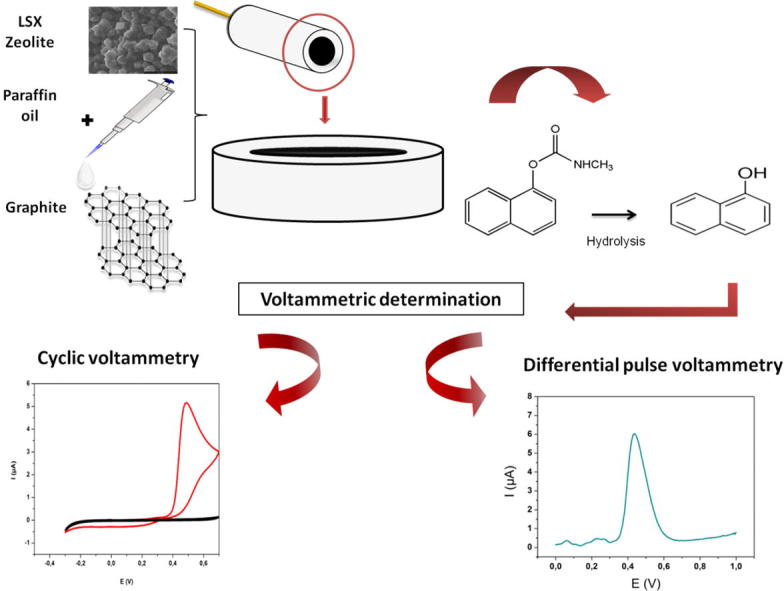
Graphical abstract
Keywords: Carbaryl (CBR), Differential pulse voltammetric technique (DPV), Low silica X zeolite, Pesticide, Carbon paste electrode, Sensor
Abstract
A new and simple approach for carbaryl determination in natural sample was proposed using Low Silica X (LSX) zeolite modified carbon paste electrode. LSX zeolite with a porous structure was incorporated into carbon paste electrode in the appropriate portion. The prepared electrode was then characterized using scanning electron microscopy, cyclic voltammetry and electrochemical impedance spectroscopy. Various experimental parameters as the zeolite amounts, pH, accumulation time, and differential pulse voltammetric parameters were optimized. Under optimal conditions, a linear response was obtained in the range of 1–100 µM of carbaryl using differential pulse voltammetry with detection limit of 0.3 µM (S/N = 3). The sensors showed good selectivity, stability, and reproducibility and has been successfully applied for detection of carbaryl in tomato samples with good recoveries.
Introduction
Pesticides and their degradation products residue are a major pollutant and represent a potential menace to the ecosystem and human health. The non-rational uses of these agricultural inputs have many negative consequences and lead to the pollution of soil, fruits, vegetables, surface water, and groundwater. Moreover, these compounds are characterized by their persistence, their toxicity and known to bioaccumulate in the environment [1], [2]. The use and impact of pesticides are an increasingly noticeable concern of the community. Consequently, there is an urgent need to develop new procedures for the determination of low amounts of these pollutants in different matrices. Carbaryl (CBR) is the widespread name for a compound known as 1-naphthyl methylcarbamate. It is a type of most frequently carbamate insecticide used to control a wide variety of pests, such as moths, beetles, cockroaches, ants, ticks, and mosquitoes by the inhibition of cholinesterase, one of the most important enzymes in the nervous systems of pests, vertebrates, and humans (WHO, 1994); [3]. The excessive and indiscriminate use of this pesticide is a major preoccupation because of the potential damage that this compound could cause to the environment and human. For instance, the harm caused to the major systems of the body, immune, nervous, and endocrine system [4], [5]. During the last decades, several analytical methods were employed to analyze pesticides such as gas chromatography-mass spectrometry, Electro-Fenton technology, amperometric detection, Raman spectroscopy, acoustic technologies, and fluorescence methods [6], [7], [8], [9], [10], [11]. Electrochemical techniques known as rapid and inexpensive methods are a good alternative to the classical methods used to determine trace level of pesticides and organic compounds [12], [13], [14], [15], [16]. Electrochemical studies concerning detection of carbamate pesticide, mainly carbaryl, were reported in the literature based on amperometric or voltammetric methods using non-enzymatic and enzymatic sensors [17], [18], [19], [20], [21], [22], [23]. During the last few years, detection of this pesticide with electrochemical biosensors based on enzymes was considered as a promising tool [24]. However, the enzymatic ways need long analysis time and pre-treatment steps. Non-enzymatic methods remain a very attractive option, capable to provide quantitative detection of carbaryl with a low cost and short analysis time. Zeolites, identified as microporous crystalline aluminosilicate materials [25], widely used for their high surface area, ion exchange capability, adsorptive capacity, and molecular sieving ability, are considered as interesting materials, which can be exploited in the development of modified electrodes. Zeolite modified electrodes were used as sensors for different reasons explained in the paper of Walcarius [26]. In fact, zeolite modified electrodes combine the advantage of ion exchange voltammetry with the molecular sieving property of zeolites. The properties of zeolites, such as size selectivity, high chemical and thermal stability could be coupled with the high sensitivity of voltammetric techniques [27], [28], [29]. Indeed, zeolite modified electrodes were previously used to detect some kind of pesticides like linuron and paraquat as reported by Siara et al., and Walcarius et al. [30], [31]. In the literature, only the photodecomposition of carbaryl was investigated using zeolite and silver as a catalyst [33], [34]. The potential of both modified and unmodified carbon paste electrodes in electrochemical applications and in modern electroanalysis of inorganic ions has been studied by many authors [35], [36]. Zeolite modified carbon paste electrodes are a kind of modified carbon paste electrodes used to detect several molecules. However, to our best knowledge, the use of zeolite doped carbon paste electrode was not studied for the detection of carbaryl. In this work, new sensor was made from LSX zeolite modified carbon paste for the indirect detection of carbaryl. The electrode was characterized using microscopic, voltammetric and electrochemical spectroscopic techniques. The analytical performances of the resulting sensor were investigated after the optimization of experimental parameters. The obtained electrochemical electrode was used to determinate carbaryl in tomato sample using differential pulse voltammetry.
Experimental
Regents and materials
The chemical reagents used in the preparation of all solutions were analytical reagent grade. Graphite was supplied from Sigma Aldrich, St. Louis, Mo., USA. Chemical products used in synthesis of low silica X zeolite are: sodium aluminate (NaAlO2, 50–56 wt%, Sigma Aldrich, Saint-Quentin Fallavier, France) as source of Al, sodium metasilicate nonahydrate (Na2SiO3·9H2O, ≥98 wt% Merck, Fontenay Sous Bois, France) as source of Si, sodium hydroxide (NaOH, 98 wt%, Sigma Aldrich, Saint-Quentin Fallavier, France), potassium hydroxide (KOH, 85 wt%, Merck, Fontenay Sous Bois, France) and bidistilled water. Carbaryl (CBR, C12H11NO2, 99.8% purity) was purchased from Sigma-Aldrich, St. Louis, Mo., USA.
Synthesis of low silica X zeolite (LSXZ)
The low silica X zeolite powder was hydrothermally synthesized. Based on Kühl method, the molar composition of synthesis batch was Al2O3:2.2 SiO2:5.5 Na2O:1.65 K2O:400 H2O. Solution of sodium aluminate (SA) was prepared by dissolving sodium hydroxide and sodium aluminate in water. A sodium silicate (SB) solution was obtained by adding potassium and sodium hydroxide, and sodium silicate to water. Solutions SA and SB were carefully mixed under strong stirring at room temperature for at least 1 h to form aluminosilicate hydrogel. The mixture was poured into Teflon autoclave and then put in the stove for hydrothermal crystallization of zeolite that was carried out at a temperature of 90 °C for 24 h. After crystallization, synthesized powder was cooled down to 20 °C and washed with bidistilled water until the pH value of washing water became neutral. Then, the obtained powder was finally dried at 100 °C in the stove during 6 h.
Instrumentation
Cyclic voltammetric, electrochemical impedance spectroscopy and differential pulse voltammetric experiments were performed using AUTOLAB PGSTAT302N (Metrohm Autolab B.V., Utrecht, The Netherlands) Potentiostat/Galvanostat controlled by GPES 4.9 software. The three-electrode system consisted of a saturated calomel electrode (SCE) as reference electrode (which can be replaced by Ag/AgCl/KClsat), a platinum disk as auxiliary electrode, and a modified carbon paste as working electrode. The pH was measured using pH meter Fisher Scientific Accumet AB15 Basic, Waltham, MA USA. All the potentials reported in this work were given against SCE 3M KCl reference electrode at a laboratory temperature. X-ray diffraction (XRD) patterns were measured by using a Philips X'Pert PRO (PANalytical B.V, Limeil-Brevannes, France) with CuKα1 radiation source (α = 1.5406 Å). Scanning Electron Microscopy (SEM) measurements were carried out by using a FEI Company model (Mérignac, France), Quanta 200, 10 kV.
Preparation of working electrode
The powder of graphite and zeolite were hand mixed with different proportions (w(LSXZ)/w(G)). Then, the paste was packed vigorously into the cavity (2 mm, Ф = 3 mm) of cylindrical Teflon-PTFE tube electrode and electrical contact was established with a copper rod. The resultant electrode is hereby denoted as zeolite X modified carbon paste electrode (ZXCPE). The unmodified electrode (carbon paste-CPE) was prepared with similar way without adding zeolite. The electrodes were renewed by simple extrusion of a small quantity of the paste from the electrode surface.
Procedure
Standard solution of carbaryl was daily prepared in acetonitrile. Aliquots of this solution were mixed with 0.5 mol L−1 sodium hydroxide solution in a 20 mL volumetric flask to hydrolyze the pesticide. The supporting electrolyte solution was added to the mixture for the voltammetric experiments. The solution of carbaryl hydrolyzed derivative was pipetted quantitatively into an electrochemical cell. The electrode was dipped into the electrolyte with a suitable concentration of CBR at open circuit. The studied supporting electrolytes were phosphate buffer, acetate buffer and hydrochloric acid. Voltammetric experiments were performed in electrolyte without agitation at room temperature. Cyclic voltammetry (CV) was used in the range of −0.3 and 0.7 V at scan rate of 50 mV/s. Differential pulse voltammetry (DPV) was carried out between 0.3 and 0.8 V with pulse period of 0.2 s under optimized conditions (pulse amplitude, modulation time and step potential).
Sample preparation
Tomato purchased from local market was cut into small pieces and put into a stainless steel blender to be mixed and homogenized. The electrolyte was added and the mixture was stirred using magnetic stirrer. The sample was vigorously shaken by ultrasonication for 1 h. Afterward, the sample was centrifuged and then the supernatant was collected. The analysis of carbaryl was carried out using the standard addition method. The obtained results were taken from an average of three parallel experiments.
Results and discussion
Characterization of the electrode
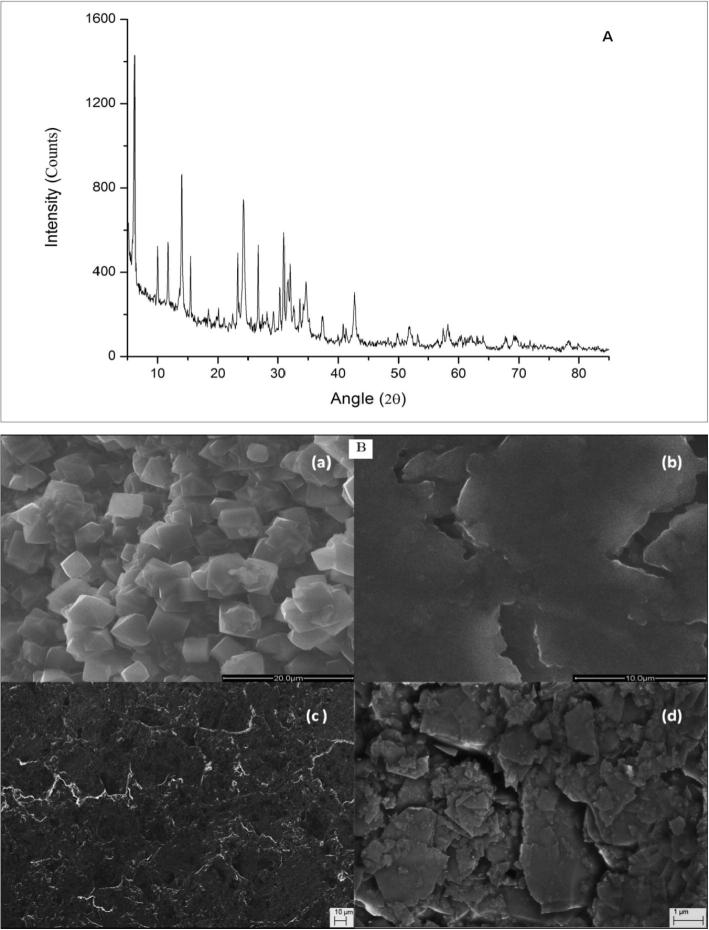
The synthesized LSX zeolite was analyzed by XRD analyses. The XRD pattern, in the 2θ range of 5–85 shown in Fig. 1A, presents intense diffractions peaks at 2θ-values equal to 6.12°, 13.96°, 24.31°, 26.69° and 30.97° which corresponds to the characteristic peaks of zeolite X [37], [38]. This is in agreement with the standard spectra of zeolite X (JCPDS No. 01-089-8235) and since the ratio Si/Al used in this study is lower than 1.1, the obtained results confirm that the synthesis process produced successfully LSX zeolite [39]. The scanning electron microscopy (SEM) micrograph of crystalline phase is a useful technique that can identify the morphology and size of resulted crystals. The morphological structure of the Low Silica X zeolite, the ZXCPE and CPE was investigated by SEM. As can be seen from Fig. 1B, (a) the micrograph image of LSX zeolite demonstrated grains with octahedral morphology and very smooth surface. The particle size distribution of synthesized zeolite was displayed in Fig. S1. The average size of grains was about 3 µm. These results were in accordance with the results obtained in the work of Hui et al. [39]. The specific surface area of LSX zeolite was estimated about 830 m2g−1 [40]. CPE was characterized with a compact surface (b). The resulting ZXCPE (c and d) exhibited very different morphology compared to CPE, indicating the effect of zeolite incorporation even at low percentage.
Fig. 1.
(A) X-ray diffraction patterns of Low Silica X Zeolite (LSXZ), (B) SEM images for (a) low silica X zeolite, (b) CPE and (c, d) ZXCPE.
Electrochemical characterization of ZXCPE
Cyclic voltammetry (CV) was used to investigate the electrochemical behavior of the ZXCPE in potassium hexacyanoferrate(III)/(II)" solution as redox probes. Fig. 2A represents the responses obtained by cyclic voltammetry between −0.2 and +0.7 V (vs. SCE) at CPE, and ZXCPE recorded in 0.1 M KCl solution containing 1 mM [Fe(CN)6]3−/4− (1:1) at 50 mV/s. The ratio between anodic and cathodic peaks was about 1 both for ZXCPE and CPE demonstrating the reversibility of the system. At CPE (curve b), a couple of defined oxidation and reduction peaks were observed with peak currents Ipa = 18 µA and Ipc = −19 µA. When the electrode was modified with zeolite (curve a), a slight decrease in (Ep) and an evident increase in (Ip) were observed (Ipa = 26 µA et Ipc = −26 µA) which implies that the electron transfer rate at ZXCPE was improved. The CV scans are recorded on the ZXCPE surface at different scan rates. The process on the surface of ZXCPE is investigated in the same solution of [Fe(CN)6]3−/4− in the range of 25–300 mV s−1 and is depicted in Fig. 2B. As shown in the figure, significant increment in peak current was obtained with rising the scan rate. The plot of the peak current versus the square root of the scan rate indicates a linear relationship expressed by the regression equation below:
Fig. 2.
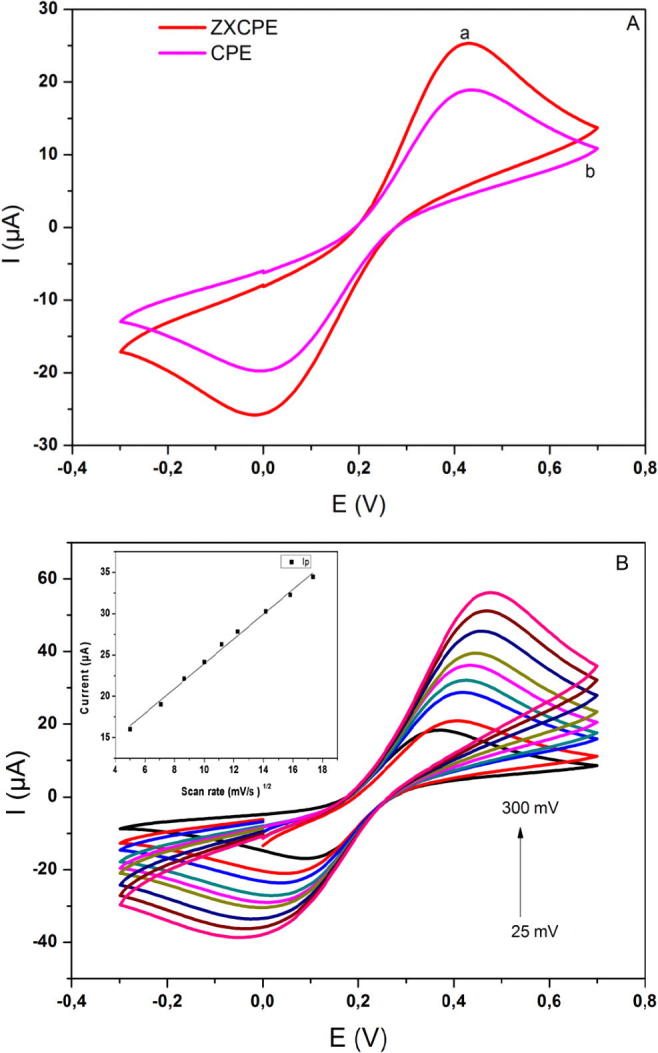
(A) Cyclic voltammograms of unmodified and modified electrodes in 1.0 mM [Fe(CN)6]3−/4−/0.1 M KCl at 50 mV s− 1; ZXCPE (a) and CPE (b), (B) Cyclic voltammograms of the ZXCPE electrode in 1.0 mM [Fe(CN)6]3−/4−/0.1 M KCl at scan rates 25–300 mV/s. Insert presents linearity curve at scan rates 25–300 mV/s.
It suggests that the reaction on the surface of electrode is approximately reversible and also involve that the phenomenon in the electrode double-layer is diffusion controlled [30], [41].
The effective surface area for ZXCPE and CPE were determined using the [Fe(CN)6]3−/4−redox system and applying the Randles–Sevcik equation [42]:
where n is the number of electrons, C is the concentration of [Fe(CN)6]3−/4− (mol L−1), D is the diffusion coefficient of [Fe(CN)6]3− in solution (cm2 s−1), υ is the scan rate (V s−1), and A is the electrode area (cm2). The effective surface areas for ZXCPE and CPE were calculated as 0.067 and 0.026 cm2, respectively. These results demonstrate that the ZXCPE has the largest effective surface area and would be expected to perform better.
Electrochemical impedance spectroscopic characterization of ZXCPE
It is well known that the Electrochemical Impedance Spectroscopy (EIS) is an effective technique for the characterization of the electrochemical process that occurs on the electrode-solution interface. Herein, EIS was employed for further characterization of the modified electrode as well as confirmation of the results found previously by CV. Fig. 3 shows the Nyquist diagrams of carbon paste and zeolite modified carbon paste electrodes, in 1 mM [Fe(CN)6]3−/4− containing 0.1 M KCl. In Nyquist spectra, at higher frequencies the semicircle presents the electron transfer process, whereas the linear part at lower frequencies presents the diffusion process [30], [43]. A large semicircle with an almost straight tail line for bare CPE confirms the high charge transfer resistance occurring at the surface of carbon paste electrode due to the presence of paraffin oil (curve a). ZXCPE displayed smaller semicircle and linear portion suggesting the mixed charge transfer and diffusion kinetics controlled reaction (curve b). By fitting the data using a suitable equivalent circuit, the Rct value of 9.65 kΩ and 0.42 µF for constant phase element were obtained at bare CPE. After the modification of the electrode by zeolite, the charge transfer resistance value (Rct = 4.69 kΩ) decreased with an increase of the constant phase element (0.84 µF) suggesting that low silica X zeolite accelerates the electron transfer between the electrochemical probe redox and the electrode surface. The obtained results are in agreement with the results of cyclic voltammetry.
Fig. 3.
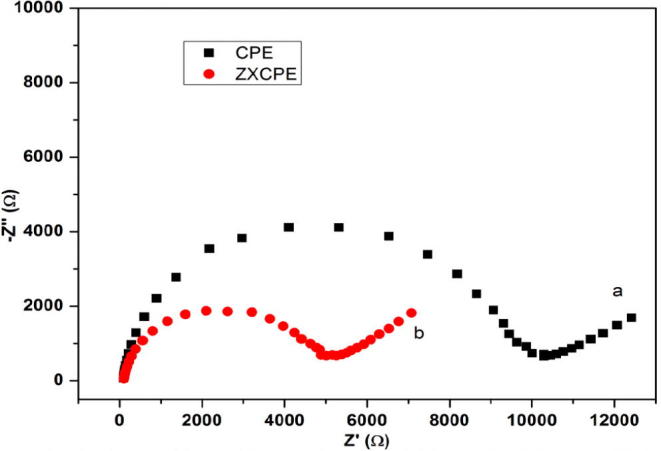
Nyquist plots of impedance spectra at (a) bare CPE, (b) ZXCPE in 1 mM [Fe(CN)6]3−/4− solution containing 0.1 mol L−1 KCl over the frequency range from 50 Hz to 10 kHz and amplitude of 10 mV.
Electrochemical behavior of carbaryl on ZXCPE
In order to characterize the modified electrode and before any analysis, the ZXCPE and CPE were immersed in acetate buffer and tested using CV in the absence of carbaryl. It was observed that a background current obtained at ZXCPE was similar to CPE. Fig. S2 in supplementary demonstrates the cyclic voltammograms in the absence (blank) and presence of 100 µM of carbaryl at ZXCPE and CPE. Both electrodes give sensitive responses in presence of 100 µM of CBR, an irreversible peak appeared at 487 mV and 506 mV vs. SCE on ZXCPE and CPE, respectively, attributed to the oxidation of CBR as mentioned by other authors [22]. Comparing ZXCPE with CPE, the current recorded at ZXCPE was 34% higher than that on CPE under the same conditions. The peak current increased and the oxidation potential shifted negatively leading to electrocatalytic enhancement of carbaryl oxidation on ZXCPE. This result was owed to the fact that the LSX zeolite has large micropores size with a diameter of 7.4 Å defined by twelve membered oxygen rings, which plays an important role in the shape selectivity towards the carbaryl diffusion. Besides, the higher specific surface area, the large external surface area with the considerable amount of Si—OH and Al—OH groups, which are presents on its surface, as well as intercrystalline pores facilitate the analyte diffusion. A possible explanation of the response of ZXCPE toward carbaryl oxidation is that low silica X zeolite has active structure and Si—OH and Al—OH species at the surface that can form hydrogen bonds with the —OH groups of the hydrolyzed derivative of carbaryl and abates the —OH bond energies. The O⋯H—O would transfer the electrons. These clarifications are consistent with those given for —COOH functionalized carbon nanotubes materials and —OH nanocrystalline zeolite Beta [44], [45].
Optimization studies of carbaryl determination
In order to improve the analytical performance of the modified electrode and before conducting the voltammetric detection of carbaryl, some experimental parameters will be optimized.
Effect of supporting electrolyte
The first parameter studied is the supporting electrolyte which is an essential parameter in electroanalytical analysis [46]. The modified electrode by zeolite was investigated in different supporting electrolytes using cyclic voltammetry to select the best medium for the detection of CBR on ZXCP electrode. The cyclic voltammograms (figure not shown) of CBR with ZXCPE in 0.1 M solution of Hydrochloric acid (HCl), potassium chloride (KCl), acetate buffer (ABS), phosphate buffer (PBS) were studied. The best result on current response and shape of the peak of CBR was found in 0.1 M acetate buffer solution. This result is in agreement with those obtained by Moraes et al. [21]. Hence, acetate buffer was selected as the appropriate supporting electrolyte in all following voltammetric experiments.
Effect of zeolite ratio
The proportion of zeolite in the carbon paste was examined by varying the percentage between 1 and 50%. This parameter can affect the voltammetric responses as well as the properties of the sensor. Thus, different amounts of zeolite were used to prepare modified carbon pastes. The current responses of carbaryl increased up to 10% of zeolite. The maximum currents were obtained with 5 and 10% of zeolite with best response at 5% as observed from Fig. 4. Quantities exceeding 10% of zeolite reduced dramatically the current response. This is probably a result of the diminution of conductive area (carbon particles) at the surface of electrode. The increase in peak current at 5% may be due to the presence of optimal quantities of Si-OH and Al-OH groups on the surface of electrode as suggested by Siara et al. [30]. Similar results have demonstrated that the percentage of zeolite affects the charge transport and the ion exchange of the electrode [27]. High content of zeolite cause saturation of the surface of electrode and reduce the oxidation current of carbaryl response [45]. Therefore, an electrode containing 5% of LSX zeolite was chosen for the other experiments of this work.
Fig. 4.
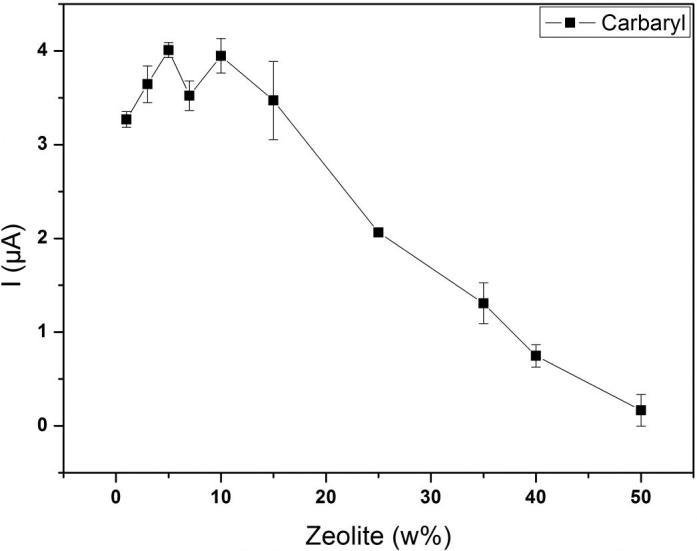
The plot of peak current of carbaryl versus Low Silica X zeolite amounts ratio. Proportions of zeolite: 1, 3, 5, 7, 10, 15, 25, 35, 40, and 50%.
Effect of pH
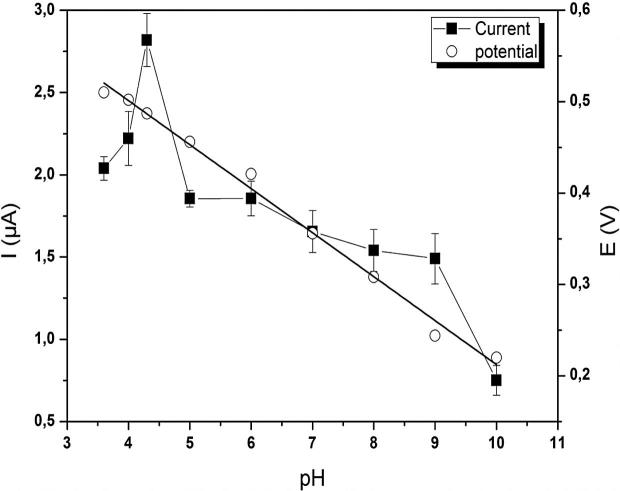
The effect of pH on the carbaryl response at ZXCPE was performed in the range between 3.5 and 10 using 0.1 M acetate buffer containing 100 µM of carbaryl. The peak potential of carbaryl (Ep) shifted to less positive potentials with the increment of pH values as illustrated in Fig. 5. The relationship between Ep and pH is described by the following equation:
Fig. 5.
Effect of pH on the peak potential (○) and peak current (■) for carbaryl oxidation on the ZXCP electrode using buffer supporting electrolyte 0.1 M containing 100 µmol L−1 of carbaryl.
The slope of the equation was 48 mV per pH, which implies that carbaryl oxidation follows the Nernst equation requiring identical number of protons and electrons. In addition, the pH affects the peak current (Ip) as demonstrated in Fig. 5. The peak current decreases for lower and higher pH values, while the best response was observed at pH of 4.3. The obtained results could be explained by the fact that at low pH, the naphthol molecules can be protonated. This will cause the diminution of peak current and affect the oxidation process. In alkaline medium, naphthoxide ion was formed from the hydrolyzed derivative of carbaryl (1-naphtol). In fact, OH− extracts –H from 1-naphthol, then, the electrochemical properties and the voltammetric current responses of the hydrolyzed derivative of carbaryl were influenced. These results are similar to some previous reports in the literature [21], [45]. A value of pH 4.3 was chosen as the optimal pH for the next measurements.
Effect of potential and time preconcentration
Since the response of differential pulse voltammetry is related to the preconcentration potential, so, this parameter was studied in the range from −0.4 to 0.2 V (vs. SCE) at ZXCPE in ABS pH 4.3. The peak current was not changed with the variation of potential (figure not shown) [22]. As a result, an open-circuit was chosen to determinate CBR with ZXCPE [31]. The preconcentration time was investigated in the range from 2 s to 300 s. As can be seen in supplementary Fig. S3, the current responses increased gradually with the increasing of preconcentration time from 2 to 180 s and only had a little change if a longer accumulation time was applied. Therefore, a preconcentration time of 120 s was employed in further studies to decrease the time of analysis.
Effect of differential pulse voltammetry parameters
The response of DPV can be affected by the pulse amplitude, modulation time and step potential. Therefore, these parameters were explored to find the optimum experimental conditions for the detection of carbaryl [47]. The influence of pulse amplitude was examined by changing it between 10 and 100 mV. The best pulse amplitude was 60 mV. The modulation time was also evaluated from 10 to 100 ms. A well defined peak with high current was seen at modulation time of 50 ms. Hence, the modulation time of 50 ms was selected as the optimal value. The step potential was varied from 1 to 10 mV and highest peak current was obtained at 6 mV. After fixing these parameters, the corresponding scan rate was found at 30 mV s−1.
Effect of interferences
The detection of the sensor was examined in the presence of other interfering species on the signals of the carbaryl. It was found that 100-fold of Na+, K+, Mg2+, Ca2+, Al3+, Cl−, CO32−, SO42−, NO3− did not interfere with the carbaryl signal (<10%). By introducing hydroquinone or ascorbic acid, the response of carbaryl was unaffected. However, the presence of some phenols as catechol or bisphenol exerted an influence on the peak current of carbaryl. After the addition of catechol, a small increase was observed at the peak current of carbaryl, it is probably due to the appearance of catechol oxidation peak at the same peak potential of carbaryl. The bisphenol showed an oxidation peak at 0.6 V and slightly affect the peak current of carbaryl.
Calibration curve
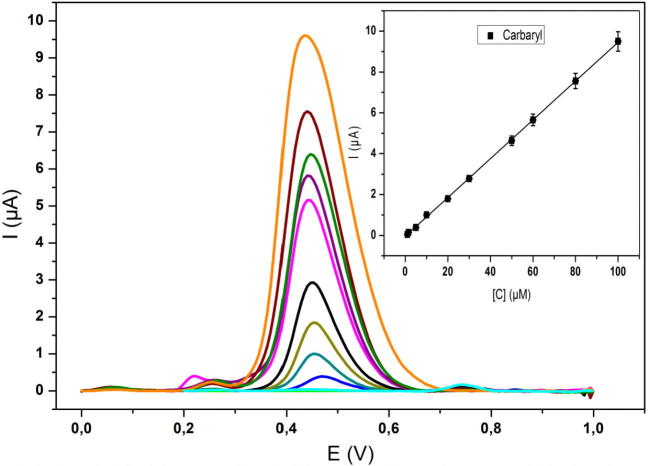
After the optimization of parameters, the DPV was carried out to examine the relationship between current response and concentration of CBR under the optimized parameters. Fig. 6 shows the differential pulse voltammetric curves for various concentrations of CBR at ZXCPE in ABS at pH 4.3. Well-defined peaks, proportional to the concentration of CBR were noted from 1 to 100 μM. The linear regression equation can be expressed as ipa (μA) = 0.09473 C (μM) − 0.04728, with a correlation coefficient of 0.9993. The detection limit was 0.3 μM (S/N = 3). A comparison between electroanalytical results of different methods for CBR determination by means of various techniques was summarized in Table 1. The results exhibited that the ZXCPE has a satisfactory performance, which could lead to practical application. The reproducibility of the approach was investigated through series of 5 prepared electrodes under the same conditions at short time interval with RSD of 3.4%. The RSD value indicated the good reproducibility of the method. The electrode was stored for 3 months, after that the stability was investigated. The oxidation peak current of carbaryl remains to 83% of its initial current. This result revealed the stability of the sensor at long-term.
Fig. 6.
Differential pulse voltammetric curves of CBR oxidation for different concentrations 0, 1, 5, 10, 20, 30, 50, 60, 70, 80, and 100 µM (from bottom to top) at ZXCPE in 0.1 mol L−1 acetate buffer solution pH (4.3). Insert: calibration plot of peak currents on CBR concentrations, under the optimized parameters.
Table 1.
Comparison of the proposed method with published methods for electrochemical determination of CBR.
| Electrodes | Analyte | Linear range (µM) | LOD (µM) | Ref. |
|---|---|---|---|---|
| Carbon Nanotube/Cobalt Phthalocyanine/GCE | CBR | 0.33–6.61 | 0.005 | [21] |
| Cobalt(II)oxide/reduced Graphene Oxide/GCE | CBR (indirect) | 0.5–200 | 0.03 | [22] |
| Graphene oxide-ionic liquid/GCE | CBR | 0.10–12 | 0.02 | [20] |
| Glassy carbon electrode | CBR (indirect) | 0.5–100 | 2 | [48] |
| AChE /cobalt phtalocyanine/ Carbon paste electrode | CBR | 50–750 | 4 | [17] |
| AChE/PANI/MWCNT/GCE | CBR | 9.9–49.6 | 1.4 | [18] |
| Low Silica X zeolite modified carbon paste electrode | CBR (indirect) | 1–100 | 0.3 | This work |
LOD: limit of detection, GCE: glassy carbon electrode, AChE: Acetylcholinesterase, CBR: carbaryl, PANI: Polyaniline, MWCNT: Multi-walled carbon nanotubes.
Analysis of real samples
The developed method was used for the carbaryl analysis in natural sample spiked with the pesticide. To evaluate the accuracy and the applicability of the suggested method, standard addition method and extrapolation in the linear regression were used for analysis of carbaryl in tomato sample. As represented in Table 2, good recovery values from 95 to 104% were resulted for tomato samples. The recoveries confirm that the ZXCPE is a sensitive sensor for analyzing real samples. The results signified that there are no effects of matrix of the tomato sample and also that this method is precise and suitable for detection of CBR.
Table 2.
Determination of Carbaryl in tomato samples with carbaryl amounts added and recovered, recovery percentages, and RSD.
| Original (µM) | Added (µM) | Found (µM) | Recovery (%) | RSDb (%) | |
|---|---|---|---|---|---|
| TOMATO | NDa | 10 | 10.09 | 100.9 | 2.04 |
| ND | 50 | 52.06 | 104.12 | 3.40 | |
| ND | 60 | 57.26 | 95.43 | 5.66 | |
| ND | 100 | 100.65 | 100.65 | 5.53 |
Not detected.
Relative standard deviation.
Conclusions
In summary, a simple and sensitive sensor for the determination of carbaryl based on a zeolite modified electrode was successfully developed and applied to detect carbaryl in real samples. The modified electrode could significantly enhance the response of carbaryl suggesting that the rich surface of Si-OH and Al-OH can improve the response of analyte. The calibration curve of this electrode exhibited linear correlation from 1 to 100 µM with a low limit of detection and offers a good reproducibility and stability. Moreover, the proposed sensor was applied to determinate carbaryl in tomato sample. The main advantages of the sensor are easy preparation, low cost, reduced time consumption and long electrode life-time.
Acknowledgements
This work was supported by MESRSFC (Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et de la Formation des Cadres, Morocco) and CNRST (Centre National pour la Recherche Scientifique et Technique, Morocco) Project No.: PPR/2015/72.
Acknowledgments
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animals subjects.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jare.2017.08.002.
Appendix A. Supplementary material
References
- 1.Teixeira H., Proença P., Alvarenga M., Oliveira M., Marques E.P., Vieira D.N. Pesticide intoxications in the Centre of Portugal: three years analysis. Forensic Sci Int. 2004;143:199–204. doi: 10.1016/j.forsciint.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 2.Van Toan P., Sebesvari Z., Bläsing M., Rosendahl I., Renaud F.G. Pesticide management and their residues in sediments and surface and drinking water in the Mekong Delta, Vietnam. Sci Total Environ. 2013;452–453:28–39. doi: 10.1016/j.scitotenv.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Nollet LML, Rathore HS, editors. Handbook of pesticides: methods of pesticide residues analysis. Boca Raton (FL): CRC Press; 2010.
- 4.Lima M.P.R., Cardoso D.N., Soares A.M.V.M., Loureiro S. Carbaryl toxicity prediction to soil organisms under high and low temperature regimes. Ecotoxicol Environ Saf. 2015;114:263–272. doi: 10.1016/j.ecoenv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Lee D.-Y., Kang H.-W., Kaneko S., Kwon Y.-S., Muramatsu H. Direct monitoring of paraquat induced cell death using quartz crystal sensor. Thin Solid Films. 2009;518:707–710. [Google Scholar]
- 6.Alamgir Zaman Chowdhury M., Fakhruddin ANM., Nazrul Islam M., Moniruzzaman M., Gan SH., Khorshed Alam M. Detection of the residues of nineteen pesticides in fresh vegetable samples using gas chromatography–mass spectrometry. Food Control. 2013;34:457–465. [Google Scholar]
- 7.Çelebi M.S., Oturan N., Zazou H., Hamdani M., Oturan M.A. Electrochemical oxidation of carbaryl on platinum and boron-doped diamond anodes using electro-Fenton technology. Sep Purif Technol. 2015;156:996–1002. [Google Scholar]
- 8.Cheng X., Wang Q., Zhang S., Zhang W., He P., Fang Y. Determination of four kinds of carbamate pesticides by capillary zone electrophoresis with amperometric detection at a polyamide-modified carbon paste electrode. Talanta. 2007;71:1083–1087. doi: 10.1016/j.talanta.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Fan Y., Lai K., Rasco B.A., Huang Y. Determination of carbaryl pesticide in Fuji apples using surface-enhanced Raman spectroscopy coupled with multivariate analysis. LWT - Food Sci Technol. 2015;60:352–357. [Google Scholar]
- 10.García J.V., Rocha M.I., March C., García P., Francis L.A., Montoya A. Love mode surface acoustic wave and high fundamental frequency quartz crystal microbalance immunosensors for the detection of carbaryl pesticide. Proc Eng. 2014;87:759–762. [Google Scholar]
- 11.Xie D.-D., Han R.-Y., Shen J.-C., Xiao C.-G., Zheng Z.-K., Wang Z.-W. Determination of trace carbaryl in water using europium-diallyl phthalate as fluorescent probe. Chin J Anal Chem. 2015;43:1069–1074. [Google Scholar]
- 12.Anandhakumar S., Dhanalakshmi K., Mathiyarasu J. Non-enzymatic organophosphorus pesticide detection using gold atomic cluster modified electrode. Electrochem Commun. 2014;38:15–18. [Google Scholar]
- 13.Guzsvány V., Papp Z., Zbiljić J., Vajdle O., Rodić M. Bismuth modified carbon-based electrodes for the determination of selected neonicotinoid insecticides. Molecules. 2011;16:4451–4466. doi: 10.3390/molecules16064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dyk J.S., Pletschke B. Review on the use of enzymes for the detection of organochlorine, organophosphate and carbamate pesticides in the environment. Chemosphere. 2011;82:291–307. doi: 10.1016/j.chemosphere.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Zeng Y., Yu D., Yu Y., Zhou T., Shi G. Differential pulse voltammetric determination of methyl parathion based on multiwalled carbon nanotubes–poly(acrylamide) nanocomposite film modified electrode. J Hazard Mater. 2012;217–218:315–322. doi: 10.1016/j.jhazmat.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Medany S.S., Ismail K.M., Badawy W.A. Kinetics of the electropolymerization of aminoanthraquinone from aqueous solutions and analytical applications of the polymer film. J Adv Res. 2012;3:261–268. [Google Scholar]
- 17.Caetano J., Machado S.A.S. Determination of carbaryl in tomato “in natura” using an amperometric biosensor based on the inhibition of acetylcholinesterase activity. Sens Actuators B Chem. 2008;129:40–46. [Google Scholar]
- 18.Cesarino I., Moraes F.C., Lanza M.R.V., Machado S.A.S. Electrochemical detection of carbamate pesticides in fruit and vegetables with a biosensor based on acetylcholinesterase immobilised on a composite of polyaniline–carbon nanotubes. Food Chem. 2012;135:873–879. doi: 10.1016/j.foodchem.2012.04.147. [DOI] [PubMed] [Google Scholar]
- 19.Song Y., Chen J., Sun M., Gong C., Shen Y., Song Y. A simple electrochemical biosensor based on AuNPs/MPS/Au electrode sensing layer for monitoring carbamate pesticides in real samples. J Hazard Mater. 2016;304:103–109. doi: 10.1016/j.jhazmat.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 20.Liu B., Xiao B., Cui L. Electrochemical analysis of carbaryl in fruit samples on graphene oxide-ionic liquid composite modified electrode. J Food Compost Anal. 2015;40:14–18. [Google Scholar]
- 21.Moraes F.C., Mascaro L.H., Machado S.A.S., Brett C.M.A. Direct electrochemical determination of carbaryl using a multi-walled carbon nanotube/cobalt phthalocyanine modified electrode. Talanta. 2009;79:1406–1411. doi: 10.1016/j.talanta.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Wang M., Huang J., Wang M., Zhang D., Chen J. Electrochemical nonenzymatic sensor based on CoO decorated reduced graphene oxide for the simultaneous determination of carbofuran and carbaryl in fruits and vegetables. Food Chem. 2014;151:191–197. doi: 10.1016/j.foodchem.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L., Zhao F., Zeng B. Electrochemical determination of carbaryl by using a molecularly imprinted polymer/graphene-ionic liquid-nano Au/chitosan-AuPt alloy nanoparticles composite film modified electrode. Int J Electrochem Sci. 2014;9:1366–1377. [Google Scholar]
- 24.Hatefi-Mehrjardi A. Bienzyme self-assembled monolayer on gold electrode: an amperometric biosensor for carbaryl determination. Electrochim Acta. 2013;114:394–402. [Google Scholar]
- 25.Zhang L., Huang Y. An investigation into the crystallization of low-silica X zeolite. J Porous Mater. 2015;22:843–850. [Google Scholar]
- 26.Walcarius A. Zeolite-modified electrodes in electroanalytical chemistry. Anal Chim Acta. 1999;384:1–16. [Google Scholar]
- 27.Cao L., Jia J., Wang Z. Sensitive determination of Cd and Pb by differential pulse stripping voltammetry with in situ bismuth-modified zeolite doped carbon paste electrodes. Electrochim Acta. 2008;53:2177–2182. [Google Scholar]
- 28.Rolison D.R. Zeolite-modified electrodes and electrode-modified zeolites. Chem Rev. 1990;90:867–878. [Google Scholar]
- 29.Azizi S.N., Ranjbar S., Raoof J.B., Hamidi-Asl E. Preparation of Ag/NaA zeolite modified carbon paste electrode as a DNA biosensor. Sens Actuators B Chem. 2013;181:319–325. [Google Scholar]
- 30.Siara L.R., de Lima F., Cardoso C.A.L., Arruda G.J. Electrochemically pretreated zeolite-modified carbon-paste electrodes for determination of linuron in an agricultural formulation and water. Electrochim Acta. 2015;151:609–618. [Google Scholar]
- 31.Walcarius A., Rozanska S., Bessière J., Wang J. Screen-printed zeolite-modified carbon electrodes. Analyst. 1999;124:1185–1190. [Google Scholar]
- 33.Kanan S.M., Tripp C.P., Austin R.N., Patterson H.H. Photoluminescence and Raman spectroscopy as probes to investigate silver and gold dicyanide clusters doped in A-zeolite and their photoassisted degradation of carbaryl. J Phys Chem B. 2001;105:9441–9448. [Google Scholar]
- 34.Kanan M.C., Kanan S.M., Austin R.N., Patterson H.H. Photodecomposition of carbaryl in the presence of silver-doped zeolite Y and Suwannee River natural organic matter. Environ Sci Technol. 2003;37:2280–2285. doi: 10.1021/es026136+. [DOI] [PubMed] [Google Scholar]
- 35.Salih F.E., Ouarzane A., El Rhazi M. Electrochemical detection of lead (II) at bismuth/poly(1,8-diaminonaphthalene) modified carbon paste electrode. Arab J Chem. 2017;10:596–603. [Google Scholar]
- 36.Oularbi L., Turmine M., El Rhazi M. Electrochemical determination of traces lead ions using a new nanocomposite of polypyrrole/carbon nanofibers. J Solid State Electrochem. 2017;21(11):3289–3300. [Google Scholar]
- 37.Balkus K.J., Ly K.T. The preparation and characterization of an X-type zeolite: an experiment in solid-state chemistry. J Chem Educ. 1991;68:875. [Google Scholar]
- 38.Hashemi H., Nezamzadeh-Ejhieh A., Karimi-Shamsabadi M. A novel cysteine sensor based on modification of carbon paste electrode by Fe(II)-exchanged zeolite X nanoparticles. Mater Sci Eng C Mater Biol Appl. 2016;58:286–293. doi: 10.1016/j.msec.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 39.Hui H., Gao J., Wang G., Liu P., Zhang K. Effects of Na and K ions on the crystallization of low-silica X zeolite and its catalytic performance for alkylation of toluene with methanol. J Braz Chem Soc. 2013 [Google Scholar]
- 40.Romero M.D., Gómez J.M., Ovejero G., Rodríguez A. Synthesis of LSX zeolite by microwave heating. Mater Res Bull. 2004;39:389–400. [Google Scholar]
- 41.Rooney M.B., Coomber D.C., Bond A.M. Achievement of near-reversible behavior for the [Fe(CN)6]3−/4− redox couple using cyclic voltammetry at glassy carbon, gold, and platinum macrodisk electrodes in the absence of added supporting electrolyte. Anal Chem. 2000;72:3486–3491. doi: 10.1021/ac991464m. [DOI] [PubMed] [Google Scholar]
- 42.Švancara I., Kalcher K., Walcarius A., Vytras K. vol. 315. CRC Press and Taylor & Francis Group, LLC; Boca Raton: 2012. (Electroanalysis with Carbon Paste Electrodes). [Google Scholar]
- 43.Samanta S., Srivastava R. Simultaneous determination of epinephrine and paracetamol at copper-cobalt oxide spinel decorated nanocrystalline zeolite modified electrodes. J Colloid Interf Sci. 2016;475:126–135. doi: 10.1016/j.jcis.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 44.Ding Y.-P., Liu W.-L., Wu Q.-S., Wang X.-G. Direct simultaneous determination of dihydroxybenzene isomers at C-nanotube-modified electrodes by derivative voltammetry. J Electroanal Chem. 2005;575:275–280. [Google Scholar]
- 45.Kaur B., Srivastava R. Selective, nanomolar electrochemical determination of environmental contaminants dihydroxybenzene isomers found in water bodies using nanocrystalline zeolite modified carbon paste electrodes. Electroanalysis. 2014;26:1739–1750. [Google Scholar]
- 46.Wei H., Sun J.-J., Wang Y.-M., Li X., Chen G.-N. Rapid hydrolysis and electrochemical detection of trace carbofuran at a disposable heated screen-printed carbon electrode. Analyst. 2008;133:1619-1624. doi: 10.1039/b806750c. [DOI] [PubMed] [Google Scholar]
- 47.de Figueiredo-Filho L.C.S., dos Santos V.B., Janegitz B.C., Guerreiro T.B., Fatibello-Filho O., Faria R.C. Differential pulse voltammetric determination of paraquat using a bismuth-film electrode. Electroanalysis. 2010;22:1260–1266. [Google Scholar]
- 48.Guiberteau A., Diaz T.G., Salinas F., Ortiz J.M. Indirect voltammetric determination of carbaryl and carbofuran using partial least squares calibration. Anal Chim Acta. 1995;305:219–226. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.