Abstract
Background
Medical management of acute pain among hospital inpatients may be enhanced by mind-body interventions.
Objective
We hypothesized that a single, scripted session of mindfulness training focused on acceptance of pain or hypnotic suggestion focused on changing pain sensations through imagery would significantly reduce acute pain intensity and unpleasantness compared to a psychoeducation pain coping control. We also hypothesized that mindfulness and suggestion would produce significant improvements in secondary outcomes including relaxation, pleasant body sensations, anxiety, and desire for opioids, compared to the control condition.
Methods
This three-arm, parallel-group randomized controlled trial conducted at a university-based hospital examined the acute effects of 15-min psychosocial interventions (mindfulness, hypnotic suggestion, psychoeducation) on adult inpatients reporting “intolerable pain” or “inadequate pain control.” Participants (N = 244) were assigned to one of three intervention conditions: mindfulness (n = 86), suggestion (n = 73), or psychoeducation (n = 85).
Key Results
Participants in the mind-body interventions reported significantly lower baseline-adjusted pain intensity post-intervention than those assigned to psychoeducation (p < 0.001, percentage pain reduction: mindfulness = 23%, suggestion = 29%, education = 9%), and lower baseline-adjusted pain unpleasantness (p < 0.001). Intervention conditions differed significantly with regard to relaxation (p < 0.001), pleasurable body sensations (p = 0.001), and desire for opioids (p = 0.015), but all three interventions were associated with a significant reduction in anxiety (p < 0.001).
Conclusions
Brief, single-session mind-body interventions delivered by hospital social workers led to clinically significant improvements in pain and related outcomes, suggesting that such interventions may be useful adjuncts to medical pain management.
Trial registration
Trial Registry: ClinicalTrials.gov; registration ID number: NCT02590029
KEY WORDS: acute pain, analgesia, hypnosis, mindfulness, non-opioid therapy, opioid
INTRODUCTION
Half of all inpatients experience acute pain during hospitalization.1 , 2 Effective hospital-based pain management is compromised by a number of barriers, including patient perceived helplessness, lack of education and non-pharmacological interventions, and significant time delay in analgesic administration.3 Poorly managed acute pain can lead to increased hospitalization costs,3 reduced patient satisfaction,4 and risk of persistent pain.5 For alleviation of pain, more than half of patients in the U.S. are treated with opioids during hospitalization,6 and 2–14% of these patients experience at least one opioid-related adverse event.7 Further, opioid receipt in the hospital is associated with a nearly fivefold increase in the odds of chronic opioid use 12 months after discharge.8
As opioid-related deaths and opioid addiction continue to climb, national efforts to stem the opioid epidemic underscore the need for non-pharmacological interventions for pain management. Psychological factors including maladaptive cognitions (e.g., catastrophizing, fear of pain) and negative emotions can exacerbate pain perception via cortico-limbic brain circuitry,9 – 11 providing a mechanistic basis for the potential efficacy of psychosocial interventions for acute pain. Although logistical constraints pose a challenge to the provision of integrated inpatient medical and psychological services, social workers may provide psychosocial support to allay pain and distress among inpatients. As such, the present study examined the acute impact of three brief psychosocial interventions provided by hospital social workers: mindfulness training, hypnotic suggestion, and psychoeducation.
Mindfulness consists in cultivating metacognitive awareness and acceptance of thoughts, feelings, and sensations.12 Randomized trials indicate that mindfulness alleviates pain by facilitating a shift from affective to sensory processing of pain sensations13 and reducing thalamic amplification of nociceptive signals via prefrontal cognitive control mechanisms.14 Brief mindfulness interventions have been reported to reduce experimentally-induced pain in the laboratory,15 , 16 but have not been studied for acute clinical pain in hospital settings. By contrast, suggestive interventions have been shown to reduce pain and distress in the hospital.17 These interventions consist in providing suggestions to modify thoughts, emotions, and sensations. Meta-analysis indicates that suggestive interventions, including hypnosis and suggestion without hypnotic induction, result in significant, small effect size reductions in postoperative pain and anxiety.18 Psychoeducational interventions have also been shown to improve coping in hospital settings.19 No study to date has examined the efficacy of mindfulness and hypnotic suggestion compared to psychoeducation for hospital inpatients with intolerable pain. Moreover, it is not known whether a single session of mindfulness or hypnosis is of sufficient dose to outperform an active control in reducing acute clinical pain and secondary outcomes such as desire for opioids and anxiety. The present study addresses these knowledge gaps.
We hypothesized that a single session of mindfulness or hypnotic suggestion would significantly reduce acute pain intensity and unpleasantness compared to a psychoeducation control condition. We also hypothesized that mindfulness and suggestion would produce significant increases in relaxation and pleasant body sensations, and reduce anxiety and desire for opioids, compared to the control condition.
METHODS
Trial Design and Randomization
This was a single-site, three-arm, parallel-group randomized controlled trial (RCT). The randomization sequence was generated by computer before the start of the trial via simple random allocation to the study conditions. The principal investigator (E.G.), who directed the randomization, was provided with random numbers to assign to a condition, and he never interacted with participants before or after assignment to a condition. Individuals were the unit of randomization. The University of Utah institutional review board approved all procedures. Participants provided informed consent after reviewing an informed consent cover letter with study personnel.
Setting and Participants
The study was conducted in Salt Lake City from October 2015 through October 2016. The hospital where the study took place had historically performed below the national average in patient ratings of their acute pain management when compared to other academic medical centers, prompting providers at this institution to seek new non-opioid options for addressing acute pain. English-speaking adult inpatients (≥18 years) at a public hospital reporting “intolerable pain” or “inadequate pain control” (on the Clinically Aligned Pain Assessment tool,20 a clinical assessment of pain employed at this hospital) were included in this trial. Patients with altered mental status due to delirium, psychosis, or pharmacological sedation as determined by nursing assessment were excluded. Potential participants were screened for eligibility via medical record review and asked by clinical social workers whether they were interested in receiving psychosocial pain management services as part of a research evaluation.
Procedures
Consenting participants (who were pre-randomized to intervention condition via simple random allocation) then privately completed a brief self-report assessment of patient-reported outcomes (PROs), consisting of validated numeric rating scales (0–10) of pain intensity, pain unpleasantness, relaxation, pleasurable body sensations, and desire for opioids. Following this PRO assessment, participants received one of three 15-min psychosocial interventions delivered by a clinical social worker, after which they privately completed the same self-report assessment to assess patient-reported outcomes during the period immediately following the intervention. Social workers and participants were not blinded. Participants were not compensated for their participation.
Interventions
Mindfulness Training
The mindfulness intervention consisted in a single, scripted 15-min training session in focused attention on breathing and body sensations, with concomitant metacognitive monitoring and acceptance of discursive thoughts, negative emotions, and pain. This mindfulness script (see supplementary materials) closely followed a mindfulness induction script validated in prior mindfulness research.21
Hypnotic Suggestion
The suggestion intervention consisted in a single, scripted 15-min self-hypnosis session which invited patients to roll their eyes upward, close their eyes, and breathe deeply, focus on sensations of floating, and imagine the visual, auditory, olfactory, and tactile details of a pleasant scene of their choosing. The script provided suggestions for transforming pain into sensations of warmth, coolness, or tingling. This script closely followed a standardized self-hypnotic induction script (see supplementary materials) validated in prior research on self-hypnosis during acute medical procedures.22 The mindfulness and hypnotic suggestion scripts were exactly equal in number of words.
Psychoeducation
The psychoeducation condition consisted in a single 15-min session in which a social worker provided empathic responses to the patient and then attempted to increase perception of pain control by reviewing common behavioral pain coping strategies (e.g., stretching, using hot and cold compresses). The psychoeducation condition followed a pain coping education brochure utilized throughout the university hospital.
Study interventions were implemented by clinical social workers, each of whom was trained to deliver all three interventions throughout the study. Training comprised 3 h of instruction in empathic responses, mindfulness, and hypnotic suggestion under the supervision of the first author, a clinician with more than 10 years of experience in providing mind-body interventions for patients with pain in medical settings.
Outcome and Assessments
Self-assessments were administered immediately before and after the intervention, which comprised a 15-min interval. Primary outcomes of pain intensity and unpleasantness were measured with two items rated on a numeric rating scale (0–10)—a widely used and validated approach to measuring clinical pain.23 Participants who reported ≥30% reduction in pain intensity were identified as “responders” based on a validated threshold for “moderate clinical benefit” identified in best practice guidelines for pain research.24 Secondary outcomes were measured via validated single items assessing relaxation,25 anxiety,22 pleasant body sensations,26 and desire for opioids,27 all rated on a numeric rating scale (0–10) to minimize patient confusion. There was no follow-up measure of potential impacts of the brief interventions, because we reasoned that no residual benefit of a single intervention session could be expected multiple hours, days, or weeks later.
Power Calculation
A priori power analysis was conducted in G*Power. An estimated total sample size of 244 was needed to detect an overall between-group effect on baseline-adjusted post-treatment pain (f = 0.20, or of small size) with 80% power, two-sided p < 0.05.28
Statistical Analysis
Hypothesis testing was conducted via SPSS version 23 software (IBM Corp., Armonk, NY) using an analysis of covariance (ANCOVA) strategy29 for primary (i.e., pain intensity and pain unpleasantness) and secondary outcomes (anxiety, relaxation, pleasant body sensations, desire for opioids), adjusted for baseline differences. In accordance with a classical ANCOVA approach for analyzing clinical trial outcomes,29 co-varying of baseline values ensures that comparisons of post-intervention values by treatment group are independent of baseline differences. In ANCOVA models, post-intervention values of outcome variables were regressed on intervention group (mindfulness or suggestion vs. education control) after co-varying pre-intervention values. We used linear mixed models with random intercepts under maximum likelihood estimation for intention-to-treat (ITT) analyses of the entire randomized sample. Because opioids might influence pain outcomes, we also controlled for morphine equivalent daily dose (calculated via the Washington State Agency Medical Directors’ Group opioid dose calculator) in the 24-h period prior to intervention as a covariate in a sensitivity analysis. Because significant between-group differences in gender were observed (see Table 1), we controlled for participant gender as a covariate in a sensitivity analysis. Though this study was not powered to be a comparative effectiveness or non-inferiority trial with respect to the two mind-body interventions (mindfulness vs. hypnotic suggestion), we conducted exploratory post hoc contrasts to test for between-group differences. Multiple comparisons were adjusted using the Bonferroni-Holm method.
Table 1.
Baseline Demographic and Clinical Characteristics by Intervention Group and Total Sample
| Variable | Total Sample (N = 244) | Suggestion (n = 73) | Mindfulness (n = 86) | Education (n = 85) |
|---|---|---|---|---|
| Age, mean (SD), years | 51.1 (16.6) | 50.6 (16.5) | 51.4 (15.7) | 51.2 (17.9) |
| Female sex, no. (%) | 140 (57.4) | 30 (41.1) | 57 (66.3) | 53 (62.4) |
| Race/ethnicity, no. (%) | ||||
| White | 230 (94.3) | 70 (95.9) | 79 (91.9) | 81 (95.3) |
| Hispanic/Latino | 6 (2.5) | 2 (2.7) | 2 (2.3) | 2 (2.4) |
| Unknown | 8 (3.3) | 1 (1.4) | 5 (5.8) | 2 (2.4) |
| Pain intensity, mean (SD) | 5.5 (2.7) | 5.7 (2.6) | 5.7 (2.7) | 5.0 (2.7) |
| Morphine equivalent opioid dose in past 24 h, mean (SD) | 93.3 (209.8) | 115.6 (299.3) | 70.9 (123.2) | 96.7 (185.3) |
| ICD-10 diagnostic category, no. (%) | ||||
| Injury, poisoning, and certain other consequences of external causes | 44 (18.0) | 16 (21.9) | 18 (20.9) | 10 (11.8) |
| Diseases of the digestive system | 32 (13.1) | 9 (12.3) | 8 (9.3) | 15 (17.6) |
| Diseases of the musculoskeletal system and connective tissue | 29 (11.9) | 10 (13.7) | 8 (9.3) | 11 (12.9) |
| Symptoms, signs, and abnormal clinic and laboratory findings, not classified elsewhere | 23 (9.4) | 6 (8.2) | 13 (15.1) | 4 (4.7) |
| Diseases of the circulatory system | 19 (7.8) | 4 (5.5) | 6 (7.0) | 9 (10.6) |
| Certain infectious and parasitic diseases | 18 (7.4) | 4 (5.5) | 5 (5.8) | 9 (10.6) |
| Diseases of the genitourinary system | 14 (5.7) | 6 (8.2) | 4 (4.7) | 4 (4.7) |
| Diseases of the skin and subcutaneous tissue | 13 (5.3) | 7 (9.6) | 4 (4.7) | 2 (2.4) |
| Diseases of the respiratory system | 11 (4.5) | 2 (2.7) | 3 (3.5) | 6 (7.1) |
| Factors influencing health status and contact with health services | 11 (4.5) | 2 (2.7) | 6 (7.0) | 3 (3.5) |
| Diseases of the nervous system | 7 (2.9) | 2 (2.7) | 4 (4.7) | 1 (1.2) |
| Endocrine, nutritional, and metabolic diseases | 7 (2.9) | 2 (2.7) | 2 (2.3) | 3 (3.5) |
| Pregnancy, childbirth, and the puerperium | 6 (2.5) | 1 (1.4) | 1 (1.2) | 4 (4.7) |
| Other | 5 (2.0) | 1 (1.4) | 2 (2.3) | 2 (2.4) |
| Unknown | 5 (2.0) | 1 (1.4) | 2 (2.3) | 2 (2.4) |
| Hospital unit, no. (%) | ||||
| Internal medicine | 104 (42.6) | 27 (36.9) | 40 (46.5) | 37 (43.5) |
| Orthopedic trauma | 37 (15.2) | 14 (19.2) | 10 (11.6) | 13 (15.3) |
| Cardiovascular medicine | 28 (11.5) | 6 (8.2) | 8 (9.3) | 14 (16.5) |
| Neurological acute care | 27 (11.1) | 9 (12.3) | 11 (12.8) | 7 (8.2) |
| Surgical specialty and transplants | 18 (7.4) | 8 (11.0) | 4 (4.7) | 6 (7.1) |
| Burn center | 15 (6.1) | 6 (8.2) | 6 (7.0) | 3 (3.5) |
| Obstetrics | 8 (3.3) | 2 (2.7) | 3 (3.5) | 3 (3.5) |
| Other | 7 (2.9) | 1 (1.4) | 4 (4.7) | 2 (2.4) |
RESULTS
Participant Flow and Characteristics
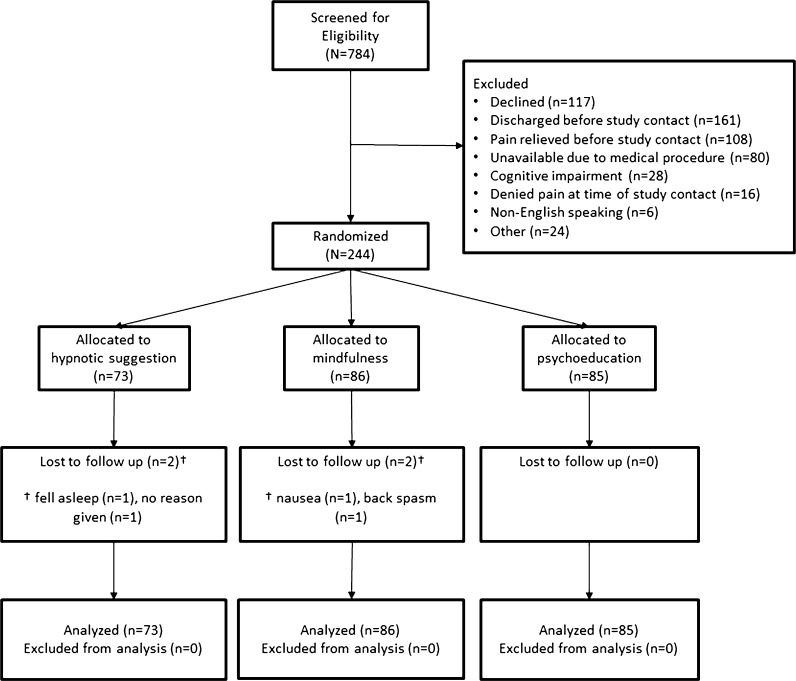
Participant flow through enrollment, randomization, and analysis phases of the trial is shown in Figure 1. Of 784 potential patients screened for eligibility, over the course of 1 year, 244 individuals were deemed eligible, agreed to participate, and were assigned to one of the intervention conditions: mindfulness (n = 86), suggestion (n = 73), or psychoeducation (n = 85). Differences in random allocation were purely due to random chance. Among the most common reasons for exclusion were patients who declined to participate (n = 117) or were discharged (n = 161), those whose pain was relieved (n = 108), or those who were unavailable due to medical procedures (n = 80) at the time of study contact. No adverse events related to participation in the study were reported.
Figure 1.
CONSORT Flow Diagram of the Randomized Clinical Trial of Mindfulness, Suggestion, and Education for Acute Inpatient Pain. *Reasons for exclusion were as follows: patient had a cognitive impairment, was discharged before study assessment and intervention could be initiated, was unavailable or uncooperative, was under age 18, had a language barrier, was a prisoner, denied experiencing pain at the time of the intervention, was intubated, or had a hearing impairment.
Table 1 lists the summary descriptive statistics for the groups at baseline. None of the baseline variables differed across intervention conditions by chance (p > 0.05), except for gender (p = 0.003). By chance, fewer female participants were assigned to the suggestion condition (41%) than the mindfulness (66%) or education (62%) conditions. The mean (SD) age of participants was 51.1 (SD = 16.6 years, range 18–93 years), and 57% (140 of 244) were female. The mean pre-intervention (SD) pain intensity rating of 5.5 (2.7) indicated a sample with moderate or greater pain level. By chance, participants in the psychoeducation condition had lower pre-intervention pain scores than those randomized to the other conditions, though this difference was not statistically significant, F(2,237) = 1.98, p = 0.14. Examination of International Classification of Diseases (ICD)-10 diagnostic category indicated that at the time of enrollment, the majority of study participants were receiving medical treatment for injuries (18%), followed by diseases of the digestive system (13%), musculoskeletal system (12%), and circulatory system (8%), and infectious diseases (7%), among others.
Four participants did not complete the intervention and were missing post-intervention data (see Fig. 1 for CONSORT diagram). Those lost to post-intervention assessment showed pain ratings statistically equivalent to those of the retained sample, indicating that the level of pain was independent of dropout. Given the few missing data points (< 2% missing for any outcome variable), we conducted ANCOVA for analyses of the treated sample (N = 240), and used linear mixed models for intention-to-treat (ITT) analyses of the entire randomized sample (N = 244). Within-group effects are reported in Table 2.
Table 2.
Primary and Secondary Outcomes as a Function of Intervention Condition and Time
| Suggestion (N = 71) |
Mindfulness (N = 84) |
Education (N = 85) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Pre | Post | t | ES | Pre | Post | t | ES | Pre | Post | t | ES |
| Pain intensity | 5.69 (2.59) | 4.38 (2.90) | 6.71*** | 0.48 | 5.74 (2.73) | 4.56 (2.79) | 6.48*** | 0.43 | 5.00 (2.67) | 4.67 (2.84) | 2.67* | 0.12 |
| Pain unpleasantness | 5.87 (2.83) | 4.42 (3.07) | 6.21*** | 0.49 | 5.77 (3.11) | 4.06 (2.83) | 7.29*** | 0.58 | 4.98 (2.87) | 4.72 (3.05) | 1.87** | 0.09 |
| Anxiety | 4.85 (3.17) | 3.08 (2.97) | 5.33*** | 1.27 | 3.93 (3.37) | 2.74 (2.90) | 4.46*** | 0.98 | 3.74 (3.08) | 3.09 (2.91) | 2.36* | 0.51 |
| Relaxation | 4.58 (2.85) | 6.51 (2.48) | 6.13*** | 0.72 | 5.11 (3.04) | 6.80 (2.67) | 5.40*** | 0.59 | 5.40 (2.89) | 5.69 (2.82) | −1.35 | 0.10 |
| Pleasant sensations | 2.85 (2.88) | 5.00 (3.14) | 7.03*** | 0.71 | 2.89 (2.81) | 4.65 (3.15) | 5.07*** | 0.59 | 3.59 (2.95) | 4.01 (2.98) | −1.62 | 0.14 |
| Desire for opioids | 4.39 (3.52) | 3.07 (3.17) | 4.04*** | 0.40 | 4.15 (3.86) | 3.49 (3.79) | 2.44* | 0.17 | 3.71 (3.52) | 3.60 (3.71) | 0.52 | 0.03 |
Data are given as mean (SE). ES = within-group effect size (Cohen’s d)
* p < 0.05, ** p ≤ 0.01, *** p ≤ 0.001
Primary Outcome
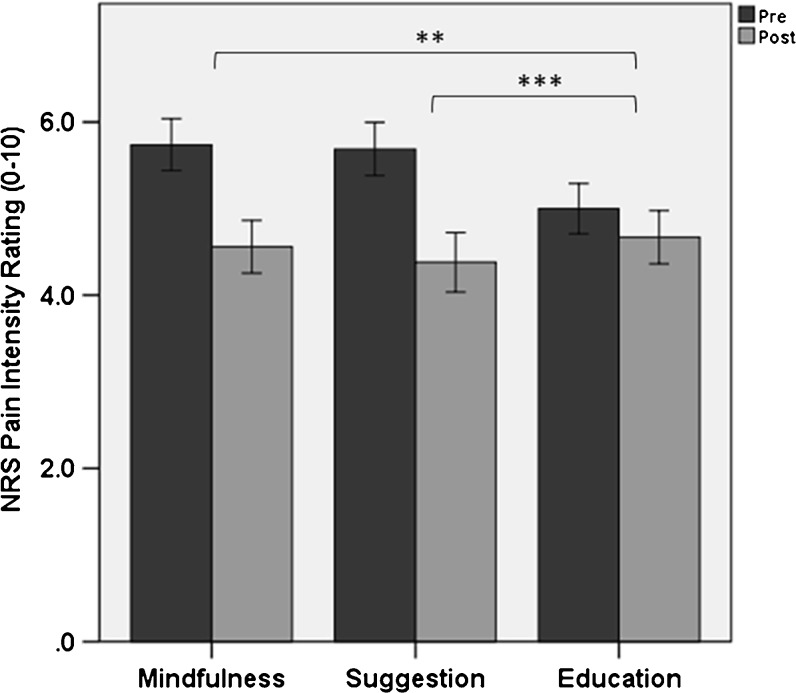
The ANCOVA model for pain severity revealed a statistically significant effect of intervention condition (p < 0.001, η2 partial = 0.07), with participants assigned to mindfulness (B = −0.78, p = 0.001, 95% CI: −1.23, −0.33) and suggestion (B = −0.91, p < 0.001, 95% CI: −1.38, −0.45) reporting significantly lower baseline-adjusted pain severity post-intervention than those assigned to the education condition (see Fig. 2). We observed significant mean reductions in pain intensity ratings during mindfulness meditation (23%; p < 0.001, d = 0.43), suggestion (29%; p < 0.001, d = 0.48), and education conditions (9%; p = 0.009, d = 0.12). Intervention conditions significantly differed in the proportion of “responders” who achieved at least a clinically significant 30% reduction in pain intensity, χ2 = 11.56, p = 0.003, with 27%, 39%, and 15% of responders in the mindfulness, suggestion, and education conditions, respectively. Though the mindfulness and suggestion groups did not significantly differ in morphine equivalent dose in the 24 h prior to intervention, the presence of sizable mean differences in opioid dosing suggested that pre-intervention differences in this variable should be controlled in a sensitivity analysis along with participant gender. In this sensitivity analysis, intervention condition remained a significant predictor of baseline-adjusted pain severity (p < 0.001, η2 partial = 0.11).
Figure 2.
Numeric rating scale (NRS) pain intensity ratings (± 95% confidence intervals). Participants assigned to mindfulness (p = 0.001) and hypnotic suggestion (p < 0.001) reported significantly lower baseline-adjusted pain intensity ratings post-intervention compared with a psychoeducation control condition. Within-subject pain intensity reduction: * p < 0.05, ** p < 0.01, *** p < 0.001.
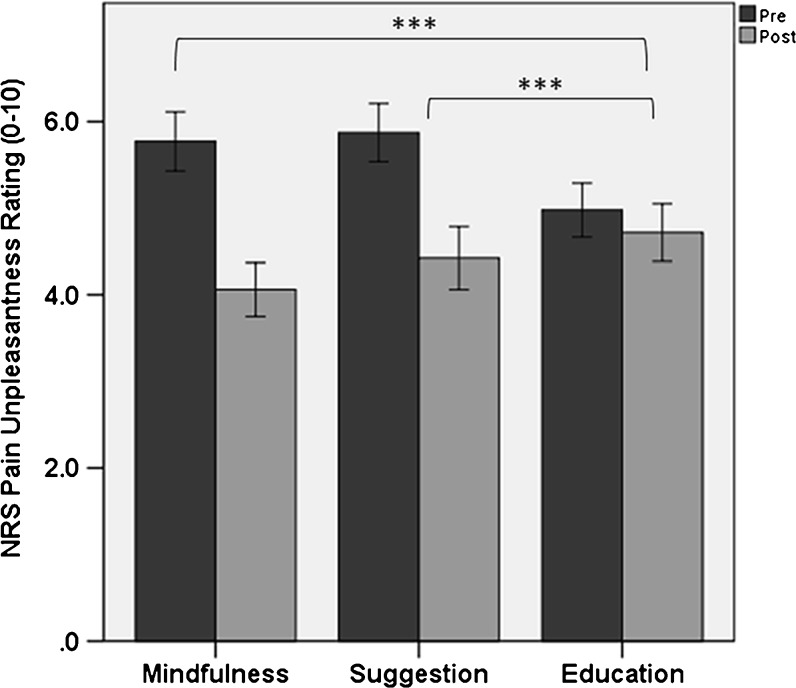
The ANCOVA model for pain unpleasantness revealed a statistically significant effect of intervention condition (p < 0.001, η2 partial = 0.10), with participants assigned to mindfulness (B = −1.31, p < 0.001, 95% CI: −1.84, −0.78) and suggestion (B = −1.03, p < 0.001, 95% CI: −1.59, −0.47) reporting significantly lower baseline-adjusted pain unpleasantness post-intervention than those assigned to education (see Fig. 3).
Figure 3.
Numeric rating scale (NRS) pain unpleasantness ratings (± 95% confidence intervals). Participants assigned to mindfulness (p < 0.001) and hypnotic suggestion (p < 0.001) reported significantly lower baseline-adjusted pain unpleasantness ratings post-intervention compared with a psychoeducation control condition. Within-subject pain unpleasantness reduction: * p < 0.05, ** p < 0.01, *** p < 0.001.
ITT analysis through linear mixed modeling including all randomized participants identified significant effects of intervention condition on pain intensity (p < 0.001) and pain unpleasantness (p < 0.001).
Secondary Outcomes
The ANCOVA models for relaxation (p < 0.001, η2 partial = 0.08), pleasant body sensations (p = 0.001, η2 partial = 0.06), and desire for opioids (p = 0.015, η2 partial = 0.04) revealed statistically significant effects of intervention condition. Post hoc contrasts revealed that participants assigned to mindfulness and suggestion reported significantly higher baseline-adjusted relaxation (p < 0.001) and pleasurable body sensations (p < 0.001) than those assigned to the education condition. In contrast, participants in the suggestion condition reported significantly lower desire for opioids than those in the education condition (p = 0.004), whereas participants assigned to mindfulness did not (p = 0.19). There was no significant between-group difference in anxiety, (p = 0.15, η2 partial = 0.02), though participants across all three intervention conditions reported a significant decrease in anxiety (p < 0.001).
DISCUSSION
Findings indicate that a single, scripted 15-min session of mindfulness training or hypnotic suggestion delivered in the hospital setting can result in immediate reductions in acute pain intensity. Though the mean pain reduction was modest in size, approximately one-third of patients treated with mind-body interventions achieved at least a 30% reduction in pain intensity—a clinically significant level of pain relief comparable to 5 mg oxycodone.30 Mind-body interventions produced ancillary benefits, including improvements in pain unpleasantness and relaxation. Though all three interventions reduced anxiety, the palliative effects of mind-body therapies exceeded those derived from psychoeducation.
Hypothetically, brief mindfulness training and hypnotic suggestion produce therapeutic benefits via unique and overlapping mechanisms of action. Whereas mindfulness is a self-regulatory process that purportedly relieves pain by fostering non-reactive acceptance of aversive somatovisceral sensations and attendant negative emotions, hypnotic suggestion is usually delivered by a facilitator and employs visual, kinesthetic, and interoceptive imagery to alter the pain sensations themselves. These differences in psychological mechanism may parallel neuroimaging findings demonstrating that mindfulness alleviates acute pain intensity by increasing activation in the cingulate and insular cortices, whereas both mindfulness and hypnosis alleviate pain intensity by decreasing activation of somatosensory cortical representations of pain.15 , 31 More mechanistic research is needed to elucidate commonalities and differences between these two types of mind-body therapy for relief of acute clinical pain.
The study had some limitations. First, without follow-up data, the duration of the observed therapeutic effects is unknown, although it is unlikely that a brief single-session intervention would result in long-lasting pain relief. Additional research is needed to determine whether effects can be prolonged or intensified with larger or repeated doses. Second, the suggestion and mindfulness interventions contained some overlapping instructions for focused attention and monitoring of body sensations, including a similar introduction that framed both interventions as a form of “concentration”; this overlap was intended to engender similar levels of perceived credibility between the two experimental conditions. Overlap between these two mind-body interventions is practically inevitable, given that both techniques involve attention regulation.32 The mind-body interventions in this study differed in their emphasis on acceptance of versus change in pain sensations.
Third, the psychoeducation condition in the present study controlled for non-specific therapeutic factors (e.g., attention from a caring professional), but did not completely control for the possibility that the observed effects of mind-body interventions might be due to placebo or suggestibility. Psychoeducation was associated with significant reductions in pain intensity that were comparable to those achieved by placebo as reported in a systematic review of opioid analgesic trials,30 suggesting that placebo effects may have played a role in any of the three interventions. This study did not include a sham, and we were unable to assess for suggestibility because of the need to minimize subject burden. To reduce the influence of expectations with respect to mindfulness and hypnotic suggestion, we informed participants only that the study was designed to test the effects of psychosocial support on acute pain. Not highlighting mindfulness and suggestion in the study information and introducing the techniques with the same language may have attenuated between-groups differences in participant expectations, though we cannot know this is the case in the absence of direct measurement of therapeutic expectancy. It is possible that patients who expected the most benefit self-selected into the study, whereas those with low expectations declined to participate. Limited participation in the present study might also reflect low acceptability of mind-body therapies among certain religious and cultural groups. Additional screening may be needed to select patients whose beliefs do not conflict with such therapies.
Fourth, the study was limited in that the hypnotic suggestion condition was not hypnosis per se—it was not delivered by hypnotherapists, and was a scripted self-hypnosis protocol that lacked patient-tailored, formal hypnotic induction. Nonetheless, we opted for a highly reproducible, pragmatic approach that could be exported to a medical setting without experts in hypnosis. That said, study interventionists were trained by a mind-body therapy expert certified in clinical hypnosis; such expertise may not be available in all medical settings, thereby limiting study generalizability.
Lastly, the study was not powered to investigate the comparative efficacy of mindfulness vs. hypnotic suggestion. After dose-response studies identify optimal dosing for larger and longer-lasting effects, future adequately powered head-to-head trials could reveal the differential outcomes of these two mind-body therapies.
Conclusions
Delivery of a single, scripted, brief mind-body intervention session by clinical social workers with minimal training produced immediate benefits for hospitalized patients reporting unmanageable pain. As such, scripted mind-body interventions may be a useful adjunct to inpatient pain management, and might be cost-effectively integrated into standard medical care. Our study strongly suggests that the role of clinical social workers needs to be expanded to include provision of mind-body therapies in healthcare settings.
Acknowledgements
E.L.G. and Y.N. were supported during the preparation of this manuscript by grant numbers R01DA042033 and R61AT009296 from the National Institutes of Health (PI: Garland). We would also like to acknowledge the following social workers for their assistance with the study: Rebecca Ablad, Mary Andolsek, Reyna Barragan, Lisa Boice, Lora Bonham, Ann Cook, Drue Didier, Andrea Gomes, Stephen Hoffman, Sarah Leymaster, Lindsey Painter, Kristen Quinn, Suzy Ricker, Nate Rose, Amanda Russell, and Heather Smith.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
References
- 1.Whelan CT, Jin L, Meltzer D. Pain and satisfaction with pain control in hospitalized medical patients: no such thing as low risk. Arch. Intern. Med. 2004;164:175–180. doi: 10.1001/archinte.164.2.175. [DOI] [PubMed] [Google Scholar]
- 2.Conway Morris A, Howie N. Pain in medical inpatients: an under-recognised problem? J. R. Coll. Physicians Edinb. 2009;39:292–295. doi: 10.4997/JRCPE.2009.401. [DOI] [PubMed] [Google Scholar]
- 3.Lin RJ, Reid MC, Liu LL, Chused AE, Evans AT. The Barriers to High-Quality Inpatient Pain Management A Qualitative Study. Am J Hosp Palliat Med. 2014;1049909114530491. [DOI] [PMC free article] [PubMed]
- 4.Glowacki D. Effective pain management and improvements in patients’ outcomes and satisfaction. Crit. Care Nurse. 2015;35:33–41. doi: 10.4037/ccn2015440. [DOI] [PubMed] [Google Scholar]
- 5.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. The Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 6.Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J. Hosp. Med. 2014;9:73–81. doi: 10.1002/jhm.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kane-Gill SL, Rubin EC, Smithburger PL, Buckley MS, Dasta JF. The cost of opioid-related adverse drug events. J. Pain Palliat. Care Pharmacother. 2014;28:282–293. doi: 10.3109/15360288.2014.938889. [DOI] [PubMed] [Google Scholar]
- 8.Calcaterra SL, et al. Opioid prescribing at hospital discharge contributes to chronic opioid use. J. Gen. Intern. Med. 2016;31:478–485. doi: 10.1007/s11606-015-3539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9:463–463. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp. Brain Res. 2008;186:79–85. doi: 10.1007/s00221-007-1206-7. [DOI] [PubMed] [Google Scholar]
- 11.Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009;47:987–994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y-Y, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015;16:213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 13.Garland EL, et al. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J. Behav. Med. 2012;35:591–602. doi: 10.1007/s10865-011-9391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeidan F, Vago DR. Mindfulness meditation–based pain relief: a mechanistic account. Ann. N. Y. Acad. Sci. 2016;1373:114–127. doi: 10.1111/nyas.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeidan F, et al. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J. Neurosci. 2011;31:5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeidan F, et al. Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. J. Neurosci. 2015;35:15307–15325. doi: 10.1523/JNEUROSCI.2542-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kekecs Z, Varga K. Positive suggestion techniques in somatic medicine: A review of the empirical studies. Interv. Med. Appl. Sci. 2013;5:101–111. doi: 10.1556/IMAS.5.2013.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kekecs Z, Nagy T, Varga K. The effectiveness of suggestive techniques in reducing postoperative side effects: a meta-analysis of randomized controlled trials. Anesth. Analg. 2014;119:1407–1419. doi: 10.1213/ANE.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 19.Devine EC. Effects of psychoeducational care for adult surgical patients: a meta-analysis of 191 studies. Patient Educ. Couns. 1992;19:129–142. doi: 10.1016/0738-3991(92)90193-M. [DOI] [PubMed] [Google Scholar]
- 20.Gordon DB. Acute pain assessment tools: let us move beyond simple pain ratings. Curr. Opin. Anesthesiol. 2015;28:565–569. doi: 10.1097/ACO.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 21.Garland EL, Hanley A, Farb NA, Froeliger B. State mindfulness during meditation predicts enhanced cognitive reappraisal. Mindfulness. 2015;6:234–242. doi: 10.1007/s12671-013-0250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang EV, et al. Adjunctive self-hypnotic relaxation for outpatient medical procedures: a prospective randomized trial with women undergoing large core breast biopsy. Pain. 2006;126:155–164. doi: 10.1016/j.pain.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 24.Dworkin RH, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain Off. J. Am. Pain Soc. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Rainville P, Hofbauer RK, Bushnell MC, Duncan GH, Price DD. Hypnosis modulates activity in brain structures involved in the regulation of consciousness. J Cogn Neurosci. 2002;14:887–901. doi: 10.1162/089892902760191117. [DOI] [PubMed] [Google Scholar]
- 26.Seymour B, et al. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat. Neurosci. 2005;8:1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- 27.Garland EL, et al. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. J. Consult. Clin. Psychol. 2014;82:448. doi: 10.1037/a0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosier EM, Iadarola MJ, Coghill RC. Reproducibility of pain measurement and pain perception. Pain. 2002;98:205–216. doi: 10.1016/S0304-3959(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 29.Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat. Med. 1992;11:1685–1704. doi: 10.1002/sim.4780111304. [DOI] [PubMed] [Google Scholar]
- 30.Gaskell H, Derry S, Moore RA, McQuay HJ. Single dose oral oxycodone and oxycodone plus paracetamol (acetaminophen) for acute postoperative pain in adults. Cochrane Database Syst Rev. 2009;CD002763. doi:10.1002/14651858.CD002763.pub2. [DOI] [PMC free article] [PubMed]
- 31.Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J. Neurophysiol. 2001;86:402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- 32.Lifshitz M, Raz A. Hypnosis and meditation: Vehicles of attention and suggestion. J. Mind-Body Regul. 2012;2:3–11. [Google Scholar]