Abstract
Macrophages (Mϕs) of patients with Alzheimer’s disease and mild cognitive impairment (MCI) are defective in amyloid-β1-42 (Aβ) phagocytosis and have low resistance to apoptosis by Aβ. Omega-3 fatty acids (ω-3s) in vitro and in vivo and the ω-3 mediator, resolvin D1, in vitro increase Aβ phagocytosis by Mϕs of patients with MCI. We have investigated the unfolded protein response (UPR) to endoplasmic reticulum (ER) stress by Mϕs in a longitudinal study of fish-derived, ω-3–supplemented patients with MCI. Patients in the apolipoprotein E (ApoE)e3/e3 subgroup over time exhibited an increase of protein kinase RNA-like ER kinase (PERK) expression, Aβ phagocytosis, intermediate M1-M2 Mϕ type, and a Mini-Mental State Examination (MMSE) rate of change of +1.8 points per year, whereas patients in the ApoEe3/e4 subgroup showed individually divergent results with an MMSE rate of change of −3.2 points per year. In vitro treatment of Mϕs by fish-derived ω-3 emulsion increased Aβ phagocytosis, PERK expression, and UPR RNA signature, and decreased ER stress signature. Augmented genes in the UPR signature included chaperones, lectins, foldases, and N-linked glycosylation enzymes. In summary, fish-derived ω-3s increase cytoprotective genes and decrease proapoptotic genes, improve immune clearance of Aβ, and are associated with an improved MMSE rate of change in ApoEe3/e3 vs. ApoEe3/e4 patients.—Olivera-Perez, H. M., Lam, L., Dang, J., Jiang, W., Rodriguez, F., Rigali, E., Weitzman, S., Porter, V., Rubbi, L., Morselli, M., Pellegrini, M., Fiala, M. Omega-3 fatty acids increase the unfolded protein response and improve amyloid-β phagocytosis by macrophages of patients with mild cognitive impairment.
The brains of patients with Alzheimer’s disease (AD), Parkinson’s disease, and frontotemporal lobar degeneration (1) as well as the spinal cords of patients with amyotrophic lateral sclerosis (2) display endoplasmic reticulum (ER) stress. Potential stress inducers include oxidative stress, abnormal glycosylation, mitochondrial dysfunction, depletion of ER calcium stores, inflammation, and accumulation of misfolded proteins, such as amyloid-β1-42 (Aβ), hyperphosphorylated τ, synuclein-α, mutant superoxide dismutase-1, RNA-binding protein fused in sarcoma/translocated in sarcoma, and TAR DNA-binding protein 43 (3). ER stress may cause neuronal apoptosis, the main pathogenic outcome. In addition, autophagy plays an essential role in the clearance of aggregated toxic proteins and ameliorates ER stress but is impaired in neurodegenerative diseases (4).
ER stress activates an unfolded protein response (UPR) (5, 6), which is initiated by 3 ER membrane–associated proteins: protein kinase RNA-like ER kinase (PERK; also called protein kinase R–like eukaryotic initiation factor 2α kinase), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6). The luminal domains of IRE1 and PERK are bound by the chaperone BIP (also called GRP78), which prevents their activation. The first branch of UPR, IRE1 kinase, activates the transcription factor, X-box binding protein-1 (XBP1), which stimulates the chaperones that respond to ER stress as well as the inflammatory kinases, JNK and IκB. XBP1 prevents the accumulation of free calcium in the cytosol by down-regulating a specific isoform of the ryanodine Ca2+ channel, RyR3, and prevents Aβ neurotoxicity (7). In contrast, the downstream effect of IRE1 increased Aβ deposition in an animal model (8). Thus, the downstream effects and benefits or harms for patients have to be determined in relevant human cells.
The second branch is mediated by PERK phosphorylation, which leads to phosphorylation of eukaryotic initiation factor 2α (eIF2α), resulting in the down-regulation of global protein synthesis as well as transactivation of UPR responses by transcription factors, activating transcription factor 4 (ATF4), nuclear factor–like 2, and NF-κB. ATF4 may induce either a proapoptotic branch with CHOP [C/EBP homologous protein; DNA damage inducible transcript 3 (DDIT3)]-mediated transactivation of the proapoptotic Bcl-2 family member, PUMA (9), or a cytoprotective branch with the chaperones BIP/GRP78, GRP94, the lectins calnexin and calreticulin, carbohydrate-processing enzymes, and foldases (10). In neurodegenerative diseases, the PERK pathway may be activated in a maladaptive fashion (1).
The third branch’s ATF6 is translocated and activated in the Golgi apparatus into a transcription factor, which stimulates the expression of genes that contain ER stress elements, UPR element, and cAMP response elements in their promoters, which boosts ER folding by BIP/GRP78 and degradation of misfolded proteins by ER-associated degradation (11).
ER stress proteins in the CNS tissues have been therapeutic targets in neurodegenerative disorders (3, 4). Type II inhibitors of IRE1 inhibit all signaling by inhibiting both kinase and RNase activities (12). Salubrinal (12–15) targets the regulatory subunits of the eukaryotic translation initiation factor 2α protein, phosphatase 1c, and protects against ER stress-induced cell death. Other approaches against ER stress include progesterone (16), which attenuates ER stress in astrocytes, and natural products, such as resveratrol and withaferin A (17). Omega-3 fatty acids (ω-3s) and specialized proresolving mediators play an increasing role in the immunotherapy of AD (18) and traumatic brain injury (19). In neural cells that are differentiated from pluripotential stem cells, Aβ oligomers accumulate and cause ER stress, which is alleviated by docosahexaenoic acid (DHA) treatment (20).
Defects of AD macrophages (Mϕs) in Aβ phagocytosis and susceptibility to Aβ-induced apoptosis (21, 22) interfere with brain clearance of Aβ. ω-3s, specialized proresolving mediator resolvin D1 (RvD1), curcuminoids, and vitamin D3 stimulate the phagocytosis of Aβ and protect against apoptosis in vitro (23) and in vivo (24, 25). Here, we investigate the hypothesis that ER stress inhibits Mϕ function but that ω-3–induced UPR enhances Aβ phagocytosis and decreases apoptosis, thus improving clearance of Aβ by Mϕs.
MATERIALS AND METHODS
Study design and population
The study population included 11 ω-3–supplemented patients with mild cognitive impairment (MCI) or subjective cognitive impairment (SCI), patients with AD, and normal controls who were observed for a mean of 19.9 mo (Table 1) (25). MCI was diagnosed by using the Petersen criteria (26), and SCI was diagnosed according to subjective complaints and subtle cognitive defects (27). Cognitive results were evaluated in 11 patients; immune tests were performed in 7 patients (Fig. 1). The conduct of this study as an observational study and the testing of blood samples were approved by the University of California, Los Angeles, Institutional Review Board.
TABLE 1.
MMSE rate of change per year in ApoEe3/e3 and ApoEe3/e4 patients with MCI
| ApoE | Patient | Initial | Final | Initial date | Final date | Follow-up (mo) | Rate of change |
|---|---|---|---|---|---|---|---|
| ε3/ε3 | |||||||
| MCI | 2 | 24 | 28 | 9/23/2013 | 10/9/2016 | 37 | 0.11 |
| SCI | 4 | 30 | 30 | 12/22/2013 | 1/18/2016 | 25 | 0.00 |
| SCI | 8 | 26 | 30 | 5/26/2014 | 3/20/2016 | 10 | 0.40 |
| SCI | 10 | 28 | 30 | 6/18/2014 | 2/21/2016 | 20 | 0.10 |
| SCI | 17 | 28 | 30 | 7/4/2015 | 6/30/2016 | 12 | 0.17 |
| Mean, 20.8 | Mean/mo, 0.15 | ||||||
| Mean/yr, 1.86 | |||||||
| ε3/ε4 | |||||||
| MCI | 9 | 26 | 9 | 5/26/2014 | 10/10/2016 | 29 | −0.59 |
| SCI | 12 | 29 | 27 | 9/22/2014 | 3/13/2017 | 30 | −0.07 |
| MCI | 14 | 25 | 25 | 9/14/2014 | 1/28/2017 | 28 | 0.00 |
| SCI | 15 | 30 | 30 | 5/5/2015 | 6/28/2016 | 13 | 0.00 |
| MCI | 16 | 20 | 14 | 4/30/2015 | 10/13/2015 | 6 | −1.00 |
| SCI | 18 | 30 | 30 | 2/16/2016 | 11/30/2016 | 9 | 0.00 |
| Mean, 19.1 | Mean/mo, −0.28 | ||||||
| Mean (all), 19.9 | Mean/yr, −3.31 |
MMSE results in the longitudinal study of ω-3–supplemented patients.
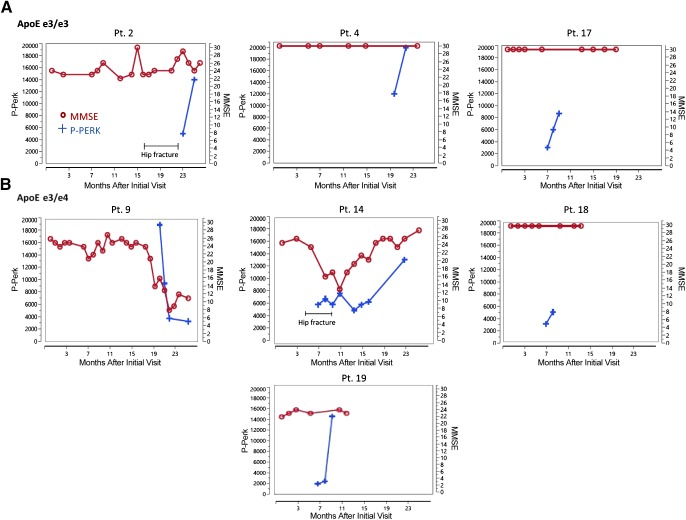
Figure 1.
MMSE and phospho-PERK results on successive visits of patients that received supplementation with the ω-3 drink. At each visit, cognition was tested by MMSE and on two successive visits, the expression of phospho-PERK in Mϕs was determined by immunofluorescence. Crosses indicate phospho-PERK, circles indicate MMSE score. A) ApoEe3/e3 patients. B) ApoEe3/e4 patients.
Isolation of peripheral blood mononuclear cells and Mϕ cultures
Peripheral blood mononuclear cells were isolated by using the Ficoll-Hypaque gradient method, and Mϕs were differentiated from 50,000 mononuclear cells by culture in Iscove’s modified Dulbecco’s medium with 10% autologous serum for 10–14 d in 8-well culture slides (Becton Dickinson Discovery Labware, Bedford, MA, USA).
ω-3 preparations and RvD1
In vivo, participants self-supplemented daily under caregiver supervision with 200 ml of the fish ω-3 suspension, Smartfish (SMF) with resveratrol (SMF/Res; Smartfish AS, Oslo, Norway) drink, which contained 1000 mg DHA and 1000 mg eicosapentaenoic acid (EPA) from cod and salmon, botanical antioxidants (pomegranate and chokeberry),10 μg vitamin D3, and 150 mg resveratrol. For in vitro testing that compared different preparations, we used the SMF/Res emulsion or SMF without resveratrol. We diluted all preparations in fetal calf serum, sonicated, and then diluted in Iscove’s modified Dulbecco’s medium to the final concentration of 27 µg/ml DHA. We used RvD1 (Cayman Chemical, Ann Arbor, MI, USA) at a concentration of 27 ng/ml. Concentrations of 27 µg/ml DHA and 27 ng/ml RvD1 were shown to be equipotent (28).
Immunofluorescence microscopy
Mϕs were fixed with 4% paraformaldehyde, washed with 0.02% Triton X-100, permeabilized with 0.2% Triton X-100, and stained by rabbit Ab to phospho-PERK (Thr981; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or with caspase-3 (active; GeneTex, Irvine, CA, USA). Finally, secondary Abs donkey Alexa Fluor 568 anti-rabbit IgG and phalloidin-FITC (Sigma-Aldrich, St. Louis, MO, USA) were applied and a coverslip was mounted with ProLong Gold (Thermo Fisher Scientific, Waltham, MA, USA). Preparations were examined by using ×20 and 40 objectives in an Olympus B-max microscope (Olympus, Tokyo, Japan). Images obtained by the Hamamatsu camera (Hamamatsu City, Japan) were scanned in 3 fields by using Image Pro software (Media Cybernetics, Rockville, MD, USA). Data are shown as means ± sem integrated optical density.
Phagocytosis of Aβ
Mϕs were treated with ω-3 preparations or RvD1 and exposed for 18 h to fluorescent HiLyte Aβ (2 μg/ml; Anaspec, Fremont, CA, USA), washed, fixed by 4% paraformaldehyde, and stained using phalloidin-Texas Red, then images were scanned.
RNA sequencing analysis and Mϕ signature
We stimulated Mϕs with ω-3s or RvD1 and sequenced Mϕ and peripheral blood mononuclear cell libraries by using an Illumina HiSeq 4000 (Illumina, San Diego, CA, USA) to obtain 50-bp reads. Reads were aligned to the University of California, Santa Cruz (UCSC; Santa Cruz, CA, USA) hg19 reference genome, and read counts were produced by using HTSeq (29). DESeq was used to normalize the counts (30). A gene expression signature score was calculated as previously described. (31). In brief, normalized gene counts were log10 transformed and the arithmetic mean of the transformed counts was calculated across a select set of genes for each sample. Sets of genes are representative of a signaling pathway or molecular signature of interest. In our expression analysis, the ER stress signature is based on 14 genes: ATF4, CASP3, CDYL, CMTR2, DDIT3, EIF2AK3, ERN1, HSPA5, HYOU1, MEX3B, RBM39, SNHG1, SNHG12, and XBP1 (32). The UPR gene list was downloaded from the molecular signatures database (10, 33–35).
Statistical methods
The Mini-Mental State Examination (MMSE) rate of change was computed as the rate of change per year = 12 × (last value − first value)/n mo follow-up. We analyzed the results of phospho-PERK and Aβ phagocytosis by repeated measures ANOVA using JMP software (SAS Institute, Cary, NC, USA). Unadjusted Mann-Whitney Wilcoxon test P values were calculated by comparing Mϕ RNA sequencing signature scores between control and treatment groups. Unadjusted Wilcoxon signed-rank test P values were calculated by comparing gene counts between SMF- and control-exposed Mϕ pairs that were derived from the same culture. Differential gene expression analysis was performed on the Mϕ RNA sequencing data on paired samples with the ibb R package, which uses an inverted β-binomial test (36).
RESULTS
Patients with ω-3–supplemented apolipoprotein E e3/e3 maintain MMSE score and increase phospho-PERK; some patients with apolipoprotein E e3/e4 display parallel decline of MMSE score and phospho-PERK
We extended the longitudinal study of the previous cohort (25) of 11 patients with MCI and SCI who voluntarily supplemented with ω-3s, with a mean follow-up of 19.9 mo. In agreement with the previous study, Aβ phagocytosis increased in patients in both apolipoprotein E (ApoE)e3/e3e3/e3 and ApoEe3/e4 subgroups who received ω-3 supplementation. Furthermore, the subgroups differed in the mean MMSE rate of change: in the ApoEe3/e3 subgroup, MMSE score increased by +1.86 points per year, and in the ApoEe3/e4 subgroup, mean MMSE rate of change declined by −3.3 points per year (Table 1).
Patients in the ApoEe3/e3 subgroup showed individual clinical and immunologic differences compared with patients in the ApoEe3/e4 subgroups. Patients in the ApoEe3/e3 subgroup with MCI and SCI had no overall decline of MMSE scores. Patient 2 suffered recurrent urinary tract infections and hip fracture, and his MMSE score showed small increases and decreases but no overall decline. However, in the ApoEe3/e4 subgroup, 2 patients with MCI had large parallel decreases of phospho-PERK and MMSE. Patient 14 with MCI had a fluorodeoxyglucose scan, the result of which was consistent with AD-type dementia, and her initial MMSE score was 25 points. In her course, she stopped ω-3 supplementation during hospitalization for a hip fracture, declined into a dementia stage with an MMSE score of 11 in parallel with phospho-PERK decline, but recovered her cognitive state (MMSE score, 26) and phospho-PERK after resumption of supplementation. Patient 9 with MCI with an initial MMSE score of 26 points suffered a drastic parallel decline in both phospho-PERK and cognition, with a loss of 9 points despite continuing supplementation with SMF/Res. The result of his fluorodeoxyglucose scan was consistent with Lewy body dementia. A patient with AD had a parallel decline of MMSE and phospho-PERK despite supplementation (data not shown).
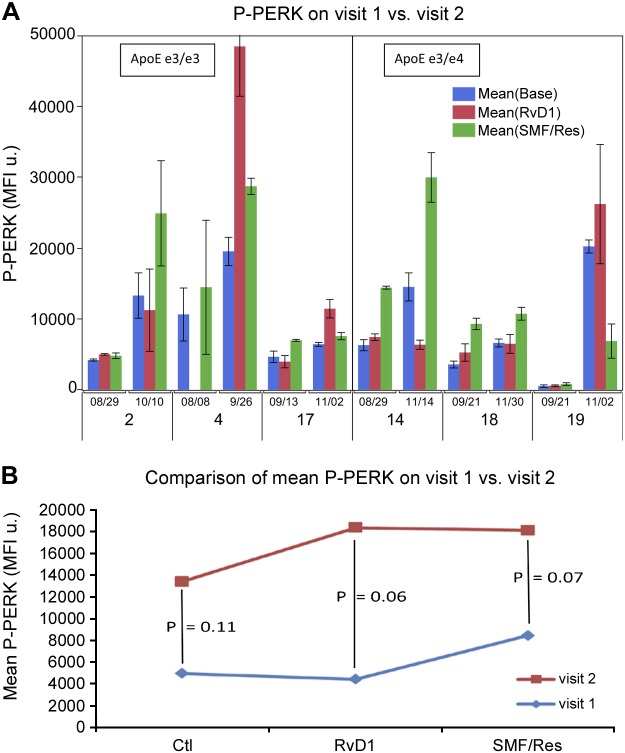
A comparison of phospho-PERK expression in the whole cohort on the first vs. the second visit demonstrated that phospho-PERK expression increased over time with ω-3 supplementation. The increase occurred in Mϕs that were treated in vitro with a placebo (P = 0.11), RvD1 (P = 0.06), or SMF/Res (P = 0.07), and seemed to be increased by in vitro RvD1 or SMF/Res treatment (not significant; Fig. 2).
Figure 2.
ω-3 emulsion supplementation stimulates phospho-PERK expression in Mϕs. A) Phospho-PERK was determined by immunofluorescence. Replicate Mϕ cultures were treated for 18 h by medium control (Ctl), ω-3 emulsion, or RvD1. B) Comparison of mean phospho-PERK on 2 successive visits by repeated measures ANOVA (patient 9 with possible Lewy body disease not included). MFI, mean fluorescence intensity.
Tunicamycin, but not Aβ, increases phospho-PERK expression
We compared the effects of putative stressors in Mϕs, the glycation inhibitor tunicamycin, and the pathogenic peptide Aβ on phospho-PERK expression. Tunicamycin increased phospho-PERK expression compared with placebo (P = 0.08). Surprisingly, soluble Aβ had no significant effect on phospho-PERK (P = 0.83; Fig. 3).
Figure 3.
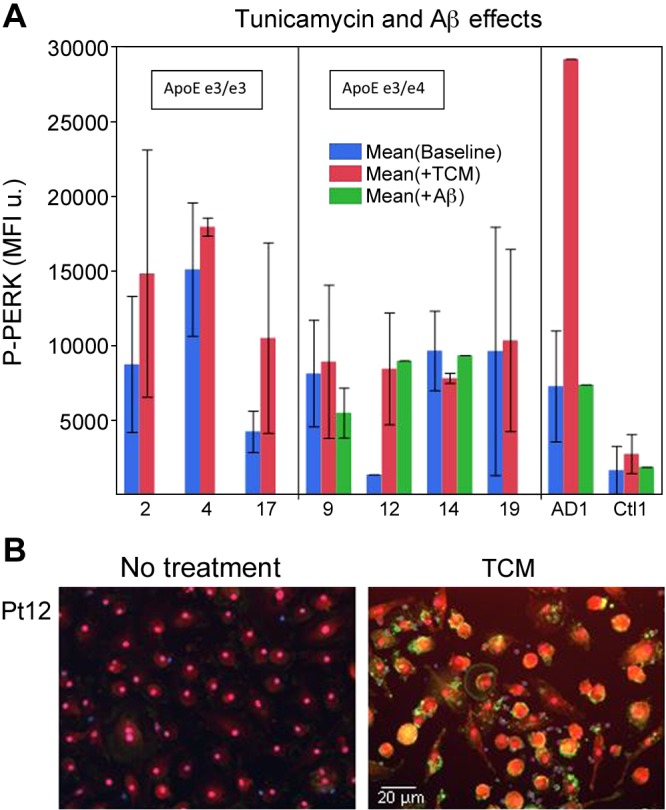
Tunicamycin (TCM) stimulates phospho-PERK expression, but soluble Aβ does not. A) Mϕs were treated 18 h with TCM or soluble Aβ, and the expression of phospho-PERK in Mϕs was determined by immunofluorescence and scanning. B) Immunofluorescence of phospho-PERK in Mϕs of patient 12 on no treatment and after TCM treatment. Repeated measures ANOVA: no treatment vs. TCM, P = 0.08; no treatment vs. Aβ, P = 0.83. MFI, mean fluorescence intensity.
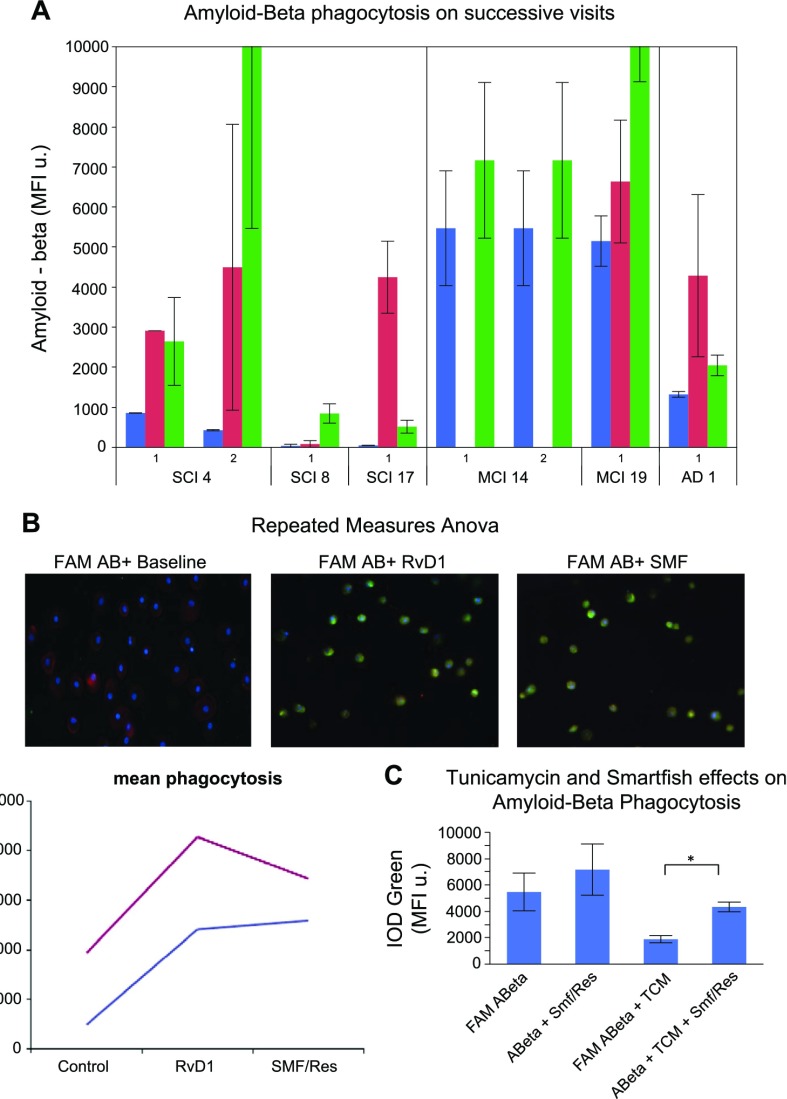
ω-3s increase Aβ phagocytosis and decrease apoptosis
ω-3 supplementation has the goal of immune system repair and cognition improvement via recovery of effective Aβ phagocytosis by monocytes and Mϕs (21) and increase of resistance to Aβ-induced apoptosis (22, 25). Our previous study showed a significant increase of phagocytosis (25). To investigate the combined effects of ω-3 supplementation in vivo and stimulation in vitro on Mϕ phagocytosis, we treated Mϕs of patients with MCI and SCI, obtained at successive visits, with RvD1 or SMF/Res, and exposed them to fluorescent Aβ for 18 h. Mean Aβ phagocytosis increased at the second visit compared with placebo after treatment with RvD1 and SMF/Res (not significant; Fig. 4A, B). Treatment of Mϕs with tunicamycin decreased Aβ phagocytosis, but SMF/Res recovered phagocytosis (Fig. 4C).
Figure 4.
Longitudinal study of Aβ phagocytosis in the Mϕs of ω-3–supplemented patients. A) Mϕs isolated at 2 visits were treated with RvD1 or SMF/Res and exposed for 18 h to fluorescent Aβ. Comparison of mean phagocytosis by repeated measures ANOVA (not significant). B) Immunofluorescence of Mϕs (patient 14) exposed to fluorescent Aβ at baseline (blue) and after treatment by RvD1 or SMF/Res (red) for 18 h. C) Tunicamycin decreased phagocytosis of fluorescent Aβ. SMF/Res recovered phagocytosis. IOD, integrated optical density; MFI, mean fluorescence intensity. Error bars indicate sem.
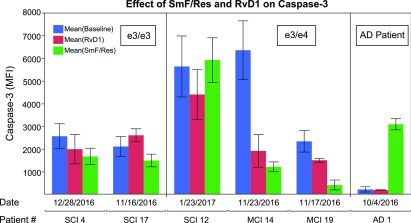
RvD1 and ω-3 emulsion seemed to decrease the expression of caspase-3 in Mϕs of patients with MCI, but the decrease was not significant (Fig. 5).
Figure 5.
RvD1 or ω-3 effects on Mϕ apoptosis. Mϕs isolated at each visit were treated with RvD1 or ω-3 emulsion, and caspase-3 expression was determined by immunofluorescence. MFI, mean fluorescence intensity. Error bars indicate sem.
ω-3s increase UPR signature genes and decrease ER stress signature genes
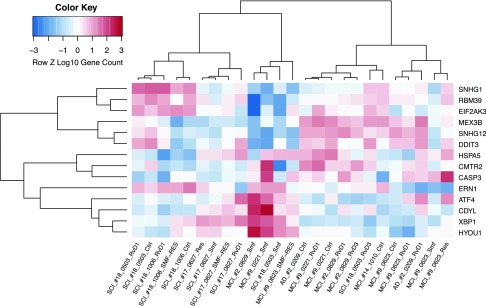
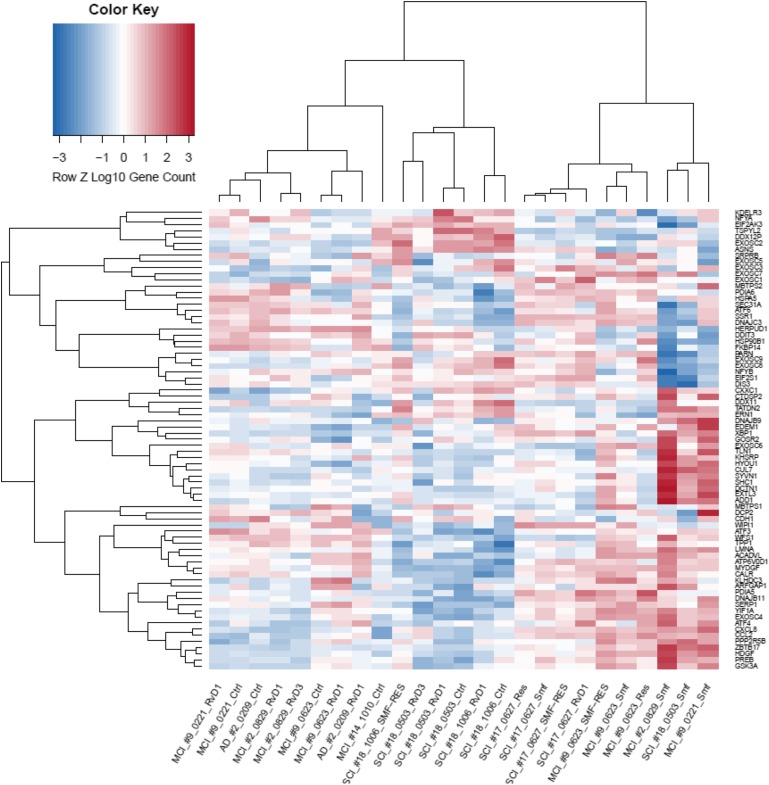
To evaluate the transcriptional effects of ω-3s, we used the ER stress gene set that has been shown to be induced by ER stress across different mouse strains (32) and a UPR gene set for the unfolded protein regulatory response system (33). We computed the ER stress and UPR signature gene expression in the Mϕ transcriptome and displayed their values as a hierarchical clustering dendrogram. Analysis of the top 14 ER stress signature genes (Fig. 6) showed down-regulation of the apoptosis promoters CHOP/DDIT3 and CASP3 (caspase-3), except in patient 9, who failed to respond to ω-3s. Analysis of the top 75 UPR signature genes (Fig. 7) revealed that SMF/Res regulated mRNAs of the 3 canonical UPR pathway proteins: 1) the endoribonuclease IRE1 (ERN1; stimulating the translation of XBP1; data not shown); 2) ATF6 (up-regulating ER chaperones and ER-associated degradation proteins); and 3) ATF4 (cAMP-response element binding protein 2). Up-regulated genes with chaperone functions included EXTL3 (the glycosyltransferase catalyzing the transfer of N-acetylglucosamine to glycosaminoglycan chain), DCTN1 (important for ER to Golgi apparatus transfer), and CALR (calreticulin; calcium–binding chaperone).
Figure 6.
Hierarchical cluster and heatmap of ER stress genes in stimulated Mϕs. Heatmap and dendrogram of Mϕ expression levels exposed to different compounds: RvD1, RvD3, resveratrol (Res), SMF, SMF/Res, or negative control (Ctrl). Expression measured by their relative expression level (z score) after log10 transformation for ER stress genes (rows) across Mϕ samples (columns). Each cell represents relative expression level with respect to other samples for a single gene, where red indicates a higher expression level and blue indicates a lower expression level. MFI, mean fluorescence intensity. *P < 0.01.
Figure 7.
Hierarchical cluster and heatmap of UPR genes in stimulated Mϕs. Heatmap and dendrogram of Mϕ expression levels exposed to different compounds: RvD1, RvD3, resveratrol (Res), SMF, SMF/Res, or negative control (Ctrl). Expression measured by their relative expression level (z score) for UPR genes (rows) across Mϕ samples (columns). Each cell represents relative expression level with respect to other samples for a single gene, where red indicates a higher expression level and blue indicates a lower expression level.
To analyze the most significant genes that were differentially expressed among the signature gene sets, we calculated the inverted β-binomial P value between control- and SMF/Res-treated Mϕs for paired samples. SMF/Res down-regulated genes in the ER stress signature, including CHOP/DDIT3 and the translational regulator MEX3B (Supplemental Fig. 1). SMF/Res up-regulated genes in the UPR signature: ATF4 (cAMP response element-binding transcription factor 2), chemokines CCL2 and CXCL8, HYOU1 (heat shock protein 70 family), and SERP1 (stress associated endoplasmic reticulum protein 1; Supplemental Fig. 2). The comparison of different treatments in all study participants by Mann-Whitney Wilcoxon test showed that SMF significantly (P < 0.01) down-regulated ER stress genes (Supplemental Fig. 3A) and significantly (P < 0.01) up-regulated UPR signature genes (Supplemental Fig. 3B).
DISCUSSION
In our previous studies, supplementation with fish-derived ω-3 SMF/Res had positive cognitive effects in the patients in the ApoEe3/e3 subgroup and modulated the unfavorable M1 or M2 Mϕ phenotype into a favorable intermediate M1-M2 Mϕ phenotype with increased Aβ phagocytosis; whereas, in individual patients in the ApoEe3/e4 subgroup, the results were heterogeneous (24, 25). Here, we again observed the beneficial functional effects of ω-3s on MMSE in patients in the ApoEe3/e3 subgroup (Table 1).
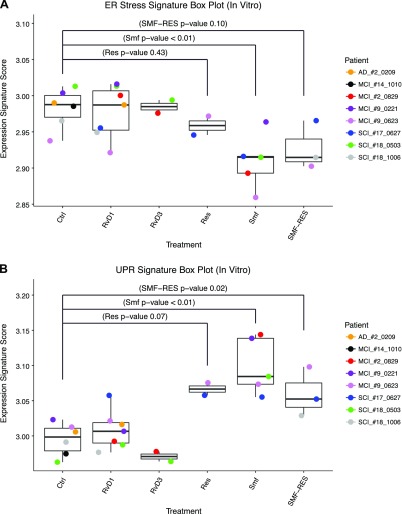
The main objective of our study was to analyze the in vivo and in vitro effects of ω-3s on phospho-PERK expression and ER stress and UPR RNA signatures. Surgical, medical, and lifestyle complications significantly impacted the clinical course and immunity during the long follow-up (mean follow-up 19.1 mo). SMF/Res supplementation was associated with an increase in phospho-PERK in the second visit compared with the first visit (Fig. 2). In patients in the ApoEe3/e3 subgroup, MMSE status and phospho-PERK expression improved, but phospho-PERK expression and MMSE either both improved or both declined in parallel in patients in the ApoEe3/e4 subgroup (Figs. 1 and 2). The latter result suggests that ω-3s may not overcome ER stress and improve cognition in certain patients, possibly in relation to ω-3 catabolism (37). SMF/Res significantly increased in vitro the UPR RNA signature score and significantly down-regulated genes in the ER stress signature (Fig. 8). SMF/Res and SMF alone up-regulated ATF4 more consistently than did RvD1 and down-regulated CHOP/DDIT3 more consistently than did RvD1 (Figs. 6–8). Thus, SMF/Res seems to have a broader cytoprotective spectrum of activity than RvD1. These results support the hypothesis that ω-3s and secialized proresolving mediators have beneficial but heterogeneous effects in Mϕs from patients with AD by increasing the cytoprotective and immunoenhancing branch of phospho-PERK and decreasing the proapoptotic branch of phospho-PERK mediated by CHOP/DDIT3.
Figure 8.
ER stress signature gene expression box plot (Mann-Whitney Wilcoxon). Replicate Mϕ cultures were exposed to different compounds: RvD1, RvD3, resveratrol (Res), SMF, SMF/Res, or negative control (Ctrl). Box plots of ER stress signature scores under different treatment conditions. Mann-Whitney Wilcoxon test of ER stress signatures scores between ctrl samples to the Res, SMF, and SMF/Res groups produced unadjusted values of P < 0.43, P < 0.01, and P < 0.10, respectively. B) UPR signature gene expression box plot (Mann-Whitney Wilcoxon). P < 0.07, P < 0.01, P < 0.02, calculated between Res, SMF, and SMF/Res groups, respectively, to control samples.
Both tunicamycin and ω-3s increased phospho-PERK in Mϕs (Fig. 3). Tunicamycin is an inhibitor of glycosylation with negative effects on phagocytosis (Fig. 4), whereas ω-3s are lipid mediators with positive effects on phagocytosis. Thus, tunicamycin and ω-3s have opposite effects on phagocytosis, likely as a result of the stimulation of different responses downstream from phospho-PERK, and possibly the effects of ω-3s on faulty glycosylation (21). Unexpectedly, soluble Aβ did not increase phospho-PERK, and therefore, we plan to test oligomeric and fibrillary Aβ.
Our study has important limitations because of the uncontrolled design of the study, voluntary caregiver supervision of ω-3 supplementation, small sample size with a greater proportion of patients with SCI in the ApoEe3/e3 group vs. ApoEe3/e4 group (80 vs. 50%), interruption of supplementation during hospitalizations, supervision, and impact of intercurrent infections, fractures, and lifestyle events. Future studies should to analyze the switch between prosurvival and proapoptotic UPR functions, including IRE-1 signaling by XBP1 (38) and an important question concerning ω-3 effects on defective autophagy (4).
In summary, fish-derived ω-3s increase the cytoprotective phospho-PERK pathway, decrease ER stress, and increase UPR in association with increased Aβ phagocytosis and decreased Mϕ apoptosis. Patients in the ApoEe3/e3 subgroup showed parallel immunologic and cognitive improvement or stabilization, whereas some patients in the ApoEe3/e4 subgroup had parallel immunologic and cognitive decline. Our previous immunochemical results demonstrated that Mϕs migrate across the blood-brain barrier and upload Aβ but are defective in phagocytosis and Aβ degradation and suffer apoptosis (22). The results of this study suggest that fish-derived ω-3s may induce UPR mechanisms in the innate immune system that increase Aβ phagocytosis and protect against apoptosis, thus repairing the clearance of Aβ in the AD brain.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Institute of General Medical Sciences Grant 5P01GM099134-03 (to M.P.) and a donation from Smartfish AS (to M.F.). Smartfish AS supplied the drink to the patients who requested it. The authors thank Leonard Haller, Angela Kim, Janteshpreet Sandhu, Jessica Xu, Gigi Arnone, Sheila Hou, Peter Cho, and Yasmine Alhoch (all from University of California, Los Angeles, Los Angeles, CA, USA) for assistance with data collection. The authors thank R. Rao (the Buck Institute, Novato, CA, USA) for testing the APOE genotype of study patients. The authors are grateful to Fawaz George Haj and Bruce Hammock (both from University of California, Davis, Davis, CA, USA) for helpful discussions of lipid mediators and ER stress. Smartfish was not involved in the design, analysis, interpretation of the data, or the decision to submit the manuscript for publication. M.F. received paid travel to meetings and honoraria from Smartfish.
Glossary
- ω-3
omega-3 fatty acid
- Aβ
amyloid-β1–42
- AD
Alzheimer’s disease
- ApoE
apolipoprotein E
- ATF4
activating transcription factor 4
- ATF6
activating transcription factor 6
- CHOP
C/EBP homoogous protein (DDIT3)
- DDIT3
DNA damage inducible transcript 3 (CHOP)
- DHA
docosahexaenoic acid
- ER
endoplasmic reticulum
- IRE1
inositol-requiring enzyme 1
- Mϕ
macrophage
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- PERK
protein kinase RNA-like ER kinase
- RvD1
resolvin D1
- SCI
subjective cognitive impairment
- SMF
Smartfish
- SMF/Res
Smartfish with resveratrol
- UPR
unfolded protein response
- XBP1
X-box binding protein-1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
H. M. Olivera-Perez, J. Dang, W. Jiang, and F. Rodriguez performed the bulk of the Mϕ experiments; L. Lam analyzed RNA data and RNA signatures and performed bioinformatics analysis; L. Rubbi and M. Morselli performed RNA sequencing; E. Rigali and S. Weitzman analyzed clinical and protein data; V. Porter performed clinical examinations; M. Pellegrini supervised RNA testing, directed RNA sequencing, and wrote the manuscript; M. Fiala developed the hypothesis, designed research, directed the project, analyzed data, wrote the manuscript, and supervised the peer review process.
REFERENCES
- 1.Scheper W., and Hoozemans J. J. (2015) The unfolded protein response in neurodegenerative diseases: a neuropathological perspective. Acta Neuropathol. 130, 315–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker A. K., Soo K. Y., Sundaramoorthy V., Parakh S., Ma Y., Farg M. A., Wallace R. H., Crouch P. J., Turner B. J., Horne M. K., and Atkin J. D. (2013) ALS-associated TDP-43 induces endoplasmic reticulum stress, which drives cytoplasmic TDP-43 accumulation and stress granule formation. PLoS One 8, e81170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle K. M., Kennedy D., Gorman A. M., Gupta S., Healy S. J., and Samali A. (2011) Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J. Cell. Mol. Med. 15, 2025–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Y., Arikkath J., Yang L., Guo M. L., Periyasamy P., and Buch S. (2016) Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy 12, 225–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernales S., Papa F. R., and Walter P. (2006) Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 22, 487–508 [DOI] [PubMed] [Google Scholar]
- 6.Wang S., and Kaufman R. J. (2012) The impact of the unfolded protein response on human disease. J. Cell Biol. 197, 857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casas-Tinto S., Zhang Y., Sanchez-Garcia J., Gomez-Velazquez M., Rincon-Limas D. E., and Fernandez-Funez P. (2011) The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum. Mol. Genet. 20, 2144–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duran-Aniotz C., Cornejo V. H., Espinoza S., Ardiles A. O., Medinas D. B., Salazar C., Foley A., Gajardo I., Thielen P., Iwawaki T., Scheper W., Soto C., Palacios A. G., Hoozemans J. J., and Hetz C. (2017) IRE1 signaling exacerbates Alzheimer’s disease pathogenesis [E-pub ahead of print]. Acta Neuropathol. doi:10.1007/s00401-017-1694-x [DOI] [PubMed] [Google Scholar]
- 9.Galehdar Z., Swan P., Fuerth B., Callaghan S. M., Park D. S., and Cregan S. P. (2010) Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J. Neurosci. 30, 16938–16948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schröder M., and Kaufman R. J. (2005) The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 [DOI] [PubMed] [Google Scholar]
- 11.Li M., Baumeister P., Roy B., Phan T., Foti D., Luo S., and Lee A. S. (2000) ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol. Cell. Biol. 20, 5096–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh R., Wang L., Wang E. S., Perera B. G., Igbaria A., Morita S., Prado K., Thamsen M., Caswell D., Macias H., Weiberth K. F., Gliedt M. J., Alavi M. V., Hari S. B., Mitra A. K., Bhhatarai B., Schürer S. C., Snapp E. L., Gould D. B., German M. S., Backes B. J., Maly D. J., Oakes S. A., and Papa F. R. (2014) Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158, 534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyce M., Bryant K. F., Jousse C., Long K., Harding H. P., Scheuner D., Kaufman R. J., Ma D., Coen D. M., Ron D., and Yuan J. (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307, 935–939 [DOI] [PubMed] [Google Scholar]
- 14.Feldman H. C., Tong M., Wang L., Meza-Acevedo R., Gobillot T. A., Lebedev I., Gliedt M. J., Hari S. B., Mitra A. K., Backes B. J., Papa F. R., Seeliger M. A., and Maly D. J. (2016) Structural and functional analysis of the allosteric inhibition of IRE1α with ATP-competitive ligands. ACS Chem. Biol. 11, 2195–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maly D. J., and Papa F. R. (2014) Druggable sensors of the unfolded protein response. Nat. Chem. Biol. 10, 892–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong Y., Wang X., Sun S., Xue G., Li J., and Hou Y. (2016) Progesterone exerts neuroprotective effects against Aβ-induced neuroinflammation by attenuating ER stress in astrocytes. Int. Immunopharmacol. 33, 83–89 [DOI] [PubMed] [Google Scholar]
- 17.Pereira D. M., Valentão P., Correia-da-Silva G., Teixeira N., and Andrade P. B. (2015) Translating endoplasmic reticulum biology into the clinic: a role for ER-targeted natural products? Nat. Prod. Rep. 32, 705–722 [DOI] [PubMed] [Google Scholar]
- 18.Fiala M., Terrando N., and Dalli J. (2015) Specialized pro-resolving mediators from omega-3 fatty acids improve amyloid-β phagocytosis and regulate inflammation in patients with minor cognitive impairment. J. Alzheimers Dis. 48, 293–301 [DOI] [PubMed] [Google Scholar]
- 19.Begum G., Harvey L., Dixon C. E., and Sun D. (2013) ER stress and effects of DHA as an ER stress inhibitor. Transl. Stroke Res. 4, 635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo T., Asai M., Tsukita K., Kutoku Y., Ohsawa Y., Sunada Y., Imamura K., Egawa N., Yahata N., Okita K., Takahashi K., Asaka I., Aoi T., Watanabe A., Watanabe K., Kadoya C., Nakano R., Watanabe D., Maruyama K., Hori O., Hibino S., Choshi T., Nakahata T., Hioki H., Kaneko T., Naitoh M., Yoshikawa K., Yamawaki S., Suzuki S., Hata R., Ueno S., Seki T., Kobayashi K., Toda T., Murakami K., Irie K., Klein W. L., Mori H., Asada T., Takahashi R., Iwata N., Yamanaka S., and Inoue H. (2013) Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell 12, 487–496 [DOI] [PubMed] [Google Scholar]
- 21.Fiala M., Liu P. T., Espinosa-Jeffrey A., Rosenthal M. J., Bernard G., Ringman J. M., Sayre J., Zhang L., Zaghi J., Dejbakhsh S., Chiang B., Hui J., Mahanian M., Baghaee A., Hong P., and Cashman J. (2007) Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc. Natl. Acad. Sci. USA 104, 12849–12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaghi J., Goldenson B., Inayathullah M., Lossinsky A. S., Masoumi A., Avagyan H., Mahanian M., Bernas M., Weinand M., Rosenthal M. J., Espinosa-Jeffrey A., de Vellis J., Teplow D. B., and Fiala M. (2009) Alzheimer disease macrophages shuttle amyloid-beta from neurons to vessels, contributing to amyloid angiopathy. Acta Neuropathol. 117, 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizwicki M. T., Liu G., Fiala M., Magpantay L., Sayre J., Siani A., Mahanian M., Weitzman R., Hayden E. Y., Rosenthal M. J., Nemere I., Ringman J., and Teplow D. B. (2013) 1α,25-Dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-β phagocytosis and inflammation in Alzheimer’s disease patients. J. Alzheimers Dis. 34, 155–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiala M., Halder R. C., Sagong B., Ross O., Sayre J., Porter V., and Bredesen D. E. (2015) ω-3 supplementation increases amyloid-β phagocytosis and resolvin D1 in patients with minor cognitive impairment. FASEB J. 29, 2681–2689 [DOI] [PubMed] [Google Scholar]
- 25.Famenini S., Rigali E. A., Olivera-Perez H. M., Dang J., Chang M. T., Halder R., Rao R. V., Pellegrini M., Porter V., Bredesen D., and Fiala M. (2017) Increased intermediate M1-M2 macrophage polarization and improved cognition in mild cognitive impairment patients on ω-3 supplementation. FASEB J. 31, 148–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen R. C., Doody R., Kurz A., Mohs R. C., Morris J. C., Rabins P. V., Ritchie K., Rossor M., Thal L., and Winblad B. (2001) Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992 [DOI] [PubMed] [Google Scholar]
- 27.Duara R., Loewenstein D. A., Greig M. T., Potter E., Barker W., Raj A., Schinka J., Borenstein A., Schoenberg M., Wu Y., Banko J., and Potter H. (2011) Pre-MCI and MCI: neuropsychological, clinical, and imaging features and progression rates. Am. J. Geriatr. Psychiatry 19, 951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G., Fiala M., Mizwicki M. T., Sayre J., Magpantay L., Siani A., Mahanian M., Chattopadhyay M., La Cava A., and Wiedau-Pazos M. (2012) Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: inhibition of inflammation by resolvin D1. Am. J. Neurodegener. Dis. 1, 60–74 [PMC free article] [PubMed] [Google Scholar]
- 29.Anders S., Pyl P. T., and Huber W. (2015) HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anders S., and Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol. 11, R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam L., Chin L., Halder R. C., Sagong B., Famenini S., Sayre J., Montoya D., Rubbi L., Pellegrini M., and Fiala M. (2016) Epigenetic changes in T-cell and monocyte signatures and production of neurotoxic cytokines in ALS patients. FASEB J. 30, 3461–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow C. Y., Wang X., Riccardi D., Wolfner M. F., and Clark A. G. (2015) The genetic architecture of the genome-wide transcriptional response to ER stress in the mouse. PLoS Genet. 11, e1004924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., and Mesirov J. P. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyerovich K., Ortis F., Allagnat F., and Cardozo A. K. (2016) Endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. J. Mol. Endocrinol. 57, R1–R17 [DOI] [PubMed] [Google Scholar]
- 35.Eizirik D. L., Cardozo A. K., and Cnop M. (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29, 42–61 [DOI] [PubMed] [Google Scholar]
- 36.Pham T. V., Piersma S. R., Warmoes M., and Jimenez C. R. (2010) On the beta-binomial model for analysis of spectral count data in label-free tandem mass spectrometry-based proteomics. Bioinformatics 26, 363–369 [DOI] [PubMed] [Google Scholar]
- 37.Yassine H. N., Feng Q., Azizkhanian I., Rawat V., Castor K., Fonteh A. N., Harrington M. G., Zheng L., Reed B. R., DeCarli C., Jagust W. J., and Chui H. C. (2016) Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol. 10, 1208–1216 [DOI] [PubMed] [Google Scholar]
- 38.Yassine H. N., Braskie M. N., Mack W. J., Castor K. J., Fonteh A. N., Schneider L. S., Harrington M. G., and Chui H. C. (2017) Association of docosahexaenoic acid supplementation with Alzheimer disease stage in apolipoprotein E ε4 carriers: a review. JAMA Neurol. 74, 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.