Abstract
Background
The emergence of multidrug-resistant bacteria is a worldwide concern and in order to find an alternative to this problem, the occurrence of antimicrobial compounds in Plectranthus amboinicus essential oil was investigated. Thus, this study aims to determine susceptibility of Staphylococcus aureus isolated from food to antibiotics, P. amboinicus essential oil (PAEO) and carvacrol.
Methods
Leaves and stem of P. amboinicus were used for extraction of essential oil (PAEO) by hydrodistillation technique and EO chemical analysis was performed by gas chromatography coupled to a mass spectrometer. S. aureus strains (n = 35) isolated from food and S. aureus ATCC 6538 were used to evaluate the antimicrobial and antibiofilm activity of PAEO and carvacrol. All strains (n = 35) were submitted to antimicrobial susceptibility profile by disk diffusion method. Determination of MIC and MBC was performed by microdilution technique and antibiofilm activity was determined by microtiter-plate technique with crystal violet assay and counting viable cells in Colony Forming Units (CFU).
Results
Carvacrol (88.17%) was the major component in the PAEO. Antibiotic resistance was detected in 28 S. aureus strains (80%) and 12 strains (34.3%) were oxacillin and vancomycin-resistant (OVRSA). From the 28 resistant strains, 7 (25%) showed resistance plasmid of 12,000 bp. All strains (n = 35) were sensitive to PAEO and carvacrol, with inhibition zones ranging from 16 to 38 mm and 23 to 42 mm, respectively. The lowest MIC (0.25 mg mL−1) and MBC (0.5 mg mL−1) values were observed when carvacrol was used against OVRSA. When a 0.5 mg mL−1 concentration of PAEO and carvacrol was used, no viable cells were found on S. aureus biofilm.
Conclusion
The antibacterial effect of carvacrol and PAEO proves to be a possible alternative against planktonic forms and staphylococcal biofilm.
Keywords: Staphylococcus aureus, Drug-resistant bacteria, Plectranthus amboinicus
Background
Staphylococcus aureus is a Gram-positive cocci often associated with gastroenteritis acquired from contaminated foods such as milk [1] and shrimp. The occurrence of multidrug-resistant bacteria in food is a worldwide concern [2], and S. aureus drug-resistant to beta-lactams has been isolated from dairy products [1] and shrimp [3].
Some factors act for selecting drug-resistant bacteria pathogens, such as inappropriate use of antibiotics, and/or the inadequate disposal of antimicrobial drugs in the environment [4, 5]. Thus, the inappropriate use of antibiotics in aquaculture [6, 7] and livestock [8] has contributed to the selection of resistant microbial species, and, consequently, increased food contamination level of animal origin and derivatives.
As an alternative to mitigate the occurrence of multidrug-resistant bacteria, the study of antimicrobial compounds in phanerogams has been proposed [9]. In this context, the plants of genus Plectranthus - 3000 recognized species, spread along countries in Africa, South America, Asia and Australia – are widely recognized in folk and popular medicine, being employed in digestive treatments as well as in infectious, inflammatory and respiratory problems [10, 11]. Species of Plectrantus [12, 13], including P. amboinicus [14], has been studied due to its pharmacological properties in order to validate its popular use.
Bioactivity of P. amboinicus is related to the occurrence of 76 volatiles and 30 non-volatile compounds belonging to different classes of phytochemicals (monoterpenoids, diterpenoids, triterpenoids, sesquiterpenoids, phenolics, flavonoids, esters, alcohols and aldehydes) [15]. Studies about the pharmacological activities of P. amboinicus are conducted from extracts or essential oil, i.e., complex volatile compounds, synthesized naturally in different plant parts during the process of secondary metabolism with great potential in the field of biomedicine [16]. In this sense, P. amboinicus crude essential oils could be used as a tool for the developing novel and more efficacious antimicrobial agents against S. epidermidis and Candida species [17].
The susceptibility of methicillin-resistant S. aureus (MRSA) clinical isolates to extracts of P. amboinicus has been reported [18], however, studies about the action of this oil and its major component against planktonic and biofilm of drug-resistant S. aureus from food are still incipient.
Thus, this study aims to determine the susceptibility of drug-resistant S. aureus strains isolated from milk and shrimp to P. amboinicus essential oil (PAEO) and its major component - carvacrol.
Methods
Strains origin
Thirty five Staphylococcus aureus strains isolated from pasteurized milk (n = 12) and shrimp (n = 23) were used. All strains are part of the bacterial catalogue available at Center for Applied Molecular Bioprospecting and Experimentation (NUBEM - INTA College) and had the following biochemical characteristics: Gram-positive cocci, catalase-positive, manitol-positive and coagulase-positive. The strains, maintained in Tryptone Soy Broth with 20% glycerol, were plated onto Mannitol Salt Agar (35 °C for 48 h) and isolated Tryptone Soy Agar for performing microbiological analyzes.
Antibiogram
As screening for the selection of drug-resistant strain for Minimum Inhibitory Concentration (MIC) and minimum bactericidal concentration (MBC) and antibiofilm activity, all strains (n = 35) were submitted to antimicrobial susceptibility profile by disk diffusion method using Mueller Hinton Agar [16]. The following antibiotics discs were used: oxacillin (1 μg), penicillin G (10 U), ampicillin (10 μg), cefotaxime (30 μg), ceftriaxone (30 μg), cefepime (30 μg), imipenem (10 μg), vancomycin (30 μg), gentamicin (10 μg), and chloramphenicol (30 μg). Antimicrobial susceptibility was detected by analyzing the inhibition zones, measured and compared according the standards set by the CLSI (2012) [19] which classify the strains as sensitive, intermediate or resistant.
Plasmid DNA extraction
S. aureus resistant strains were inoculated in Brain Heart Infusion broth and cultured for 24 h at 35 °C. Then, 2.0 mL of each culture was retrieved for plasmid DNA extraction with a commercially available extraction kit (GeneJET Plasmid Miniprep Kit). A 3.0 μL aliquot of each sample of extracted DNA was submitted to 1.5% agarose gel electrophoresis in TBE buffer for 60 min at 120 V, 200 mA and 100 w. The plasmid DNA was viewed under UV light with a Spectrolinetransilluminator and photographed with a Kodak EDAS 290.
Plant material
Leaves and stem of P. amboinicus were collected in October 2014, on a farm (3.49′52,16″S, 40.24′38,38″W) located in Cariré (Northest Brazil). The botanical identification was made at Herbarium Professor Francisco José de Abreu Matos – (HUVA), Acaraú Valley State University (Sobral-CE). The dried specimens were deposited in the HUVA and registered under specific numbering 18,416.
Extraction of Plectranthus amboinicus essential oil (PAEO)
Extraction of PAEO was made at the NUBEM (INTA College), using the hydrodistillation technique. Four extractions were performed, using 7.690 Kg of plant in order to obtain 5.2 mL essential oil, approximately. PAEO was transferred to sterile amber vial, which was covered with aluminum foil and maintained at 2 to 8 °C. An aliquot of approximately 20 μL was used for chemical analysis.
Chemical analysis of the essential oil (PAEO)
The essential oil chemical analysis was performed by gas chromatography coupled to a mass spectrometer (GC-MS) using a gas chromatograph (Shimadzu QP2010 Plus), and helium (He) as carrier gas. A capillary column Factor Four/ VF-5 ms, 30 m length, 0.25 mm internal diameter, 0.25 mm film thickness was used. The carrier gas flow rate was 1 mL min−1. The initial oven temperature was 60 °C. After heating for 2 min, it increased at a constant rate of 2 °C per minute up to 110 °C; then 3 °C per minute to 150 °C; and 15 °C per minute to 290 °C to a final, isotherm 290 °C for 17 min. The injector and detector temperatures were respectively 250 °C and 310 °C. The mode of injection was split and the injection volume was 1 mL. Mass spectra were produced by electron impact (70 eV). Quantitative analysis of the essential oil composition was made on a gas chromatograph, coupled with HP5890 Series II ionization detector, using the same operating conditions and the same type of column as the CG/EM analysis (except for the injector and detector temperatures, which were of 220 °C and 250 °C, respectively). The percentage of each constituent is calculated by the integral of the area under the respective peaks to the total area of all the constituents of the sample. The various constituents of the essential oil were identified by visual comparison of their mass spectra with those in the literature [20] and with actual standards in Nist08 library computer system, as well as by comparing retention rates with those in the literature [20]. A standard solution of n-alkanes (C8-C20) was injected under the same chromatographic conditions used to provide samples and to get retention rates.
PAEO and carvacrol antimicrobial effect
Antimicrobial potential of OEPA and carvacrol was determined by: (1) antibiogram, (2) determination of minimum inhibitory concentration (MIC), (3) minimum bactericidal concentration (CBM) and (4) antibiofilm activity.
Antibiogram to PAEO and carvacrol
The antimicrobial activity of PAEO and its major component was performed from the disk diffusion test on Mueller Hinton Agar, as detailed on the Antibiogram item. Sterile paper discs (6 mm in diameter) were soaked with PAEO (20 μL) and 98% Carvacrol (20 μL) (Sigma). The antimicrobial effect was detected by the formation of inhibition zones around the disks soaked with PAEO and carvacrol. The halos were measured in millimeters, labeled as bioactive those larger than 15 mm. As positive and negative control, tetracycline (30 μg) and disks soaked with 20 μLTween 80 solution 3% were used, respectively.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
Determination of MIC and MBC was performed by microdilution technique in Tryptone Soy Broth (TSB) [19], using polystyrene plates of 96 wells and two strains: S. aureus ATCC 6538, and oxacillin- and vancomycin-resistant S. aureus (OVRSA).Concentration of both strains was adjusted in TSB to 1.25 × 107 Colony Forming Units (CFU mL−1).20 replicates of each strain were tested with PAEO and carvacrol in concentrations of 4 mg mL−1, 2 mg mL−1, 1 mg mL−1, 0.5 mg mL−1, 0.25 mg mL−1, 0.125 mg mL−1 and 0.0625 mg mL−1. MIC was determined from visual reading and was considered the concentration able to inhibit microbial growth. In order to determine the MBC, a pool was made of 10 wells for each concentration, and then plating was performed in 10 μL in triplicate Tryptone Soy Agar. The concentration which was not verified microbial growth was considered CBM.
Antibiofilm activity
Antibiofilm activity was determined by microtiter-plate technique [21] with crystal violet (CV) assay, counting viable cells in Colony Forming Units (CFU) [22]. In order to determine the PAEO and carvacrol action in the biofilm, plates were subjected to optical density microplate reading (Molecular Devices -SpectraMaxParadigmMulti-Mode) at a wavelength of 570 nm. Activity was found in concentrations that failed to display the adhesion of crystal violet in any of the wells.
Controls
For CIM, CBM and antibiofilm activity, TSB containing the test substance (PAEO or carvacrol) in concentrations of 4 mg mL−1, 2 mg mL−1, 1 mg mL −1, 0.5 mg mL -1, 0.25 mg mL -1, 0.125 mg mL−1 and 0.0625 mg mL−1 was used as turbidity control. For contamination control, only the culture medium (TSB) in three wells on the plates we used. Negative control was done by inoculating 100 μL of strain (1.25 × 107 CFU mL−1) in wells containing culture medium (TSB). As positive control, tetracycline in the same concentration of test substances was used: 4 mg mL−1, 2 mg mL−1, 1 mg mL −1, 0.5 mg mL -1, 0.25 mg mL -1, 0.125 mg mL−1 and 0.0625 mg mL−1.
Statistical analysis
Data analysis in GraphPad Prism 5 with ANOVA statistical, followed by a Student-Newman-Keuls test for significant variability comparisons (p < 0.001) between different concentrationswere used to conduct the analysis.
Results
Antibiotic resistance was observed in 28 S. aureus strains (80%) and ten resistance profiles were observed: three profiles of monoresistance (n = 5, 17.8%), three cross-resistance to beta-lactams (n = 8; 28.6%) and four multidrug resistant (n = 15; 53.6%) (Table 1). From the 28 resistant strains, 7 (25%) showed plasmid of 12,000 bp (Fig. 1).
Table 1.
Antimicrobial resistance profile of 28 Stapylococcus aureus strains isolated from milk and shrimp
| Profile | Drugs | N° of Isolates | Total (%) | |
|---|---|---|---|---|
| Shrimp | Milk | |||
| Monoresistance | Pen | 1 | 0 | 5 (17.8) |
| Oxa | 3 | 0 | ||
| Cro | 1 | 0 | ||
| Cross-resistanceto beta-lactam | Pen + Amp | 2 | 0 | 8 (28.6) |
| Oxa + Amp | 3 | 0 | ||
| Pen + Amp + Oxa + Cro + Ctx + Cpm | 3 | 0 | ||
| Multidrug resistance | Amp + Van | 1 | 0 | 15 (53.6) |
| Pen + Amp + Gen | 1 | 0 | ||
| Pen + Amp + Oxa + Van | 1 | 11 | ||
| Pen + Amp + Oxa + Ctx + Van | 0 | 1 | ||
| Total | 16 | 12 | 28 (100) | |
PEN penicillin G, OXA oxacillin, CRO Ceftriaxone, AMP ampicillin, CTX cefotaxime, CPM cefepime, VAN vancomycin, GEN gentamicin
Fig. 1.
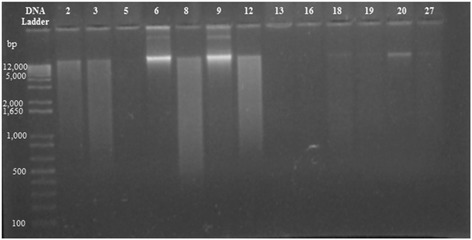
DNA Plasmid electrophoresed on 1.5% agarose gel. Lane 1: DNA Ladder. DNA Plasmid 12,000 bp instrains2 (Lane 2), 3 (Lane 3), 6 (Lane 5), 8 (Lane 6), 9 (Lane 7), 12 (Lane 8) and 20 (Lane 13)
Carvacrol (88.17%) was the major component in the essential oil of P. amboinicus, followed by caryophyllene oxide, 1,8-cineole, 4-terpinenol, alpha-cisbergamoteno, p-cymene and gamma- terpinene (Table 2, Fig. 2).
Table 2.
Chemical characterization of the essential oil extracted from leaves of Plectranthus amboinicus
| Components | CRI | RILIT | RTCG/FID | % |
|---|---|---|---|---|
| p-cymene | 1029 | 1026 | 11.757 | 0.72 |
| 1,8-cineol | 1037 | 1031 | 12.181 | 2.01 |
| Gamma-terpinene | 1063 | 1059 | 13.589 | 0.45 |
| 4-terpineol | 1184 | 1177 | 21.256 | 1.78 |
| Carvacrol | 1303 | 1299 | 29.335 | 88.17 |
| Alpha-cisbergamoteno | 1420 | 1412 | 35.765 | 0.84 |
| NI | 1435 | – | 36.470 | 0.17 |
| Caryophyllene oxide | 1591 | 1583 | 42.640 | 5.85 |
| Total | 99.99% |
CRI calculated retention index, RIlit retention index of the literature, RT retention time of CG-FID, NI unidentified
Fig. 2.
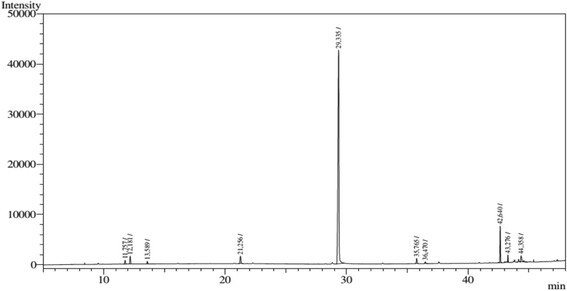
CG-MS chromatogram analysis of Plectranthus amboinicus essential oil
All strains (n = 35) of S. aureus were sensitive to PAEO and carvacrol with inhibition zones ranging from 16 to 38 mm and 23 to 42 mm, respectively (Table 3). The standard strain of S. aureus ATCC 6538 showed a inhibitory halo of 34 and 47, respectively, for PAEO and carvacrol.
Table 3.
Distribution of the Staphylococcus aureus strains according to inhibition zone size against the essential oil Plectranhus amboinicus, carvacrol and tetracycline
| Inhibition halos interval (mm) | Shrimp (n = 23) | Milk (n = 12) | ||||
|---|---|---|---|---|---|---|
| PAEO | Carvacrol | TCY | PAEO | Carvacrol | TCY | |
| 16–20 | 0 | 0 | 1 | 4 | 0 | 1 |
| 21–25 | 4 | 2 | 3 | 3 | 1 | 11 |
| 26–30 | 16 | 12 | 6 | 5 | 3 | 0 |
| 31–35 | 2 | 4 | 12 | 0 | 8 | 0 |
| 36–40 | 1 | 3 | 1 | 0 | 0 | 0 |
| 41–42 | 0 | 2 | 0 | 0 | 0 | 0 |
PAEO Plectranthus amboinicus essential oil. TCY tetracycline 30 μg. n number of bacterial strains. mm millimeters
MIC value of 0.25 mg mL−1 was observed when PAEO were used against S. aureus ATCC 6538 and carvacrol was used against S. aureus ATCC 6538 and OVRSA. The lowest MBC value (0.5 mg mL−1) was detected when carvacrol was used against OVRSA (Table 4).
Table 4.
Minimum Inhibitory Concentration (MIC mg mL−1) and Minimum Bactericidal Concentration (MBC mg mL−1) of Plectranthus amboinicus essential oil and carvacrol
| Substances | Activity antimicrobial | Staphylococcus aureus | |
|---|---|---|---|
| ATCC 6538 | OVRSA | ||
| PAEO | MIC | 0.25 | 0.5 |
| MBC | 2 | 1 | |
| Carvacrol | MIC | 0.25 | 0.25 |
| MBC | 1 | 0.5 | |
ATCC American Type Culture Collection, OVRSA Oxacillin and vancomycin-resistant Staphylococcus aureus
Biofilm activities against S. aureus ATCC 6538 and OVRSA were observed in concentrations of 0.5 mg mL−1 (OEPA) and 0.25 mg mL−1 (carvacrol) (Fig. 3). However, inhibition potential was detected in all tested concentrations of carvacrol and OEPA, in comparison to the negative control. Tetracycline inhibits biofilm formation (S. aureus ATCC 6538 and OVRSA) in all concentrations tested (0.062, 0.125, 0.25, 0.5, 1, 2 and 4 mg mL−1).
Fig. 3.
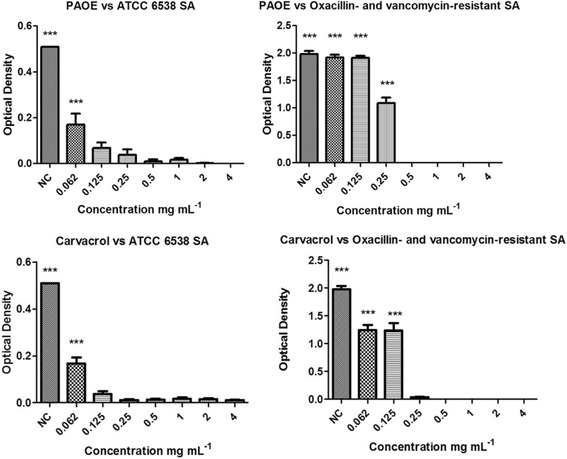
Optical density of bacterial biofilm in S. aureus ATCC 6538 and S. aureus isolated from food, treated with OEPA and carvacrol in varying concentrations (0.062, 0.125, 0.25, 0.5, 1, 2 and 4 mg mL−1). ANOVA, followed by Student-Newman-Keulstest ***p < 0.001 vs. all variables.*SA: Staphylococcus aureus. NC: negative control
Discussion
Multidrug-resistant profile was detected in 15 strains (53.6%) (Table 1). Some studies have described the incidence of multidrug-resistant strains of S. aureus in foods and in those who handle them, confirming the assertion that one of the main sources of food contamination by S. aureus is the handler [23, 24]. Contamination of foods by S. aureus multiresistant are related to care in handling, processing and transport of food, as well as correlation of bacterial resistance to the indiscriminate use of antimicrobials in agriculture and aquaculture [2, 25].
In addition to the considerable rate of penicillin resistance, it is valuable to mention the high incidence of strains resistant to oxacillin (n = 22; 78.6%) (Table 1). Oxacillin resistance is associated of the mecA gene, which encodes a protein with low binding affinity to β-lactam. Thus, in vitro resistance to oxacillin indicated resistance to all other β-lactam drugs [26, 27].
In case of infection caused by strains resistant to oxacillin, the clinically chosen therapy is vancomycin. Thus, another fact worth mentioning in this study is that 14 (50%) of 28 all the resistant strains would persist against vancomycin (Table 1). The present research corroborate studies of Akindolire et al. (2015) [28] and Nunes et al. (2016) [29], that reporting a high incidence (60–100%) of S. aureus strains resistant to vancomycin in isolated samples of milk or its derivatives. Baumgartner et al. (2014) [23] reported the presence of S. aureus carriers of beta-lactam resistance genes, methicillin and vancomycin in foods, including dairy products.
Of the 28 resistant strains, 7 (25%) showed plasmid of 12,000 bp (Fig. 1) that are probably related to resistance, since these seven strains were isolated from milk and presented multi-drug resistance profile (Pen + Amp + Oxa + Van). For McCarthy and Lindsay (2012) [30], plasmids are responsible for the spread of virulence and resistance genes in S. aureus populations. The authors assigned a total of 39 plasmid groups of Staphylococcus aureus (pGSA) based on the combination of the rep genes each plasmid had and revealed 28 plasmid groups with large genomes (>15Kb),which carried a diverse range of genes.
Our findings corroborate several studies when reporting carvacrol as a major component of Plectranthus amboinicus essential oil. On the other hand, the concentration of carvacrol (88.17%) in the PAEO obtained in the present study (Table 2) was greater than the variation already described in literature - from 28 to 70%. Compounds such as γ-terpinene, ρ-cement and caryophyllene oxide have been found in smaller amounts when compared to data from this study [17, 31, 32]. Phytocompounds variation in essential oils may be related to the time of botanical material collection, region, soil and seasons [33]. Although detected in small quantities (Table 2), it is known that p-cymene boosts the pharmacological effects of carvacrol [34].
All strains (n = 35) of S. aureus were sensitive to PAEO and carvacrol. Most strains isolated from milk showed sensitivity to tetracycline with halos of 21–25 mm (n = 11; 91.6%) (Table 3). When subjected to the test with carvacrol, 100% (n = 12) demonstrated halos size of an equal sensitivity or greater than those of positive control. Moreover, inhibition halos of an equal or greater than those of positive control were observed in 66.6% of the tested strains from milk with OEPA.S. aureus strains isolated from shrimp showed sensitivity to tetracycline and most of them presented halos between 31 to 40 mm (n = 13, 56.52%) in size (Table 3). When tested with carvacrol and OEPA, 39.13% (n = 9) and 13.04% (n = 3) strains showed, respectively, halos sizes greater than or equal to control. Thus, the carvacrol was also more effective when used against the strains of shrimp samples. In this study, the antimicrobial activity of PAEO seems to be related to the concentration of carvacrol (88.17%), whereas all strains were susceptible to both compounds tested and the largest halos were observed when using carvacrol.
Providing support to the findings of this research, Ajitha et al. (2014) [35] found potential inhibition of the species P. amboinicus against Staphylococcus spp., Escherichia coli, Pseudomonas spp. and Bacillus spp. The authors describe halos measuring around 10 mm, i.e., values lower than those found in the present study.
Rodrigues et al. (2013) [36] evaluated the antimicrobial activity of OEPA containing 67.9% carvacrol, and found that the MIC against S. aureus ATCC 12692 (0.128 mg mL−1) was higher than MIC against the strain of multidrug-resistant S .aureus (0.032 mg mL−1). In the present study, it was observed that the MIC for OEPA OVRSA strain (0.5 mg mL−1) was higher than the MIC for S. aureus ATCC 6538 (0.25 mg mL−1). The difference between the two studies may be related to the chemical composition of the oils, since the OEPA obtained by Rodrigues et al., (2013) [36] had 13 compounds including thymol (1.3%) and thymol methyl ether (1.6%), while the OEPA used inthe present study contained seven constituents, with a concentration of carvacrol (88.17%) higher.
Regarding the CIM carvacrol, the same value (0.5 mg mL−1) was observed when both strains (S. aureus ATCC 6538 and OVRSA) were tested (Table 4). Carvacrol and thymol present the ability to dissolve themselves in the cytoplasmic membrane, aligning the fatty acid chains and providing an increase in the passive permeability of the membrane.
Considering the values of CBM, OEPA and carvacrol showed better results against the OVRSA strain (Table 4). This finding is of utmost importance, since it demonstrates a promising potential of those two bioactive substances as antimicrobial agents tested against medically resistant strain. Hosseinkhani et al. (2016) [37] also obtained promising results when testing the antibacterial activity of an essential oil rich in monoterpenes against multidrug resistant strains of S. aureus. According to the authors, the results may indicate the possibility of using monoterpenes incorporated into antimicrobial formulations in order to treat infections caused by multi-resistant bacteria.
In the present study, the isolated carvacrol demonstrated a bactericidal activity against both strains (ATCC and OVRSA) twice as effective when compared to OEPA. This fact may be explained considering that biological action of essential oils is probably related to the synergy between all its compounds, and not only to the action of their main constituent [38]. Sokovic et al. (2010) [39] tested several essential oils with therapeutic potential and also noted that the carvacrol compound had higher activity against S. aureus when compared with essential oils.
Souza et al. (2013) [40] investigated the influence of carvacrol on S. aureus strains isolated from food, and the monoterpenestrongly interfered in the permeability of the plasmic membrane, in halotolerance, in coagulase activities, and in the production of enterotoxins. In addition, the authors report that the damage caused by carvacrol in the membrane is accompanied by important changes in the surface cells of S. aureus.
Figure 3 shows the biofilm reduction of two strains of S. aureus caused by the use of OEPA and carvacrol. All OEPA and carvacrol concentrations had inhibitory effect on the biofilm of ATCC strains, as a statistically significant difference (p < 0.001) was observed when compared to the negative control. Regarding the OVRSA strain, all carvacrol concentrations showed an inhibitory effect on the biofilm, but complete inhibition was detected at a concentration of 0.25 mg mL−1. The OEPA had inhibitory effect against biofilm OVRSAwhen used concentration of 0.25 mg mL−1, but only completely inhibited biofilm at a concentration of 0.5 mg mL−1.Studies often relate the effect of inhibiting biofilm formation OEPA and carvacrol to: (1) damage to the cell envelope, leading to cell death and to the ability to attach biofilms; and (2) a reduction in the expression of quorum sensing activation of genes, due to decreased bacterial density caused by a delay in the development of cell growth in planktonic cells [41, 42]. Yadav et al. (2015) [43] found activity of carvacrol in combination with eugenol against against Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus biolfim.
Arfa et al. (2006) [44] relate antibiofilm activity of carvacrol with the presence of free hydroxyl group, as carvacrol without this composition does not show antibacterial activity. In addition, the mixture of free hydroxyl with other chemical compounds enhances the effect against biofilm carvacrol [45]. Although its mechanism is not fully understood, evidences indicate that its antimicrobial activity is due to: (1) Increase the permeability of the cytoplasmic membrane, silted H+ into the cell by binding to the hydroxyl group and (2) modification of fatty membrane acids [34].
Conclusions
Our results show the antibacterial and antibiofilm potential of PAEO and carvacrol against the S. aureus standard strain and, especially, against oxacillin- and vancomycin-resistant S. aureus. Considering that P. amboinicus is a plant used in popular medicine practices in Brazil, more studies on the in vivo antibacterial and toxicity are recommended.
Acknowledgments
Funding
The first author received a Scholarship from the from the National Foundation - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Availability of data and materials
All data has been provided in the paper.
Authors’ contributions
HMM collected the plants, was involved in the preparation of the essential oil. SECBV and TTAC were involved in the microbiological analysis as well writing of the manuscript. FEACJ and MGC were involved in the chemical analysis of the essential oil (PAEO). FGM and OVS were involved in the extraction and analysis of plasmids. RAC did the conception, supervised the work and corrected the final manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sara Edwirgens Costa Benício Vasconcelos, Email: sara.benicio@hotmail.com.
Hider Machado Melo, Email: hidermbio@gmail.com.
Theodora Thays Arruda Cavalcante, Email: theodorathays@gmail.com.
Francisco Eduardo Aragão Catunda Júnior, Email: cearajr@gmail.com.
Mário Geraldo de Carvalho, Email: mgeraldo@ufrrj.br.
Francisca Gleire Rodrigues Menezes, Email: gleirerodrigues@yahoo.com.br.
Oscarina Viana de Sousa, Email: oscarinasousa@yahoo.com.br.
Renata Albuquerque Costa, Email: renata.albuq@gmail.com.
References
- 1.Ayele Y, Gutema FD, Edao BM, Girma R, Tufa TB, Beyene TJ, Tadesse F, Geloye M, Beyi AF. Assessment of Staphylococcus aureus along milk value chain and its public health importance in Sebeta, central Oromia, Ethiopia. BMC Microbiol. 2017;17:141. doi: 10.1186/s12866-017-1048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song M, Bai Y, Xu J, Carter MQ, Shi C, Shi X. Genetic diversity and virulence potential of Staphylococcus aureus isolates form raw and processed food commodities in shanghai. Int J Food Microbiol. 2015;195:1–8. doi: 10.1016/j.ijfoodmicro.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Arfatahery N, Davoodabadi A, Abedimohtasab T. Characterization of toxin genes and antimicrobial susceptibility of Staphylococcus aureus isolates in fishery products in Iran. Sci Rep. 2016;6:34216. doi: 10.1038/srep34216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng VC, Wong SC, Ho PL, Yuen KY. Strategic measures for the control of surging antimicrobial resistance in Hong Kong and mainland of China. Emerg Microbes Infect. 2015;4:e8. doi: 10.1038/emi.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S, Cohen J, Findlay D, Gyssens I, Heuer OE, Kahlmeter G, Kruse H, Laxminarayan R, Liébana E, López-Cerero L, MacGowan A, Martins M, Rodríguez-Baño J, Rolain JM, Segovia C, Sigauque B, Tacconelli E, Wellington E, Vila J. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Cesare A, Luna GM, Vignaroli C, Pasquaroli S, Tota S, Paroncini P, Biavasco F. Aquaculture can promote the presence and spread of antibiotic-resistant Enterococci in marine sediments. PlosOne. 2013;8:e62838. doi: 10.1371/journal.pone.0062838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tajbakhsh E, Khamesipour F, Ranjbar R, Ugwu IC. Prevalence of class 1 and 2 Integrons in multi-drug resistant Escherichia coli isolated from aquaculture water in ChaharmahalVaBakhtiari province, Iran. Ann Clin Microbiol Antimicrob. 2015;14:27. doi: 10.1186/s12941-015-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Q, Tian T, Niu T, Wang P. Molecular characterization of antibiotic resistance in cultivable multidrug-resistant bacteria from livestock manure. Environ Pollut. 2017;229:188–198. doi: 10.1016/j.envpol.2017.05.073. [DOI] [PubMed] [Google Scholar]
- 9.Atanasov AG, Waltenberger B, Pferschy-Wenzing EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Pollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovilovic MD, Kopp B, Bauer R, Dirsch VM, Stuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldia S, Joshi BC, Pathak U, Joshi MC. The genus Plecthanthus in India and its chemistry. Chem Biodivers. 2011;8:244–252. doi: 10.1002/cbdv.201000048. [DOI] [PubMed] [Google Scholar]
- 11.Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23:174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Kiraithe MN, Nguta JM, Mbaria JM, Kiama SG. Evaluation of the use of Ocimum Suave Willd. (Lamiaceae), Plectranthus barbatus Andrews (Lamiaceae) and Zanthoxylum chalybeum Engl. (Rutaceae) as antimalarial remedies in Kenyan folk medicine. J Ethnopharmacol. 2016;178:266–271. doi: 10.1016/j.jep.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Crevelin EJ, Caixeta SC, Dias HJ, Groppo M, Cunha WR, Martins CH, Crotti AE. Antimicrobial activity of the essential oil of Plectranthus neochilus against Cariogenic bacteria. Evid Based Complement Alternat Med. 2015;2015:102317. doi: 10.1155/2015/102317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swamy MK, Arumugam G, Kaur R, Ghasemzadeh A, Yusoff MM, Sinniah UR. GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves. Evid Based Complement Alternat Med. 2017;2017:1517683. doi: 10.1155/2017/1517683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arumugam G, Swamy MK, Sinniah UR. Plectranthus amboinicus (lour.) Spreng: botanical, Phytochemical, pharmacological and nutritional significance. Molecules. 2016;21:369. doi: 10.3390/molecules21040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swamy MK, Akhtar MS, Sinniah UR. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Alternat Med. 2016;3012462 [DOI] [PMC free article] [PubMed]
- 17.Santos NO, Mariane B, Lago JHG, Sartorelli P, Rosa W, Soares MG, Silva AM, Lorenzi H, Vallim MA, Pascon RC. Assessing the chemical composition and antimicrobial activity of essential oils from Brazilian plants—Eremanthus erythropappus (Asteraceae), Plectrantuns barbatus, and P. amboinicus (Lamiaceae) Molecules. 2015;20:8440–8452. doi: 10.3390/molecules20058440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira FF, Torres AF, Gonçalves TB, Santiago GM, Carvalho CB, Aguiar MB, Camara LM, Rabenhorst SH, Martins AM, Valença Junior JT, Nagao-Dias AT. Efficacy of Plectranthus amboinicus (Lour.) Spreng in a murine model of methicillin-resistant Staphylococcus aureus skin abscesses. Evid Based Complement Alternat Med. 2013;2013:291592. [DOI] [PMC free article] [PubMed]
- 19.CLSI . Clinical and laboratory standards institute. 2012. pp. 25–23. [Google Scholar]
- 20.Adams RP. Identification of essential oils by gas chromatography/mass spectrometry. 4. Carol Stream: Allured Publishing Corporation; 2007. [Google Scholar]
- 21.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40:175–179. doi: 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 22.Cavalcante TT, Anderson Matias da Rocha B, Alves Carneiro V, Vassiliepe Sousa Arruda F, Fernandes do Nascimento AS, Cardoso Sá N, do Nascimento KS, Sousa Cavada B, Holanda Teixeira E. Effect of Lectins from DiocleinaeSubtribe against oral Streptococci. Molecules. 2011;16:3530–3543. doi: 10.3390/molecules16053530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgartner A, Niederhauser I, Johler S. Virulence and resistance gene profiles of Staphylococcus aureus strains isolated from ready-to-eat foods. J Food Prot. 2014;77:1232–1236. doi: 10.4315/0362-028X.JFP-14-027. [DOI] [PubMed] [Google Scholar]
- 24.Patchanee P, Tadee P, Arjkumpa O, Love D, Chanachai K, Alter T, Hinjoy S, Tharavichitkul P. Occurrence and characterization of livestock-associated methicillin-resistant Staphylococcus aureus in pig industries of northern Thailand. J Vet Sci. 2014;15:529–536. doi: 10.4142/jvs.2014.15.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noor R, Hasan MF, Rahman MM. Molecular characterization of the virulent microorganisms along with their drug-resistance traits associated with the export quality frozen shrimps in Bangladesh. Springerplus. 2014;3:469. doi: 10.1186/2193-1801-3-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumitrescu O, Dauwalder O, Boisset S, Reverdy MÉ, Tristan A, Vandenesch F. Résistance aux antibiotiques chez Staphylococcus aureus les points-clésen 2010. Med Sci. 2010;26:943–949. doi: 10.1051/medsci/20102611943. [DOI] [PubMed] [Google Scholar]
- 27.Spagnolo AM, Orlando P, Panatto D, Amicizia D, Perdelli F, Cristina ML. Staphylococcus aureus with reduced susceptibility to vancomycin in healthcare settings. J Prev Med Hyg. 2014;55:137–144. [PMC free article] [PubMed] [Google Scholar]
- 28.Akindolire MA, Babalola OO, Ateba CN. Detection of antibiotic resistant Staphylococcus aureus from milk: a public health implication. Int J Environ Res Public Health. 2015;12:10254–10275. doi: 10.3390/ijerph120910254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nunes RS, Souza CP, Pereira KS, Del Aguila EM, Paschoalin VM. Identification and molecular phylogeny of coagulase-negative staphylococci isolates from minas Frescal cheese in southeastern Brazil: Superantigenic toxin production and antibiotic resistance. J Dairy Sci. 2016;16:00084–00089. doi: 10.3168/jds.2015-9693. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy AJ, Lindsay JA. The distribution of plasmids that carry virulence and resistance genes in Staphylococcus aureus is lineage associated. BMC Microbiol. 2012;12:104. doi: 10.1186/1471-2180-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murthy PS, Ramalakshmi K, Srinivas P. Fungitoxic activity of Indian borage (Plectranthusamboinicus) volatiles. Food Chem. 2009;114:1014–1018. doi: 10.1016/j.foodchem.2008.10.064. [DOI] [Google Scholar]
- 32.Senthilkumar A, Venkatesalu V. Chemical composition and larvicidal activity of the essential oil of Plectranthus amboinicus (lour.) Spreng against Anopheles stephensi: a malarial vector mosquito. Parasitol Res. 2010;107:1275–1278. doi: 10.1007/s00436-010-1996-6. [DOI] [PubMed] [Google Scholar]
- 33.Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr J. 2008;23:213–226. doi: 10.1002/ffj.1875. [DOI] [Google Scholar]
- 34.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012;3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajitha B, Ashok Kumar Reddy Y, Sreedhara Reddy P. Biosynthesis of silver nanoparticles using Plectranthus amboinicus leaf extract and its antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2014;128:257–262. doi: 10.1016/j.saa.2014.02.105. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues FFG, Costa JGM, Rodrigues, FFG, Campos, AR. Study of the interference between Plectranthus species essential oils from Brazil and aminoglycosides. J Evid Based Complementary Altern Med.2013;2013:724161. [DOI] [PMC free article] [PubMed]
- 37.Hosseinkhani F, Jabalameli F, Banar M, Abdellahi N, Taherikalani M, Leeuwen WB, Emaneini M. Monoterpene isolated from the essential oil of Trachyspermum ammi is cytotoxic to multidrug-resistant Pseudomonas aeruginosa and Staphylococcus aureus strains. Rev Soc Bras Med Trop. 2016;49:172–176. doi: 10.1590/0037-8682-0329-2015. [DOI] [PubMed] [Google Scholar]
- 38.Yap PSX, Yiap BC, Ping HC, Lim SHE. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol J. 2014;8:6–14. doi: 10.2174/1874285801408010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soković M, Glamočlija J, Marin PD, Brkić D, Griensven LJLD. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souza EL, Oliveira CEV, Stamford TLM, Conceição ML, Gomes Neto NJ. Influence of carvacrol and thymol on the physiological attributes, enterotoxin production and surface characteristics of Staphylococcus aureus strains isolated from foods. Braz J Microbiol. 2013;44:29–35. doi: 10.1590/S1517-83822013005000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonçalves TB, Braga MA, Oliveira F, Santiago GMP, Carvalho CBM, Cabral PB, Santiago T, Sousa JS, Barros E, Nascimento RF. Effect of subinihibitory and inhibitory concentrations of Plectranthus amboinicus (lour.) Spreng essential oil on Klebsiella pneumoniae. Phytomedicine. 2012;19:962–968. doi: 10.1016/j.phymed.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Espina L, Pagán R, López D, García-Gonzalo D. Individual constituents from essential oils inhibit biofilm mass production by multi-drug resistant Staphylococcus aureus. Molecules. 2015;20:11357–11372. doi: 10.3390/molecules200611357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadav MK, Chae S-W, Im GJ, Chung J-W, Song J-J. Eugenol: a phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. Plos One. 2015;10(3):e0119564. doi: 10.1371/journal.pone.0119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arfa BA, Combes S, Preziosi-Belloy L, Gontard N, Chalier P. Antimicrobial activity of carvacrol related to its chemical structure. Lett Appl Microbiol. 2006;43:149–154. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 45.Cacciatore I, Di Giulio M, Fornasari E, Di Stefano A, Cerasa LS, Marinelli L, et al. Carvacrol Codrugs: a new approach in the antimicrobial plan. PLos One. 2015;10:e0120937. doi: 10.1371/journal.pone.0120937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data has been provided in the paper.


