Abstract
Liver regeneration is a highly organized tissue regrowth process and is the most important reaction of the liver to injury. The overall process of liver regeneration includes three phases: priming stage, proliferative phase, and termination phase. The initial step aims to induce hepatocytes to be sensitive to growth factors with the aid of some cytokines, including TNF-α and IL-6. The proliferation phase promotes hepatocytes to re-enter G1 with the stimulation of growth factors. While during the termination stage, hepatocytes will discontinue to proliferate to maintain normal liver mass and function. Except for cytokine- and growth factor-mediated pathways involved in regulating liver regeneration, new substances and technologies emerge to influence the regenerative process. Here, we reviewed novel and important signaling molecules involved in the process of liver regeneration to provide a cue for further research.
1. Introduction
The liver, composed of parenchymal cells—hepatocytes—and nonparenchymal cells including endothelial cells, Kupffer cells, lymphocytes, and stellate cells, has a unique capacity to precisely regulate its growth and mass, which is particularly remarkable since hepatocytes are stable cells and rarely divide in the normal state, as they are quiescent in the G0 phase of the cell cycle [1]. However, their proliferative capacity is initiated in the case of liver tissue loss. There are two different regenerative models. Partial hepatectomy (two-thirds of the liver is removed) initiates a unique response, during which the remaining diploid hepatocytes enter into the cell cycle to compensate for the loss of liver tissue, taking about a week [2]. Another pattern of the regenerative model is established by insult, such as toxins and viral infection, during which all hepatocytes are hurt and oval cells are considered as potent stem cells to differentiate into hepatocytes and biliary cells. Both of the two patterns of liver regeneration will be involved in the review.
Findings of past several decades have revealed that liver regeneration is a complex network regulated by various growth factors and cytokines expressed at the site of injury or migrated to the liver via the circulatory system. To sum it up, the regenerative process includes three critical steps [3]: firstly, quiescent hepatocytes convert from G0 to G1 of the cell cycle when faced with multiple stimulations (the priming phase); secondly, with the help of mitogens, hepatocytes progress beyond the restriction point to the G1 phase and then the mitosis (the proliferation phase); and then the last, cells terminate proliferation under the control of negative factors (the termination phase), such as transforming growth factor beta (TGF-β) and activin (Figure 1). In these three phases, various cytokines or growth factors exhibit a pivotal role through cell signaling pathways of multiple biological effects. Here, we endeavor to summarize some classical and novel signaling molecules participating in the process.
Figure 1.
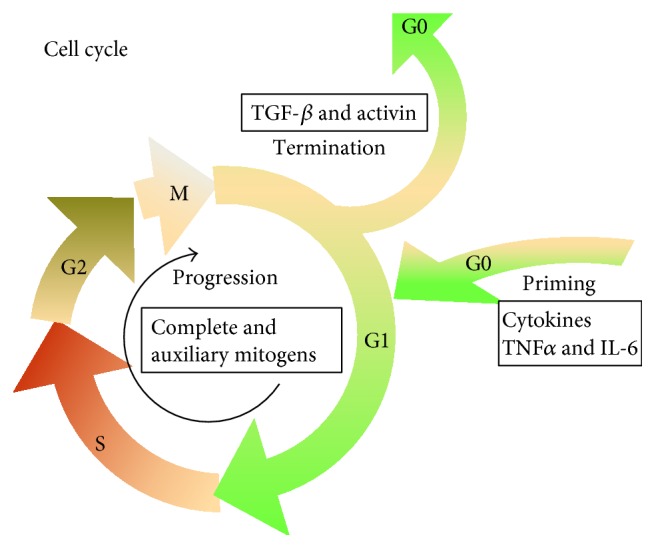
The outline of liver regeneration process.
2. The Priming Phase: The Primary Molecules Tumor Necrosis Factor-α (TNF-α) and IL-6
Inflammation is a complex biological response and is characterized by recruitment, proliferation, and activation of a series of inflammatory cells and immune cells, and it aims to alleviate infections, eliminate damaged cells, and initiate tissue repair and regeneration [4]. Inflammation goes through the whole process of liver damage and promotes regeneration of the injured liver. Inflammation-induced regeneration primarily is triggered by cytokines and growth factors released from inflammatory cells. The most widely studied proinflammatory cytokines are TNF-α and IL-6.
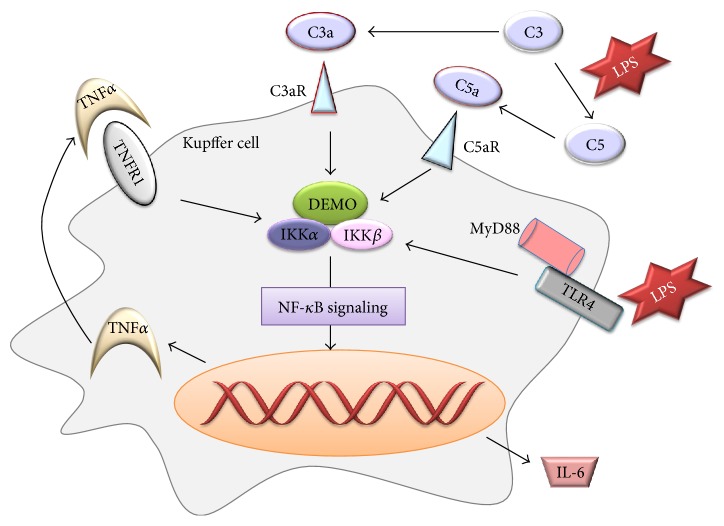
Kupffer cells are known to produce a group of cytokines and immunomodulating mediators that have stimulatory and inhibitory effects on hepatic injury. Hepatic macrophages are the main source of TNF-α and IL-6 through the NF-κB signaling pathway triggered either by Gut-derived factor lipopolysaccharide (LPS)/Toll-like receptor4 (TLR4) signaling or by C3a and C5a, components of the complement system (Figure 2()). They prime hepatocytes to re-enter into the cell cycle in the first stage. Loss of either TNF-α or IL-6 could delay liver regeneration [5]. TLR4 recognizes its ligand LPS and then recruits and activates myeloid differentiation factor 88 (MyD88), triggering signal transduction downstream to promote the release of proinflammatory factors. In the view of C3 and C5, part of innate immune response which works in the process of liver injury to fight with multiple pathogens, they exert their effects on hepatocyte proliferation by activation of the bioactive peptides C3a and C5a with the stimulation of LPS [6] (Figure 2()). C3a not only mediates signals to the downstream C5a but also affects hepatocyte proliferation in a C5-independent fashion [7]. Mice deficient of either C3 or C5 showed impaired liver regeneration [8]. A complement inhibitor, CR2-CD59, targeting the site of complement activation and specially inhibiting the membrane attack complex (MAC), was used to study the complement-dependent balance between liver damage and regeneration and the results showed that CR2-CD59 not only has no effect on the production of C3a and C5a but enhances liver regeneration and remarkably improves the long-term survival, partly because of the increased level of hepatic TNF-α and IL-6 via STAT3 and Akt activation [6].
Figure 2.
The production of TNF-α and IL-6 in Kupffer cell through NF-κB signaling in the early phase of liver regeneration.
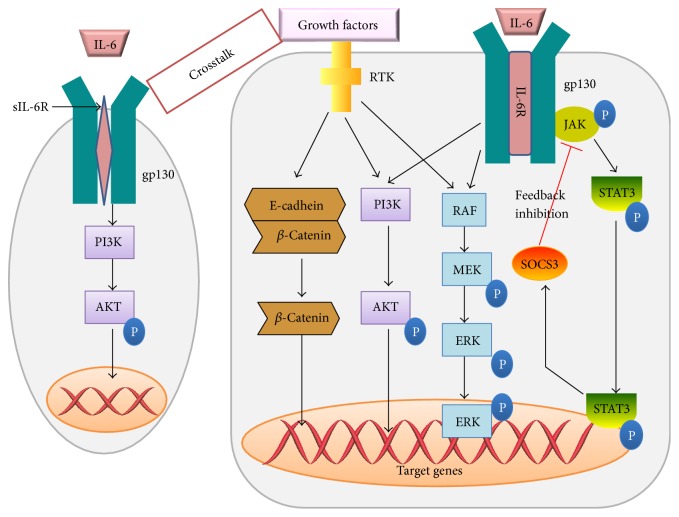
IL-6 is a pleiotropic cytokine and is secreted during inflammatory conditions upon LPS stimulation in a TNF-α-dependent/-independent manner (Figure 2). In response to liver injury, IL-6 mediates the acute-phase response and induces both cytoprotective and mitogenic functions. IL-6-induced signaling pathways are critical to the early onset as well as the progression and maintenance of the regenerative process [9]. Conventionally, IL-6 binds to the interleukin-6 receptor (IL-6R) and the IL-6/IL-6R complex initiates a coreceptor, glycoprotein (gp) 130, leading to JAk/STAT, MAPK, and PI3K/AKT activation [10]. STAT3 is able to upregulate the expression of suppressors of cytokine signaling (SOCS), an important negative regulator of cytokine signaling, leading to the downregulation of gp130 signals [10, 11]. Surprisingly, either IL-6 or IL-6R alone has no affinity to gp130 and only when the IL-6/IL-6R complex is formed, interaction with gp130 would occur.
Although gp130 is present in almost all cells, IL-6R is only expressed in limited cell types, for example, hepatocyte; thus, it seems that the effect of IL-6 is restricted to these cells. However, a soluble form of IL-6R (sIL-6R) was found and could still bind IL-6 to trigger intracellular signals, being called IL-6 trans-signaling [12]. SIL-6R is mostly generated by the proteolytic cleavage of membrane-bound receptor or by alternative splicing of the transmembrane domain coding exon [13]. The event of IL-6 trans-signaling not only could occur on cells short of IL-6R but also affects hepatocytes expressing IL-6R to prolong STAT3 phosphorylation and enhances the effect of IL-6 in liver regeneration [14, 15]. Blockade of IL-6 trans-signaling would deteriorate CCL4-induced liver damage [16]. It has been speculated that sIL-6R and sgp130, a soluble form of gp130, may constitute a buffer in the blood and once secreted, IL-6 will bind sIL-6R and then the complex IL-6/sIL-6R will bind sgp130 with a high affinity. Only when the concentration of IL-6 is very high, exceeding the level of sIL-6R, IL-6 could bind to membrane-bound IL-6R [17].
Of note, researches recently found that gp130, independent of the gp130 effector STAT3, initiates the activation of YAP and Notch, controlling the tissue growth and regeneration in intestinal epithelial cells upon mucosal injury [18]. Besides, YAP overexpression has been found in several solid tumors and elevated YAP levels contribute to tumor growth. YAP-mediated induction of Jag-1 was able to activate Notch signaling in HCC and mouse hepatocytes [19]. The role of this novel signaling in liver injury, repair, and regeneration remains to be charted.
3. The Proliferation Phase: Complete Mitogens and Auxiliary Mitogens
The proliferation phase, also called the second phase or progression phase, converts cells from G1 phase to mitosis. The molecules involved in the second phase were mainly separated into two groups, that is, complete mitogens and auxiliary mitogens [20] (Table 1). The former refers to these factors mitogenic in both primary-cultured hepatocytes and in animal experiments, including hepatocyte growth factor (HGF), transforming growth factor- (TGF-) α, epidermal growth factor (EGF), heparin-binding-EGF (HB-EGF), and their common receptor EGFR. They could activate secondary or delayed gene responses to stimulate DNA synthesis and cell proliferation. The latter, although they are not mitogenic in hepatocytes, may contribute to the regenerative process partially by magnifying or accelerating the effects of complete mitogens.
Table 1.
Common complete and auxiliary mitogens.
| Factor | Origin | Target |
|---|---|---|
| Complete mitogens | ||
| HGF | Mainly stellate cells | HGF directly regulates hepatocyte DNA synthesis and cell proliferation by blinding to its receptor c-met. |
| EGF | Brunner's gland in the duodenum | They provoke hepatocyte proliferation mainly through the Ras-MAPK signaling pathway by binding to their identical receptor and may compensate for each other to some degree in the process. |
| TGF-α | Hepatocytes | |
| Auxiliary mitogens | ||
| Bile acids | Hepatocytes and cholangicytes | Appropriate concentration of BAs may promote liver regeneration mainly via farnesoid X receptor (FXR) signaling pathways to stimulate the expression of FoxM1b, a key regulator of cell cycle, to participate in cells proliferation [31]. |
| NE | Nerve system | NE may amplify the effect of EGF and HGF by acting on the α1-adrenergic receptor associated with Gαh, a G protein [32, 33], and besides, it could induced the expression of Smad7 to abolish activin A-induced growth inhibition of hepatocyte by activation of NF-κB [34]. |
| VEGF | Hepatocytes | VEGF family, particularly VEGF-A, is strongly upregulated in hepatocytes during the regenerative process and may facilitate proliferation of sinusoidal endothelial cells and hepatocytes 48 h following PHx [35]. |
| Insulin | Pancreatic islets | Insulin could contribute to liver regeneration despite not being a primary mitogen and its proliferative effect mainly mediated through insulin receptors (IRs) that shift to nucleus to activate inositol 1,4,5,-trisphosphate- (InsP3-) dependent Ca2+ signaling pathways [36]. |
| IGF-1 | Liver | IGF-I works as a booster to liver regeneration by upregulation of HGF and downregulation of transforming growth factor beta 1(TGF-β1), a repressor of proliferation, and decreased level of IGF-I could impair the regenerative process [37]. |
| Estrogen | Reproductive system | Estrogen has been shown to promote hepatocyte proliferation mainly through estrogen receptor alpha (ERα) [38]. Moreover, the estrogen level could be influenced by IL-6 and there may be crosstalk between estrogen signaling and IL-6 signaling pathways [39]. |
| Serotonin (5-hydroxytryptamine, 5HT) | Enterochromaffin cells | Serotonin, via HT receptor 2 (HTR2), has been reported to contribute to liver regeneration [40]. And it was found that liver regeneration would be arrested when ketanserin was administrated to block 5-HT2, a subtype of 5-HT, approximately at the G1/S transition point [41]. |
3.1. Complete Mitogens
Complete mitogens exhibit direct hepatotrophic effects, which is defined that they could lead DNA synthesis in serum-free media in vitro and cause liver enlargement when injected in vivo. HGF and ligands of EGFR, including EGF, TGF-α, and HB-EGF, acting as the major complete mitogens for hepatocytes, could provoke hepatocyte proliferation mainly through the Ras-MAPK signaling and PI3K/AKT signaling pathway by binding to corresponding receptors, c-met and EGFR [21, 22] (Figure 3). Based on the research conducted by Huh et al. [23], mice knockout of the c-met gene showed hypersensitivity to Fas-mediated apoptosis and may retard the development of the liver after injury. The similar condition was also observed when EGFR was suppressed by silencing RNAs [24].
Figure 3.
Growth factors, along with some cytokines, guide the progression of liver regeneration through expression of some cell cycle-related proteins mainly by PI3K/AKT, wnt-independent/β-catenin, Ras/MAPK, and JAK/STAT signaling pathways.
3.2. Auxiliary Mitogens
Auxiliary mitogens, such as bile acids (BAs) [25], norepinephrine (NE) [26], endothelial growth factor (VEGF) [27], insulin-like growth factor system (IGF system) [28], estrogen [29], and serotonin [30] (Table 1), although not mitogenic in cultured hepatocytes, may delay liver regeneration in their absence.
Blood platelets, not just functioning in the hematologic system, actually fulfill a wider role in health and diseases [42]. Platelets may be part of the innate immune system and also fight with infection, including bacteria, viruses, and microorganisms. Mediators provided by platelets not only recruit leukocytes to the site of vascular injury and inflammation but also aid in tissue repair and regeneration. Being recruited to the sinusoids after PHx and releasing molecules, such as HGF, VEGF, insulin-like growth factor-1 (IGF-1), and serotonin, platelets are described as a positive factor involved in liver regeneration [43]. Patients suffering from 70% PHx would improve the regenerative capacity of the liver if provided with plasma rich in platelets [44]. Conversely, administration of antiplatelet antibodies would depress liver regeneration [45]. However, it does not mean that administration of platelet concentrates and thrombopoietin receptor agonists could be widely used on clinical operations to support liver regeneration and alleviate outcomes of patients with liver failure or small-for-size syndrome owing to the severely undesirable side effects brought by the strategy, for example, venous or portal vein thrombosis, or even fatal transfusion-related acute lung injury [46]. Thus, more works are needed to ascertain its beneficial effects and to minimize potential side effects at the same time.
3.3. Wnt Proteins
Wnt ligands are secreted glycoproteins and are produced primarily by hepatic nonparenchymal cell compartment, especially Kupffer cells and endothelial cells [47]. They are beneficial and necessary for liver regeneration. Wnts may activate the chief downstream effector, β-catenin, and initiate the classic wnt/β-catenin signaling cascade and finally express target genes, such as c-myc and cyclinD1 [48]. Other than wnt proteins, β-catenin can also be stimulated through a non-wnt fashion, that is, wnt-independent signaling. β-Catenin forms the bridge between the cytoplasmic tail of E-cadherin and actin cytoskeleton, through which β-catenin may act as a mediator of tyrosine kinase signaling [49]. At the membrane, β-catenin could be phosphorylated at tyrosine residues 654 and 670 by different kinases including c-met, EGFR, and others [50], which induce the dissociation of β-catenin from E-cadherin, and subsequently, β-catenin translocates to the nucleus to control the expression of target genes (Figure 3()). Although both classic wnt/β-catenin signaling and wnt-independent signaling are advantageous to the regenerative process, the positive role of the former is more remarkable. When knocked out of LRP5/6, coreceptor of wnt proteins, mice showed impaired classic wnt/β-catenin signaling and retarded regenerative process after PHx despite that the non-wnt pathways remained intact [51].
3.4. Exosomes
Other than hormones, cytokines, and growth factors contributing to liver regeneration, exosomes are found to improve the regenerative process as well. Exosomes are membrane-enclosed nanovesicles possessing a variety of physiological properties and function as important vesicles involved in intercellular communication [52]. Exosomes are released by several types of cells and carry active signals to target cell within adjacent and remote areas. Recently, exosomes derived from hepatocytes were reported to improve liver regeneration owing to the production of intracellular sphingosine-1-phosphate (S1P) [53]. S1P is indispensable for hepatocyte exosome-induced proliferation. Only hepatocyte-derived exosomes, not other liver cells, contain neutral ceramidase and sphingosine kinase 2 (SK2) required for S1P synthesis. Exosomes fuse with and deliver synthetic machinery to target hepatocyte. And within the target hepatocyte, sphingosine-1-phosphate (S1P) is produced to promote cell proliferation. Besides, the number of hepatocyte-derived exosomes increased after liver injury. Similarly, MSC-derived exosomes exert hepatoprotective effects and relieve drug-induced liver injury through activation of proliferative and regenerative responses [54], underlining the tremendous potential of the exosome-based therapies for liver disease.
4. Termination of Liver Regeneration
When the normal liver mass/body mass ratio of 2.5% has been restored, liver regeneration would be terminated. However, mechanisms of controlling the hepatocyte apoptosis to correct an overshooting of regenerative response have not been well investigated. Thus far, the most well-known antiproliferative factors are transforming growth factor beta (TGF-β) and related TGF-β family members [55].
4.1. TGF-β-Mediated Pathways
TGF-β, especially TGF-β1, puts a brake on liver regeneration and works as an inducer of cell apoptosis in vitro and in vivo, being active in G1 phase of the cell cycle [56]. TGF-β1 exerts its function mainly through binding to its receptors type I receptor (TβRI) and type II receptor (TβRII) to encode related protein expression. Whereas, researchers recently have proved the lack of TGF-β gene upregulation in the termination stage and concluded that intact signaling by TGF-β may not be required for the termination phase of liver regeneration [57]. They also found that some genes were upregulated in the termination regulation and may have potential negative effect on the cell cycle and promotion of cell apoptosis, such as the zinc finger protein gene (ZNF490) and caspase recruitment domain-containing protein 11 (CARD11) gene. Therefore, more details are needed to verify and elucidate molecules that participated in the termination phase and the signaling they involved.
Apart from liver cells, TGF-β1 is also synthesized in extrahepatic tissues, including platelet and the spleen [58, 59]. The spleen, known as an immune organ, would secret TGF-β1 to end the liver regeneration. Splenectomy significantly increased the number of proliferating cells 48 h after PHx [60]. Recently, the spleen was proved to not only increase TGF-β1and its receptor TβRII but also downregulate HGF and its receptor c-met to exhibit growth inhibitory effects on cell proliferation, indicating that the spleen could remotely influence and regulate liver regeneration [59].
4.2. Other Relevant TGF-β Family Members
Other TGF-β family members, primarily activins and bone morphogenetic proteins (BMPs), were revealed to be implicated in numerous biological processes, including liver regeneration. Activin A, an activin subtype, is increased by 12 h in response to partial hepatectomy and considered as a negative regulator of liver regeneration and induces hepatocyte growth arrest and apoptosis in vitro and in vivo [61, 62]. Administration of the activin A antagonist follistatin enhanced DNA synthesis and prolonged hepatocyte proliferation [63]. Apart from the inhibitory effect on DNA synthesis, activin A also significantly affects the production of fibronectin, component of extracellular matrix (ECM) which is essential for liver regeneration [64]. With regard to BMP, unlike TGF-β1 and activin A, it is quite complicated. Major BMPs bind to their receptors mainly to phosphorylate Smad1/5/8 rather than Smad2/3 to exert repressive effects on liver regeneration [65]. However, different subunits of BMPs exhibit different or even reverse effects, for example, BMP7 that promotes hepatocyte proliferation, whereas BMP4 represses proliferation in the hepatoma cell line Huh7 [66]. One main possible reason may be that BMP7 and BMP4 act through pathways of opposite effects [67]. Thus, the accurate functions of other BMPs in the liver regeneration process remain to be undetermined.
5. The Future Perspectives in the Fields of Liver Regeneration
In spite of having been investigated for so many years, the actual mechanisms of liver regeneration are still obscure and far from practical application to solve clinical liver disease. However, the embarrassing situation is greatly improved by the appearance of new-fashioned technologies—cell transplantation therapy and liver bioengineering—aimed at alleviating the dilemma caused by insufficient liver regeneration or shortage of liver donors, and they are becoming hotspots in the research field. Cell transplantation, mainly referring to stem cells or progenitor cells, has been extensively studied on liver regeneration owing to the potential of differentiation into hepatocytes [68]. Studies have demonstrated that mesenchymal stem cells (MSC) [69], fetal progenitor cell [70], and embryonic stem cells [71] could improve liver injury to some extent. However, the source of transplanted cells and the livability and immune rejection after being transplanted may limit the application of cell therapy on clinical operations. Whereas, liver bioengineering, namely, three-dimensional matrix liver scaffolds, was first reported in 2010 and it includes two parts: decellularization and recellularization [72]. A decellularized liver scaffold (DLS) is characterized by retaining intact vasculature system and a fine web of matrix, providing necessary environment similar to a normal liver for cells to grow, proliferate, and differentiate [73]. After that, the DLS would be repopulated with functional human cells, mainly autologous liver progenitor cells. Furthermore, perfusion of the recellularized liver scaffold with positive molecules for cell regeneration or differentiation, for example, granulocyte colony stimulating factor (G-CSF), may facilitate liver regeneration [74]. Taken together, cell therapy, along with liver bioengineering, may be a new path for liver regeneration development.
6. Conclusions
Despite having been studied for so many years, the passion and energy for liver regeneration never fade out, since the demands for it is urgent from the past till now because of liver transplantation or liver failure or other end-stage liver diseases. It is a multifactor and multipath network, and the exact mechanisms are incompletely understood. Although the appearance of the new technologies opens our thoughts and horizons and, together with the previous results of researches, may drive us closer to clinical application, we still have a long way to go as we always operated studies on animals and the conclusions we deduced could not be applied to human directly because of species differences.
Acknowledgments
This work was supported by the Science and Technology Support Program of Sichuan Province, China (no. 2015SZ0049 and no. 2016SZ0042).
Abbreviations
- TGF-β:
Transforming growth factor beta
- TNF-α:
Tumor necrosis factor-α
- TLR:
Toll-like receptor
- MyD88:
Myeloid differentiation factor 88
- AP-1:
Activating protein1
- LPS:
Lipopolysaccharide
- SOCS:
Suppressors of cytokine signaling
- HGF:
Hepatocyte growth factor
- TGF:
Transforming growth factor
- EGF:
Epidermal growth factor
- BAs:
Bile acids
- NE:
Norepinephrine
- VEGF:
Endothelial growth factor
- IGF:
Insulin-like growth factor
- ER:
Estrogen receptors
- ZNF490:
Zinc finger protein gene
- CARD11:
Caspase recruitment domain-containing protein 11
- ECM:
Extracellular matrix
- S1P:
Sphingosine-1-phosphate
- SK2:
Sphingosine kinase 2
- MSC:
Mesenchymal stem cells
- DLS:
Decellularized liver scaffold
- G-CSF:
Granulocyte-colony stimulating factor.
Conflicts of Interest
The authors have no financial conflict of interests.
References
- 1.Michalopoulos G. K., DeFrances M. C. Liver regeneration. Science. 1997;276(5309):60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Sun G. P., Irvine K. D. Control of growth during regeneration. Current Topics in Developmental Biology. 2014;108:95–120. doi: 10.1016/B978-0-12-391498-9.00003-6. [DOI] [PubMed] [Google Scholar]
- 3.Pahlavan P. S., Feldmann R. E., Zavos C., Kountouras J. Prometheus’ challenge: molecular, cellular and systemic aspects of liver regeneration. Journal of Surgical Research. 2006;134(2):238–251. doi: 10.1016/j.jss.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Karin M., Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529(7586):307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang L. I., Mars W. M., Michalopoulos G. K. Signals and cells involved in regulating liver regeneration. Cell. 2012;1(4):1261–1292. doi: 10.3390/cells1041261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall K. M., He S., Zhong Z., Atkinson C., Tomlinson S. Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy. The Journal of Experimental Medicine. 2014;211(9):1793–1805. doi: 10.1084/jem.20131902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastellos D., Papadimitriou J. C., Franchini S., Tsonis P. A., Lambris J. D. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. Journal of Immunology. 2001;166(4):2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 8.Strey C. W., Markiewski M., Mastellos D., et al. The proinflammatory mediators C3a and C5a are essential for liver regeneration. The Journal of Experimental Medicine. 2003;198(6):913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujiyoshi M., Ozaki M. Molecular mechanisms of liver regeneration and protection for treatment of liver dysfunction and diseases. Journal of Hepato-Biliary-Pancreatic Surgery. 2011;18(1):13–22. doi: 10.1007/s00534-010-0304-2. [DOI] [PubMed] [Google Scholar]
- 10.Schaper F., Rose-John S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine & Growth Factor Reviews. 2015;26(5):475–487. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Campbell J. S., Prichard L., Schaper F., et al. Expression of suppressors of cytokine signaling during liver regeneration. The Journal of Clinical Investigation. 2001;107(10):1285–1292. doi: 10.1172/JCI11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nechemia-Arbely Y., Shriki A., Denz U., et al. Early hepatocyte DNA synthetic response posthepatectomy is modulated by IL-6 trans-signaling and PI3K/AKT activation. Journal of Hepatology. 2011;54(5):922–929. doi: 10.1016/j.jhep.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70(1):11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Peters M., Blinn G., Jostock T., et al. Combined interleukin 6 and soluble interleukin 6 receptor accelerates murine liver regeneration. Gastroenterology. 2000;119(6):1663–1671. doi: 10.1053/gast.2000.20236. [DOI] [PubMed] [Google Scholar]
- 15.Drucker C., Gewiese J., Malchow S., Scheller J., Rose-John S. Impact of interleukin-6 classic- and trans-signaling on liver damage and regeneration. Journal of Autoimmunity. 2010;34(1):29–37. doi: 10.1016/j.jaut.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Gewiese-Rabsch J., Drucker C., Malchow S., Scheller J., Rose-John S. Role of IL-6 trans-signaling in CCl4 induced liver damage. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2010;1802(11):1054–1061. doi: 10.1016/j.bbadis.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Arras D., Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. Journal of Hepatology. 2016;64(6):1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi K., Wu L. W., Grivennikov S. I., et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519(7541):57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschaharganeh D. F., Chen X., Latzko P., et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144(7):1530–1542.e12. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michalopoulos G. K. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. The American Journal of Pathology. 2010;176(1):2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao S. A., Glorioso J. M., Nyberg S. L. Liver regeneration. Translational Research. 2014;163(4):352–362. doi: 10.1016/j.trsl.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong F., Nguyen V. A., Shen X., Kunos G., Gao B. Rapid activation of protein kinase B/Akt has a key role in antiapoptotic signaling during liver regeneration. Biochemical and Biophysical Research Communications. 2000;279(3):974–979. doi: 10.1006/bbrc.2000.4044. [DOI] [PubMed] [Google Scholar]
- 23.Huh C. G., Factor V. M., Sanchez A., Uchida K., Conner E. A., Thorgeirsson S. S. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paranjpe S., Bowen W. C., Tseng G. C., Luo J. H., Orr A., Michalopoulos G. K. RNA interference against hepatic epidermal growth factor receptor has suppressive effects on liver regeneration in rats. American Journal of Pathology. 2010;176(6):2669–2681. doi: 10.2353/ajpath.2010.090605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang W. D., Ma K., Zhang J., et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312(5771):233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 26.Cruise J. L. Alpha-1-adrenergic receptors in liver-regeneration. Digestive Diseases and Sciences. 1991;36(4):485–488. doi: 10.1007/BF01298880. [DOI] [PubMed] [Google Scholar]
- 27.Bockhorn M., Goralski M., Prokofiev D., et al. VEGF is important for early liver regeneration after partial hepatectomy. The Journal of Surgical Research. 2007;138(2):291–299. doi: 10.1016/j.jss.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Enguita-German M., Fortes P. Targeting the insulin-like growth factor pathway in hepatocellular carcinoma. World Journal of Hepatology. 2014;6(10):716–737. doi: 10.4254/wjh.v6.i10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biondo-Simoes Mde L., Erdmann T. R., Ioshii S. O., Matias J. E., Calixto H. L., Schebelski D. J. The influence of estrogen on liver regeneration: an experimental study in rats. Acta Cirurgica Brasileira / Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia. 2009;24(1):3–6. doi: 10.1590/s0102-86502009000100002. [DOI] [PubMed] [Google Scholar]
- 30.Clavien P. A. Liver regeneration: a spotlight on the novel role of platelets and serotonin. Swiss Medical Weekly. 2008;138(25-26):361–370. doi: 10.4414/smw.2008.12231. [DOI] [PubMed] [Google Scholar]
- 31.Fan M. J., Wang X. C., Xu G. Y., Yan Q., Huang W. Bile acid signaling and liver regeneration. Biochimica et Biophysica Acta-Gene Regulatory Mechanisms. 2015;1849(2):196–200. doi: 10.1016/j.bbagrm.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindroos P. M., Zarnegar R., Michalopoulos G. K. Hepatocyte growth-factor (hepatopoietin A) rapidly increases in plasma before DNA-synthesis and liver-regeneration stimulated by partial-hepatectomy and carbon-tetrachloride administration. Hepatology. 1991;13(4):743–750. [PubMed] [Google Scholar]
- 33.Ohtake Y., Kobayashi T., Maruko A., et al. Norepinephrine modulates the zonally different hepatocyte proliferation through the regulation of transglutaminase activity. American Journal of Physiology Gastrointestinal and Liver Physiology. 2010;299(1):G106–G114. doi: 10.1152/ajpgi.00365.2009. [DOI] [PubMed] [Google Scholar]
- 34.Kanamaru C., Yasuda H., Takeda M., et al. Smad7 is induced by norepinephrine and protects rat hepatocytes from activin A-induced growth inhibition. The Journal of Biological Chemistry. 2001;276(49):45636–45641. doi: 10.1074/jbc.M105302200. [DOI] [PubMed] [Google Scholar]
- 35.Taniguchi E., Sakisaka S., Matsuo K., Tanikawa K., Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. The Journal of Histochemistry and Cytochemistry : Official Journal of the Histochemistry Society. 2001;49(1):121–130. doi: 10.1177/002215540104900112. [DOI] [PubMed] [Google Scholar]
- 36.Amaya M. J., Oliveira A. G., Guimaraes E. S., et al. The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology. 2014;59(1):274–283. doi: 10.1002/hep.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallek G., Friedrich N., Ittermann T., et al. IGF-1 and IGFBP-3 in patients with liver disease. Laboratoriumsmedizin. 2013;37(1):13–20. [Google Scholar]
- 38.Uebi T., Umeda M., Imai T. Estrogen induces estrogen receptor alpha expression and hepatocyte proliferation in the livers of male mice. Genes to Cells : Devoted to Molecular & Cellular Mechanisms. 2015;20(3):217–223. doi: 10.1111/gtc.12214. [DOI] [PubMed] [Google Scholar]
- 39.Chiu E. J., Lin H. L., Chi C. W., Liu T. Y., Lui W. Y. Estrogen therapy for hepatectomy patients with poor liver function? Medical Hypotheses. 2002;58(6):516–518. doi: 10.1054/mehy.2001.1496. [DOI] [PubMed] [Google Scholar]
- 40.Lesurtel M., Clavien P. A. Serotonin: a key molecule in acute and chronic liver injury! Clinics and Research in Hepatology and Gastroenterology. 2012;36(4):319–322. doi: 10.1016/j.clinre.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Papadimas G. K., Tzirogiannis K. N., Mykoniatis M. G., Grypioti A. D., Manta G. A., Panoutsopoulos G. I. The emerging role of serotonin in liver regeneration. Swiss Medical Weekly. 2012;142, article w13548 doi: 10.4414/smw.2012.13548. [DOI] [PubMed] [Google Scholar]
- 42.Nurden A. T. Platelets, inflammation and tissue regeneration. Thrombosis and Haemostasis. 2011;105(Supplement 1):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 43.Meyer J., Lejmi E., Fontana P., Morel P., Gonelle-Gispert C., Bühler L. A focus on the role of platelets in liver regeneration: do platelet-endothelial cell interactions initiate the regenerative process? Journal of Hepatology. 2015;63(5):1263–1271. doi: 10.1016/j.jhep.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Matsuo R., Nakano Y., Ohkohchi N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Annals of Surgery. 2011;253(4):759–763. doi: 10.1097/SLA.0b013e318211caf8. [DOI] [PubMed] [Google Scholar]
- 45.Murata S., Ohkohchi N., Matsuo R., Ikeda O., Myronovych A., Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World Journal of Surgery. 2007;31(4):808–816. doi: 10.1007/s00268-006-0772-3. [DOI] [PubMed] [Google Scholar]
- 46.Lisman T., Porte R. J. Mechanisms of platelet-mediated liver regeneration. Blood. 2016;128(5):625–629. doi: 10.1182/blood-2016-04-692665. [DOI] [PubMed] [Google Scholar]
- 47.Ding B. S., Nolan D. J., Butler J. M., et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468(7321):310–U240. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monga S. P. S. Role of Wnt/β-catenin signaling in liver metabolism and cancer. The International Journal of Biochemistry & Cell Biology. 2011;43(7):1021–1029. doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monga S. S. Role and regulation of β-catenin signaling during physiological liver growth. Gene Expression. 2014;16(2):51–62. doi: 10.3727/105221614X13919976902138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nejak-Bowen K. N., Monga S. P. S. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Seminars in Cancer Biology. 2011;21(1):44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang J., Mowry L. E., Nejak-Bowen K. N., et al. Beta-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 2014;60(3):964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lou G., Chen Z., Zheng M., Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Experimental & Molecular Medicine. 2017;49(6, article e346) doi: 10.1038/emm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nojima H., Freeman C. M., Schuster R. M., et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. Journal of Hepatology. 2016;64(1):60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan C. Y., Lai R. C., Wong W., Dan Y. Y., Lim S. K., Ho H. K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Research & Therapy. 2014;5(3):p. 76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derynck R., Zhang Y. E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 56.Romero-Gallo J., Sozmen E. G., Chytil A., et al. Inactivation of TGF-β signaling in hepatocytes results in an increased proliferative response after partial hepatectomy. Oncogene. 2005;24(18):3028–3041. doi: 10.1038/sj.onc.1208475. [DOI] [PubMed] [Google Scholar]
- 57.Nygard I. E., Mortensen K. E., Hedegaard J., et al. The genetic regulation of the terminating phase of liver regeneration. Comparative Hepatology. 2012;11(1):p. 3. doi: 10.1186/1476-5926-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi K., Murata S., Ohkohchi N. Novel therapy for liver regeneration by increasing the number of platelets. Surgery Today. 2013;43(10):1081–1087. doi: 10.1007/s00595-012-0418-z. [DOI] [PubMed] [Google Scholar]
- 59.Lee S. C., Jeong H. J., Choi B. J., Kim S. J. Role of the spleen in liver regeneration in relation to transforming growth factor-β1 and hepatocyte growth factor. Journal of Surgical Research. 2015;196(2):270–277. doi: 10.1016/j.jss.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 60.Ueda S., Yamanoi A., Hishikawa Y., Dhar D. K., Tachibana M., Nagasue N. Transforming growth factor-β1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats. Laboratory Investigation. 2003;83(11):1595–1603. doi: 10.1097/01.lab.0000095686.10639.c8. [DOI] [PubMed] [Google Scholar]
- 61.Takamura K., Tsuchida K., Miyake H., Tashiro S., Sugino H. Activin and activin receptor expression changes in liver regeneration in rat. Journal of Surgical Research. 2005;126(1):3–11. doi: 10.1016/j.jss.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Chen L., Zhang W., Liang H. F., et al. Activin A induces growth arrest through a SMAD-dependent pathway in hepatic progenitor cells. Cell Communication and Signaling: CCS. 2014;12:p. 18. doi: 10.1186/1478-811X-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kogure K., Omata W., Kanzaki M., et al. A single intraportal administration of follistatin accelerates liver-regeneration in partially hepatectomized rats. Gastroenterology. 1995;108(4):1136–1142. doi: 10.1016/0016-5085(95)90212-0. [DOI] [PubMed] [Google Scholar]
- 64.Date M., Matsuzaki K., Matsushita M., Tahashi Y., Sakitani K., Inoue K. Differential regulation of activin A for hepatocyte growth and fibronectin synthesis in rat liver injury. Journal of Hepatology. 2000;32(2):251–260. doi: 10.1016/S0168-8278(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 65.Tsugawa D., Oya Y., Masuzaki R., et al. Specific activin receptor-like kinase 3 inhibitors enhance liver regeneration. The Journal of Pharmacology and Experimental Therapeutics. 2014;351(3):549–558. doi: 10.1124/jpet.114.216903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kan N. G., Junghans D., Belmonte J. C. I. Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. The FASEB Journal. 2009;23(10):3516–3525. doi: 10.1096/fj.09-131730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Do N., Zhao R., Ray K., et al. BMP4 is a novel paracrine inhibitor of liver regeneration. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;303(11):G1220–G1227. doi: 10.1152/ajpgi.00105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Z., Wang F. S. Stem cell therapies for liver failure and cirrhosis. Journal of Hepatology. 2013;59(1):183–185. doi: 10.1016/j.jhep.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 69.Esrefoglu M. Role of stem cells in repair of liver injury: experimental and clinical benefit of transferred stem cells on liver failure. World Journal of Gastroenterology. 2013;19(40):6757–6773. doi: 10.3748/wjg.v19.i40.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kisseleva T., Gigante E., Brenner D. A. Recent advances in liver stem cell therapy. Current Opinion in Gastroenterology. 2010;26(4):395–402. doi: 10.1097/MOG.0b013e32833a6bec. [DOI] [PubMed] [Google Scholar]
- 71.Ezzat T., Dhar D. K., Malago M., Olde Damink S. W. Dynamic tracking of stem cells in an acute liver failure model. World Journal of Gastroenterology. 2012;18(6):507–516. doi: 10.3748/wjg.v18.i6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uygun B. E., Soto-Gutierrez A., Yagi H., et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nature Medicine. 2010;16(7):814–U120. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tapias L. F., Ott H. C. Decellularized scaffolds as a platform for bioengineered organs. Current Opinion in Organ Transplantation. 2014;19(2):145–152. doi: 10.1097/MOT.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Booth C., Soker T., Baptista P., et al. Liver bioengineering: current status and future perspectives. World Journal of Gastroenterology. 2012;18(47):6926–6934. doi: 10.3748/wjg.v18.i47.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]