Abstract
Current healthcare systems and guidelines are not designed to adapt to care for the large and growing number of patients with complex care needs and those with multimorbidity. Minimally disruptive medicine (MDM) is an approach to providing care for complex patients that advances patients’ goals in health and life while minimizing the burden of treatment. Measures of treatment burden assess the impact of healthcare workload on patient function and well-being. At least two of these measures are now available for use with patients living with chronic conditions. Here, we describe these measures and how they can be useful for clinicians, researchers, managers, and policymakers. Their work to improve the care of high-cost, high-use, complex patients using innovative patient-centered models such as MDM should be supported by periodic large-scale assessments of treatment burden.
KEY WORDS: treatment burden, multimorbidity, quality measures, chronic disease, minimally disruptive medicine
MULTIMORBIDITY AND TREATMENT BURDEN
People are increasingly living with multiple chronic conditions.1 – 3 In the United States, their care accounts for two-thirds of healthcare spending, and out-of-pocket costs are disproportionally high for patients with lower income, poor health, and activity limitations.4 , 5 Patients and caregivers must also shoulder the workload of incorporating treatments into their daily routines and of interacting with healthcare systems over extended periods of their lives.6 While their situation calls for holistic, patient-centered care to improve health and function, patients with multimorbidity must navigate poorly coordinated healthcare silos, each implementing disease-specific guidelines and care pathways to meet narrowly defined performance measures. Living as a patient presents numerous demands: seeking help by choosing and enrolling in health insurance that they can afford; overcoming frustrating administrative barriers; adjusting to arbitrary schedules and communication gaps; traveling to and participating in appointments; making sense of instructions, test results, and bills; obtaining and renewing prescription drugs; paying bills; and more. Such guideline-concordant care overwhelms patients with multimorbidity and their caregivers;7 – 11 they, in turn, opt out of some or all of their prescribed treatments.12 This nonadherence affects patient outcomes, wastes resources, and complicates patient–clinician relationships, adding work to their encounters.13 , 14 Some nonadherence results from the failure of the healthcare system to identify overburdened patients unable to take on the work and costs of implementing prescribed treatments.6 , 13 Designing high-quality, high-value, patient-centered care requires an awareness of the burden of treatment.
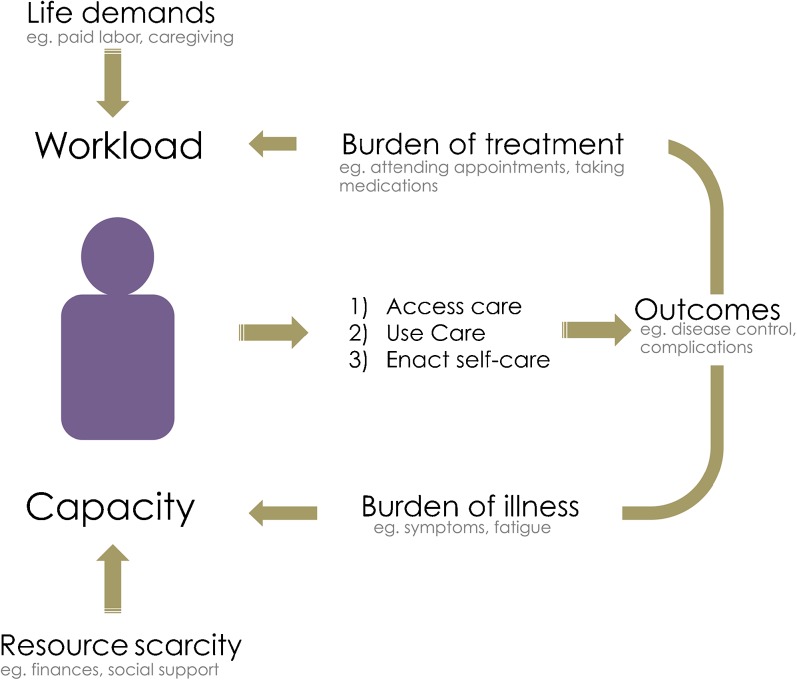
The Cumulative Complexity Model (CuCoM) proposes that accessing and using care, as well as enacting self-care, require sufficient capacity (time, energy, and resources) to shoulder the treatment workload (Fig. 1).15 , 16 The impact of patient work will depend on each person’s dynamic context. The burden of treatment is necessarily reported by patients; third-party measures of healthcare “workload” (e.g., personal spending) do not take into account the subjective experience of accomplishing the work of being a patient while ill, stressed, or poor. Some patients see their own watchful administration of treatment as an empowering action that improves their situation and prevents disease exacerbation and complications.17 Yet patients with complex social situations can be made particularly vulnerable, as the demands of life draw on the same finite capacity they have to attend to the work of being a patient. Indeed, patients must do work to implement treatments while also laboring to meet meaningful obligations to self, family, community, and job. When overwhelmed patients present with poor outcomes, healthcare professionals, pressured by incentives and regulations to follow guidelines, may respond with treatment intensification, i.e., with more work assigned to the patient. As the increased treatment workload further exceeds patient capacity, it leads to nonadherence, exhaustion with self-care,12 and poorer outcomes. Further intensification exacerbates this vicious cycle.15 , 18
Figure 1.
Cumulative Complexity Model (CuCoM). The model describes how clinical and social factors affect care in patients with chronic conditions and multimorbidity. Workload comprises patients’ life demands and healthcare demands with their associated burden of treatment. Capacity refers to patients’ resources and abilities. A balance between workload and capacity should support patients’ ability to access and use care and to enact self-care in order to achieve improved health outcomes. When patients experience poor health outcomes, the burden of illness increases, and the clinical response is often to intensify treatment. This combination can exacerbate workload/capacity imbalance, resulting in a cycle of deterioration. Sources: Shippee et al. (2012),15 Leppin et al. (2015).16
Patient-centered frameworks—such as minimally disruptive medicine (MDM)—aim to minimize the treatment burden by optimizing the workload necessary to achieve patient goals while boosting capacity. The principles of MDM include determining the burden of treatment, coordinating clinical services, recognizing comorbidity in clinical evidence, and centering on the patient’s perspective.13 Examples of strategies for achieving MDM include goal-elicitation, shared decision making, reduction of workload (e.g., de-prescribing, streamlining of appointment schedules and prescription refills), and enhancement of capacity (close collaboration with community entities for material support; physical, psychological, and occupational therapy; palliative care).16 Considering the burden of treatment and modifying treatments to take this into account has the potential to improve outcomes. A reanalysis of 25 years of randomized trials involving interventions to prevent hospital readmission, for example, found that interventions that provided greater support for improving the capacity for self-care among patients and their families after hospital discharge were more efficacious.19 Supporting their capacity reduced the burden of treatment and the need for inpatient care.
Efforts to reduce treatment burden will depend on practice and policy-level commitments to support care that is individualized to the patient’s existing burden, capacity, and situation, and that attends not just to the clinical but also to the personal and social context of their lives. Adopting the principles of MDM may help determine a course of action that patients, particularly those with multimorbidity and at risk of becoming overwhelmed, can implement.13 , 20 For this to succeed in an appreciable way, we must reliably assess and chronicle the burden of treatment.
MEASURING THE BURDEN OF TREATMENT
By “burden of treatment,” we refer to the workload of healthcare and how it impacts patients’ functioning and well-being.12 Measuring this aspect of healthcare can offer insights that enable one to take practical steps to improve the care of patients with chronic conditions. In accordance with the U.S. Department of Health and Human Services’ strategic framework for patients with multiple chronic conditions,21 population-based assessments of treatment burden can serve as indicators of the healthcare system’s ability to provide high-quality care while minimizing the effort healthcare requires from patients.20 To this end, treatment burden can serve as a patient-reported indicator of the effect that patient work has on the “role, social, physical, and psychological functioning” of patients with chronic conditions.12
Several disease-specific questionnaires are available for assessing self-reported treatment burden; other measures assess aspects of treatment burden as part of broader scales.22 At least two general self-reported measures of treatment burden for patients with any chronic condition(s) have recently been developed, the Patient Experience with Treatment and Self-management (PETS) measure and the Treatment Burden Questionnaire (TBQ).12 , 23 , 24 To our knowledge, these tools have not yet been used in large-scale surveys of nationally representative populations in order to gauge healthcare quality, except for an ongoing nationally representative study in Switzerland25 and a large cohort in the UK.26 Table 1 describes their characteristics. Table 2 describes their psychometric properties and the domains each measure considers.12 , 24 The PETS measure comprises nine multi-item scales (48 items total) covering domains such as medical information and role/social activity limitations, while the TBQ is a unidimensional instrument of treatment burden. Both measures have good reliability and demonstrated construct validity against other scales.12 , 24 The TBQ global score was higher for patients receiving physical therapy and needing equipment such as wheelchairs, and the PETS score was higher for patients with lower health literacy and lower medication adherence. Higher treatment burden is associated with the number of chronic conditions patients have, less knowledge about treatment, financial difficulty due to treatment and health problems, and exhaustion associated with self-care.12 , 24 These measures should be adapted to context and purpose; cost issues may not apply in countries with universal healthcare coverage, and certain conditions may require adding condition-specific burdens.
Table 1.
Description of Validation Methods
| Measure | No. of items | Conceptual evidence | Mode and method of administration | Setting and participants | Considerations |
|---|---|---|---|---|---|
| Treatment Burden Questionnaire (TBQ)23 , 24 | 15 | Interviews, open-ended questions, and literature review | Self-administered; paper and pencil, Web | French language: France; 6 clinical sites; 502 with at least 1 condition requiring medical follow-up in last 6 months English language: Multi-country; members of PatientsLikeMe internet platform; 610 adults with 1 or more chronic conditions |
Currently being adapted for use in other languages |
| Patient Experience with Treatment and Self-management (PETS)20 , 26 | 48 | Semi-structured interviews and focus groups with chronically ill patients; systematic literature review of measures | Self-administered; paper and pencil | USA; 2 clinical sites; 332 adults patients with 2 or more chronic conditions | Can be adapted for computer administration; number of items may change with further testing |
Table 2.
Domains and Psychometric Properties
| TBQ* | PETS | |
|---|---|---|
| Domains | ||
| Health behavior change | X | |
| Healthcare expenses | X | X |
| Healthcare services | X | X |
| Interpersonal challenges | X | X |
| Medical appointments | X | X |
| Medical information | X | |
| Medications | X | X |
| Mental toll/exhaustion | X | X† |
| Monitoring health | X | X |
| Physical toll/exhaustion | X† | |
| Role and social activities | X | X |
| Psychometric properties | ||
| Cronbach’s alpha | 0.9 | 0.79–0.95 |
| Test-retest | 0.77 (0.70–0.82) | N/A |
| Item-total correlation | 0.47–0.71 | NR |
| Factor analysis | Unidimensional instrument | 9-factor model |
| Convergent/discriminant validity | Positive association with MMAS-8/ Negative association with PLMQOL, knowledge about treatment | Positive association with CCDS, TSQM side effects/ Negative association with TSQM convenience, self-efficacy, PROMIS-10 |
| Known group validity | Higher TBQ global score for patients who needed equipment (e.g., wheelchair), who received physical therapy, and those with GI and skin disease | Greater burden in 8 of 9 domains in patients with lower health literacy and patients with lower medication adherence. More burden in all domains for patients with more financial difficulty |
| Related variables | Number of different conditions, drug administration and quantity, medical follow-up | Non-statistically significant correlation with number of diagnoses, encounters with providers, role/social activity limitation, and physical/mental exhaustion with self-care |
| Sensitivity to change | N/A | N/A |
*Data for the English-language version
†Constitute one domain
N/A: not applicable, NR: not reported, MMAS-8: 8-item Morisky Medication Adherence Scale, PLMQOL: PatientsLikeMe Quality of Life, CCDS: Chronic Condition Distress Scale, TSQM: Treatment Satisfaction Questionnaire for Medication, PROMIS-10: Patient-Reported Outcomes Measurement Information System Global-10, GI: gastrointestinal
THE PROMISE OF MEASURING THE BURDEN OF TREATMENT
Integrating treatment burden measures in healthcare supports a transition to a model of care that prioritizes quality and efficacy while minimizing disruption to patients. While several disease-specific measures with high validity and responsiveness to change are available,22 treatment burden is not being measured routinely in population health programs or in the evaluation of new models of care such as patient-centered medical homes or accountable care organizations. Nor are these disease-specific treatment burden measures used to inform the delivery of care, improve patient capacity to engage in effective chronic disease management, or improve patient outcomes. These measurements will be crucial as the value of the care that healthcare organizations provide to their communities is shaped by the context in which they exist.27 , 28 As a quality indicator, treatment burden can challenge healthcare organizations to improve both the content of their care and the administrative protocols that routinely shift work to patients and caregivers. These measures can transform the way we assess care by going beyond the short-lived post-encounter impressions inherent in patient experience assessments. Unlike most performance measures, which tend to favor overtreatment to achieve markers of disease control, burden of treatment—a patient-reported measure—can be combined with a measure of poor care (e.g., percentage of patients with diabetes with HbA1c > 9%) to promote high-quality patient-centered care. Health systems can identify items or domains where patients are consistently reporting high treatment burden in order to modify the way they deliver care.
Medical practices can benefit from implementing MDM. Practice-based studies show that patients and clinicians differ in their assessments of adherence and perceptions of medication importance,29 and clinicians fail to explore contextual factors that may impact patient health.30 Valid measures of treatment burden, serving as outcome measures, can also help gauge the effectiveness of alternative models of healthcare that align with MDM—e.g., care coordination, telemedicine (designed to reduce the burden of accessing healthcare services)— for complex or high-need patients.12 Better indicators may orient these efforts not to “high utilizers” or “high-cost” patients (a healthcare-centered approach), but to preventing and alleviating high treatment burden in patients at highest risk for decompensation (a patient-centered approach).16 These assessments can also provide empirical data for guideline panelists to evaluate the practical trade-offs influencing medical recommendations.31
Because these measures are relatively new, there are limited data on the ability of PETS and TBQ to detect changes over time (across critical periods in the life span that are characterized by more or less healthcare usage).12 , 24 To fill this gap, validated measures of treatment burden can be deployed to large samples of the population over time through existing national surveys such as the National Health Interview Survey (NHIS), or longitudinal surveys that focus specifically on older adult populations with a high prevalence of multimorbidity, such as the Health and Retirement Study (HRS)32 or the National Health and Aging Trends Study (NHATS).33 These can create a patient-centered picture of the epidemiology of multimorbidity and of healthcare’s response to it across groups, regions, health systems, and time. A longitudinal, periodically assessed measure of treatment burden, a sort of “healthcare footprint,” can indicate the success of interventions tested in trials and implemented in policies in improving health while respecting the functioning and well-being of populations.12 If reform toward MDM and other patient-centered models is successful, periodic measures of treatment burden should improve over time.
Assessment of treatment burden will advance our understanding of the interactions between the demands life makes on each patient and the work of being one. This understanding should support the design, implementation, and evaluation of MDM and other patient-centered care models for patients with multimorbidity.9 Ultimately, the value of care for patients should reflect the health outcomes achieved and the degree of burden that patients and their caregivers must bear to achieve those outcomes. We now have the opportunity to make the burden of treatment visible and useful.
Acknowledgements
We would like to thank the International Minimally Disruptive Medicine workgroup, especially David T. Eton and Viet Thi Tran, for their input on earlier versions of this manuscript. The workgroup members include Summer Allen, Kasey Boehmer, Juan Pablo Brito, Ian Hargraves, Katie Gallacher, Michael R. Gionfriddo, Aaron Leppin, Frances Mair, Marc R. Matthews, Carl May, Victor M. Montori, Elizabeth Rogers, Nilay Shah, Nathan Shippee, Kate Vickery, and Kathleen Yost.
Compliance with Ethical Standards
Funding
GSB and VMM were supported by CTSA grant numbers TL1 TR000137 and UL1 TR000135, respectively, from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). ARQ is supported by an American Diabetes Association career development award (ADA 7–13-CD-08). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
References
- 1.Wu S-Y, Green A. Projection of chronic illness prevalence and cost inflation. Santa Monica, CA: RAND Health. 2000;18.
- 2.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 3.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- 4.Anderson G. Chronic Care: Making the Case for Ongoing Care. Princeton, NJ: Robert Wood Johnson Foundation; 2010. Available at: http://www.rwjf.org/content/dam/farm/reports/reports/2010/rwjf54583. Accessed Jun 12, 2017.
- 5.Banthin JS, Bernard DM. Changes in financial burdens for health care: national estimates for the population younger than 65 years, 1996 to 2003. JAMA. 2006;296(22):2712–2719. doi: 10.1001/jama.296.22.2712. [DOI] [PubMed] [Google Scholar]
- 6.Wolff JL, Boyd CM. A Look at Person- and Family-Centered Care Among Older Adults: Results from a National Survey [corrected] J Gen Intern Med. 2015;30(10):1497–1504. doi: 10.1007/s11606-015-3359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerr EA, Heisler M, Krein SL, et al. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients’ treatment priorities and self-management? J Gen Intern Med. 2007;22(12):1635–1640. doi: 10.1007/s11606-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogeli C, Shields AE, Lee TA, et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(Suppl 3):391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition—multimorbidity. JAMA. 2012;307(23):2493–2494. [DOI] [PMC free article] [PubMed]
- 10.Quinones AR, Markwardt S, Botoseneanu A. Multimorbidity Combinations and Disability in Older Adults. J Gerontol A Biol Sci Med Sci. 2016;71(6):823–830. doi: 10.1093/gerona/glw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran VT, Barnes C, Montori VM, Falissard B, Ravaud P. Taxonomy of the burden of treatment: a multi-country web-based qualitative study of patients with chronic conditions. BMC Med. 2015;13:115. doi: 10.1186/s12916-015-0356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eton DT, Yost KJ, Lai JS, et al. Development and validation of the Patient Experience with Treatment and Self-management (PETS): a patient-reported measure of treatment burden. Qual Life Res. 2016. [DOI] [PMC free article] [PubMed]
- 13.May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. doi: 10.1136/bmj.b2803. [DOI] [PubMed] [Google Scholar]
- 14.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002;288(22):2880–2883. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 15.Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65(10):1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Leppin A, Montori V, Gionfriddo M. Minimally Disruptive Medicine: A Pragmatically Comprehensive Model for Delivering Care to Patients with Multiple Chronic Conditions. Healthcare. 2015;3(1):50. doi: 10.3390/healthcare3010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridgeway JL, Egginton JS, Tiedje K, et al. Factors that lessen the burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Prefer Adherence. 2014;8:339–351. doi: 10.2147/PPA.S58014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sav A, King MA, Whitty JA, et al. Burden of treatment for chronic illness: a concept analysis and review of the literature. Health Expect. 2015;18(3):312–324. doi: 10.1111/hex.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107. doi: 10.1001/jamainternmed.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mair FS, May CR. Thinking about the burden of treatment. BMJ. 2014;349:g6680. doi: 10.1136/bmj.g6680. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. Multiple Chronic Conditions: A Strategic Framework Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions 2010; http://www.hhs.gov/sites/default/files/ash/initiatives/mcc/mcc_framework.pdf. Accessed Jun 12, 2017.
- 22.Eton DT, Elraiyah TA, Yost KJ, et al. A systematic review of patient-reported measures of burden of treatment in three chronic diseases. Patient Relat Outcome Meas. 2013;4:7–20. doi: 10.2147/PROM.S44694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran VT, Montori VM, Eton DT, Baruch D, Falissard B, Ravaud P. Development and description of measurement properties of an instrument to assess treatment burden among patients with multiple chronic conditions. BMC Med. 2012;10:68. doi: 10.1186/1741-7015-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran VT, Harrington M, Montori VM, Barnes C, Wicks P, Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med. 2014;12:109. doi: 10.1186/1741-7015-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deruaz-Luyet A, N’Goran AA, Tandjung R, et al. Multimorbidity in primary care: protocol of a national cross-sectional study in Switzerland. BMJ Open. 2015;5(10):e009165. doi: 10.1136/bmjopen-2015-009165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.University of Southampton. Horizons: Understanding the impact of cancer diagnosis and treatment on everyday life. 2017; http://www.horizons-hub.org.uk/index.html. Accessed Jun 12, 2017.
- 27.Hu J, Nerenz D. Relationship Between Stress Rankings and the Overall Hospital Star Ratings: An Analysis of 150 Cities in the United States. JAMA Intern Med. 2017;177(1):136–137. doi: 10.1001/jamainternmed.2016.7068. [DOI] [PubMed] [Google Scholar]
- 28.Gu Q, Koenig L, Faerberg J, Steinberg CR, Vaz C, Wheatley MP. The Medicare Hospital Readmissions Reduction Program: potential unintended consequences for hospitals serving vulnerable populations. Health Serv Res. 2014;49(3):818–837. doi: 10.1111/1475-6773.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidorkiewicz S, Tran V-T, Cousyn C, Perrodeau E, Ravaud P. Discordance between drug adherence as reported by patients and drug importance as assessed by physicians. Ann Fam Med. 2016;14(5):415–421. [DOI] [PMC free article] [PubMed]
- 30.Weiner SJ, Schwartz A, Weaver F, et al. Contextual errors and failures in individualizing patient care: a multicenter study. Ann Intern Med. 2010;153(2):69–75. doi: 10.7326/0003-4819-153-2-201007200-00002. [DOI] [PubMed] [Google Scholar]
- 31.Andrews JC, Schunemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. Journal of clinical epidemiology. 2013;66(7):726–735. doi: 10.1016/j.jclinepi.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Hodes R, Suzman R. Growing older in America: The health and retirement study. Bethesda: National Institute on Aging, National Institute of Health, US Department of Health and Human Services. 2007.
- 33.Montaquila J, Freedman V, Edwards B, Kasper J. National Health and Aging Trends Study Round 1 Sample Design and Selection. NHATS Technical Paper# 1. Baltimore: Johns Hopkins University School of Public Health; 2012. [Google Scholar]