Abstract
As platelets are rich in growth factors for tissue regeneration, autologous platelet-rich plasma (PRP) has been used to treat some refractory corneal defects. Although PRP is effective, the cost of its preparation is very high. This article presents three cases of refractory corneal ulcer under the prescription of autologous PRP. The autologous PRP used in these cases was easily prepared in the blood bank laboratory. In this paper, we collected three patients with refractory corneal ulcer who were unresponsive to conventional treatment. The patients presented with neurotrophic ulcer, exposure corneal ulcer, and limbal deciency with corneal ulcer after hepatitic keratitis. Although we easily prepared autologous PRP eye drops using simple laboratory centrifugation, this preparation still had a clinical effect on corneal defect. The mean intervention time was 24 ± 6.9 days. The case with exposure corneal ulcer had significant wound healing and the other two cases felt subjective symptom relief. There were some clinical improvements of refractory corneal ulcers in our three cases. We present the clinical results of three cases and report an easy procedure for the preparation of autologous PRP. Autologous PRP prepared simply in the laboratory, it may be an alternative option for treating refractory corneal ulcer.
Keywords: corneal ulcer, platelet-rich plasma, autologous
1. Introduction
The treatment of refractive corneal ulcer still poses a challenge to many ophthalmologists. Previous therapeutic modalities include the use of bandage contact lenses, topical artificial tears, sodium hyaluronate, and autologous serum with fibronectin, substance P, or insulin-like growth factor 1.1 Topical application of serum eye drops has been reported to accelerate healing of persistent ocular surface defects.
Autologous platelet-rich plasma (PRP) has also been proven to beneficial in cell proliferation and wound healing.2 The difference between autologous PRP and autologous serum is the platelets preserved in the autologous PRP. Platelets are great sources of growth factors such as platelet-derived growth factors (PDGFs) aa, bb, and ab, transforming growth factors (TGFs) β1 and β2, vascular endothelial growth factor, and epithelial growth factor. Platelets also adhere to the damaged vascular endothelium and start a healing reaction that includes the release of numerous cytokines and growth factors.3 Autologous PRP is rich in growth factors known for healing epithelial and internal wounds. Clinically, some ocular surface defects such as dormant corneal ulcer, dry eye, and ocular surface syndrome have been treated with autologous PRP.2,3,4,5,6,7 As a complement to tissue regeneration procedures, PRP also supports the wound healing process in other specialties such as oral and maxillofacial surgery, reconstructive surgery, orthopedics, cardiovascular surgery, and plastic surgery.8,9,10,11,12,13,14,15
The conventional production of autologous PRP is complicated, involving large volumes of whole blood (500 mL), and a hospital environment with sophisticated and expensive equipment. This article presents three cases of refractory corneal ulcer under the prescription of autologous PRP that was easily prepared in the laboratory.
2. Study design and methods
2.1. Participants
Four eyes of three patients with refractory corneal ulcers were treated at the Department of Ophthalmology Shin-Kong Wu Ho-Su Memorial Hospital in Taipei, Taiwan. This research was conducted with the approval of the Institutional Review Board.
2.2. Patients and examinations
In this study, we included three patients with refractory corneal ulcer. The mean age of the patients was 45.67 ± 30.17 years (range, 11–66 years). Their respective histories are briefly described as follows.
Case A was a 66-year-old woman who had underlying history of uremia on hemodialysis, diabetes mellitus, and hypertension for many years. She initially presented with bilateral refractory neurotrophic corneal ulcers: 9.3 mm × 5.2 mm diffuse corneal ulcer in the right eye and a 7.7 mm × 3.2 mm band form corneal ulcer in the left. Vision was only counting fingers at 30 cm in both eyes. The patient suffered from persistent ocular irritation and mucus discharge for about 1 month. Bacterial culture of the cornea showed no growth. We had already used nonpreserved artificial tears and antibiotic eye drops to protect the cornea. We also used therapeutic contact lenses for the neurotrophic ulcers. However, the corneal wound was persistent, so we added autologous PRP every 2 hours except sleep for 17 days.
Case B was a 60-year-old woman who had cataract surgery previously. Several months earlier she had received surgery for skull base meningioma, but she complained of an impairment in closing the right eye. The neurosurgeon prescribed lubrication for her. She visited our ophthalmology department because of vision change, severe eye congestion, and pain for 2 weeks. Her vision in the right eye was changed from 6/10 to 6/60. The right eye presented a diffuse corneal epithelial defect with severe infiltration almost in whole cornea. She refused tarsorraphy for exposure corneal ulcer. We used autologous PRP every 2 hours and topical antibiotic medication for 21 days.
Case C was a 9-year-old girl who had history of right eye amblyopia. She also had suffered from persistent herpetic keratitis 4 years earlier on the same-side right eye. After the infection, the right eye developed macular cornea, corneal scar, and neo-vascularization. Vision was only light perception in the right eye. Because of limbal deficiency, she suffered from recurrent ocular irritation, corneal ulcer, and stromal infiltration. Although we have used many lubricants to the irregular ocular surface, she still often complained of burning pain and discharge. Trying to relieve the symptom, she received autologous PRP every 2 hours for 35 days.
All the three patients were refractory to conventional treatment for corneal ulcer, such as preservative-free artificial tears, sodium hyaluronate eye drops, patching, or soft therapeutic contact lens applications.
2.3. Preparation of autologous PRP
In our blood bank, 20 mL of whole blood from each patient was taken by venipuncture. Before venipuncture, we used Sindine solution (povidone iodine 10% 200 mL/BT) and alcohol swab (isopropyl alcohol 70%) to scrub the area at least 4 cm in all directions from the intended site of venipuncture three times. Blood samples were drawn into 20-mL syringes. Then we transferred 2 mL into a tube (K2-EDTA) for complete blood count (Sysmex XE5000), and 18 mL blood into sterile glass tube with 2 mL of anticoagulant CPDA-1 in preparation for autologous PRP. Samples were gently agitated to mix the anticoagulant thoroughly with the whole blood and centrifuged for 10 minutes at 800 rpm (KA1000A Kubota). After centrifugation, the upper fraction of each sample was 2–3 mL PRP. Plasma was separated carefully in a sterile manner under clean bench conditions. Then 1.0 mL plasma was counted for platelet data with Sysmex XE5000 (Sysmex Corporation, Europe GmbH, Born-barch 1, 22848 Norderstedt, Germany), and the remaining 1.0–2.0 mL plasma was collected as the final autologous PRP product. To minimize contamination, we discarded all 5 mL of antibiotic eye drops from its sterile bottle and used this emptied bottle for storage of autologous PRP.
Patients were instructed to keep their current bottles in a dark, cool place under refrigeration at +4°C. Every week, we prepared a new bottle for each patient to keep autologous PRP fresh and avoid patient infection.
Autologous PRP was administered to patients once every 2 hours daily as required in addition to preservative-free artificial tears for at least 1 week. All topical medications were discontinued with the resolution of the corneal lesions or limited response after 35 days.
3. Results
3.1. Laboratory test of autologous PRP
The results of laboratory tests are shown in Table 1. The result of microbiology tests were: the autologous PRP of Cases B and C was normal, but autologous PRP eye drops of Case A on second examination were found to contain Stenotrophomonas maltophilia.
Table 1.
Platelet count in whole blood and platelet-rich plasma (PRP).
| Platelet count in whole blood | Platelet count in PRP | |
|---|---|---|
| Case A | 134.67 ± 6.11 × 109/L | 377.67 ± 76.79 × 109/L |
| Case B | 215.67 ± 5.86 × 109/L | 580.653 ± 52.56 ×109/L |
| Case C | 366 ± 42.22 × 109/L | 795.33 ± 16.04 × 109/L |
All patient data including prothrombin time and activated partial thromboplastin time tests were normal.
3.2. Clinical features
Refractory corneal ulcer of diabetic Case A had chronic stromal infiltration and corneal scarring for a long time. The neurotrophic ulcer showed limited response to autologous PRP. Clinically, the epithelial defect still persisted and stromal infiltration had little improvement after 17 days treatment with autologous PRP. However, the patient mentioned that foreign body sensation improved subjectively. The mucus discharge and ocular congestion were decreased after autologous PRP treatment (Fig. 1).
Fig. 1.
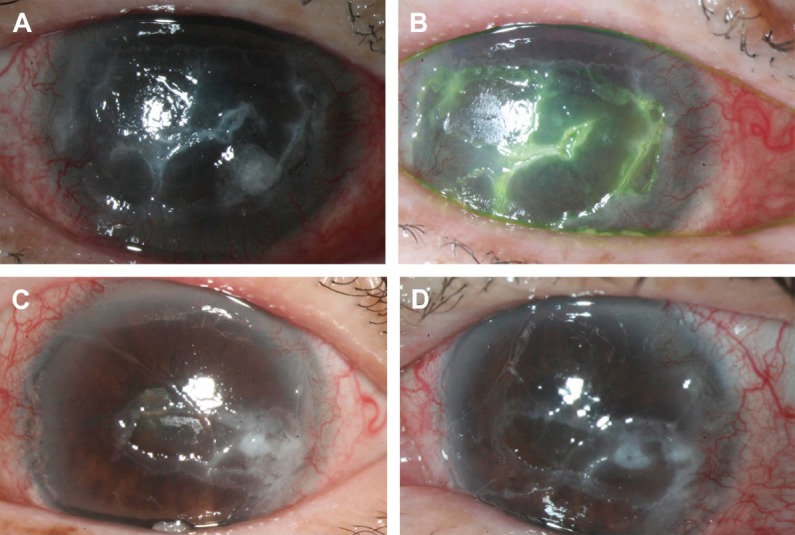
Case A. Diabetic corneal erosions in both eyes (A: right eye; B: left eye) before treatment by topical autologous platelet-rich plasma. Note the diffuse corneal defect and haziness. The corneal epithelial defect healed subclinically after 17 days, with a notable decrease in foreign body sensation (C: right eye; D: left eye).
The diffuse epithelial defect in Case B was almost healed after 21 days of PRP treatment (Fig. 2). The patient reported a gradual decrease of ocular discomfort and felt that vision improved week-by-week. Her vision recovered to 6/10. The exposure keratopathy subsided without the need for a more aggressive surgical procedure.
Fig. 2.
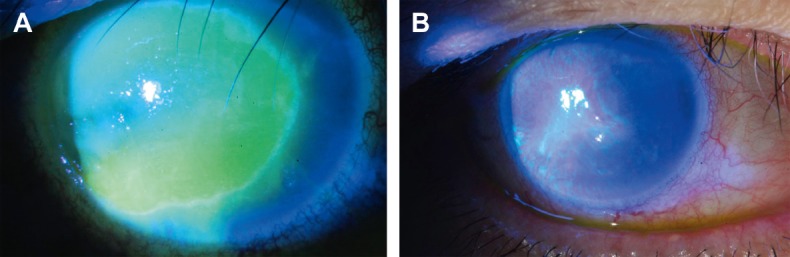
Case B. (A) Exposure keratopathy under fluorescence stain before treatment. (B) The corneal epithelial defect almost healed with a notable decrease in corneal haziness within 21 days.
The refractory corneal ulcer of Case B with stromal infiltration induced by herpetic keratitis shows mild improvement (Fig. 3). The subjective symptoms such as burning pain and discharge were relieved. Meanwhile, the corneal neovascularization and conjunctival congestion declined moderately. The girl was happy to return to her daily activities because of ocular symptom improvement.
Fig. 3.
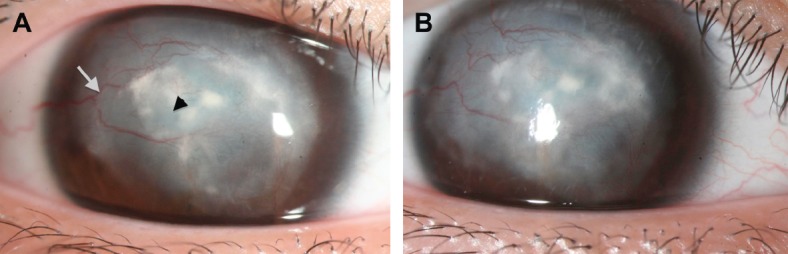
Case C. (A) Persistent herpetic keratitis before treatment. Note the stromal infiltration (arrowhead) and neovascularization (white arrow). (B) The corneal epithelial defect and stromal infiltration moderately decreased after 35 days of platelet-rich plasma treatment.
In summary, the corneal epithelial defect of exposure keratopathy (Case B) improved significantly after 21 days of application of autologous PRP. However, in diabetic Case A, refractory corneal ulcer with corneal scarring and stromal infiltration showed the least response to autologous PRP in this case series.
4. Discussion
Autologous PRP has been proven to be very effective for tissue regeneration and wound healing.2,3,4,5,6,7,16,17,18 Because PRP is developed from autologous blood, it is inherently safe and free from transmissible diseases such as HIV and hepatitis. With PRP, the increased number of platelets delivers an increased number of growth factors to the surgical area. The seven known growth factors in PRP are: PDGFs aa, bb, and ab, TGF-β1 and -β2, vascular endothelial growth factor, and epithelial growth factor. These are native growth factors in their biologically determined ratios.16,17,18,19 As with other tissues, wound healing in the cornea is controlled by a variety of growth factors and other substances influencing proliferation, differentiation, and migration of corneal epithelial cells, which in parts reach the ocular surface via the natural tear film. Epithelial growth factor supports proliferation and migration of epithelial cells and seems to inhibit apoptosis. PDGF, hepatocyte growth factor, and fibroblast growth factor are stimuli of cell proliferation, whereas TGF-β seems to inhibit corneal epithelial cell proliferation and enhances apoptosis.14,15 Generally, these biologic products of PRP are manufactured through a particular kit under good manufacturing practice conditions. When manufacturing a biologic PRP, it is essential to have established specifications to assure safety, purity, and potency. The cost expansion of manufacturing PRP is very high, so PRP possibly could be used only in some higher economic populations.
As far as we know, this is the first report to present a process that was not through a particular kit and manufacture cabinet, but provided a preparation regimen of autologous PRP in the laboratory. We analyzed our easy-to-prepare autologous PRP, which resulted in a two- to three-fold enrichment of platelet. The quality was good enough to approach the treatment level. Costs seem to be lower without requiring specific equipment and high-cost disposable kit.18 For topical ocular surface defects, easily preparing growth factors of PRP in a blood bank laboratory is attractive. However, there are concerns about safety and traceability because of the risk of bacterial contamination. To avoid the risk of infection, we put PRP in an empty bottle of antibiotic eye drops and stored it in the refrigerator at 4°C.19 At each weekly visit, we also monitored the condition of PRP including the platelet count and bacterial culture. In all 11 PRP preparations from three patients, only one positive bacterial culture was found from the diabetic patient (Case A). From the second PRP sample of Case A, we cultured Steno-trophomonas maltophilia; however, blood culture of this patient revealed no bacteremia. This implies that the bacteria strains may have been the result of contamination. Although it is possible that this was a false-positive culture result, there are no data provided to support this conclusion. Absolutely, we should perform aseptic procedures more carefully to prevent sample contamination in laboratory. The patients need more health education for PRP usage at home.17,18
PRP is defined as a portion of the plasma fraction of autologous blood having a platelet concentration above baseline.20 PRP has more concentrated platelets than normal plasma (approximately 150–400 × 109/L). In such conditions, PRP contains not only a high level of platelets, but also the full complement of clotting factors, the latter of which typically remain at their normal physiologic levels. In blood cell analysis, Lee et al21 reported that the platelet count of the PRP group was approximately 4.25 times higher than that of the whole blood group using a manufacturing process. Although using a centrifuge in our easy-to-prepare method did not give a platelet count as high as previously reported, this method still provided platelet preparations that were concentrated two- to three-fold over total blood values.21 According to the clinical results, the easy-to-prepare autologous PRP still worked.
Although there are only three cases reports here, we found that autologous PRP has a potential role for treating refractive corneal ulcer. Compared to diffuse superficial corneal ulcer, PRP has limited effect on corneal scar or deep stromal infiltration. The platelet concentration of the easily prepared autologous PRP is lower than those of specialized procedures, but this autologous PRP from our laboratory could be used in situations where manufacturing a biologic PRP is not practical.
Footnotes
Conflicts of interest: The authors have no financial interests to disclose.
References
- 1.Matsumoto Y, Dogru M, Goto E, Ohashi Y, Kojima T, Ishida R, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthal mology. 2004;111:1115–1120. doi: 10.1016/j.ophtha.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Hartwig D, Harloff S, Liu L, Schlenke P, Wedel T, Geerling G. Epitheliotrophic capacity of a growth factor preparation produced from platelet concentrates on corneal epithelial cells: a potential agent for the treatment of ocular surface defects. Transfusion. 2004;44:1724–1731. doi: 10.1111/j.0041-1132.2004.04079.x. [DOI] [PubMed] [Google Scholar]
- 3.Alio JL, Abad M, Artola A, Rodriguez-Prats JL, Pastor S, Ruiz-Colecha J. Use of autologous platelet-rich plasma in the treatment of dormant corneal ulcers. Ophthalmology. 2007;114:1286–1293. doi: 10.1016/j.ophtha.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 4.Yazawa M, Ogata H, Nakajima T, Mori T, Watanabe N, Handa M, et al. Basic studies on the clinical applications of platelet-rich plasma. Cell Transpl. 2003;12:509–518. doi: 10.3727/000000003108747073. [DOI] [PubMed] [Google Scholar]
- 5.Bettina K, Heather S. Growth factors in the anterior segment: role in tissue maintenance, wound healing and ocular pathology. Exp Eye Res. 2004;79:677–688. doi: 10.1016/j.exer.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Alio JL, Colecha JR, Pastor S, Rodriguez AE, Artola A. Symptomatic dry eye treatment with autologous platelet-rich plasma. Ophthalmic Res. 2007;39:124–129. doi: 10.1159/000100933. [DOI] [PubMed] [Google Scholar]
- 7.Alio JL, Arnalich-Montiel F, Rodriguez AE. The role of “eye platelet rich plasma” (E-PRP) for wound healing in ophthalmology. Curr Pharm Biotechnol. 2012;13:1257–1265. doi: 10.2174/138920112800624355. [DOI] [PubMed] [Google Scholar]
- 8.Sammartino G, Tia M, Marenzi G, di Lauro AE, D’Agostino E, Claudio PP. Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg. 2005;63:766–770. doi: 10.1016/j.joms.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Choi BH, Im CJ, Huh JY, Suh JJ, Lee SH. Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg. 2004;33:56–59. doi: 10.1054/ijom.2003.0466. [DOI] [PubMed] [Google Scholar]
- 10.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Englert SJ, Estep TH, Ellis-Stoll CC. Autologous platelet gel applications during cardiovascular surgery: effect on wound healing. J Extra Corpor Technol. 2005;37:148–152. [PMC free article] [PubMed] [Google Scholar]
- 12.Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229–237. doi: 10.1097/00006534-200101000-00037. [DOI] [PubMed] [Google Scholar]
- 13.Bhanot S, Alex JC. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002;18:27–33. doi: 10.1055/s-2002-19824. [DOI] [PubMed] [Google Scholar]
- 14.Thorn JJ, Sørensen H, Weis-Fogh U, Andersen M. Autologous fibrin glue with growth factors in reconstructive maxillofacial surgery. Int J Oral Maxillofac Surg. 2004;33:95–100. doi: 10.1054/ijom.2003.0461. [DOI] [PubMed] [Google Scholar]
- 15.Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84:434–439. doi: 10.1177/154405910508400507. [DOI] [PubMed] [Google Scholar]
- 16.Hoppenreijs VP, Pels E, Vrensen GF, Treffers WF. Basic fibroblast growth factor stimulates corneal endothelial cell growth and endothelial wound healing of human corneas. Invest Ophthalmol Vis Sci. 1994;35:931–944. [PubMed] [Google Scholar]
- 17.Klenkler B, Sheardown H. Growth factors in the anterior segment: role in tissue maintenance, wound healing and ocular pathology. Exp Eye Res. 2004;79:677–688. doi: 10.1016/j.exer.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Akhundov K, Pietramaggiori G, Waselle L, Darwiche S, Guerid S, Scaletta C, et al. Development of a cost-effective method for platelet-rich plasma (PRP) prep aration for topical wound healing. Ann Burns Fire Disasters. 2012;25:207–213. [PMC free article] [PubMed] [Google Scholar]
- 19.Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med. 2001;11:403–417. doi: 10.1046/j.1365-3148.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- 20.Arnoczky SP, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21:180–185. doi: 10.1097/JSA.0b013e3182999712. [DOI] [PubMed] [Google Scholar]
- 21.Lee JW, Kwon OH, Kim TK, Cho YK, Choi KY, Chung HY, et al. Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch Plast Surg. 2013;40:530–535. doi: 10.5999/aps.2013.40.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]


