Abstract
Synthetic pyrethroid—fenvalerate—is one of the most widespread toxic pollutants and has adverse effect on living systems. However, little is known about its biotransformation mechanism in different microorganisms. To elucidate the pathway that might be involved in the catabolism of fenvalerate, we used Bacillus flexus strain XJU-4 (3-nitrobenzoate degrading organism) as an ideal fenvalerate degrading bacterium. Thin layer chromatography, high performance liquid chromatography and gas chromatography–mass spectrometry analysis results revealed that 3-phenoxybenzoate, protocatechuate, and catechol are the three main by-products of fenvalerate metabolism. Additionally, the bacterial cell-free enzymes showed the activities of fenvalerate hydrolyzing esterase, 3-phenoxybenzaldehyde dehydrogenase, 3-phenoxybenzoate dioxygenase, phenol hydroxylase, protocatechuate 2,3-dioxygenase and catechol-2,3-dioxygenase. Thus, in strain XJU-4, protocatechuate and catechol were further metabolized through meta-cleavage pathway. Moreover, laboratory-scale soil experiments results suggest that B. flexus strain XJU-4 is a suitable contender for bioremediation of pyrethroid fenvalerate-contaminated sites.
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0957-5) contains supplementary material, which is available to authorized users.
Keywords: Biodegradation, Bacillus flexus strain XJU-4, Microcosms, Fenvalerate, 3-Phenoxybenzoate
Introduction
For the past few decades, pesticides are continuously being used for both agricultural and industrial purposes (Eqani et al. 2012; Tallur et al. 2015; Talwar et al. 2014). Besides their effectiveness, these pesticides posed several potential health threats to the ecosystem including microorganisms present in the soil (Pandey and Singh 2004) and other wildlife (Eqani et al. 2012). Fenvalerate (a synthetic pyrethroid), is also known as a chiral pesticide and reported to be used nearly 1 kiloton per annum worldwide (Chen et al. 2011a).
Even though, fenvalerate has higher toxicity against pests, but, it was observed that it has lower toxic effect toward mammals, birds, and plants (Garey and Wolff 1998). Nevertheless, several studies shown that fenvalerate has endocrine toxicity, genotoxic effects, neurotoxicological effects and as a tumour promoter (Fei et al. 2010; Gu et al. 2010; Hemming et al. 1993; Qu et al. 2008; Wang et al. 2017; Wolansky and Harrill 2008; Xia et al. 2004). This synthetic pesticide has been mainly used in agricultural sector, as well as in the home for sanitation purposes and also on cattle to control pests. Given consideration to its widespread use, several studies revealed that fenvalerate has been often detected into the soil, sediment and water (Ismail and Maznah 2005; McKinlay et al. 2008; Xue and Xu 2006). Fenvalerate half-life in soil ranged between 360 and 1440 h; however, it depends on microorganisms, moisture, temperature, pH, soil properties (Ismail and Maznah 2005). Typically, transformation of fenvalerate proceeds through several ways, including volatilization, photolysis, hydrolysis and microorganisms in the eco-geological system (Chen et al. 2011a). Generally, in the environment, 3-phenoxybenzoate has been identified as a common intermediate of pyrethroids including fenvalerate and has higher toxic effects than parent (pyrethroids) compounds (Xia et al. 2004; Yuan et al. 2010; Zhu et al. 2016).
It has been widely reported in the literature that the microorganisms played an essential role in the degradation and detoxification of fenvalerate and other pyrethroid residues in the environment (Chen et al. 2011a; Yu et al. 2013). There are several reports on the degradation of fenvalerate by various microorganisms like Achromobacter sp., Acinetobacter sp. strain JN8, Bacillus cereus, Cladosporium strain HU, Owenweeksia hongkongensis, genus of Pseudomonas, Sphingomonas sp. F-7 and Stenotrophomonas sp. strain ZS-S-01(Boricha and Fulekar, 2010; Chen et al. 2011a, b; Deborah et al. 2013; Fulekar 2009; Jin et al. 2014; Maloney et al. 1988; Yu et al. 2013). However, it is necessary to understand the mechanism of fenvalerate metabolism in different bacteria, which is a critical step for enhancing existing bioremediation techniques for fenvalerate removal in the eco-geological system. In this paper, we proposed a pathway for the degradation of fenvalerate by Bacillus flexus strain XJU-4 under aerobic condition. Furthermore, we have also investigated the bioremediation of fenvalerate in the soil using bacterium; B. flexus strain XJU-4.
Materials and methods
Chemicals and media
Fenvalerate, phenol, 4-hydroxy-3-phenoxybenzoic acid, 3-phenoxybenzoic acid, protocatechuic acid, gentisic acid, 4-chlorocatechol, catechol, 4-nitrocatechol and 3-methylcatechol with more than 97% purity were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemical compounds were of highest (analytical) grade obtained by commercial sources. The stock solutions of substrates like 3-Phenoxybenzoate and fenvalerate were prepared at 100 mM concentration by dissolving in methanol and acetone, respectively. The individual stock solutions were sterilized by membrane filtration and added into autoclaved enrichment medium under sterile condition to get the required concentrations. The enrichment medium (mineral-salts medium, MSM 1) that contained K2HPO4, 6.30; KH2PO4, 1.82; NH4NO3, 1.00; MgSO4·7H2O, 0.20; CaCl2·2H2O, 0.10; Na2MoO4·2H2O, 0.006; MnSO4·H2O, 0.06, and FeSO4·7H2O, 0.10 gl−1. The bacterial cell suspension was measured by plate-count technique (Mulla et al. 2012).
Microorganism and growth condition
The 3-nitrobenzoate degrading B. flexus strain XJU-4 used in the current study was formerly isolated and identified in our laboratory (Mulla et al. 2011a, b). For growth study, 5 ml (OD600 nm of 0.7) of organism was initially inoculated into 95 ml of MSM 1 (in 500 ml Erlenmeyer flask) supplemented with fenvalerate (2 mM) as well as 3-phenoxybenzoate (3 mM), respectively, and kept on a rotary shaker (150 rpm) under dark condition at 30 °C. B. flexus strain XJU-4 growth at different intervals was quantified at 600 nm by spectrophotometer (Hitachi U-2800, Tokyo, Japan). The bacterial culture was preserved on fenvalerate-mineral salts agar slants as well as 3-phenoxybenzoate-mineral salts agar slants, respectively.
Utilization of fenvalerate and other aromatic compounds
The B. flexus strain XJU-4 ability to consume individual substrates (supplemented at initial concentration of 2 mM) such as fenvalerate, 3-phenoxybenoate, 4-hydroxy-3-phenoxybenzoate, phenol, protocatechuate, gentisate, catechol, 3-methylcatechol, 4-chlorocatechol, 3-chlorocatechol and 4-nitrocatechol as a sole source of carbon was studied by quantifying B. flexus strain XJU-4 growth under liquid MSM 1 as described above. Degradation of fenvalerate and 3-phenoxybenzoate during growth of strain XJU-4 was determined at different incubation periods by high performance liquid chromatography (HPLC) analysis. Uninoculated controls were used to measure any conversion of fenvalerate (2 mM) as well as 3-phenoxybenzoate (3 mM) affected by abiotic factors. The effect of various concentration of fenvalerate (1–4 mM) as well as 3-phenoxybenzoate (1–4 mM) on the growth of B. flexus strain XJU-4 was observed after 6 days of incubation.
Analytical methods
The degradation by-products were isolated from culture supernatants of strain XJU-4 grown on fenvalerate by extraction using diethyl ether and verified by thin layer chromatography (TLC) on glass plates coated with silica gel G using two different solvent systems: (a) chloroform–acetic acid (95:5, vol/vol) and (b) benzene–methanol–acetic acid (45:8:4, vol/vol). The by products were observed at 254 nm under ultraviolet (UV) chamber and/or by exposing iodine vapours on TLC glass plates in TLC chamber. Phenolic compounds were confirmed by their characteristic colour on spraying with 2% solution of 2,6-dichloroquinone-4-chlorimide in methanol (Gibbs reagent) or using diazotized p-nitroaniline (Mulla et al. 2011b). ortho-Dihydroxy compounds were confirmed by spraying with Arnow’s reagent (1937). GC–MS analysis was performed using a Shimadzu QP2010 Plus (Tokyo, Japan) as described previously (Mulla et al. 2011b). The by-products were analyzed by HPLC (Shimadzu, Kyoto, Japan) equipped with SPD-10AVP UV-Detector. The separation was executed on Silica gel-packed C18 column (4.6 × 250 mm) of particle size (5 μm) (Phenomenex) by isocratic condition using 50 mM phosphate buffer (pH 7.0) plus acetonitrile (3:7) for isolated metabolite I and 3-phenoxybenzoate whereas 40 mM acetic acid plus methanol (1:1) for fenvalerate, isolated metabolite II as well as isolated metabolite III. The flow rate of both mobile phase were kept at 1 ml min−1. UV–Visible spectra were obtained using spectrophotometer (Hitachi U-2800, Tokyo, Japan).
Enzyme assays
The bacterial culture was grown on MSM 1 supplemented with appropriate concentration of individual substrate like fenvalerate and glucose were harvested by centrifugation at 6500×g for 12 min at 4 °C. The bacterial cell pellets were washed and resuspended in two volume of 50 mM phosphate buffer (pH 7.0). The bacterial culture cell-free extracts were prepared by sonication (Ultrasonic processor, model XL 2010) for 5 min and centrifugation (at 11,000×g) was carried out at 4 °C up to 42 min. The supernatant free from cell debris was used for further enzymatic studies.
Fenvalerate hydrolyzing esterase activity was assessed spectrophotometrically by determining with decrease in absorbance at 239 nm due to disappearance of substrate, fenvalerate. 3-Phenoxybenzaldehyde dehydrogenase activity was assessed spectrophotometrically by determining with increase in absorbance at 340 nm, due to the formation of NADPH. 3-Phenoxybenzoate dioxygenase activity was assessed spectrophotometrically by determining with increase in absorbance at 295 nm (Tallur et al. 2008). 4-Hydroxy-3-phenoxybenzoate hydroxylase was assessed spectrophotometrically at 30 °C by determining with decrease in absorbance at 340 nm, due to the substrate dependence oxidation of NADPH (Tallur et al. 2008). Phenol hydroxylase activity was quantified according to Neujahr and Gaal (1973). Protocatechuate 2,3-dioxygenase, protocatechuate 3,4-dioxygenase and protocatechuate 4,5-dioxygenase activities were quantified according to Mulla et al. (2011b). Catechol-1,2-dioxygenase activity was quantified according to Hayaishi et al. (1957). Catechol-2,3-dioxygenase activity was quantified according to Kim et al. (1992). Protein was quantified by the method of Lowry et al. (1951).
Microcosm experiments
Soil used in microcosm experiment was collected from agriculture field around Dharwad, India. The soil contained 23.0% clay, 21.0% silt, 37.0% sand, 0.67% organic carbon, 0.068% total nitrogen and had a pH of 7.40. Microcosm experiments were carried out as described previously (Chen et al. 2011a). 100 g of soil were placed in 500 ml beakers. Water content of each beaker was adjusted into 40% (maximum water holding capacity) and was maintained whole experimental study by adding double distilled water whenever necessary. Further, fenvalerate was added into each beaker at a final concentration of 2.5 mM in acetone solution, thoroughly mixed and kept for a while to evaporate acetone. Four different types of microcosms experimental set up were made (a) examination microcosm with non-sterile soil, (b) examination microcosms with sterile soil, (c) control microcosms with sterile soil, and (d) control microcosms with non-sterile soil. Sterilization was performed for the collected soil samples by autoclaving at 121.5 °C for 40 min. Examination microcosms with sterile and non-sterile soils were inoculated with microbial suspension (B. flexus strain XJU-4) by drip irrigation method to give a final concentration of 2 × 107 cells colony-forming units (CFUs) g−1 soil, whereas the control microcosms (without strain XJU-4) with non-sterile and sterile soils were left non-bioaugmented. All the microcosms were concealed using perforated aluminium foil and finally kept at 30 °C for incubation up to 10 days. Soils samples (5 g) were collected at regular intervals and extracted for fenvalerate residual analysis by HPLC (as described above method).
Statistical analysis
In this study all experiments were carried out by triplicate and their results are provided as mean ± standard deviation (SD). Experimental data obtained were evaluated statistically using one-way ANOVA (SPSS. 7.5). A P ≤ 0.05 was recorded as statistically significant (Mulla et al. 2011b).
Results and discussion
Utilization of fenvalerate and other aromatic compounds by bacterial culture
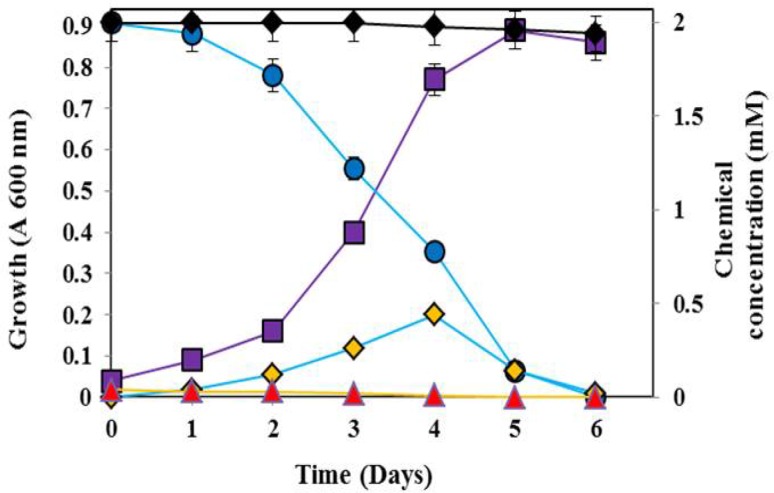
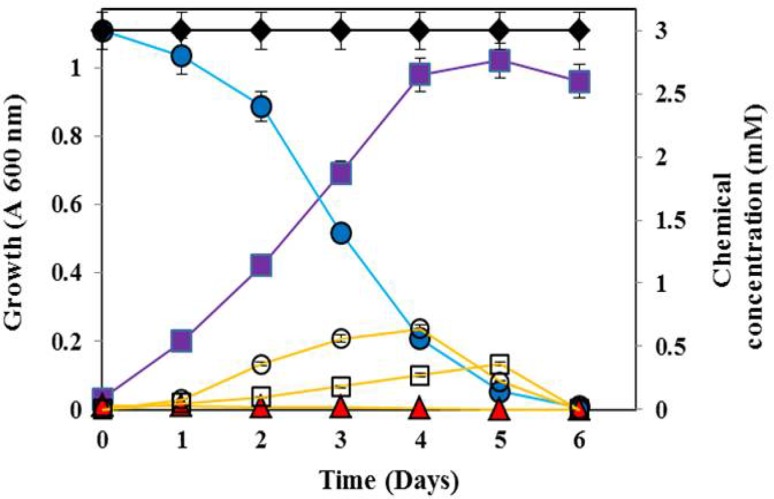
The strain XJU-4 utilized fenvalerate, 3-phenoxybenzoate, phenol, catechol, protocatechuate as a sole source of carbon and energy but not 4-hydroxy-3-phenoxybenzoate, gentisate, 3-chlorocatechol, 4-chlorocatechol, 4-nitrocatechol and 3-methylcatechol. The bacterium, B. flexus strain XJU-4 growth on fenvalerate (2 mM) as a sole source of carbon is shown in Fig. 1. The bacterial culture was completely degraded 2 mM concentration of fenvalerate within 6 days of incubation (Fig. 1). The accumulation of 3-phenoxybenzoate in the culture medium during growth of B. flexus strain XJU-4 on fenvalerate is shown in Fig. 1. Similarly, the fenvalerate degrading bacterium was also utilized 3-phenoxybenzoate (3 mM) as a sole source of carbon and energy (Fig. 2). The accumulation of protocatechuate and catechol in the culture medium during growth of B. flexus strain XJU-4 on 3-phenoxybenzoate is shown in Fig. 2. The effect of various concentrations of fenvalerate and 3-phenoxybenzoate on strain XJU-4 showed maximum growth at 2 and 3 mM, respectively (data was not shown).
Fig. 1.
Utilization of fenvalerate (2 mM) ( ) during growth (
) during growth ( ) of Bacillus flexus strain XJU-4 with the accumulation of 3-phenoxybenzoate in the culture filtrate (
) of Bacillus flexus strain XJU-4 with the accumulation of 3-phenoxybenzoate in the culture filtrate ( ). Uninoculated (with 2 mM fenvalerate) (
). Uninoculated (with 2 mM fenvalerate) ( ) and inoculated (without 2 mM fenvalerate) controls (
) and inoculated (without 2 mM fenvalerate) controls ( ) in the mineral-salts medium. Data values represent the averages of triplicate determinations
) in the mineral-salts medium. Data values represent the averages of triplicate determinations
Fig. 2.
Utilization of 3-phenoxybenzoate (3 mM) ( ) during growth (
) during growth ( ) of Bacillus flexus strain XJU-4 with the accumulation of protocatechuate (
) of Bacillus flexus strain XJU-4 with the accumulation of protocatechuate ( ) as well as catechol (
) as well as catechol ( ) in the culture filtrate. Uninoculated (with 3 mM 3-phenoxybenzoate) (
) in the culture filtrate. Uninoculated (with 3 mM 3-phenoxybenzoate) ( ) and inoculated (without 3 mM 3-phenoxybenzoate) controls (
) and inoculated (without 3 mM 3-phenoxybenzoate) controls ( ) in the mineral salts medium. Data values represent the averages of triplicate determinations
) in the mineral salts medium. Data values represent the averages of triplicate determinations
Identification of metabolites
The analysis of culture supernatants of B. flexus strain XJU-4 grown on fenvalerate by UV, TLC and HPLC revealed that the presence of three metabolites (I–III). Their R f values and λmax were found to be identical to that of authentic 3-phenoxybenzoate, protocatechuate and catechol (Table S1, Supplementary Information, SI). The mass spectrum of isolated metabolite I (Fig. S1A, SI) showed molecular peak M+ at m/z 214, is in good agreement with empirical formula C13H10O3. The spectral data were identical with that of 3-phenoxybenzoate (Fig. S1B, SI). The mass spectrum of isolated metabolite II (Fig. S1C, SI) showed molecular peak M+ at m/z 154, is in good agreement with empirical formula C7H6O4. The spectral data corresponded well with that of authentic protocatechuate (Fig. S1D, SI). The mass spectrum of isolated metabolite III (Fig. S1E, SI) showed molecular peak M+ at m/z 110, is in good agreement with empirical formula C6H6O2. The spectral data corresponded well with that of authentic catechol (Fig. S1F, SI). On the basis of these results, the presences of three by-products of fenvalerate biodegradation were identified as 3-phenoxybenzoate (first metabolite), protocatechuate (second metabolite) and catechol (third metabolite) with their authentic compounds (Fig. S1, SI).
Bacterial cell-free enzymes involved in fenvalerate catabolism
The activities of various enzymes involved in the degradation of fenvalerate are given in Table 1. The cell-free extract of the B. flexus strain XJU-4 grown on fenvalerate contained the activities of fenvalerate hydrolyzing esterase, 3-phenoxybenzaldehyde dehydrogenase, 3-phenoxybenzoate dioxygenase, phenol hydroxylase, protocatechuate 2,3-dioxygenase and catechol 2,3-dioxygenase. The activities of 4-hydroxy-3-phenoxybenzoate, protocatechuate 3,4-dioxygenase, protocatechuate 4,5-dioxygenase and catechol 1,2-dioxygenase were not detected in the cell-free extract. The overall results revealed that a meta-cleavage pathway was involved in fenvalerate degradation. On the other hand, the cell-free extract of glucose grown cells (B. flexus strain XJU-4) were not contained any of these enzyme activities. Therefore, these results suggest that the enzymes involved in the transformation mechanism were induced by the growth of bacterium on fenvalerate. More studies are needed to further support oxygenase enzymes that are responsible for fenvalerate degradation.
Table 1.
Specific activities of enzymes in the cell-free extract of Bacillus flexus strain XJU-4 grown on fenvalerate
| Enzyme | Specific activitya (units/mg of protein) |
|---|---|
| Fenvalerate hydrolyzing esterase | 0.164 ± 0.002 |
| 3-Phenoxybenzaldehyde dehydrogenase | 0.242 ± 0.002 |
| 3-Phenoxybenzoate dioxygenase | 0.354 ± 0.004 |
| 4-Hydroxy-3-phenoxybenzoate hydroxylase | 0.000 ± 0.00 |
| Phenol hydroxylase | 0.398 ± 0.002 |
| Protocatechuate 2,3-dioxygenase | 0.476 ± 0.003 |
| Protocatechuate 3,4-dioxygenase | 0.000 ± 0.00 |
| Protocatechuate 4,5-dioxygenase | 0.000 ± 0.00 |
| Gentisate-1,2-dioxygenase | 0.000 ± 0.00 |
| Catechol-1,2-dioxygenase | 0.000 ± 0.00 |
| Catechol-2,3-dioxygenas | 0.487 ± 0.004 |
Glucose-grown cells did not contain these activities
Values are the mean ± standard deviation (SD) of triplicates
a One unit is defined as the formation or consumption of 1 µmol of the product or substrate, respectively, per min
Microcosm experiments
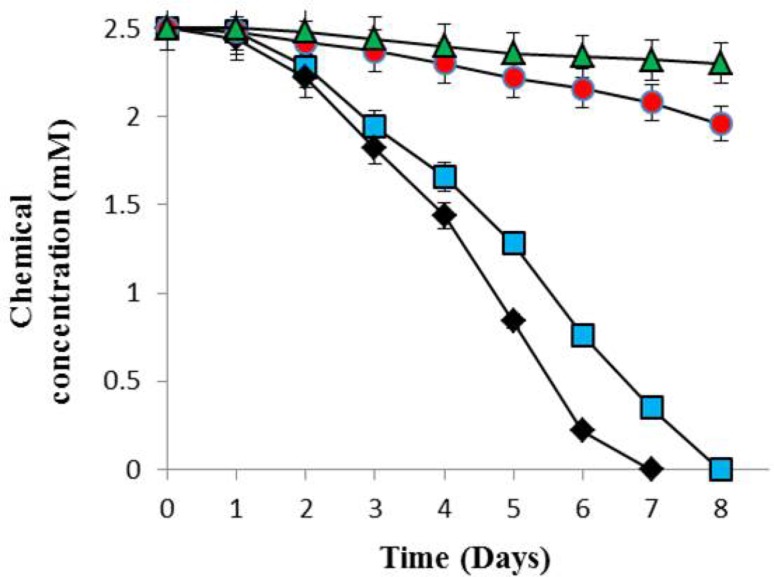
To observe the capacity of B. flexus strain XJU-4 to degrade fenvalerate in the soil, we studied microcosm experiments (Fig. 3). The experiment conducted using microcosms with sterile soil; we observed that the complete removal of fenvalerate (2.5 mM) was achieved by strain XJU-4 within 8 days. On the 2nd day, 0.22 mM (8.8%) degradation was detected and degradation was 0.84 mM (33.6%) by 4th day. The degradation was 1.74 mM (69.6%) by 6th day. Almost complete degradation of fenvalerate was observed at 8th day. In other tested microcosms with non-sterile soil, complete fenvalerate degradation by bacterium was observed within 7 days. On the other hand, in control experiments (non-sterile and sterile soil without B. flexus strain XJU-4), the degradation of fenvalerate was observed only by 21.6 and 8%, respectively, after 8 days of incubation (Fig. 3).
Fig. 3.
Dynamics of degradation of fenvalerate (2.5 mM) by strain XJU-4 during microcosm studies [a microcosm (inoculated) with sterile soil ( ), b microcosm (inoculated) with non-sterile soil (
), b microcosm (inoculated) with non-sterile soil ( ), c control (uninoculated) with sterile soil (
), c control (uninoculated) with sterile soil ( ) and d control (uninoculated) with non-sterile soil (
) and d control (uninoculated) with non-sterile soil ( )]. Data values represent the averages of triplicate determinations
)]. Data values represent the averages of triplicate determinations
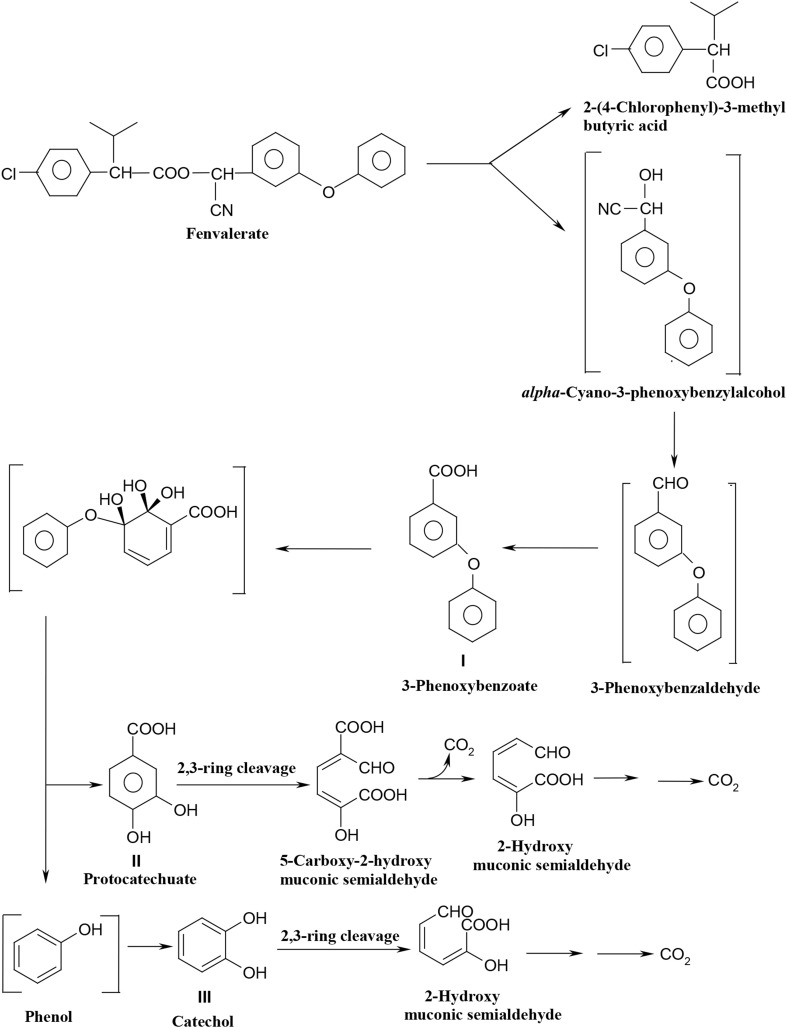
From the results it is confirmed that the B. flexus strain XJU-4 degraded synthetic pyrethroid pesticide (i.e., fenvalerate) by hydrolysis of ester linkage to form 3-phenoxybenzoate via 3-phenoxybenzaldehyde. The 3-phenoxybenzoate was further metabolized into phenol as well as protocatechuate. UV, TLC, HPLC, GC–MS analysis and enzymatic studies have confirmed that 3-phenoxybenzoate, phenol, protocatechuate and catechol were the major by-products of fenvalerate metabolism in the presence of strain XJU-4. Additionally, the presence of higher activities of protocatechuate 2,3-dioxygenase and catechol 2,3-dioxygenase in the fenvalerate-grown cells has suggested that protocatechuate and catechol were further metabolized via meta-cleavage pathway under oxidation condition. Looking into Fig. 4, it can be seen the pathway for the degradation of phenoxybenzoate moiety of fenvalerate in B. flexus strain XJU-4.
Fig. 4.
Proposed pathway for the degradation of fenvalerate in Bacillus flexus strain XJU-4
The initial step of fenvalerate degradation into 3-phenoxybenzoate (in B. flexus strain XJU-4) was similar to that described in other organisms such as Bacillus circus, Pseudomonas fluorescens, Achromobacter sp., Sphingomonas sp. F-7 and Stenotrophomonas sp. strain ZS-S-01 (Chen et al. 2011a; Maloney et al. 1988; Yu et al. 2013). However, later pathway of fenvalerate metabolite, 3-phenoxybenzoate proceeds in B. flexus strain XJU-4 was vary from those reported for other organisms. For example, B. flexus strain XJU-4 catabolized 3-phenoxybenzoate into protocatechuate and phenol, and similar to that reported for the genus of Pseudomonas (Halden et al. 1999, 2000) and Micrococcus (Tallur et al. 2008). On the other hand, in Bacillus cercus, Pseudomonas fluorescens and Achromobacter sp., 3-phenoxybenzoate is biotransformed into 4-hydroxy-3-phenoxybenzoate (Maloney et al. 1988). Though, in strain XJU-4, protocatechuate and catechol were proceeded via meta-cleavage pathway under oxidation condition, whereas in strain CPN 1, protocatechuate and catechol are oxidized through ortho-ring cleavage (Tallur et al. 2008). Other than bacteria, fungal culture (Cladosporium strain HU) was transformed fenvalerate into 3-phenoxybenzaldehyde and α-hydroxy-3-phenoxy-benzeneacetonitrile (Chen et al. 2011b). In another study, Zhu et al. (2016) reported in Aspergillus oryzae (a filamentous fungus M-4 strain), 3-phenoxybenzoate was gradually transformed into various metabolites such as phenol, 3-hydroxy-5-phenoxy benzoate, protocatechuate, catechol and gallic acid.
Additionally, strain XJU-4 has also showed its ability to degrade 3-phenoxybenzoate, which is a major degradation by-product, and common to most pyrethroids. Hence, in this study, both fenvalerate and 3-phenoxybenzoate degradation was accomplished by same bacterium; B. flexus strain XJU-4. However, further study is necessary to determine fenvalerate degradation pathway proceeds through, 2-(4-chlorophenyl)-3-methyl butyric acid, which might also have an impact on environment.
Moreover, the bioremediation potential of Gram-positive bacterium (i.e., B. flexus strain XJU-4) was also explored in soil with fenvalerate (2.5 mM) spiked sterile and non-sterile soil using microcosms approach. B. flexus strain XJU-4 significantly degraded fenvalerate in microcosms with sterile and non-sterile soils. However, it was observed that the degradation of fenvalerate in non-sterile soil was slightly faster than sterile soil. These results suggest that indigenous bacteria and biotic conditions could assist the degradation of fenvalerate with strain XJU-4. Hence, the organism was favourable for the use in bioremediation of fenvalerate-contaminated sites. Previously, Chen et al. (2011a), also reported bioremediation of 95% of fenvalerate (~0.12 mM) by a Gram-negative bacterium, Stenotrophomonas sp. strain ZS-S-01 within 9 days. From the results of our study, it indicates that more than 75% of fenvalerate was degraded from contaminated soil than controls. However, it is difficult to assess accurately as bioremediation was significantly influenced by various parameters in the environment (Chen et al. 2011a). But, still bioremediation method is considered to be a primary choice for the remediation of contaminated sites due to its eco-friendly and cost-effectiveness.
Conclusion
Our investigation results suggest that the degradation pathway of fenvalerate by B. flexus strain XJU-4 proceeds via 3-phenoxybenzoate to phenol and protocatechuate. Phenol was further biotransformed into catechol. Finally, both protocatechuate and catechol were entered into the meta-cleavage pathway. Furthermore microcosm investigations support for the organism could be useful for the decontamination of fenvalerate-contaminated sites. The results would be useful for the environmental authorities towards the management of fenvalerate (a pyrethroid pesticide) contaminated soils/environment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The first author is thankful to the University Grants Commission for providing a Research Fellowship in Sciences for Meritorious Students. The authors also extend their gratitude to University Scientific and Instruments Center, Karnatak University and Deanship of Scientific Research, King Saud University for providing speciality to perform experimental study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0957-5) contains supplementary material, which is available to authorized users.
Contributor Information
Sikandar I. Mulla, Email: sikandar.mulla7@yahoo.com
Fuad Ameen, Email: fhasan@ksu.edu.sa.
References
- Arnow LE. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine-tyrosine mixtures. J Biol Chem. 1937;118:531–537. [Google Scholar]
- Boricha H, Fulekar MH. Identification of Owenweeksia honkongenesis as a novel organism for the remediation of pesticide-fenvalerate. Rom Biotechnol Lett. 2010;15:5104–5110. [Google Scholar]
- Chen S, Yang L, Hu M, Liu J. Biodegradation of fenvalerate and 3-phenoxybenzoic acid by a novel Stenotrophomonas sp. strain ZS-S-01 and its use in bioremediation of contaminated soils. Appl Microbiol Biotechnol. 2011;90:755–767. doi: 10.1007/s00253-010-3035-z. [DOI] [PubMed] [Google Scholar]
- Chen S, Hu Q, Hu M, Luo J, Weng Q, Lai K. Isolation and characterization of a fungus able to degrade pyrethroids and 3-phenoxybenzaldehyde. Bioresour Technol. 2011;102:8110–8116. doi: 10.1016/j.biortech.2011.06.055. [DOI] [PubMed] [Google Scholar]
- Deborah GSA, Thatheyus AJ, Vidhya R. Biodegradation of the Synthetic pyrethroid, fenvalerate by Pseudomonas viridiflava. Am J Microbiol Res. 2013;1:32–38. doi: 10.12691/ajmr-1-2-4. [DOI] [Google Scholar]
- Eqani SAMAS, Malik RN, Katsoyiannis A, Zhang G, Chakraborty P, Mohammad A, Jones KC. Distribution and risk assessment of organochlorine contaminants in surface water from River Chenab, Pakistan. J Environ Monit. 2012;14:1645–1654. doi: 10.1039/c2em11012a. [DOI] [PubMed] [Google Scholar]
- Fei J, Qu JH, Ding XL, Xue K, Lu CC, Chen JF, Song L, Xia YK, Wang SL, Wang XR. Fenvalerate inhibits the growth of primary cultured rat preantral ovarian follicles. Toxicology. 2010;267:1–6. doi: 10.1016/j.tox.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Fulekar MH. Bioremediation of fenvalerate by Pseudomonas aeruginosa in a scale up bioreactor. Rom Biotechnol Lett. 2009;14:4900–4905. [Google Scholar]
- Garey J, Wolff MS. Estrogenic and antiprogestagenic activities of pyrethroid insecticides. Biochem Biophys Res Commun. 1998;251:855–859. doi: 10.1006/bbrc.1998.9569. [DOI] [PubMed] [Google Scholar]
- Gu A, Shi X, Yuan C, Ji G, Zhou Y, Long Y, Song L, Wang S, Wang X. Exposure to fenvalerate causes brain impairment during zebrafish development. Toxicol Lett. 2010;197:188–192. doi: 10.1016/j.toxlet.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Halden RU, Tepp SM, Halden BG, Dwyer DF. Degradation of 3-phenoxybenzoic acid in soil by Pseudomonas pseudoalcaligenes POB310(pPOB) and two modified Pseudomonas strains. Appl Environ Microbiol. 1999;65:3354–3359. doi: 10.1128/aem.65.8.3354-3359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halden RU, Peters EG, Halden BG, Dwyer DF. Transformation of mono- and dichlorinated phenoxybenzoates by phenoxybenzoate-dioxygenase in Pseudomonas pseudoalcaligenes POB310 and a modified diaryl ether-metabolizing bacterium. Biotechnol Bioeng. 2000;69:107–112. doi: 10.1002/(SICI)1097-0290(20000705)69:1<107::AID-BIT13>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Hayaishi O, Katagiri M, Rothberg S. Studies on oxygenases; pyrocatechase. J Biol Chem. 1957;229:905–920. [PubMed] [Google Scholar]
- Hemming H, Flodstrom S, Warngard L. Enhancement of altered hepatic foci in rat liver and inhibition of intercellular communication in vitro by the pyrethroid insecticides fenvalerate, flucythrinate and cypermethrin. Carcinogenesis. 1993;14:2531–2535. doi: 10.1093/carcin/14.12.2531. [DOI] [PubMed] [Google Scholar]
- Ismail BS, Maznah Z. Persistence of fenvalerate in three Malaysian agricultural soils. Bull Environ Contam Toxicol. 2005;75:789–796. doi: 10.1007/s00128-005-0820-y. [DOI] [PubMed] [Google Scholar]
- Jin Z, Guo Q, Zhang Z, Yan T. Biodegradation of type II pyrethroids and major degraded products by a newly isolated Acinetobacter sp. strain JN8. Can J Microbiol. 2014;60:541–545. doi: 10.1139/cjm-2014-0104. [DOI] [PubMed] [Google Scholar]
- Kim Y, Choi B, Lee J, Chang H, Min KR. Characterization of catechol 2,3-dioxygenases. Biochem Biophys Res Commun. 1992;183:77–82. doi: 10.1016/0006-291X(92)91611-S. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maloney SE, Maule A, Smith AR. Microbial transformation of the pyrethroid insecticides: permethrin, deltamethrin, fastac, fenvalerate, and fluvalinate. Appl Environ Microbiol. 1988;54:2874–2876. doi: 10.1128/aem.54.11.2874-2876.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay R, Plant JA, Bell JN, Voulvoulis N. Endocrine disrupting pesticides: implications for risk assessment. Environ Int. 2008;34:168–183. doi: 10.1016/j.envint.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Mulla SI, Hoskeri RS, Shouche YS, Ninnekar HZ. Biodegradation of 2-nitrotoluene by Micrococcus sp. strain SMN-1. Biodegradation. 2011;22:95–102. doi: 10.1007/s10532-010-9379-3. [DOI] [PubMed] [Google Scholar]
- Mulla SI, Manjunatha TP, Hoskeri RS, Tallur PN, Ninnekar HZ. Biodegradation of 3-Nitrobenzoate by Bacillus flexus strain XJU-4. World J Microb Biotechnol. 2011;27:1587–1592. doi: 10.1007/s11274-010-0611-4. [DOI] [Google Scholar]
- Mulla SI, Talwar MP, Hoskeri RS, Ninnekar HZ. Enhanced degradation of 3-nitrobenzoate by immobilized cells of Bacillus flexus strain XJU-4. Biotechnol Bioproc E. 2012;17:1294–1299. doi: 10.1007/s12257-012-0211-2. [DOI] [Google Scholar]
- Neujahr HY, Gaal A. Phenol hydroxylase from yeast. Purification and properties of the enzyme from Trichosporon cutaneum. Eur J Biochem. 1973;35:386–400. doi: 10.1111/j.1432-1033.1973.tb02851.x. [DOI] [PubMed] [Google Scholar]
- Pandey S, Singh DK. Total bacterial and fungal population after chlorpyrifos and quinalphos treatments in groundnut (Arachis hypogaea L.) soil. Chemosphere. 2004;55:197–205. doi: 10.1016/j.chemosphere.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Qu JH, Hong X, Chen JF, Wang YB, Sun H, Xu XL, Song L, Wang SL, Wang XR. Fenvalerate inhibits progesterone production through cAMP-dependent signal pathway. Toxicol Lett. 2008;176:31–39. doi: 10.1016/j.toxlet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Tallur PN, Megadi VB, Ninnekar HZ. Biodegradation of cypermethrin by Micrococcus sp. strain CPN 1. Biodegradation. 2008;19:77–82. doi: 10.1007/s10532-007-9116-8. [DOI] [PubMed] [Google Scholar]
- Tallur PN, Mulla SI, Megadi VB, Talwar MP, Ninnekar HZ. Biodegradation of cypermethrin by immobilized cells of Micrococcus sp. strain CPN 1. Braz J Microbiol. 2015;46:667–672. doi: 10.1590/S1517-838246320130557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar MP, Mulla SI, Ninnekar HZ. Biodegradation of organophosphate pesticide quinalphos by Ochrobactrum sp. strain HZM. J Appl Microbiol. 2014;117:1283–1292. doi: 10.1111/jam.12627. [DOI] [PubMed] [Google Scholar]
- Wang B, Liu J-J, Wang Y, Fu L, Shen R, Yu Z, Wang H, Chen Y-H, Zhang C, Meng X-H, Xu D-X. Maternal fenvalerate exposure induces fetal intrauterine growth restriction through disrupting placental thyroid hormone receptor signaling. Toxicol Sci. 2017;157:377–386. doi: 10.1093/toxsci/kfx052. [DOI] [PubMed] [Google Scholar]
- Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol. 2008;30:55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Xia Y, Bian Q, Xu L, Cheng S, Song L, Liu J, Wu W, Wang S, Wang X. Genotoxic effects on human spermatozoa among pesticide factory workers exposed to fenvalerate. Toxicology. 2004;203:49–60. doi: 10.1016/j.tox.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Xue N, Xu X. Composition, distribution, and characterization of suspected endocrine-disrupting pesticides in Beijing GuanTing Reservoir (GTR) Arch Environ Contam Toxicol. 2006;50:463–473. doi: 10.1007/s00244-005-1097-1. [DOI] [PubMed] [Google Scholar]
- Yu FB, Shan SD, Luo LP, Guan LB, Qin H. Isolation and characterization of a Sphingomonas sp. strain F-7 degrading fenvalerate and its use in bioremediation of contaminated soil. J Environ Sci Heal B. 2013;48:198–207. doi: 10.1080/03601234.2013.730299. [DOI] [PubMed] [Google Scholar]
- Yuan C, Wang C, Gao SQ, Kong TT, Chen L, Li XF, Song L, Wang YB. Effects of permethrin, cypermethrin and 3-phenoxybenzoic acid on rat sperm motility in vitro evaluated with computer-assisted sperm analysis. Toxicol In Vitro. 2010;24:382–386. doi: 10.1016/j.tiv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Li J, Yao K, Zhao N, Zhou K, Hu X, Zou L, Han X, Liu A, Liu S. Degradation of 3-phenoxybenzoic acid by a filamentous fungus Aspergillus oryzae M-4 strain with self-protection transformation. Appl Microbiol Biotechnol. 2016;100:9773–9786. doi: 10.1007/s00253-016-7847-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.