Abstract
Fifty-four macromycetes, isolated from southeastern Mexico, were used in order to evaluate their capacity for degradation and tolerance to the herbicide paraquat. Ten of these strains were capable of growing in a solid culture medium in the presence of 200 ppm paraquat. Subsequently, assays to evaluate the degradation of the xenobiotic in a liquid medium were carried out. Of the ten strains evaluated, three presented the highest levels of degradation of the compound, which were Trametes pavonia (54.2%), Trametes versicolor (54.1%) and Hypholoma dispersum. They presented the highest overall degradation percentage (70.7%) after 12 days culture. The presence of ligninolytic enzymes in these strains was evaluated. H. dispersum only presented aryl alcohol oxidase activity; however, with the data obtained, it was not possible to conclude whether this specific enzyme is responsible for paraquat degradation. The level of degradation obtained is above the one reported for Pseudomonas putida, one of the few reports on paraquat degradation. This is the first report on the contaminant degradation capacity of H. dispersum.
Keywords: Pesticide, Ligninolytic enzymes, Mycoremediation, Paraquat
Introduction
Paraquat (N, N′-dimethyl-4, 4′-bipyridinium dichloride) is one of the most frequently used herbicides in agriculture. It is a cationic non-systematic, non-selective contact compound that instantaneously interferes with the photosynthesis processes of plants. Once the compound comes into contact with the plants' leaves, where the reaction occurs, it has an immediate effect (Kumar et al. 2016; PMEP 2017). The world health organization (WHO) estimates that the minimum lethal dose in humans is 35 mg/kg of concentrated paraquat (Tsai 2013). This compound results in neurological damage, renal and hepatic insufficiency, lacerations in mouth, nose and throat and severe damage to the lungs (Blanco-Ayala et al. 2014). In the soil, paraquat is absorbed by organic matter and depending on soil composition, can remain there for up to 5 years (Pateiro-Moure et al. 2009; Muhamad et al. 2011; Conde-Cid et al. 2017).
The transformation of paraquat in soil can take place through a variety of mechanisms, including photodegradation, chemical degradation, and microbial metabolism. Photo degradation reactions occur on the surface, a few centimetres within the soil (Rongchapo et al. 2016; Jaiswal et al. 2017). Soil surface photodegradation depends on the intensity of ultraviolet light within the wavelength range of 285–310 nm (Jafarinejad 2015; Zahedi et al. 2015).
There are several groups of microorganisms capable of using paraquat as a nitrogen source (Gondar et al. 2012; Devashree et al. 2014), including bacteria and actinomycetes. Pseudomonas putida was used to evaluate paraquat degradation using activated charcoal as an intermediary. In the absence of activated charcoal, paraquat degradation levels have reached 47.29%, while 95% degradation was attained when activated charcoal was present; this is due to the strong absorption of paraquat by this component (Kopytko et al. 2002).
The capacity of fungi to degrade paraquat has not yet been evaluated; however, there are many reports that indicate its potential in the biodegradation processes of other similar compounds. For example, ligninolytic or white rot fungi (WRF) have developed a unique non-specific enzymatic system that functions in an extracellular environment. This mechanism is based on the production of free radicals and allows participant enzymes to be catalytically active on a wide diversity of organic substrates, including those that are recalcitrant for the environment (Rhodes 2014). Phanerochaete chrysosporium and Trametes versicolor are among the most studied fungi and have been evaluated for the degradation of aromatic hydrocarbons, colourants and agrochemicals, among others (Fulekar et al. 2013; Ding et al. 2013; Bautista et al. 2015). WRF possess unique characteristics that allow the degradation of such compounds and the most studied enzymes from these organisms include laccases, manganese peroxide and arylalcohol oxidase (Rhodes 2014).
Another group of fungi that has been evaluated for degrading contaminants is the brown rot fungi (BRF). These fungi degrade cellulose and hemicellulose present in trees and plants and the production of free radicals by the Fenton reaction promotes the degradation of contaminant compounds, the most important including chlorofenol and 2,4,6, trinitrotoluene (Newcombe et al. 2002; Zhu et al. 2017). Other studies include the degradation of 1,1,1-trichloro-2,2-bis (4-chlorophenyl) ethane (DDT) by brown rot fungi such as Fomitopsis pinicola and Daedalea dickinsii. These fungi can transform DDT to DDE and DDD via the Fenton reaction (Purnomo et al. 2010a, b, 2011).
The present study aims to identify both the degradation and tolerance capacity of macromycetes isolated from the southern part of Chiapas Mexican state, to the paraquat herbicide. Furthermore, the study evaluates the influence of the ligninolytic enzymes system in the removal of this harmful compound from the culture medium.
Materials and methods
Biological material
Fifty-four strains of fungi, collected in the southeastern region of the state of Chiapas, Mexico, were used in this study. The collected fungi were isolated in a selective medium and taxonomically identified by using their particular macro and microscopic characteristics. All of them were subsequently deposited in the mycology collection of ECOSUR. The fungi were grown in Petri dishes on malt extract agar (MEA) and were maintained at 28 °C, until complete colonization. Among them, ten strains were selected for the degradation in liquid medium experiments and were identified by using specific initiators for the ITS-4 and ITS-5 regions (Ma et al. 2009). The PCR products were analyzed in a capillary sequencer (MACROGEN, inc., Seoul, Korea) while the sequences acquired were analyzed by means of BLAST type algorithms provided by the National Centre of Biotechnology Information (NCBI BLAST). The strains tested were identified as: strain ECS-49 Fomitopsis meliae; ECS-50 Trametes maxima; ECS-56, ECS-58 and ECS-59 Polyporus tricholoma; ECS-66 T. Villosa; ECS-67 T. Pavonia; ECS-68 T. Ellipsospora; ECS-79 T. versicolor and ECS-705 Hypholoma dispersum.
Mycelial growth in the presence of paraquat
The tolerance to paraquat, by the 54 fungal strains that were previously isolated, was analyzed. In order to determine the radial extension rate (RER) of each strain in presence of the herbicide, an agar disc (5 mm diameter) with mycelium was placed in the centre of a Petri plate (9 cm diameter) with the minimum salt and peptone medium (MSPM, in g/l: potassium monobasic phosphate 5, magnesium sulphate 0.4, ammonium sulphate 10, sodium chloride 10, casein peptone 2, dextrose 5 and bacteriological agar 16). 200 mg/l paraquat were added (sterilized with Acrodisc® filters; 0.2 µm pore size). Three repetitions per treatment were carried out and the petri dishes were maintained at 28 °C. The controls were monitored using MSPM, without paraquat. Growth was measured daily until total colonization of the Petri dish (4–30 days, depending on the strain).
Liquid culture with paraquat
With regard to the degradation of paraquat in liquid medium, pre-inocula of the tolerant strains were prepared by adding eight agar with mycelium discs (5 mm diameter) to each 125 ml flask, containing 30 ml ME liquid medium. Flasks were maintained at 28 °C and were shaken constantly at 125 rpm for 10 days. Afterwards, mycelium produced in the flasks was filtered (under aseptic conditions) and crushed in 30 ml sterile distilled water until attaining a homogenous suspension. Subsequently, 1 ml of this suspension was used to inoculate 125 ml Erlenmeyer flasks with 30 ml MSPM. The material was agitated at 125 rpm and 28 °C. After 3 days incubation, paraquat (100 ppm final concentration) was added and flasks were incubated for 15 days total. All experiments were repeated three times.
Samples were taken at 3, 6, 9 and 12 days after seeding. Two controls were used: (1) heat-inactivated mycelium (by autoclaving the flasks at 121 °C, 30 min) was used as an adsorption control and (2) a non-inoculated sample was included as an abiotic control for other types of losses. Both controls with same paraquat concentration were prepared according to each strain. All samples were filtered (Whatman filters, grade 6) and centrifuged (5000 rpm, 10 min), at 4 °C. Filtered mycelium was used to determine biomass through dry weight while supernatant was used to determine the remaining concentration of paraquat and the enzymatic activity (laccase, manganese peroxidase, lignin peroxidase, aryl alcohol oxidase and versatile peroxidase).
Measured variables
Radial Extension Rate (RER): this index measured daily mycelial growth along two perpendicular directions in each Petri dish, using a Vernier scale (Mitutoyo®, ±0.05) until the complete colonization of the dish. The RER was calculated by applying the linear growth function Y = krX + C (where Y is distance and X is time); the results were expressed in mm/day.
Rate of growth (rg) was calculated for each strain through the following formula:
Inhibition of mycelial growth (%) due to paraquat was determined by means of the following formula:
Strains presenting less than 50% inhibition were considered as tolerant to the herbicide and were used for later assays.
Determination of paraquat
In order to determine the concentration of paraquat, an enzymatic immunoassay was carried out, using the Paraquat Elisa Kit KA1424 (Abnova: Taipei, Taiwan).The test is based on the competition between paraquat contained in the sample and the horseradish peroxidase to combine the antibody directly against the paraquat, immobilized in the micropores of the Elisa dish.
Enzymatic activity
Laccase (Lac): A reaction blend with 2,6 dimethoxiphenol (DMP) 50 mM in a buffer solution of sodium 200 mM pH 5, H2O and enzymatic extract was used. The reaction was measured over two minutes and DMP oxidation was monitored by an increase in absorbance at 469 nm (ε469 = 49,600/M/cm) (Tinoco et al. 2001).
Aryl alcohol oxidase (AAO): This was determined through the oxidation of 25 Mm veratryl alcohol in a buffer solution of 500 Mm sodium phosphate pH 6, H2O and enzymatic extract. The reaction was measured for two minutes at 310 nm (ε310 = 9300/M/cm) (Guillén et al. 2000).
Manganese peroxidase (MnP): A reaction blend consisting of 20 mM manganese sulphate tetrahydrate (MnSO4 4H2O), 100 mM sodium malonate pH 4.5, H2O2 10 mM, H2O and enzymatic extract. Oxidation was spectrophotometrically monitored for one minute at 270 nm (ε 270 = 1159 M/M/cm) (Wariishi et al. 1992).
Lignin peroxidase (LiP): This was estimated using a reaction solution consisting of 50 mM veratrilic alcohol in a buffer solution of 200 Mm sodium tartrate pH 3, H2O2 100 mM, H2O and enzymatic extract. Changes in absorbance at 310 nm (ε310 = 9300/M/cm) were monitored during 2 min (Tien and Kirk 1984).
Versatile peroxidase (VP): Determined by the oxidation of 1 Mm black five reactive in a buffer solution of 200 mM sodium malonate pH 3, H2O2 10 mM, H2O and enzymatic extract. Changes in absorbance at 598 nm (ε598 = 30,000/M/cm) were monitored over a period of 5 min (Rodríguez et al. 2004).
In all cases, enzymatic unity (U) was defined as the quantity of enzyme required for the transformation of 1 µmol of substrate per minute.
Experimental design
A completely random design was used. All the experiments were carried out three times, under identical conditions. The IBM SPSS Statistics software was used for the statistical analysis; in all cases a multivariate or unifactorial ANOVA was applied and the data was analyzed through a comparison between the Tukey and Bonferroni tests, both with a significance level of 0.05.
Results and discussion
Collection and isolation of macromycetes
Seven collections were carried out in the south of the state of Chiapas in southeast Mexico (municipalities of Tapachula, Unión Juárez, Cacahoatán and Motozintla). A total of 126 specimens of macromycetes were collected and subsequently taken to the laboratory for identification, morphological analysis and isolation. A total of 54 strains were isolated (Table 1).
Table 1.
Radial Extension Rate (mm/day) of the strains grown in MMSP with 200 mg/l paraquat at 28 °C
| Strain | Control (mm/day) | Paraquat (mm/day) | Inhibition (%) | Groupa | Strain | Control (mm/day) | Paraquat (mm/day) | Inhibition (%) | Groupa |
|---|---|---|---|---|---|---|---|---|---|
| ECS-46 | 9.74d | 4.82cde | 50.5 | NT | ECS-74 | 3.1efg | 0 | 100 | NG |
| ECS-47 | 7.1de | 2.2fg | 61.3 | NT | ECS-75 | 5.1ef | 0 | 100 | NG |
| ECS-48 | 2.3 fg | 0 | 100 | NG | ECS-76 | 9.3cde | 2.6efg | 72.1 | NT |
| ECS-49 | 3.1efg | 1.7fg | 45.1 | T | ECS-77 | 2.8efg | 0 | 100 | NG |
| ECS-50 | 9.4cde | 5.7cd | 39.3 | T | ECS-78 | 8.1de | 3ef | 63 | NT |
| ECS-51 | 5.2ef | 2.1fg | 59.6 | NT | ECS-79 | 5.9def | 3.1ef | 47.4 | T |
| ECS-52 | 1.7 g | 0.2h | 88.2 | NT | ECS-80 | 7.9de | 1.9 fg | 76 | NT |
| ECS-53 | 9.2cde | 3.2ef | 65.2 | NT | ECS-81 | 21.8b | 4.1de | 81.1 | NT |
| ECS-54 | 4.4ef | 2.3fg | 47.7 | NT | ECS-82 | 19.1bc | 3.1ef | 83.7 | NT |
| ECS-55 | 5.5ef | 1.9fg | 65.4 | NT | ECS-85 | 1.6 g | 0 | 100 | NG |
| ECS-56 | 3.5f | 1.9fg | 45.7 | T | ECS-86 | 5.3ef | 0 | 100 | NG |
| ECS-57 | 4.4ef | 1.3fg | 70.4 | NT | ECS-87 | 4.4ef | 0 | 100 | NG |
| ECS-58 | 12.5cd | 6.4c | 48.8 | T | ECS-88 | 13.1bcd | 4.4de | 72 | NT |
| ECS-59 | 22.9b | 12.4b | 45.8 | T | ECS-89 | 14.2c | 4.2de | 70.4 | NT |
| ECS-60 | 8.2de | 4.1de | 50 | NT | ECS-90 | 18.4bc | 0 | 100 | NG |
| ECS-61 | 4.8ef | 0 | 100 | NG | ECS-91 | 16.1bc | 1.1g | 93.1 | NT |
| ECS-62 | 6.3e | 1.5fg | 76.1 | NT | ECS-92 | 2.1 fg | 0 | 100 | NG |
| ECS-63 | 7.6de | 2.3fg | 69.7 | NT | ECS-93 | 22.9b | 4.6cde | 80 | NT |
| ECS-64 | 8.2de | 3.6e | 56.1 | NT | ECS-94 | 4.2ef | 0 | 100 | NG |
| ECS-65 | 12.1 cd | 0 | 100 | NG | ECS-95 | 10d | 0 | 100 | NG |
| ECS-66 | 35.2a | 18.3a | 48.1 | T | ECS-96 | 2.6efg | 1.4fg | 80 | NT |
| ECS-67 | 19.6bc | 10.7bc | 45.4 | T | ECS-97 | 14.9c | 2.5efg | 83.2 | NT |
| ECS-68 | 8.1de | 5.5cd | 32.1 | T | ECS-100 | 4ef | 0 | 100 | NG |
| ECS-70 | 8.3de | 2.6f | 68.7 | NT | ECS-703 | 1.5g | 0 | 100 | NG |
| ECS-71 | 11.3cd | 0 | 100 | NG | ECS-705 | 1.7g | 1.3fg | 23.5 | T |
| ECS-72 | 2.2fg | 1.1g | 50 | NT | ECS-706 | 22.1b | 4.9d | 77.8 | NT |
| ECS-73 | 4.1ef | 1.6fg | 60.9 | NT | ECS-707 | 2.3fg | 1.1g | 52.1 | NT |
Incubation 15 days
Mean of three repetitions
MSPM minimum salt and peptone mediums
a T tolerant strain (<50% inhibition), NT non-tolerant strain (≥50% inhibition) and NG strain that did not experience growth in the presence of paraquat (100% inhibition).The control corresponds to the grown sample without paraquat. Different small letters in the same column indicate that there are significant differences between treatments, according to the Tukey test (α = 0.05)
Evaluation of paraquat tolerant strains
Growth rate in the presence of the herbicide and the resulting inhibition in each of the strains evaluated is presented in Table 1. Growth fluctuated between 0 (16 strains) and 18.3 mm/day (strain ECS-66). Inhibition by paraquat varied between 23.5% (strain ECS-705) and 100% (16strains). The statistical analysis determined significant differences between strains (P = 0.05); strain ECS-66 obtained the highest RER, for both with or without paraquat (18.3 and 35.2 mm/day, respectively).
Consistent with the level of herbicide tolerance established for this study (<50% inhibition) three main groups were evident: (a) 10 strains tolerant to paraquat (T), (b) 29 strains not tolerant to paraquat (NT), presenting an RER on paraquat containing medium that was less than half the one observed on the control without paraquat, and (c) strains presenting no growth in presence of paraquat (NG). The ten strains that were tolerant to paraquat were selected for subsequent degradation assays.
Paraquat degradation assays
Table 2 displays the increase in biomass in each of the strains cultivated in MSMP with100 ppm paraquat, at 28 °C and 120 rpm during 12 days. At the start of the experiments, all the samples contained an initial biomass concentration of 1 g/l. F. meliae ECS- 49, T. maxima ECS-50 and the three strains of P. tricholoma (ECS-56, 58 and 59) did not demonstrate an increase in biomass after day 6. Taking into consideration that the addition of paraquat took place on day 3, the lack of growth of these strains could be due to inhibition caused by the presence of paraquat (results not shown). In contrast, the strains T. villosa ECS-66, T. pavonia ECS-67, T. ellipsospora ECS-68, T. versicolor ECS-79 and H. dispersum ECS-705, experienced an increase in biomass. T. versicolor ECS-79 and H. dispersum ECS-705 presented the largest amount of biomass at 12 days growth (5.89 and 6.2 g/l respectively). T. maxima ECS-50 demonstrated the lower growth with 3.5 g/l biomass produced over 12 days. H. dispersum ECS-705 demonstrated the highest rate of growth, (0.57 g/day, α < 0.05) while the strains P. tricholoma ECS-56, F. meliae ECS-49 and T. maxima displayed the lowest ones (0.22, 0.22 and 0.23 g/day respectively, statistical group “a”, α < 0.05).
Table 2.
Growth rate and biomass production of the selected strains, grown in MSPM with 100 ppm of paraquat, at 28 °C, 120 rpm during 12 days
| Strain | Growth rate (g/day) | Biomass (g/l) |
|---|---|---|
| Fomitopsis meliae (ECS-49) | 0.22a | 4.10b |
| Trametes maxima (ECS-50) | 0.23a | 3.50c |
| Polyporus tricholoma (ECS-56) | 0.22a | 4.20b |
| Polyporus tricholoma (ECS-58) | 0.33c | 4.23b |
| Polyporus tricholoma (ECS-59) | 0.29bc | 5.02b |
| Trametes villosa (ECS-66) | 0.41d | 5.30b |
| Trametes pavonia (ECS-67) | 0.42d | 4.82b |
| Trametes ellipsospora (ECS-68) | 0.34c | 4.79b |
| Trametes versicolor (ECS-79) | 0.34c | 5.89a |
| Hypholoma dispersum (ECS-705) | 0.57e | 6.20a |
Identical small letters in the same column indicate that there are no significant differences between strains, according to the Tukey’s test (P < 0.05)
Figure 1 shows the concentration of residual paraquat for each fungi strain tested at this point. Degradation percentages vary between 15 and 22% for F. meliae ECS-49, T. villosa ECS-66, T. maxima ECS-50 and the three strains of P. tricholoma ECS-56, ECS-58 and ECS-59, coinciding with low biomass production for at least five of them. Growth inhibition is reflected in a low percentage of paraquat degradation. The degradation percentages for T. ellipsospora ECS-68 and P. tricholoma ECS-56, ECS-58 and ECS-59 were also around 28–29%, while they were higher for the strains T. pavonia ECS-67, T. versicolor ECS-79 and H. dispersum ECS-705 (54%–70% after 12 days growth). H. dispersum ECS-705 achieved the greatest degradation of paraquat with 70.7% (29.23 ppm) after 12 days growth.
Fig. 1.
Degradation of 100 ppm paraquat by different fungal strains in a liquid culture at 28 °C, 120 rpm, and 3, 6, 9 and 12 days culture. C 1 MMSP without inoculation; C 2 heat-inactivated mycelium 3; both controls were treated as with the samples (100 mg/l de paraquat). Same small letter in two bars indicate that there are no significant differences between strains on time 12, according to the Tukey's test (P < 0.05). The experiments were triplicated
The data obtained does not reveal if the compound was totally degraded and incorporated into the energetic metabolism of the fungi; however, two controls were included in the experiments: C1 corresponding to the culture medium with 100 mg/l paraquat without mycelium, and C2 presenting sterile mycelium with paraquat, in order to identify the possible adhesion of paraquat to the fungal structures as well as eliminating the possibility of a decrease in the concentration of the compound due to physical (shaking, evaporation, light, absorption) or chemical processes, through interaction with the culture medium. In both controls, the concentration of paraquat remained constant over time, demonstrating that its decrease in the treatments corresponded to degradation by the strains tested. Thus, it is possible to observe the capacity of the strains in transforming the compound, although the degradation pathway remains to be clarified. Several reports refer to the presence of intermediaries that have been analyzed during the photocatalytic degradation of paraquat, such aspyridine paraquat, protonated dipyridine paraquat and 4-carboxy-1-methyl pyridine (Cantavenera et al. 2007). The metabolites associated with the degradation of paraquat by microorganisms or macromycetes have yet to be identified. In the case of the oxidation pathway, there are no reports on the intermediaries produced during the degradation of the compound (Florêncio et al. 2004; Cantavenera et al. 2007; Zahedi et al. 2015).
The above-mentioned samples were also used for the measurement of enzymatic activity to ascertain any possible association with paraquat degradation.
Five ligninolytic activities were determined: Lac, MnP, AAO, LiP and VP. In the majority of the strains, only the first three enzyme activities were observed.
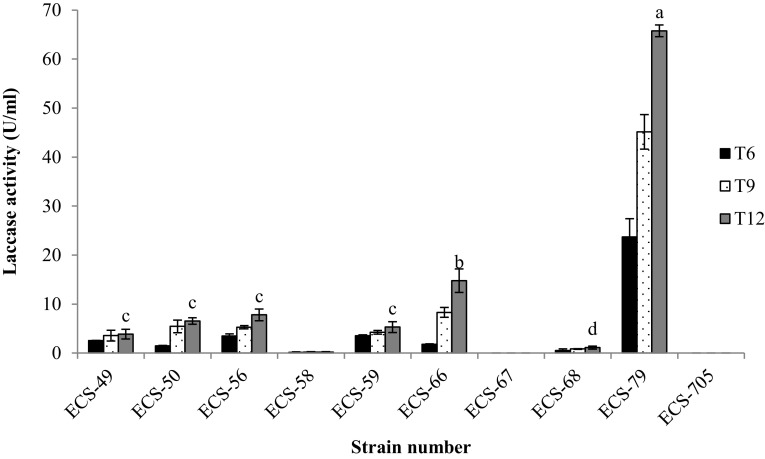
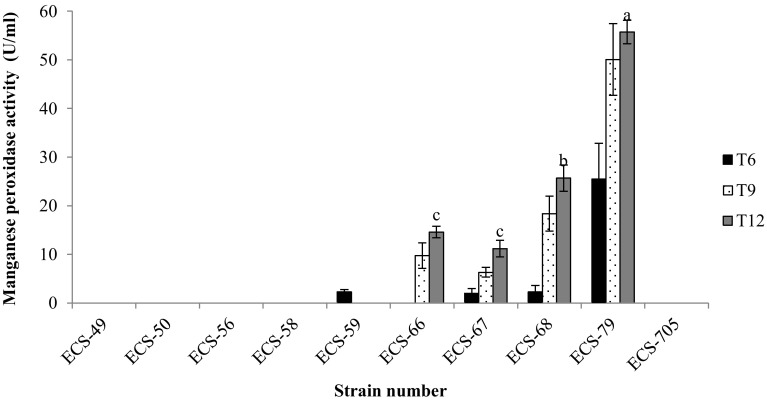
Both LiP and VP did not present activity in any of the extracts analyzed. T. versicolor ECS-79 presented the highest Lac activity (Fig. 2) (65.78 U/ml) on day 12. The strain H. dispersum ECS-705, which presented the highest percentage of paraquat degradation, did not demonstrate any Lac activity. Manganese peroxidase activity (Fig. 3), reported as responsible for several processes of xenobiotic degradation, also demonstrated degradation percentages between 28 and 50% for T. pavonia ECS-67, T. ellipsospora ECS-68 and T. versicolor ECS-79. H. dispersum ECS-705 did not present Lac and MnP activity. Finally, AAO activity was evident in all isolates (Fig. 4); AAO presented the highest activity in P. tricholoma ECS-56 and T. villosa ECS-66. These strains did not demonstrate a considerable degradation of paraquat; however, T. pavonia ECS-67 and T. ellipsospora ECS-68 presented acceptable levels of this enzyme with values of 16 and 24 U/ml respectively, coinciding with paraquat degradation. AAO was the only enzymatic activity identified for H. dispersum ECS-705, the strain that presented the highest percentage of paraquat degradation, although the level of activity was intermediate when compared with the other isolated strains. It remains to be established whether this enzyme is responsible for paraquat degradation.
Fig. 2.
Laccase activity of the extracellular culture medium (substrate DMP, pH 5 at 26 °C) during paraquat degradation. Activity was measured on day 6, 9 and 12 of the culture. Same small letters in each bar indicate that there are no significant differences between strains after the culture was 12 days old, according to the Tukey test (P < 0.05). The experiments were triplicated and the mean comparisons for each strain were shown
Fig. 3.
Activity of the manganese peroxidase enzyme in the extracellular culture medium (H2O2 substrate with MnSO4), pH 4.5 at 26 °C during paraquat degradation. Activity was measured at day 6, 9 and 12 of the culture. Identical small letters in each bar indicate that there are no significant differences between strains after the culture was 12 days old, according to the Tukey test (P < 0.05). The experiments were triplicated
Fig. 4.
Activity of the aryl alcohol oxidase (AAO) enzyme from the extracellular culture medium (veratrylic alcohol substrate, pH 6, 26 °C) during paraquat degradation. Activity was measured at day 6, 9 and 12 of the culture. Identical small letters in each bar indicate that there are no significant differences between strains after the culture was 12 days old, according to the Tukey test (P < 0.05). The experiments were triplicated
Discussion
Fifty-four strains of macromycetes were analyzed for paraquat degradation. Ten of those strains were considered as tolerant to paraquat. It is worth to point out the high concentration of paraquat (200 mg/l) used for the solid medium experiments. Anasonye et al. (2015) found that the growth of the fungi Phanerochaete chrysosporium was inhibited at concentrations of 24 ppm TNT when malt extract agar was used as a base medium. In 2005, Mukherjee and Mittal (Mukherjee and Mittal 2005), clearly identified inhibition of Aspergillus terreus and Cladosporium oxysporum, when exposed to the insecticide endosulfan at concentration of 100 µg/l.
However, the quantities used in degradation experiments vary considerably, depending on the compound used, fluctuating between 1 and 500 mg/l (Kästner et al. 1999; Sasaki et al. 2005; Lee et al. 2014). Aspergillus flavus was capable of degrading 800 ppm (99.6%) of glyphosate in a liquid medium after 16 days growth (Eman et al. 2013). The above implies that the concentrations of contaminants that fungi can tolerate, varies significantly. In nature, when a herbicide is added to the soil, microorganisms demonstrate different response times; a part of the soil microbiota is poisoned, resulting in cellular lysis (Bending et al. 2002). However, several microorganisms are resistant and tolerant to the contaminant, increasing their biomass in order to reduce competition (Gondar et al. 2012; Rongchapo et al. 2016). Only a few of them can grow and degrade the compound efficiently. Many of the strains used in this study present similar patterns of intoxication, tolerance and degradation when in contact with the herbicide paraquat.
Three strains demonstrated the highest level of degradation in the experiments carried out in liquid mediums: T. versicolor ECS- 79 and T. pavonia ECS- 67 presented 55% degradation, while H. dispersum ECS-705 presented 71% degradation after 15 days of growth. We have no knowledge of any reported study on paraquat degradation by macromycetes; there are only two reports concerning microorganisms, one is with P. putida and the other with Lipomyces sp. P. putida attained 47.29% degradation in a culture medium that was free of an absorbent agent used to increase percentage degradation to a maximum of 95% (Kopytko et al. 2002). In contrast, the yeast Lipomyces sp. attained 100% degradation of paraquat in a liquid culture after 3 days incubation; however, the contaminant concentration was only 27 ppm. When the paraquat concentration was increased two fold (54 ppm), biomass and paraquat degradation decreased notably to less than 10% (Hata et al. 1986). The degradation obtained by H. dispersum ECS-705 (71%) is less than previously reported for P. putida. In our study, the concentration of paraquat was five times greater. A similar effect was reported by Hua Fang (Fang et al. 2008), for Verticillium sp on chlorpyrifos, which experienced a degradation percentage of 88.5%, although the paraquat concentrations were much lower (50 mg/l). This also coincides with a study by Da Silva Coelho (da Silva Coelho et al. 2010), where levels of diuron and bentazon degradation were 74 and 61%, respectively, after 10 days culture, under conditions similar to those evaluated in this study.
The strain ECS-79 was identified as Trametes versicolor. There are several reports on the degradation potential of this species and together with P. chrysosporium, is one of the most frequently studied macromycete species regarding the degradation of xenobiotic compounds such as aromatic polycyclic hydrocarbons (anthracene and naphthalene) (Marco-Urrea et al. 2010; Jelic et al. 2012; Wolfand et al. 2016; Kamei 2017). This paper represents the first study that analyses the breakdown of the herbicide paraquat by both of these strains. There is only one study of the strain ECS- 67, T. pavonia; examining the degradation of ferulic acid (Tanruean et al. 2013). Other studies have analyzed the productive capacity of ligninolytic enzymes such as laccase and manganese peroxidase (Kenkebashvili et al. 2012; Singh et al. 2013; Sari et al. 2016). This is associated with the degradation of recalcitrant compounds and coincides with the observations made in this study where this strain demonstrated a high percentage of degradation.
This is the first study on the potential for xenobiotic degradation by the species H. dispersum. Fungi of the genus Hypholoma play an important role in decomposition of woody materials, in particular wood polymers such as lignin, cellulose and hemicellulose. Species in this genus are easily recognizable because the dark spores create a distinctive greenish effect on the yellow cap underside. Hypholoma means mushrooms with threads, because of the threads-like veil that connects the cap to the stem when young (Parker 1933; Cortez and Silveira 2007).
H. fasciculare is the only species belonging to the Hypholoma genus that has been studied for the degradation of chlorpyrifos and the pesticides atrazine and diuron. It also has shown ability to degrade over 88% of terbuthylazine, a triazine herbicide in liquid culture after 42 days (Gramss et al. 1999; Bending et al. 2002).
Only one of the three strains that presented the highest degradation percentages presented an increase in laccase enzyme activity (T. versicolor ECS-79). This is consistent with a study by Mougin (Mougin et al. 2002), who also reported an increase in laccase activity for T. versicolor ECS-79, in the presence of different environmental contaminants. However, the absence of this enzyme in the strain T. pavonia ECS-67, contrasts with the results presented by Arana-Cuenca (Arana-Cuenca et al. 2004), who reported the presence of laccase in this species. The absence of Lac and other ligninolytic enzymes during the assays does not provide conclusive proof that these strains are incapable of producing them. The lack of these enzymes could be due to diverse factors, primarily culture medium composition, in addition to time and incubation conditions (Saparrat et al. 2002; Zucca et al. 2011).
The ligninolytic enzymes that constitute the fungi degradation systems vary for each species. Some rot fungi selectively degrade lignin; consequently, these species lack one or two ligninolytic enzymes (Yang et al. 2013; Rouches et al. 2016). It should be emphasised that the conditions evaluated at the laboratory level are different from those found in nature. The synthesis and secretion of ligninolytic enzymes to the extracellular medium occur under conditions of limited levels of carbon or nitrogen; however, this is suppressed by agitation in a submerged liquid medium (Elisashvili et al. 2009; Zhuo et al. 2017). Therefore, the precise conditions for each species and strain must be assessed in order to make the most of their potential for degradation.
Activity could only be detected in three of the five ligninolytic enzymes monitored and only in the case of the strain T. versicolor ECS-79, did there appear to be a relationship between paraquat degradation and the production of these enzymes. However, the strain that presented the highest percentage of degradation, demonstrated only a low level of aryl alcohol oxidase activity. Jauregui, Kües and Shin (Jauregui et al. 2003; Kües 2015; Shin et al. 2017), found the presence of an intracellular system of enzymes that corresponds to the cytochrome P-450 system, which could be linked to contaminant degradation. This system may explain paraquat degradation in the absence of several evaluated ligninolytic enzymes; however, results obtained so far do not provide sufficient evidence to conclude if this is attributable to this type of intracellular system.
In summary, the strain H. dispersum ECS-705 presented the highest degradation of the herbicide. This coincides with the greatest production of biomass (6.2 g/l) and high speed growth when paraquat is present (0.57 g/day); however, when ligninolytic activity was analyzed, only AAO activity was observed. The strain T. versicolor ECS-79 attained 54.9% paraquat degradation and presented three of the five evaluated activities, with a note worthy laccase activity of 65.8 U/ml. This strain also demonstrated the highest MnP activity, with 55.7 U/ml.
The strains that demonstrated high levels of degradation capability have potential as bioremediators of the herbicide paraquat. In future studies, the degradation and culture conditions for the strain H. dispersum ECS-705 could be optimized in order to increase the degradation rate and favour the production of enzymes that may be involved in the degradation process. Furthermore, it is essential that other types of emerging contaminants be evaluated.
Acknowledgements
The first author is grateful to the Consejo Nacional de Ciencia y Tecnología (CONACYT) (National Council for Science and Technology) for providing the scholarship PDNAL-227794. We thank Lilia Moreno Ruiz, René Humberto Andrade Gallegos and Luz Verónica García Fajardo, for their valuable help during this study.
Compliance with ethical standards
Conflict of interest
Authors report no conflict of interest.
References
- Anasonye F, Winquist E, Räsänen M, et al. Bioremediation of TNT contaminated soil with fungi under laboratory and pilot scale conditions. Int Biodeterior Biodegrad. 2015;105:7–12. doi: 10.1016/j.ibiod.2015.08.003. [DOI] [Google Scholar]
- Arana-Cuenca A, Roda A, Téllez A, et al. Comparative analysis of laccase-isozymes patterns of several related Polyporaceae species under different culture conditions. J Basic Microbiol. 2004;44:79–87. doi: 10.1002/jobm.200310324. [DOI] [PubMed] [Google Scholar]
- Bautista LF, Morales G, Sanz R. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by laccase from Trametes versicolor covalently immobilized on amino-functionalized SBA-15. Chemosphere. 2015;136:273–280. doi: 10.1016/j.chemosphere.2015.05.071. [DOI] [PubMed] [Google Scholar]
- Bending GD, Friloux M, Walker A, et al. Degradation of contrasting pesticides by white rot fungi and its relationship with ligninolytic potential. FEMS Microbiol Lett. 2002;212:59–63. doi: 10.1111/j.1574-6968.2002.tb11245.x. [DOI] [PubMed] [Google Scholar]
- Blanco-Ayala T, Andérica-Romero AC, Pedraza-Chaverri J. New insights into antioxidant strategies against paraquat toxicity. Free Radic Res. 2014;48:623–640. doi: 10.3109/10715762.2014.899694. [DOI] [PubMed] [Google Scholar]
- Cantavenera MJ, Catanzaro I, Loddo V, et al. Photocatalytic degradation of paraquat and genotoxicity of its intermediate products. J Photochem Photobiol A Chem. 2007;185:277–282. doi: 10.1016/j.jphotochem.2006.06.021. [DOI] [Google Scholar]
- Conde-Cid M, Paradelo R, Fernández-Calviño D, et al. Retention of quaternary ammonium herbicides by acid vineyard soils with different organic matter and Cu contents. Geoderma. 2017;293:26–33. doi: 10.1016/j.geoderma.2017.01.027. [DOI] [Google Scholar]
- Cortez VG, da Silveira RMB. Species of Hypholoma (Fr.) P. Kumm. (Strophariaceae, Agaricales) in Rio Grande do Sul State, Brazil. Acta Bot Brasilica. 2007;21:609–621. doi: 10.1590/S0102-33062007000300008. [DOI] [Google Scholar]
- da Silva Coelho J, de Oliveira AL, Marques de Souza CG, et al. Effect of the herbicides bentazon and diuron on the production of ligninolytic enzymes by Ganoderma lucidum. Int Biodeterior Biodegrad. 2010;64:156–161. doi: 10.1016/j.ibiod.2009.12.006. [DOI] [Google Scholar]
- Devashree Y, Dutta BK, Paul SB, Choudhury S. The effect of Paraquat and Fipronil on the soil and rh izosphere microflora of tea (Camellia sinensis (L) O. kuntze) Int J Innov Appl Stu d. 2014;7:1534–1543. [Google Scholar]
- Ding J, Chen B, Zhu L. Biosorption and biodegradation of polycyclic aromatic hydrocarbons by Phanerochaete chrysosporium in aqueous solution. Chinese Sci Bull. 2013;58:613–621. doi: 10.1007/s11434-012-5411-9. [DOI] [Google Scholar]
- Elisashvili V, Kachlishvili E, Tsiklauri N, et al. Lignocellulose-degrading enzyme production by white-rot Basidiomycetes isolated from the forests of Georgia. World J Microbiol Biotechnol. 2009;25:331–339. doi: 10.1007/s11274-008-9897-x. [DOI] [Google Scholar]
- Eman A, Abdel-megeed A, Suliman AA, Sadik MW. Biodegradation of Glyphosate by fungal strains isolated from herbicides polluted-soils in Riyadh area. Int J Curr Microbiol App Sci. 2013;2:359–381. [Google Scholar]
- Fang H, Qin Xiang Y, Jie Hao Y, et al. Fungal degradation of chlorpyrifos by Verticillium sp. DSP in pure cultures and its use in bioremediation of contaminated soil and pakchoi. Int Biodeterior Biodegrad. 2008;61:294–303. doi: 10.1016/j.ibiod.2007.10.001. [DOI] [Google Scholar]
- Florêncio MH, Pires E, Castro AL, et al. Photodegradation of Diquat and Paraquat in aqueous solutions by titanium dioxide: evolution of degradation reactions and characterisation of intermediates. Chemosphere. 2004;55:345–355. doi: 10.1016/j.chemosphere.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Fulekar MH, Pathak B, Fulekar J, Godambe T. Bioremediation of organic pollutants using Phanerochaete chrysosporium. In: Goltapeh EM, Danesh YR, Varma A, editors. Fungi as bioremediators. Berlin: Springer; 2013. pp. 135–157. [Google Scholar]
- Gondar D, Lopez R, Antelo J, et al. Adsorption of paraquat on soil organic matter: Effect of exchangeable cations and dissolved organic carbon. J Hazard Mater. 2012;235:218–223. doi: 10.1016/j.jhazmat.2012.07.044. [DOI] [PubMed] [Google Scholar]
- Gramss G, Voigt K-D, Kirsche B. Degradation of polycyclic aromatic hydrocarbons with three to seven aromatic rings by higher fungi in sterile and unsterile soils. Biodegradation. 1999;10:51–62. doi: 10.1023/A:1008368923383. [DOI] [PubMed] [Google Scholar]
- Guillén F, Gómez-Toribio V, Martínez MJ, Martínez AT. Production of hydroxyl radical by the synergistic action of fungal laccase and aryl alcohol oxidase. Arch Biochem Biophys. 2000;383:142–147. doi: 10.1006/abbi.2000.2053. [DOI] [PubMed] [Google Scholar]
- Hata S, Shirata K, Takagishi H. Degradation fo paraquat and diquat by the yeast Lipomyces starkei. J Gen Appl Microbiol. 1986;32:193–202. doi: 10.2323/jgam.32.193. [DOI] [Google Scholar]
- Jafarinejad S. Recent advances in determination of herbicide paraquat in environmental waters and its removal from aqueous solutions: a review. Int Res J Appl Basic Sci. 2015;9:1758–1774. [Google Scholar]
- Jaiswal DK, Verma JP, Yadav J. Microbe induced degradation of pesticides in agricultural soils. In: Singh SN, editor. Microbe-induced degradation of pesticides. Cham: Springer International Publishing; 2017. pp. 167–189. [Google Scholar]
- Jauregui J, Valderrama B, Albores A, Vazquez-Duhalt R. Microsomal transformation of organophosphorus pesticides by white rot fungi. Biodegradation. 2003;14:397–406. doi: 10.1023/A:1027316610450. [DOI] [PubMed] [Google Scholar]
- Jelic A, Cruz-Morató C, Marco-Urrea E, et al. Degradation of carbamazepine by Trametes versicolor in an air pulsed fluidized bed bioreactor and identification of intermediates. Water Res. 2012;46:955–964. doi: 10.1016/j.watres.2011.11.063. [DOI] [PubMed] [Google Scholar]
- Kamei I. Co-culturing effects of coexisting bacteria on wood degradation by Trametes versicolor. Curr Microbiol. 2017;74:125–131. doi: 10.1007/s00284-016-1162-1. [DOI] [PubMed] [Google Scholar]
- Kästner M, Streibich S, Beyrer M, et al. Formation of Bound Residues during microbial degradation of [(14)C]Anthracene in soil. Appl Environ Microbiol. 1999;65:1834–1842. doi: 10.1128/aem.65.5.1834-1842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkebashvili N, Elisashvili V, Wasser SP. Effect of carbon, nitrogen sources, and copper concentration on the ligninolytic enzyme production by Coriolopsis gallica. J Waste Convers Bioprod Biotechnol. 2012;1:22–27. [Google Scholar]
- Kopytko M, Chalela G, Zauscher F. Biodegradation of two commercial herbicides (Gramoxone and Matancha) by the bacteria Pseudomonas putida. Electron J Biotechnol. 2002;5(2):182–195. doi: 10.2225/vol5-issue2-fulltext-1. [DOI] [Google Scholar]
- Kües U. Fungal enzymes for environmental management. Curr Opin Biotechnol. 2015;33:268–278. doi: 10.1016/j.copbio.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Kumar H, Singh VB, Meena BL, et al. Paraquat poisoning: a case report. J Clin Diagn Res. 2016;10:OD10–OD11. doi: 10.7860/JCDR/2016/15858.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Jang Y, Choi Y-S, et al. Biotechnological procedures to select white rot fungi for the degradation of PAHs. J Microbiol Methods. 2014;97:56–62. doi: 10.1016/j.mimet.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Ma XL, Kong P, You MP, et al. Molecular variation among isolates belonging to eight races of Phytophthora clandestina. Australas Plant Pathol. 2009;38:608–616. doi: 10.1071/AP09047. [DOI] [Google Scholar]
- Marco-Urrea E, Pérez-Trujillo M, Cruz-Morat C, et al. Degradation of the drug sodium diclofenac by Trametes versicolor pellets and identification of some intermediates by NMR. J Hazard Mater. 2010;176:836–842. doi: 10.1016/j.jhazmat.2009.11.112. [DOI] [PubMed] [Google Scholar]
- Mougin C, Kollmann A, Jolivalt C. Enhanced production of laccase in the fungus Trametes versicolor by the addition of xenobiotics. Biotechnol Lett. 2002;24:139–142. doi: 10.1023/A:1013802713266. [DOI] [Google Scholar]
- Muhamad H, Ismail BS, Sameni M, Mat N. Adsorption study of 14C-paraquat in two Malaysian agricultural soils. Environ Monit Assess. 2011;176:43–50. doi: 10.1007/s10661-010-1565-6. [DOI] [PubMed] [Google Scholar]
- Mukherjee I, Mittal A. Bioremediation of Endosulfan Using Aspergillus terreus and Cladosporium oxysporum. Bull Environ Contam Toxicol. 2005;75:1034–1040. doi: 10.1007/s00128-005-0853-2. [DOI] [PubMed] [Google Scholar]
- Newcombe D, Paszczynski A, Gajewska W, et al. Production of small molecular weight catalysts and the mechanism of trinitrotoluene degradation by several Gloeophyllum species. Enzyme Microb Technol. 2002;30:506–517. doi: 10.1016/S0141-0229(02)00014-5. [DOI] [Google Scholar]
- Parker CS. A taxonomic study of the genus Hypholoma in North America. Mycologia. 1933;25:160–212. doi: 10.2307/3754210. [DOI] [Google Scholar]
- Pateiro-Moure M, Nóvoa-Muñoz JC, Arias-Estévez M, et al. Quaternary herbicides retention by the amendment of acid soils with a bentonite-based waste from wineries. J Hazard Mater. 2009;164:769–775. doi: 10.1016/j.jhazmat.2008.08.071. [DOI] [PubMed] [Google Scholar]
- PMEP (2017) Pesticide Management Education Program. In: Cornell Univ. http://pmep.cce.cornell.edu/. Accessed 3 Aug 2017
- Purnomo AS, Mori T, Kamei I, et al. Application of mushroom waste medium from Pleurotus ostreatus for bioremediation of DDT-contaminated soil. Int Biodeterior Biodegrad. 2010;64:397–402. doi: 10.1016/j.ibiod.2010.04.007. [DOI] [Google Scholar]
- Purnomo AS, Mori T, Kondo R. Involvement of Fenton reaction in DDT degradation by brown-rot fungi. Int Biodeterior Biodegrad. 2010;64:560–565. doi: 10.1016/j.ibiod.2010.06.008. [DOI] [Google Scholar]
- Purnomo AS, Mori T, Takagi K, Kondo R. Bioremediation of DDT contaminated soil using brown-rot fungi. Int Biodeterior Biodegrad. 2011;65:691–695. doi: 10.1016/j.ibiod.2011.04.004. [DOI] [Google Scholar]
- Rhodes CJ. Mycoremediation (bioremediation with fungi)—growing mushrooms to clean the earth. Chem Speciat Bioavailab. 2014;26:196–198. doi: 10.3184/095422914X14047407349335. [DOI] [Google Scholar]
- Rodríguez E, Nuero O, Guillén F, et al. Degradation of phenolic and non-phenolic aromatic pollutants by four Pleurotus species: the role of laccase and versatile peroxidase. Soil Biol Biochem. 2004;36:909–916. doi: 10.1016/j.soilbio.2004.02.005. [DOI] [Google Scholar]
- Rongchapo W, Khamdahsag P, Grisdanurak N, et al. Photocatalytic degradation OF Paraquat by using titanium dioxide on rice husk silica and zeolite y in sodium form. Suranaree J Sci Technol. 2016;23:343–350. [Google Scholar]
- Rouches E, Herpoël-Gimbert I, Steyer JP, Carrere H. Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: A review. Renew Sustain Energy Rev. 2016;59:179–198. doi: 10.1016/j.rser.2015.12.317. [DOI] [Google Scholar]
- Saparrat MCN, Martínez MJ, Cabello MN, Arambarri AM. Screening for ligninolytic enzymes in autochthonous fungal strains from Argentina isolated from different substrata. Rev Iberoam Micol. 2002;19:181–185. [PubMed] [Google Scholar]
- Sari AA, Yasin H, Tachibana S, Hadibarata T. Effects of Mediators for ligninolytic enzyme production and kinetic studies on degradation of pentachlorobenzene by Trametes versicolor U80. Water Air Soil Pollut. 2016;227:317. doi: 10.1007/s11270-016-3006-9. [DOI] [Google Scholar]
- Sasaki M, Maki J, Oshiman K, et al. Biodegradation of bisphenol A by cells and cell lysate from Sphingomonas sp. strain AO1. Biodegradation. 2005;16:449–459. doi: 10.1007/s10532-004-5023-4. [DOI] [PubMed] [Google Scholar]
- Shin JY, Bui DC, Lee Y, et al. Functional characterization of cytochrome P450 monooxygenases in the cereal head blight fungus Fusarium graminearum. Environ Microbiol. 2017;19:2053–2067. doi: 10.1111/1462-2920.13730. [DOI] [PubMed] [Google Scholar]
- Singh MP, Vishwakarma SK, Srivastava AK, et al. Bioremediation of Direct Blue 14 and extracellular ligninolytic enzyme production by white rot fungi: Pleurotus Spp. Biomed Res Int. 2013;2013:1–4. doi: 10.1155/2013/180156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanruean K, Chandet N, Rakariyatham N. Bioconversion of ferulic acid into high value metabolites by White rot fungi isolated from fruiting-body of the polypore mushroom. J Med Bioeng. 2013;2:168–172. [Google Scholar]
- Tien M, Kirk TK. Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci USA. 1984;81:2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco R, Pickard MA, Vazquez-Duhalt R. Kinetic differences of purified laccases from six Pleurotus ostreatus strains. Lett Appl Microbiol. 2001;32:331–335. doi: 10.1046/j.1472-765X.2001.00913.x. [DOI] [PubMed] [Google Scholar]
- Tsai W-T. A review on environmental exposure and health risks of herbicide paraquat. Toxicol Environ Chem. 2013;95:197–206. doi: 10.1080/02772248.2012.761999. [DOI] [Google Scholar]
- Wariishi H, Valli K, Gold MH. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J Biol Chem. 1992;267:23688–23695. [PubMed] [Google Scholar]
- Wolfand JM, LeFevre GH, Luthy RG. Metabolization and degradation kinetics of the urban-use pesticide fipronil by white rot fungus Trametes versicolor. Environ Sci Process Impacts. 2016;18:1256–1265. doi: 10.1039/C6EM00344C. [DOI] [PubMed] [Google Scholar]
- Yang S, Hai FI, Nghiem LD, et al. Understanding the factors controlling the removal of trace organic contaminants by white-rot fungi and their lignin modifying enzymes: a critical review. Bioresour Technol. 2013;141:97–108. doi: 10.1016/j.biortech.2013.01.173. [DOI] [PubMed] [Google Scholar]
- Zahedi F, Behpour M, Ghoreishi SM, Khalilian H. Photocatalytic degradation of paraquat herbicide in the presence TiO2 nanostructure thin films under visible and sun light irradiation using continuous flow photoreactor. Sol Energy. 2015;120:287–295. doi: 10.1016/j.solener.2015.07.010. [DOI] [Google Scholar]
- Zhu Y, Xue J, Cao J, Xiao H. A potential mechanism for degradation of 4,5-dichloro-2-(n-octyl)-3[2 H]-isothiazolone (DCOIT) by brown-rot fungus Gloeophyllum trabeum. J Hazard Mater. 2017;337:72–79. doi: 10.1016/j.jhazmat.2017.04.072. [DOI] [PubMed] [Google Scholar]
- Zhuo R, Yuan P, Yang Y, et al. Induction of laccase by metal ions and aromatic compounds in Pleurotus ostreatus HAUCC 162 and decolorization of different synthetic dyes by the extracellular laccase. Biochem Eng J. 2017;117:62–72. doi: 10.1016/j.bej.2016.09.016. [DOI] [Google Scholar]
- Zucca P, Rescigno A, Olianas A, et al. Induction, purification, and characterization of a laccase isozyme from Pleurotus sajor-caju and the potential in decolorization of textile dyes. J Mol Catal B Enzym. 2011;68:216–222. doi: 10.1016/j.molcatb.2010.11.008. [DOI] [Google Scholar]