Abstract
T cells are critical effectors of host immunity that target intracellular pathogens, such as the causative agents of HIV, tuberculosis, and malaria. The development of vaccines that induce effective cell-mediated immunity against such pathogens has proved challenging; for tuberculosis and malaria, many of the antigens targeted by protective T cells are not known. Here, we report a novel approach for screening large numbers of antigens as potential targets of T cells. Malaria provides an excellent model to test this antigen discovery platform because T cells are critical mediators of protection following immunization with live sporozoite vaccines and the specific antigen targets are unknown. We generated an adenovirus array by cloning 312 highly expressed pre-erythrocytic Plasmodium yoelii antigens into adenovirus vectors using high-throughput methodologies. The array was screened to identify antigen-specific CD8+ T cells induced by a live sporozoite vaccine regimen known to provide high levels of sterile protection mediated by CD8+ T cells. We identified 69 antigens that were targeted by CD8+ T cells induced by this vaccine regimen. The antigen that recalled the highest frequency of CD8+ T cells, PY02605, induced protective responses in mice, demonstrating proof of principle for this approach in identifying antigens for vaccine development.
Keywords: vaccine, T cell, malaria, adenovirus, antigen, screen, vector, antigen discovery, viral vector
Introduction
Almost all licensed vaccines are thought to mediate protection through antibody production; therefore, antigen discovery research and development has focused largely on the identification of antigens that induce protective antibodies.1 The availability of serum, the ease of working with antibodies, and, more recently, advances in microarray technology have facilitated these efforts. However, vaccine development for some of the most devastating infectious diseases, such as malaria, tuberculosis (TB), and HIV, has met with limited success, partially because these organisms have intracellular life cycle stages that are not targeted by antibodies, and they have developed sophisticated mechanisms to avoid clearance by host immune responses.2 Since T cells have been implicated in protection from these diseases,3, 4, 5, 6, 7 considerable efforts have been directed at developing vaccines that induce protective T cell responses. However, for infectious agents with large genomes that express many potential T cell antigens such as parasites and bacteria, many of the specific antigens that are targeted by protective CD8+ T cells are not known. Identification of the target antigens of protective T cell responses would greatly facilitate vaccine development.
Malaria killed approximately 429,000 people in 2015,8 most of them children, in sub-Saharan Africa. Despite decades of effort, a highly effective malaria vaccine is not available. Immunization with attenuated Plasmodium sporozoites can provide high levels of protection in mice, non-human primates, and humans.9, 10, 11, 12 Protection is mediated by CD8+ T cells, which target a set of mostly unknown pre-erythrocytic stage antigens.13, 14, 15, 16, 17, 18 Activated CD8+ T cells can kill infected hepatocytes, thereby preventing blood-stage infection, which is responsible for the clinical symptoms of the disease. However, substantial delivery issues are a considerable barrier to licensure of live sporozoite-based vaccines, and broad protection against circulating strains has not been demonstrated. An alternative approach is to identify the targets of these protective CD8+ T cell responses and formulate them into a multivalent subunit vaccine designed to induce sustained T cell immunity.
The two P. falciparum sporozoite vaccines that are associated with high levels of protection in humans are radiation-attenuated sporozoites (RAS) and live sporozoites with concomitant chloroquine treatment to kill newly emerging blood-stage parasites (SPZ+CQ). Immunization with RAS leads to infection of hepatocytes and expression of a set of early liver-stage genes, but these attenuated sporozoites do not develop into late liver and blood stages.19 In BALB/c mice, the protective T cell response following vaccination with RAS is dominated by CD8+ T cells specific for the major surface protein on the sporozoite, the circumsporozoite protein (CSP), although T cell responses specific for other antigens can also contribute to protection.20 In humans, T cell responses specific for several antigens have been observed following RAS immunization.21, 22, 23 In contrast to RAS, vaccination with SPZ+CQ allows expression of the full repertoire of liver-stage genes and replication of the parasite in hepatocytes.24 Unlike RAS, where protection requires approximately 1,000 bites from infected mosquitoes, SPZ+CQ can provide durable protection in volunteers with as few as 30–45 bites.25 This robust protection is strictly dependent on CD8+ T cells26 and immune response to CSP is not required, highlighting the fact that the specific antigen targets of protective immunity are not known.27
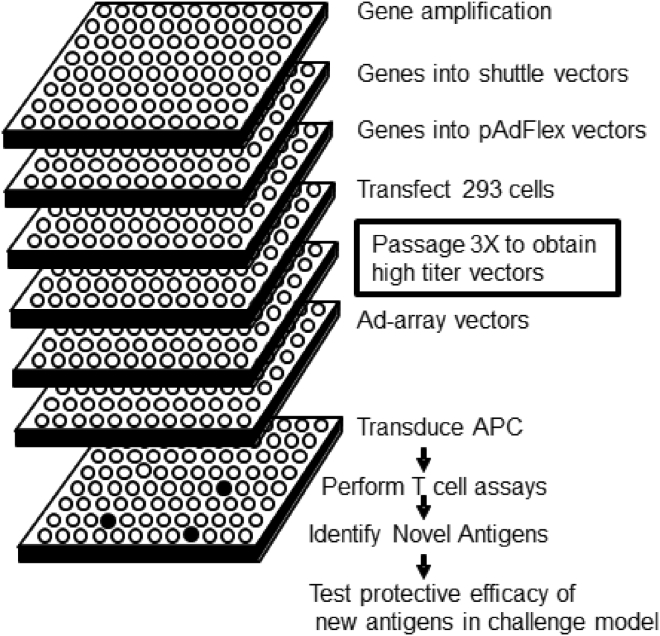
In this report, we describe a novel platform for the discovery of antigens that are the targets of T cell responses to infection (Figure 1). Using this system, we identified 69 pre-erythrocytic antigens that were targeted by CD8+ T cell responses in mice immunized with protective regimens of P. yoelii SPZ+CQ. Moreover, we demonstrated that the antigen that recalled the highest frequency of interferon gamma (IFNγ)-expressing CD8+ T cells, PY02605, provided sterile protection in mice when delivered in a DNA prime-adenovector boost regimen.
Figure 1.
Schematic View of High-Throughput Ad-Array Generation and Antigen Identification Assays
The general steps involved in generating a defined array of adenovectors and their use in antigen discovery screens using high-throughput technology are indicated.
Results
Generation of an Array of Adenovectors That Express a Panel of Highly Expressed P. yoelii Pre-erythrocytic Antigens
Pre-erythrocytic antigens, which are expressed in the sporozoite and liver stages of the Plasmodium spp. life cycle, are particularly promising targets for malaria vaccine development, with great potential to prevent infection and transmission.28 The pre-erythrocytic stages of the parasitic life cycle are vulnerable to vaccine intervention because their antigens are expressed at a time when low numbers of sporozoites are transmitted by the mosquito to the human host and only a few hepatocytes become infected. We selected P. yoelii pre-erythrocytic genes with identifiable P. falciparum orthologs for generation of an adenovector array (Ad-array) based on their level of expression in microarray29, 30, 31 and protein mass spectrometry29 datasets. Gene selection was made without regard to protein function or subcellular localization. In total, 312 P. yoelii genes were amplified from genomic DNA and cloned into E1/E3-deleted adenovirus type 5 (Ad5) vector genomes (Figure 2).
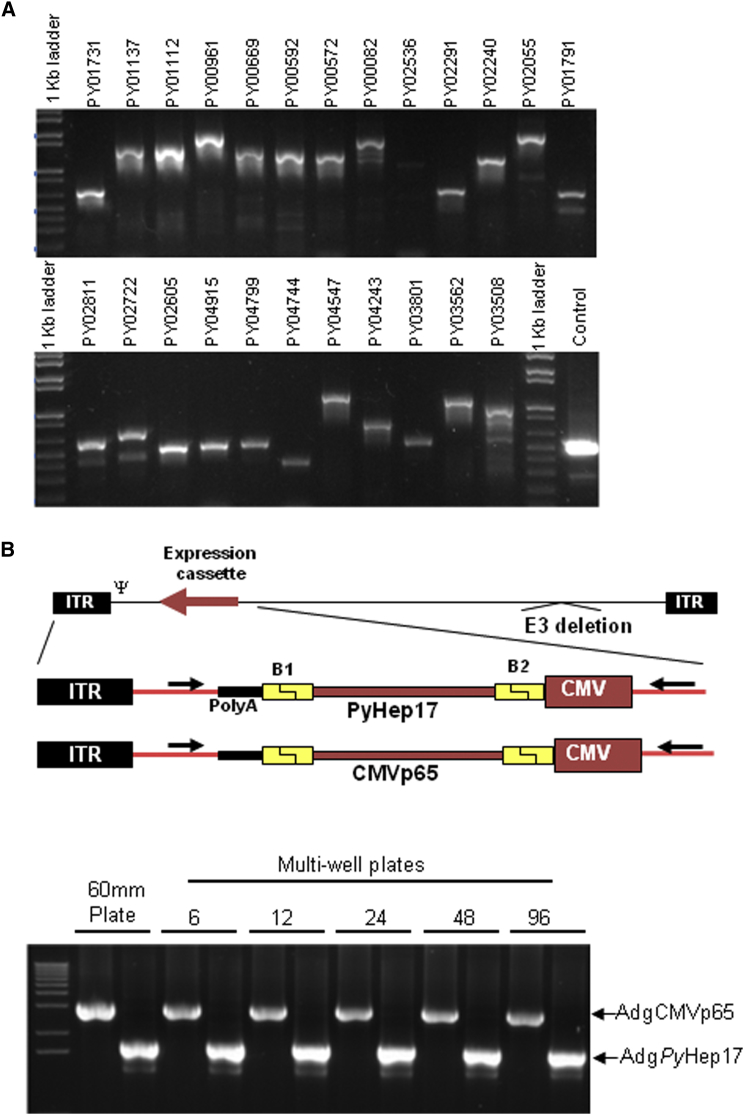
Figure 2.
Generating the Ad-Array
(A) >300 P. yoelii pre-erythrocytic genes were amplified using P. yoelii genomic DNA and gene-specific primers. PCR products were electrophoresed on 1% agarose gels, showing a subset of 24 genes. The control is a pair of oligonucleotide primers used to amplify the E1 region of Ad5 DNA. (B) Parallel generation of two Ad-array vectors in multi-well plates. The schematic indicates two Ad-array vectors, AdgPyHep17 and AdgCMVp65. The inverted terminal repeats (ITRs), CMV promoter, SV40 poly(A), and the 25-bp-long attB recombinase sequences that flank the transgene (B1 and B2) are indicated. Two plasmids, encoding PyHep17 and CMVp65, were AdFlex linearized with Pac I and transfected into 293 cells in 60-mm, 6-well, 12-well, 24-well, 48-well, and 96-well plates. Following two passages in 293 cells in the same plate size, CPE was observed in all wells. Viral DNA was obtained, and PCR analysis was performed using primers that flank the expression cassette as indicated by the arrows in the schematic. The products of the PCR reaction were loaded into a 1% agarose gel and electrophoresed. Arrows next to AdgPyHep17 and AdgCMVp65 indicate the expected size for the PCR products.
To facilitate high-throughput production of the Ad-array, we compared the efficiency of adenovector generation in multi-well plates of different sizes. The adenovector plasmid had to convert into an adenovirus vector in sufficient quantities and quality to function in the antigen screening assay. Initially, we tested conversions of two pAdFlex plasmids that expressed the P. yoelii Hep17 antigen (AdgHep17) and the cytomegalovirus p65 antigen (AdgCMVp65). These large plasmids were transfected into 293 cells in 60-mm, 6-well, 12-well, 24-well, 48-well, and 96-well plates, and the cells were passaged to increase the adenovector titer. We observed efficient adenovector conversion in all of the wells as indicated by full cytopathic effect (CPE) at passage 2. Vector identity was verified by PCR using oligonucleotides that spanned the expression cassette (Figure 2B). Vector titers from each of the CPE wells (Table S1) demonstrated equivalent yields per infected cell. These results indicated that multiple adenovectors can be generated from pAdFlex adenovector plasmids in a parallel process in multi-well plates and that 96-well plates were suitable for the generation of the Ad-array.
The Screening System
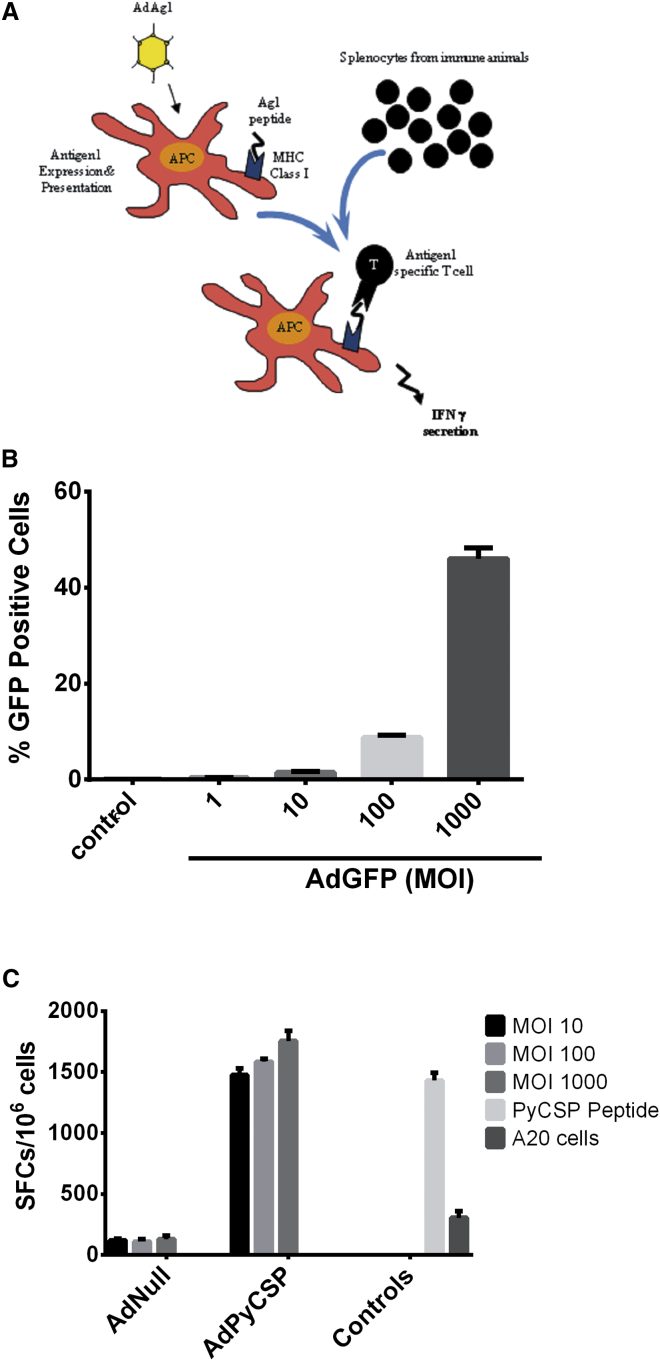
The overall design of our antigen screening system is shown in Figure 3A. To test the elements of the screen, we first determined the MOI necessary to efficiently infect A20 cells. Cells were infected with various doses of AdGFP, an Ad5 vector expressing GFP, and the percentage of infected cells was measured 48 hr post-infection (Figure 3B). MOIs of 10, 100, or 1,000 focal forming units (ffus)/cell were required to infect approximately 2%, 10%, or 50% of the cells, respectively. To determine if adenovirus vectors could efficiently present antigen following infection of antigen presenting cells (APCs), we immunized BALB/c mice with a PyCSP-expressing plasmid, stimulated splenocytes from these mice with APCs infected with an Ad5 vector expressing PyCSP (AdPyCSP), and measured activated T cells by the enzyme linked immunosorbent spot (ELISpot) assay. We observed strong recall responses to the AdPyCSP-infected cells, even at a low MOI, comparable to those generated by pulsing APCs with a peptide containing the PyCSP immunodominant epitope (Figure 3C). Very low responses were seen in the negative controls. These results demonstrate that A20 cells (which express both major histocompatibility complex [MHC] class I and class II alleles) infected with AdPyCSP are able to present antigen to immune T cells. This process was highly efficient, as strong T cell responses were observed even at an MOI of 10, a multiplicity that resulted in transduction of approximately 2% of the target cells. Increasing the MOI resulted in substantially increased A20 cell transduction (Figure 3B) but only marginally increased functional activity in the ELISpot assay (Figure 3C). Thus, low-level target cell transduction is sufficient for optimal activity to detect T cell responses in the ELISpot assay.
Figure 3.
Adenovector-Expressed Antigen Is Effective at Recalling T Cell Responses from Immunized Mice
(A) Schema for in vitro antigen discovery. First, A20 APCs are infected with each adenovector in the array. After 24 hr, the specific antigen of interest is expressed and presented on MHC class I molecules. The infected APCs are incubated with splenocytes isolated from immune animals to identify CD8+ T cells with specificity for the antigens expressed in the Ad-array. These antigen-specific CD8+ T cells are identified by either ICS and flow cytometry or ELISpot assays measuring IFNγ secretion. (B) Ad5 vector effectively transduces APCs. A20 cells were infected with AdGFP at the indicated MOI. The percentage of GFP+ cells was determined by flow cytometry. (C) AdPyCSP-infected APCs can recall CD8+ T cell responses from immune mice. Target A20 cells were infected with various MOIs of an Ad5 vector expressing PyCSP (AdPyCSP). Control targets were uninfected A20 cells, A20 cells infected with various MOIs of an Ad5 vector that does not express a transgene (AdNull), and A20 cells stimulated with an immunodominant PyCSP peptide (amino acids 280–288, SYVPSAEQI). These targets were used to stimulate splenocytes from BALB/c mice immunized with a PyCSP DNA vaccine. IFNγ-expressing cells were measured by ELISpot. SFC (spot-forming cells); error bars indicate the SEM (n = 3). SFC, spot-forming cell.
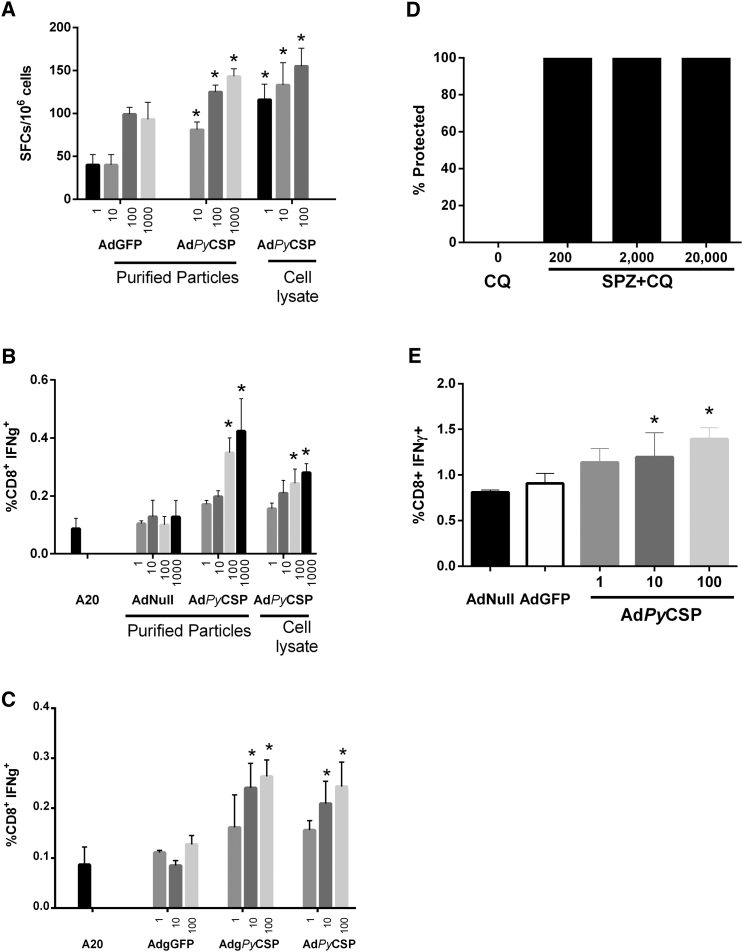
To determine whether lower-frequency T cell responses from mice immunized with sporozoite vaccines could be identified using our approach, we assayed CD8+ T cell responses specific for PyCSP from mice immunized with protective regimens of RAS and SPZ+CQ. We chose PyCSP as the test antigen because it is the most well-characterized target of T cell responses from mice immunized with these regimens.20, 27 First, we assayed splenocytes from mice immunized with a highly protective three-dose regimen of RAS for the presence of PyCSP-specific T cells. We were able to recall PyCSP-specific T cells in splenocytes from these mice using AdPyCSP-infected A20 cells in both ELISpot (Figure 4A) and intracellular cytokine staining (ICS) assays (Figure 4B). Low background responses were observed in the negative controls.
Figure 4.
Adenovector-Expressed Antigen Is Effective at Recalling CD8+ T Cell Responses from Mice Immunized with Protective Regimens of Sporozoite Vaccines
Target A20 cells were infected at the indicated MOIs with AdPyCSP (either triple CsCl2 purified adenovector or unpurified cell lysate from adenovector infected cells) and incubated with splenocytes from RAS or SPZ+CQ-immunized mice. Control targets were A20 cells infected with AdNull, AdGFP, and uninfected A20 cells. (A) IFNγ-secreting cells from mice immunized with RAS were measured by ELISpot. (B) CD8+ IFNγ+ cells from mice immunized with RAS were measured by ICS and flow cytometry. (C) Comparison of Ad-array PyCSP vector (AdgPyCSP) with AdPyCSP, which does not contain the recombination motifs flanking the expression cassette. CD8+ IFNγ+ cells from mice immunized with RAS were measured by ICS and flow cytometry. (D) Dose-response analysis for efficacy of SPZ+CQ vaccine regimen following immunization with 0, 200, 2,000, or 20,000 P. yoelii sporozoites with chloroquine treatment. Mice were assessed for sterile protection by blood smear. (E) AdPyCSP-infected cells can recall CD8+ T cell responses from mice immunized with 2,000 SPZ+CQ. Target A20 cells were infected with cell lysates containing the indicated Ad5 vectors and antigen-specific CD8+ T cell responses were measured by ICS and flow cytometry. Error bars indicate the SEM (n = 3). Asterisks indicate statistically significant differences compared with AdGFP (A), A20 (B and C), or AdNull (E) controls (p < 0.05 by ANOVA with the Bonferroni mean-comparisons test).
It was important to assess the degree of purity of the adenovector preparation necessary for the screen because if unpurified adenovectors were suitable, this would greatly simplify generation of the Ad-array. Accordingly, we compared highly purified AdPyCSP (purified over three successive CsCl gradients) with cell lysates containing unpurified recombinant adenovector. PyCSP-specific CD8+ T cell responses were detected with both purified and unpurified vectors using ELISpot (Figure 4A) and ICS (Figure 4B) assays. Our results indicated that vector purification is not required to identify antigens that recall CD8+ T cell responses in mice immunized with RAS.
Ad-array vectors contain 25bp-long attB sequences flanking the transgene (Figure 2B), which are remnants of the recombinase cloning reaction. We compared Ad-array vectors directly with our vaccine adenovectors, which do not carry the flanking attB sequences. Our results indicate that the attB sequences did not inhibit the capacity to recall T cell responses in mice (Figure 4C), indicating that Ad-array vectors are suitable for screening.
Mice immunized with a two-dose regimen of 200, 2,000, and 20,000 SPZ+CQ were completely protected from P. yoelii sporozoite challenge (Figure 4D). Figure 4E shows that PyCSP-specific T cells were induced by immunizing mice with a highly protective 2,000 SPZ+CQ regimen. Splenocytes from immunized mice had a high background of activated CD8+ T cells. When incubated with A20 cells infected with the negative control vectors AdNull and AdGFP, 0.8%–0.9% of the CD8+ T cells were activated. A20 cells infected with AdPyCSP recalled PyCSP-specific T cell responses that were more frequent than the negative controls. Statistically significant results were observed with MOIs of 10 and 100 ffu/cell. These data suggested that it would be possible to utilize our Ad-array technology to identify new antigen targets of protective T cell responses following immunization of mice with SPZ+CQ.
Identification of the Antigen Targets of CD8+ T Cells Induced following Vaccination with Protective Regimens of SPZ+CQ
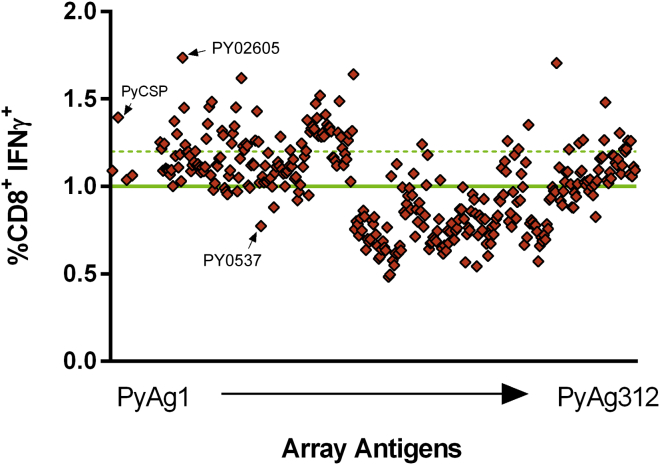
To generate protective T cells for the identification of antigens, we used the 2,000 SPZ+CQ regimen and harvested splenocytes 2 weeks after the last sporozoite immunization. The full array was screened simultaneously, in triplicate, against these freshly isolated splenocytes by ICS to identify pre-erythrocytic stage antigens able to recall IFNγ-expressing CD8+ T cells. A20 cells infected with 100 ffu/cell AdgPyCSP were included as a positive control. Negative controls included uninfected A20 cells and A20 cells infected with 100 ffu/cell of AdNull and AdGFP vectors. The mean of the negative controls was 1% IFNγ-expressing CD8+ T cells (Figure 5). We defined antigens with responses greater than 2 SD of the mean of the negative controls (>1.2% CD8+ IFNγ+ cells) as positive hits in our screen. By this definition, 69 of the antigens in the array were positive and were targeted by CD8+ T cells induced in mice immunized with SPZ+CQ (Figure 5; Table 1). Thirteen of these antigens recalled higher-frequency CD8+ T cell responses than PyCSP. The antigen that recalled the highest response was PY02605. We also analyzed CD4+ T cell responses and tumor necrosis factor (TNF)-α and interleukin (IL)-2 cytokines by ICS. CD4+ T cell responses were not observed in this system. CD8+ TNF-α-expressing T cells were observed and tended to mirror the CD8+ IFNγ responses. Very low levels of IL-2-expressing cells were observed (data not shown).
Figure 5.
Identification of Targets of CD8+ T Cell Responses Induced by a Highly Protective SPZ+CQ Vaccine Regimen
Splenocytes from BALB/c mice immunized with SPZ+CQ were screened for CD8+ recall responses stimulated by 312 pre-erythrocytic antigens and evaluated by ICS and flow cytometry. The mean of the negative controls is indicated by the horizontal green line. The dotted green line indicates responses that are ≥2 SD above the mean of the negative controls. Arrows indicate two antigens tested for protection shown in Table 2.
Table 1.
P. yoelii Genes that Recalled T Cell Responses >2 SDs Higher than the Mean of the Negative Controls
| SPZ+CQ Rank | P. yoelii Gene ID | CD8+ IFNγ+ (%) | Size (nt) | GenVec ID | SPZ+CQ Rank | P. yoelii Gene ID | CD8+ IFNγ+ (%) | Size (nt) | GenVec ID |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PY02605 | 1.74 | 522 | GV0014 | 36 | PY02240 | 1.30 | 1,131 | GV0011 |
| 2 | PY02989 | 1.71 | 1,292 | GV0350 | 37 | PY02076 | 1.29 | 1,095 | GV0041 |
| 3 | PY02326 | 1.64 | 624 | GV0139 | 38 | PY00463 | 1.29 | 972 | GV0112 |
| 4 | PY02030 | 1.62 | 1,485 | GV0054 | 39 | PY01621 | 1.29 | 2,958 | GV0117 |
| 5 | PY00629 | 1.52 | 2,877 | GV0113 | 40 | PY00808 | 1.28 | 1,359 | GV0114 |
| 6 | PY07062 | 1.49 | 939 | GV0129 | 41 | PY05019 | 1.28 | 1,134 | GV0084 |
| 7 | PY00361 | 1.48 | 1,332 | GV0033 | 42 | PY00142 | 1.28 | 1,950 | GV0130 |
| 8 | PY04421 | 1.48 | 390 | GV0402 | 43 | PY06454 | 1.27 | 525 | GV0030 |
| 9 | PY05048 | 1.47 | 1,008 | GV0110 | 44 | PY04001 | 1.26 | 444 | GV0372 |
| 10 | PY07825 | 1.46 | 726 | GV0032 | 45 | PY02435 | 1.26 | 408 | GV0401 |
| 11 | PY05748 | 1.45 | 5,319 | GV0050 | 46 | PY01556 | 1.26 | 1,836 | GV0135 |
| 12 | PY04475 | 1.43 | 894 | GV0066 | 47 | PY03629 | 1.26 | 1,509 | GV0065 |
| 13 | PY02148 | 1.41 | 972 | GV0118 | 48 | PY05566 | 1.26 | 1,116 | GV0446 |
| 14 | PY03168 | 1.39 | 1,194 | CSP | 49 | PY04605 | 1.26 | 645 | GV0294 |
| 15 | PY05088 | 1.39 | 630 | GV0111 | 50 | PY05369 | 1.26 | 1,308 | GV0451 |
| 16 | PY06246 | 1.38 | 1,209 | GV0104 | 51 | PY01620 | 1.26 | 2,151 | GV0067 |
| 17 | PY01731 | 1.37 | 708 | GV0009 | 52 | PY00082 | 1.25 | 1,716 | GV0001 |
| 18 | PY07531 | 1.36 | 438 | GV0031 | 53 | PY03102 | 1.25 | 360 | GV0369 |
| 19 | PY02175 | 1.35 | 1,377 | GV0119 | 54 | PY04293 | 1.25 | 1,086 | GV0099 |
| 20 | PY03289 | 1.35 | 894 | GV0326 | 55 | PY04306 | 1.24 | 633 | GV0046 |
| 21 | PY01848 | 1.34 | 744 | GV0107 | 56 | PY03138 | 1.24 | 1,680 | GV0058 |
| 22 | PY04028 | 1.34 | 1,398 | GV0049 | 57 | PY00592 | 1.24 | 1,410 | GV0003 |
| 23 | PY03601 | 1.34 | 1,524 | GV0122 | 58 | PY02214 | 1.24 | 525 | GV0196 |
| 24 | PY03098 | 1.34 | 2,058 | GV0121 | 59 | PY02722 | 1.24 | 705 | GV0015 |
| 25 | PY01182 | 1.33 | 1,716 | GV0106 | 60 | PY00920 | 1.23 | 1,239 | GV0059 |
| 26 | PY01906 | 1.33 | 2,643 | GV0108 | 61 | PY03879 | 1.23 | 1,875 | GV0055 |
| 27 | PY00858 | 1.32 | 1,419 | GV0040 | 62 | PY00382 | 1.22 | 633 | GV0131 |
| 28 | PY02102 | 1.32 | 696 | GV0138 | 63 | PY00723 | 1.21 | 936 | GV0439 |
| 29 | PY04079 | 1.31 | 828 | GV0123 | 64 | PY00572 | 1.21 | 1,326 | GV0002 |
| 30 | PY00935 | 1.31 | 1,506 | GV0105 | 65 | PY01758 | 1.21 | 825 | GV0320 |
| 31 | PY06529 | 1.31 | 1,847 | GV0128 | 66 | PY07442 | 1.21 | 852 | GV0436 |
| 32 | PY01558 | 1.31 | 2,547 | GV0116 | 67 | PY03674 | 1.21 | 1,686 | GV0360 |
| 33 | PY00196 | 1.30 | 738 | GV0431 | 68 | PY03081 | 1.21 | 3,657 | GV0057 |
| 34 | PY03814 | 1.30 | 3,690 | GV0048 | 69 | PY03150 | 1.21 | 891 | GV0092 |
| 35 | PY01863 | 1.30 | 954 | GV0137 |
nt, nucleotide.
PY02605 Is a Protective Antigen
Since the SPZ+CQ regimen induces protective T cell responses directed against antigens expressed in the pre-erythrocytic stages of the parasite life cycle,26, 27, 32 we hypothesized that a subset of antigens identified in the SPZ+CQ screen would induce protective immune responses when delivered using a potent vaccine regimen designed to optimize CD8+ T cell responses. Accordingly, we compared antigens that ranked at the two extremes of our screening strategy, PY02605 and PY05837, for their capacity to protect mice against a sporozoite challenge. PY02605 recalled the highest frequency of CD8+ T cells and PY05837 did not recall antigen-specific T cell responses in our screen. We tested the protective capacity of these antigens using a DNA prime-Ad5 boost regimen in BALB/c mice.33 Mice were immunized with 100 μg of DNA vector expressing the specific antigen and then boosted 6 weeks later with 1 × 1010 particle units (PUs) of an Ad5 vector expressing the same antigen. Two weeks after the Ad5 boost, mice were challenged with P. yoelii sporozoites and protection was monitored by microscopic examination of Giemsa-stained blood smears. 43% of the PY02605 immunized mice were sterilely protected (6 of 14 mice), indicating that PY02605 can provide protection in mice (Table 2). Only 1 mouse from the group of 14 immunized with the PY05837 antigen was negative for blood-stage parasitemia following challenge, suggesting that this antigen is not protective. The positive control group, which was immunized with PyCSP-expressing DNA and Ad5 vectors, protected 100% of the mice. The negative control group, immunized with DNA and Ad5 vectors that did not express any transgene (Null) did not protect any mice (Table 2). These data indicate that our antigen discovery system is capable of identifying protective antigens.
Table 2.
Immunization with PY02605 Partially Protects BALB/c Mice from P. yoelii Sporozoite Challenge
| Vaccine Antigen | Protected/Total | Protection (%) | p Valuea |
|---|---|---|---|
| Null | 0/14 | 0 | NA |
| PyCSP | 14/14 | 100 | <0.001 |
| PY02605 | 6/14 | 43 | 0.016 |
| PY05837 | 1/14 | 7 | 1 |
Fisher’s exact test (two-sided) versus null. Alpha < 0.05.
PF3D7_0932900 Is Immunogenic in Mice
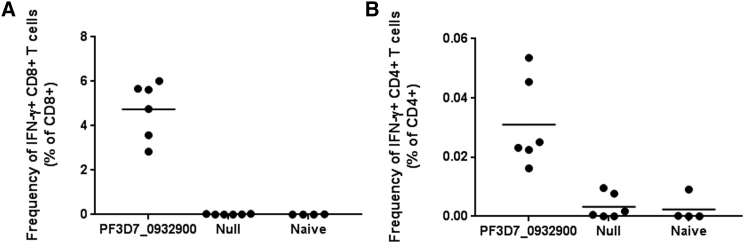
To begin evaluation of a selected pre-erythrocytic antigen as a vaccine candidate, we cloned the P. falciparum ortholog of PY02605 into a highly immunogenic and low seroprevalent gorilla adenovector (GC46)34, 35 and tested immunogenicity in mice. PF3D7_0932900 was codon optimized for expression in mammals, synthesized, and used to produce GC46.PF3D7_0932900. We immunized BALB/c mice (n = 6/group) with a single intramuscular (IM) administration of GC46.PF3D7_0932900 (1 × 109 PU). GC46.Null immunized and naive mice were included as control groups. At 21 days post-immunization, mice were euthanized for T cell studies. Antigen-specific T cell responses were measured from splenocytes by flow cytometry after stimulation with overlapping peptide pools and staining for cytokines and cell surface markers (Figure 6). GC46.PF3D7_0932900 was highly immunogenic, inducing robust antigen-specific CD8+ and low-level CD4+ T cell responses.
Figure 6.
The P. falciparum Ortholog of PY02605, PF3D7_0932900, Is Immunogenic in BALB/c Mice
Mice were immunized with 1 × 109 PU of GC46.PF3D7_0932900 or a control adenovector that does not express a transgene, GC46.Null. Three weeks post-immunization, mice were euthanized and PF3D7_0932900-specific CD8+ (A) and CD4+ (B) T cell responses were measured by ICS and flow cytometry following 4-hr stimulation using pooled overlapping 15-mer peptides.
Discussion
Identification of CD8+ T cell targets of infectious disease has the potential to increase our understanding of cellular immunity and to facilitate the development of vaccines, diagnostics, and therapeutics. In this report, we describe a novel antigen discovery system and demonstrate its capacity to identify the antigen targets of protective T cell responses to infection. Although we demonstrated the feasibility of this system for malaria, we expect that the Ad-array technology and screens similar to the one employed here may also be useful for identifying the antigens targeted by T cell responses to other infectious agents or associated with other disease states. Arrayed adenoviral expression libraries have been produced previously and used to identify gene products involved in disease pathways36; however, to our knowledge, this work is the first demonstration of the use of Ad-array technology to identify the antigen targets of cellular immunity. We show that low titers of unpurified adenovirus vector, quantities that transduce only 2% of autologous APCs, are sufficient to recall antigen-specific T cell responses in mice immunized with highly protective regimens of live sporozoite vaccines. Our ability to use low quantities of unpurified vector in the antigen discovery screen simplifies high-throughput Ad-array production and indicates potential for producing large Ad-arrays encoding the full transcriptome of complex pathogens with large genomes.
Advantages of the Ad-array technology over other T cell antigen discovery efforts are that adenoviruses efficiently infect many cell types including APCs37 and that, following infection, full-length proteins are expressed and multiple undefined epitopes are presented, similar to what occurs during a natural infection. The Ad-array technology was as effective as the immunodominant PyCSP peptide in detecting PyCSP-specific CD8+ T cell responses. Moreover, this technology was highly sensitive, detecting low-level PyCSP-specific CD8+ T cell responses following immunization with RAS and SPZ+CQ. Other T cell antigen discovery efforts utilized in silico algorithms to predict epitopes with high MHC binding affinity and then generated libraries of peptides corresponding to the selected epitopes.21, 22, 23, 38, 39, 40, 41 Although these methods are improving, they are expensive and limited in their capacity to predict protective T cell epitopes.42, 43 In the mouse malaria models, epitope prediction algorithms have supported the identification of several pre-erythrocytic antigens that are targeted by T cell responses induced following immunization with P. yoelii and P. berghei RAS or SPZ+CQ; however, these antigens have not demonstrated protective efficacy when tested in challenge experiments.38, 39, 40
We focused on identifying T cell responses specific for pre-erythrocytic antigens following immunization with a highly protective SPZ+CQ vaccine regimen because of the potential for increased diversity of antigen responses associated with this regimen relative to RAS.44, 45 Using this regimen, we identified a set of 69 pre-erythrocytic stage antigens that were targeted by vaccine-induced CD8+ T cells. Thirteen of these antigens recalled more robust responses than PyCSP and were expressed in the liver stage of the P. yoelii life cycle (Table S2).29 The antigen that recalled the most frequent CD8+ T cell responses, PY02065, is a 173 amino acid conserved Plasmodium protein with unknown function that is upregulated throughout the liver stage of the P. yoelii life cycle.29 Since protection induced by the SPZ+CQ regimen is dependent on CD8+ T cells, we hypothesized that a subset of the 69 antigen-specific CD8+ T cells that we identified may contribute to the robust protection observed with this regimen. We tested this hypothesis by evaluating the protective efficacy induced by a DNA prime-Ad5 boost regimen expressing the antigen that recalled the most frequent CD8+ T cells, PY02065. This robust T cell vaccine regimen was partially protective, inducing sterile protection (absence of blood-stage infection) in 6 of 14 mice following P. yoelii sporozoite challenge. This finding strongly suggests that our antigen discovery system is effective at identifying the targets of protective T cell responses to malarial infection, although additional antigens that recalled CD8+ T cells in our screen will need to be tested to further confirm our hypothesis.
Our malaria vaccine development efforts focus on improving DNA-Ad5-CA, a first-generation vaccine candidate that induced an encouraging 27% sterile protection in human volunteers challenged with controlled human malaria infection (CHMI).40 DNA-Ad5-CA is a DNA prime-Ad5 boost regimen that expresses two P. falciparum antigens: PfCSP and apical membrane antigen 1 (PfAMA1). CD8+ T cell responses specific for PfAMA1 were associated with protection, and 2 of the 4 protected volunteers had the highest frequency of CD8+ T cell responses specific for PfCSP. Our goal is to increase vaccine efficacy to meet the preferred product characteristic target of 75% efficacy set forth by the World Health Organization (WHO).46 Since overall efficacy of DNA-Ad5-CA was limited by pre-existing immunity to the Ad5 vector,40 we have selected a highly immunogenic gorilla-based adenovector, GC46,34, 35, 47 for advancement and have recently demonstrated superior immunogenicity and protection with this vector relative to Ad5 in a malaria model.47 In animal models, GC46 performance is similar to chimpanzee adenovirus type 3,48 and given the excellent safety, immunogenicity, and efficacy data that have been achieved with the chimpanzee-based adenovirus vectors in humans,49, 50, 51, 52, 53, 54 we are enthusiastic about the potential to test GC46 vectors expressing CSP and AMA-1 in the clinic. Additional improvements in efficacy can be obtained by increasing the number of antigens in this vaccine regimen.55, 56 In this regard, PF3D7_0932900, the P. falciparum ortholog of PY02065, and the P. falciparum orthologs of other antigens identified in this work have potential to increase the efficacy of the DNA-Ad5-CA vaccine candidate.
Materials and Methods
Primer Design, Gene Amplification, and Plasmid Cloning
Highly abundant P. yoelii pre-erythrocytic antigens with identifiable P. falciparum orthologs were selected for inclusion in the Ad-array. We included genes encoding proteins ≥130 amino acids in length because larger proteins have a greater probability of containing T cell epitopes. The DNA sequences of the selected genes used for amplification were obtained from the PlasmoDB database.57 All of the single exon genes were amplified directly from P. yoelii (17XNL) genomic DNA using a pair of gel purified primers: the forward (fwd) primer contains the Kozak consensus CACC upstream of the starting codon ATG, the reverse (rev) primer retains the native stop codon at the end of the oligonucleotide, and both of the primers were designed to have the same melting temperature (Tm) at 66°C. PCR amplification was performed using high-fidelity platinum Taq polymerase (Invitrogen, Carlsbad, CA) or Hot Star polymerase (QIAGEN, Gaithersburg, MD). For multi-exon genes, we amplified individual exons first, and then assembled individual exons into an intact gene product using the cDNA synthesis PCR.58 All PCR-amplified gene products were cloned into a shuttle vector, pCR8/GW/TOPO (Invitrogen, Carlsbad, CA). The correct orientation of the clones was confirmed by DNA sequencing using M13 fwd and M13 rev oligonucleotides. The pAdFlex plasmids pACE1(3511rfC)E3(10X) and pACE1(t.rfC.MCS)E3(10X) contain the full-length Ad5 genome with deletions in the E1 and E3 regions. Within the E1 region, we inserted attB1 and attB2 elements for lambda recombination and a CMV promoter and poly(A) sequences for antigen expression. The genes in the shuttle vector were moved into these destination gateway-converted adeno plasmids with high efficiency using the Gateway LR reaction59 (Invitrogen, Carlsbad, CA), in 96-well plate format to generate an array of pAdFlex plasmids expressing pre-erythrocytic genes from P. yoelii (pAdgPy1-360).
Ad-Array Viral Vector Construction
The Ad-array is an array of adenovirus vectors expressing P. yoelii antigen genes constructed from an array of pAdFlex plasmids. Briefly, the recombinant Ad5 genomes containing an antigen gene expression cassette in the E1 region were liberated from pAdFlex plasmids by digestion with Pac I or I-CeuI restriction endonuclease, which cleaves adjacent to the inverted terminal repeats (ITRs) of the Ad5 genome. This vector DNA (60 ng) was transfected into monolayer 293 cells seeded at 70,000 cells per well in 96-well plates using 0.7 μL polyfect transfection reagent (QIAGEN, Gaithersburg, MD). Three days later, transfected cells were frozen and thawed three times to lyse the cells, releasing infectious recombinant virus, and 18 μL of these lysates was used to infect fresh 293 cells in another 96-well plate. Cell lysates were serially passaged in this manner every 3 days until CPE was observed. The identity of the adenovector was determined using primers Ad5s278 (CGCGGGAAAACTGAATAAGA) and Ad5a3598 (GCTGCTGCAAAACAGATACA) as indicated in Figure 2B. Vector yields were determined by using the ffu assay60 on a subset of approximately 10% of the array to ensure that sufficient quantities of vector were being produced.
Production of Adenovirus Vectors for Protection Studies in Mice
To generate sufficient vector for mouse protection experiments, residual CPE lysates were expanded in four 10-cm plates and the cell lysates were harvested 3 days later. The expanded cell lysates were analyzed to determine infectious vector particle concentration, were analyzed by PCR to ensure vector genomic integrity, and were used to seed a production run in suspension 293-ORF6 cells in shaker flasks using serum-free media. Three days after infection, the recombinant vectors were released from infected cells by three cycles of freeze-thawing, treated with benzonase (EMD Millipore, Billerica, MA), purified by banding on two successive CsCl gradients, dialyzed into final formulation buffer and stored at −80°C. Physical PUs were determined by absorbance at 260 nm following disruption of the capsid with SDS.
Mice, Parasites, and Cells
The study protocols were reviewed and approved by the Walter Reed Army Institute of Research/Naval Medical Research Center (NMRC) and GenVec Institutional Animal Care and Use Committees in compliance with all applicable federal regulations governing the protection of animals in research. Female (6- to 8-week-old) BALB/c mice were purchased from Harlan Laboratories (http://www.envigo.com) or Charles River Laboratories (Wilmington, MA). CD1 outbred mice (5–6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). P. yoelii (17XNL non-lethal strain, clone 1.1) parasites were maintained by alternating passage in Anopheles stephensi mosquitoes and female CD1 mice. HEK293 cells, a human embryonic kidney cell line transformed by sheared adenovirus type 5 DNA, were obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained in DMEM supplemented with 10% calf serum. The A20.2J (ATCC clone HB-98) B cell line derived from BALB/c mice, which expresses both class I and class II MHC genes,61 was purchased from ATCC and maintained in RPMI-1640 medium supplemented with 20% fetal bovine serum (FBS) and 1% glutamine.
Immunization Regimens
RAS were generated by exposing salivary gland P. yoelii sporozoites to 10,000 rads. To obtain splenocytes for testing recall responses to PyCSP, female BALB/c mice were immunized, via tail vein injection, with three doses of RAS (10,000, 5,000, and 5,000) at 3-week intervals.
For SPZ+CQ immunizations, female BALB/c mice were immunized with two administrations (1 month apart) of live infectious P. yoelii sporozoites. Various doses of sporozoites were tested (group 1 = 20,000, group 2 = 2,000, group 3 = 200, group 4 = 0). Infected mice received a 0.1mL intraperitoneal injection of a solution of chloroquine hydrochloride (Sigma-Aldrich, St. Louis, MO) 8 mg/mL diluted in PBS, to kill newly emerging blood-stage parasites, starting on the same day as sporozoite immunizations and continuing for 10 consecutive days following each sporozoite immunization. For both SPZ+CQ and RAS immunizations, most of these mice were euthanized 2 weeks after the last dose to obtain splenocytes for T cell analysis. However, a subset of the mice was infected with P. yoelii sporozoites to insure that 100% sterile protection had been achieved, as described below.
For plasmid DNA immunizations, BALB/c mice were immunized with 100 μg DNA vector, pPyCSP, in a 0.1 mL volume by intramuscular immunization as described previously.62 Two weeks later, mice were euthanized to obtain splenocytes for analysis. For DNA-Ad5 immunizations, BALB/c mice were immunized with 100 μg of DNA vector, pcDNA3.2-Dest (Invitrogen, Carlsbad, CA) in a 0.1 mL volume by intramuscular immunization. Six weeks later, these mice were boosted with 1 × 1010 PU of Ad5 vector in a 0.1 mL volume. For both DNA and Ad5 administrations, we performed bilateral injections into the tibialis anterior muscles with a 0.3-mL syringe and a 29G1/2 needle (Becton Dickinson Co., Franklin Lakes, NJ).
For GC46.PF3D7_0932900 immunizations, BALB/c mice were immunized with a single dose of 1 × 109 PU of GC46.PF3D7_0932900 by the intramuscular route with a 1-mL syringe and a 30G needle (Becton Dickinson Co., Franklin Lakes, NJ). At 21 days post-immunization, mice were euthanized for splenocyte harvest and assessment of immune responses by ICS and flow cytometry.
Protection Studies
Protection studies were performed as previously described.55 Mice were challenged intravenously in the tail vein with 200 P. yoelii sporozoites using a 1-mL syringe and 26G1/2 needle. Sporozoites were hand dissected from infected mosquito salivary glands63 and diluted for challenge in M199 medium containing 5% normal mouse serum (Gemini Bio-Products, West Sacramento, CA). The development of parasitemia was monitored over the next 2 weeks by microscopic examination of Giemsa-stained blood smears. Mice were considered protected if no parasites were observed in any sample at day 6, day 9, or day 14 post-challenge.
Infection of A20 Cells
A20.J2 cells (ATCC, Manassas, VA), also referred to as A20 cells, are a B cell line derived from BALB/c mice that expresses both class I and class II major histocompatability complex genes. A20 cells were grown in 15 mL fresh RPMI-1640 media plus 20% FBS and 1% L-glutamine in 25-mL T-flasks. The T-flasks were kept upright and incubated at 37°C in a 5% CO2 incubator. When the cells reached a density of 1.2−1.8 × 106 cells/mL, they were used to seed 12-well plates at a density of 5.0 × 105 cells/well. The following day, the cells were infected with AdGFP, an adenovirus vector that expresses GFP, for 2 hr in a volume of 200 μL. After infection, cells were washed with PBS, overlaid with 1 mL fresh media, and incubated at 37°C and 5% CO2 for 48 hr. The percentage of the GFP+ cells was analyzed by flow cytometry. For the array screening, 6 × 105 A20.2J cells were infected with 150 μL CPE lysate from each of the Ad-array vectors in a volume of 350 μL in 24-well plates for 2 hr. After infection, 0.25 mL fresh media were added to each well and the infected cells were incubated at 37°C and 5% CO2 for 24 hr before plating in the stimulations described below.
ICS and Flow Cytometry Analysis
Stimulation by Ad5-Infected A20 Cells
Splenocytes harvested from SPZ+CQ or RAS-immunized animals were stimulated by co-culture with infected/irradiated A20 cells in 96-well plates. Spleens were gently crushed using the flat end of a 3-cc or 10-cc syringe plunger, and the cell suspension was passed through a 70-μm filter. The splenocytes were washed with ice-cold 2% FBS/10 mM HEPES/1× Hank’s balanced salt solution (HBSS), resuspended in RPMI, counted, diluted to 1 × 107 cells/mL in RPMI medium, and plated at 1 × 106 cells/well in 96-well plates. At 24 hr after infection, A20 cells were irradiated in a Pantak X-Rad 320 irradiator at 16,666 rads. After irradiation, the viable cell concentration was adjusted to 1.5 × 106 cells per mL and 1.5 × 105 infected cells were transferred to each well of U-bottom 96-well plates preloaded with 1 × 106 splenocytes from vaccinated or naive mice, in triplicate, and incubated for 5 hr at 37°C. BD Golgi Plug (BD Bioscience, San Jose, CA) was added 1 hr into the incubation to block cytokine release. Cells were centrifuged at 1,200 rpm for 5 min, the supernatant was flicked, and the cell pellets were resuspended by gentle vortexing. Live and dead cells were first stained with LIVE/DEAD Fixable Aqua stain kit (BD Biosciences, San Diego, CA), then the cells were blocked with FC Block (BD Biosciences, San Diego, CA). After blocking, cell surface markers were stained with the following antibodies (fluorochromes): CD4-eFluor-450 (clone RM4-5; eBioscience, San Diego, CA) and CD8a-PerCP-Cy5.5 (clone 53-6.7; BD Biosciences, San Diego, CA). Following fixation/permeabilization steps, the samples were stained intracellularly with the following antibodies (fluorochromes): IFN-γ-PE (clone XMG 1.2), TNF-α APC (clone MP6 XT22), and IL-2-Alexa488 (clone JES6-5H4) (BD Biosciences, San Diego, CA). The frequency of CD4+ and CD8+ T cells, as well as antigen-specific IFNγ, TNF-α, and IL-2 intracellular cytokine-positive T cells was determined in an 8-color upgraded FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA) with a 96-well Automated Micro-sampling System (AMS) (Cytek, Fremont, CA). Data were analyzed using FloJo software (Treestar, Inc., Ashland, OR).
ICS Flow Cytometry Analysis
Stimulation by PF3D7_0932900 Peptide Pools
Splenocytes from GC46.PF3D7_0932900 immunized mice were harvest and plated at 2 × 106 cells per well in a 96-well v-bottom plate. Cells were stimulated for 4 hr in the presence of 20 μg/mL brefeldin A (Sigma-Aldrich, St. Louis, MO) with either 15-mer peptides for the PF3D7_0932900 antigen at 2 μg/mL, overlapping by 10 amino acids (Mimotopes, Victoria, Australia), or 1% DMSO as a negative control. Subsequently, cells were stained with the LIVE/DEAD Fixable Blue Dead Cell Stain Kit, for UV excitation (Invitrogen, Grand Island, NY), surface stained with CD14 phycoerythrin (PE) (clone Sa14-2; Life Technologies, Grand Island, NY), CD19 Brilliant Violet 650 (clone 6D5; Biolegend, San Diego, CA), CD3 Alexa 700 (clone 17A2; Biolegend, San Diego, CA), CCR7 PerCPCy5.5 (clone 4B12; eBioscience, San Diego, CA), CD44 Pacific Blue (clone IM7; Biolegend, San Diego, CA), and CD62L Brilliant Violet 786 (clone MEL-14; BD Biosciences, San Diego, CA) and permeabilized using Cytofix/Cytoperm reagent (BD Biosciences, San Diego, CA). Cells were then intracellularly stained with CD4 Brilliant Violet 605 (clone RM4-5; Biolegend, San Diego, CA), together with CD8 Horizon V500 (clone 53-6.7), TNF Cy7PE (clone MP6-XT22), IFNγ allophycocyanin (clone XMG1.2), and IL-2 FITC (clone JES6-5H4; BD Biosciences, San Diego, CA). Samples were acquired using an LSR Fortessa (Becton Dickinson Immunocytometry Systems, San Jose, CA) and data were analyzed using FlowJo version 9.4.11 (TreeStar Inc., Ashland, OR). To identify antigen-specific responses, cells were gated on forward scatter (threshold), exclusion of aggregates, and subsequently to include singlets, viable cells, CD14−, CD3+, CD19−, CD3+, lymphocytes, and either CD4+ or CD8+ populations.
ELISpot Assay
RAS splenocytes were stimulated in vitro with Ad5-transduced or PyCSP peptide-pulsed APCs and the number of P. yoelii antigen-specific IFNγ-secreting spot-forming cells was evaluated after a 36-hr culture period. Splenocytes were tested at 400,000, 200,000, and 100,000 per well, while APCs were tested at 100,000 per well. Assays were performed in triplicate and the number of IFNγ-secreting cells, recognized as spot-forming cells (SFCs), was counted using an automated ELISpot Reader manufactured by AutoImmun Diagnostika (AID) GmbH (Strassberg, Germany). Data were presented as the number of IFNγ-secreting SFCs per million spleen cells.
Author Contributions
Conceptualization, J.T.B. and D.E.B.; Methodology, P.C. and M.S.; Formal Analysis, P.C., C.A.L., and J.T.B.; Investigation, P.C., C.A.L., G.E., B.A.M., J.B., and E.C.S; Writing – Original Draft, J.T.B.; Writing – Review & Editing, J.T.B., J.C.A, D.L.D., and D.E.B.; Visualization, J.T.B., C.A.L., and E.C.S.; Supervision, J.T.B., D.E.B., D.L.D., and J.C.A.; Project Administration, C.R.K, T.L.R., and E.V.; Funding Acquisition, J.T.B., C.R.K., and D.L.D.
Conflicts of Interest
D.E.B. is employed at GenVec, Inc. J.T.B., P.C., B.A.M., G.E., and C.A.L. were employed at GenVec, Inc. at the time this work was performed.
Acknowledgments
We thank Randy Osborn, Holly Torano, Damodar Ettyreddy, Elena Semenova, A’drian Pineda, Esteban Abot, Arnel Belmonte, Dianne Litilit, Rebecca Danner, Nonenipha Rangel, and Kyosuke Oda for their excellent technical assistance. We thank Rena Cohen for copy editing and assistance with submission of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. M.S. and E.V. are employees of the U.S. Government. T.L.R. was a U.S. military officer at the time this work was performed. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. P.C., G.E., B.A.M., C.R.K., D.E.B., and J.T.B. are/were employees of GenVec Inc., a for-profit corporation. This work was supported by the NIH (grants 1R43AIO84269-01 and 1R43AI100467-01 to J.T.B.), the Department of Defense (grant A043-189-0667 to J.T.B.), and the NMRC Military Infectious Disease Research Program (work unit number 602236N.04427.B27.A0242).
Footnotes
Supplemental Information includes two tables and can be found with this article online at http://dx.doi.org/10.1016/j.omtm.2017.08.003.
Supplemental Information
References
- 1.Davies D.H., Liang X., Hernandez J.E., Randall A., Hirst S., Mu Y., Romero K.M., Nguyen T.T., Kalantari-Dehaghi M., Crotty S. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. USA. 2005;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nabel G.J. Designing tomorrow’s vaccines. N. Engl. J. Med. 2013;368:551–560. doi: 10.1056/NEJMra1204186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krzych U., Zarling S., Pichugin A. Memory T cells maintain protracted protection against malaria. Immunol. Lett. 2014;161:189–195. doi: 10.1016/j.imlet.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuji M. A retrospective evaluation of the role of T cells in the development of malaria vaccine. Exp. Parasitol. 2010;126:421–425. doi: 10.1016/j.exppara.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overstreet M.G., Cockburn I.A., Chen Y.C., Zavala F. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol. Rev. 2008;225:272–283. doi: 10.1111/j.1600-065X.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann S.H. Fact and fiction in tuberculosis vaccine research: 10 years later. Lancet Infect. Dis. 2011;11:633–640. doi: 10.1016/S1473-3099(11)70146-3. [DOI] [PubMed] [Google Scholar]
- 7.Appay V., Douek D.C., Price D.A. CD8+ T cell efficacy in vaccination and disease. Nat. Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization (2016). World Malaria Report. http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/.
- 9.Nussenzweig R.S., Vanderberg J., Most H., Orton C. Protective immunity produced by the injection of X-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 10.Clyde D.F., Most H., McCarthy V.C., Vanderberg J.P. Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci. 1973;266:169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman S.L., Goh L.M., Luke T.C., Schneider I., Le T.P., Doolan D.L., Sacci J., de la Vega P., Dowler M., Paul C. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 12.Gwadz R.W., Cochrane A.H., Nussenzweig V., Nussenzweig R.S. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull. World Health Organ. 1979;57(Suppl 1):165–173. [PMC free article] [PubMed] [Google Scholar]
- 13.Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 14.Weiss W.R., Sedegah M., Beaudoin R.L., Miller L.H., Good M.F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. USA. 1988;85:573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman S.L., Isenbarger D., Long G.W., Sedegah M., Szarfman A., Waters L., Hollingdale M.R., van der Meide P.H., Finbloom D.S., Ballou W.R. Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science. 1989;244:1078–1081. doi: 10.1126/science.2524877. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues M.M., Cordey A.S., Arreaza G., Corradin G., Romero P., Maryanski J.L., Nussenzweig R.S., Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 1991;3:579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 17.Romero P., Maryanski J.L., Corradin G., Nussenzweig R.S., Nussenzweig V., Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 18.Weiss W.R., Jiang C.G. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS ONE. 2012;7:e31247. doi: 10.1371/journal.pone.0031247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menard R., Tavares J., Cockburn I., Markus M., Zavala F., Amino R. Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol. 2013;11:701–712. doi: 10.1038/nrmicro3111. [DOI] [PubMed] [Google Scholar]
- 20.Kumar K.A., Sano G., Boscardin S., Nussenzweig R.S., Nussenzweig M.C., Zavala F., Nussenzweig V. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444:937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 21.Doolan D.L., Southwood S., Freilich D.A., Sidney J., Graber N.L., Shatney L., Bebris L., Florens L., Dobano C., Witney A.A. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc. Natl. Acad. Sci. USA. 2003;100:9952–9957. doi: 10.1073/pnas.1633254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doolan D.L., Hoffman S.L., Southwood S., Wentworth P.A., Sidney J., Chesnut R.W., Keogh E., Appella E., Nutman T.B., Lal A.A. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA-B supertype alleles. Immunity. 1997;7:97–112. doi: 10.1016/s1074-7613(00)80513-0. [DOI] [PubMed] [Google Scholar]
- 23.Doolan D.L., Southwood S., Chesnut R., Appella E., Gomez E., Richards A., Higashimoto Y.I., Maewal A., Sidney J., Gramzinski R.A. HLA-DR-promiscuous T cell epitopes from Plasmodium falciparum pre-erythrocytic-stage antigens restricted by multiple HLA class II alleles. J. Immunol. 2000;165:1123–1137. doi: 10.4049/jimmunol.165.2.1123. [DOI] [PubMed] [Google Scholar]
- 24.Yayon A., Ginsburg H. Chloroquine inhibits the degradation of endocytic vesicles in human malaria parasites. Cell Biol. Int. Rep. 1983;7:895. doi: 10.1016/0309-1651(83)90207-2. [DOI] [PubMed] [Google Scholar]
- 25.Roestenberg M., McCall M., Hopman J., Wiersma J., Luty A.J., van Gemert G.J., van de Vegte-Bolmer M., van Schaijk B., Teelen K., Arens T. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 2009;361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 26.Belnoue E., Costa F.T., Frankenberg T., Vigário A.M., Voza T., Leroy N., Rodrigues M.M., Landau I., Snounou G., Rénia L. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 2004;172:2487–2495. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 27.Mauduit M., Tewari R., Depinay N., Kayibanda M., Lallemand E., Chavatte J.M., Snounou G., Rénia L., Grüner A.C. Minimal role for the circumsporozoite protein in the induction of sterile immunity by vaccination with live rodent malaria sporozoites. Infect. Immun. 2010;78:2182–2188. doi: 10.1128/IAI.01415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speake C., Duffy P.E. Antigens for pre-erythrocytic malaria vaccines: building on success. Parasite Immunol. 2009;31:539–546. doi: 10.1111/j.1365-3024.2009.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarun A.S., Peng X., Dumpit R.F., Ogata Y., Silva-Rivera H., Camargo N., Daly T.M., Bergman L.W., Kappe S.H. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc. Natl. Acad. Sci. USA. 2008;105:305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y., Ramachandran V., Kumar K.A., Westenberger S., Refour P., Zhou B., Li F., Young J.A., Chen K., Plouffe D. Evidence-based annotation of the malaria parasite’s genome using comparative expression profiling. PLoS ONE. 2008;3:e1570. doi: 10.1371/journal.pone.0001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams C.T., Azad A.F. Transcriptional analysis of the pre-erythrocytic stages of the rodent malaria parasite, Plasmodium yoelii. PLoS ONE. 2010;5:e10267. doi: 10.1371/journal.pone.0010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bijker E.M., Bastiaens G.J., Teirlinck A.C., van Gemert G.J., Graumans W., van de Vegte-Bolmer M., Siebelink-Stoter R., Arens T., Teelen K., Nahrendorf W. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A. 2013;110:7862–7867. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruder J.T., Semenova E., Chen P., Limbach K., Patterson N.B., Stefaniak M.E., Konovalova S., Thomas C., Hamilton M., King C.R. Modification of Ad5 hexon hypervariable regions circumvents pre-existing Ad5 neutralizing antibodies and induces protective immune responses. PLoS ONE. 2012;7:e33920. doi: 10.1371/journal.pone.0033920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McVey D., Cranfield M.R., Torano H., Kuete H.M., Lee G.P., Glenn A., Bruder J.T., Rangel D., Brough D.E., Gall J.G. Adenoviruses isolated from wild gorillas are closely related to human species C viruses. Virology. 2013;444:119–123. doi: 10.1016/j.virol.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 35.Johnson T.R., Rangel D., Graham B.S., Brough D.E., Gall J.G. Genetic vaccine for respiratory syncytial virus provides protection without disease potentiation. Mol. Ther. 2014;22:196–205. doi: 10.1038/mt.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michiels F., van Es H., van Rompaey L., Merchiers P., Francken B., Pittois K., van der Schueren J., Brys R., Vandersmissen J., Beirinckx F. Arrayed adenoviral expression libraries for functional screening. Nat. Biotechnol. 2002;20:1154–1157. doi: 10.1038/nbt746. [DOI] [PubMed] [Google Scholar]
- 37.Arthur J.F., Butterfield L.H., Roth M.D., Bui L.A., Kiertscher S.M., Lau R., Dubinett S., Glaspy J., McBride W.H., Economou J.S. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther. 1997;4:17–25. [PubMed] [Google Scholar]
- 38.Mishra S., Rai U., Shiratsuchi T., Li X., Vanloubbeeck Y., Cohen J., Nussenzweig R.S., Winzeler E.A., Tsuji M., Nussenzweig V. Identification of non-CSP antigens bearing CD8 epitopes in mice immunized with irradiated sporozoites. Vaccine. 2011;29:7335–7342. doi: 10.1016/j.vaccine.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hafalla J.C., Bauza K., Friesen J., Gonzalez-Aseguinolaza G., Hill A.V., Matuschewski K. Identification of targets of CD8+ T cell responses to malaria liver stages by genome-wide epitope profiling. PLoS Pathog. 2013;9:e1003303. doi: 10.1371/journal.ppat.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chuang I., Sedegah M., Cicatelli S., Spring M., Polhemus M., Tamminga C., Patterson N., Guerrero M., Bennett J.W., McGrath S. DNA prime/adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS ONE. 2013;8:e55571. doi: 10.1371/journal.pone.0055571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguiar J.C., Bolton J., Wanga J., Sacci J.B., Iriko H., Mazeika J.K., Han E.T., Limbach K., Patterson N.B., Sedegah M. Discovery of novel Plasmodium falciparum pre-erythrocytic antigens for vaccine development. PLoS ONE. 2015;10:e0136109. doi: 10.1371/journal.pone.0136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaves F.A., Lee A.H., Nayak J.L., Richards K.A., Sant A.J. The utility and limitations of current Web-available algorithms to predict peptides recognized by CD4 T cells in response to pathogen infection. J. Immunol. 2012;188:4235–4248. doi: 10.4049/jimmunol.1103640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleri W., Paul S., Dhanda S.K., Mahajan S., Xu X., Peters B., Sette A. The Immune Epitope Database and Analysis Resource in epitope discovery and synthetic vaccine design. Front. Immunol. 2017;8:278. doi: 10.3389/fimmu.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butler N.S., Schmidt N.W., Vaughan A.M., Aly A.S., Kappe S.H., Harty J.T. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe. 2011;9:451–462. doi: 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doll K.L., Butler N.S., Harty J.T. CD8 T cell independent immunity after single dose infection-treatment-vaccination (ITV) against Plasmodium yoelii. Vaccine. 2014;32:483–491. doi: 10.1016/j.vaccine.2013.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization (2014). WHO Preferred Product Characteristics (PPC) for Malaria Vaccines. http://www.euvaccine.eu/sites/default/files/uploads/Files/3-WHO_IVB_14.09_eng.pdf.
- 47.Limbach K., Stefaniak M., Chen P., Patterson N.B., Liao G., Weng S., Krepkiy S., Ekberg G., Torano H., Ettyreddy D. New gorilla adenovirus vaccine vectors induce potent immune responses and protection in a mouse malaria model. Malar. J. 2017;16:263. doi: 10.1186/s12936-017-1911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quinn K.M., Da Costa A., Yamamoto A., Berry D., Lindsay R.W., Darrah P.A., Wang L., Cheng C., Kong W.P., Gall J.G. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 2013;190:2720–2735. doi: 10.4049/jimmunol.1202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnes E., Folgori A., Capone S., Swadling L., Aston S., Kurioka A., Meyer J., Huddart R., Smith K., Townsend R. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ewer K.J., O’Hara G.A., Duncan C.J., Collins K.A., Sheehy S.H., Reyes-Sandoval A., Goodman A.L., Edwards N.J., Elias S.C., Halstead F.D. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat. Commun. 2013;4:2836. doi: 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ledgerwood J.E., Sullivan N.J., Graham B.S. Chimpanzee adenovirus vector Ebola vaccine--preliminary report. N. Engl. J. Med. 2015;373:776. doi: 10.1056/NEJMc1505499. [DOI] [PubMed] [Google Scholar]
- 52.Ogwang C., Kimani D., Edwards N.J., Roberts R., Mwacharo J., Bowyer G., Bliss C., Hodgson S.H., Njuguna P., Viebig N.K., MVVC group Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci. Transl. Med. 2015;7:286re5. doi: 10.1126/scitranslmed.aaa2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ewer K., Rampling T., Venkatraman N., Bowyer G., Wright D., Lambe T., Imoukhuede E.B., Payne R., Fehling S.K., Strecker T. A monovalent chimpanzee adenovirus ebola vaccine - preliminary report. N. Engl. J. Med. 2015;374:1635–1646. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tapia M.D., Sow S.O., Lyke K.E., Haidara F.C., Diallo F., Doumbia M., Traore A., Coulibaly F., Kodio M., Onwuchekwa U. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2016;16:31–42. doi: 10.1016/S1473-3099(15)00362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Limbach K., Aguiar J., Gowda K., Patterson N., Abot E., Sedegah M., Sacci J., Richie T. Identification of two new protective pre-erythrocytic malaria vaccine antigen candidates. Malar. J. 2011;10:65. doi: 10.1186/1475-2875-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doolan D.L., Sedegah M., Hedstrom R.C., Hobart P., Charoenvit Y., Hoffman S.L. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J. Exp. Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aurrecoechea C., Brestelli J., Brunk B.P., Dommer J., Fischer S., Gajria B., Gao X., Gingle A., Grant G., Harb O.S. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37:D539–D543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibson D.G., Young L., Chuang R.Y., Venter J.C., Hutchison C.A., 3rd, Smith H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 59.Katzen F. Gateway(®) recombinational cloning: a biological operating system. Expert Opin. Drug Discov. 2007;2:571–589. doi: 10.1517/17460441.2.4.571. [DOI] [PubMed] [Google Scholar]
- 60.Bruder J.T., Chen P., Semenova E., Thomas C.A., Konovalova S., Ekberg G., Ettyreddy D., McVey D., Gall J.G., King C.R., Brough D.E. Identification of a suppressor mutation that improves the yields of hexon-modified adenovirus vectors. J. Virol. 2013;87:9661–9671. doi: 10.1128/JVI.00462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kappler J., White J., Wegmann D., Mustain E., Marrack P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc. Natl. Acad. Sci. USA. 1982;79:3604–3607. doi: 10.1073/pnas.79.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sedegah M., Rogers W.O., Belmonte A., Belmonte M., Banania G., Patterson N., Ferrari M., Kaslow D.C., Carucci D.J., Richie T.L., Doolan D.L. Vaxfectin enhances immunogenicity and protective efficacy of P. yoelii circumsporozoite DNA vaccines. Vaccine. 2006;24:1921–1927. doi: 10.1016/j.vaccine.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 63.Doolan D.L., Hoffman S.L. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J. Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.