ANALYSES
Table 5 presents the HRs for the individual components of reproductive duration, age at menarche, and age at menopause, both for the entire sample and stratified by surgical versus natural menopause. The association between shorter reproductive duration and increased risk of incident HF was related to an earlier age at menopause (adjusted HR: 0.99 per additional year before menopause; 95% CI: 0.98 to 0.99 per additional year), and particularly among women with natural menopause (adjusted HR: 0.97 per year before natural menopause; 95% CI: 0.96 to 0.99 per year). There was no evidence of statistically significant effect modification by race/ethnicity (p = 0.13).
Table 5.
Ages at Menarche and Menopause and Incident HF
| Age-Adjusted
|
Multivariable-Adjusted*
|
|||||
|---|---|---|---|---|---|---|
| Hazard Ratio† | 95% Cl | p Value Across Group | Hazard Ratio† | 95% Cl | p Value Across Group | |
| Overall (n = 25,084) | ||||||
| Age at menarche | 0.96 | 0.93–0.99 | 0.001 | 0.98 | 0.95–1.00 | 0.23 |
| Age at menopause | 0.98 | 0.97–0.98 | <0.001 | 0.99 | 0.98–0.99 | 0.02 |
|
| ||||||
| Natural menopause‡ (n = 13,969) | ||||||
| Age at menarche | 0.95 | 0.90–0.99 | 0.02 | 0.97 | 0.02–1.02 | 0.19 |
| Age at menopause | 0.98 | 0.96–0.99 | 0.002 | 0.97 | 0.96–0.99 | 0.002 |
|
| ||||||
| Surgical menopause‡ (n = 11,057) | ||||||
| Age at menarche | 0.98 | 0.94–1.02 | 0.22 | 0.99 | 0.95–1.04 | 0.68 |
| Age at menopause | 0.99 | 0.98–0.99 | 0.03 | 1.00 | 0.99–1.01 | 0.52 |
Multivariable models were adjusted for: age at screening, household income, education level, ethnicity, U.S. region, body mass index, hypertension, diabetes, hyperlipidemia, smoking status, breastfeeding, history of pregnancy loss, and usage of oral contraception or menopausal hormone therapy.
Per additional year.
Surgical menopause includes women who reported hysterectomy and/or oophorectomy. Natural menopause includes neither hysterectomy nor oophorectomy.
Abbreviations as in Table 2.
Early age at first pregnancy (age <20 years) was significantly associated with increased risk of HF hospitalization among women without a history of oral contraceptive or menopausal hormone therapy (Table 6) and among women with natural menopause (Table 7), but not surgical menopause (Table 8).
Table 6.
Reproductive Factors and Incident Total HF Among Women Without Use of Oral Contraceptive Pills or Menopausal Hormone Therapy
| Number of Women (n = 10.337) | Incide Heart Failure Events | Incide Heart Failure Events Per 100 Person-Yrs | Age-Adjusted
|
Multivariable-Adjusted*
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p Value Across Group | Hazard Ratio | 95% CI | p Value Across Group | ||||
| Number of Live births | 0.95 | 0.98 | |||||||
| 0 | 92 | 7 | 6.9 | 1.37 | 0.63–2.98 | 1.34 | 0.53–3.38 | ||
| 1 | 1,177 | 64 | 4.6 | Reference | N/A | Reference | N/A | ||
| 2 | 2,378 | 149 | 5.0 | 1.10 | 0.82–1.48 | 1.09 | 0.79–1.51 | ||
| 3 | 2,407 | 155 | 5.1 | 1.04 | 0.77–1.39 | 1.07 | 0.77–1.48 | ||
| 4 | 1,895 | 131 | 5.6 | 1.10 | 0.81–1.49 | 1.06 | 0.76–1.49 | ||
| $ 5 | 2,388 | 167 | 5.9 | 1.08 | 0.81–1.45 | 1.02 | 0.74–1.42 | ||
|
| |||||||||
| Age at first pregnancy, yrs | <0.0001 | 0.03 | |||||||
| <20 | 2,376 | 168 | 6.2 | 1.90 | 1.40–2.59 | 1.61 | 1.14–2.27 | ||
| 20–24 | 4,570 | 290 | 5.1 | 1.34 | 1.01–1.79 | 1.25 | 0.92–1.70 | ||
| 25–29 | 2,336 | 156 | 5.2 | 1.23 | 0.91–1.67 | 1.22 | 0.88–1.70 | ||
| $ 30 | 1,055 | 59 | 4.4 | Reference | N/A | Reference | N/A | ||
|
| |||||||||
| Reproductive duration (per additional yr) | 10,337 | 673 | 5.3 | 0.98 | 0.97–0.99 | 0.004 | 0.99 | 0.98–1.01 | 0.34 |
Multivariable models were adjusted for: age at screening, household income, education level, ethnicity, U.S. region, body mass index, hypertension, diabetes, hyperlipidemia, smoking status, breastfeeding, history of pregnancy Loss, and prior hysterectomy.
Abbreviations as in Table 2.
Table 7.
Reproductive Factors and Incident Total HF Among Women With Natural Menopause*
| Number of Women (n = 15,675) | Incident HF Events | Incident HF Events Per 100 Person-Yrs | Age-Adjusted
|
Multivariable-Adjusted†
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Cl | p Value Across Group | Hazard Ratio | 95% Cl | p Value Across Group | ||||
| Number of Live births | 0.20 | 0.65 | |||||||
| 0 | 92 | 7 | 6.3 | 2.33 | 1.06–5.13 | 1.74 | 0.62–4.88 | ||
| 1 | 1,679 | 52 | 2.4 | Reference | N/A | Reference | N/A | ||
| 2 | 3,988 | 161 | 3.0 | 1.18 | 0.86–1.62 | 1.27 | 0.91–1.79 | ||
| 3 | 3,955 | 162 | 3.0 | 1.03 | 0.75–1.42 | 1.15 | 0.81–1.62 | ||
| 4 | 2,830 | 141 | 3.8 | 1.21 | 0.87–1.68 | 1.26 | 0.87–1.78 | ||
| $ 5 | 3,131 | 167 | 4.2 | 1.22 | 0.89–1.68 | 1.14 | 0.78–1.63 | ||
|
| |||||||||
| Age at first pregnancy, yrs | 0.0001 | 0.02 | |||||||
| <20 | 3,157 | 148 | 3.8 | 1.81 | 1.34–2.45 | 1.59 | 1.13–2.22 | ||
| 20–24 | 7,031 | 283 | 3.0 | 1.20 | 0.91–1.58 | 1.21 | 0.90–1.62 | ||
| 25–29 | 3,793 | 191 | 3.7 | 1.26 | 0.95–1.67 | 1.35 | 0.99–1.83 | ||
| $ 30 | 1,694 | 68 | 3.1 | Reference | N/A | Reference | N/A | ||
|
| |||||||||
| Reproductive duration (per additional yr) | 15,675 | 690 | 3.3 | 0.98 | 0.96–0.99 | 0.0003 | 0.98 | 0.96–0.99 | 0.002 |
Table 8.
Reproductive Factors and Incident Total HF Among Women With Surgical Menopause*
| Number of Women (n = 12,773) | Incident HF Events | Events Per100 Person-Yrs | Age-Adjusted
|
Multivariable-Adjusted†
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p Value Across Group | Hazard Ratio | 95% CI | p Value Across Group | ||||
| Number of Live births | 0.63 | 0.43 | |||||||
| 0 | 94 | 9 | 9.0 | 1.66 | 0.83–3.30 | 1.75 | 0.87–3.53 | ||
| 1 | 1,544 | 85 | 4.5 | Reference | N/A | Reference | N/A | ||
| 2 | 3,112 | 170 | 4.3 | 0.97 | 0.75–1.27 | 1.02 | 0.76–1.36 | ||
| 3 | 3,072 | 195 | 5.0 | 1.06 | 0.82–1.37 | 1.20 | 0.90–1.59 | ||
| 4 | 2,210 | 146 | 5.3 | 1.10 | 0.84–1.44 | 1.16 | 0.86–1.57 | ||
| $ 5 | 2,741 | 196 | 5.9 | 1.09 | 0.84–1.41 | 1.16 | 0.87–1.55 | ||
|
| |||||||||
| Age at first pregnancy, yrs | 0.01 | 0.28 | |||||||
| <20 | 3,791 | 228 | 5.0 | 1.04 | 0.78–1.39 | 0.84 | 0.62–1.15 | ||
| 20–24 | 5,759 | 360 | 4.9 | 0.86 | 0.66–1.13 | 0.78 | 0.59–1.04 | ||
| 25–29 | 2,401 | 145 | 4.8 | 0.74 | 0.55–0.99 | 0.75 | 0.55–1.03 | ||
| $ 30 | 822 | 68 | 6.6 | Reference | N/A | Reference | N/A | ||
|
| |||||||||
| Reproductive duration (per additional yr) | 12,773 | 801 | 5.0 | 0.99 | 0.98–1.00 | 0.12 | 1.00 | 0.99–1.01 | 0.60 |
DISCUSSION
Overall, our study demonstrated an association between selected reproductive factors and incident HF. Specifically, a shorter total reproductive duration resulted in a modestly increased risk of any HF, likely driven by a younger age at menopause. Moreover, our findings suggested that the association between total reproductive duration and HF is more pronounced in women with natural menopause compared with surgical menopause. Additionally, nulliparity was associated with an increased risk of HFpEF, even after adjustment for cardiovascular and sociodemographic confounders. However, higher parity was not associated with HF risk.
SHORTER REPRODUCTIVE DURATION AND HF
Prior data have suggested that women with early menopause, and consequently a shorter reproductive period with fewer reproductive cycles and lower cumulative exposure to endogenous sex hormones, have an increased risk of CHD, stroke, and possibly HF (4–7). An analysis of the Framingham Heart Study population suggested that the increased CHD risk with early menopause may be influenced by a loss of the protective effects of endogenous sex hormones and an increase in thrombotic risk (27). Estrogens have beneficial effects on nitric oxide synthesis and signaling, coronary artery calcification, myocardial contractile reserve, insulin resistance, and lipid metabolism, and progesterone has been demonstrated to decrease blood pressure through vasodilation and decreased angiotensin-II vasoresponsiveness (2,28–33). Unfortunately, randomized trials of sex hormone supplementation have failed to uniformly support the hypothesis that cardiovascular protection can be restored with exogenous estrogen or estrogen/progesterone supplementation (1,2,30,34–36).
Our finding that a shorter total reproductive duration was associated with a modestly increased risk of HF, even after adjustment for traditional cardiovascular risk factors, and particularly among women with natural menopause, might be due to the increased CHD risk that accompanies early menopause. We did not demonstrate a significant association between a shorter reproductive period and HFrEF that would corroborate this hypothesis, but a substantial proportion of HF events had unknown ejection fraction (27%), suggesting that HFrEF was likely under-represented. Importantly, another analysis of the Framingham population raised the possibility of reverse causation, demonstrating that premenopausal CHD risk factors, including higher serum cholesterol levels, blood pressure, and weight, were each associated with earlier age at menopause (37). These findings warrant ongoing evaluation of the potential cardioprotective mechanisms of endogenous sex hormone exposure in women.
NUMBER OF PREGNANCIES LEADING TO LIVE BIRTHS AND HF
Our findings that nulliparity was associated with a higher risk of incident HFpEF were also consistent with prior data from the Swedish Population Registers, which suggested a J-shaped association between the number of pregnancies and subsequent cardiovascular disease (including HF). We did not observe a statistically significant increased risk of HFpEF with higher numbers of live births in this study.
To our knowledge, the association between nulliparity and HFpEF has not been reported previously. The continuous ovulatory cycles uninterrupted by pregnancy that correlate with nulliparity have been reported to increase the risk of breast, ovarian, and uterine cancers (38), but are generally believed to be protective against adverse cardiac remodeling and diastolic dysfunction (39). We hypothesized that the association between nulliparity and HFpEF might be driven by the established association between infertility, a correlate of nulliparity, and CVD (17,40). Exact causes of infertility have not been closely examined in terms of their association with CVD, and a large cohort analysis did not implicate fertility therapy as a cause of subsequent cardiovascular disease (41). We repeated the analysis controlling for a reported history of infertility, and the association between nulliparity and HFpEF was still present (adjusted HR: 2.62; 95% CI: 1.24 to 5.55), suggesting that the association could not be explained by infertility. It remains possible that residual confounding by hypertension or sociodemographic factors associated with hypertension is still present, despite their inclusion as covariates in the regression model, and this association merits further investigation.
STUDY LIMITATIONS
The availability of reproductive data and adjudicated HF outcomes in the UNC HF cohort of WHI allowed us to uniquely evaluate the association between total reproductive duration and number of pregnancies. Only the first occurrence of HF hospitalization was included as an outcome in the initial WHI study, and details such as LV ejection fraction were not uniformly reported. We had a large sample size that was well represented along racial/ethnic, socioeconomic, and geographic lines. A substantial proportion of women in the cohort had missing reproductive data and were excluded from the analysis, which would likely diminish our power to identify true associations. We also relied upon self-reporting in determining our primary exposures and we did not have biomarker-confirmed menopause status. Additional information regarding pregnancy complications, such as pregnancy-induced hypertension, pre-eclampsia, and peripartum cardiomyopathy, would have been helpful and informative to include in the model, but were not available in the WHI cohort at the time of our analysis. A substantial percentage of HF cases had undetermined EF (27%), and more detailed echocardiographic findings or biomarkers, such as brain natriuretic peptide, would have been helpful to confirm the diagnoses. Finally, our decision to analyze HFpEF and HFrEF outcomes individually required additional statistical tests, and our results should be interpreted as hypothesis generating, with the need for corroboration in other populations. We did not account for multiple tests in determining statistical significance.
CONCLUSIONS
In this study, we found that a shorter total reproductive duration was associated with higher risk of incident total HF hospitalization in postmenopausal women, and nulliparity was associated with higher risk of incident hospitalized HFpEF. Whether exposure to endogenous sex hormones underlies this relationship should be investigated in future studies.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Postmenopausal women with longer reproductive duration have a lower risk of hospitalization for HF, and nulliparity is associated with a higher risk of developing HFpEF.
TRANSLATIONAL OUTLOOK
Further studies are needed to determine the mechanisms linking endogenous sex hormone exposure during a woman’s reproductive years to cardiovascular risk after menopause.
Acknowledgments
This work was supported by the American College of Cardiology Merck Award (to Dr. Hall), American Heart Association Grant 13CRP17350002 (to Dr. Parikh), and National Institutes of Health grant 7R21HL115398 (to Dr. Parikh). The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services, through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
ABBREVIATIONS AND ACRONYMS
- ADHF
acute decompensated heart failure
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
Footnotes
Listen to this manuscript’s audio summary by JACC Editor-in-Chief Dr. Valentin Fuster.
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Harvey RE, Coffman KE, Miller VM. Women-specific factors to consider in risk, diagnosis and treatment of cardiovascular disease. Womens Health (Lond) 2015;11:239–57. doi: 10.2217/whe.14.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011;109:687–96. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tunstall-Pedoe H. Myth and paradox of coronary risk and the menopause. Lancet. 1998;351:1425–7. doi: 10.1016/S0140-6736(97)11321-6. [DOI] [PubMed] [Google Scholar]
- 4.Appiah D, Schreiner PJ, Demerath EW, Loehr LR, Chang PP, Folsom AR. Association of age at menopause with incident heart failure: a prospective cohort study and meta-analysis. J Am Heart Assoc. 2016;5:e003769. doi: 10.1161/JAHA.116.003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebong IA, Watson KE, Goff DC, Jr, et al. Age at menopause and incident heart failure: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2014;21:585–91. doi: 10.1097/GME.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman I, Akesson A, Wolk A. Relationship between age at natural menopause and risk of heart failure. Menopause. 2015;22:12–6. doi: 10.1097/GME.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 7.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;19:1081–7. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams JQ, Alexander AM., Jr Alterations in cardiovascular physiology during labor. Obstet Gynecol. 1958;12:542–9. [PubMed] [Google Scholar]
- 9.Bernstein IM, Ziegler W, Badger GJ. Plasma volume expansion in early pregnancy. Obstet Gynecol. 2001;97:669–72. doi: 10.1016/s0029-7844(00)01222-9. [DOI] [PubMed] [Google Scholar]
- 10.Carbillon L, Uzan M, Uzan S. Pregnancy, vascular tone, and maternal hemodynamics: a crucial adaptation. Obstet Gynecol Surv. 2000;55:574–81. doi: 10.1097/00006254-200009000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Chung E, Leinwand LA. Pregnancy as a cardiac stress model. Cardiovasc Res. 2014;101:561–70. doi: 10.1093/cvr/cvu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. Am J Obstet Gynecol. 2016;215:484.e1–14. doi: 10.1016/j.ajog.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 13.Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367–80. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ness RB, Harris T, Cobb J, et al. Number of pregnancies and the subsequent risk of cardiovascular disease. N Engl J Med. 1993;328:1528–33. doi: 10.1056/NEJM199305273282104. [DOI] [PubMed] [Google Scholar]
- 15.Ngo AD, Roberts CL, Figtree G. Association between interpregnancy interval and future risk of maternal cardiovascular disease-a population-based record linkage study. BJOG. 2016;123:1311–8. doi: 10.1111/1471-0528.13729. [DOI] [PubMed] [Google Scholar]
- 16.Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later-life maternal cardiovascular disease. Am Heart J. 2010;159:215–21.e6. doi: 10.1016/j.ahj.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Parikh NI, Cnattingius S, Mittleman MA, Ludvigsson JF, Ingelsson E. Subfertility and risk of later life maternal cardiovascular disease. Hum Reprod. 2012;27:568–75. doi: 10.1093/humrep/der400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh NI, Lloyd-Jones DM, Ning H, et al. Association of number of live births with Left ventricular structure and function. The Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2012;163:470–6. doi: 10.1016/j.ahj.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker DR, Lu B, Sands-Lincoln M, et al. Risk of cardiovascular disease among postmenopausal women with prior pregnancy loss: the Women’s Health Initiative. Ann Fam Med. 2014;12:302–9. doi: 10.1370/afm.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steenland K, Lally C, Thun M. Parity and coronary heart disease among women in the American Cancer Society CPS II population. Epidemiology. 1996;7:641–3. doi: 10.1097/00001648-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg EO, Thienelt CD, Katz SE, et al. Gender differences in molecular remodeling in pressure overload hypertrophy. J Am Coll Cardiol. 1999;34:264–73. doi: 10.1016/s0735-1097(99)00165-5. [DOI] [PubMed] [Google Scholar]
- 22.Parikh NI, Jeppson RP, Berger JS, et al. Reproductive risk factors and coronary heart disease in the Women’s Health Initiative Observational Study. Circulation. 2016;133:2149–58. doi: 10.1161/CIRCULATIONAHA.115.017854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JS, Jung I, Youn JC, Cho HY. Impact of adolescent pregnancy on hypertension in postmenopausal women. J Hypertens. 2016;34:47–53. doi: 10.1097/HJH.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 24.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–8. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 25.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 26.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannel WB, Levy D. Menopause, hormones, and cardiovascular vulnerability in women. Arch Intern Med. 2004;164:479–81. doi: 10.1001/archinte.164.5.479. [DOI] [PubMed] [Google Scholar]
- 28.Petre RE, Quaile MP, Rossman EI, et al. Sex-based differences in myocardial contractile reserve. Am J Physiol Regul Integr Comp Physiol. 2007;292:R810–8. doi: 10.1152/ajpregu.00377.2006. [DOI] [PubMed] [Google Scholar]
- 29.Seed M, Knopp RH. Estrogens, Lipoproteins, and cardiovascular risk factors: an update following the randomized placebo-controlled trials of hormone-replacement therapy. Curr Opin Lipidol. 2004;15:459–67. doi: 10.1097/01.mol.0000137231.84772.80. [DOI] [PubMed] [Google Scholar]
- 30.Shlipak MG, Chaput LA, Vittinghoff E, et al. Lipid changes on hormone therapy and coronary heart disease events in the Heart and Estrogen/progestin Replacement Study (HERS) Am Heart J. 2003;146:870–5. doi: 10.1016/S0002-8703(03)00412-5. [DOI] [PubMed] [Google Scholar]
- 31.Christian RC, Liu PY, Harrington S, Ruan M, Miller VM, Fitzpatrick LA. Intimal estrogen receptor (ER)beta, but not ERalpha expression, is correlated with coronary calcification and atherosclerosis in pre- and postmenopausal women. J Clin Endocrinol Metab. 2006;91:2713–20. doi: 10.1210/jc.2005-2672. [DOI] [PubMed] [Google Scholar]
- 32.Kanaya AM, Herrington D, Vittinghoff E, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/Progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 33.Thomas P, Pang Y. Protective actions of progesterone in the cardiovascular system: potential role of membrane progesterone receptors (mPRs) in mediating rapid effects. Steroids. 2013;78:583–8. doi: 10.1016/j.steroids.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 35.Maric-Bilkan C, Arnold AP, Taylor DA, et al. Report of the National Heart, Lung, and Blood Institute Working Group on Sex Differences Research in Cardiovascular Disease: scientific questions and challenges. Hypertension. 2016;67:802–7. doi: 10.1161/HYPERTENSIONAHA.115.06967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 37.Kok HS, van Asselt KM, van der Schouw YT, et al. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976–83. doi: 10.1016/j.jacc.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 38.Britt K, Short R. The plight of nuns: hazards of nulliparity. Lancet. 2012;379:2322–3. doi: 10.1016/S0140-6736(11)61746-7. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L. Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H628–40. doi: 10.1152/ajpheart.00859.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of Family Growth. Natl Health Stat Report. 2013:1–18. [PubMed] [Google Scholar]
- 41.Udell JA, Lu H, Redelmeier DA. Long-term cardiovascular risk in women prescribed fertility therapy. J Am Coll Cardiol. 2013;62:1704–12. doi: 10.1016/j.jacc.2013.05.085. [DOI] [PubMed] [Google Scholar]


