Abstract
Mimetics of Glucagon-like peptide 1 (GLP-1) are becoming a useful alternative or complementary treatment choice to insulin in the treatment of diabetes mellitus. The lack of hypoglycemia as a side effect when GLP-1 receptor agonists are used along with the tendency of these therapeutic agents to prevent or even reduce weight gain makes them valuable targets in therapy development. However, native GLP-1 and many of its early analogues have very short half-lives, requiring repeated treatment to maintain therapeutic levels. Since all current treatments are injected subcutaneously, a large focus has been made on trying to extend the half-lives of GLP-1 analogues while retaining bioactivity. Most success in this regard has been achieved with the use of peptide-protein fusions, which are not as well suited for oral administration. However, recent work focused on the development of non-fusion peptides with increased half-lives may be more appropriate for oral administration. This mini-review discusses the structural characteristics of past and present analogues as well as the recent work that has been done towards developing novel GLP-1 receptor agonists.
Keywords: Peptide-mimetics, Glucagon-like peptide 1, GLP-1, Diabetes Mellitus, T2DM, T1DM
INTRODUCTION
Diabetes mellitus is a metabolic disorder associated with highly elevated blood glucose levels as a result of insulin deficiency and has been categorized into two types [Ashcroft and Rorsman 2012]. Type I diabetes mellitus (T1DM) is an auto-immune disease wherein β cells are destroyed, resulting in the loss of the ability to produce insulin. T1DM is typically diagnosed in childhood and requires treatment throughout the patient’s lifetime. Type II diabetes mellitus (T2DM) is more common. It is an acquired disorder that is strongly influenced by lifestyle and genetic predisposition. Once acquired, it too requires constant treatment. Both types carry a burden in the form of a wide array of complications including hematologic abnormalities, cataracts, microangiopathies, neuropathies, and macroangiopathies in the kidney and retina [Brownlee and Cerami 1981].
Glucagon-like peptide 1 (GLP-1) is an incretin hormone produced by the enzymatic degradation of pro-glucagon [Kim and Egan 2008; Lopez et al. 1983]. Full length GLP-1 is 36 amino acids long and contains a C-terminal amide. However, its active form (the form that is secreted) is truncated at the N-terminus to produce GLP-1 (7–36 amide). The secreted (7–36 amide) peptide is what is typically referred to as GLP-1 [Williams 2016]. The secretion of GLP-1 in the ileum is induced after meals. β cells contain the most GLP-1 receptors; however, adipose cells and cells in the central nervous system (CNS) also express GLP-1 receptors. In the brain, GLP-1 decreases appetite and increases satiety, resulting in lower water and food intake. In the stomach, it reduces acid production and decreases gastric emptying. In adipose tissues, GLP-1 increases glucose uptake and glycogen production. In the pancreas, it is responsible for inducing insulin synthesis and secretion in β cells while increasing β cell neogenesis and regeneration, and reducing apoptosis. GLP-1 also acts on α and γ cells in the pancreas, reducing glucagon secretion and increasing somatostatin secretion, respectively [Drucker and Nauck 2006]. Because the mechanism of action of GLP-1 on β cells is of particular importance to diabetes mellitus, it will be discussed briefly in more detail. GLP-1 activates the GLP-1R G-protein coupled receptor in β cells, resulting in production of cAMP, which in turn leads to an increase in glucose-stimulated insulin secretion. This activation has been found to occur through phosphorylation of synaptotagmin-7 by protein kinase A (PKA), resulting in an increase in Ca2+-activated insulin exocytosis [Wu et al. 2015]. An alternative, PKA and cAMP-independent mechanism for insulin response has also been identified wherein phospholipase C (PLC) mediates activation of transient receptor potential family receptors TRPM4 and TRPM5 resulting in sensitization of β cells to glucose [Kolic and MacDonald 2015].
Due to the ability of GLP-1 to reduce apoptosis and to induce neogensis and regeneration in β cells, as well as to induce insulin production and secretion, GLP-1 and its mimetics are primarily used as alternative first line therapies to insulin-based therapeutics or in combination therapies for T2DM [Drucker and Nauck 2006]. However, native GLP-1 has a very limited plasma half-life of only a few minutes before it is degraded. Thus, mimetics of GLP-1 have been and continue to be developed in order to decrease the frequency with which treatments, generally delivered through subcutaneous injection, need to be administered. GLP-1 mimetics primarily come in two forms, GLP-1 receptor agonists, which exogenously activate the GLP-1 receptor to upregulate insulin production and secretion, and dipeptidyl peptidase IV (DPP-IV) inhibitors, which slow GLP-1 degradation by binding to DPP-IV and preventing it from degrading natively produced GLP-1 [Drucker and Nauck 2006]. This mini-review will focus on recent advances in the development of GLP-1 receptor agonists intended for the treatment of diabetes mellitus.
Several GLP-1 mimetic drugs are already in use clinically (Table 1), including Exenatide (a synthetically produced version of the Exendin-4 sequence originally found in the venom of Heloderma suspectum and used as a starting scaffold for several mimetics) [Eng et al. 1992; Gedulin et al. 2003], Liraglutide [Ostawal et al. 2016], Dulaglutide [Jimenez-Solem et al. 2010], Albiglutide [Stewart et al. 2008], and Lixisenatide [Christensen et al. 2009]. Long acting versions of many of these drugs are also available, most typically prepared by changing their formulations to slow absorption after injection or to increase their affinity to serum proteins, which in turn increases their plasma half-lives substantially [van Witteloostuijn et al. 2016]. These mimetics all target the GLP-1 receptor in β cells and are approved for treatment of T2DM and in some cases, such as Liraglutide, for treatment of obesity. Newer mimetics have longer half-lives, decreasing the frequency with which they need to be administered. For example, Exenatide is dosed twice daily, but its long acting version is dosed once weekly. In comparison, the original formulation of Liraglutide is given either once-daily or once weekly [Bayes et al. 2003; Gedulin et al. 2003]. Recent progress in developing new mimetic drugs has been made by continuing the effort to increase the plasma half-life of GLP-1 through pendant modifications. Research groups have also looked more closely at the activity of the degradation products of GLP-1 to identify peptides with additional therapeutic potential, although these peptides are suspected to act independently from the GLP-1 receptor pathway [Sun et al. 2015a; Tomas et al. 2011]. Table 1 summarizes the different GLP-1 peptide mimics that have seen recent development, and includes those peptides approved for clinical use and some peptides that either failed or have been withdrawn from clinical trials for comparison.
Table 1.
Clinical Status and Sequences of GLP-1 Receptor Peptide Agonists
| Peptide | Clinical Status |
Sequence | Ref. |
|---|---|---|---|
| GLP-1 (7–36) | HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG-NH2 | [Lopez et al. 1983] | |
| Exendin-4 | Approved | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS-NH2 | [Eng et al. 1992] |
| Liraglutide (Victoz, Saxenda) | Approved | HAEGTFTSDVSSYLEGQAA-(K-P1)-EFIAWLVRGRG | [Bayes et al. 2003; Ostawal et al. 2016] |
| Exenatide (Byetta, Bydureon) | Approved | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS-NH2 | [Gedulin et al. 2003] |
| CJC-1131 | Preclinical (No longer in development) | HaEGTFTSDVSSYLEGQAAKEFIAWLVKGR-P2 | [Kim et al. 2003] |
| Albiglutide (Eperzan, Tanzeum) | Approved | HGEGTFTSDVSSYLEGQAAKEFIAWLVKGRHGEGTFTSDVSSYLEGQAAKEFIAWLVKGR-Albumin | [Stewart et al. 2008] |
| Taspoglutide | Failed Phase III (Hypersensitivity) | H-Aib-EGTFTSDVSSYLEGQAAKEFIAWLVK-Aib-R-NH2 | [Sebokova et al. 2010] |
| CJC-1134 | Canceled Phase II (Competition) | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPP-P3 | [Baggio et al. 2008] |
| Lixisenatide | Approved | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPSKKKKKK-NH2 | [Christensen et al. 2009] |
| Exenatide-XTEN | Phase I | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS-XTEN | [Schellenberger et al. 2009] |
| Efpeglenatide (LAPS CA Exendin-4, LAPS Exendin-4, HM11260C) | Phase II | 4-imidazoacetyl-GEGTFTSDLSKQMEEEAVRLFIEWL-(K-PEG-Fc)-NGGPSSGAPPPS-NH2 | [Choi et al. 2014] |
| Dulaglutide (Trulicity) | Approved | HGEGTFTSDVSSYLEEQAAKEFIAWLVKGG-Fc-Fc-GGKVLWAIFEKAAQEELYSSVDSTFTGEGH | [Jimenez-Solem et al. 2010] |
| Semaglutide (NN9924/OG217SC) | Phase III | H-Aib-EGTFTSDVSSYLEGQAA-(K-P4)-EFIAWLVRGRG-NH2 | [Lund et al. 2011] |
| Amphiphile Mimetic | Preclinical | HSEGTFTSDGEEEAAVV-(K-P5)-NH2 | [Khan et al. 2012] |
| Coumaglutide (13c) | Preclinical | HGEGTFTSDV-(C-P6)-SYLEGQAAKEFIAWLVKGR-NH2 | [Sun et al. 2015b] |
| I-3 | Preclinical | HGEGTFTSDLS-(C-P7)-QMEEEAVRLFIEWLKNGGPSSGAPPPS-NH2 | [Sun et al. 2016] |
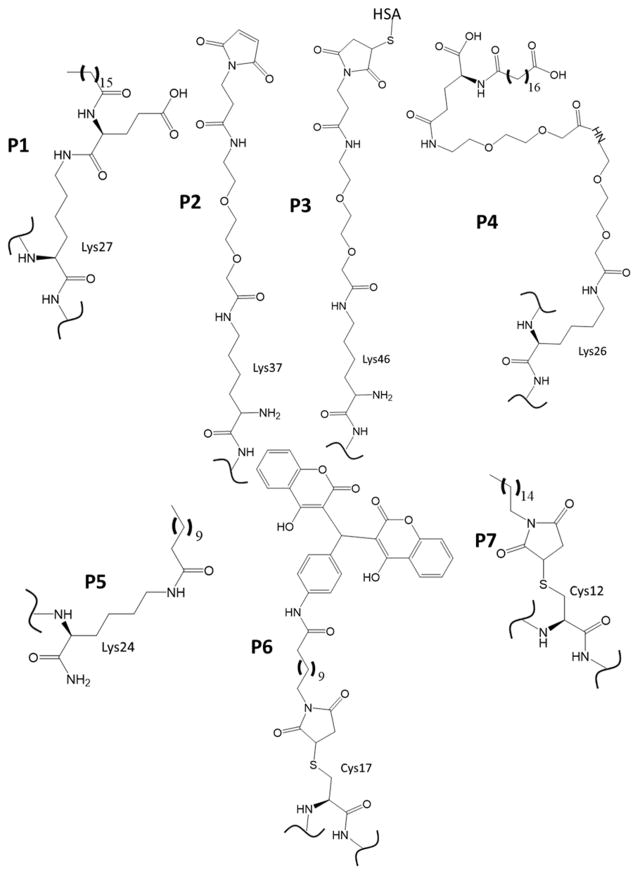
Text that is bolded indicates modification from the native GLP-1 (7-36 amide) sequence. See Fig. 2 for the chemical structures represented by pendant groups P1-7.
A common strategy for improving the plasma half-life of GLP-1 mimetic peptides has been to increase their association with blood-borne proteins such as albumin through the inclusion of pendant groups with affinity to those proteins. In some cases the protein or a fragment of the protein is used directly as the pendant modification. Both human serum albumin and the Fc fragment of IgG (which also reduces peptide immunogenicity) have been used to increase the plasma half-life [Kratz and Elsadek 2012; van Witteloostuijn et al. 2016]. Incorporation of the pendant groups is generally accomplished through attachment via a linker, either to an amino acid side chain, or to one of the peptide’s termini. In cases where the pendant group does not directly increase the plasma half-life, the pendant modification associates with the targeted protein noncovalently, and thereby increases the half-life.
COVALENTLY BINDING PENDANT MODIFICATIONS
Efpeglenatide (HM11260C, Langlenatide, LAPS CA Exendin-4, LAPS Exendin-4) is a modified version of Exendin-4 [Choi et al. 2015; Choi et al. 2014]. The N-terminus histidine is replaced with a 4-imidazoacetyl group (Fig. 1) (effectively removing the terminal amine) to improve the analog’s resistance to degradation [Jung et al. 2016; Song et al. 2009]. In addition, this analogue also has an internal flexible PEG linker at lysine 27 that attaches an aglycosylated Fc fragment of IgG to increase the plasma half-life and to lower the immunogenicity of the peptide [Watkins et al. 2015; Yoon et al. 2015]. A trial with diabetic participants receiving either 6 mg once weekly or 16 mg once monthly of Efpeglenatide compared with 1.8 mg Liraglutide daily or placebo was conducted for 90 days. The results showed that Efpeglenatide was comparable to Liraglutide in its ability to reduce blood glucose levels; however, it was better at stimulating insulin secretion. It was generally well tolerated with some gastrointestinal (GI)-related side effects [Watkins et al. 2015]. Another 12-week study that followed diabetic subjects found that relatively higher doses of Efpeglenatide (3 – 4 mg, compared with 0.8, 1, and 2 mg) administered once weekly lowered HbA1c levels (a measure of the 3 month average blood glucose levels) as well or better than 1.8 mg of Liraglutide given once daily [Yoon et al. 2015]. A 20-week study comparing once weekly dosing to every other week dosing given to obese subjects who did not have diabetes found that all dosing types were effective at lowering body mass [Pratley et al. 2015]. Experiments have suggested that Efpeglenatide exhibits super-agonistic activity due to an approximately 2- to 4-fold larger Kd when binding to the GLP-1 receptor, which can help explain its improved ability to stimulate insulin secretion [Choi et al. 2015; Choi et al. 2016]. It is intended to be a weekly or monthly subcutaneous injection treatment for T2DM or obesity.
Fig. 1.
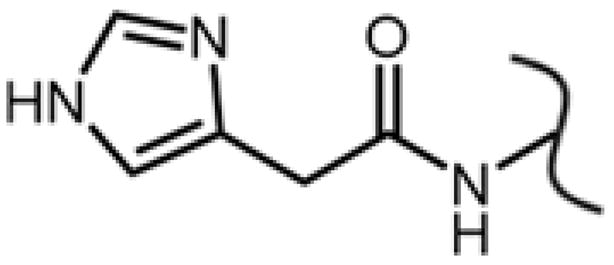
The substitution used in Efpeglenatide to remove the N-terminal amine from the N-terminus histidine.
CJC-1131 is a GLP-1-based peptide that utilizes a C-terminal-linked maleimide to react with a cysteine residue on human serum albumin in vivo to form a covalent bond resulting in extended plasma half-life. CJC-1131 also has a D-Ala substitution at its second residue to increase its resistance to enzymatic degradation by DPP-IV [Kim et al. 2003]. Although this analogue was active in diabetic animal models, further work was not conducted [Kratz and Elsadek 2012]. Instead, work was continued using CJC-1134.
CJC-1134 (CJC-1134-PC) has a recombinant human serum albumin attached to the lysine-extended C-terminus of Exendin-4 using the same thiol-coupling strategy used for in vivo coupling in CJC-1131 (attachment via the reaction between a cysteine and a maleimide reactive group) to increase the plasma half-life [Baggio et al. 2008]. This peptide showed similar potency to Exenatide in in vitro studies. In mice feeding studies, CJC-1134 reduced food intake more than Exenatide. It performed similarly to Exenatide in reducing GLP-1R-dependent gastric emptying. A 4-week experiment where mice were fed with a high fat diet and treated either once or twice daily with either Exenatide or CJC-1134 showed significant weight loss, eventually bringing weights down to be comparable to those of mice on a normal diet. However, serum HbA1c levels were only significantly reduced in mice treated with CJC-1134, suggesting that CJC-1134 can lower average blood glucose levels faster than Exenatide. Cholesterol levels were also lowered only in CJC-1134-treated mice over the period of the study. Both Exenatide and CJC-1134 caused increases in triglycerides in the plasma due to reduction in transcription for fatty acid binding proteins 1 and 2. Levels of triglycerides have been observed to drop in humans after long-term treatment of Exenatide, suggesting that this may be a difference between the effect in mice and in humans [Simo et al. 2015]. CJC-1134 also performed well in diabetes models and showed promise as a once a week therapeutic in phase I/II clinical trials, but faced competition in the marketplace from already established drugs, such as Liraglutide, with similar treatment regimens that limited its predicted market share, and thus clinical trials with this analogue were terminated [Christensen and Knop 2010; Kratz and Elsadek 2012].
Exenatide-XTEN (VRS 859, E-XTEN) is a fusion between the Exendin-4 peptide and the engineered protein XTEN that was developed recombinantly in Escherichia coli [Schellenberger et al. 2009; Tomlinson et al. 2016]. The addition of the XTEN protein is projected to both increase the plasma half-life to approximately 139 h and also slow down the subcutaneous absorption rate (to approximately 100 h), making this mimetic a candidate for once-a-month treatment. Exenatide-XTEN showed low immunogenicity and performed similarly to Exenatide in glucose challenge experiments in mice models [Schellenberger et al. 2009]. In a 30-day dose-ranging clinical trial, Exenatide-XTEN showed promise as a once-a-month treatment to provide glycemic control and appeared to only have minor GI side effects [Cleland et al. 2012]
NONCOVALENTLY BINDING PENDANT MODIFICATIONS
Semaglutide (NN9924/OG217SC) is an Exendin-4 derivative that incorporates a very long, flexible linker (Fig. 2, P4) at lysine 26 and ends with a 16 carbon chain terminating in a carboxyl group. This functionalization was done to increase the association with human serum albumin to increase the plasma half-life. Semaglutide is currently undergoing phase III clinical trials for use as a once-weekly subcutaneous injection treatment for T2DM. It has also started phase I and II clinical trials for oral administration. It has shown increased weight loss and decreases in HbA1c compared to Liraglutide at higher doses, but also had an increased occurrence of side effects, primarily GI-tract-related, at those dosages. Its plasma half-life is also greater than liraglutide’s [Lund et al. 2014; Tomlinson et al. 2016].
Fig. 2.
The linkers and pendant groups for non-fusion peptides used in established and recently developed GLP-1 receptor agonists to increase the plasma half-life and/or to reduce the immunogenicity of the peptides. The sequences in Table 1 indicate where these groups are incorporated. HSA is human serum albumin.
The Self-Assembling Amphiphilic GLP-1 mimetic discussed here has been developed primarily for potential use in aiding β cell transplants when treating T1DM [Khan et al. 2012]. The peptide contains a long carbon chain group (Fig. 2, P5) attached via a C-terminal lysine, similarly to Semaglutide and Liraglutide. However, rather than being used to extend plasma half-life, this pendant modification allows the GLP-1 analogue to self-assemble into 1-dimensional nanofibers that in turn develop into gel structures capable of stabilizing transplanted β cells. This process is aided by acidic conditions, which drives the formation of an α-helical conformation in the peptide. Calcium chloride is used for rapid gel-assembly. This peptide gel structure was also shown to have insulin upregulation activity comparable to that of Exendin-4. The peptides in the gel activate insulin release by the PKA-dependent pathway, but also, at higher concentrations, via a lipid-raft dependent pathway hypothesized to be mediated through Epac2. When cells were encapsulated in the gel, its insulinotropic effect was amplified by approximately 8-fold.
Coumaglutide is a GLP-1-based peptide containing a D8G substitution and incorporating a dicoumarol group by substituting cysteine for serine 17 (Fig. 2 P6) and attachment of the pendent group to the substituted cysteine using maleimide (compound 13c in reference) [Han et al. 2013]. It utilizes a linker length of 10 carbons between the dicoumarol group and the maleimide group used for attachment to the peptide. The role of the dichomarol group is to improve the affinity of the peptide to albumin in order to increase its half-life. Coumaglutide was comparable to Liraglutide and Exendin-4 in its ability to lower blood glucose and induce the secretion of insulin. The duration of its effects was found to be superior (with a half-life of 68 h) to those of Liraglutide and Exendin-4 in diabetic mouse models. Once daily injections increased glucose tolerance and gave lower HbA1c levels. Preliminary long-term treatment results in mouse models were comparable to those from long-term treatment of Liraglutide. Further mouse model and cell studies showed Coumaglutide to be comparable to Liraglutide in terms of weight management, glucose levels and tolerance, and cell stability [Sun et al. 2015b].
I-3 is an alkyl chain-functionalized Exenatide-based analogue with a K12C substitution (Fig. 2 P7) that permits reaction with a 16-carbon alkyl chain-functionalized maleimide group intended to increase the plasma half-life through interaction with human serum albumin [Sun et al. 2016]. The alkyl chain aids in increased binding affinity with albumin, and I-3 demonstrated a superior half-life (35 – 36 h) compared with liraglutide (15 – 16 h) while retaining similar activity levels to native GLP-1. Experiments with INS-1 cells showed that I-3 has similar cell-protective activity against glucolipotoxic and oxidative stresses to Exenatide and Liraglutide, reducing apoptosis as well as maintaining cell viability. It also performed similarly to Exenatide and Liraglutide in glucose challenge studies in SD rats. In long-acting effect studies with diabetic mouse models, I-3 performed similarly or better than Exenatide and Liraglutide.
A recent patent filing [Wieczorek et al. 2017] indicates some interest in the development of GLP-1 mimetics incorporating two linker/pendant groups to further enhance non-covalent binding to human serum albumin. These linkers are incorporated into lysine 27 and another lysine substituted at any site save 18 or 27. The linkers described are of the form |-NH-(CH2)2-(O-CH2)2)1-5-O-(CH2)1-5-CO-| with human serum albumin binding moieties of the form of either HOOC-(CH2)6-16-CO-| or HOOC-C6H4-O-(CH2)3-17-CO-|. The peptide sequences themselves are to be limited to no more than ten modifications from native GLP-1. The peptides appear to be compatible with oral administration.
DISCUSSION OF TRENDS
As can be seen in Fig. 2, most GLP-1 mimetics that extend their half-lives through noncovalent interactions have incorporated hydrophobic groups with long methylene chains terminating with either methyl or carboxyl groups. This appears to be sufficient to gain greatly increased plasma half-lives compared to that of the native peptide. However, there remains an interest in further extending the plasma half-life to enable once-monthly subcutaneous injections. Thus far, mimetics that are capable of potentially being administered once-monthly have all incorporated covalent attachment to the desired carrier protein. However, there is also an interest in developing orally administered treatments [Tomlinson et al. 2016], and this approach may limit a peptide’s oral bioavailability [Martinez and Amidon 2002], which means that developing modified peptides with long half-lives and smaller molecular weights will remain of interest with alternative strategies for increasing the peptide half-life such as multi-functionalization or the incorporation of more complicated groups such as dicoumarol likely becoming more prevalent in the future.
Few of the modifications made to these peptides have substantially altered their activities, which generally have similar magnitudes as the effects of those peptide mimetics that have already been clinically approved. The similar activities demonstrate that GLP-1 sequences are permissive to modification, and even large modifications such as the fusion of entire proteins can be conducted without significant loss of activity. This trend also emphasizes the major focus to increase the duration of effects rather than on developing more potent or active analogues.
CONCLUSIONS
There are several promising peptides developed that act as GLP-1 receptor agonists. A few are in mid to late clinical stage trials (Semaglutide and Efpeglenatide), while most of the rest are in preclinical or early clinical trials. Efforts to increase the plasma half-life of new mimetics have been predominantly successful, with once-monthly dosing now within reach. This has been accomplished without sacrificing activity, and in some cases, the activity actually improved. Future efforts will likely continue to focus on half-life extension with greater efforts going towards the development of oral therapeutics for diabetes mellitus.
Acknowledgments
The Nevada INBRE (NIH GM103440) is acknowledged for the generous support of research in our laboratory.
References
- Ashcroft FM, Rorsman P. Diabetes Mellitus and the beta Cell: The Last Ten Years. Cell. 2012;148(6):1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Cao X, Drucker DJ. An albumin-exendin-4 conjugate engages central and peripheral circuits regulating murine energy and glucose Homeostasis. Gastroenterology. 2008;134(4):1137–1147. doi: 10.1053/j.gastro.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Bayes M, Rabasseda X, Prous JR. Gateways to clinical trials - July/August 2003. Methods and Findings in Experimental and Clinical Pharmacology. 2003;25(6):483–506. [PubMed] [Google Scholar]
- Brownlee M, Cerami A. The Biochemistry of the Complications of Diabetes-Mellitus. Annual Review of Biochemistry. 1981;50:385–432. doi: 10.1146/annurev.bi.50.070181.002125. [DOI] [PubMed] [Google Scholar]
- Choi I, Park S, Trautmann M, Kim J, Lee Y, Kang J, Hompesch M, Kwon S. Superagonistic mechanism of increased glucodynamic and weight loss effects of (LAPS)CA-exendin-4 (efpeglenatide) Diabetologia. 2015;58:S379–S379. [Google Scholar]
- Choi IY, Park SH, Lee GH, Choi SM, Kim SJ, Kim YH, Kang JH, Trautmann M, Hompesch M, Son JW, et al. A Long-Acting Exendin-4 Analog Conjugate to the Human Fc Fragment Reveals Low Immunogenic Potential. San Francisco, CA: American Diabetes Association; 2014. Jun 13, [Google Scholar]
- Choi IY, Park SH, Trautmann M, Moon MJ, Kim JY, Lee YM, Hompesch M, Kwon SC. Underlying Superagonistic Mechanisms of Efpeglenatide in glycaemic control and Weight Loss Potency. American Diabetes Association; 2016. Jun 10, [Google Scholar]
- Christensen M, Knop FK. Once-Weekly GLP-1 Agonists: How Do They Differ from Exenatide and Liraglutide? Current Diabetes Reports. 2010;10(2):124–132. doi: 10.1007/s11892-010-0102-x. [DOI] [PubMed] [Google Scholar]
- Christensen M, Knop FK, Holst JJ, Vilsboll T. Lixisenatide, a novel GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. Idrugs. 2009;12(8):503–513. [PubMed] [Google Scholar]
- Cleland JL, Aronson R, Humphriss E, Shore C, Zhou R, Kipnes MS. Safety, Pharmacokinetics, and Pharmacodynamics of a Single Subcutaneous Dose of VRS-859 in Patients with Type 2 Diabetes. American Diabetes Association; 2012. Jun 9, [Google Scholar]
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- Eng J, Kleinman W, Singh L, Singh G, Raufman J. Isolation and Characterization of Exendin-4, an Exendin-3 Analog, from Heloderma-Suspectum Venom - Further Evidence for an Exendin Receptor on Dispersed Acini from Guinea-Pig Pancreas. Journal of Biological Chemistry. 1992;267(11):7402–7405. [PubMed] [Google Scholar]
- Gedulin B, Smith P, Prickett K, Tryon M, Barnhill S, Reynolds J, Parkes D, Yeo T, Young A. Dose-response for 28-day glycemic improvement following a single injection of long-acting-release exenatide (Synthetic exendin-4) in diabetic fatty Zucker (ZDF) rats. Diabetes. 2003;52:A109–A109. [Google Scholar]
- Han J, Sun L, Chu Y, Li Z, Huang D, Zhu X, Qian H, Huang W. Design, Synthesis, and Biological Activity of Novel Dicoumarol Glucagon-like Peptide 1 Conjugates. Journal of Medicinal Chemistry. 2013;56(24):9955–9968. doi: 10.1021/jm4017448. [DOI] [PubMed] [Google Scholar]
- Jimenez-Solem E, Rasmussen MH, Christensen M, Knop FK. Dulaglutide, a long-acting GLP-1 analog fused with an Fc antibody fragment for the potential treatment of type 2 diabetes. Current Opinion in Molecular Therapeutics. 2010;12(6):790–797. [PubMed] [Google Scholar]
- Jung Sy, Lim CK, Song DH, Bae SM, Kim YH, Kwon SC, Lee GS Hanmi Science Co., Ltd, assignee. US9422349 B2 N-terminal modified exendin-4 patent. 2016 Aug 23;
- Khan S, Sur S, Newcomb CJ, Appelt EA, Stupp SI. Self-assembling glucagon-like peptide 1-mimetic peptide amphiphiles for enhanced activity and proliferation of insulin-secreting cells. Acta Biomaterialia. 2012;8(5):1685–1692. doi: 10.1016/j.actbio.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, Baggio LL, Bridon DP, Castaigne JP, Robitaille MF, Jette L, Benquet C, Drucker DJ. Development and characterization of a glucagon-like peptide 1-albumin conjugate - The ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes. 2003;52(3):751–759. doi: 10.2337/diabetes.52.3.751. [DOI] [PubMed] [Google Scholar]
- Kim W, Egan JM. The Role of Incretins in Glucose Homeostasis and Diabetes Treatment. Pharmacological Reviews. 2008;60(4):470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolic J, MacDonald PE. cAMP-independent effects of GLP-1 on beta cells. Journal of Clinical Investigation. 2015;125(12):4327–4330. doi: 10.1172/JCI85004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz F, Elsadek B. Clinical impact of serum proteins on drug delivery. Journal of Controlled Release. 2012;161(2):429–445. doi: 10.1016/j.jconrel.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Lopez LC, Frazier ML, Su CJ, Kumar A, Saunders GF. Mammalian pancreatic preproglucagon contains three glucagon-related peptides. Proceedings of the National Academy of Sciences. 1983;80(18):5485–5489. doi: 10.1073/pnas.80.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund A, Knop FK, Vilsboll T. Emerging GLP-1 receptor agonists. Expert Opinion on Emerging Drugs. 2011;16(4):607–618. doi: 10.1517/14728214.2011.616493. [DOI] [PubMed] [Google Scholar]
- Lund A, Knop FK, Vilsboll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: Differences and similarities. European Journal of Internal Medicine. 2014;25(5):407–414. doi: 10.1016/j.ejim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: A review of fundamentals. Journal of Clinical Pharmacology. 2002;42(6):620–643. doi: 10.1177/00970002042006005. [DOI] [PubMed] [Google Scholar]
- Ostawal A, Mocevic E, Kragh N, Xu W. Clinical Effectiveness of Liraglutide in Type 2 Diabetes Treatment in the Real-World Setting: A Systematic Literature Review. Diabetes Therapy. 2016;7(3):411–438. doi: 10.1007/s13300-016-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratley RE, Kang JH, Kim PK, Kwak EH, Han OP, Gee KH, Choi IY, Kwon SC, Trautmann M, Hompesch M. Significant Effects of HM11260C (efpeglenatide) on Body Weight over 20 weeks in Obese Subjects without Diabetes: a Randomized, Double-blind, Placebo Controlled study. European Association for the Study of Diabetes; 2015. Sep 14, [Google Scholar]
- Schellenberger V, Wang C-w, Geething NC, Spink BJ, Campbell A, To W, Scholle MD, Yin Y, Yao Y, Bogin O, et al. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nature Biotechnology. 2009;27(12):1186–U155. doi: 10.1038/nbt.1588. [DOI] [PubMed] [Google Scholar]
- Sebokova E, Christ AD, Wang H, Sewing S, Dong JZ, Taylor J, Cawthorne MA, Culler MD. Taspoglutide, an Analog of Human Glucagon-Like Peptide-1 with Enhanced Stability and in Vivo Potency. Endocrinology. 2010;151(6):2474–2482. doi: 10.1210/en.2009-1459. [DOI] [PubMed] [Google Scholar]
- Simo R, Guerci B, Schernthaner G, Gallwitz B, Rosas-Guzman J, Dotta F, Festa A, Zhou M, Kiljanski J. Long-term changes in cardiovascular risk markers during administration of exenatide twice daily or glimepiride: results from the European exenatide study. Cardiovascular Diabetology. 2015;14:116. doi: 10.1186/s12933-015-0279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DH, Lim CK, Kim YH, Kwon SC, Lee GS, Bae SM, Jung SY Hanmi Science Co., Ltd, editor. CA2789123 A1 Insulinotropic peptide derivative wherein its n-terminal amino acid is modified patent. 2009 Jan 22;
- Stewart MW, Matthews J, De Boever EH, Dobbins RL, Hodge RJ, Walker SE, Holland CH, Bush MA. Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide (Syncria (R)), a long-acting GLP-1 mimetic, in subjects with type 2 diabetes. Diabetes. 2008;57:A154–A154. [Google Scholar]
- Sun L, Dai Y, Wang C, Chu Y, Su X, Yang J, Zhou J, Huang W, Qian H. Novel Pentapeptide GLP-1 (32-36) Amide Inhibits beta-Cell Apoptosis In Vitro and Improves Glucose Disposal in Streptozotocin-Induced Diabetic Mice. Chemical Biology & Drug Design. 2015a;86(6):1482–1490. doi: 10.1111/cbdd.12615. [DOI] [PubMed] [Google Scholar]
- Sun L, Huang X, Han J, Cai X, Dai Y, Chu Y, Wang C, Huang W, Qian H. Site-specific fatty chain-modified exenatide analogs with balanced glucoregulatory activity and prolonged in vivo activity. Biochemical Pharmacology. 2016;110:80–91. doi: 10.1016/j.bcp.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Sun L, Wang C, Dai Y, Chu Y, Han J, Zhou J, Cai X, Huang W, Qian H. Coumaglutide, a novel long-acting GLP-1 analog, inhibits beta-cell apoptosis in vitro and invokes sustained glycemic control in vivo. European Journal of Pharmacology. 2015b;767:211–219. doi: 10.1016/j.ejphar.2015.10.028. [DOI] [PubMed] [Google Scholar]
- Tomas E, Wood JA, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28-36)amide inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Regulatory Peptides. 2011;169(1–3):43–48. doi: 10.1016/j.regpep.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Tomlinson B, Hu M, Zhang Y, Chan P, Liu Z-M. An overview of new GLP-1 receptor agonists for type 2 diabetes. Expert Opinion on Investigational Drugs. 2016;25(2):145–158. doi: 10.1517/13543784.2016.1123249. [DOI] [PubMed] [Google Scholar]
- van Witteloostuijn SB, Pedersen SL, Jensen KJ. Half-Life Extension of Biopharmaceuticals using Chemical Methods: Alternatives to PEGylation. Chemmedchem. 2016;11(22):2474–2495. doi: 10.1002/cmdc.201600374. [DOI] [PubMed] [Google Scholar]
- Watkins E, Kang J, Trautmann M, Choi S, Han O, Hompesch M. LAPS CA-Exendin-4 (efpeglenatide) Enhances Insulin Secretion and Beta Cell Responsiveness in Subjects with Type 2 Diabetes. European Association for the Study of Diabetes; 2015. Sep 14, [Google Scholar]
- Wieczorek B, Spetzler J, Kruse T, Linderoth L, Kofoed J Novo Nordisk A/S (Bagsvaerd, DK), assignee 20170058014 Kind Code: A1 Double-Acylated GLP-1 Derivatives patent United States Patent Application. 2017 Mar 2;
- Williams JA. GLP-1 mimetic drugs and the risk of exocrine pancreatic disease: Cell and animal studies. Pancreatology. 2016;16(1):2–7. doi: 10.1016/j.pan.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Wu B, Wei S, Petersen N, Ali Y, Wang X, Bacaj T, Rorsman P, Hong W, Suedhof TC, Han W. Synaptotagmin-7 phosphorylation mediates GLP-1-dependent potentiation of insulin secretion from beta-cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(32):9996–10001. doi: 10.1073/pnas.1513004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K-H, Kang JH, Han OP, Gee KH, Choi IY, Kwon SC, Trautmann M, Hompesch M. Once Weekly HM11260C (efpeglenatide) Significantly Improves Glycemic Control and Reduced Body Weight in Patients with Type 2 Diabetes: A Phase II Dose Finding Study. European Association for the Study of Diabetes; 2015. Sep 14, [Google Scholar]