Abstract
Silver nanoparticles (AgNPs) are employed in a variety of consumer products; however, in vivo rodent studies indicate that AgNPs can cause lung inflammation and toxicity in a strain- and particle type–dependent manner, but mechanisms of susceptibility remain unclear. The aim of this study was to assess the variation in AgNP-induced lung inflammation and toxicity across multiple inbred mouse strains and to use genome-wide association (GWA) mapping to identify potential candidate susceptibility genes. Mice received doses of 0.25 mg/kg of either 20-nm citrate-coated AgNPs or citrate buffer using oropharyngeal aspiration. Neutrophils in bronchoalveolar lavage fluid (BALF) served as markers of inflammation. We found significant strain- and treatment-dependent variation in neutrophils in BALF. GWA mapping identified 10 significant single-nucleotide polymorphisms (false discovery rate, 15%) in 4 quantitative trait loci on mouse chromosomes 1, 4, 15, and 18, and Nedd4l (neural precursor cell expressed developmentally downregulated gene 4-like; chromosome 18), Ano6 (anocatmin 6; chromosome 15), and Rnf220 (Ring finger protein 220; chromosome 4) were considered candidate genes. Quantitative RT-PCR revealed significant inverse associations between mRNA levels of these genes and neutrophil influx. Nedd4l, Ano6, and Rnf220 are candidate susceptibility genes for AgNP-induced lung inflammation that warrant additional exploration in future studies.—Scoville, D. K., Botta, D., Galdanes, K., Schmuck, S. C., White, C. C., Stapleton, P. L., Bammler, T. K., MacDonald, J. W., Altemeier, W. A., Hernandez, M., Kleeberger, S. R., Chen, L.-C., Gordon, T., Kavanagh, T. J. Genetic determinants of susceptibility to silver nanoparticle-induced acute lung inflammation in mice.
Keywords: genome-wide association, nanosilver, Nedd4l, Ano6, Rnf220
Silver nanoparticles (AgNPs) are currently incorporated into a variety of consumer products that range from athletic wear to toothbrushes (1). Because the number of AgNP-containing products continues to grow, an increased likelihood of exposure to AgNPs among workers and consumers is expected. In facilities that produce varied types of engineered nanomaterial (ENM), there are indications that employees who manufacture ENM may be at increased risk for adverse respiratory and cardiovascular outcomes (2–4). The current U.S. Occupational Safety and Health Administration–permitted exposure limit for silver dust and soluble compounds is 0.01 mg/m3 (5, 6); however, on the basis of more recent inhalation studies in rats, Weldon et al. (6) propose an occupational exposure limit of 0.19 μg/m3 for AgNPs on the basis of bile duct hyperplasia. This occupational exposure limit is also predicted to be protective of adverse lung effects that are caused by AgNP inhalation.
In vitro studies have shown that AgNPs are cytotoxic in a cell-, dose-, size-, and coating-dependent manner. A study that was conducted in mouse embryonic fibroblasts suggested that oxidative stress, mitochondrial dysfunction, apoptosis, and autophagy are mechanisms of AgNP toxicity (7). In human lung cell lines, BEAS-2B and A549, size, dose, and coating have been shown to modify AgNP-induced cellular toxicity (8–10). In BEAS-2B cells, 10-nm citrate-coated AgNPs significantly reduced cell viability at 20 μg/ml and increased necrosis at 50 µg/ml compared with 40- and 75-nm citrate-coated AgNPs (8). Similar results were also found wherein 20-nm citrate- and polyvinylpyrrolidone (PVP)-coated AgNPs decreased BEAS-2B viability to a greater degree than 110-nm citrate-coated AgNPs (10). In contrast, 110-nm PVP-coated AgNPs did not significantly decrease cell viability at doses up to 50 μg/ml (10). Compared with 20- and 100-nm PVP-coated AgNPs and 110-nm citrate-coated AgNPs, 20-nm citrate-coated AgNPs induced the highest levels of oxidative stress and necrosis (10). Furthermore, particle coating (polyethylene glycol vs. branched polyethyleneimine) was also observed to modify toxicity of AgNPs in the Hepa1c1c7 mouse liver cell line (11).
The size of AgNPs also modulates in vivo acute proinflammatory potential. In a recent study, 20-nm AgNPs induced higher numbers of neutrophils in bronchoalveolar lavage fluid (BALF) in Brown Norway (BN) rats than did 110-nm AgNPs at 1 d after instillation, regardless of outer coating type (12). Neutrophils are a major component of the acute inflammatory response and are a commonly used marker of inflammation in ENM and other pulmonary toxicity studies (13). Increases in total protein concentrations in BALF—indicative of compromised capillary/alveolar barrier function—were also observed in BN rats at 1 and 7 d after exposure to 20-nm citrate- or PVP-coated AgNPs, but not 110-nM AgNPs of either coating (12, 14). Given that oxidative stress and cellular necrosis can trigger and exacerbate inflammation, in vitro observations that 20-nm citrate-coated AgNPs induce higher levels of oxidative stress and cellular necrosis are consistent with in vivo studies in which 20-nm citrate-coated particles induced the highest levels of acute neutrophilic inflammatory responses (10, 12, 15, 16).
AgNP studies in Sprague-Dawley (SD) rats provide additional evidence that size may also modify the timing of the inflammatory response and that size and coating affect the quantity and localization of AgNPs (17–20). Furthermore, in C57BL/6 mice, size modified the dose response and timing of citrate-coated AgNP-induced lung inflammation, and surface coating and size altered AgNP effects on lung surfactant proteins and lung mechanics after instillation (10, 21).
Test animal strain has also been observed to impact AgNP-induced inflammation and toxicity (12). Previous studies have shown that BN rats are more susceptible to ovalbumin-induced allergic or T-helper (Th)2-type inflammatory responses than SD rats (22). BN rats also showed increases in markers of Th2-type inflammation compared with SD rats in response to AgNP exposure, which is characterized by increased numbers of eosinophils and concentrations of eotaxin, IL-13, and total IgE in BALF (12). Additional strain differences included significant increases relative to control in total BALF protein and bronchial hyper-responsiveness in BN rats compared with SD rats after exposure to 20-nm citrate- and PVP-coated AgNPs. Thus, in vivo studies indicate that particle size, coating, and animal strain influence AgNP-induced lung inflammation and toxicity (10, 12, 17–21).
Given the multitude of factors that can influence the toxicity of AgNPs, thorough mechanistic studies for all AgNP formulations would be a resource- and time-intensive undertaking; however, if genes and pathways that are associated with susceptibility to AgNP-induced lung inflammation and toxicity were identified by using systems-level unbiased methodologies, such as genome-wide association (GWA) mapping in model organisms, this could help to focus and prioritize those mechanistic studies. In addition, findings from such studies could provide information about human susceptibility and population variability for the purposes of risk assessment and the regulation of AgNPs, which is of importance, given that there is currently no unique regulatory standard for AgNP exposure.
GWA mapping studies using panels of multiple inbred mouse strains and primary cells from inbred mouse strains have succeeded in identifying novel susceptibility genes and regulatory mechanisms in a variety of toxicity and disease models (23–29). Some examples of phenotypes for which candidate genes have been successfully identified by using GWA mapping include susceptibility to acetaminophen and isoniazid-induced liver injury (23, 24), rotenone-induced cytotoxicity (27), hyperoxia-induced lung injury (28), Aspergillus fumigatus and Ebola virus infection (25, 29), high-fat diet–induced obesity and microbiota composition (30), atherosclerotic plaque formation (31), and identification of a novel regulatory protein for lung neutrophil influx (26). Variants in the human ortholog of the candidate gene CD44—discovered in a mouse GWA study of acetaminophen-induced liver toxicity—were associated with differences in susceptibility in two separate human cohorts (23). Identification of gene variants that explain differences in human susceptibility on the basis of differential responses across mouse strains highlights the relevance of mouse GWA mapping studies.
In this study, we exposed mice from 25 different inbred strains to 20-nm citrate-coated AgNPs or citrate buffer, measured neutrophils in BALF as a marker of lung inflammation, and used GWA mapping to identify novel candidate genes that are associated with AgNP-induced neutrophil influx into the lung.
MATERIALS AND METHODS
AgNP characterization
AgNPs that were used in this study were nominally 20 nm and coated in citrate for stabilization. AgNPs were suspended in 1 mM citrate buffer. These particles are one of several nanomaterials that are available to the National Institute of Environmental Health Sciences Centers for Nanotechnology Health Implications Research Consortium and were initially characterized by the Nanotechnology Characterization Laboratories at the National Cancer Center. These particles have been thoroughly characterized by Shannahan and colleagues (32).
Animals and dosing
All mice were males and were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The T.J.K. laboratory phenotyped 11 inbred strains (CBA/J, C57L/J, MRL/MpJ, NOD/ShiLtJ, NZB/BlNJ, NZO/HlLtJ, NZW/LacJ, PL/J, PWD/PhJ, PWK/PhJ, TALLYHO/JngJ, and WSB/EiJ), and the T.G./L.-C.C. laboratory phenotyped 10 different strains (BALB/cJ, BTBRT+tf/J, C3H/HeJ, C57BL/10J, DBA/2J, FVB/NJ, SJL/J, SM/J, and SWR/J). Both labs also assayed an additional 4 strains (129S1/SvImJ, A/J, AKR/J, and C57BL/6J mice) for interlaboratory comparison purposes. The number of replicates for each strain and treatment group are outlined in Table 1. Mice were housed in polycarbonate cages, at least 2 mice per cage, with corncob bedding in temperature (20–23°C)- and humidity-controlled rooms with a 12-h light/dark cycle. Animals were provided with standard rodent chow and water ad libitum. All animal procedures and handling were performed under National Institutes of Health and Animal Welfare Act guidelines for the ethical treatment of animals, and under protocols approved by the University of Washington and New York University School of Medicine Institutional Animal Care and Use Committees. Mice were anesthetized by using isoflurane and were treated with a dose of 0.25 mg/kg body weight of either 20-nm citrate-coated AgNPs or 1 mM citrate buffer alone using oropharyngeal aspiration. Mice were humanely killed 24 h after oropharyngeal aspiration.
TABLE 1.
AgNP QTL details
| QTL location/SNP rs number | SNP location | −log10 [P (rank order)] | Effect size (Cohen’s d) |
|---|---|---|---|
| Chromosome 18: 63.5–66.5 Mb | |||
| rs29865915 | 64292584 | 7.69 (1) | 1.58 |
| rs29959933 | 64837330 | 6.30 (2) | 1.67 |
| rs29778747 | 64837532 | 5.09 (4) | 1.2 |
| Chromosome 15: 90–100 Mb | |||
| rs31581766 | 95827212 | 5.44 (3) | 0.90 |
| rs3688273 | 96200660 | 4.87 (6) | 1.06 |
| Chromosome 4 72.5–135 Mb | |||
| rs27482013 | 117407198 | 4.93 (5) | 1.59 |
BALF
BAL was performed in the whole lung in each mouse twice sequentially (1.2 ml followed by 0.6 ml) with PBS. For strains with small body weights (PWK/PhJ and WSB/EiJ), lavage volumes were adjusted to 0.7 ml followed by 0.35 ml.
Cytospins
Lavage aliquots of 100 μl were deposited onto microscope slides by using a Shandon Cytospin (Thermo Fisher Scientific, Waltham, MA, USA) and stained with Diff-Quik (Thermo Fisher Scientific). The percentage of neutrophils in BALF was determined by the number of neutrophils present in either 500 counted cells (T.J.K. laboratory) or 100 counted cells (T.G./L.-C.C. laboratory).
Tissue collection
Tissues were either flash frozen in liquid nitrogen and stored at −80°C or fixed in 10% neutral-buffered formalin. Before freezing, a portion of the right lung was preserved in 150 μl RNAlater (Thermo Fisher Scientific) and stored at −80°C.
RNA isolation
Lung tissue was thawed, removed from RNALater, and homogenized at 50 Hz for 5 min in 700 μl of Qiazol (Qiagen, Hilden, Germany) by using 5-mm stainless steel beads (Qiagen) in 2-ml Eppendorf tubes in a TissueLyser LT (Qiagen). Total RNA was extracted by using the miRNEasy kit (Qiagen) according to manufacturer instructions for total RNA.
Quantitative RT-PCR
A PCR mixture (12 µl) that consisted of cDNA, primers, a labeled TaqMan probe, 1× TaqMan Gene Expression Master Mix, and water underwent a quantitative real-time PCR reaction. Species- and gene-specific primers and probes were designed by Applied Biosystems (Foster City, CA, USA). Amplification and fluorescence detection were measured using the ABI 7900HT Fast Real-Time PCR System with the following PCR reaction profile: 1 cycle of 95°C for 10 min, followed by 40 cycles of 95°C for 20 s and 62°C for 1 min. The threshold cycle (Ct) number at which the concentration threshold for fluorescence detection was reached was calculated by using SDS 2.4 software (Applied Biosystems). The ΔΔCt method was used to calculate differences in the expression of Nedd4l (neural precursor cell expressed developmentally down-regulated gene 4-like), Ano6 (anocatmin 6), and Rnf220 (Ring finger protein 220) mRNA between AgNP and citrate-treated mice after normalizing to the average expression of reference genes, β-actin and glyceraldehyde 3-phosphate dehydrogenase, within each sample. The anti-log base 2 of the ΔΔCt values was then used to derive fold-change in mRNA expression.
GWA mapping and other statistical analyses
Two-way ANOVA was used to determine the impact of AgNP treatment and mouse strain on BALF neutrophils in the 25 inbred strains and whether there were interactions between the 2 factors. We also calculated the broad sense heritability for percentage of neutrophils in BALF by using variance component analysis with a linear mixed model. We used R packages (https://www.rproject.org/), nlme and ape, to calculate between- and within-strain variance components while adjusting for AgNP treatment (33–37). The efficient mixed-model association (EMMA) R package was used for GWA mapping (38). Single-nucleotide polymorphisms (SNPs) for the 25 strains (National Center for Biotechnology Information Build 37/University of Santa Clara build mm9) were obtained from the Mouse Haplotype Project (Broad Institute, Cambridge, MA, USA). A linear regression that was forced through the origin derived from the mean values in AgNP- and citrate-treated mice from the 4 strains that the two laboratories had in common was used to derive an adjustment factor (0.4592) to percentage of neutrophil values from the T.J.K. laboratory to account for interlaboratory differences. All associations between BALF neutrophils and genotype were adjusted by using the EMMA program for genetic relatedness among strains (Supplemental Fig. 1). The purpose of this adjustment was to account for population structure and reduce the number of false positives (38). Individual animal data were run using the emma.REML.t algorithm, and AgNP or citrate treatments were incorporated as covariates. Significance of associations was determined by using a false discovery rate of 15%. Size effects of candidate SNPs were calculated by using Cohen’s d statistic (23, 39, 40). To account for control mice, we first subtracted the mean percentage of neutrophils in control mice from the mean of AgNP-treated mice within each strain, then assessed allele-specific differences in mean percentage of neutrophils. The pooled sd was also calculated after subtraction of control values from AgNP mice. The University of Santa Clara genome browser (Santa Clara, CA, USA) was used to explore candidate genes in regions that were indicated by significant SNPs (41). Manhattan plots were generated by using modified source code from the qqman R package (42). Other plots were created using base R, lattice, or ggplot2 packages (43–45)
To determine whether our candidate genes were differentially expressed in lung tissue between AgNP and control mice, we used a 1-sided Student’s t test to determine if strain-specific mean ΔΔCt values were different than 0. Resulting significance values from Student’s t tests for each gene were adjusted by using the Bonferroni method. Simple linear regression was used to investigate relationships between candidate gene mRNA expression and BALF neutrophils. Correlations with values of P < 0.05 were considered statistically significant.
RESULTS
Lung inflammation
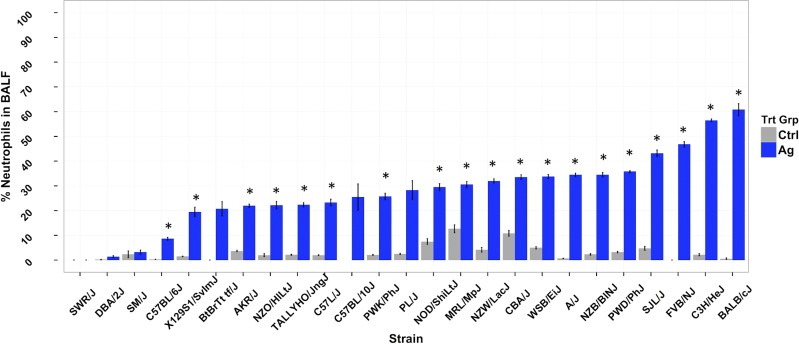
Neutrophils are key cells in acute inflammatory responses, and, accordingly, we have used neutrophil influx as a primary marker of ENM-induced lung inflammation in previously published studies (46, 47). Therefore, we used neutrophil influx as the phenotype of interest in this study, which was focused on identifying genetic determinants of AgNP-induced lung inflammation. We found significant strain-dependent increases in percentage of neutrophils in BALF in AgNP-treated mice compared with citrate-treated strain-matched controls (Fig. 1). There was a wide range of responses in AgNP-treated mice across the 25 inbred strains, ranging from 0 (SWR/J) to 60% (BALB/c) neutrophils in BALF with coefficients of variation of 166% for AgNP-treated mice and 49% for citrate controls. Of importance, statistically significant AgNP-induced lung inflammation was found in most strains, with the exception of SWR/J, DBA/2J, SM/J, BTBRT+tf/J, and C57BL/10J strains. AgNP treatment- and strain-related effects, along with a significant interaction between them (by 2-way ANOVA), strongly suggest a role for genetic background in determining susceptibility to AgNP-induced acute lung inflammation.
Figure 1.
Plot of percentage of BALF neutrophils for each strain for AgNP- and citrate buffer (Ctrl)–treated mice. Bars are mean ± sem. Asterisk denotes significant difference between AgNP-treated and control for each strain by Student’s t test with correction for multiple comparisons (P < 0.05), with the exception of the C57BL/10J strain, where n = 1 for control.
Heritability of AgNP-induced lung inflammation
We calculated the broad sense heritability for percentage of neutrophils in BALF to determine the genetic contribution to the large amount of variability we observed across strains. We found that heritability for percentage of BALF neutrophils was 0.37 when AgNP was included and adjusted for treatment in the mixed model. When evaluated separately, heritability estimates were 0.43 for citrate-treated animals and 0.73 in AgNP-treated animals. These results also demonstrate that genetic background is an important determinant of interstrain variation in AgNP-induced lung inflammation.
GWA mapping
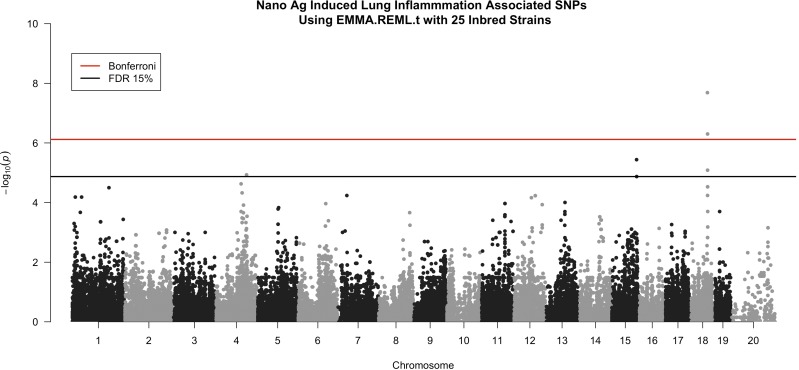
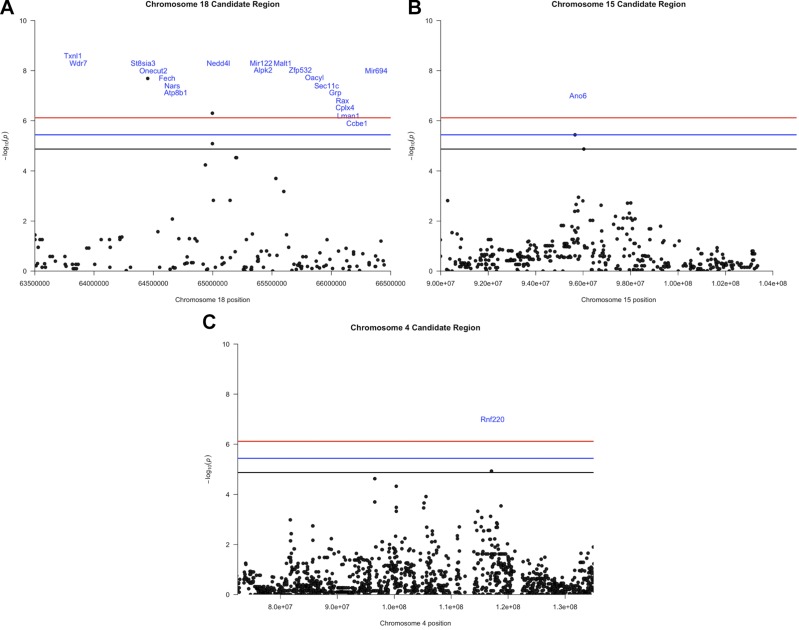
We performed GWA mapping to identify specific genomic regions and candidate genes that may contribute to interstrain differences in sensitivity to AgNPs. After filtering for SNPs with missing genotype calls, 65,493 SNPs were used in EMMA to assess their associations with percentage of BALF neutrophils (Fig. 2). We found 6 significant SNPs in quantitative trait loci (QTL) peaks on chromosomes 4, 15, and 18 (Fig. 3 and Table 1). The strongest QTL spanned 3 Mb in the qE1 region of chromosome 18. This region held 3 of the 6 significant SNPs with −log10(P) rank orders of #1 [rs29865915, close to St8sia3 (ST8 α-N-acetyl-neuraminide α-2,8-sialyltransferase 3)], #2 (rs29959933, close to Nedd4l), and #4 (rs29778747, close to Nedd4l; Fig. 3A and Table 1). This region also contained 17 other known genes and 2 microRNAs (Fig. 3A). QTL on chromosome 15 spanned 10 Mb across the distal qE3 and proximal qF1 regions and contained 1 significant SNP with rank order of #3 (rs31581766, intragenic in Ano6) and a SNP with rank order of #6 (rs3688273, not close to a gene). This QTL region also contained more than 100 other known genes (Fig. 3B and Table 1). The QTL peak region on chromosome 4 was 62.5-Mb wide and covered the qC3 region through part of the proximal portion of qD3 and contained 1 significant SNP with rank order of #5 (rs27482013, intragenic in Rnf220), and also contained more than 100 known genes (Fig. 3C and Table 1). QTL boundaries and −log10 (P values) for the 6 significant SNPs are also listed in Table 1.
Figure 2.
Manhattan plot showing the results from GWA with EMMA. Each SNP tested is plotted by its chromosome and position on the x axis. The y axis shows the −log(P) value for each tested SNP. Significance lines for Bonferroni correction and for a false discovery rate (FDR) of 15% are also plotted.
Figure 3.
Candidate QTL regions on chromosomes 18 (A), 15 (B), and 4 (C).
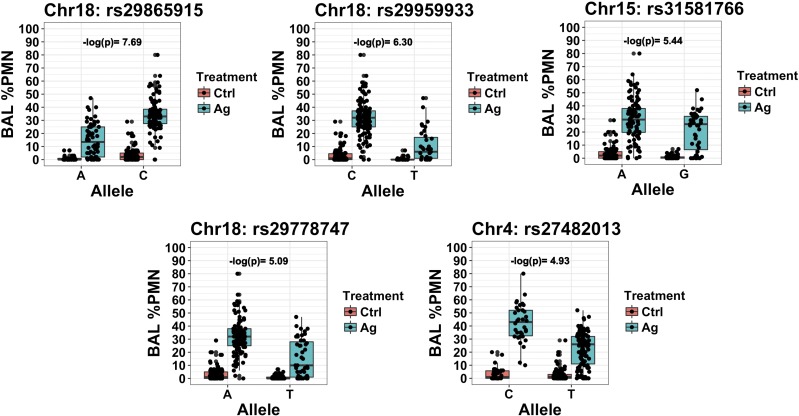
We also assessed distribution of the percentage of BALF neutrophils by treatment group and SNP allele for the 6 significant SNPs to visualize the effects that alleles and AgNP had on BALF neutrophils. Plots for 5 of 6 SNPs that were either in or near a gene are shown in Fig. 4. Cohen’s effect size d statistics for these same 5 SNPs were ≥0.9, which indicates moderate to large effects on AgNP-induced neutrophil influx (Table 1) (40). Allele distributions by strain were also examined and were all different for these 5 SNPs (Supplemental Fig. 3).
Figure 4.
Plot of percentage of BALF neutrophils for significant SNPs either in or near a candidate gene by allele and treatment group. Chr, chromosome; Ctrl, control; PMN, polymorphonuclear leukocytes.
Candidate gene exploration using the Mouse Phenome Database
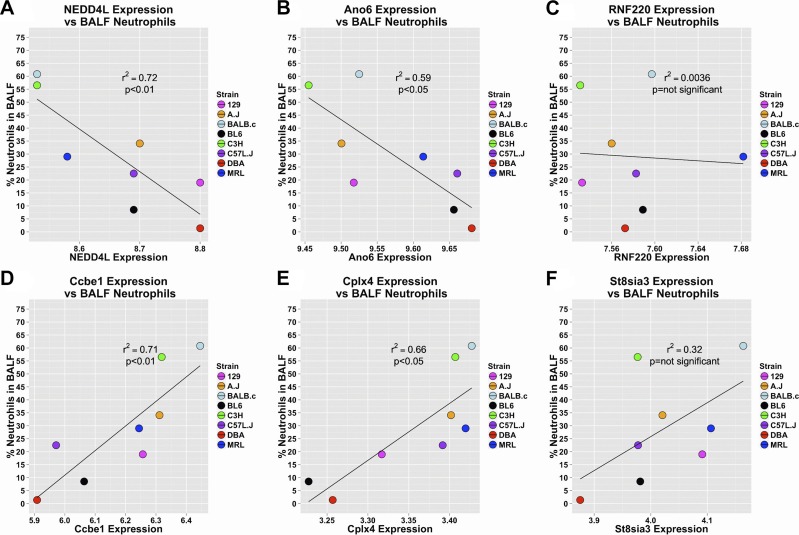
As an initial screening step for potential candidate genes, mRNA expression values for potential candidate genes that were identified in the chromosome 18 QTL and for Ano6 and Rnf220 were obtained from a 9-strain microarray-based gene expression study on untreated female mice (n = 3 per strain) submitted by Berndt and Stearns (48) to the Mouse Phenome Database (MPD). As the QTL regions on chromosomes 15 and 4 contained more than 100 genes, we focused on the expression of Ano6 and Rnf220, given that the most significant SNPs in these regions were intragenic. Expression values from the MPD were compared with strain means of percentage of BALF neutrophils from AgNP-treated mice from 8 overlapping strains used in our study. We found both positive and inverse correlations between reported baseline expression levels of some of the potential candidate genes and percentage of BALF neutrophils from our study. The most inversely correlated genes were Nedd4l on chromosome 18 and Ano6 on chromosome 15 (Fig. 5A, B). Despite containing a significant intragenic SNP, expression of Rnf220 on chromosome 4 was not correlated with percentage of BALF neutrophils (Fig. 5C). The most positively correlated genes were Ccbe1 and Cplx4, which are both on chromosome 18 (Fig. 5D, E). St8sia3, which is also on chromosome 18, was moderately correlated but was not statistically significant (Fig. 5F).
Figure 5.
Correlations between percentage of BALF neutrophils and mRNA levels from the MPD for top candidate genes. Correlations for Nedd4l (A), Ano6 (B), Rnf220 (C), Ccbe1 (D), Cplx4 (E), and St8sia3 (F) are shown.
Quantitative RT-PCR on candidate genes
To determine whether candidate genes were differentially expressed in lungs that were exposed to AgNP, we performed quantitative RT-PCR (qRT-PCR) on right lung tissue from a subset of our strains that represented low (C57BL/6J, 129S1/SvImJ), and moderate to high (MRL/MpJ, A/J) neutrophilia (Fig. 6A). In addition to the list of genes with significant intragenic SNPs and/or those that are significantly correlated or inversely correlated with percentage of BALF neutrophils using the data from Berndt and Stearns (48), we also performed qRT-PCR on St8sia3, as it is adjacent to the most significant SNP (rs26865915).
Figure 6.
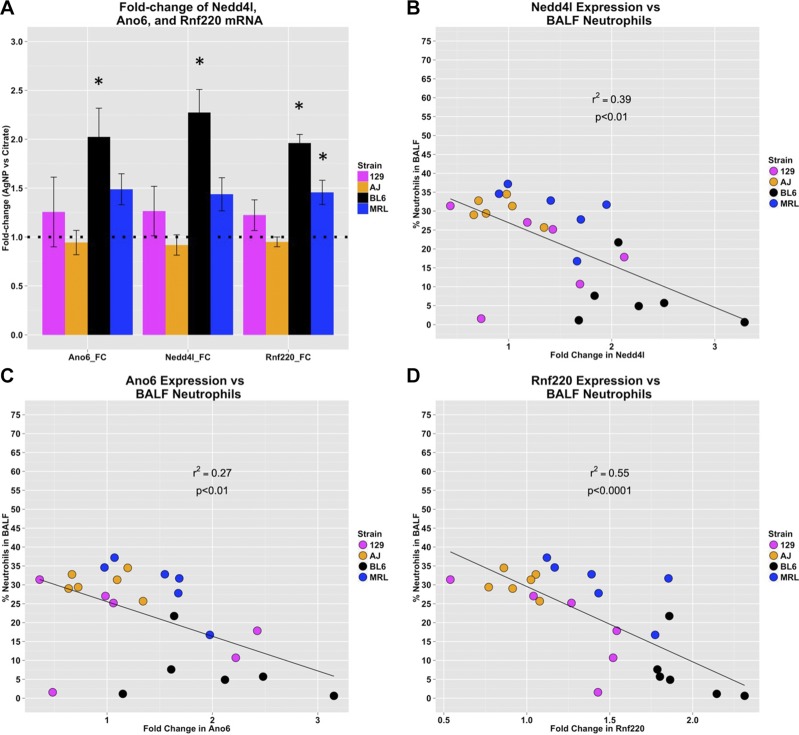
Expression of Nedd4l, Ano6, and Rnf220 mRNA (A) and correlations between percentage of BALF neutrophils and fold-change of mRNA levels between individual AgNP mice over the mean of strain-matched control mice from qRT-PCR for top candidate genes Nedd4l (B), Ano6 (C), and Rnf220 (D). *P < 0.05 for 1-sided Student’s t test after Bonferroni adjustment.
We found that the fold-changes in mRNA expression of Nedd4l, Ano6, and Rnf220 between AgNP-treated and control mice were all significantly inversely correlated with percentage of BALF neutrophils in AgNP-treated mice (Fig. 6B–D). Of interest, we found that 3 of the genes from the chromosome 18 QTL that were either significantly correlated with percentage of BALF neutrophils using mRNA expression data from the MPD (Ccbe1 and Cplx4) (48), or that were adjacent to a highly significant SNP (St8sia3), were not expressed in lung tissue that was taken from either AgNP- or citrate-treated mice.
DISCUSSION
In this study, we used GWA mapping with a panel of 25 inbred mouse strains to identify 3 candidate genes in QTLs on chromosomes 4, 15, and 18 that were associated with a 0.25-mg/kg dose of 20-nm citrate-coated AgNP-induced lung inflammation. Mouse strains were chosen to maximize genetic diversity to improve resolution of GWA mapping (49–51). As such, we included members of the Collaborative Cross founder mouse strains in our panel (52). Some strains were selected because of their genetic similarity (e.g., C57BL10/J and C57BL/6J mice) to determine how closely related strains would respond to AgNP exposure. Dose selection was focused on maximizing contrast in percentage of neutrophils in BALF across mouse strains to increase power for GWA mapping. Preliminary dose response data in genetically divergent BALB/cJ, FVB/NJ, and C57BL/6J mouse strains demonstrated that a dose of 0.25 mg/kg resulted in the greatest variability across these 3 mouse strains (Supplemental Fig. 4). By using a calculation published by Wang and colleagues (10) to translate human airborne AgNP exposures to bolus doses, 0.25 mg/kg in mice is equivalent to a human aggregate dose of 219 μg/m3 air for 8 h/d, 5 d/wk for 4 wk—this is within the 5–289 μg/m3 air observed for occupational exposures. Whereas inhalation exposures are different than bolus doses, intratracheal instillation has been acknowledged as a valid screening tool (53). Oropharyngeal aspiration—the exposure method used in this study—has been shown to have superior distribution in the lungs compared with intratracheal instillation (54). Furthermore, equivalent doses of multiwalled carbon nanotubes delivered by using aspiration and inhalation produced similar pathologies in mice (55).
Using EMMA allowed us to reduce the number of false positives by adjusting association values for genetic relatedness between strains. We gained statistical power via the capacity of EMMA to incorporate individual animal measures within a strain. Another advantage with EMMA was the ability to incorporate AgNP treatment as a covariate and use all of our data, rather than adjusting for controls by using fold-change or ignoring control animals entirely.
We found 3 significant SNPs and 20 potential candidate genes in the chromosome 18 QTL. We used the presence of intragenic SNPs, proximity to significant SNPs, and the correlation of expression data from the data published by Berndt and Stearns (48) with percentage of BALF neutrophils to further refine the list of candidate genes. We performed qRT-PCR on St8sia3 on the basis of its proximity to the most significant SNP. We chose Nedd4l as it had proximity to the second and fourth most significant SNPs (Table 1). In addition, Nedd4l expression from the Berndt and Stearns (48) data set at MPD had the strongest correlation coefficient with percentage of BALF neutrophils. Ccbe1 and Cplx4 were chosen as candidates for qRT-PCR solely on the basis of the high correlation of expression with percentage of BALF neutrophils. We found that, of the 4 genes that were assessed, only Nedd4l mRNA was significantly expressed in the lungs of our mice. We observed a significant inverse correlation with Nedd4l mRNA levels and percentage of BALF neutrophils. Nedd4l is an E3 ubiquitin ligase that is known to target the epithelial sodium channel (ENAC) for ubiquitination (56). Of interest, overexpression of ENAC in mice is known to result in severe lung inflammation in mice with phenotypic similarities to cystic fibrosis (57). ENAC-overexpressing mice experience increased mucus obstruction, decreased airway surface liquid volume, increased neutrophils, and reduced bacterial clearance (57). Mice that lack Nedd4l in lung epithelial cells also develop severe lung inflammation (58). Given that Nedd4l has been shown to inhibit ENAC, and that increased expression of ENAC is associated with lung inflammation, it is reasonable that Nedd4l could modulate susceptibility to AgNP-induced lung inflammation. Decreased levels of Nedd4l, which lead to increased ENAC, could result in a lung that is more prone to inflammation after a toxic insult, which is consistent with the inverse association we observed between Nedd4l mRNA and percentage of BALF neutrophils. Furthermore, human GWA studies have suggested that NEDD4L may be associated with variability in asthma susceptibility (P < 1 × 10−6), blood neutrophils (P < 1 × 10−5), and lung function (P < 1 × 10−4) (59).
On chromosome 15, Ano6 was considered a candidate gene as it contained an intragenic statistically significant SNP (rs31581766), and lung mRNA expression levels for Ano6 published by Berndt and Stearns (48) were inversely correlated with percentage of BALF neutrophils that were we found (Fig. 5B). Our qRT-PCR results confirmed an inverse association between Ano6 mRNA and percentage of BALF neutrophils. Ano6 is a transmembrane protein that has been shown to have ion channel activity and to influence phospholipid scrambling (60–62). The ion channel activity of Ano6 has been illustrated in several studies, but its ion selectivity is not completely understood (61). Phospholipid scramblases mediate the bidirectional transport of phospholipids between the inner and outer leaflets of the cell membrane lipid bilayer (62). Asymmetric distribution of specific phospholipids to either the inner or outer leaflet is mediated by flippase and floppase proteins, respectively (62). Scramblase-mediated relocation of phosphatidylserine (PS) to the outer leaflet of the cell membrane is important in diverse signaling mechanisms in multiple cell types (61, 62). In Ano6, the region between 529 and 559 aa, which spans transmembrane domains 4 and 5, is thought to be responsible for phospholipid scramblase activity (63). Loss of function mutations in ANO6 have been associated with Scott syndrome, which is a condition wherein individuals have defective PS scrambling and associated reductions in blood clotting (64, 65). Ano6-knockout mice recapitulate symptoms of Scott syndrome (66).
The presence of PS in the outer leaflet can also signal for phagocytosis of apoptotic cells, a process that is known as efferocytosis (61, 62). It is understood that efferocytosis of apoptotic cells is important for the resolution of inflammation. Many chronic lung diseases are associated with ineffective efferocytosis (67). Furthermore, recognition of dying cells is thought to influence the polarization of alveolar macrophages (Mϕs) toward a more M2 or inflammation-resolving state (68, 69). In human kidney HEK293 cells, overexpression of ANO6 increased PS on the cell surface (63). If reduced expression of ANO6 has the opposite effect, then apoptotic inflammatory cells with lower ANO6 expression could have less surface PS and be more resistant to efferocytosis, which potentially leads to prolonged and more severe inflammation. This hypothesis is in keeping with our observed inverse association of Ano6 mRNA and BALF neutrophils and with recently reported evidence that indicates that peritoneal Mϕs from Ano6-knockout mice have reduced phagocytic capacity, which could work in conjunction with reduced apoptotic neutrophil PS scrambling (60). There is also some evidence that Ano6 may also participate in PS scrambling in the context of mast cell degranulation (62). In addition, human GWA studies have suggested that ANO6 may be associated with variability in levels of C-reactive protein, a biomarker of inflammation (P < 3 × 10−6).
Given the many demonstrated functions of Ano6, AgNP-induced lung inflammation could be impacted by a number of different mechanisms, including ion transport–induced changes in airway surface fluid volume and viscosity, changes in PS signaling–related apoptosis and phagocytosis, and/or mast cell function (57, 62, 70).
In the QTL on chromosome 4, Rnf220 was considered a candidate gene because the most significant SNP (rs27482013) is intragenic in this gene. In addition, we observed that, among 8 strains in common between our study and those in the microarray data set published on the MPD by Berndt and Stearns (48), lung Rnf220 mRNA was inversely correlated with percentage of BALF neutrophils (Fig. 5C). We also found that when we performed qRT-PCR for Rnf220 mRNA in lung tissue from a subset of 4 strains in our study, mRNA levels again were significantly inversely correlated with percentage of BALF neutrophils (Fig. 5C). Rnf220 is an E3 ubiquitin ligase with a RING finger domain that has been shown to stabilize β-catenin and, therefore, enhance canonical Wnt signaling (71). β-Catenin stabilization was shown to occur via deubiquitination by a complex of Rnf220 and the ubiquitin-specific protease 7 (71). Promotion of Wnt/β-catenin signaling by canonical ligand, Wnt3a, and inhibition of Wnt antagonist, Dkk1, has been shown to decrease LPS-induced neutrophil influx and cytokine production (72). In addition, ghrelin has been shown to rescue LPS-induced inhibition of β-catenin, LPS-induced apoptosis in alveolar Mϕs, and outcomes in a model of the acute respiratory distress syndrome in rats (73). Thus, it is plausible that Rnf220 could modulate the susceptibility to AgNP-induced lung inflammation via increased stabilization of β-catenin and increased Wnt/β-catenin signaling that leads to a less severe inflammatory response. The inverse association we observed between Rnf220 mRNA and percentage of BALF neutrophils supports this hypothesis. In addition, human GWA studies have suggested that RNF220 may be associated with variability in lung function (P ≤ 1 × 10−4) (59).
Acknowledging that GWA study results may be influenced by model assumptions and the choice of mouse strains, we performed a sensitivity analysis. Because the phenotype data in our study was collected in two laboratories, we explored an alternate method for interlaboratory adjustment in our sensitivity analysis than in our initial analysis. Instead of adjusting the T.J.K. laboratory data using linear regression with the four strains that the two laboratories had in common, we tried using unscaled phenotype data in the EMMA model and included laboratory as an additional covariate. We also explored the effects of running the data from the T.G./L.-C.C. and T.J.K. laboratories separately, as well as excluding different combinations of wild-derived strains WSB/EiJ, PWK/PhJ, and PWD/PhJ. PWK/PhJ and PWD/PhJ strains are highly genetically related to each other but divergent from the other strains. The influence of closely related classic inbred strains was also explored by excluding C57BL/10J from the primary analysis, as they are closely related to C57BL/6J mice (Supplemental Fig. 1). After running the alternate analyses, we compared the 6 SNPs with the highest −log(P) values from the initial and alternate analyses (Supplemental Fig. 2). We observed that the most significant SNPs on chromosomes 18 (rs29865915, near St8sia3; rs29778747, near Nedd4l; rs29959933, near Nedd4l) from the initial analysis were among the top 6 SNPs in all analyses combining strains from both laboratories, regardless of interlaboratory adjustment method or whether different combinations of wild-derived strains or C57BL/10J mice were included in the analysis. We adjusted between labs by using laboratory as a covariate and when different combinations of wild WSB/EiJ and PW (PWK/PhJ and PWD/PhJ) strains were excluded, regardless of how we adjusted between labs. The most significant SNP on chromosome 15 (rs31581766, intragenic in Ano6) from the initial analysis was also among the top 6 SNPs in the analysis when adjusting for laboratory as a covariate and PW strains were excluded and all alternate analyses where linear regression was used to adjust for interlaboratory differences. The most significant SNP on chromosome 4 (rs27482013, intragenic in Rnf220) from the primary analysis was also among the top 6 SNPs of all alternate analyses where linear regression was used to adjust for interlaboratory differences. The other significant SNP on chromosome 15 (rs3688273, not close to any genes) from the initial analysis was also in the top 6 SNPs in analyses where linear regression adjustment was used for interlaboratory adjustment when either PW or C57BL/10J mice were excluded. In general, the importance of the significant SNPs and associated candidate genes that were identified in the initial analysis seem to be a relatively robust choice of mouse strain and methods for interlaboratory adjustment of percentage of BALF neutrophils. In conclusion, our study evaluated AgNP-induced lung inflammation in 25 inbred strains of mice and used GWA mapping to search for susceptibility genes that corresponded with the large range of observed responses.
In summary, we identified 3 promising candidate genes, Nedd4l, Rnf220, and Ano6, for which mRNA levels were inversely correlated with AgNP-induced lung inflammation, which is likely a complex polygenic trait. To varying degrees, Nedd4l and Rnf220 have also been associated with human variation in lung function in human GWA studies; Ano6 has been moderately associated with C-reactive protein and, thus, overall inflammation (59, 74). Because differences in human susceptibility to drug exposure have been associated with polymorphisms in genes identified by using mouse GWA mapping, the role of these genes in modulating the susceptibility to AgNP-induced lung inflammation must be further explored. By showing that genetic background contributes to variation in the inflammatory response observed across our 25 inbred mouse strains and identifying candidate susceptibility genes for additional studies, we have generated information that should guide future AgNP mechanistic studies; however, such experiments are beyond the scope of the current study. In addition, information on interstrain variation in mice that differ in sensitivity to AgNP-induced lung inflammation could be useful for gauging intraspecies uncertainty factors in AgNP risk assessments, thus assuring adequate protection for vulnerable individuals who may be genetically predisposed to AgNP-induced lung inflammation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Institute of Environmental Health Sciences (NIEHS) Grants U01-ES019545, U01-ES0200126, T32-ES015459, P30-ES007033, and P30-ES000260. S.R.K. was supported by the NIH NIEHS Intramural Research Program. The silver nanomaterials used in these studies were procured, characterized, and provided by NIEHS Centers for Nanotechnology Health Implications Research Consortium.
Glossary
- AgNP
silver nanoparticle
- Ano6
anocatmin 6
- BALF
bronchoalveolar lavage fluid
- BN
Brown Norway
- EMMA
efficient mixed-model association
- ENAC
epithelial sodium channel
- ENM
engineered nanomaterial
- GWA
genome-wide association
- Mϕ
macrophage
- MPD
Mouse Phenome Database
- Nedd4l
neural precursor cell expressed developmentally down-regulated gene 4-like
- PS
phosphatidylserine
- PVP
polyvinylpyrrolidone
- qRT-PCR
quantitative RT-PCR
- QTL
quantitative trait loci
- Rnf220
Ring finger protein 220
- SD
Sprague-Dawley
- SNP
single-nucleotide polymorphism
- St8sia3
ST8 α-N-acetyl-neuraminide α-2,8-sialyltransferase 3
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. K. Scoville, D. Botta, K. Galdanes, M. Hernandez, S. R. Kleeberger, L.-C. Chen, T. Gordon, and T. J. Kavanagh designed research; D. K. Scoville, D. Botta, K. Galdanes, S. C. Schmuck, C. C. White, P. L. Stapleton, and M. Hernandez performed research; D. K. Scoville, D. Botta, K. Galdanes, S. C. Schmuck, C. C. White, P. L. Stapleton, T. K. Bammler, J. W. MacDonald, M. Hernandez, T. Gordon, and T. J. Kavanagh analyzed data; and D. K. Scoville, W. A. Altemeier, S. R. Kleeberger, T. Gordon, and T. J. Kavanagh wrote the paper.
REFERENCES
- 1. The Project on Emerging Nanotechnologies. Consumer products inventory. Accessed March 23, 2016 at: http://www.nanotechproject.org/cpi.
- 2.Cui L. (2013) Exposure assessment and inflammatory response among workers producing calcium carbonate nanomaterials. Doctoral dissertation, University of Washington, July 25, 2013
- 3.Lee J. S., Choi Y. C., Shin J. H., Lee J. H., Lee Y., Park S. Y., Baek J. E., Park J. D., Ahn K., Yu I. J. (2015) Health surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology 9, 802–811 [DOI] [PubMed] [Google Scholar]
- 4.Liao H.-Y., Chung Y.-T., Lai C.-H., Wang S.-L., Chiang H.-C., Li L.-A., Tsou T.-C., Li W.-F., Lee H.-L., Wu W.-T., Lin M.-H., Hsu J.-H., Ho J.-J., Chen C.-J., Shih T.-S., Lin C.-C., Liou S.-H. (2014) Six-month follow-up study of health markers of nanomaterials among workers handling engineered nanomaterials. Nanotoxicology 8 (Suppl 1), 100–110 [DOI] [PubMed] [Google Scholar]
- 5. The National Institute for Occupational Safety and Health. (May 1994) Immediately dangerous to life or health. Accessed March 23, 2016 at: http://www.cdc.gov/niosh/idlh/7440224.html.
- 6.Weldon B. A., M Faustman E., Oberdörster G., Workman T., Griffith W. C., Kneuer C., Yu I. J. (2016) Occupational exposure limit for silver nanoparticles: considerations on the derivation of a general health-based value. Nanotoxicology 10, 945–956 [DOI] [PubMed] [Google Scholar]
- 7.Lee Y.-H., Cheng F.-Y., Chiu H.-W., Tsai J.-C., Fang C.-Y., Chen C.-W., Wang Y.-J. (2014) Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials 35, 4706–4715 [DOI] [PubMed] [Google Scholar]
- 8.Gliga A. R., Skoglund S., Wallinder I. O., Fadeel B., Karlsson H. L. (2014) Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 11, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W., Wu Y., Wang C., Li H. C., Wang T., Liao C. Y., Cui L., Zhou Q. F., Yan B., Jiang G. B. (2010) Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology 4, 319–330 [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Ji Z., Chang C. H., Zhang H., Wang M., Liao Y.-P., Lin S., Meng H., Li R., Sun B., Winkle L. V., Pinkerton K. E., Zink J. I., Xia T., Nel A. E. (2014) Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small 10, 385–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang C., Brunelli A., Zhu C., Hristozov D., Liu Y., Semenzin E., Wang W., Tao W., Liang J., Marcomini A., Chen C., Zhao B. (2016) Demonstrating approaches to chemically modify the surface of Ag nanoparticles in order to influence their cytotoxicity and biodistribution after single dose acute intravenous administration. Nanotoxicology 10, 129–139 [DOI] [PubMed] [Google Scholar]
- 12.Seiffert J., Hussain F., Wiegman C., Li F., Bey L., Baker W., Porter A., Ryan M. P., Chang Y., Gow A., Zhang J., Zhu J., Tetley T. D., Chung K. F. (2015) Pulmonary toxicity of instilled silver nanoparticles: influence of size, coating and rat strain. PLoS One 10, e0119726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolaczkowska E., Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 [DOI] [PubMed] [Google Scholar]
- 14.Holter J. F., Weiland J. E., Pacht E. R., Gadek J. E., Davis W. B. (1986) Protein permeability in the adult respiratory distress syndrome. Loss of size selectivity of the alveolar epithelium. J. Clin. Invest. 78, 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan M. J., Liu Z. G. (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21, 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallach D., Kang T.-B., Kovalenko A. (2014) Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat. Rev. Immunol. 14, 51–59 [DOI] [PubMed] [Google Scholar]
- 17.Anderson D. S., Patchin E. S., Silva R. M., Uyeminami D. L., Sharmah A., Guo T., Das G. K., Brown J. M., Shannahan J., Gordon T., Chen L. C., Pinkerton K. E., Van Winkle L. S. (2015) Influence of particle size on persistence and clearance of aerosolized silver nanoparticles in the rat lung. Toxicol. Sci. 144, 366–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson D. S., Silva R. M., Lee D., Edwards P. C., Sharmah A., Guo T., Pinkerton K. E., Van Winkle L. S. (2015) Persistence of silver nanoparticles in the rat lung: influence of dose, size, and chemical composition. Nanotoxicology 9, 591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva R. M., Anderson D. S., Franzi L. M., Peake J. L., Edwards P. C., Van Winkle L. S., Pinkerton K. E. (2015) Pulmonary effects of silver nanoparticle size, coating, and dose over time upon intratracheal instillation. Toxicol. Sci. 144, 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva R. M., Anderson D. S., Peake J., Edwards P. C., Patchin E. S., Guo T., Gordon T., Chen L. C., Sun X., Van Winkle L. S., Pinkerton K. E. (2016) Aerosolized silver nanoparticles in the rat lung and pulmonary responses over time. Toxicol. Pathol. 44, 673–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botelho D. J., Leo B. F., Massa C. B., Sarkar S., Tetley T. D., Chung K. F., Chen S., Ryan M. P., Porter A. E., Zhang J., Schwander S. K., Gow A. J. (2016) Low-dose AgNPs reduce lung mechanical function and innate immune defense in the absence of cellular toxicity. Nanotoxicology 10, 118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Careau E., Sirois J., Bissonnette E. Y. (2002) Characterization of lung hyperresponsiveness, inflammation, and alveolar macrophage mediator production in allergy resistant and susceptible rats. Am. J. Respir. Cell Mol. Biol. 26, 579–586 [DOI] [PubMed] [Google Scholar]
- 23.Harrill A. H., Watkins P. B., Su S., Ross P. K., Harbourt D. E., Stylianou I. M., Boorman G. A., Russo M. W., Sackler R. S., Harris S. C., Smith P. C., Tennant R., Bogue M., Paigen K., Harris C., Contractor T., Wiltshire T., Rusyn I., Threadgill D. W. (2009) Mouse population-guided resequencing reveals that variants in CD44 contribute to acetaminophen-induced liver injury in humans. Genome Res. 19, 1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Church R. J., Wu H., Mosedale M., Sumner S. J., Pathmasiri W., Kurtz C. L., Pletcher M. T., Eaddy J. S., Pandher K., Singer M., Batheja A., Watkins P. B., Adkins K., Harrill A. H. (2014) A systems biology approach utilizing a mouse diversity panel identifies genetic differences influencing isoniazid-induced microvesicular steatosis. Toxicol. Sci. 140, 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen A. L., Okumura A., Ferris M. T., Green R., Feldmann F., Kelly S. M., Scott D. P., Safronetz D., Haddock E., LaCasse R., Thomas M. J., Sova P., Carter V. S., Weiss J. M., Miller D. R., Shaw G. D., Korth M. J., Heise M. T., Baric R. S., de Villena F. P., Feldmann H., Katze M. G. (2014) Host genetic diversity enables Ebola hemorrhagic fever pathogenesis and resistance. Science 346, 987–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutledge H., Aylor D. L., Carpenter D. E., Peck B. C., Chines P., Ostrowski L. E., Chesler E. J., Churchill G. A., de Villena F. P.-M., Kelada S. N. P. (2014) Genetic regulation of Zfp30, CXCL1, and neutrophilic inflammation in murine lung. Genetics 198, 735–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki O. T., Frick A., Parks B. B., Trask O. J. Jr., Butz N., Steffy B., Chan E., Scoville D. K., Healy E., Benton C., McQuaid P. E., Thomas R. S., Wiltshire T. (2014) A cellular genetics approach identifies gene-drug interactions and pinpoints drug toxicity pathway nodes. Front. Genet. 5, 272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols J. L., Gladwell W., Verhein K. C., Cho H.-Y., Wess J., Suzuki O., Wiltshire T., Kleeberger S. R. (2014) Genome-wide association mapping of acute lung injury in neonatal inbred mice. FASEB J. 28, 2538–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durrant C., Tayem H., Yalcin B., Cleak J., Goodstadt L., de Villena F. P., Mott R., Iraqi F. A. (2011) Collaborative Cross mice and their power to map host susceptibility to Aspergillus fumigatus infection. Genome Res. 21, 1239–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parks B. W., Nam E., Org E., Kostem E., Norheim F., Hui S. T., Pan C., Civelek M., Rau C. D., Bennett B. J., Mehrabian M., Ursell L. K., He A., Castellani L. W., Zinker B., Kirby M., Drake T. A., Drevon C. A., Knight R., Gargalovic P., Kirchgessner T., Eskin E., Lusis A. J. (2013) Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 17, 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colinayo V. V., Qiao J. H., Wang X., Krass K. L., Schadt E., Lusis A. J., Drake T. A. (2003) Genetic loci for diet-induced atherosclerotic lesions and plasma lipids in mice. Mamm. Genome 14, 464–471 [DOI] [PubMed] [Google Scholar]
- 32.Shannahan J. H., Lai X., Ke P. C., Podila R., Brown J. M., Witzmann F. A. (2013) Silver nanoparticle protein corona composition in cell culture media. PLoS One 8, e74001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paradis E., Claude J., Strimmer K. (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 [DOI] [PubMed] [Google Scholar]
- 34. Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D.; R Core Team. nlme: linear and nonlinear mixed effects models. Accessed February 11, 2015 at: http://cran.r-project.org/package=nlme.
- 35.Almasy L., Blangero J. (2010) Variance component methods for analysis of complex phenotypes. Cold Spring Harb. Protoc. 2010, pdb.top77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felix T. M., Hughes K. A., Stone E. A., Drnevich J. M., Leips J. (2012) Age-specific variation in immune response in Drosophila melanogaster has a genetic basis. Genetics 191, 989–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visscher P. M., Hill W. G., Wray N. R. (2008) Heritability in the genomics era--concepts and misconceptions. Nat. Rev. Genet. 9, 255–266 [DOI] [PubMed] [Google Scholar]
- 38.Kang H. M., Zaitlen N. A., Wade C. M., Kirby A., Heckerman D., Daly M. J., Eskin E. (2008) Efficient control of population structure in model organism association mapping. Genetics 178, 1709–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen J. (1977) Statistical Power Analysis for the Behavioral Sciences, pp. 19–74, Academic Press, New York: [Google Scholar]
- 40.Labots M., Laarakker M. C., Ohl F., van Lith H. A. (2016) Consomic mouse strain selection based on effect size measurement, statistical significance testing and integrated behavioral z-scoring: focus on anxiety-related behavior and locomotion. BMC Genet. 17, 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kent W. J., Sugnet C. W., Furey T. S., Roskin K. M., Pringle T. H., Zahler A. M., Haussler D. (2002) The human genome browser at UCSC. Genome Res. 12, 996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner S. D. (2014) qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. Accessed August 3, 2015 at: http://biorxiv.org/content/early/2014/05/14/005165
- 43.Wickham H. (2009) ggplot2: Elegant Graphics for Data Analysis, Springer, New York [Google Scholar]
- 44.R Core Team (2014) R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 45.Deepayan S. (2008) Lattice: Multivariate Data Visualization With R, Springer, New York [Google Scholar]
- 46.Scoville D. K., White C. C., Botta D., McConnachie L. A., Zadworny M. E., Schmuck S. C., Hu X., Gao X., Yu J., Dills R. L., Sheppard L., Delaney M. A., Griffith W. C., Beyer R. P., Zangar R. C., Pounds J. G., Faustman E. M., Kavanagh T. J. (2015) Susceptibility to quantum dot induced lung inflammation differs widely among the Collaborative Cross founder mouse strains. Toxicol. Appl. Pharmacol. 289, 240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McConnachie L. A., Botta D., White C. C., Weldy C. S., Wilkerson H. W., Yu J., Dills R., Yu X., Griffith W. C., Faustman E. M., Farin F. M., Gill S. E., Parks W. C., Hu X., Gao X., Eaton D. L., Kavanagh T. J. (2013) The glutathione synthesis gene Gclm modulates amphiphilic polymer-coated CdSe/ZnS quantum dot-induced lung inflammation in mice. PLoS One 8, e64165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The Jackson Laboratory. (2011) Stearns1: Lung Gene Expression in Females of 9 Inbred Strains of Mice. MPD: Stearns1. Accessed November 5, 2015 at: http://phenome.jax.org/projects/Stearns1
- 49.Petkov P. M., Ding Y., Cassell M. A., Zhang W., Wagner G., Sargent E. E., Asquith S., Crew V., Johnson K. A., Robinson P., Scott V. E., Wiles M. V. (2004) An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 14, 1806–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aylor D. L., Valdar W., Foulds-Mathes W., Buus R. J., Verdugo R. A., Baric R. S., Ferris M. T., Frelinger J. A., Heise M., Frieman M. B., Gralinski L. E., Bell T. A., Didion J. D., Hua K., Nehrenberg D. L., Powell C. L., Steigerwalt J., Xie Y., Kelada S. N. P., Collins F. S., Yang I. V., Schwartz D. A., Branstetter L. A., Chesler E. J., Miller D. R., Spence J., Liu E. Y., McMillan L., Sarkar A., Wang J., Wang W., Zhang Q., Broman K. W., Korstanje R., Durrant C., Mott R., Iraqi F. A., Pomp D., Threadgill D., de Villena F. P., Churchill G. A. (2011) Genetic analysis of complex traits in the emerging Collaborative Cross. Genome Res. 21, 1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valdar W., Flint J., Mott R. (2006) Simulating the collaborative cross: power of quantitative trait loci detection and mapping resolution in large sets of recombinant inbred strains of mice. Genetics 172, 1783–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Threadgill D. W., Churchill G. A. (2012) Ten years of the Collaborative Cross. Genetics 190, 291–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Driscoll K. E., Costa D. L., Hatch G., Henderson R., Oberdorster G., Salem H., Schlesinger R. B. (2000) Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol. Sci. 55, 24–35 [DOI] [PubMed] [Google Scholar]
- 54.Lakatos H. F., Burgess H. A., Thatcher T. H., Redonnet M. R., Hernady E., Williams J. P., Sime P. J. (2006) Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp. Lung Res. 32, 181–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kinaret P., Ilves M., Fortino V., Rydman E., Karisola P., Lähde A., Koivisto J., Jokiniemi J., Wolff H., Savolainen K., Greco D., Alenius H. (2017) Inhalation and oropharyngeal aspiration exposure to rod-like carbon nanotubes induce similar airway inflammation and biological responses in mouse lungs. ACS Nano 11, 291–303 [DOI] [PubMed] [Google Scholar]
- 56.Kamynina E., Debonneville C., Bens M., Vandewalle A., Staub O. (2001) A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J. 15, 204–214 [DOI] [PubMed] [Google Scholar]
- 57.Mall M., Grubb B. R., Harkema J. R., O’Neal W. K., Boucher R. C. (2004) Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 10, 487–493 [DOI] [PubMed] [Google Scholar]
- 58.Kimura T., Kawabe H., Jiang C., Zhang W., Xiang Y.-Y., Lu C., Salter M. W., Brose N., Lu W.-Y., Rotin D. (2011) Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc. Natl. Acad. Sci. USA 108, 3216–3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramos E. M., Hoffman D., Junkins H. A., Maglott D., Phan L., Sherry S. T., Feolo M., Hindorff L. A. (2014) Phenotype-Genotype Integrator (PheGenI): synthesizing genome-wide association study (GWAS) data with existing genomic resources. Eur. J. Hum. Genet. 22, 144–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ousingsawat J., Wanitchakool P., Kmit A., Romao A. M., Jantarajit W., Schreiber R., Kunzelmann K. (2015) Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7 receptors in macrophages. Nat. Commun. 6, 6245 [DOI] [PubMed] [Google Scholar]
- 61.Pedemonte N., Galietta L. J. V. (2014) Structure and function of TMEM16 proteins (anoctamins). Physiol. Rev. 94, 419–459 [DOI] [PubMed] [Google Scholar]
- 62.Rysavy N. M., Shimoda L. M. N., Dixon A. M., Speck M., Stokes A. J., Turner H., Umemoto E. Y. (2014) Beyond apoptosis: the mechanism and function of phosphatidylserine asymmetry in the membrane of activating mast cells. BioArchitecture 4, 127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu K., Whitlock J. M., Lee K., Ortlund E. A., Cui Y. Y., Hartzell H. C. (2015) Identification of a lipid scrambling domain in ANO6/TMEM16F. eLife 4, e06901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toti F., Satta N., Fressinaud E., Meyer D., Freyssinet J. M. (1996) Scott syndrome, characterized by impaired transmembrane migration of procoagulant phosphatidylserine and hemorrhagic complications, is an inherited disorder. Blood 87, 1409–1415 [PubMed] [Google Scholar]
- 65.Suzuki J., Umeda M., Sims P. J., Nagata S. (2010) Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468, 834–838 [DOI] [PubMed] [Google Scholar]
- 66.Yang H., Kim A., David T., Palmer D., Jin T., Tien J., Huang F., Cheng T., Coughlin S. R., Jan Y. N., Jan L. Y. (2012) TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 151, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morimoto K., Janssen W. J., Terada M. (2012) Defective efferocytosis by alveolar macrophages in IPF patients. Respir. Med. 106, 1800–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korns D., Frasch S. C., Fernandez-Boyanapalli R., Henson P. M., Bratton D. L. (2011) Modulation of macrophage efferocytosis in inflammation. Front. Immunol. 2, 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mares C. A., Sharma J., Li Q., Rangel E. L., Morris E. G., Enriquez M. I., Teale J. M. (2011) Defect in efferocytosis leads to alternative activation of macrophages in Francisella infections. Immunol. Cell Biol. 89, 167–172 [DOI] [PubMed] [Google Scholar]
- 70.Martins J. R., Faria D., Kongsuphol P., Reisch B., Schreiber R., Kunzelmann K. (2011) Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc. Natl. Acad. Sci. USA 108, 18168–18172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma P., Yang X., Kong Q., Li C., Yang S., Li Y., Mao B. (2014) The ubiquitin ligase RNF220 enhances canonical Wnt signaling through USP7-mediated deubiquitination of β-catenin. Mol. Cell. Biol. 34, 4355–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo Y., Mishra A., Howland E., Zhao C., Shukla D., Weng T., Liu L. (2015) Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood 126, 2220–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li B., Zeng M., He W., Huang X., Luo L., Zhang H., Deng D. Y. (2015) Ghrelin protects alveolar macrophages against lipopolysaccharide-induced apoptosis through growth hormone secretagogue receptor 1a-dependent c-Jun N-terminal kinase and Wnt/β-catenin signaling and suppresses lung inflammation. Endocrinology 156, 203–217 [DOI] [PubMed] [Google Scholar]
- 74.Pepys M. B., Hirschfield G. M. (2003) C-reactive protein: a critical update. J. Clin. Invest. 111, 1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.