Abstract
Background
The sites of origin, causes and outcomes of severe hematochezia have not been compared between cirrhotics and non-cirrhotics. In cirrhotics versus non-cirrhotics presenting with severe hematochezia, we aimed at (1) identifying the site and etiology of gastro-intestinal bleeding and independent predictors of bleeding from the upper gastrointestinal tract versus small bowel or the colon, (2) comparing 30-day clinical outcomes, and (3) proposing an algorithm for management of severe hematochezia.
Methods
In this cohort study from two university-based medical centers, 860 consecutive patients with severe hematochezia admitted from 1995 to 2011 were prospectively enrolled with 160 (18.6 %) cirrhotics. We studied (a) general clinical and laboratory characteristics of cirrhotics versus non-cirrhotics, (b) predictors of bleeding sites in each patient group by multiple variable regression analysis, and compared (c) 30-day outcomes, including rebleeding, surgery and deaths.
Results
Cirrhosis independently predicted an upper gastrointestinal source of bleeding (OR 3.47; 95 % CI 2.01–5.96) as well as history of hematemesis, melena in the past 30 days, positive nasogastric aspirate, prior upper gastrointestinal bleeding or use of aspirin or non-steroidal anti-inflammatory. The most prevalent diagnoses were esophageal varices (20 %) in cirrhotics and colon diverticular bleeding (27.1 %) in non-cirrhotics. Thirty-day rates of rebleeding, surgical interventions and deaths were 23.1 versus 15 % (P = 0.01), 14.4 versus 6.4 % (P < 0.001), and 17.5 versus 4.1 % (P < 0.001), in cirrhotics versus non-cirrhotics, respectively.
Conclusions
Cirrhosis predicted an upper gastrointestinal site of bleeding in patients presenting with severe hematochezia. The 30-day rates of rebleeding, surgery, and death were significantly higher in cirrhotics than in non-cirrhotics.
Keywords: Cirrhosis, Hematochezia, Upper gastrointestinal bleeding, Lower gastrointestinal bleeding
Introduction
Gastrointestinal (GI) bleeding is common in cirrhotic and non-cirrhotic patients. Several algorithms and guidelines have been formulated for the treatment of GI bleeding, including by the American Association for the Study of Liver Disease (AASLD) for variceal hemorrhage, the American College of Gastroenterology (ACG) for non-variceal upper GI hemorrhages, and lower GI bleeding, and by the American Society of Gastrointestinal Endoscopy (ASGE) for upper and lower GI bleeding [1, 2]. However, no guidelines have been published for the management of severe hematochezia, i.e. the rectal passage of red blood, or clots, or both, in presence or in absence of cirrhosis. Hematochezia is a clinical manifestation of one or more lesion(s) that may be located anywhere between the upper GI tract and the rectum [3, 4]. While the type of lesion and their location in the GI tract may guide the management of patients presenting with severe hematochezia, reliable predictors of bleeding site may help to guide subsequent management and to choose appropriate endoscopic examination.
The clinical guideline’s focus in cirrhotic patients has generally been on variceal and, more recently, on non-variceal upper GI bleeding sites [5–7]. These observations may be useful to risk stratify and manage cirrhotics presenting with upper GI bleeding. However, the location, causes and outcomes, such as rebleeding, surgical interventions and deaths associated with severe hematochezia have not been compared in cirrhotics versus non-cirrhotics [4]. With the worldwide increase in chronic liver disease and the unique pathophysiology of cirrhosis and portal hypertension in GI hemorrhage, a specific assessment of cirrhotic as opposed to non-cirrhotic patients seems important. We hypothesized that the (a) sites of bleeding, (b) etiologies of hemorrhage and (c) the 30-day outcomes of severe hematochezia would differ for cirrhotic and non-cirrhotic patients. In patients hospitalized for severe hematochezia our specific objectives were to: (1) describe the lesion location (upper gastrointestinal tract [UGI] vs. colon or small bowel) and disease etiology based on endoscopic evaluations, (2) identify independent predictors of gut location of the bleed site, (3) report the 30-day clinical outcomes of patients with or without cirrhosis, and (4) develop a new management algorithm for hematochezia in cirrhotic versus non-cirrhotic patients.
Methods
This two-center observational study was approved by the institutional review boards (IRB) of Ronald Reagan University of California Los Angeles Medical Center and the Veterans Affairs Medical Center of Greater Los Angeles. Data were collected prospectively and reviewed retrospectively.
Patients
Consecutive patients admitted to the hospital for management of severe hematochezia, or who developed this after hospitalization for another reason, between June 1, 1995 and June 12, 2011, and who met the criteria for inclusion in the study, were prospectively enrolled by one of the endoscopist co-investigators of the CURE Hemostasis Research Group (DMJ, GVO, TOK, RJ, KAG, GAM, GSD) before undergoing urgent, diagnostic colonoscopy, esophago-gastro-duodenoscopy (EGD) or push-enteroscopy. If a diagnosis was not made, technetium-labeled red blood cell scans, angiography, or capsule endoscopy were also performed, as needed.
The criteria for inclusion in the study were: (1) age >18 years, (2) diagnosis of hematochezia, defined as the passage of clots with some shade of red or blood per rectum, and (3) clinical manifestations of major hemorrhage, such as recurrent hematochezia, hypotension, syncope, or orthostatic hypotension with or without tachycardia, and either a ≥2 g decrease in hemoglobin from baseline or transfusion of ≥2 units of packed red blood cells. Patients were excluded from the study if they were (1) unable to grant written informed consent, (2) mentally impaired or unable or unwilling to follow instructions, (3) clinically unstable or unable to safely undergo urgent endoscopy or colonoscopy, or (4) if they had a history of ongoing alcoholism, drug abuse, or non-compliance with medical recommendations.
Data Collection, Outcomes and Follow-up
The (a) demographic and clinical characteristics, (b) medications, (c) history of concomitant disorder(s), (d) manifestations of the present illness, including melena, hematemesis and positive or negative NG aspirate, (e) severity of the GI bleed, including hypotension, syncope or shock, and (f) etiology and severity of the cirrhosis, including Child-Pugh status were recorded. Positive NG aspirate was defined by red blood, clots, or coffee grounds that did not clear with 250 ml of lavage. Intravenous proton pump inhibitors (PPI) were given to all patients with suspected UGI bleeding and were started prior to endoscopy. In cirrhotics, octreotide was also started prior to GI procedures. Cirrhosis was diagnosed histologically or on the basis of radiologic and laboratory observations, by the hepatologists or gastroenterologists who managed these patients. Diagnosis of diverticular hemorrhage was either definitive (active bleeding, visible vessel, or adherent clot) or presumptive (diverticular without stigmata seen and no other lesions or stigmata by anoscopy, push enteroscopy, and/or capsule endoscopy) [8]. The clinical and laboratory observations, transfusion requirements, endoscopic findings, including stigmata of recent hemorrhage and type and efficacy of endoscopic hemostasis, and the clinical outcomes, including length of hospitalization, transfusions, recurrent bleeding, surgical interventions and death, were prospectively recorded on standard CURE forms by the research coordinator and/or by a co-investigator. The outcomes were ascertained from the date of diagnosis until the patient’s discharge from the hospital and at 30 days after the endoscopic diagnosis. All missing data were identified and retrieved from the medical records, co-investigators, or patients in accordance with the IRB and Health Insurance Portability and Accountability Act’s (HIPAA) regulations. All data from standard forms were coded and entered into a digital database by experienced data managers at the CURE Hemostasis Research Unit.
Statistical Analysis
Categorical data are expressed as numbers and percentages, and continuous data as mean ± SD. Fisher’s exact test was used to compare discrete variables, and Wilcoxon’s rank-sum test was used to compare continuous variables. To identify predictors of site of bleeding, the association between selected categorical variables and bleeding site was examined, using the chi-square test. Multiple variable analyses were performed with adjustments for covariates by logistic regression and by classification and regression trees (CART). We adjusted for up to ten covariates [age >65 vs. ≤65 years, history of bleeding from the (a) upper GI tract, (b) colon, (c) upper GI tract and colon, (d) undetermined source, aspirin or NSAID use, out-of-hospital versus in-hospital onset of bleeding, history of melena or hematemesis in the last 30 days, positive NG aspirate, major comorbidities, hemoglobin <8 versus ≥8 g/dl, hypotension or shock], and analyzed separately the following for gut location: (1) upper GI tract versus colon, (2) upper GI tract versus small bowel and (3) small bowel versus colon pairs of bleeding sites, as the predictors of one location versus another may vary among pairs of locations. Since the predictors may differ for the patient subsets with and without cirrhosis, we allowed for the potential effect of interactions between cirrhosis and each of the other factors, by including the appropriate terms in the logistic model to distinguish between upper GI and colon sites. Since only 40 patients had sources of bleeding in the small bowel, the logistic models to distinguish this from each of the other two locations assumed that all the effects were additive. Final models were selected, using a backwards, stepwise procedure with P < 0.1 as retention criterion for the interaction effects, and P < 0.25 as retention criterion for the main effects. Odd ratios (OR) and 95 % confidence intervals (CI) were calculated. P values <0.05 were considered statistically significant. The prospectively collected data were managed and analyzed retrospectively, using the SAS software, version 9.1 (SAS Institute, Cary, NC, USA).
Results
Among the 860 consecutive patients with severe hematochezia admitted and prospectively enrolled in this study between June 1, 1995 and June 12, 2011, 160 (18.6 %) had cirrhosis. The demographic characteristics, laboratory data, and transfusion requirements for cirrhotic versus non-cirrhotic patients at the time of presentation are shown in Table 1. Compared with the non-cirrhotics, the cirrhotic group was significantly younger, more often began to bleed in the hospital, and was more likely to have a history of melena and hematemesis within 30 days before admission, or a positive NG aspirate upon admission to the hospital. They were less likely to have been treated with aspirin, non-steroidal anti-inflammatory drugs (NSAID) or oral anticoagulants before admission to the hospital than the non-cirrhotics. The manifestations of major hemorrhage, such as hypotension, syncope or shock and the prevalence of major chronic concomitant disorders were similar in both groups.
Table 1.
Characteristics of cirrhotic versus non-cirrhotic patients hospitalized for management of severe hematochezia
| Clinical characteristics | Cirrhotics N = 160 |
Non-cirrhotics N = 700 |
P value |
|---|---|---|---|
| Age (years) | 57.9 ± 11.9 | 65.8 ± 16.1 | <0.0001 |
| Men | 98 (61) | 449 (64.1) | 0.5065 |
| In-hospital onset of bleeding | 75 (47) | 150 (21.4) | <0.0001 |
| Major concomitant chronic disorder | 79 (49.7) | 290 (41.4) | 0.0558 |
| Non-steroidal anti-inflammatories | 10 (6.3) | 91 (13) | 0.0170 |
| Aspirin | 17 (10.6) | 266 (38) | <0.0001 |
| Anticoagulation | 13 (8.1) | 113 (16.1) | 0.0098 |
| Antiplatelet agent | 3 (1.9) | 31 (4.4) | 0.1355 |
| Bleeding manifestations | |||
| Hypotension | 47 (29.4) | 164 (23.4) | 0.1126 |
| Shock | 2 (1.3) | 16 (2.3) | 0.4101 |
| Syncope | 8 (5) | 48 (6.9) | 0.3925 |
| History of | |||
| Melena | 48 (30.2) | 141 (20.1) | 0.0064 |
| Hematemesis | 21 (13.1) | 26 (3.7) | <0.0001 |
| Positive nasogastric aspirate | 14 (17.7) | 12 (3.3) | <0.0001 |
| Laboratory measurements | |||
| Hemoglobin (g/dl) | 8.7 ± 1.7 | 8.9 ± 2.3 | 0.02 |
| Platelet count (cell/mm3) | 105,563 ± 74,666 | 204,004 ± 104,081 | <0.001 |
| Creatinine (mg/dl) | 1.9 ± 3.4 | 1.6 ± 2.8 | 0.29 |
| Partial thromboplastin time (s) | 35.7 11.4 | 28.7 ± 6.8 | <0.0001 |
| Hematocrit (%) | 26 ± 5 | 29 ± 6 | <0.0001 |
| Blood urea nitrogen (mg/dl) | 31.8 ± 23.6 | 27.3 ± 25.0 | 0.09 |
| International normalized ratio | 1.6 ± 0.5 | 1.3 ± 0.76 | 0.01 |
| Number of transfusions | |||
| Packed red blood cell | 4.3 ± 4.9 | 2.9 ± 3.6 | <0.0001 |
| Fresh frozen plasma | 2.2 ± 4.9 | 0.7 ± 2.3 | <0.0001 |
| Platelets | 1.2 ± 2.8 | 0.5 ± 3.0 | <0.0001 |
Values are mean ± SD or numbers (%) of observations
The etiology of cirrhosis was alcoholic in 56 patients (35 %), hepatitis C in 50 (31.3 %), hepatitis B in 8 (5 %), nonalcoholic fatty liver disease in 6 (3.8 %), primary biliary cirrhosis in 6 (3.8 %), primary sclerosing cholangitis in 6 (3.8 %), autoimmune hepatitis in 5 (3.1 %), cryptogenic in 9 (5.6 %) and undetermined in 14 (8.8 %) patients. The Child-Pugh score was A in 10.7 %, B in 40.4 %, and C in 48.9 % of the cirrhotic patients.
Bleeding Site and Causes of Severe Hematochezia
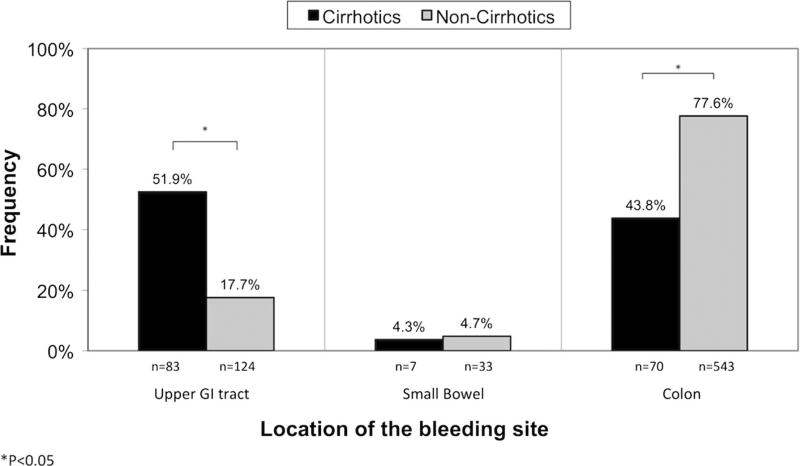
The proportion of upper GI bleeding sites was significantly higher in the cirrhotic (51.9 %) than in the non-cirrhotic (17.7 %) group (P < 0.05), whereas the proportion of colon hemorrhage was significantly higher in non-cirrhotics versus cirrhotics (77.6 vs. 43.8 %, P < 0.05). The proportion of small bowel bleeding was similar in the two groups (3.7 vs. 4.78 %).
Among the ten most frequent causes of hematochezia, five were upper GI in the cirrhotic group, versus two in the non-cirrhotic group (Table 2). In nearly 1/3 of cirrhotics, the bleeding was due to either an upper GI lesion due to portal hypertension, such as esophageal or gastric varices or portal hypertensive gastropathy, or to a gastro-duodenal ulcer. In non-cirrhotics, the most common source of hematochezia was diverticular disease of the colon, observed in 190 of the 700 (27.1 %). Other common causes of colon bleeding included ischemic colitis, internal hemorrhoids, rectal ulcer, and colon angiodysplasia. The prevalences of these causes were similar in both groups of patients.
Table 2.
Prevalence of GI bleeding etiologies in cirrhotics and non-cirrhotics
| Location/etiology | Cirrhotics N = 160 |
Non-cirrhotics N = 700 |
|---|---|---|
| Upper gastro-intestinal tract | ||
| Esophageal varices | 32 (20) | 5 (0.7) |
| Gastro-duodenal ulcer | 18 (11.2) | 69 (9.8) |
| Gastric varices | 9 (5.6) | 5 (0.6) |
| Portal versus other gastropathy | 6 (3.8) | 2 (0.3) |
| Dieulafoy’s lesion | 3 (1.9) | 2 (0.3) |
| Cancer | 0 (0) | 8 (1.1) |
| Angiomas | 2 (1.3) | 7 (1.1) |
| Anastamotic ulcer | 0 (0) | 7 (1.1) |
| Esophagitis | 3 (1.9) | 5 (0.7) |
| Others | 10 (6.2) | 14 (2.0) |
| Lower gastro-intestinal tract | ||
| Internal hemorrhoids | 14 (8.7) | 51 (7.3) |
| Rectal ulcer | 9 (5.6) | 45 (6.4) |
| Diverticulosis [presumptive/definitive] | 6 (3.8) [6/0] | 190 (27.1) [115/75] |
| Angiodysplasia | 4 (2.5) | 25 (3.6) |
| Post polypectomy | 4 (2.5) | 36 (5.1) |
| Anastamotic ulcer | 3 (1.9) | 12 (1.7) |
| Cancer | 3 (1.9) | 17 (1.7) |
| Rectal varices | 3 (1.9) | 0 (0) |
| Colitis (infectious or other) | 3 (1.9) | 12 (1.7) |
| Ischemic colitis | 12 (7.5) | 64 (9.1) |
| IBD (ulcerative colitis or Crohn’s) | 1 (0.6) | 22 (3.1) |
| Other LGI | 3 (1.9) | 19 (2.7) |
| Polyps | 1 (0.6) | 15 (2.2) |
| Radiation proctitis | 0 (0) | 15 (2.2) |
| Other etiologies | 11 (6.8) | 53 (7.7) |
Values are numbers (%) of observations
Predictors of Bleeding Sites
Patients presenting with colon hemorrhage were more likely to be >65-year-old (P < 0.0001), non aspirin or NSAID users (P = 0.0183), and to present with isolated hematochezia, i.e. in absence of a history of melena or hematemesis and in presence of a negative NG aspirate, than patients presenting with upper GI bleeding or small bowel hemorrhages (P < 0.0001). Patients whose source of bleeding was in the upper GI significantly more often (a) began to bleed after their admission to the hospital (P = 0.0011), (b) had a history of melena or hematemesis in the last 30 days (P < 0.0001), (c) had a positive NG aspirate (P < 0.0001), and (d) developed shock or hypotension at the time of presentation (P = 0.0187) than patients whose source of bleeding was elsewhere. The patients with small bowel bleeding significantly more often had a history of GI bleeding of unknown origin (P < 0.0001) than the patients with upper or lower GI sites of bleeding.
The predictors of bleeding sites identified by multiple variable, logistic regression analysis are shown in Table 3. Cirrhosis was an independent predictor of bleeding in the upper GI tract, and was significantly associated with a bleeding site located in the upper GI as opposed to the colon (OR 3.47; 95 % CI 2.01–5.96; P < 0.0001). Histories of hematemesis or melena within the last 30 days, a positive NG aspirate, a history of upper GI bleeding, and use of aspirin or NSAID were also significantly associated with upper GI as opposed to colon bleeding. Likewise, cirrhosis was significantly associated with upper GI as opposed to small bowel bleeding (OR 4.15; 95 % CI 1.57–11.00; P < 0.0004). Additional covariates included use of aspirin or NSAID (OR 3.51; 95 % CI 1.45–8.53; P = 0.006) and absence of prior hemorrhage of undetermined origin (OR 0.32; 95 % CI 0.11–0.96; P = 0.043). However, cirrhosis was not a reliable predictor of small bowel versus colon hemorrhage regardless of adjustments made for covariates. A history of bleeding of unknown source (OR 3.04; 95 % CI 1.10–8.41; P = 0.033), non-isolated hematochezia (OR 0.14; 95 % CI 0.06–0.35; P < 0.0001), age <65 years (OR 0.38; 95 % CI 0.18–0.79; P = 0.010), and a hemoglobin <8.0 g/dl (OR 0.38; 95 % CI 0.15–0.95; P = 0.09) were all associated with a small bowel as opposed to colon location in the logistic model.
Table 3.
Predictors of bleeding site by multiple variable logistic regression analysis
| Location and history with predictor | Odds ratio | 95 % CI | P value |
|---|---|---|---|
| Upper GI tract versus colon | |||
| Hematemesis in past 30 days | 14.46 | 4.53–46.14 | <0.0001 |
| Melena in past 30 days | 8.29 | 5.22–13.18 | <0.0001 |
| Positive nasogastric aspirate | 6.45 | 3.12–13.36 | <0.0001 |
| Cirrhosis | 3.47 | 2.01–5.96 | <0.0001 |
| Prior upper gastrointestinal bleeding | 2.2 | 1.08–4.46 | 0.029 |
| Use of aspirin, non-steroidal anti-inflammatory or both | 1.75 | 1.09–2.81 | 0.02 |
| Upper GI tract versus small bowel | |||
| Cirrhosis | 4.15 | 1.57–11.00 | 0.004 |
| Use of aspirin, non-steroidal anti-inflammatory or both | 3.51 | 1.45–8.53 | 0.006 |
| Prior hemorrhage of unknown origin | 0.32 | 0.11–0.96 | 0.043 |
| Small bowel versus colon | |||
| Prior hemorrhage of unknown origin | 3.04 | 1.10–8.41 | 0.033 |
| Isolated hematochezia | 0.14 | 0.06–0.35 | <0.0001 |
| Age >65 years | 0.38 | 0.18–0.79 | 0.01 |
| Hemoglobin >8 g/dl | 0.38 | 0.15–0.95 | 0.039 |
The CART model confirmed that cirrhosis was a predictor of upper GI location as opposed to the colon and the small bowel. CART identified four of the six variables detected by the logistic regression model as predictors of upper GI as opposed to colon source of bleeding, including history of melena, cirrhosis and hematemesis and a positive NG aspirate. CART identified cirrhosis, age <65 years, aspirin or NSAID treatment, and a history of bleeding of unknown source, hematemesis or melena within 30 days as predictor of upper GI as opposed to a small bowel location, confirming the outcomes of the logistic regression analysis. To distinguish small bowel from colon hemorrhage, eight variables were needed in the CART model, including melena, age <65 years, NG aspirate, inpatient status, shock or hypotension, aspirin or NSAID treatment and a history of lower GI tract bleeding.
The rates of accuracy of the logistic regression analysis versus the CART model in predicting the bleeding sources were 81 versus 78 % for upper GI tract versus colon, 72 versus 77 % for upper GI tract versus small bowel, and 76 versus 77 % for small bowel versus colon.
Endoscopic Observations and Treatment
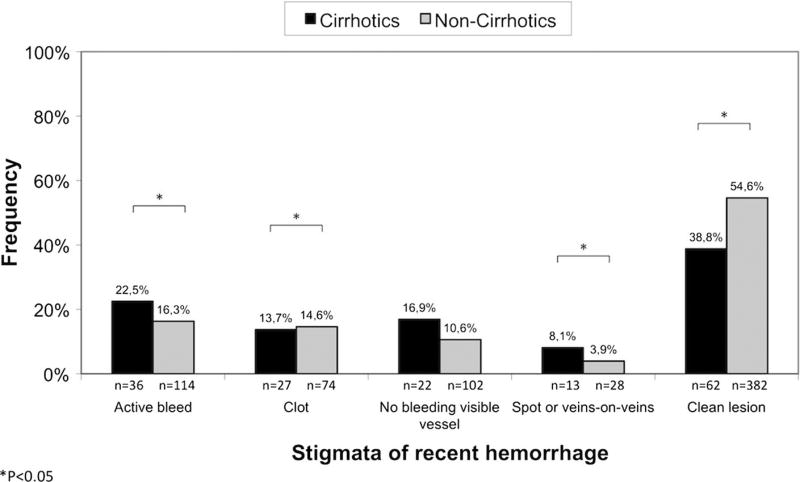
Major stigmata of recent hemorrhage, such as actively bleeding, non-bleeding visible vessel, and flat spots were significantly more prevalent in cirrhotic patients (Fig. 1). The requirement of an upper GI tract (50.0 vs. 41.9 %; P = 0.2515) and colon (30.0 vs. 28.7 %; P = 0.8252) hemostatic treatment during endoscopic examinations was similar in cirrhotics and non-cirrhotics, as were the rates of initial successful hemostasis (99.3 vs. 99.6 %).
Fig. 1.
Location in bleeding sites in cirrhotics versus non-cirrhotics presenting with severe hematochezia
Clinical Outcomes at 30 Days
At 30 days, 16.5 % of all patients had rebleeding, 7.9 % underwent surgery, and 6.5 % of patients died. At 30 days, the rates of rebleeding (23.1 vs. 15.0 %; P = 0.0122), surgery (14.4 vs. 6.4 %; P = 0.0008) and death (17.5 vs. 4.1 %; P < 0.0001) were significantly higher in cirrhotics than non-cirrhotics. The most frequently performed operations were liver transplantation in the cirrhotic (65.2 %) and colectomy in the non-cirrhotic (71.1 %) group. The most common cause of death was a major concomitant illness or comorbidity in cirrhotics (92.9 %) and in non-cirrhotics (79.3 %; P = 0.3384).
Algorithm for Management of Severe Hematochezia
The manifestations of upper GI tract hemorrhage and history of isolated hematochezia in cirrhotic and non-cirrhotic patients with upper GI hemorrhage are listed in Table 4 and were similar in both groups. In the entire sample population, 207 patients (24.1 %) had an upper GI tract source of bleeding, of whom 76 (36.7 %) had isolated hematochezia without a history of melena and hematemesis within 30 days or a positive NG aspirate on admission (Table 5). Among these 76 patients presenting with isolated hematochezia who had upper GI bleeding, a high proportion (38.2 %, 29/76) were cirrhotics. As reported earlier, cirrhosis was a predictor of upper GI tract as opposed to colon and small bowel bleeding. Other predictors of an upper GI location were hematemesis, melena, a positive NG aspirate, prior upper GI bleeding and aspirin or NSAID use.
Table 4.
Manifestations of upper GI tract hemorrhage and history of isolated hematochezia in cirrhotic and non-cirrhotic patients
| History of manifestation | All patients N = 207 |
Cirrhotics N = 83 |
Non-cirrhotics N = 124 |
P value |
|---|---|---|---|---|
| Melena in the last 30 days | 107 (51.7) | 39 (46.9) | 68 (54.8) | 0.2596 |
| Hematemesis in the last 30 days | 42 (20.3) | 19 (22.9) | 23 (18.5) | 0.4863 |
| Positive nasogastric aspirate | 21 (10.1) | 11 (13.2) | 10 (8.1) | 0.2523 |
| Witnessed hematemesis or positive nasogastric aspirate | 54 (26.1) | 26 (31.3) | 28 (22.6) | 0.1989 |
| Isolated hematochezia | 76 (36.7) | 30 (36.1) | 47 (37.9) | 0.7716 |
Values are numbers (%) of observations
Table 5.
Characteristics and 30-day outcomes of 76 patients with upper GI bleeding presenting with isolated hematochezia
| Characteristic | Cirrhotics N = 29 |
Non-cirrhotics N = 47 |
P value |
|---|---|---|---|
| Age (years) | 60.3 ± 10.9 | 63.7 ± 17.2 | 0.1289 |
| Men (%) | 51.7 | 61.7 | 0.3921 |
| In-hospital onset of hemorrhage | 16 (55.2) | 16 (34.0) | 0.0699 |
| Major concomitant chronic illness | 12 (41.4) | 26 (55.3) | 0.7779 |
| Aspirin or non-steroidal anti-inflammatory use 30-day rate of | 3 (10.3) | 27 (57.4) | <0.0001 |
| Recurrent bleeding | 1 (3.4) | 14 (29.8) | 0.0051 |
| Surgical interventions | 4 (13.8) | 3 (6.4) | 0.4170 |
| Deaths | 7 (24.1) | 5 (10.6) | 0.1936 |
Values are numbers (%) of observations
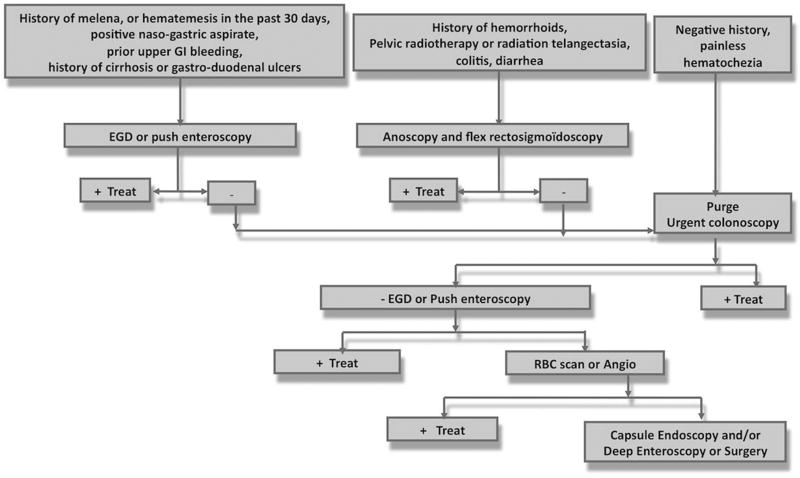
Therefore, we propose a new algorithm that includes the presence of cirrhosis and other predictors of upper GI tract bleeding at an early stage in the clinical management of hematochezia (Fig. 2). In the patients without cirrhosis, 6.7 % (47/700) had upper GI hemorrhage and presented with isolated hematochezia. Among them, only 12 patients had none of the predictors described earlier (hematemesis, melena, a positive NG aspirate, prior upper GI bleeding, history of ulcer and aspirin or NSAID use). If we retrospectively applied this algorithm in our study population, these 12 patients, which represented only 1.3 % of all patients, would have had a delayed EGD or enteroscopy of a few hours by being performed during the same session of colonoscopy. In contrast, if we applied current guidelines that recommend performing an urgent EGD or enteroscopy only in presence of a positive NG aspirate in case of hematochezia, the percentage of delayed EGD in patients with upper GI hemorrhage would have been higher, namely, 4.3 %.
Fig. 2.
Stigmata of recent hemorrhage in cirrhotics versus non-cirrhotics presenting with severe hematochezia
Discussion
This is the first detailed study of the bleeding sites and diagnoses in cirrhotic versus non-cirrhotic patients presenting with severe hematochezia. The causes of bleeding were significantly different in the two groups of patients. Among the ten most frequent causes of hematochezia, five were upper GI tract lesions in the cirrhotic versus only two in the non-cirrhotic group. In nearly 1/3 of cirrhotics, bleeding was secondary to an upper GI tract lesion due to portal hypertension, whereas in non-cirrhotics the most common sources of hematochezia were colo-rectal lesions. Rectal varices were the cause of hematochezia in <2 % of cirrhotics. Portal colopathy was not a common cause of bleeding among our cirrhotic patients.
Previous guidelines for the management of lower GI bleeding, including those issued by the American College of Gastroenterology and by the American Society for Gastrointestinal Endoscopy, recommend performing an urgent upper endoscopy only in the presence of a positive NG aspirate [1, 2] in case of hematochezia. However, in this study, only about 10 % of patients with upper GI bleeding had a positive NG aspirate. Despite the absence of relevant symptoms and despite a negative NG aspirate the current guidelines should be revised based upon our results and those of two earlier studies, where an upper GI source of bleeding was identified in up to 15 % of patients presenting with severe hematochezia [9, 10].
Severe hematochezia remains a common medical problem, for which a management consensus or practice guidelines have not been formulated [4, 11]. In this study, we confirmed with two separate multiple variable analytical models, that cirrhosis is an independent predictor of bleeding from an upper GI site, as opposed to the colon or the small bowel. Therefore, in patients with known or suspected cirrhosis who present with severe hematochezia, we highly recommend an upper GI endoscopy or push enteroscopy as a first means of diagnosing and treating the source of hemorrhage. While this strategy is probably considered by experienced clinicians who suspect that cirrhosis predicts an upper GI tract source of bleeding in patients presenting with hematochezia, this hypothesis was not supported by prior objective evidence-based medicine. We propose a new CURE algorithm for the management of these patients, which takes into account the presence of cirrhosis at the time of presentation (Fig. 2). Morbidity and mortality may be reduced by shortening the delays in diagnostic and therapeutic upper endoscopy in this subset of patients, as evidenced by our similar initial success in achieving upper GI hemostasis and similar 30-day recurrent bleeding rates in both groups of patients.
Overall, <40 % of non-cirrhotic patients presenting with upper GI bleeding had isolated hematochezia. Other predictors need to be identified to (a) facilitate the identification of the site of bleeding in non-cirrhotic patients at high risk of an upper GI source of hematochezia and (b) recommend early upper endoscopy. In this study, since a history of upper GI bleeding and the use of aspirin or NSAID were independent predictors of an upper GI site of hemorrhage, we included them in our algorithm, as criteria to proceed initially with EGD or push enteroscopy. We also recommend, by extension, the initial performance of an upper GI examination in patients presenting with a history of peptic ulcer, since it was the leading cause of upper GI hemorrhage in non-cirrhotic patients.
If we consider all cirrhotic or non-cirrhotic patients presenting with hematochezia, the rates of recurrent bleeding and death in this study were 16 and 6.5 %, respectively. The only two available randomized trials (with limited sample sizes) have reported 14 and 30 % recurrent bleeding and 2 and 5.6 % death rates, respectively, at 30 days in patients presenting with severe hematochezia [10, 12]. While these rates of recurrent bleeding are similar to ours, the mortality rate was higher in our study, perhaps because of its approximately tenfold greater sample size, our less stringent inclusion criteria, and our inclusion of patients with more severe concomitant disorders, including cirrhosis (Fig. 3).
Fig. 3.
Algorithm recommended by the CURE Hemostasis Research Group for the management of severe hematochezia
Limitations of Our Study
A potential limitation of our study is the referral bias that might have been introduced by a patient recruitment from a large tertiary care center that has an active liver transplantation program and large proportion of cirrhotic patients. This was, however, mitigated by the participation of the West Los Angeles Veterans Affairs hospital, where no liver transplants are performed. Our results might not be applicable to non-university based medical centers, which may have neither the resources nor the manpower to constitute a GI hemostasis team, nor the experience to perform urgent colonoscopies in all patients presenting with severe hematochezia.
In conclusion, cirrhosis, a history of hematemesis or melena, positive NG aspirate, prior upper GI bleeding and aspirin or NSAID use were predictors of an upper GI tract site of bleeding. Emergent upper endoscopy or push enteroscopy should be strongly considered in such patients. Cirrhotics who had a colonic source of GI bleeding had significantly higher rates of recurrent bleeding, surgical interventions, and deaths at 30 days. In 36.5 % of patients presenting with isolated hematochezia, the source of hemorrhage was in the upper GI tract. Our new algorithm is highly recommended for the clinical management of cirrhotics and non-cirrhotics presenting with severe hematochezia.
Acknowledgments
The authors thank Jeff Gornbein DPH and Daniela Markovic, Ph.D. for their contributions to the statistical analyses, Mary Ellen Jensen, MLS and Nan Sun, M.S., for the management of the data, and Martha Carrico, RN as a research coordinator. This study was funded, in part, by a NIH41301 CURE Digestive Diseases Research Center Grant—Human Studies CORE, and a Veterans Affairs Clinical Merit Review Grant (Dennis M. Jensen, M.D., Principal Investigator). Marine Camus, M.D., received a grant from the Philippe Foundation in support of a research exchange program between France and the United States of America.
Footnotes
Conflict of interest The authors declare they have no competing interests.
References
- 1.Davila RE, Rajan E, Adler DG, et al. ASGE guideline: the role of endoscopy in the patient with lower-GI bleeding. Gastrointest Endosc. 2005;62:656–660. doi: 10.1016/j.gie.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 2.Zuccaro G., Jr Management of the adult patient with acute lower gastrointestinal bleeding. American College of Gastroenterology. Practice Parameters Committee. Am J Gastroenterol. 1998;93:1202–1208. doi: 10.1111/j.1572-0241.1998.00395.x. [DOI] [PubMed] [Google Scholar]
- 3.Savides TJ, Jensen DM. GI bleeding. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology/Diagnosis/Management. 10. Philadelphia: Saunders Elsevier Saunders; 2016. pp. 297–335. [Google Scholar]
- 4.Jensen DM. Management of patients with severe hematochezia—with all current evidence available. Am J Gastroenterol. 2005;100:2403–2406. doi: 10.1111/j.1572-0241.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 5.González-González JA, García-Compean D, Vázquez-Elizondo G, Garza-Galindo A, Jáquez-Quintana JO, Maldonado-Garza H. Nonvariceal upper gastrointestinal bleeding in patients with liver cirrhosis. Clinical features, outcomes and predictors of in-hospital mortality. A prospective study. Ann Hepatol. 2011;10:287–295. [PubMed] [Google Scholar]
- 6.Lecleire S, Di Fiore F, Merle V, et al. Acute upper gastrointestinal bleeding in patients with liver cirrhosis and in noncirrhotic patients: epidemiology and predictive factors of mortality in a prospective multicenter population-based study. J Clin Gastroenterol. 2005;39:321–327. doi: 10.1097/01.mcg.0000155133.50562.c9. [DOI] [PubMed] [Google Scholar]
- 7.D’Amico G, De Franchis R. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 8.Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78–82. doi: 10.1056/NEJM200001133420202. [DOI] [PubMed] [Google Scholar]
- 9.Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology. 1988;95:1569–1574. doi: 10.1016/s0016-5085(88)80079-9. [DOI] [PubMed] [Google Scholar]
- 10.Laine L, Shah A. Randomized trial of urgent vs. elective colonoscopy in patients hospitalized with lower GI bleeding. Am J Gastroenterol. 2010;105:2636–2641. doi: 10.1038/ajg.2010.277. [DOI] [PubMed] [Google Scholar]
- 11.Zuckerman GR, Prakash C. Acute lower intestinal bleeding. Part II: etiology, therapy, and outcomes. Gastrointest Endosc. 1999;49:228–238. doi: 10.1016/s0016-5107(99)70491-8. [DOI] [PubMed] [Google Scholar]
- 12.Green BT, Rockey DC, Portwood G, et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100:2395–2402. doi: 10.1111/j.1572-0241.2005.00306.x. [DOI] [PubMed] [Google Scholar]