Abstract
Mosasauroid squamates represented the apex predators within the Late Cretaceous marine and occasionally also freshwater ecosystems. Proper understanding of the origin of their ecological adaptations or paleobiogeographic dispersals requires adequate knowledge of their phylogeny. The studies assessing the position of mosasauroids on the squamate evolutionary tree and their origins have long given conflicting results. The phylogenetic relationships within Mosasauroidea, however, have experienced only little changes throughout the last decades. Considering the substantial improvements in the development of phylogenetic methodology that have undergone in recent years, resulting, among others, in numerous alterations in the phylogenetic hypotheses of other fossil amniotes, we test the robustness in our understanding of mosasauroid beginnings and their evolutionary history. We re-examined a data set that results from modifications assembled in the course of the last 20 years and performed multiple parsimony analyses and Bayesian tip-dating analysis. Following the inferred topologies and the ‘weak spots’ in the phylogeny of mosasauroids, we revise the nomenclature of the ‘traditionally’ recognized mosasauroid clades, to acknowledge the overall weakness among branches and the alternative topologies suggested previously, and discuss several factors that might have an impact on the differing phylogenetic hypotheses and their statistical support.
Keywords: Mosasauroidea, Phylogeny, Parsimony analysis, Bayesian inference, Phylogenetic nomenclature, Fossilized birth-death model, Late Cretaceous
Introduction
Mosasauroidea was a species-rich clade of squamates adapted to an aquatic lifestyle, with evolutionary history being recorded exclusively in the Upper Cretaceous strata (e.g., Russell, 1967; Bell, 1997; Polcyn et al., 2014). During their history, mosasauroids distributed globally and evolved different ecological strategies (Polcyn et al., 2014; Bardet et al., 2015). Although the most distinguishable mosasauroid lineages, such as tylosaurines, plioplatecarpines, and derived mosasaurines, have been adequately recognized decades ago (Russell, 1967), the knowledge of mosasauroid origins and interrelationships is far from exhaustive. The studies of mosasauroid beginnings have long suffered from conflicting results of large-scale phylogenetic analyses (e.g., Lee, 1998; Conrad, 2008; Conrad et al., 2011; Gauthier et al., 2012), leading to the rise of considerable uncertainties surrounding the phylogenetic placement of Mosasauria within Squamata. However, recent analyses integrating morphological and molecular data show a good support for close relationships of Mosasauria and Serpentes within Toxicofera (Reeder et al., 2015).
The hypotheses of mosasauroid interrelationships appear to be less problematic. Phylogenetic studies frequently reconstruct the clades Halisaurinae, Mosasaurinae, Tylosaurinae, and Plioplatecarpinae (e.g., Bardet et al., 2005; Bell & Polcyn, 2005; Cuthbertson et al., 2007; Bullard & Caldwell, 2010; Fanti, Cau & Negri, 2014; Leblanc, Caldwell & Bardet, 2012; Jiménez-Huidobro & Caldwell, 2016), and a lineage of early-branching mosasaurids consisting of two branches that have recently been named Tethysaurinae and Yaguarasaurinae (Makádi, Caldwell & Ösi, 2012; Palci, Caldwell & Papazzoni, 2013; respectively).
Throughout the last two decades, the phylogenetic relationships within Mosasauroidea have been inferred using modified versions of a single data set. The data set was first introduced in Bell’s (1993) PhD thesis and formally published four years later (Bell, 1997). It was subsequently modified by inclusion of additional taxa and revision of characters and their states (see, e. g., Christiansen & Bonde, 2002; Dortangs et al., 2002; Bell & Polcyn, 2005; Polcyn & Bell, 2005; Bullard, 2006; Dutchak & Caldwell, 2006; Schulp, 2006; Schulp et al., 2006; Caldwell & Palci, 2007; Cuthbertson et al., 2007; Polcyn & Everhart, 2008; Dutchak & Caldwell, 2009; Fernandez & Martin, 2009; Leblanc, Caldwell & Bardet, 2012; Makádi, Caldwell & Ösi, 2012; Grigoriev, 2013; Palci, Caldwell & Papazzoni, 2013; Fanti, Cau & Negri, 2014; Jiménez-Huidobro & Caldwell, 2016; Otero et al., 2017; Simões et al., 2017).
The aim of this study is to estimate the robustness in our understanding of mosasauroid phylogenetic relationships by reevaluation of a recent version of that data set, published by Simões et al. (2017), that represents an effect of 20 years of detailed modifications regarding both taxon and character sampling. In particular, in this study we (1) focus on the implications of selection (or omission) among the tree-search strategies available for inferring phylogenetic relationships, a methodological bias that is often overlooked in phylogenetic systematics of fossil taxa, (2) revise the nomenclature of mosasauroid clades to assure that the applied clade names reflect differing tree topologies inferred by this and other studies, and to maintain the use of the names for the ‘traditional’ content, (3) discuss the factors that might have an impact on the differing phylogenetic hypotheses and their statistical support, and (4) suggest further modifications that may improve the resolution of the mosasauroid phylogenetic tree.
Methods
Considering that all recent assessments of mosasauroid interrelationships are based on slightly modified versions of the same data set, we decided to refrain from incorporating substantial changes to the data of Simões et al. (2017) without extensive personal observations. Instead, we provide recommendations regarding further modifications and applied methodology (see ‘Discussion’).
However, slight modifications were provided regarding the binomial nomenclature. Aigialosaurus bucchichi was placed back within Opetiosaurus (following the potential non-monophyletic nature of the dalmaticus-bucchichi grouping, as inferred by some studies; e.g., Simões et al., 2017; our results), Pannoniasaurus ‘osii’ is ‘renamed’ P. inexpectatus (the original name established by Makádi, Caldwell & Ösi, 2012), Halisaurus ‘sternbergi’ is placed within Eonatator, as E. sternbergii (Bardet et al., 2005; Konishi et al., 2016), and Platecarpus planifrons is included within Plesioplatecarpus (Konishi & Caldwell, 2011).
We analyzed the data set under both, parsimony and Bayesian inference, the latter integrating morphological and stratigraphic data (using the method of Lee et al., 2014a, implemented by Lee et al., 2014b; Gavryushkina et al., 2017), to simultaneously infer topology and timing of evolutionary events (splitting of branches or placement of ancestors along lineages) of particular mosasauroid subclades. Note that Simões et al. (2017) performed both parsimony and Bayesian analyses, but did not integrate stratigraphic information in the Bayesian inference of their morphological data set.
Parsimony analyses
Parsimony analyses were performed using TNT 1.5 (Goloboff, Farris & Nixon, 2008). In all analyses, we run 100 ‘New Technology’ search replicates, using default settings, saving all shortest trees inferred. Subsequently, for each analysis, we performed ‘Traditional Search’ heuristic search analyses exploring the tree islands inferred by the first round of analyses.
We performed three types of analyses: (1) with all characters having equal weight; first, keeping all multistate characters as unordered and, second, setting a subset of the multistate characters as ordered (listed below), (2) using the Implied Weighting option of TNT 1.5 (Goloboff, 1993; Goloboff, 1995; Goloboff et al., 2008) with three runs performed for both, ‘unordered’ and ‘ordered’ settings (K = 3, 6, and 9), and (3) with the same setting as in Simões et al. (2017) but using different ‘dolichosaur-grade’ taxa as sole outgroups.
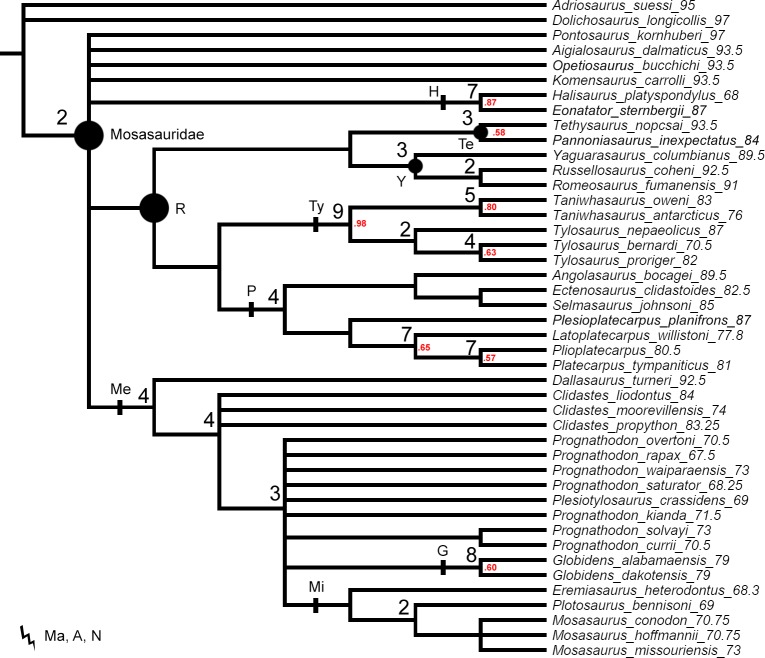
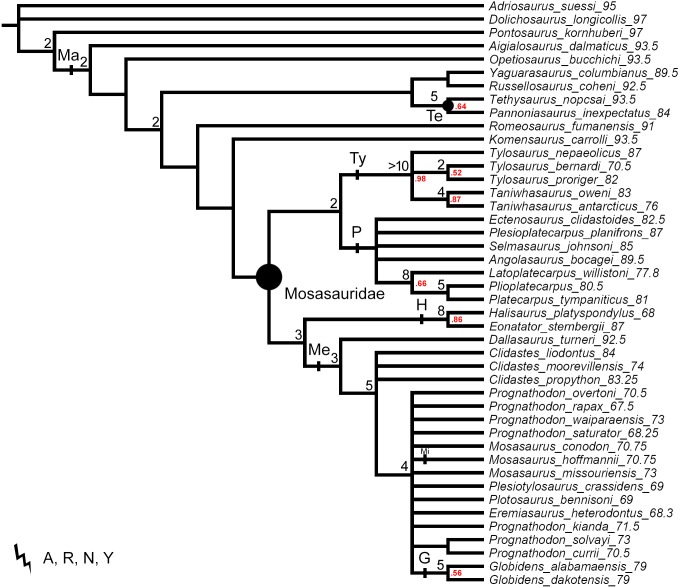
The original data set of Simões et al. (2017) set all multistate characters as unordered. These settings were replicated for the first parsimony analysis to provide a better comparison of the Decay Index and bootstrap values behind the tree topologies resulting from ‘unweighted-unordered’ parsimony analysis (Fig. 1) and our ‘unweighted-ordered’ parsimony analysis (Fig. 2).
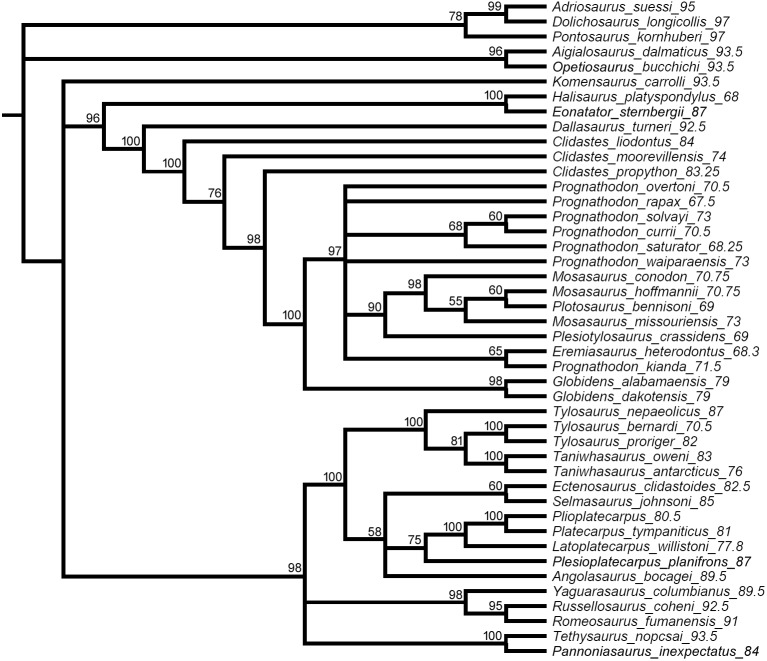
Figure 1. The strict consensus tree of 84 MPTs of length 445 inferred from unweighted parsimony analysis with all characters set as unordered (CI: 0.3640, RI: 0.7100).
Values at nodes indicate Decay Index >1 and bootstrap >0.5. In this and subsequent figures the number following each species name indicates the mean value of the tip prior (in Mya). Points on nodes indicate the extents of node-based clade names: R, Russellosaurina; Te, Tethysaurinae; Y, Yaguarasaurinae. Lines on branches indicate the extents of branch-based clade names: A, Aigialosauridae; G, Globidensini; H, Halisaurinae; Ma, Mosasauroidea; Me, Mosasaurinae; Mi, Mosasaurini; N, Natantia; P, Plioplatecarpinae; Ty, Tylosaurinae. The lightning bolt symbol indicates the names that self-destruct under the topology provided.
Figure 2. The strict consensus tree of 125 MPTs of length 465 inferred from unweighted parsimony analysis with a subset of multistate characters set as ordered (CI: 0.3484, RI: 0.7100).
Values at nodes indicate Decay Index >1 and bootstrap >0.5. Points on nodes indicate the extents of node-based clade names: R, Russellosaurina; Te, Tethysaurinae; Y, Yaguarasaurinae. Lines on branches indicate the extents of branch-based clade names: A, Aigialosauridae; G, Globidensini; H, Halisaurinae; Ma, Mosasauroidea; Me, Mosasaurinae; Mi, Mosasaurini; N, Natantia; P, Plioplatecarpinae; Ty, Tylosaurinae. The lightning bolt symbol indicates the names that self-destruct under the topology provided.
The decision to keep all characters unordered was not discussed neither justified, although it represents an implicit hypothesis on character-state transitions (see e.g., Wilkinson, 1992; Brazeau, 2011). We note that 19 among the multistate character statements in the character list of Simões et al. (2017) describe additive transformation series of nested states, and thus should be considered as ordered (the character statements: 1, 8, 10, 18, 20, 29, 30, 32, 37, 41, 53, 54, 55, 63, 72, 88, 96, 102, and 110). The setting of the above listed characters as unordered artificially excludes potential synapomorphies from the character sample, and may lead to the inference of spurious relationships (Brazeau, 2011). It is noteworthy that Simões et al. (2017) apparently recognized that some of these characters may be considered as ordered, but then left those characters as unordered (e.g., Simões et al., 2017, Supplemental Information 1, see definition of state (1) of character 8, and comment on character statement 55).
The Decay Index and bootstrap values were calculated only in the two parsimony analyses with all characters having equal weight (‘unordered’ and ‘ordered’). The support values for the results inferred through the six runs of weighted parsimony (3 runs of ‘unordered’ settings for K = 3, 6, and 9; and 3 of ‘ordered’ settings for the same values of K) and the analyses with only one ‘dolichosaur’ included were not calculated. Rather, the inferred topologies resulting from these analyses are intended to visualize the effects of the use of different tree-search strategies (also, see ‘Discussion’ for comments on ‘Potential issues resulting from application of the Implied Weighting function’ and the ‘Outgroup selection’, that is particularly relevant when assessing the present results of parsimony analyses with only a single ‘dolichosaur’ included).
Bayesian inference
Bayesian phylogenetic analysis integrating morphological and stratigraphic information was performed following the method discussed by Lee et al. (2014a), using implementations discussed by Lee et al. (2014b) and the Fossilized Birth–Death tree model sampling ancestors (FBDSA) introduced by Gavryushkina et al. (2014) and Gavryushkina et al. (2017). Bayesian inference analyses were performed in BEAST 2.4.4. (Drummond et al., 2012; Bouckaert et al., 2014), implemented with the packages for the analysis of morphological characters, using the model of Lewis (2001), and for sampling potential ancestors among the ingroup (Gavryushkina et al., 2014). The morphological matrix was the same as used in the parsimony analysis (see ‘Parsimony analyses’ above), with all characters set as unordered, to reproduce the settings used by Simões et al. (2017). Contrary to the outgroup used by previous analyses of mosasauroid affinities (‘composite’ outgroup and Varanus, see below and ‘Discussion’), Simões et al. (2017) added three early Late Cretaceous non-mosasauroid squamates, Adriosaurus suessi Seeley, 1881, Dolichosaurus longicollis Owen, 1850, and Pontosaurus kornhuberi Caldwell, 2006, and selected A. suessi as the root of the topologies. This outgroup selection is more realistic than the strategy followed in other recent analyses of Mosasauroidea, that use the extant and distantly-related Varanus (e.g., Palci, Caldwell & Papazzoni, 2013; Jiménez-Huidobro & Caldwell, 2016; Otero et al., 2017), since it assumes that the ancestral mosasauroid morphology is likely represented by the simplesiomorphies shared by penecontemporary semi-aquatic squamates close to the mosasauroid root. Furthermore, the use of Cenomanian squamates as mosasauroid outgroups does not violate uniform sampling rate required by the use of the FBDSA model. However, see the ‘Outgroup selection’ paragraph of ‘Discussion’ for further comments.
Since the character matrix did not include autapomorphies of the sampled taxa, the Lewis’s (2001) model was conditioned to variable characters only using the implementation included in BEAST 2.4.4. Stratigraphic information for the mosasauroid taxa was taken from the literature, and converted to geochronological ages. Stratigraphic data and age constraints for each terminal were obtained mainly from Polcyn et al. (2014) and integrated with information from the Paleobiology Database (http://paleobiodb.org/). The ages for Romeosaurus fumanensis and Prognathodon kianda were obtained from Palci, Caldwell & Papazzoni (2013) and Strganac et al. (2014), respectively. For the Bayesian analyses they performed, Simões et al. (2017) discussed the use of alternative distributions of the rate heterogeneity and rate frequency parameters, in particular, they suggested the use of a lognormal distribution instead of the more frequently used gamma distribution. In our analysis, rate variation across traits was modeled using the multi-gamma parameter (default model and unique implemented for the analysis of morphological data in BEAST 2). The rate variation across branches was modeled using the relaxed log-normal clock model, with the number of discrete rate categories that approximate the rate distribution set as n − 1 (with n the number of branches), the mean clock rate using default setting, and not setting to normalize the average rate. Particularly relevant for the taxonomic purposes of this study, the FBDSA tree model allows for testing whether one or more of the included taxa are sampled ancestors of one or more other included taxa, as it discriminates between cladogenetic and anagenetic patterns in macroevolution (Gavryushkina et al., 2014; Cau, 2017 and reference therein). We used two tree models included in the BEAST package: the Sampled Ancestor Fossilized Birth Death Skyline Model (Gavryushkina et al., 2014) and the FBDSA model (Gavryushkina et al., 2017). Convergence (stationarity) in numerical parameters among the different analyses was identified using Tracer (Rambaut & Drummond, 2009): the results showed broadly overlapping, non-trending traces across all replicate runs for every parameter, with effective sample sizes (ESS) of every parameter exceeding 100. Since all taxa included in the analysis are extinct, the rho parameter of Gavryushkina et al. (2014), which defines the probability to sample among extant taxa, was set as 0. The root age of the tree model was conservatively set as a uniform prior spanning between the age of the oldest ingroup taxa and 200 Mya (near the Triassic-Jurassic boundary: this age falls within the estimated range of the origin of the crown clade Squamata (Jones et al., 2013) though consistently pre-dates all known crown squamates (Conrad, 2008; Gauthier et al., 2012), and thus defines a time range that likely includes the age of the last common ancestor of all terminal taxa included). A first round of the analysis used four replicate runs of 10 million generations, with sampling every 1,000 generations, that were subsequently combined using LogCombiner 1.7.3 (included in the BEAST package). Then, we replicated the same analysis performing a single run of 40 million generations. In both analyses, burnin was set at 20%, and the Maximum Clade Credibility Tree (MCCT) used as framework for phyletic reconstruction. Convergence of parameters among the different runs was evaluated using Tracer. Exploration of the results of the alternative analyses produced identical topologies and did not indicate any significant differences in age inference. Given the overall overlap among the results of the alternative Bayesian analyses, for brevity, the following discussion refers to the analysis based on the single run of 40 million replications and using the FBDSA model. Although the MCCT is the topology with the maximum product of clade posterior probabilities, and is used for summarizing posterior distributions of trees (e.g., Lee et al., 2014b), it is necessary to remark that (1) not all relationships supported by the posterior distribution inferred are depicted in the MCCT, and (2) the most weakly-supported nodes included in the MCCT usually are recovered in small subsets of the posterior distribution. The half-compact consensus of the post-burnin topologies inferred (equivalent to a 50% majority rule consensus of the shortest trees, used in parsimony analyses) has been included for comparison with the MCCT (see Cau, 2017).
Results
All parsimony analyses (Figs. 1–4) and the Bayesian inference using the FBDSA model (Figs. 5–7) reconstruct most of the ‘traditionally’ recognized mosasaurid groups (Halisaurinae, Mosasaurinae, Plioplatecarpinae, Tethysaurinae, and Tylosaurinae) with the exception of Yaguarasaurinae which breaks down under the ‘unweighted-ordered’ parsimony analysis (Fig. 2) and two ‘weighted-ordered’ parsimony analyses (K = 6 and 9; Figs. 3D and 3F). However, the support behind the inferred nodes is generally poor with only a limited number of clades being strongly supported. The bootstrap and Decay Index (DI) values, which were calculated only in the ‘unweighted-unordered’ and ‘unweighted-ordered’ parsimony analyses using the full data set (i.e., when all three ‘dolichosaurs’ were included; Figs. 1 and 2), were highest for the clade Tylosaurinae (DI = 9 and >10, respectively; and bootstrap = 0.98) and the two species of the tylosaurine Taniwhasaurus (DI = 5 and 4; bootstrap = 0.80 and 0.87), and the clade Halisaurinae (DI = 7 and 8; bootstrap = 0.87 and 0.86). High values of DI were further calculated for the clade of advanced plioplatecarpines formed by Latoplatecarpus willistoni, Platecarpus tympaniticus, and Plioplatecarpus spp. (DI = 7 and 8, respectively), the clade of P. tympaniticus and Plioplatecarpus spp. (DI = 7 and 5), and the two species of the mosasaurine Globidens (DI = 8 and 5). However, the bootstrap values are <0.70 in all these groupings.
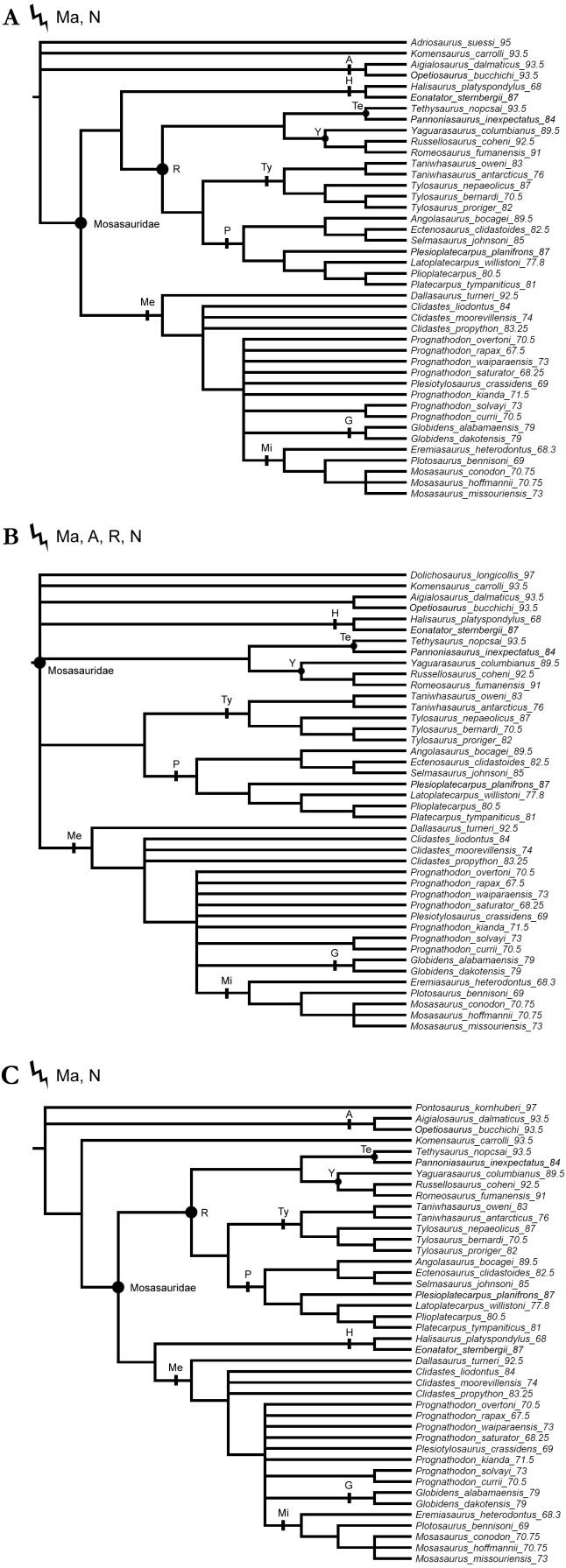
Figure 4. Strict consensus trees produced by the alternative tests using a single ‘dolichosaur’ taxon as outgroup.
Trees rooted on (A) Adriosaurus suessi (40 MPTs), (B) Dolichosaurus longicollis (140 MPTs), and (C) Pontosaurus kornhuberi (20 MPTs) Points on nodes indicate the extents of node-based clade names: R, Russellosaurina; Te, Tethysaurinae; Y, Yaguarasaurinae. Lines on branches indicate the extents of branch-based clade names: A, Aigialosauridae; G, Globidensini; H, Halisaurinae; Ma, Mosasauroidea; Me, Mosasaurinae; Mi, Mosasaurini; N, Natantia; P, Plioplatecarpinae; Ty, Tylosaurinae. The lightning bolt symbol indicates the names that self-destruct under the topology provided.
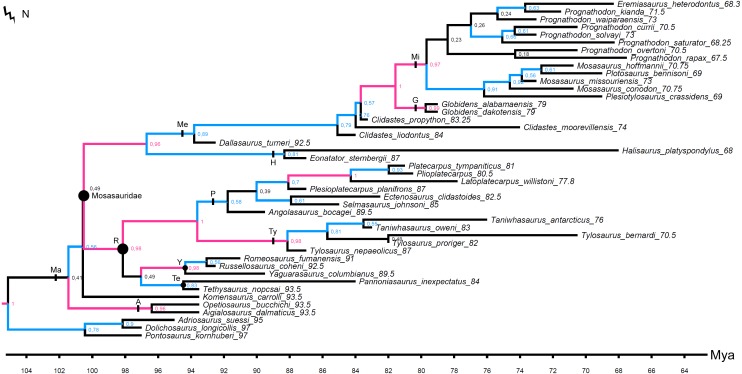
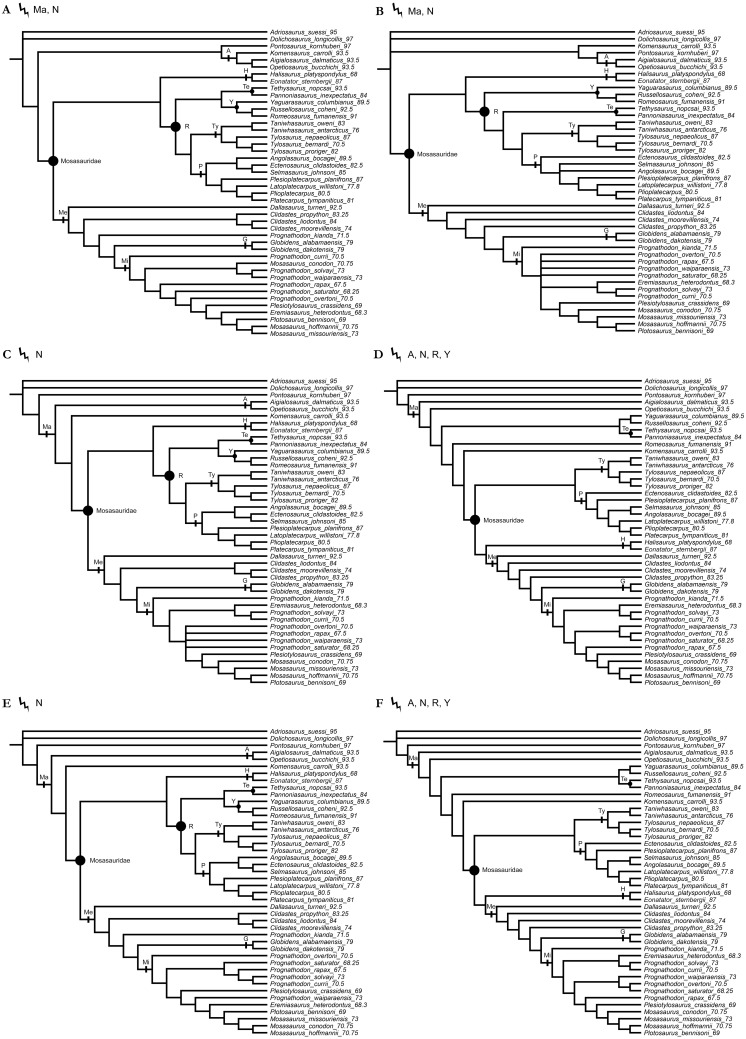
Figure 5. MCCT inferred by the Bayesian analysis.
Branches colored according to posterior probability (pp) values: black, pp < 0.5; blue, 0.5 ≤ pp < 0.95; pink, pp ≥ 0.95. Points on nodes indicate the extents of node-based clade names: R, Russellosaurina; Te, Tethysaurinae; Y, Yaguarasaurinae. Lines on branches indicate the extents of branch-based clade names: A, Aigialosauridae; G, Globidensini; H, Halisaurinae; Ma, Mosasauroidea; Me, Mosasaurinae; Mi, Mosasaurini; N, Natantia; P, Plioplatecarpinae; Ty, Tylosaurinae. The lightning bolt symbol indicates the names that self-destruct under the topology provided.
Figure 7. Half compact (majority rule) consensus of the topologies inferred among the post-burnin trees saved by the Bayesian analysis.
Branch lengths not to scale. Numbers at nodes indicate % of sampled trees inferring those nodes.
Figure 3. The strict consensus trees of the shortest topologies inferred from weighted parsimony analyses with all characters unordered (UO) and a subset of multistate characters set as ordered (O).
(A) UO with K = 3 (1 MPT), (B) O with K = 3 (4 MPTs), (C) UO with K = 6 (2 MPTs), (D) O with K = 6 (1 MPT), (E) UO with K = 9 (1 MPT), (F) O with K = 9 (1 MPT). Points on nodes indicate the extents of node-based clade names: R, Russellosaurina; Te, Tethysaurinae; Y, Yaguarasaurinae. Lines on branches indicate the extents of branch-based clade names: A, Aigialosauridae; G, Globidensini; H, Halisaurinae; Ma, Mosasauroidea; Me, Mosasaurinae; Mi, Mosasaurini; N, Natantia; P, Plioplatecarpinae; Ty, Tylosaurinae. The lightning bolt symbol indicates the names that self-destruct under the topology provided.
The Bayesian analysis strongly supports the monophyly of Tylosaurinae (posterior probability [pp] value = 0.98), the clade formed by L. willistoni, P. tympaniticus, and Plioplatecarpus spp. (pp = 1), and the monophyly of Globidens (pp = 0.99). However, the other groupings that were well supported by the parsimony analyses, have pp values below 0.95 (Halisaurinae: pp = 0.81; Taniwhasaurus: pp = 0.55). Interestingly, the Bayesian analysis strongly supports groupings that were not reconstructed by some parsimony analyses or only poorly supported, such as the Yaguarasaurinae (pp = 0.98) or the connection of Halisaurinae with Mosasaurinae (pp = 0.96). It also infers strong support for the grouping of advanced mosasaurines, including Globidens, the species attributed to Prognathodon, Mosasaurus, Eremiasaurus, Plesiotylosaurus, and Plotosaurus (pp = 1). In both parsimony analyses, for which the DI and bootstrap values were calculated, this grouping was reconstructed monophyletic as well but bootstrap was <0.50 (DI = 3 for ‘unweighted-unordered’ parsimony analysis and 4 for ‘unweighted-ordered’ parsimony analysis). Additionally, the Bayesian analysis strongly supports the grouping of tethysaurines, yaguarasaurines, plioplatecarpines, and tylosaurines (pp = 0.98) and a clade formed by plioplatecarpines and tylosaurines (pp = 1). In parsimony analyses, the former grouping was reconstructed only under the ‘unweighted-unordered’ settings but the DI was <2 and the bootstrap was <0.50. The latter grouping was inferred by both parsimony analyses but only the result of the ‘unweighted-ordered’ parsimony analysis showed the DI >1 (2). The bootstrap values were <0.50 in both cases. The Bayesian analysis also strongly supports the monophyly of Aigialosaurus dalmaticus and Opetiosaurus bucchichi (pp = 0.96), a grouping not inferred by the two parsimony analyses.
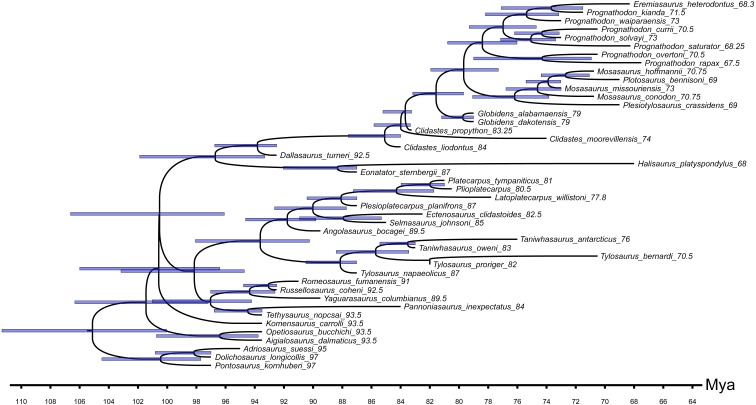
The Bayesian analysis inferred the age (and relative confidence interval) for each node (Figs. 5 and 6). The analysis estimated the divergence of the mosasauroids relative to the ‘dolichosaur’ outgroup during the Albian age (∼105 Mya), thus constraining the origin of the mosasauroid root during the last 6 million years of the Early Cretaceous. Focusing on the most robustly supported nodes in the MCCT (pp not less than 0.95), the mean age inferred for the Aigialosaurus + Opetiosaurus node is dated at ∼96 Mya (95% CI [94–100 Mya]), the mosasaurine-russellosaurinan divergence is dated at 100 Mya (95% CI [96–106.5 Mya]), the divergence of the Tylosaurinae and Plioplatecarpinae lineages is dated at ∼93.6 Mya (95% CI [90–98 Mya]), the origin of the last common ancestor of the included tylosaurine species is dated at 88 Mya (95% CI [87–90.5 Mya]), the lineage including Latoplatecarpus willistoni, Plioplatecarpus spp., and Platecarpus tympanicus originated at ∼84 Mya (95% CI [81.5–87 Mya]), the last common ancestor of mosasaurines and halisaurines is dated at ∼96.7 Mya (95% CI [93–102] Mya), the last common ancestor of Mosasaurini and Globidensini is dated at ∼81.6 Mya (95% CI [80–83 Mya]), the age of the last common ancestor of the two Globidens species included is dated at ∼80 Mya (95% CI [79–81 Mya]), and the last common ancestor of all mosasaurines closer to M. hoffmannii than Globidens is dated at ∼80 Mya (95% CI [77.3–82 Mya]).
Figure 6. MCCT indicating the 95% confidence age range estimated for each node.
‘Weak spots’ in the phylogeny of mosasauroids
The support and resolution is particularly poor near the base of the inferred trees. The ‘unweighted-unordered’ parsimony analysis shows an extensive basal polytomy and does not support the monophyly of mosasaurids exclusive of the ‘aigialosaurs’ (Aigialosaurus dalmaticus and Opetiosaurus bucchichi) and ‘dolichosaurs’ (Fig. 1). The ‘unweighted-ordered’ parsimony analysis groups halisaurines, mosasaurines, plioplatecarpines, tylosaurines, tethysaurines, and yaguarasaurines, but the support is weak (DI <2; bootstrap < 0.50). At the same time, it keeps tethysaurines outside ‘traditional’ mosasaurids (halisaurines, mosasaurines, plioplatecarpines, and tylosaurines) and does not support the monophyly of Yaguarasaurinae (Fig. 2). The Bayesian analysis, nevertheless, infers the monophyly of Mosasasauridae, consisting of monophyletic tethysaurines and yaguarasaurines, but the support is very low (pp = 0.49).
The weighted parsimony analyses and the analyses with a single ‘dolichosaur’ taxon included do not add much to the resolution either. Interestingly, however, there is a tendency, under some ‘ordered’ settings, to move the tethysaurines and yaguarasaurines (the latter being non-monophyletic) outside the ‘traditional’ mosasaurids when halisaurines are reconstructed as the sister taxon to mosasaurines (Figs. 2, 3D and 3F). There is also an apparent lack of resolution within the more advanced mosasaurines (the clade formed by Globidens, the species attributed to Prognathodon, Mosasaurus, Eremiasaurus, Plesiotylosaurus, and Plotosaurus), which are, nevertheless, inferred monophyletic by all analyses (Figs. 1–7; see also above for the support of this grouping). The most striking is the non-monophyly of Prognathodon (inferred also by other authors; e.g., Leblanc, Caldwell & Bardet, 2012; Simões et al., 2017). Some analyses unite certain taxa assigned to Prognathodon, but only the monophyly of P. solvayi and P. currii is reconstructed consistently (Figs. 1–7; except for Fig. 3A), though still poorly supported (DI < 2; bootstrap <0.50; pp = 0.61).
Further, the monophyly of Clidastes is supported only by ‘weighted-unordered’ parsimony analyses; regardless of the value of K (Figs. 3A, 3C, 3E). All other analyses, including the Bayesian inference, keep Clidastes paraphyletic relative to other mosasaurines.
Phylogenetic nomenclature
Inferred phylogenetic relationships are further discussed within the context of mosasauroid systematics and used as the primary basis for nomenclatural revision of the main mosasauroid clades.
The recommended phylogenetic definitions applied for the taxon names follow the International Code of Phylogenetic Nomenclature, or PhyloCode, hereafter ICPN (Cantino & De Queiroz, 2010). They are summarized in Table 1. Likewise, the taxon names are attributed to the authors that introduced them (following the ICPN: Art. 9.8, Note 9.8A.2), and not according to the Principle of Coordination (ICZN, 1999: Art. 36). This approach is preferred due to its more transparent account of the original literature.
Table 1. Recommended phylogenetic definitions applied to mosasauroid taxon names.
| Clade name | Internal specifier(s) | External specifier(s) | Type of phylogenetic definition | Authorship |
|---|---|---|---|---|
| Mosasauroidea | Mosasaurus hoffmannii, Aigialosaurus dalmaticus | Dolichosaurus longicollis, Adriosaurus suessi, Pontosaurus lesinensis | Branch-based | New |
| Aigialosauridae | Aigialosaurus dalmaticus, Opetiosaurus bucchichi | Dolichosaurus longicollis, Adriosaurus suessi, Pontosaurus lesinensis, Mosasauridae = (Mosasaurus hoffmannii, Halisaurus platyspondylus, Tylosaurus proriger) | Branch-based | New |
| Mosasauridae | Mosasaurus hoffmannii, Halisaurus platyspondylus, Tylosaurus proriger | Node-based | Madzia & Conrad (in press) | |
| Halisaurinae | Halisaurus platyspondylus | Mosasaurus hoffmannii, Tylosaurus proriger, Tethysaurus nopcsai, Yaguarasaurus columbianus | Branch-based | New |
| Natantia | Mosasaurus hoffmannii, Tylosaurus proriger, Plioplatecarpus marshii | Halisaurus platyspondylus | Branch-based | Conrad (2008) |
| Mosasaurinae | Mosasaurus hoffmannii | Tylosaurus proriger, Plioplatecarpus marshii, Halisaurus platyspondylus, Tethysaurus nopcsai, Yaguarasaurus columbianus | Branch-based | New |
| Mosasaurini | Mosasaurus hoffmannii | Globidens alabamaensis | Branch-based | New |
| Globidensini | Globidens alabamaensis | Mosasaurus hoffmannii | Branch-based | New |
| Russellosaurina | Russellosaurus coheni, Tylosaurus proriger, Plioplatecarpus marshii | Mosasaurus hoffmannii | Node-based | New |
| Tethysaurinae | Tethysaurus nopcsai, Pannoniasaurus inexpectatus | Halisaurus platyspondylus, Mosasaurus hoffmannii, Tylosaurus proriger, Plioplatecarpus marshii, Yaguarasaurus columbianus | Node-based | New |
| Yaguarasaurinae | Yaguarasaurus columbianus, Russellosaurus coheni, Romeosaurus fumanensis | Tethysaurus nopcsai, Halisaurus platyspondylus, Tylosaurus proriger, Plioplatecarpus marshii, Mosasaurus hoffmannii | Node-based | New |
| Plioplatecarpinae | Plioplatecarpus marshii | Mosasaurus hoffmannii, Tylosaurus proriger, Tethysaurus nopcsai, Yaguarasaurus columbianus | Branch-based | New |
| Tylosaurinae | Tylosaurus proriger | Plioplatecarpus marshii, Mosasaurus hoffmannii | Branch-based | Conrad (2008) |
Even though the majority of the preferred phylogenetic definitions is labeled as ‘new’ (see Table 1), most of them merely represent modified versions of the definitions proposed by other authors. We attempted to provide only the necessary changes to maintain the traditional meaning of the clade names and to maximize their stability given the inferred ‘weak spots’ in the mosasauroid phylogenetic tree.
Mosasauroidea Camp, 1923
Preferred phylogenetic definition
The most inclusive clade containing Mosasaurus hoffmannii Mantell, 1829 and Aigialosaurus dalmaticus Kramberger, 1892, but not Dolichosaurus longicollis Owen, 1850, Adriosaurus suessi Seeley, 1881, or Pontosaurus lesinensis Kornhuber, 1873. This definition is branch-based.
Remarks
Mosasauroidea traditionally includes mosasaurids and ‘aigialosaurs’ (e.g., Bell, 1997; Bell & Polcyn, 2005; Conrad, 2008). Proper delimitation of the extent of the name Mosasauroidea, however, requires adequate knowledge of the early evolution of Mosasauria and reappraisal of the phylogenetic positions of potential non-mosasauroid mosasaurs (e.g., the species belonging to Adriosaurus, Pontosaurus, Dolichosaurus). These taxa, or their subset, have been hypothesized to be either more closely related to snakes (see e.g., Palci & Caldwell, 2007; Caldwell & Palci, 2010; Palci & Caldwell, 2010) or to mosasaurids (e.g., Reeder et al., 2015). Considering that (1) the ‘dolichosaurs’ are traditionally regarded as non-mosasauroids, and (2) ‘aigialosaurs’ and mosasaurids are frequently inferred more closely related to each other than either is to the ‘dolichosaurs’, we propose a new definition that seems to adhere to the traditional use of Mosasauroidea (i.e., ‘aigialosaurs’ plus mosasaurids, but not ‘dolichosaurs’) and reflects the uncertainties surrounding the phylogenetic placements of near-mosasaurids and early mosasaurids as inferred, among others, in the present study (see Figs. 1–7).
Aigialosauridae Kramberger, 1892
Preferred phylogenetic definition
The most inclusive clade containing Aigialosaurus dalmaticus Kramberger, 1892 and Opetiosaurus bucchichi Kornhuber, 1901 but not Dolichosaurus longicollis Owen, 1850, Adriosaurus suessi Seeley, 1881, Pontosaurus lesinensis Kornhuber, 1873, or the clade originating with the most recent common ancestor of Halisaurus platyspondylus Marsh, 1869, Mosasaurus hoffmannii Mantell, 1829, and Tylosaurus proriger (Cope, 1869). This definition is branch-based.
Remarks
Aigialosauridae has a long and problematic history. The last thorough review of the interrelationships of early Mosasauria, i.e., those species associated with the evolutionary transition to aquatic lifestyle, was published by Dutchak (2005) who concluded that “redescriptions of the key taxa (Aigialosaurus dalmaticus, Opetiosaurus bucchichi and ‘the Trieste aigialosaur’) are essential to further investigations into re-testing the most recent hypotheses” (p. 228). Although A. dalmaticus and O. bucchichi have since been redescribed (Dutchak & Caldwell, 2006; Dutchak & Caldwell, 2009; respectively), and ‘the Trieste aigialosaur’ was assessed and given the name Komensaurus carrolli (Caldwell & Palci, 2007), the status of Aigialosauridae did not change. Indeed, Dutchak & Caldwell (2009) argued that O. bucchichi should be assigned to Aigialosaurus (as A. bucchichi), suggesting close relationships of the two taxa. Still, their analysis does not necessarily support this conclusion (see Dutchak & Caldwell, 2009: Fig. 4).
While it is certainly possible that A. dalmaticus and O. bucchichi are more closely related to one another than either is to other mosasauroids, such a result is currently not strongly supported statistically. The ‘full’ parsimony analyses (with all ‘dolichosaurs’ included and A. suessi selected as outgroup) reconstruct the taxa in a basal polytomy with other mosasauroid subclades (Fig. 1) or as successively more closely related to mosasaurids, with A. dalmaticus being the more basal of the two (Fig. 2). The Bayesian inference, majority of the weighted parsimony analyses (except for Figs. 3D and 3F), and parsimony analyses using different ‘dolichosaurs’ as outgroups, nevertheless, reconstruct a clade formed by both these species (Figs. 3–5), though their position on the mosasauroid tree is unstable.
Considering the problematic nature of mosasauroid origins, we admit that Aigialosauridae might be of use in the future. In this case, however, we strongly encourage using a complex self-destructive phylogenetic definition to reflect the history of the name as well as its unstable contents (see ICPN: Art. 11.9). The self-destructive branch-based definition that is proposed here keeps Aigialosauridae in use only if A. dalmaticus and O. bucchichi are more closely related to each other than either is to ‘dolichosaurs’ or Mosasauridae sensu Madzia & Conrad (in press). Also, it does not allow the use of the name in the cases when A. dalmaticus and O. bucchichi are reconstructed within Mosasauridae.
Mosasauridae Gervais, 1853
Preferred phylogenetic definition
The least inclusive clade containing Mosasaurus hoffmannii Mantell, 1829, Halisaurus platyspondylus Marsh, 1869, and Tylosaurus proriger. This definition is node-based.
Remarks
The history of the name Mosasauridae, its approximate synonyms, and its application were discussed by Madzia & Conrad (in press) who also provided the phylogenetic definition for the clade name as will be recognized by the ICPN.
The Bayesian analysis and parsimony analyses using different ‘dolichosaurs’ as the outgroup maintain the monophyly of mosasaurines, plioplatecarpines, tylosaurines, tethysaurines, yaguarasaurines, and the two halisaurine species. The ‘unweighted-ordered’ parsimony analysis, however, reconstructs tethysaurines and yaguarasaurines outside Mosasauridae, with Romeosaurus being inferred as the sister taxon to Komensaurus carrolli + mosasaurids, outside tethysaurines + a clade formed by Yaguarasaurus and Russellosaurus (Fig. 2). Thus, it makes Yaguarasaurinae polyphyletic.
The mutual relationships of particular mosasaurid clades are unsettled and highly dependent on the tree-search strategies used (Figs. 1–7). Still, even though the hypotheses of mosasaurid interrelationships are differing, the definition proposed by Madzia & Conrad (in press) does not require modifications. It covers all ‘traditional’ mosasaurid taxa, including the plioplatecarpines. Though not represented in the phylogenetic definition, Plioplatecarpus and its kin are kept within Mosasauridae under all inferred topologies.
Halisaurinae Bardet et al., 2005
Preferred phylogenetic definition
The most inclusive clade containing Halisaurus platyspondylus Marsh, 1869, but not Mosasaurus hoffmannii Mantell, 1829, Tylosaurus proriger (Cope, 1869), Tethysaurus nopcsai Bardet, Suberbiola & Jalil, 2003, or Yaguarasaurus columbianus Páramo, 1994. This definition is branch-based.
Remarks
Bardet et al. (2005) defined Halisaurinae as “Mosasauridae more closely related to Halisaurus than to Mosasaurus” (p. 464). Later, Conrad (2008) used equivalent branch-based definition with type species as specifiers: “All taxa sharing a more recent common ancestor with Halisaurus platyspondylus than Mosasaurus hoffmannii” (p. 127). Because the position of the species for which the name Halisaurinae was proposed is not very stable within Mosasauroidea (see the results of the present analysis and the Natantia paragraph below), we consider the proposed branch-based definition including additional external specifiers, representing other inferred clades, to be the most appropriate one.
Nevertheless, the current data set is not fully suitable for testing the phylogenetic position of Halisaurinae within Mosasauridae as the clade is represented by only two taxa (H. platyspondylus and Eonatator sternbergii).
Natantia Owen, 1851
Preferred phylogenetic definition
The most inclusive clade containing Mosasaurus hoffmannii Mantell, 1829, Tylosaurus proriger (Cope, 1869), and Plioplatecarpus marshii Dollo, 1882, but not Halisaurus platyspondylus Marsh, 1869. This definition is branch-based.
Remarks
Bell (1997) resurrected the name Natantia from the mid-nineteenth century (Owen, 1851). It was used to unite Bell’s (1997) ‘Russellosaurinae’ (see the Russellosaurina paragraph) and Mosasaurinae, exclusive of the Halisaurus species and the ‘aigialosaurs.’ Conrad (2008: 128) proposed the following branch-based definition: “All taxa sharing a more recent common ancestor with Mosasaurus hoffmanni, Tylosaurus proriger, and Plioplatecarpus marshi than with Halisaurus platyspondylus”. When applied on some recent phylogenetic hypotheses based on the data set initially published by Bell & Polcyn (2005), that infer halisaurines to be nested within the smallest clade containing Mosasaurus, Tylosaurus, and Plioplatecarpus, Natantia self-destructs.
Our analyses do not support the concept of Natantia either (Figs. 1–7). In the ‘unweighted-ordered’ parsimony analysis (Fig. 2), some weighted parsimony analyses (Figs. 3D and 3F), parsimony analysis with Pontosaurus as the outgroup (Fig. 4C), and Bayesian analysis (Fig. 5), halisaurines form the sister taxon to mosasaurines. When Adriosaurus is used as outgroup, and other ‘dolichosaurs’ are excluded, and under some weighted parsimony analyses, halisaurines are more closely related to the clade formed by tethysaurines, yaguarasaurines, tylosaurines, and plioplatecarpines than to mosasaurines (Figs. 3A– 3C, 3E and 4A).
It is worth noting that Boas (1880) used the name Natantia for a subgroup of decapod crustaceans. Although Owen’s (1851) Natantia was published earlier, the priority issue is problematic. The ICZN (1999) does not govern the names above the family group, and Natantia, approximately corresponding to the concept of Owen (1851), had not been in use until Bell (1997). Similarly, the use of Boas (1880) is outdated (WoRMS, 2015), though it was of importance in the past (see, for example, the discussion in Felgenhauser & Abele, 1983).
We refrain from providing a lengthy discussion of the nomenclatural issue or a solution to it but since the name Natantia Owen (1851) was published earlier, we provisionally keep it as the name for the potential grouping as discussed above.
Mosasaurinae Williston, 1897
Preferred phylogenetic definition
The most inclusive clade containing Mosasaurus hoffmannii (Mantell, 1829), but not Tylosaurus proriger (Cope, 1869), Plioplatecarpus marshii Dollo, 1882, Halisaurus platyspondylus Marsh, 1869, Tethysaurus nopcsai Bardet, Suberbiola & Jalil, 2003, or Yaguarasaurus columbianus Páramo, 1994. This definition is branch-based.
Remarks
Mosasaurinae is traditionally considered to represent a species-rich clade with substantial morphological and ecological diversity (e.g., Bell, 1997; Bell & Polcyn, 2005; Bardet et al., 2015).
The first published phylogenetic definition is the following: “All taxa sharing a more recent common ancestor with Mosasaurus hoffmanni than with Tylosaurus proriger or Plioplatecarpus marshi” (Conrad, 2008: 128). This branch-based definition keeps the traditional contents of Mosasaurinae intact when applied to the majority of recent analyses. We added additional external specifiers, Halisaurus platyspondylus, Tethysaurus nopcsai, and Yaguarasaurus columbianus, to reflect the traditional contents of Mosasaurinae and the inferred overall instability in the mosasaurid interrelationships. The monophyly of mosasaurines, however, is inferred by all our analyses (Figs. 1–7).
Mosasaurini Russell, 1967
Preferred phylogenetic definition
The most inclusive clade containing Mosasaurus hoffmannii Mantell, 1829, but not Globidens alabamaensis Gilmore, 1912. This definition is branch-based.
Remarks
Bell (1997: 322) abandoned Mosasaurini on the basis of the supposed paraphyly of Mosasaurus and “expanded [Plotosaurini] to include basic taxa previously referred to Mosasaurus”. Both taxon names, Mosasaurini and Plotosaurini, were introduced in the same publication (Russell, 1967). However, it seems that the former has gained more attention (e.g., Leblanc, Caldwell & Bardet, 2012; Fanti, Cau & Negri, 2014). Leblanc, Caldwell & Bardet (2012: 101) argued to replace Plotosaurini with Mosasaurini which they used for “the group consisting of (Eremiasaurus (Mosasaurus + Plotosaurus))”. Although the close connection of these taxa is generally supported by recent phylogenetic studies (e.g., Grigoriev, 2013; Palci, Caldwell & Papazzoni, 2013; Fanti, Cau & Negri, 2014; Jiménez-Huidobro & Caldwell, 2016), analyses using multiple tree-search strategies show conflicting results (Simões et al., 2017). The grouping is maintained in the ‘unweighted-unordered’ parsimony analysis, under one ‘weighted-unordered’ parsimony analysis (Fig. 3E), and when only one of the ‘dolichosaur’ taxa is included (Fig. 4). Still, ‘unweighted-ordered’ parsimony, other weighted parsimony analyses, and the Bayesian inference fail to support such topology.
Globidensini Russell, 1967
Preferred phylogenetic definition
The most inclusive clade containing Globidens alabamaensis (Gilmore, 1912), but not Mosasaurus hoffmannii Mantell, 1829. This definition is branch-based.
Remarks
Bell (1997) used Russell’s (1967) Globidensini to unite Globidens, Prognathodon, and Plesiotylosaurus. Although such close connection of these taxa is not necessarily supported by current studies (e.g., Palci, Caldwell & Papazzoni, 2013; Fanti, Cau & Negri, 2014; Jiménez-Huidobro & Caldwell, 2016), there is indeed a tendency to keep them together under the name Globidensini (e.g., Schulp et al., 2008; Leblanc, Caldwell & Bardet, 2012). Nevertheless, forcing Prognathodon solvayi, the type species of Prognathodon, to be a globidensin (by selecting it as an internal specifier), would be potentially ineffective considering the likely para- or even polyphyletic nature of the taxa attributed to Prognathodon.
All our analyses fail to reconstruct Globidensini with more than only the two species of Globidens included (Figs. 1–7). Nevertheless, the clade name may still be useful for discussions related to mosasaurid ecology (due to the specialized dentition of Globidens and Carinodens, its potential close relative (Schulp, Jagt & Fonken, 2004)).
Russellosaurina Polcyn & Bell, 2005
Preferred phylogenetic definition
The least inclusive clade containing Russellosaurus coheni Polcyn & Bell, 2005, Tylosaurus proriger (Cope, 1869), and Plioplatecarpus marshii Dollo, 1882, but not Mosasaurus hoffmannii Mantell, 1829. This definition is node-based.
Remarks
Due to its problematic history, the name Russellosaurina is discussed here in detail. In his PhD thesis, Bell (1993) proposed a new name, Russellosaurinae, to link tylosaurines and plioplatecarpines together, and provided the following node-based definition: “The most recent common ancestor of Tylosaurus, Ectenosaurus and Plioplatecarpus and all of its descendants” (p. 183). He noted that Russellosaurinae consists of “Tylosaurus and Plioplatecarpini” (p. viii), which matched his definition. Bell’s PhD thesis was published four years later (Bell, 1997). Until that time, ‘Russellosaurinae’ was in use in an informal sense as a node-based name for a clade consisting of ‘tylosaurines’ and ‘plioplatecarpines’ (Caldwell, 1996). Because the paper by Bell (1997) was originally intended to simply be the published version of his PhD thesis, Bell (1997) again introduced ‘Russellosaurinae’ as a new taxon name. However, its extent seems to be different as the name was introduced “in anticipation of formally designating the taxon and describing a new taxon, Russellosaurus, from new Turonian material from Texas” (p. 322). Although there was no explicit information about how closely related Russellosaurus was to ‘russellosaurines’ (sensu Bell, 1993), and in the ‘Summary’ paragraph of Bell (1997: 324) ‘Russellosaurinae’ is again listed as consisting of “Tylosaurus and Plioplatecarpini” only, it is clear that Bell (1997) intended to anchor ‘Russellosaurinae’ on the taxon Russellosaurus. Until Polcyn & Bell (2005), where ‘Russellosaurinae’ was officially replaced with Russellosaurina, authors used the name in the traditional informal way, and always as a node-based name for a clade containing Tylosaurus and Plioplatecarpini (Christiansen & Bonde, 2002) or Plioplatecarpinae (Bardet et al., 2005); the latter two names referring to the same content.
Polcyn & Bell (2005) introduced the name Russellosaurina “to give identity to the monophyletic grouping of Tylosaurinae plus Plioplatecarpinae and closely related forms” (Polcyn & Bell, 2005: 323). What the “closely related forms” are is clear from the ‘Systematic palaeontology’ paragraph (p. 322), according to which the only non-mosasaurine mosasaurid taxa listed there as Russellosaurina are “[t]he subfamilies Tylosasaurinae [sic] and Plioplatecarpinae and their sister-clade containing the genera Tethysaurus, Russellosaurus and Yaguarasaurus”. Unfortunately, the composition of Russellosaurina is not that transparent in other parts of that paper. According to the abstract, Russellosaurina “includes Plioplatecarpinae, Tylosaurinae, their [most recent] common ancestor and all [of its] descendants” (p. 321), and according to the phylogenetic definition, Russellosaurina consists of “[a]ll mosasaurs more closely related to Tylosaurinae and Plioplatecarpinae, the genus Tethysaurus, their common ancestor and all descendants than to Mosasaurinae” (p. 322). This definition is clearly branch-based with “Tylosaurinae and Plioplatecarpinae, the genus Tethysaurus, their common ancestor and all descendants” being a node-based clade and an internal specifier of the definition. This wording is therefore inconsistent with all previously cited statements.
When Polcyn & Bell (2005) established the name, they gave it the rank of ‘parafamily,’ a term introduced by Olshevsky (1991) for ‘paraphyletic family’ (the prefix ‘para-’ indicates ‘paraphyly’), and not recognized by the ICZN. Therefore, it is of the same level as ‘family’. However, the suffix ‘-ina’ typically indicates a subtribe in zoological nomenclature, so when assigning the name Russellosaurina a rank, the taxon should be contained within a tribe and a subfamily. Here, Russellosaurina is considered an unranked clade name with the node-based definition provided above. In our definition, M. hoffmannii is used as a qualifying clause (ICPN: Art. 11.9). The suggested compilation is preferred for various reasons. First, it should “[supersede] previous references to ‘Russellosaurinae”’ (Polcyn & Bell, 2005: 323), thus applying to the clade originating with the most recent common ancestor of Tylosaurinae, Plioplatecarpinae, and R. coheni. Further, Russellosaurina has always been understood as a node-based name. Although Conrad (2008) “tentatively” followed the original branch-based definition, he simultaneously noted that “the definition Polcyn & Bell (2005) intended for Russellosaurina is frustratingly ambiguous” (Conrad, 2008: 129). Since R. coheni was omitted from the specifiers, the original definition violated the ICPN (Art. 11.7).
According to the new definition, Russellosaurina contains the species R. coheni, Y. columbianus, T. nopcsai, the clade Plioplatecarpinae, and the clade Tylosaurinae (as inferred, e.g., in Bell & Polcyn, 2005; Dutchak & Caldwell, 2006; Cuthbertson et al., 2007). It may also contain Halisaurinae as reconstructed in Caldwell & Palci (2007), or self-destruct under the hypothesis from Bardet et al. (2005). Russellosaurina may also contain only Plioplatecarpinae and Tylosaurinae, if R. coheni and Y. columbianus are basal members of Plioplatecarpinae as it was suggested by Polcyn & Bell (2005: 332) and inferred in Dutchak & Caldwell (2009: Fig. 5). Russellosaurina self-destructs if R. coheni, Y. columbianus and T. nopcsai form the sister taxon to the least inclusive clade including M. hoffmannii and T. proriger as reconstructed in Dutchak & Caldwell (2009: Fig. 4).
The ‘unweighted-unordered’ parsimony analysis (Fig. 1), some weighted parsimony analyses (Figs. 3A–3C and 3E), parsimony analyses with Adriosaurus and Pontosaurus used as outgroups (Figs. 4A and 4C), and Bayesian analysis (Fig. 5) support Russellosaurina. Under all other topologies, Russellosaurina self-destructs (Figs. 2, 3D, 3F and 4B).
Tethysaurinae Makádi, Caldwell & Ösi, 2012
Preferred phylogenetic definition
The least inclusive clade containing Tethysaurus nopcsai Bardet, Suberbiola & Jalil, 2003 and Pannoniasaurus inexpectatus Makádi, Caldwell & Ösi, 2012, but not Halisaurus platyspondylus Marsh, 1869, Mosasaurus hoffmannii (Mantell, 1829), Tylosaurus proriger (Cope, 1869), Plioplatecarpus marshii Dollo, 1882, or Yaguarasaurus columbianus Páramo, 1994. This definition is node-based.
Remarks
Makádi, Caldwell & Ösi (2012) introduced the name Tethysaurinae for “[t]he most recent common ancestor of Pannoniasaurus inexpectatus and Russellosaurus coheni Polcyn & Bell, 2005 […] and all its descendants”. Following the results of their phylogenetic analysis, the clade Tethysaurinae was formed by P. inexpectatus, R. coheni, Tethysaurus nopcsai, and Yaguarasaurus columbianus. However, by omitting T. nopcsai from the internal specifiers, the phylogenetic definition violates the ICPN (Art. 11.7). Later, Palci, Caldwell & Papazzoni (2013) introduced the name Yaguarasaurinae and defined it as “[t]he most recent common ancestor of Romeosaurus, gen. nov., Russellosaurus, and Yaguarasaurus, and all of its descendants”. Tethysaurinae was kept only for Pannoniasaurus and Tethysaurus, that formed the sister clade to the Yaguarasaurinae (see below for comments on this name).
We follow the node-based concept of Tethysaurinae as delimited by Palci, Caldwell & Papazzoni (2013) but considering the unstable position of the two tethysaurines on the mosasauroid tree (see Figs. 1–7), we added five external specifiers to maintain the ‘traditional’ contents.
All our analyses reconstruct monophyletic tethysaurines (Figs. 1–7).
Yaguarasaurinae Palci, Caldwell & Papazzoni, 2013
Preferred phylogenetic definition
The least inclusive clade containing Yaguarasaurus columbianus Páramo, 1994, Russellosaurus coheni Polcyn & Bell, 2005, and Romeosaurus fumanensis Palci, Caldwell & Papazzoni, 2013, but not Tethysaurus nopcsai Bardet, Suberbiola & Jalil, 2003, Halisaurus platyspondylus Marsh, 1869, Tylosaurus proriger (Cope, 1869), Plioplatecarpus marshii Dollo, 1882, or Mosasaurus hoffmannii Mantell, 1829. This definition is node-based.
Remarks
As noted above, Yaguarasaurinae was introduced by Palci, Caldwell & Papazzoni (2013) who defined it as “[t]he most recent common ancestor of Romeosaurus, gen. nov., Russellosaurus, and Yaguarasaurus, and all of its descendants”. We follow such definition but considering the weak support for the connection of Yaguarasaurinae and Tethysaurinae (Figs. 1, 2, 5 and 7), we added five external specifiers to prevent the name to cover an unintended clade.
The Bayesian analysis and majority of the parsimony analyses support the monophyly of the yaguarasaurines as delimited by Palci, Caldwell & Papazzoni (2013). Only under the topology resulting from the ‘unweighted-ordered’ parsimony analysis and two ‘weighted-ordered’ parsimony analyses Yaguarasaurinae self-destructs (Figs. 2, 3D and 3F).
Plioplatecarpinae Dollo, 1884
Preferred phylogenetic definition
The most inclusive clade containing Plioplatecarpus marshii Dollo, 1882, but not Mosasaurus hoffmannii Mantell, 1829, Tylosaurus proriger (Cope, 1869), Tethysaurus nopcsai Bardet, Suberbiola & Jalil, 2003, or Yaguarasaurus columbianus Páramo, 1994. This definition is branch-based.
Remarks
Conrad (2008: 130) defined Plioplatecarpinae as “[a]ll taxa sharing a more recent common ancestor with Plioplatecarpus marshi[i ] than with Tylosaurus proriger or Mosasaurus hoffmannii”. Such definition matches the published hypotheses; Plioplatecarpinae as sister taxon to Tylosaurinae or to Mosasaurinae (e.g., Bell, 1997; Bardet et al., 2005; Bell & Polcyn, 2005; Leblanc, Caldwell & Bardet, 2012; Palci, Caldwell & Papazzoni, 2013; Jiménez-Huidobro & Caldwell, 2016), but does not reflect the possible close connection of plioplatecarpines with yaguarasaurines (as suggested by Polcyn & Bell [2005: 332] and then inferred, together with Tethysaurus, by Dutchak & Caldwell [2009: Fig. 5]). Thus, we included two additional external specifiers, Tethysaurus nopcsai and Yaguarasaurus columbianus, that assure the adherence of the name Plioplatecarpinae to the traditional contents under alternative hypotheses.
The topologies inferred through our parsimony and Bayesian analyses support the monophyly of the traditional plioplatecarpines as delimited by Konishi & Caldwell (2011) (Figs. 1–7).
Tylosaurinae Williston, 1897
Preferred phylogenetic definition
The most inclusive clade containing Tylosaurus proriger (Cope, 1869), but not Plioplatecarpus marshii Dollo, 1882, or Mosasaurus hoffmannii Mantell, 1829. This definition is branch-based.
Remarks
The tylosaurine interrelationships have been intensively studied during the past decade (e.g., Bullard, 2006; Martin & Fernández, 2007; Caldwell et al., 2008; Bullard & Caldwell, 2010; Jiménez-Huidobro & Caldwell, 2016; Otero et al., 2017), resulting, among others, in numerous changes in binomial nomenclature. The monophyly of Tylosaurinae, nevertheless, has not been put into question.
Conrad (2008: 130) defined Tylosaurinae as “[a]ll taxa sharing a more recent common ancestor with Tylosaurus proriger than with Mosasaurus hoffmannii or Plioplatecarpus marshi[i ]”. This definition adheres to the traditional contents of Tylosaurinae under all current topologies, including these inferred by our parsimony and Bayesian analyses (Figs. 1–7).
Discussion
Inferences using the Fossilized Birth–Death model with sampled ancestors (FBDSA)
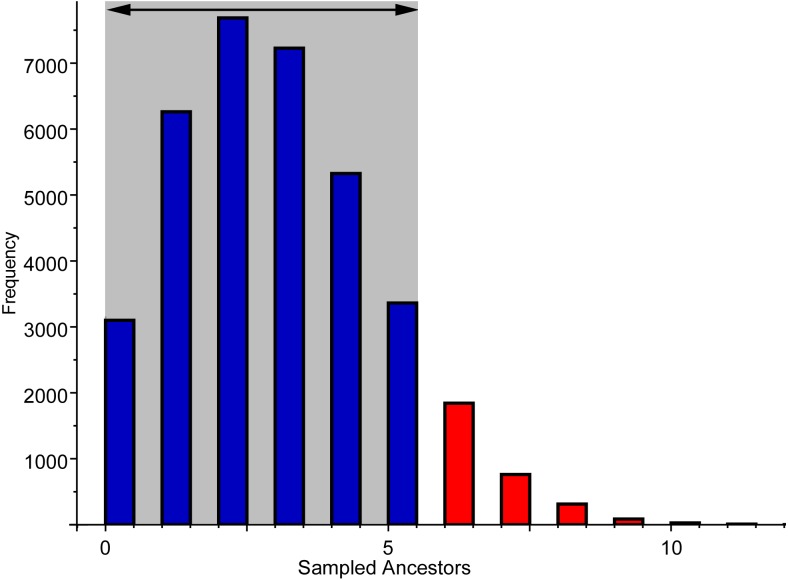
The FBDSA model, that discriminates between cladogenetic and anagenetic patterns in macroevolution (Gavryushkina et al., 2014; Gavryushkina et al., 2017), inferred several ancestral-descendent relationships, a subset of which is shown in the MCCT (see Fig. 5). Nevertheless, all of them were weakly supported and therefore are not discussed further. Instead of focusing on the consensus topologies (like the MCCT), a more accurate way for estimating the frequency of ancestor-descendant relationships obtained by the Bayesian analysis is by considering all the post-burnin topologies inferred (see Cau, 2017). In the 95% of the sampled trees using the data set of Simões et al. (2017), the number of sampled ancestors inferred ranges between 0 and 5 (Fig. 8), which suggests that up to 11% of the included mosasauroid taxa are potential direct ancestors of one or more other mosasauroids included. Nevertheless, these values probably overestimate the frequency of sampled ancestors. It should be remarked that in these analyses, the character list a priori excludes invariant characters (in particular, the autapomorphies of terminal units), as is common practice in parsimony analyses sampling exclusively potential synapomorphies. This methodological bias thus may inflate the frequency of the sampled ancestors, since it does not discriminate between actual ancestors along anagenetic lineages (that have a null terminal branch length) from spurious zero-length terminal branches due to omission of autapomorphies. In conclusion, taking into account the methodological bias due to omission of invariant characters from the morphological features included, this analysis suggests that no more than one-tenth of the inferred relationships among the actual phylogenetic tree of Mosasauroidea could be tentatively interpreted as anagenetic (direct ancestor-descendant) patterns.
Figure 8. Frequency of sampled ancestors among the alternative topologies produced by the Bayesian analysis using the FBDSA model.
Grey area indicates the 95% confidence interval of sampled trees.
Potential issues resulting from application of the Implied Weighting function
As shown by Simões et al. (2017) and our parsimony and Bayesian analyses, the structure of the mosasauroid phylogenetic tree is highly dependent on the applied tree-search strategies. Use of some phylogenetic methods may currently lead to prefer insufficiently supported phylogenetic hypotheses. For example, Simões et al. (2017) performed a single test of parsimony analysis using the Implied Weighting (IW) function, keeping the default value for the K parameter (K = 3). Compared to their unweighted parsimony analyses, which show polytomies near the base of Mosasauroidea and within Mosasaurinae (Simões et al., 2017: Figs. 1A, 1B), the topology inferred from the parsimony analysis with IW function was fully resolved (Simões et al., 2017: Fig. 1C), and represented the only unambiguous support for a single origin of the hydropedal and hydropelvic conditions that are related to the transition from semi- to a fully aquatic lifestyle (with a reversal within Tethysaurinae to plesiopelvic condition). However, the evolutionary meaning of the K parameter is currently hotly debated (e.g., O’Reilly et al., 2016; Congreve & Lamsdell, 2016; Goloboff, Torres & Arias, 2017), and a recent investigation of the effects of implied weighting on modeled phylogenetic data revealed particularly poor abilities of the method to resolve data sets with large amounts of conflicts or polytomies (Congreve & Lamsdell, 2016). Goloboff, Torres & Arias (2017) criticized some aspect of the studies by O’Reilly et al. (2016) and Congreve & Lamsdell (2016) but repeated the necessity for the investigation of proper values of K relative to the numbers of analyzed taxa (Goloboff, 1993; Goloboff, 1995) and evaluation of more than a single concavity parameter (Goloboff et al., 2008).
It is far beyond the scope of the present paper to contribute to the debate, but given that concerns regarding the ‘proper’ use of weighted parsimony still exist, we suggest that the results of parsimony analyses with the IW function are generally treated ‘conservatively’. That is, rather than preferring a single inferred topology with a particular value of K that seems to fit best for the analyzed data, trees produced by different runs should be compared in order to spot, and prioritize, the groupings that are consistently being reconstructed. For example, all weighted parsimony analyses reconstruct monophyletic Halisaurinae (Halisaurus + Eonatator) but the position of this clade on the mosasauroid tree is unstable. They are either, the sister taxon to the clade formed by tethysaurines, yaguarasaurines, tylosaurines, and plioplatecarpines (Figs. 3A–3C, and 3E) or the sister taxon to mosasaurines (Figs. 3D, 3F). We suggest that regardless of which of the two hypotheses is inferred following the use of the best-fitting value(s) of K, the position of halisaurines should be regarded as unstable and, ideally, compared to the results produced by other methods of phylogenetic inference. Therefore, in the case of the present data set, the position of halisaurines should be treated as ambiguous. The only method that infers a strong support for either hypothesis is the Bayesian analysis that reconstructs halisaurines as the sister taxon to mosasaurines (pp = 0.96).
Data sampling
Following the results of the phylogenetic analyses using multiple tree-search strategies, we discuss the factors in the data sampling that might influence the differing hypotheses of mosasauroid phylogenetic relationships and their statistical support, and suggest further changes to the explored data set that might improve the resolution of the mosasauroid phylogenetic relationships.
Outgroup selection
In the initial version of the data set introduced by Bell (1993) and Bell (1997), the outgroup was constructed following the algorithm described by Maddison, Donoghue & Maddison (1984). The final outgroup OTU was based on the characters present in eight modern squamates (Aspidoscelis sexlineata, Crotaphytus collaris, Dipsosaurus dorsalis, Gekko gecko, Gerrhonotus liocephalus, Plestiodon laticeps, Shinisaurus crocodilurus, and Varanus niloticus) and two extinct squamates (Estesia mongoliensis and Gilmoreteius chulsanensis). Such ‘composite’ operational taxonomic unit was used by most later authors (e.g., Bell & Polcyn, 2005; Caldwell & Palci, 2007; Leblanc, Caldwell & Bardet, 2012). More recently, however, some studies preferred to use only the character states present in Varanus as the outgroup (e.g., Palci, Caldwell & Papazzoni, 2013; Jiménez-Huidobro & Caldwell, 2016) “because both taxa [i.e., Mosasauroidea and Varanus] are large-bodied anguimorphs that share a number of symplesiomorphic features” (Palci, Caldwell & Papazzoni, 2013: 608).
The outgroup sampling is known to have a great effect on the structure of phylogenetic trees (e.g., Graham, Olmstead & Barrett, 2002; Spaulding, O’Leary & Gatesy, 2009; Kirchberger et al., 2014; Wilberg, 2015). Given the alternative placements of Mosasauroidea among different phylogenies published (e.g., Conrad, 2008; Gauthier et al., 2012; Reeder et al., 2015), it is not universally agreed which squamates may represent the closest sister group of mosasauroids. Therefore, outgroup selection among extant squamates may be biased by preference among the alternative placement of Mosasauroidea.
The problems with the use of the ‘composite’ OTU, then, was already commented on by Palci, Caldwell & Papazzoni (2013: 608) who noted that the “outgroup is problematic for several reasons: (1) it does not reflect the character state composition of a real organism; (2) it can produce paradoxical combinations of character states where a feature coded as absent in one character is further defined in a second character […]; and (3) lack of repeatability of the process that produced such codings”, noting that Bell (1997) “was not very explicit on how he obtained the character states for his outgroup”. The third point (lack of repeatability of the process), however, does not seem to be entirely fair. Even though Palci, Caldwell & Papazzoni (2013) are certainly correct that Bell (1997) was not particularly specific regarding the scores of his ‘composite’ OTU, that paper was supposed be the published version of his PhD thesis (Bell, 1993), which is explicitly referred to by Bell (1997: 294) and includes information on where the scores come from (Bell, 1993: 9–16, 251, 265–268).
To solve the issues with outgroup selection, Simões et al. (2017) expanded the data set by adding three ‘dolichosaur-grade’ taxa: Adriosaurus suessi Seeley, 1881, Dolichosaurus longicollis Owen, 1850, and Pontosaurus kornhuberi Caldwell, 2006, and designed A. suessi as the basalmost outgroup. Even though A. suessi constitutes a much better outgroup than the ‘composite’ OTU and Varanus, because its age and morphology more closely reflect those of the last common ancestor of all mosasauroids, such approach forces Dolichosaurus and Pontosaurus to be inferred more closely to mosasaurids than to Adriosaurus. This outgroup setting may thus lead to the construction of an artificial ‘dolichosaur grade’ as the basalmost mosasauroid condition (i.e., due to the outgroup setting in TNT used by Simões et al., 2017, ‘dolichosaurs’ are constrained to form a paraphyletic series leading to Mosasauroidea), which may lead to spurious relationships among the ingroup taxa merely based on squamate symplesiomorphies that are absent among the ‘dolichosaur’ taxa. As Simões et al. (2017) noted, some studies reconstruct these ‘dolichosaurs’ to represent snake-branch pythonomorphs (see e.g., Palci & Caldwell, 2007; Caldwell & Palci, 2010; Palci & Caldwell, 2010). Thus, all these three OTUs may be ‘equally’ distantly related to Mosasauridae. It is noteworthy that the latter hypothesis is supported by the Bayesian analysis using the FBDSA model, which reconstructed all ‘dolichosaur’ taxa as forming a clade excluding all other OTUs.
To avoid any bias due to a priori assumptions on character state transformation (because of the alternative extant squamate outgroup used and potentially incorrect outgroup/basal ingroup designation), we suggest to perform analyses using different outgroup selection or to consider the use of a ‘remote outgroup’. Perhaps, the well preserved Early Cretaceous (Aptian) squamate Huehuecuetzpalli mixtecus Reynoso, 1998, might serve as the root in a separate analysis. That taxon is universally recognized as more basal than any alternative mosasauroid outgroup used previously (Conrad, 2008; Gauthier et al., 2012), and may represent the ancestral squamate morphology regardless of the preferred closest relatives of mosasauroids. However, see also Graham, Olmstead & Barrett (2002) and Kirchberger et al. (2014) for independent tests regarding the effects of the use of phylogenetically distant outgroups in molecular studies.
Taxon sampling
As discussed above, the outgroup selection has a substantial impact on the structure of the inferred tree topology, including the statistical support of the basal branching near the root of Mosasauroidea. Still, the resolution of the rootward mosasauroids might not necessarily improve without an increased number of early mosasaurids and near-mosasaurids analyzed. The most recent version of the data set was expanded with the addition of Adriosaurus suessi, Dolichosaurus longicollis, and Pontosaurus kornhuberi, and separation of Opetiosaurus bucchichi from the Aigialosaurus OTU (even if it is assigned to Aigialosaurus as A. bucchichi; Dutchak & Caldwell, 2009; Simões et al., 2017). Still, it could also benefit, for instance, from addition of Acteosaurus tommasinii (Palci & Caldwell, 2010), Adriosaurus microbrachis (Palci & Caldwell, 2007), Adriosaurus skrbinensis (Caldwell & Palci, 2010), Aphanizocnemus libanensis (Dal Sasso & Pinna, 1997), Carsosaurus marchesettii (e.g., Caldwell, Carroll & Kaiser, 1995; Caldwell & Palci, 2007), Coniasaurus crassidens (Caldwell & Cooper, 1999), Eidolosaurus trauthi (Nopcsa, 1923), and Pontosaurus lesinensis (Pierce & Caldwell, 2004). The fact that some or most of these taxa can be more closely related to snakes than to mosasaurids (see e.g., Palci & Caldwell, 2007; Caldwell & Palci, 2010; Palci & Caldwell, 2010), is not a problem as their morphology approximates to that of the mosasaurid ancestor, and therefore supplements the knowledge of early pythonomorph evolution.
The data set of Simões et al. (2017) contains members of all well-recognized mosasauroid subclades; the taxa traditionally contained within Halisaurinae, Mosasaurinae, Plioplatecarpinae, and Tylosaurinae. It also contains all tethysaurines and yaguarasaurines (except Romeosaurus sorbinii Palci, Caldwell & Papazzoni, 2013), as these two clades were inferred in studies using recent versions of the data set (Makádi, Caldwell & Ösi, 2012; Palci, Caldwell & Papazzoni, 2013; respectively). Still, some of the clades are substantially underrepresented even though detailed descriptions of their members have been published and some of those taxa have been scored for characters in older versions of the same data set. For example, the current version of the data set includes only two halisaurine OTUs (Halisaurus platyspondylus and Eonatator sternbergii; with the latter being labeled as ‘Halisaurus sternbergi’) even though detailed studies have also been published, for example, for Halisaurus arambourgi (Bardet et al., 2005; Polcyn et al., 2012) or Phosphorosaurus ortliebi (Lingham-Soliar, 1996; Holmes & Sues, 2000; Bardet et al., 2005). Likewise, the data set could be supplemented by recently described Eonatator coellensis (Páramo-Fonseca, 2013) and Phosphorosaurus ponpetelegans (Konishi et al., 2016). Such sampling could test some of the implied relationships (the connection of E. coellensis to E. sternbergii, H. arambrourgi to H. platyspondylus, P. ponpetelegans to P. ortliebi). A phylogenetic analysis of Halisaurinae was recently published by Konishi et al. (2016). The analysis did not reconstruct monophyletic Halisaurus nor Eonatator but inferred sister-taxon relationships between P. ortliebi and P. ponpetelegans, a taxon described by these authors. However, the analysis was based on only 21 cranial characters and rooted on Platecarpus tympaniticus, a derived plioplatecarpine that might not serve best as the outgroup for such analysis due to its placement and age. Considering the unsettled relationships within Halisaurinae, and the differing position of the clade within Mosasauridae, an expansion of the data set by using more halisaurines (and modification of the characters to better reflect their morphology) might result in improving the resolution of the mosasauroid tree topology.
New reappraisals of certain tylosaurine species have also been published recently. For example, Hainosaurus pembinensis and H. bernardi, the latter being the type species of Hainosaurus, have been assigned to Tylosaurus (Bullard & Caldwell, 2010; Jiménez-Huidobro & Caldwell, 2016, respectively), and Tylosaurus kansasensis was proposed to be a juvenile of T. nepaeolicus, and thus removed from the data set (Jiménez-Huidobro, Simões & Caldwell, 2016). However, T. pembinensis is not included in the recent version of the data set, which does not enable to further test the newly proposed hypotheses. Interestingly, the ordered-unweighted parsimony analysis and the Bayesian analysis do not support the monophyly of Tylosaurus (represented by T. proriger, T. bernardi, and T. nepaeolicus) exclusive of Taniwhasaurus (Figs. 2 and 5). When only one ‘dolichosaur’ is in the data set and used as the outgroup, regardless of which one it is, Tylosaurus is monophyletic (Fig. 4). The resolution might improve with a more appropriate outgroup selection and addition of T. pembinensis and, possibly, ‘Hainosaurus’ neumilleri (Martin, 2007). Additionally, Tylosaurus ‘saskatchewanensis’ (Bullard, 2006) and ‘Hainosaurus’ ‘kenbrowni’ (Thompson, 2005; Thompson, 2011) can also be considered pending their formal descriptions.
The understanding of the plioplatecarpines, in turn, may improve by separation of the Plioplatecarpus OTU into several terminal units. Such sampling could test the monophyly of Plioplatecarpus (a taxon consisting of a few species, including P. marshii, P. houzeaui, P. primaevus, and the recently described P. peckensis; Cuthbertson & Holmes, 2015), estimate the support for the tree topology obtained by Konishi & Caldwell (2011) and Cuthbertson & Holmes (2015), test the connection of ‘Latoplatecarpus’ nichollsae and L. willistoni, or provide additional support for the separation of Plesioplatecarpus planifrons (labeled as ‘Platecarpus planifrons’ in the data set of Simões et al., 2017) from Platecarpus tympaniticus (Konishi & Caldwell, 2011).
Mosasaurines are problematic, as is apparent from differing and often poorly resolved tree topologies. The inference of the structure of the mosasaurine phylogenetic tree appears to be difficult especially due to the unstable positions of the taxa attributed to Prognathodon (e.g., Leblanc, Caldwell & Bardet, 2012; Simões et al., 2017; our study). Nevertheless, numerous derived mosasaurines are currently under revision as is apparent from Street & Caldwell (2017) that provided detailed reappraisal of Mosasaurus hoffmannii, preliminary discussion of some other taxa traditionally assigned to Mosasaurus, and reported on an ongoing research. Together with reconsideration of some species traditionally attributed to Prognathodon, the resolution of the mosasaurines might benefit from addition of some presumably rootward mosasaurine taxa that have not been included in previous ‘complete’ versions of the Bell’s data set (i.e., when the aim was to assess the interrelationships within all major clades of mosasauroids). These include, for example, Kourisodon puntledgensis (Nicholls & Meckert, 2002). This taxon, which has previously been used as an outgroup in some analyses (Konishi & Caldwell, 2011; Cuthbertson & Holmes, 2015), originates from the upper Santonian of British Columbia, Canada, and is one of the oldest known mosasaurines. Its inclusion might have an impact on the resolution of Mosasaurinae.
Character sampling
We suggest that character statements are redefined from those used in recent versions of Bell’s (1997) data set, following the recommendations in Sereno (2007) and Brazeau (2011). In particular, compound characters are suggested to be atomized; i.e., neomorphic and transformational features should be considered as distinct characters and not as alternative states of a single character. Therefore, when not resulting in loss of information, characters are suggested to be defined as binary. When multistate character statements are included, and the states form unambiguous morphoclines that describe a nested set of alternative states (e.g., marginal tooth numbers, vertebral numbers, phalangeal formulas), the corresponding character statements should be set as ordered, to avoid a priori exclusion of potential synapomorphies represented by the subset of states representing a derived condition (e.g., Wilkinson, 1992; Sereno, 2007; Brazeau, 2011). Such states, however, should be formulated to avoid marked polymorphism. For example, the current version of the data set (Simões et al., 2017) includes a six-state character dealing with the dentary tooth count: “(53) Dentary tooth number: 20–24 (0); 17–19 (1); 15–16 (2); 14 (3); 13 (4); 12 (5)”. Yet such defined states insufficiently reflect differences in taxa where the dentary tooth count is one of the few distinguishing characters. Furthermore, once set as ordered to reflect the homology among nested state-transitions, the character defined this way leads to inflating the phylogenetic importance of a feature that may be merely size-related and individually variable among the same taxon. For instance, Mosasaurus hoffmannii is often reported as having 14 dentary teeth (e.g., Street & Caldwell, 2017). However, some specimens have 15 dentary teeth (e.g., CAMSM F22228, IRSNB R 0303; D Madzia, pers. obs., 2017; Mulder, Cornelissen & Verding, 2004), or only 13 (NHMM 009002; Everhart et al., 2016). Thus, M. hoffmannii can be scored for states 2, 3, and 4. At the same time, Mosasaurus lemonnieri, which is currently considered to be distinct from M. hoffmannii (Street & Caldwell, 2017; D Madzia, 2017, unpublished data), has always 16 dentary teeth. Still, it would be covered under the same state (2).
This example demonstrates that character definitions and among-state transition settings may significantly influence relationships, and must be discussed prior to phylogenetic analyses.
‘Data handling’
As we have expressed above, we consider the current versions of the Bell’s (1997) data set to be insufficient for accurate inferences of mosasauroid phylogenetic relationships. We suggest to (1) reconsider the outgroup selection, (2) increase the number of analyzed taxa, and named some of those that we think might improve the resolution of the mosasauroid phylogenetic tree, and (3) revise the morphological characters and their states. Naturally, it is essential to note that the steps should be undertaken after careful considerations and simultaneously. Specifically, increasing the number of analyzed taxa could have an entirely opposite effect and cause more instability if the additions do not sufficiently reflect the differing morphologies of the proposed OTUs and their character evolution. Also, we suggest to consider even those taxa that might be regarded as too incomplete to be included in the data matrix (see e.g., Wiens, 2003a; Wiens, 2003b; Wiens & Morrill, 2011). The relevance of all additions might be tested, for example, following the principle of safe taxonomic reduction (Wilkinson, 1995), using TAXEQ3 (Wilkinson, 2001), or through ‘concatabominations’ (Siu-Ting et al., 2015). However, it has also been argued that “there is no justification—either a priori or a posteriori—to definitively exclude unstable taxa from the data matrix as this involves the deletion of phylogenetic information that can be relevant (or even critical) for understanding the relationships of the entire group” (Pol & Escapa, 2009: 13). Therefore, Pol & Escapa (2009) offered to use a TNT script, IterPCR, that provides a list of characters related to the instability of each unstable taxon. This script has already been implemented in TNT (Goloboff & Szumik, 2015).
Conclusions
Throughout the last two decades the phylogenetic relationships within Mosasauroidea have been inferred using modified versions of a single data set, originally published by Bell (1997). In order to estimate the robustness in our understanding of mosasauroid phylogenetic relationships, we used a recent version of that data set (published by Simões et al., 2017) and focused on the effects of tree-search strategy selection.
Parsimony and Bayesian analyses of the same data set showed considerable differences in tree topologies near the base of Mosasauroidea, suggesting that an increased number of the basal taxa and morphological characters phylogenetically informative for large-scale relationships need to be taken into account. Furthermore, the different topologies obtained by the alternative tree-search strategies suggest that one particular phylogenetic hypothesis may be significantly biased by the phylogenetic method used as suggested by Simões et al. (2017). We thus suggest to perform different analyses of the same data, using alternative tree-search strategies and tree models, and to consider as supported only those hypotheses shared consistently by the majority of analyses. Following the results of the present study, the monophyly of the traditional mosasauroid groups (Halisaurinae, Tethysaurinae, Plioplatecarpinae, Tylosaurinae, Mosasaurinae, and possibly also Yaguarasaurinae) can be currently considered supported. Yet their mutual relationships as well as the relations within these groups are still largely unsettled.
From the nomenclatural perspective, we see little or no support for the use of some binomial combinations. Specifically, our analyses often failed to reconstruct monophyly for the mosasaurine taxon Prognathodon. Although the Bayesian analysis infers some support, albeit extremely poor, for a clade formed by all taxa attributed to Prognathodon (and including Eremiasaurus), ‘Prognathodon’ requires complex reassessment and some taxa will have to be removed from it (see also, e.g., Leblanc, Caldwell & Bardet, 2012; Simões et al., 2017).
We recommend that future implementations of the mosasauroid data set will discuss the combined effects of taxon sampling, character construction, and tree-search strategy settings. For instance, in phylogenetic analysis using parsimony, and where all characters are set as having equal weight, the splitting of the multistate characters into distinct binary characters does not bias the reconstruction of the state transitions. On the contrary, in phylogenetic analysis using parsimony as tree-search strategy, and with the Implied Weighting function, multistate or compound characters, once subdivided into binary characters, are analyzed with different weighting settings. Furthermore, in Bayesian phylogenetic analyses where rate variation across morphological characters are modeled using the gamma parameter, different state transitions of the same morphocline may evolve at different rates.
We conclude that, until the data set is significantly improved by a more appropriate taxon sampling and revision of characters, the currently inferred phylogenetic relationships of mosasauroids should be seen as tentative and subject to change.
Supplemental Information
Acknowledgments
DM would like to thank Annelise Folie, Alain Drèze, and Cécilia Cousin (all Royal Belgian Institute of Natural Sciences, Belgium), and John W. M. Jagt (Natuurhistorisch Museum Maastricht, the Netherlands) for access to specimens in their care. Hallie P. Street (University of Alberta, Canada), Valentin Fischer (University of Liège, Belgium), and three anonymous reviewers provided thorough reviews and valuable suggestions that substantially improved the manuscript. The program TNT is made available with the sponsorship of the Willi Hennig Society.
Institutional abbreviations
- CAMSM
Sedgwick Museum of Earth Sciences, University of Cambridge, Cambridge, UK
- IRSNB
Royal Belgian Institute of Natural Sciences, Brussels, Belgium
- NHMM
Natuurhistorisch Museum Maastricht, Maastricht, the Netherlands.
Funding Statement
Daniel Madzia is supported by the National Science Centre (Poland) grant No. 2015/19/N/ST10/01628. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Daniel Madzia, Email: daniel.madzia@gmail.com.
Andrea Cau, Email: cauand@gmail.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Daniel Madzia and Andrea Cau conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplementary File.
References
- Bardet et al. (2015).Bardet N, Houssaye A, Vincent P, Suberbiola XP, Amaghzaz M, Jourani E, Meslouh S. Mosasaurids (Squamata) from the Maastrichtian Phosphates of Morocco: biodiversity, palaeobiogeography and palaeoecology based on tooth morphoguilds. Gondwana Research. 2015;27(3):1068–1078. doi: 10.1016/j.gr.2014.08.014. [DOI] [Google Scholar]
- Bardet et al. (2005).Bardet N, Suberbiola XP, Iarochene M, Bouyahyaoui F, Bouya B, Amaghzaz M. A new species of Halisaurus from the Late Cretaceous phosphates of Morocco, and the phylogenetical relationships of the Halisaurinae (Squamata: Mosasauridae) Zoological Journal of the Linnean Society. 2005;143(3):447–472. doi: 10.1111/j.1096-3642.2005.00152.x. [DOI] [Google Scholar]
- Bardet, Suberbiola & Jalil (2003).Bardet N, Suberbiola XP, Jalil N-E. A new mosasauroid (Squamata) from the Late Cretaceous (Turonian) of Morocco. Comptes Rendus Palevol. 2003;2:607–616. doi: 10.1016/j.crpv.2003.09.006. [DOI] [Google Scholar]
- Bell (1993).Bell GL. PhD thesis. 1993. A phylogenetic revision of Mosasauroidea (Squamata) [Google Scholar]
- Bell (1997).Bell GL. A phylogenetic revision of North American and Adriatic Mosasauroidea. In: Callaway JM, Nicholls EL, editors. Ancient marine reptiles. Academic Press; San Diego: 1997. pp. 293–332. [Google Scholar]
- Bell & Polcyn (2005).Bell GL, Polcyn MJ. Dallasaurus turneri, a new primitive mosasauroid from the Middle Turonian of Texas and comments on the phylogeny of Mosasauridae (Squamata) Netherlands Journal of Geosciences. 2005;84(3):177–194. doi: 10.1017/S0016774600020965. [DOI] [Google Scholar]
- Boas (1880).Boas JEV. Studier over Decapodernes Slaegtskabsforhold. Dansk Videnskabernes Seksjeab, Copenhagen, Skrifter, Naturvidenskabelig og Matematisek Afdeling. 1880;1:23–210. [Google Scholar]
- Bouckaert et al. (2014).Bouckaert RR, Heled J, Kuehnert D, Vaughan TG, Wu C-H, Xie D, Suchard MA, Rambaut A, Drummond AJ. BEAST 2: a software platform for Bayesian evolutionary analysis. PLOS Computational Biology. 2014;10(4):e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau (2011).Brazeau MD. Problematic character coding methods in morphology and their effects. Biological Journal of the Linnean Society. 2011;104:489–498. doi: 10.1111/j.1095-8312.2011.01755.x. [DOI] [Google Scholar]
- Bullard (2006).Bullard TS. MSc thesis. 2006. Anatomy and systematics of North American tylosaurine mosasaurs. [Google Scholar]
- Bullard & Caldwell (2010).Bullard TS, Caldwell MW. Redescription and rediagnosis of the tylosaurine mosasaur Hainosaurus pembinensis Nicholls, 1988, as Tylosaurus pembinensis (Nicholls, 1988) Journal of Vertebrate Paleontology. 2010;30(2):416–426. doi: 10.1080/02724631003621870. [DOI] [Google Scholar]
- Caldwell (1996).Caldwell MW. Ontogeny and phylogeny of the mesopodial skeleton in mosasauroid reptiles. Zoological Journal of the Linnean Society. 1996;116:407–436. doi: 10.1111/j.1096-3642.1996.tb00131.x. [DOI] [Google Scholar]
- Caldwell (2006).Caldwell MW. A new species of Pontosaurus (Squamata, Pythonomorpha) from the Upper Cretaceous of Lebanon and a phylogenetic analysis of Pythonomorpha. Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. 2006;34:1–42. [Google Scholar]
- Caldwell, Carroll & Kaiser (1995).Caldwell MW, Carroll RL, Kaiser H. The pectoral girdle and forelimb of Carsosaurus marchesetti (Aigialosauridae), with a preliminary phylogenetic analysis of mosasauroids and varanoids. Journal of Vertebrate Paleontology. 1995;15(3):516–531. doi: 10.1080/02724634.1995.10011245. [DOI] [Google Scholar]
- Caldwell & Cooper (1999).Caldwell MW, Cooper JA. Redescription, palaeobiogeography and palaeoecology of Coniasaurus crassidens Owen, 1850 (Squamata) from the Lower Chalk (Cretaceous; Cenomanian) of SE England. Zoological Journal of the Linnean Society. 1999;127(4):423–452. doi: 10.1111/j.1096-3642.1999.tb01380.x. [DOI] [Google Scholar]
- Caldwell et al. (2008).Caldwell MW, Konishi T, Obata I, Muramoto K. New species of Taniwhasaurus (Mosasauridae, Tylosaurinae) from the upper Santonian-lower Campanian (Upper Cretaceous) of Hokkaido, Japan. Journal of Vertebrate Paleontology. 2008;28(2):339–348. doi: 10.1671/0272-4634(2008)28[339:ANSOTM]2.0.CO;2. [DOI] [Google Scholar]
- Caldwell & Palci (2007).Caldwell MW, Palci A. A new basal mosasauroid from the Cenomanian (U. Cretaceous) of Slovenia with a review of mosasauroid phylogeny and evolution. Journal of Vertebrate Paleontology. 2007;27(4):863–880. doi: 10.1671/0272-4634(2007)27[863:ANBMFT]2.0.CO;2. [DOI] [Google Scholar]
- Caldwell & Palci (2010).Caldwell MW, Palci A. A new species of marine ophidiomorph lizard, Adriosaurus skrbinensis, from the Upper Cretaceous of Slovenia. Journal of Vertebrate Paleontology. 2010;30(3):747–755. doi: 10.1080/02724631003762963. [DOI] [Google Scholar]
- Camp (1923).Camp CL. Classification of the lizards. Bulletin of the American Museum of Natural History. 1923;48(11):289–480. [Google Scholar]
- Cantino & De Queiroz (2010).Cantino PD, De Queiroz K. International code of phylogenetic nomenclature. Version 4chttp://www.ohio.edu/phylocode/PhyloCode4c.pdf. [15 February 2017];2010
- Cau (2017).Cau A. Specimen-level phylogenetics in paleontology using the Fossilized Birth–Death model with Sampled Ancestors. PeerJ. 2017;5:e3055. doi: 10.7717/peerj.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen & Bonde (2002).Christiansen P, Bonde N. A new species of gigantic mosasaur from the Late Cretaceous of Israel. Journal of Vertebrate Paleontology. 2002;22(3):629–644. doi: 10.1671/0272-4634(2002)022[0629:ANSOGM]2.0.CO;2. [DOI] [Google Scholar]
- Congreve & Lamsdell (2016).Congreve CR, Lamsdell JC. Implied weighting and its utility in palaeontological data sets: a study using modelled phylogenetic matrices. Palaeontology. 2016;59(3):447–462. doi: 10.1111/pala.12236. [DOI] [Google Scholar]
- Conrad (2008).Conrad JL. Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bulletin of the American Museum of Natural History. 2008;310:1–182. doi: 10.1206/310.1. [DOI] [Google Scholar]
- Conrad et al. (2011).Conrad JL, Ast JC, Montanari S, Norell MA. A combined evidence phylogenetic analysis of Anguimorpha (Reptilia: Squamata) Cladistics. 2011;27(3):230–277. doi: 10.1111/j.1096-0031.2010.00330.x. [DOI] [PubMed] [Google Scholar]
- Cope (1869).Cope ED. Remarks on Holops brevispinus, Ornithotarsus immanis and Macrosaurus proriger. Proceedings of the Academy of Natural Sciences, Philadelphia. 1869;21:1–123. [Google Scholar]
- Cuthbertson & Holmes (2015).Cuthbertson RS, Holmes RB. A new species of Plioplatecarpus (Mosasauridae, Plioplatecarpinae) from the Bearpaw Formation(Campanian, Upper Cretaceous) of Montana, USA. Journal of Vertebrate Paleontology. 2015;35(3):e922980. doi: 10.1080/02724634.2014.922980. [DOI] [Google Scholar]
- Cuthbertson et al. (2007).Cuthbertson RS, Mallon JC, Campione NE, Holmes RB. A new species of mosasaur (Squamata: Mosasauridae) from the Pierre Shale (lower Campanian) of Manitoba. Canadian Journal of Earth Sciencies. 2007;44:593–606. doi: 10.1139/e07-006. [DOI] [Google Scholar]
- Dal Sasso & Pinna (1997).Dal Sasso C, Pinna G. Aphanizocnemus libanensis n. gen. n. sp, a new dolichosaur (Reptilia, Varanoidea) from the Upper Cretaceous of Lebanon. Paleontologia Lombarda. 1997;7:1–31. [Google Scholar]
- Dollo (1882).Dollo L. Note sur l’ostéologie des Mosasauridæ. Bulletin du Musée Royal d’Histoire Naturelle de Belgique. 1882;1:55–80. [Google Scholar]
- Dollo (1884).Dollo L. Le mosasaure. Revue des Questions Scientifiques. 1884;16:648–653. [Google Scholar]
- Dortangs et al. (2002).Dortangs RW, Schulp AS, Mulder EWA, Jagt JWM, Peeters HHG, Graaf DT. A large new mosasaur from the Upper Cretaceous of the Netherlands. Netherlands Journal of Geosciences. 2002;81(1):1–8. doi: 10.1017/S0016774600020515. [DOI] [Google Scholar]
- Drummond et al. (2012).Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutchak (2005).Dutchak AR. A review of the taxonomy and systematics of aigialosaurs. Netherlands Journal of Geosciences. 2005;84(3):221–222. doi: 10.1017/S0016774600021004. [DOI] [Google Scholar]
- Dutchak & Caldwell (2006).Dutchak AR, Caldwell MW. Redescription of Aigialosaurus dalmaticus Kramberger, 1892, a Cenomanian mosasauroid lizard from Hvar Island, Croatia. Canadian Journal of Earth Sciences. 2006;43:1821–1834. doi: 10.1139/e06-086. [DOI] [Google Scholar]
- Dutchak & Caldwell (2009).Dutchak AR, Caldwell MW. A redescription of Aigialosaurus (= Opetiosaurus) bucchichi Kornhuber, 1901 (Squamata: Aigialosauridae) with comments on mosasauroid systematics. Journal of Vertebrate Paleontology. 2009;29(2):437–452. doi: 10.1671/039.029.0206. [DOI] [Google Scholar]
- Everhart et al. (2016).Everhart M, Jagt JWM, Mulder EWA, Schulp AS. Mosasaurs—how large did they really get?. In: Kear BP, Lindgren J, Sachs S, editors. 5th triennial Mosasaur meeting—a global perspective on Mesozoic marine amniotes, Uppsala, 16–20 May 2016, Program and Abstracts, Museum of Evolution; Uppsala. 2016. pp. 8–10. [Google Scholar]
- Fanti, Cau & Negri (2014).Fanti F, Cau A, Negri A. A giant mosasaur (Reptilia, Squamata) with an unusually twisted dentition from the Argille Scagliose Complex (late Campanian) of Northern Italy. Cretaceous Research. 2014;49:91–104. doi: 10.1016/j.cretres.2014.01.003. [DOI] [Google Scholar]
- Felgenhauser & Abele (1983).Felgenhauser BE, Abele LG. Phylogenetic relationships among shrimp-like decapods. In: Schram F, editor. Crustacean issues 1, Crustacean phylogeny. A. A. Balkema; Rotterdam: 1983. pp. 291–311. [Google Scholar]
- Fernandez & Martin (2009).Fernandez M, Martin JE. Description and phylogenetic relationships of Taniwhasaurus antarcticus (Mosasauridae, Tylosaurinae) from the upper Campanian (Cretaceous) of Antarctica. Cretaceous Research. 2009;30:717–726. doi: 10.1016/j.cretres.2008.12.012. [DOI] [Google Scholar]
- Gauthier et al. (2012).Gauthier JA, Kearney M, Maisano JA, Rieppel O, Behlke ADB. Assembling the squamate tree of life: perspectives from the phenotype and the fossil record. Bulletin of the Peabody Museum of Natural History. 2012;53(1):3–308. doi: 10.3374/014.053.0101. [DOI] [Google Scholar]
- Gavryushkina et al. (2017).Gavryushkina A, Heath TA, Ksepka DT, Stadler T, Welch D, Drummond AJ. Bayesian total evidence dating reveals the recent crown radiation of penguins. Systematic Biology. 2017;66:57–73. doi: 10.1093/sysbio/syw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavryushkina et al. (2014).Gavryushkina A, Welch D, Stadler T, Drummond AJ. Bayesian inference of sampled ancestor trees for epidemiology and fossil calibration. PLOS Computational Biology. 2014;10(12):e1003919. doi: 10.1371/journal.pcbi.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais (1853).Gervais P. Observations relatives aux Reptiles fossiles de France (deuxième partie) Comptes Rendus Hebdomadaires des Séances de l’académie des Sciences. 1853;36:470–474. [Google Scholar]
- Gilmore (1912).Gilmore CW. A new mosasauroid reptile from the Cretaceous of Alabama. Proceedings of the United States National Museum. 1912;40(1870):489–484. [Google Scholar]
- Goloboff (1993).Goloboff PA. Estimating character weights during tree search. Cladistics. 1993;9:83–91. doi: 10.1111/j.1096-0031.1993.tb00209.x. [DOI] [PubMed] [Google Scholar]
- Goloboff (1995).Goloboff PA. Parsimony and weighting: a reply to Turner and Zandee. Cladistics. 1995;11:91–104. doi: 10.1111/j.1096-0031.1995.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Goloboff et al. (2008).Goloboff PA, Carpenter JM, Arias JS, Esquivel DFM. Weighting against homoplasy improves phylogenetic analysis of morphological data sets. Cladistics. 2008;24:758–773. doi: 10.1111/j.1096-0031.2008.00209.x. [DOI] [Google Scholar]
- Goloboff, Farris & Nixon (2008).Goloboff PA, Farris J, Nixon K. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24:774–786. doi: 10.1111/j.1096-0031.2008.00217.x. [DOI] [Google Scholar]
- Goloboff & Szumik (2015).Goloboff PA, Szumik C. Identifying unstable taxa: efficient implementation of triplet-based measures of stability, and comparison with Phyutility and RogueNaRok. Molecular Phylogenetics and Evolution. 2015;88:93–104. doi: 10.1016/j.ympev.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Goloboff, Torres & Arias (2017).Goloboff PA, Torres A, Arias JS. Weighted parsimony outperforms other methods of phylogenetic inference under models appropriate for morphology. Cladistics. 2017 doi: 10.1111/cla.12205. Epub ahead of print June 4 2017. [DOI] [PubMed] [Google Scholar]
- Graham, Olmstead & Barrett (2002).Graham SW, Olmstead RG, Barrett SCH. Rooting phylogenetic trees with distant outgroups: a case study from the commelinoid monocots. Molecular Biology and Evolution. 2002;19:1769–1781. doi: 10.1093/oxfordjournals.molbev.a003999. [DOI] [PubMed] [Google Scholar]
- Grigoriev (2013).Grigoriev D. Redescription of Prognathodon lutugini (Squamata, Mosasauridae) Proceedings of the Zoological Institute RAS. 2013;317(3):246–261. [Google Scholar]
- Holmes & Sues (2000).Holmes RB, Sues H-D. A partial skeleton of the basal mosasaur Halisaurus platyspondylus from the Severn Formation (Upper Cretaceous: Maastrichtian) of Maryland. Journal of Paleontology. 2000;74(2):309–316. doi: 10.1017/S0022336000031516. [DOI] [Google Scholar]
- ICZN (1999).International Commission on Zoological Nomenclature (ICZN) The international trust for zoological nomenclature. Fourth Edition. London: ICZN; 1999. International code of zoological nomenclature. 306 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Huidobro & Caldwell (2016).Jiménez-Huidobro P, Caldwell MW. Reassessment and reassignment of the early Maastrichtian mosasaur Hainosaurus bernardi Dollo, 1885, to Tylosaurus Marsh, 1872. Journal of Vertebrate Paleontology. 2016;36(3):e1096275. doi: 10.1080/02724634.2016.1096275. [DOI] [Google Scholar]
- Jiménez-Huidobro, Simões & Caldwell (2016).Jiménez-Huidobro P, Simões TR, Caldwell MW. Re-characterization of Tylosaurus nepaeolicus (Cope, 1874) and Tylosaurus kansasensis Everhart, 2005: ontogeny or sympatry? Cretaceous Research. 2016;65:68–81. doi: 10.1016/j.cretres.2016.04.008. [DOI] [Google Scholar]
- Jones et al. (2013).Jones MEH, Anderson CL, Hipsley CA, Müller J, Evans SE, Schoch RR. Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara) BMC Evolutionary Biology. 2013;13:208. doi: 10.1186/1471-2148-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberger et al. (2014).Kirchberger PC, Sefc KM, Sturmbauer C, Koblmüller S. Outgroup effects on root position and tree topology in the AFLP phylogeny of a rapidly radiating lineage of cichlid fish. Molecular Phylogenetics and Evolution. 2014;70:57–62. doi: 10.1016/j.ympev.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi & Caldwell (2011).Konishi T, Caldwell MW. Two new plioplatecarpine (Squamata, Mosasauridae) genera from the Upper Cretaceous of North America, and a global phylogenetic analysis of plioplatecarpines. Journal of Vertebrate Paleontology. 2011;31(4):754–783. doi: 10.1080/02724634.2011.579023. [DOI] [Google Scholar]
- Konishi et al. (2016).Konishi T, Caldwell MW, Nishimura T, Sakurai K, Tanoue K. A new halisaurine mosasaur (Squamata: Halisaurinae) from Japan: the first record in the western Pacific realm and the first documented insights into binocular vision in mosasaurs. Journal of Systematic Palaeontology. 2016;14(10):809–839. doi: 10.1080/14772019.2015.1113447. [DOI] [Google Scholar]
- Kornhuber (1873).Kornhuber A. Über einen neuen fossilen saurier aus Lesina. Herausgegeben Von Der K. K. Geologischen Reichsanstalt. 1873;5:75–90. [Google Scholar]
- Kornhuber (1901).Kornhuber A. Opetiosaurus bucchichi, eine neue fossile Eidechse aus der unteren Kreide von Lesina in Dalmatien. AbhandLungender Kaiserlich-Königlichen Geologischen Reichsanstalt zu Wien. 1901;17(5):1–24. [Google Scholar]
- Kramberger (1892).Kramberger KG. Aigialosaurus: eine neue Eidechse aus den Kreideschiefern der Insel Lesina mit Rücksicht auf die bereits beschriebenen Lacertiden von Comen und Lesina. Glasnik Hrvatskoga Naravoslovnoga Društva (Societas Historico-Naturalis Croatica) u Zagrebu. 1892;7:74–106. [Google Scholar]
- Leblanc, Caldwell & Bardet (2012).Leblanc ARH, Caldwell MW, Bardet N. A new mosasaurine from the Maastrichtian (Upper Cretaceous) phosphates of Morocco and its implications for mosasaurine systematics. Journal of Vertebrate Paleontology. 2012;32(1):82–104. doi: 10.1080/02724634.2012.624145. [DOI] [Google Scholar]
- Lee (1998).Lee MSY. Convergent evolution and character correlation in burrowing reptiles: towards a resolution of squamate relationships. Biological Journal of the Linnean Society. 1998;65:369–453. doi: 10.1111/j.1095-8312.1998.tb01148.x. [DOI] [Google Scholar]
- Lee et al. (2014a).Lee MSY, Cau A, Naish D, Dyke GJ. Morphological clocks in palaeontology, and a mid-Cretaceous origin of crown Aves. Systematic Biology. 2014a;63:442–449. doi: 10.1093/sysbio/syt110. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2014b).Lee MSY, Cau A, Naish D, Dyke GJ. Sustained miniaturization and anatomical innovation in the dinosaurian ancestors of birds. Science. 2014b;345(6196):562–566. doi: 10.1126/science.1252243. [DOI] [PubMed] [Google Scholar]
- Lewis (2001).Lewis PO. A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology. 2001;50(6):913–925. doi: 10.1080/106351501753462876. [DOI] [PubMed] [Google Scholar]
- Lingham-Soliar (1996).Lingham-Soliar T. The first description of Halisaurus (Reptilia Mosasauridae) from Europe, from the Upper Cretaceous of Belgium. Bulletin de l’Institut Royal des Sciences Naturelles de Belqique, Sciences de la Terre. 1996;66:129–136. [Google Scholar]
- Maddison, Donoghue & Maddison (1984).Maddison WP, Donoghue MJ, Maddison DR. Outgroup analysis and parsimony. Systematic Zoology. 1984;33:83–103. doi: 10.2307/2413134. [DOI] [Google Scholar]
- Madzia & Conrad (in press).Madzia D, Conrad JL. Mosasauridae. In: De Queiroz K, Cantino PD, Gauthier JA, editors. Phylonyms: a companion to the PhyloCode. University of California Press; Berkeley: (In Press) [Google Scholar]
- Makádi, Caldwell & Ösi (2012).Makádi LS, Caldwell MW, Ösi A. The first freshwater mosasauroid (Upper Cretaceous, Hungary) and a new clade of basal mosasauroids. PLOS ONE. 2012;7(12):e51781. doi: 10.1371/journal.pone.0051781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantell (1829).Mantell GA. A tabular arrangement of the organic remains of the county of Sussex. Transactions of the Geological Society. 1829;2:201–216. [Google Scholar]
- Marsh (1869).Marsh OC. Notice of some new mosasauroid reptiles from the Greensand of New Jersey. American Journal of Science. 1869;48:392–397. [Google Scholar]
- Martin (2007).Martin JE. A North American Hainosaunts (Squamata: Mosasauridae) from the Late Cretaceous of southern South Dakota. In: Martin JE, Parris DC, editors. The geology and paleontology of the Late Cretaceous marine deposits of the dakotas: Geological Society of America Special Paper. vol. 427. 2007. pp. 199–207. [Google Scholar]
- Martin & Fernández (2007).Martin JE, Fernández M. The synonymy of the Late Cretaceous mosasaur (Squamata) genus Lakumasaurus from Antarctica with Taniwhasaurus from New Zealand and its bearing upon faunal similarity within the Weddellian Province. Geological Journal. 2007;42(2):203–211. doi: 10.1002/gj.1066. [DOI] [Google Scholar]
- Mulder, Cornelissen & Verding (2004).Mulder EWA, Cornelissen D, Verding L. Is Mosasaurus lemonnieri a juvenile Mosasaurus hoffmanni? A discussion. In: Schulp AS, Jagt JWM, editors. First mosasaur meeting, Maastricht, 8–12 May 2004, abstract book and field guide; Maastricht. 2004. pp. 2–66. [Google Scholar]
- Nicholls & Meckert (2002).Nicholls EL, Meckert D. Marine reptiles from the Nanaimo Group (Upper Cretaceous) of Vancouver Island. Canadian Journal of Earth Science. 2002;39(11):1591–1603. doi: 10.1139/e02-075. [DOI] [Google Scholar]
- Nopcsa (1923).Nopcsa F. Eidolosaurus und Pachyophis, Zwei neue Neocom-Reptilien. Palaeontographica. 1923;55:97–154. [Google Scholar]
- Olshevsky (1991).Olshevsky G. A revision of the parainfraclass Archosauria Cope, 1869, excluding the advanced Crocodylia. 1991. p. 196. (Mesozoic Meanderings #2). [Google Scholar]
- O’Reilly et al. (2016).O’Reilly J, Puttick M, Parry L, Tanner A, Tarver J, Fleming J, Pisani D, Donoghue P. Bayesian methods outperform parsimony but at the expense of precision in the estimation of phylogeny from discrete morphological data. Biology Letters. 2016;12:20160081. doi: 10.1098/rsbl.2016.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero et al. (2017).Otero RA, Soto-Acuña S, Rubilar-Rogers D, Gutstein CS. Kaikaifilu hervei gen. et sp. nov, a new large mosasaur (Squamata, Mosasauridae) from the upper Maastrichtian of Antarctica. Cretaceous Research. 2017;70:209–225. doi: 10.1016/j.cretres.2016.11.002. [DOI] [Google Scholar]
- Owen (1850).Owen R. Description of the fossil reptiles of the chalk formation. In: Dixon F, editor. The geology and fossils of the tertiary and cretaceous formations of sussex. Longman, Brown, Green and Longmans; London: 1850. pp. 378–404. [Google Scholar]
- Owen (1851).Owen R. Section II: the fossil Reptilia of the Cretaceous period. Cassell & Company Limited; London: 1851. A history of British fossil reptiles; pp. 155–210. [Google Scholar]
- Palci & Caldwell (2007).Palci A, Caldwell MW. Vestigial forelimbs and axial elongation in a 95-million-year-old non-snake squamate. Journal of Vertebrate Paleontology. 2007;27(1):1–7. [Google Scholar]
- Palci & Caldwell (2010).Palci A, Caldwell MW. Redescription of Acteosaurus tommasinii von Meyer, 1860, and a discussion of evolutionary trends within the clade Ophidiomorpha. Journal of Vertebrate Paleontology. 2010;30:94–108. doi: 10.1080/02724630903409139. [DOI] [Google Scholar]
- Palci, Caldwell & Papazzoni (2013).Palci A, Caldwell MW, Papazzoni CA. A new genus and subfamily of mosasaurs from the Upper Cretaceous of northern Italy. Journal of Vertebrate Paleontology. 2013;33(3):599–612. doi: 10.1080/02724634.2013.731024. [DOI] [Google Scholar]
- Páramo (1994).Páramo ME. Posición sistemática de un reptil marino con base en los restos fósiles encontrados en capas del Cretácico Superior en Yaguará (Huila) Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales. 1994;19:63–80. [Google Scholar]
- Páramo-Fonseca (2013).Páramo-Fonseca ME. Eonatator coellensis nov. sp. (Squamata: Mosasauridae), nueva especie del Cretácico Superior de Colombia. Revista de la Academia Colombiana de Ciencias. 2013;37(145):499–518. [Google Scholar]
- Pierce & Caldwell (2004).Pierce SE, Caldwell MW. Redescription and phylogenetic position of the Adriatic (Upper Cretaceous; Cenomanian) dolichosaur Pontosaurus lesinensis Kornhuber, 1873. Journal of Vertebrate Paleontology. 2004;24(2):373–386. doi: 10.1671/1960. [DOI] [Google Scholar]
- Pol & Escapa (2009).Pol P, Escapa IH. Unstable taxa in cladistic analysis: identification and the assessment of relevant characters. Cladistics. 2009;25:1–13. doi: 10.1111/j.1096-0031.2008.00233.x. [DOI] [PubMed] [Google Scholar]
- Polcyn & Bell (2005).Polcyn MJ, Bell GL. Russellosaurus coheni n. gen, n. sp, a 92 million-year-old mosasaur from Texas (USA), and the definition of the parafamily Russellosaurina. Netherlands Journal of Geosciences. 2005;84:321–333. doi: 10.1017/S0016774600021107. [DOI] [Google Scholar]
- Polcyn & Everhart (2008).Polcyn MJ, Everhart MJ. Description and phylogenetic analysis of a new species of Selmasaurus (Mosasauridae: Plioplatecarpinae) from the Niobrara Chalk of western Kansas. Proceedings of the Second Mosasaur Meeting. 2008:13–28. [Google Scholar]
- Polcyn et al. (2014).Polcyn MJ, Jacobs LL, Araújo R, Schulp AS, Mateus O. Physical drivers of mosasaur evolution. Palaeogeography, Palaeoclimatology, Palaeoecology. 2014;400:17–27. doi: 10.1016/j.palaeo.2013.05.018. [DOI] [Google Scholar]
- Polcyn et al. (2012).Polcyn MJ, Lindgren J, Bardet N, Cornelissen D, Verding L, Schulp AS. Description of new specimens of Halisaurus arambourgi Bardet & Pereda Suberbiola, 2005 and the relationships of Halisaurinae. Bulletin de la Société Géologique de France. 2012;183(2):123–136. doi: 10.2113/gssgfbull.183.2.123. [DOI] [Google Scholar]
- Rambaut & Drummond (2009).Rambaut A, Drummond AJ. Tracer: MCMC trace analysis tool. v1.5http://beast.bio.ed.ac.uk/ 2009
- Reeder et al. (2015).Reeder TW, Townsend TM, Mulcahy DG, Noonan BP, Wood Jr PL, Sites JW, Wiens JJ. Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLOS ONE. 2015;10(3):e0118199. doi: 10.1371/journal.pone.0118199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynoso (1998).Reynoso V-H. Huehuecuetzpalli mixtecus gen sp. Nov: a basal squamate (Reptilia) from the Early Cretaceous of Tepexi De Rodríguez, Central México. Philosophical Transactions B: Biological Sciences. 1998;353:477–500. [Google Scholar]
- Russell (1967).Russell DA. Systematics and morphology of American mosasaurs. Bulletin of the Peabody Museum of Natural History. 1967;23:1–241. [Google Scholar]
- Schulp (2006).Schulp AS. A comparative description of Prognathodon saturator (Mosasauridae, Squamata), with notes on its phylogeny. In: Schulp AS, editor. On maastricht mosasaurs. publicaties van het natuurhistorisch genootschap in limburg. 45(1) Natuurhistorisch Genootschap in Limburg; Maastricht: 2006. pp. 19–56. [Google Scholar]
- Schulp, Jagt & Fonken (2004).Schulp AS, Jagt JWM, Fonken F. New material of the mosasaur Carinodens belgicus from the Upper Cretaceous of The Netherlands. Journal of Vertebrate Paleontology. 2004;24:744–747. doi: 10.1671/0272-4634(2004)024[0744:NMOTMC]2.0.CO;2. [DOI] [Google Scholar]
- Schulp et al. (2008).Schulp AS, Polcyn MJ, Mateus O, Jacobs LL, Morais ML. A new species of Prognathodon (Squamata, Mosasauridae) from the Maastrichtian of Angola, and the affinities of the mosasaur genus Liodon. Proceedings of the Second Mosasaur Meeting. 2008:1–12. [Google Scholar]
- Schulp et al. (2006).Schulp AS, Polcyn MJ, Mateus O, Jacobs LL, Morais ML, Da Silva Tavares T. New mosasaur material from the Maastrichtian of Angola, with notes on the phylogeny, distribution and palaeoecology of the genus Prognathodon. In: Schulp AS, editor. On Maastricht Mosasaurs. Publicaties van het Natuurhistorisch Genootschap in Limburg. 45(1) 2006. pp. 57–67. [Google Scholar]
- Seeley (1881).Seeley HG. On Remains of a small Lizard from the Neocomian Rocks of Comén, near Trieste preserved in the Geological Museum of the University of Vienna. Quarterly Journal of the Geological Society. 1881;37:52–56. doi: 10.1144/GSL.JGS.1881.037.01-04.07. [DOI] [Google Scholar]
- Sereno (2007).Sereno PC. Logical basis for morphological characters in phylogenetics. Cladistics. 2007;23:565–587. doi: 10.1111/j.1096-0031.2007.00161.x. [DOI] [PubMed] [Google Scholar]
- Simões et al. (2017).Simões TR, Vernygora O, Paparella I, Jimenez-Huidobro P, Caldwell MW. Mosasauroid phylogeny under multiple phylogenetic methods provides new insights on the evolution of aquatic adaptations in the group. PLOS ONE. 2017;12(5):e0176773. doi: 10.1371/journal.pone.0176773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu-Ting et al. (2015).Siu-Ting K, Pisani D, Creevey CJ, Wilkinson M. Concatabominations: identifying unstable taxa in morphological phylogenetics using a heuristic extension to safe taxonomic reduction. Systematic Biology. 2015;64:137–143. doi: 10.1093/sysbio/syu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding, O’Leary & Gatesy (2009).Spaulding M, O’Leary MA, Gatesy J. Relationships of Cetacea (Artiodactyla) among mammals: Increased taxon sampling alters interpretations of key fossils and character evolution. PLOS ONE. 2009;4(9):e7062. doi: 10.1371/journal.pone.0007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street & Caldwell (2017).Street HP, Caldwell MW. Rediagnosis and redescription of Mosasaurus hoffmannii (Squamata: Mosasauridae) and an assessment of species assigned to the genus Mosasaurus. Geological Magazine. 2017;154(3):521–557. doi: 10.1017/S0016756816000236. [DOI] [Google Scholar]
- Strganac et al. (2014).Strganac C, Salminen J, Jacobs LL, Polcyn MJ, Ferguson KM, Mateus O, Schulp AS, Morais ML, Da Silva Tavares T, Gonçalves AO. Carbon isotope stratigraphy, magnetostratigraphy, and 40Ar/39Ar age of the Cretaceous South Atlantic coast, Namibe Basin, Angola. Journal of African Earth Sciences. 2014;99(2):452–462. doi: 10.1016/j.jafrearsci.2014.03.003. [DOI] [Google Scholar]
- Thompson (2005).Thompson WA. MSc thesis. 2005. The first record of Hainosaurus (Reptilia: Mosasauridae) from the Pierre Shale of South Dakota, and implications for differentiating between the Tylosaurine Genera Tylosaurus and Hainosauras. [Google Scholar]
- Thompson (2011).Thompson WA. PhD thesis. 2011. The Phylogeny and Biostratigraphy of the Tylosaurine Mosasauridae (Reptilia, Squamata) [Google Scholar]
- Wiens (2003a).Wiens JJ. Incomplete taxa, incomplete characters, and phylogenetic accuracy: Is there a missing data problem? Journal of Vertebrate Paleontology. 2003a;23:297–310. doi: 10.1671/0272-4634(2003)023[0297:ITICAP]2.0.CO;2. [DOI] [Google Scholar]
- Wiens (2003b).Wiens JJ. Missing data, incomplete taxa, and phylogenetic accuracy. Systematic Biology. 2003b;52:528–538. doi: 10.1080/10635150390218330. [DOI] [PubMed] [Google Scholar]
- Wiens & Morrill (2011).Wiens JJ, Morrill MC. Missing data in phylogenetic analysis: reconciling results from simulations and empirical data. Systematic Biology. 2011;60:719–731. doi: 10.1093/sysbio/syr025. [DOI] [PubMed] [Google Scholar]
- Wilberg (2015).Wilberg EW. What’s in an outgroup? the impact of outgroup choice on the phylogenetic position of thalattosuchia (crocodylomorpha) and the origin of crocodyliformes. Systematic Biology. 2015;64(4):621–637. doi: 10.1093/sysbio/syv020. [DOI] [PubMed] [Google Scholar]
- Wilkinson (1992).Wilkinson M. Ordered versus unordered characters. Cladistics. 1992;8:375–385. doi: 10.1111/j.1096-0031.1992.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson (1995).Wilkinson M. Coping with abundant missing entries in phylogenetic inference using parsimony. Systematic Biology. 1995;44:501–514. doi: 10.1093/sysbio/44.4.501. [DOI] [Google Scholar]
- Wilkinson (2001).Wilkinson M. Department of Zoology. The Natural History Museum; London: 2001. TAXEQ3: software and documentation. [Google Scholar]
- Williston (1897).Williston SW. Range and distribution of the mosasaurs. Kansas University Quarterly. 1897;6:177–189. [Google Scholar]
- WoRMS (2015).World Register of Marine Species (WoRMS) Natantia. 2015. http://www.marinespecies.org/aphia.php?p =taxdetails&id=181484. [02 February 2017]. http://www.marinespecies.org/aphia.php?p =taxdetails&id=181484
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplementary File.