Abstract
Maternal cigarette smoke, including prenatal nicotinic exposure (PNE), is responsible for sudden infant death syndrome (SIDS). The fatal events of SIDS are characterized by severe bradycardia and life-threatening apneas. Although activation of transient receptor potential vanilloid 1 (TRPV1) of superior laryngeal C fibers (SLCFs) could induce bradycardia and apnea and has been implicated in SIDS pathogenesis, how PNE affects the SLCF-mediated cardiorespiratory responses remains unexplored. Here, we tested the hypothesis that PNE would aggravate the SLCF-mediated apnea and bradycardia via up-regulating TRPV1 expression and excitation of laryngeal C neurons in the nodose/jugular (N/J) ganglia. To this end, we compared the following outcomes between control and PNE rat pups at postnatal days 11–14: 1) the cardiorespiratory responses to intralaryngeal application of capsaicin (10 µg/ml, 50 µl), a selective stimulant for TRPV1 receptors, in anesthetized preparation; 2) immunoreactivity and mRNA of TRPV1 receptors of laryngeal sensory C neurons in the N/J ganglia retrogradely traced by 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; and 3) TRPV1 currents and electrophysiological characteristics of these neurons by using whole-cell patch-clamp technique in vitro. Our results showed that PNE markedly prolonged the apneic response and exacerbated the bradycardic response to intralaryngeal perfusion of capsaicin, which was associated with up-regulation of TRPV1 expression in laryngeal C neurons. In addition, PNE increased the TRPV1 currents, depressed the slow delayed rectifier potassium currents, and increased the resting membrane potential of these neurons. Our results suggest that PNE is capable of aggravating the SLCF-mediated apnea and bradycardia through TRPV1 sensitization and neuronal excitation, which may contribute to the pathogenesis of SIDS.—Gao, X., Zhao, L., Zhuang, J., Zang, N., Xu, F. Prenatal nicotinic exposure prolongs superior laryngeal C-fiber–mediated apnea and bradycardia through enhancing neuronal TRPV1 expression and excitation.
Keywords: SIDS, cardiorespiratory activity, IKs
Sudden infant death syndrome (SIDS) is the leading cause of infant death, often occurring in seemingly healthy male infants. The fatal events of SIDS are characterized by severe bradycardia, life-threatening apneas, and gasps (1, 2). Among multiple factors presumably responsible for SIDS, maternal cigarette smoke and exposure during the developmentally sensitive period serve as the major internal risk factors (3, 4). Cigarette smoke contains numerous compounds emitted as gases and condensed tar particles, among which nicotine is the major neurotoxic chemical (5). Prenatal nicotinic exposure (PNE) has been extensively used to reveal the adverse impacts of cigarette smoke on cardiorespiratory functions and has thereby been linked with SIDS pathogenesis (6–8). We recently found that PNE-induced death immediately after severe bradycardia and apneas during acute hypoxic exposure in rat pups at postnatal day (P)11–P14 (9). Prior to death, PNE also induced a remarkably prolonged apneic response to stimulation of bronchopulmonary C fibers (PCFs) (10). Besides PCFs, the superior laryngeal nerve (SLN) innervating the larynx (11, 12) plays an important role in causing central apnea and bradycardia (13–15). Interestingly, it has been clinically reported that one SIDS victim’s siblings with a very long apneic response to SLN stimulation died later from SIDS (16). The SLN is mainly composed of sensory fibers, especially unmyelinated superior laryngeal C fibers (SLCFs) (11, 17). The SLCF-mediated apnea and bradycardia could be triggered by capsaicin (Cap) (18), a selective stimulant to transient receptor potential vanilloid 1 (TRPV1) (19). TRPV1 channels are located at the terminals of laryngeal afferents and in their cell bodies in the nodose/jugular (N/J) ganglia (10, 20). To date, it has not been fully explored whether PNE affects SLCFs’ TRPV1 receptors to alter the apneic and bradycardia responses.
Nevertheless, changes in neural electrophysiological characteristics are able to alter the excitability and sensitivity of SLCFs in addition to TRPV1 receptors. For example, the raised resting membrane potential and/or the decreased outward potassium currents could up-regulate neuronal excitation (21). The slow rectifier outward potassium current (IKs) is attributed to the cell excitation via its dominant role in the phase of action potential duration (22). It is related to repolarization of action potential (23) and prevents generation of early afterdepolarization (24). Depressed IKs shortens action potential duration, thereby accelerating the fire rate and up-regulating the excitation of neurons. Similarly, a more depolarized membrane potential, closer to the threshold potential, allows neurons to easily be stimulated and triggered to generate effective signal propagation. Therefore, it is critical to define PNE influence on the electrophysiological properties of laryngeal C neurons in order to better understand the PNE-induced changes in SLCF sensitivity and potentiation.
Based on the lines of the information mentioned above, we tested whether PNE would augment the SLCF-mediated apnea and bradycardia via up-regulating TRPV1 expression and changing the electrophysiological properties of laryngeal C neurons. We compared the following experiments between control (Ctrl) and PNE rat pups: 1) the apneic response to intralaryngeal application of Cap in anesthetized preparation, 2) immunoreactivity and mRNA level of TRPV1 receptors in laryngeal sensory neurons of the N/J ganglia that were retrogradely traced by 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) microinjected into the SLN, and 3) TRPV1 current, resting potential, and IKs recorded in these neurons in vitro by using a whole-cell patch-clamp technique.
MATERIALS AND METHODS
Pathogen-free Sprague-Dawley rats (250–350 g) were purchased from Charles River Laboratories (Wilmington, MA, USA). Rats were housed in the animal facility at Lovelace Respiratory Research Institute in filter-top cages and provided with water and food ad libitum. The room was constantly ventilated, and the temperature was kept at 23°C. The animals were quarantined for 2 wk before the experiments. The experimental protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), Bethesda, MD, USA] and approved by the Institutional Animal Care and Use Committee, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (Frederick, MD, USA).
PNE animal preparation
PNE pretreatment was the same as previously reported (10). Briefly, adult female rats were randomly designated to receive saline (n = 7, Ctrl) and nicotine (n = 9, PNE) via a mini-osmotic pump (0.25 μl/h for 28 d) (Alza, Palo Alto, CA, USA). The pump was subcutaneously implanted in the female rats to deliver saline or nicotine tartrate (6 mg/kg/d), which produces plasma levels of free nicotine of about 24 ng/ml (roughly 150 nM) in neonates and 18 ng/ml (111 nM) in pregnant dams (25). These levels are within the range (15–45 ng/ml) observed in the blood of pregnant women described as moderate smokers (26) and in the amniotic fluid of smoke-exposed human fetuses (27). Ten days after implantation, each female rat was placed in a breeding cage with a male rat for up to 4 d. Those with vaginal plugs were considered pregnant and separated from the male rats. On the seventh day of gestation, the pump was replaced with a new one filled accordingly with saline or nicotine.
Rat pups born by spontaneous vaginal delivery were housed with their mother and siblings (24–25°C; 12:12 h light/dark cycle). In all experiments, no more than 3 male pups from each litter with a similar overall litter size were used in each study to minimize the possible effect of genetic difference between litters on the results. Male pups at P11–14 were chosen in this study because male infants are much more vulnerable than female infants in human SIDS (28) and because the pups’ brain development at this period is equivalent to newborn infants at 2–4 mo (29), when SIDS incidence is at the peak. All studies were performed between 9:00 am and 5:00 pm to avoid influence from the circadian rhythm (30). Pups from vehicle- and nicotine-treated dams were grouped as Ctrl and PNE, which were randomly assigned to 3 study series (see following section).
Experimental protocols
Study series I was performed to test whether PNE altered the cardiorespiratory responses to intralaryngeal application of Cap in Ctrl and PNE pups (n = 6 and 10, respectively). At P4–5, these pups were anesthetized and received SLN pretreatment with DiI, a retrograde tracer to mark laryngeal neurons within the N/J ganglia. Seven to 9 days later (P11–14), they were anesthetized again for recording the cardiorespiratory responses to intralaryngeal application of Cap (10 µg/ml, 50 µl).
Study series II was designed to examine PNE effect on TRPV1 expression in laryngeal C neurons. After completion of the cardiorespiratory responses to intralaryngeal application of Cap, as described for study series I, these pups were euthanized to collect the N/J ganglia as previously reported (31, 32) and prepared for the immunohistochemical processing to determine the immunoreactivity of TRPV1 on N/J ganglionic neurons, especially those colabeled with DiI. With respect to TRPV1 mRNA level (see following paragraph for primary neuron culture), laryngeal C neurons labeled by DiI with a cell size <25 µm were picked by a glass pipette from primary cultured N/J ganglionic neurons and subjected to single-cell RT-PCR examination.
Study series III was carried out to verify the influences of PNE on laryngeal sensory C-neuron electrophysiological properties. P11–14 Ctrl and PNE pups were pretreated with the retrograde tracer DiI in the SLN (n = 10/group). Seven to 9 days later, the N/J ganglia were collected for primary neuron culture. The whole-cell patch-clamp technique was used to record electrophysiological characteristics (the resting membrane potential and IKs), followed by the recording of TRPV1 currents on the laryngeal C neurons.
Retrograde labeling of laryngeal C neurons in the N/J ganglia
Pups were anesthetized with continuous inhalation of isoflurane (1–2%), administered via a nose cone connected to a vaporizing machine (SurgiVet, Waukesha, WI, USA). A midline incision (1 to ∼1.5 cm) was made in the neck to expose both sides of the SLN. As reported before (33), a beveled glass micropipette needle (10-μm tip diameter) was pulled by a DMZ universal puller (Dagon, Minneapolis, MN, USA), sharpened with a microforge (MF-900; Narishige, Tokyo, Japan), and connected to a Harvard PHD Syringe pump (Harvard Apparatus, Holliston, MA, USA). The tip of the needle was gently inserted into the isolated SLN to microinject 0.5 µl of DiI solution at a rate of 0.1 µl/min. The skin incision was sutured after bilateral microinjections.
Cardiorespiratory responses to intralaryngeal application of Cap
The animals were anesthetized with urethane (1400 mg/kg, intraperitoneally), and cardiorespiratory activity was recorded. Supplemental doses of urethane (120–240 mg/kg, intravenously) were provided as needed to suppress corneal and withdrawal reflexes. The trachea was opened with a transverse incision to expose the lumen while leaving the posterior tracheal wall intact. The caudal tracheal opening was cannulated and connected to a pneumotachograph (Frank’s Manufacturing, Albuquerque, NM, USA) for recording airflow (34). The airflow signals were integrated to generate tidal volume (VT), respiratory frequency (fR), and minute ventilatory volume (VE). An expiratory duration (TE) ≥2 s was used to define an apnea (35, 36). Raw data of these variables, heart rate (HR), and mean arterial blood pressure (MABP) were recorded by PowerLab/8sp (model ML 785; ADInstruments, Colorado Springs, CO, USA) and a computer with the LabChart Pro 7 software (ADInstruments). The rostral segment of the trachea was cannulated with a laryngeal catheter separately. Briefly (37), a laryngeal catheter (PE-190) with a premade window (∼2.5 × 1.2 mm) was inserted cranially from the rostral tracheal opening and extended out of the mouth, bypassing the pharynx and mouth. The window was positioned just caudal to the vocal cord, by which >80% of the laryngeal mucosal surface was exposed to the perfused solution. The catheter was then fixed at both ends. The solution in the catheter was perfused at a constant flow rate (10 µl/s) through the tracheal end of the catheter and out of the mouth end. Perfusion at this flow rate for 5 s did not induce detectable leaking, because the same amount of solution volume was collected from the mouth end of the catheter in our studies. After Cap delivery, the catheter was washed by delivering saline twice. The left femoral artery was cannulated for monitoring arterial blood pressure and HR. The animal was exposed to 30% O2 throughout the experiment to prevent hypoxia. The core temperature of the animal was monitored with a rectal probe and maintained at ∼36°C with a heating pad and radiant heat.
Immunohistochemistry
The rats were perfused transcardially with 30 ml of saline followed by 150 ml of 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The bilateral N/J ganglia were removed and postfixed for 2 h in the same fixative. The tissue blocks were rinsed in PBS, cytoprotected overnight in 30% sucrose in 0.1 M phosphate buffer, and then frozen in a Tissue-Tek optimal cutting temperature (OCT) embedding medium (Miles, Elkhart, IN, USA). The N/J ganglia was serially sectioned (10-µm-thick sections) by a precision cryostat, and sections were mounted on the gelatin-chromalum–coated slides and stored at −70°C until they were processed for immunohistochemical studies (38). Frozen slides were dried at room temperature for 1 h and washed 3 times in PBS, blocked for 1 h in PBS with 0.1% TritonX-100 containing 10% goat serum, and incubated overnight at 4°C with primary antibodies diluted in the blocking solution rabbit anti-TRPV1 (1:500) (Thermo Fisher Scientific, Waltham, MA, USA). The sections were then washed 3 times in PBS and incubated for 2 h at room temperature with secondary antibodies and goat anti-rabbit IgG conjugated with Alexa-488 fluorochrome (1:200 dilutions) (Thermo Fisher Scientific). Sections were then washed 3 times in PBS and sealed with coverslip by Antifade Reagent with DAPI (Thermo Fisher Scientific), a marker of the nucleus of neurons. Epifluorescent images of DiI and TRPV1 were obtained using ×10 by 40 water-immersion objective lenses, confocal laser scanning microscopes, and imaging software (LSM510 Meta; Carl Zeiss GmbH, Jena, Germany). The grayscale values of TRPV1 immunoreactivity were measured in neurons double labeled by TRPV1 and DiI (cell size < 25 µm) in Ctrl and PNE groups with ImageJ (1.48v; NIH) software, respectively.
Cultured laryngeal C neurons in the N/J ganglia
The primary N/J ganglionic neurons were cultured as reported previously (39). Briefly, after decapitation, the rat head was immediately immersed in ice-cold DMEM/F12 solution, followed by quick extraction of N/J ganglia under a dissecting microscope. Each ganglion was desheathed, cut into eight pieces, placed in a 0.08% type IV collagenase, and incubated for 60 min in 5% CO2 in air at 37°C. The ganglion suspension was centrifuged (150 g, 5 min), and the supernatant was aspirated. The cell pellet was resuspended in 0.05% trypsin for 1 min and centrifuged (150 g, 5 min); the pellet was then resuspended in a modified DMEM/F12 solution (supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μM minimum essential medium nonessential aa) and gently triturated with a small-bore fire-polished Pasteur pipette. Myelin debris was separated and discarded after centrifugation of the dispersed cell suspension (500 g, 8 min) through a layer of 15% bovine serum albumin. The cell pellet was resuspended in the modified DMEM/F12 solution, plated onto poly-l-lysine–coated glass coverslips, and incubated overnight (5% CO2 in air at 37°C).
Single-cell real-time PCR for laryngeal C neurons
Similar to our previous report (32), laryngeal C neurons were identified by retrograde labeling, and neural size (<25 μm) was determined with fluorescence microscopy. With a negative pressure, a single cell was picked up by a glass pipette (tip inner diameter, 10 μm). The pipette tip was then broken into a PCR tube containing 5 μl single-cell lysis solution/DNAase I (Single cell-to-CT kit, 4458237; Thermo Fisher Scientific) and incubated for 5 min at room temperature. After adding 0.5 μl Stop Solution, the PCR tube was immediately snap frozen. A sample of the bath solution from the vicinity of a labeled neuron was collected from each coverslip for no-template experiments (bath negative Ctrl). In accord with the kit’s instruction manual, after reverse transcription, TRPV1 (Rn00583117_m1) and β-actin (Rn00667869_m1) primers were mixed for preamplification. TaqMan real-time PCR was then conducted on an ABI PRISM 7900 HT system (Applied Biosystems, Foster City, CA, USA) to measure TRPV1 mRNA using the ΔΔCt method. Reaction conditions were carried out as follows: 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 72°C for 30 s.
Whole-cell patch-clamp recording
The electrophysiological recording techniques used were similar to those previously described (40). All recordings were made on the neurons cultured on the coverslip. Neurons were superfused (2 ml/min) continuously with standard extracellular solution containing the following chemicals (mM): 136 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 0.33 NaH2PO4, 10 glucose, and 10 HEPES. The pH was adjusted to 7.4 with NaOH. For a Ca2+-free extracellular solution, CaCl2 was replaced with 1 mM EGTA. In some experiments, capsazepine (0.1 mM) was included in the standard extracellular solution to block TRPV1 currents induced by Cap. Whole-cell patch clamp was performed by using Axopatch 200B, Digidata 1440A, and pClamp 10.5 software (Molecular Devices, Palo Alto, CA, USA). Patch pipettes were pulled from borosilicate glass capillary tubing (G-1.5; Narishige International, East Meadow, NY, USA) with a PC-10 2-stage electrode puller (Narishige International). The pipette solution had the following composition (mM): 92 potassium gluconate, 40 KCl, 8 NaCl, 1 CaCl2, 0.5 MgCl2, 10 EGTA, and 10 HEPES. The pH was adjusted to 7.2 with KOH. The pipette resistance was 3–5 MΩ when filled with the above saline. The chemical stimulants (Cap, 0.15 or 1.5 µM) were applied through a glass pipette (tip diameter, 10 μm) by a pressure-driven microinjection system (Picospritzer II; General Valve, Fairfield, NJ, USA) with consistent parameters (10 s and 2 pounds per square inch), and the volume was 1.2 to ∼1.6 nl for each application. Laryngeal C neurons were identified by retrograde labeling, and the neural size (<25 μm) (36) was determined with fluorescence microscopy (41). Neural signals were filtered at 2 kHz and sampled at 10 kHz. Series resistance (6–18 MΩ) was monitored throughout the recordings, and data were discarded if the resistance changed by >20%. The experiments were performed at room temperature (∼22°C). Data from N/J ganglionic neurons were pooled for group analysis because no difference was found between responses of the neurons obtained from these 2 ganglia.
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Tocris Bioscience (Bristol, United Kingdom) unless otherwise stated. A stock solution of capsaicin (1 mM) was prepared in 1% Tween80, 1% ethanol, and 98% saline. The stock solution of capsazepine (100 mM) was dissolved in DMSO and diluted in standard extracellular solution to the final concentration before the experiments.
Data acquisition and statistical analysis
All variables were expressed as absolute values with the exception that the apneic, HR, MABP responses to Cap, and cell population were presented as a percentage. Group data were reported as means ± se. Two-way ANOVA was used to compare TRPV1 immunoreactivity (TRPV1-IR) in N/J ganglionic neurons with and without DiI labeling between Ctrl and PNE groups, and 1-way ANOVA or t test was used to analyze the differences between the groups. If an overall test was significant, Tukey’s test was used for specific comparisons. Values of P < 0.05 were considered significant.
RESULTS
PNE fails to alter pups’ behavior and baseline cardiorespiratory activity
The pregnant rats undergoing PNE showed no discernible behavior abnormalities, such as agitation, loss of appetite, or shortness of breath. Ctrl and PNE pups were delivered vaginally at full term (gestational d 21); no dead fetuses were found. There was no significant difference in birth numbers between Ctrl and PNE groups (11.7 ± 1.5 vs. 10.4 ± 1.9; P > 0.05). PNE did not significantly alter the pups’ body weight, body temperature, or the baseline values of VE, VT, fR, TE, HR, and MABP at an anesthetized state (Table 1).
TABLE 1.
PNE effects on BW, BT, and cardiorespiratory variables
| Variables | Ctrl, n = 6 | PNE, n = 10 |
|---|---|---|
| Body weight (g) | 32.4 ± 3.4 | 29.9 ± 3.1 |
| Body temperature (°C) | 36.6 ± 0.06 | 36.5 ± 0.04 |
| VT (ml) | 0.29 ± 0.04 | 0.28 ± 0.03 |
| fR (breaths/min) | 96.2 ± 5.9 | 106.0 ± 7.0 |
| VE (ml/min) | 28.3 ± 3.3 | 29.1 ± 4.0 |
| TE (s) | 0.56 ± 0.06 | 0.41 ± 0.06 |
| HR | 389.6 ± 32.6 | 393.1 ± 8.6 |
| MABP (mmHg) | 55.4 ± 3.7 | 62.7 ± 3.0 |
PNE aggravates the SLCF-mediated apnea and bradycardia
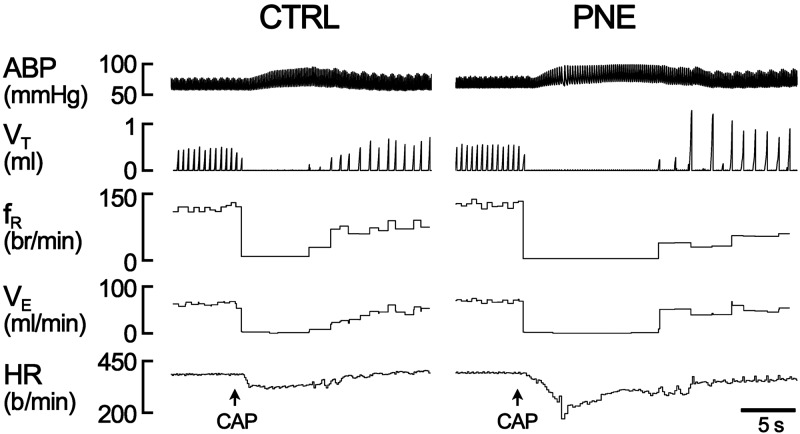
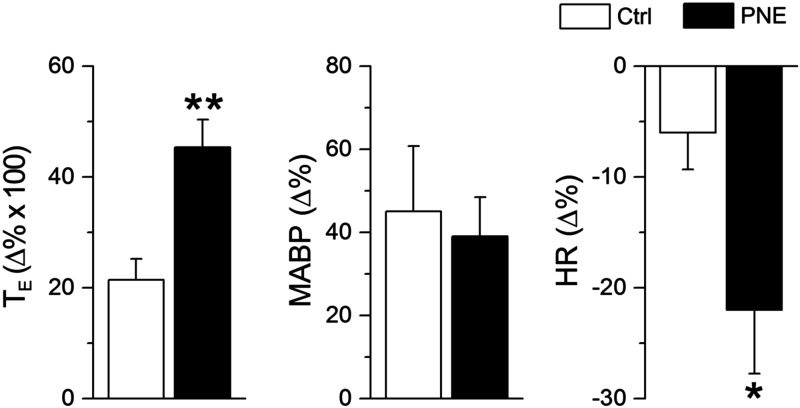
Intralaryngeal infusion of Cap (10 µg/ml, 50 µl) triggered an immediate apnea and bradycardia in all anesthetized pups. The apneic duration and the bradycardia were significantly longer and more severe in PNE pups than those in Ctrl groups. Figure 1 depicts the typical recording of the respiratory response to Cap in a Ctrl and a PNE pup, during which the Cap-induced apnea and bradycardia were markedly changed by PNE pretreatment. The corresponding group data are illustrated in Fig. 2. Clearly, PNE significantly augmented the Cap-induced apneic duration and bradycardia with little effect on the Cap-produced hypertension. These Cap-induced cardiorespiratory responses were highly reproducible by application of Cap twice in each animal, which is consistent with a previous report (42).
Figure 1.
Typical recordings of cardiorespiratory responses to intralaryngeal application of Cap (10 µg/ml, 50 µl) in a Ctrl and a PNE anesthetized and spontaneously breathing pup. In each panel, the traces from the top to bottom are arterial blood pressure (ABP), VT, fR, VE, HR, and event markers (arrows) for Cap application.
Figure 2.
Group data showing PNE effects on TE (left), MABP (middle), and HR (right) (n = 6 and 10 for Ctrl and PNE pups). Data are means ± se. *P < 0.05, **P < 0.01 vs. Ctrl.
PNE increases TRPV1 expression on N/J ganglionic neurons, especially laryngeal C neurons
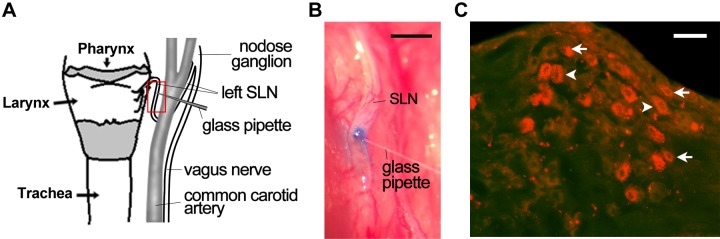
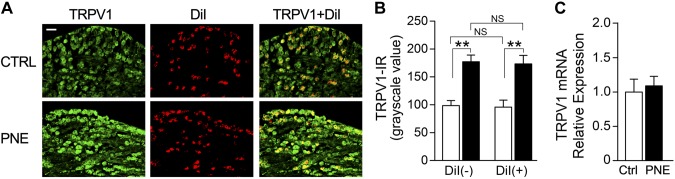
Two additional Ctrl pups were used in a pilot study to validate the feasibility of microinjection into the SLN and retrograde tracing of cell bodies of SLCFs (laryngeal C neurons). A glass pipette preloaded with Chicago Sky Blue was advanced toward the SLN in a Ctrl pup (Fig. 3A). After microinjection of Chicago Sky Blue (2%, 0.2 µl; injecting duration, 2 min), the stain was vividly visible within the SLN (Fig. 3B). The same approach was applied for microinjection of DiI in another Ctrl pup. DiI was recruited and transported to the N/J ganglionic neurons after 7 d injection (Fig. 3C). We subsequently compared the grayscale value of TRPV1 immunoreactivity in N/J ganglionic neurons and laryngeal C neurons marked by DiI with a cell size <25 μm (36) between Ctrl and PNE pups. Interestingly, PNE significantly increased TRPV1 expression in N/J ganglionic neurons, especially in laryngeal C neurons (Fig. 4A). We found only a few DiI-labeled neurons (<4%) with a cell size >25 µm in all of the pups tested in this study (Fig. 4A). The populations of DiI-labeled neurons over all of the neurons (marked by DAPI; not shown) in Ctrl and PNE pups were similar (17.63 ± 0.56 and 20.12 ± 1.52%; P > 0.05). Owing to the predominant occupation of laryngeal C neurons in the pups, we mainly focused on these neurons in the following experiments. Statistically, the grayscale value of TRPV1 expression in N/J ganglionic neurons and laryngeal C neurons were 70 and 75% higher in PNE than in Ctrl pups. Moreover, the grayscale values of TRPV1 expression in N/J ganglionic C neurons innervating the larynx (with DiI labeling) or the other organs (without DiI labeling) were not significantly different in Ctrl and PNE pups (Fig. 4B). We also found that PNE significantly elevated the population of neurons labeled by TRPV1 alone (30.83 ± 0.81% in Ctrl group vs. 44.95 ± 4.50 in the PNE group; P < 0.05) or coupled with DiI (10.26 ± 0.70 vs. 15.18 ± 1.88; P = 0.06). Therefore, PNE not only strengthened the TRPV1-IR expression in laryngeal C neurons but also tended to increase the population of these neurons expressing TRPV1. In contrast to the immunocytochemistry data, PNE did not significantly alter mRNA TRPV1 in laryngeal C neurons (n = 20 neurons for each group; P > 0.05) (Fig. 4C).
Figure 3.
Laryngeal sensory neurons marked by DiI (a retrograde tracer) injected into the SLN. A) A schematic paradigm represents the location of SLN and the SLN segment targeted by a glass pipette needle (surrounded by a red box). B) The SLN stained with Chicago Sky Blue immediately after its microinjection via the glass pipette needle in a Ctrl pup. C) Laryngeal sensory neurons (in the nodose ganglion) retrogradely traced by DiI microinjected into the SLN, in which the arrows and arrowheads point to C and Aδ neurons, respectively. The tissue section of the nodose ganglia was obtained from a Ctrl pup 7 d after DiI microinjection. Scale bars, 0.5 mm (B) and 50 µm (C).
Figure 4.
Comparison of TRPV1 expression in N/J ganglionic (especially laryngeal) C neurons between Ctrl and PNE pups. A) Examples showing nodose ganglionic neurons expressing TRPV1 (green, left) and DiI alone (red, middle) and their merging (orange, right) in a Ctrl and a PNE pup. Scale bar, 50 µm. B) Group data of grayscale value of TRPV1 immunoreactivity in all C neurons (without DiI colabeling, left pair) and laryngeal C neurons (with DiI colabeling, right pair) in the N/J ganglia from Ctrl and PNE groups (n = 6 and 10 for Ctrl and PNE pups). C) TRPV1 mRNA levels of laryngeal C neurons (with DiI labeling and diameter <25 µm) were detected in the Ctrl and PNE groups (n = 20 for each group) by single-cell RT-PCR. Data are presented as means ± se. **P < 0.01 vs. Ctrl.
PNE enhances the amplitudes of TRPV1 currents on laryngeal C neurons
After confirming the increased TRPV1 expression in laryngeal C neurons, we examined the functional effects of PNE on TRPV1 channels in these neurons. Using whole-cell patch clamp, we recorded the TRPV1 currents by application of Cap (0.15 and 1.5 µM) onto isolated laryngeal C neurons with DiI labeling and cell size <25 μm. The voltage was clamped at −70 mV, near the resting membrane potential of these neurons. As illustrated in Fig. 5, 0.15 µM Cap barely triggered effective TRPV1 currents in the Ctrl group (n = 10) but evoked significant TRPV1 currents in the PNE group (n = 10; P < 0.05). Under application of 1.5 µM Cap, a total of 22 and 25 laryngeal C neurons were recorded in 10 Ctrl and PNE pups, respectively. Among these neurons, some pups showed no response (n = 2 and 3 for Ctrl and PNE group) or a small response (<1.0 nA) (n = 6 in Ctrl and PNE pups) to Cap application. The remaining neurons (n = 14 and 16 neurons in Ctrl and PNE neurons) were sensitive to Cap with the amplitudes of TRPV1 currents much greater than 1.0 nA. The populations of the subgroups of C neurons were 13, 27, and 60% in the C neurons with no, small, and large responses in Ctrl pups and 12, 24, and 64% in PNE pups. PNE failed to significantly alter the population in each subgroup (P > 0.05). The neurons with positive response to Cap were tested as summarized here. First, the small responses were not significantly different between the groups (374.50 ± 94.95 vs. 562.33 ± 131.65 pA; P > 0.05). Interestingly, the Cap-evoked currents in the laryngeal C neurons with a large response were significantly greater in the PNE than the Ctrl group (2219.42 ± 180.07 vs. 1671.75 ± 188.06 pA; P < 0.05). The neurons with a large response were also used for the subsequent tests. Second, when applying the same concentration Cap 30 s later in the same neurons, the percentage of neurons that had effective currents was decreased to 25% in the Ctrl group but was 45% in the PNE group. Furthermore, the amplitudes of second TRPV1 currents in PNE were significantly higher than those in the Ctrl group (P < 0.01). After the application of capsazepine (0.1 mM), which is a blocker of TRPV receptors, nearly all of TRPV1 currents were abolished in the Ctrl and PNE groups. When Ca2+-free extracellular solution was superfused, TRPV1 currents in both groups were lowered, but they were still significantly greater in the PNE group than in the Ctrl group (n = 8 each group; P < 0.01).
Figure 5.
PNE effects on TRPV1 currents in laryngeal C neurons. Tracings (A, typical recordings) and graphs (B, group data), from left to right, represent TRPV1 currents in response to 0.15 µM Cap, the first and second application of 1.5 µM Cap, 1.5 µM Cap after application of capsazepine (CapZ, 0.1 mM) in the bath, and 1.5 µM Cap in the bath with Ca2+-free extracellular solution. The whole-cell patch clamp was used with voltage clamped at −70 mV. Twenty-two laryngeal C neurons from Ctrl (n = 10) and 25 laryngeal C neurons from PNE pups (n = 10) were recorded. Data are presented as means ± se. *P < 0.05 vs. Ctrl.
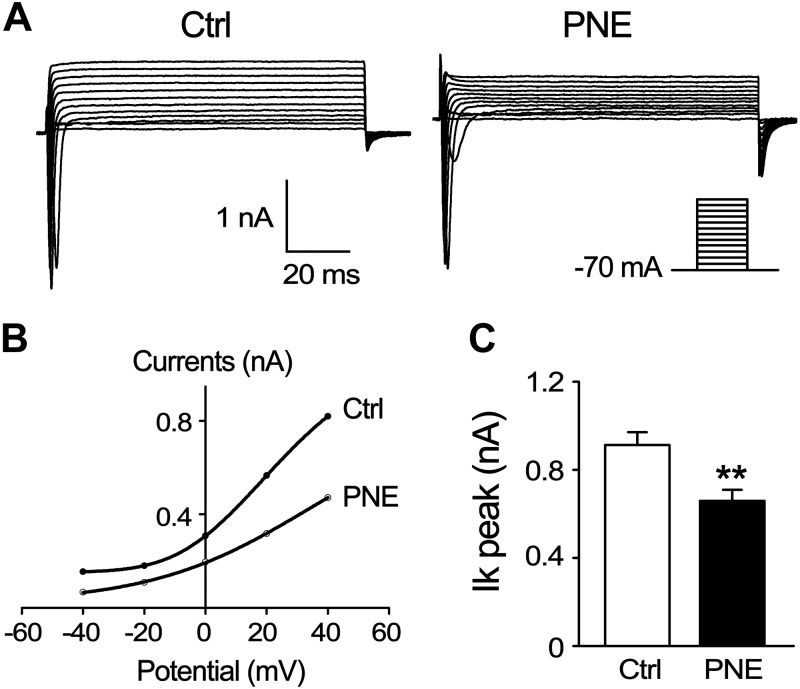
PNE depresses IKs and raises the resting potential of laryngeal C neurons
Sensory neural excitability can be affected not only by TRPV1 channel but also by electrophysiological characteristics. We found that the resting potentials were significantly higher in the laryngeal C neurons from PNE pups than those from Ctrl pups (−62.96 ± 1.87 vs. −73.30 ± 2.57 mV; P < 0.01; n = 30/group). In contrast, PNE did not change the membrane capacitance, series resistance, and action currents (Table 2). We subsequently compared IKs when the membrane potential was clamped at 12 different voltage-clamp levels in a continuous and incremental manner (−70 to 40 mV with a 10-mV increase per step and 100 ms duration). Typical recordings of IKs on a laryngeal neuron from a Ctrl and a PNE pup are represented in Fig. 6A. Statistically, PNE downshifted the current-voltage (I-V) curve of the IKs channel in laryngeal C neurons (Fig. 6B) and significantly depressed the IKs from 912.96 ± 57.33 pA (n = 31 from 10 Ctrl pups) to 659.16 ± 50.56 pA (n = 32 from 10 PNE pups) when the membrane potential was clamped at 40 mV (Fig. 6C).
TABLE 2.
Electrophysiological characteristics of SLCF-c neurons in N/J neurons
| Characteristics | Ctrl, n = 30 | PNE, n = 30 |
|---|---|---|
| Resting potential (mV) | −73.3 ± 2.6 | −63.0 ± 1.9* |
| Membrane capacitance (pF) | 31.0 ± 2.0 | 26.1 ± 2.1 |
| Series resistance (MΩ) | 6 to ∼16 | 7 to ∼15 |
| Action currents (nA) | −6.2 ± 0.5 | −6.3 ± 0.5 |
P < 0.01 vs. PNE groups.
Figure 6.
PNE effects on IKs in laryngeal C neurons. A) Typical recordings of IKs on a laryngeal C neuron (retrogradely traced) from Ctrl and PNE pups, respectively. B) I-V curve of IKs in laryngeal C neurons from the 2 groups. C) Group histogram of IKs (at −40 mV voltage clamp) in these neurons from Ctrl and PNE group.s Laryngeal C neurons from Ctrl (n = 10) and 32 laryngeal C neurons from PNE pups (n = 10) were recorded (B, C). Data are presented as means ± se. **P < 0.01 vs. Ctrl.
DISCUSSION
Previous studies have shown that PNE induces a variety of anatomic and functional abnormalities on the cardiorespiratory system, such as reduced ventilatory output, altered breathing pattern, and decreased ventilatory responses to hypoxia and/or hypercapnia (9, 43–46). The fatal events of SIDS are featured by severe bradycardia and life-threatening apneas (1, 2). Although SLCFs are known to play a key role in generating bradycardia and life-threatening apnea and have been implicated in generating SIDS, the impact of PNE on the SLCF-mediated cardiorespiratory responses remains unclear. Our major finding is that PNE significantly aggravates the SLCF-mediated apnea and bradycardia by about 2- and 4-fold, respectively. The results of this study demonstrate an augmenting effect of PNE on the SLCF-mediated apnea and bradycardia. This finding, along with our previous results showing the ability of PNE to prolong the PCF-mediated apnea (10) and the severe bradycardia and apnea occurring immediately before death in PNE rat pups (9), advances the current recognition of the toxicity of PNE on respiratory Ctrl. Most importantly, the accelerated SLCF-mediated cardiorespiratory response by PNE is potentially linked with the pathogenesis of SIDS due to the following reasons.
First, SLN-mediated apnea is more sensitive and stronger during an early age than during adulthood (47–49). This finding is in accord with the high vulnerability to SIDS seen in infants. In fact, there is a case of a SIDS victim’s sibling who showed a long-lasting apneic response to SLN stimulation and died later from SIDS (16). Second, hypoxemia is an acute precursor of SIDS (50–52) and is capable of sensitizing the SLN-mediated apnea (14, 16, 47, 53) in infants and neonatal/postnatal animals. Therefore, one can reason that when SLCFs are activated, the evoked apneic response will be greatly amplified in infants with hypoxia and maternal cigarette smoke exposure, thus contributing to SIDS pathogenesis. Third, some investigators have speculated that heat stress may be involved in the higher incidence of SIDS in prone, rather than in supine, infants (54, 55). Because TRPV1 is sensitive to heat (56), it is probable that heat stress could augment SLCF sensitization induced by PNE. Fourth, it is generally accepted that low pH triggers the SLN-mediated respiratory reflexes by acting on TRPV1 (57). The SLN-mediated apnea has long been suspected of playing a role in some SIDS victims (48). Infants regurgitate gastric contents from time to time, and the low pH of gastric acid refluxed into the larynx could trigger the apneic response (58, 59). Therefore, the SLCF-mediated apneic response to the refluxed gastric acid may be amplified by PNE, hypoxia, and hyperthermia and may thereby lead to life-threatening apneas and bradycardia as noted in SIDS victims.
An interesting result in this study is that the aggravated SLCF-mediated apnea and bradycardia by PNE is associated with up-regulation of TRPV1 expression in laryngeal C neurons (Fig. 4). We found that ∼96% of DiI-labeled N/J ganglionic neurons had small size (<25 µM) in P11–P14 rat pups, which is markedly higher than laryngeal C neurons (∼80%) in the jugular ganglia of adult rats (60). This finding suggests an age-dependent SLN development. We also found that PNE not only strengthened the TRPV1-IR expression but that this also tended to increase the population of these neurons expressing TRPV1. The lack of significant elevation of mRNA TRPV1 in laryngeal C neurons suggests an effect of PNE on synthesis of TRPV1 protein. In fact, our morphologic data also showed a parallel elevation of TRPV1-IR expression in laryngeal and nonlaryngeal sensory neurons (Fig. 4A, B). Consistent with our findings, it has been reported that PNE induces PCF plasticity, including enhancement of PCF density (10), up-regulation of neurokinin A receptor, and increase of IL-1RI in vagal pulmonary C neurons (61). Moreover, our patch-clamp data show that PNE increases the amplitudes of TRPV1 currents in laryngeal C neurons in response to Cap and sensitizes the TRPV1 receptors in low-dose Cap and Ca2+-free extracellular solution (Fig. 5), which functionally confirms the PNE effects on TRPV1. It is well known that neuronal excitability is based on firing properties that are determined by synaptic inputs and inherent membrane functions (62). In the present studies, we found that the IKs is depressed by PNE (Fig. 6), concomitant with the raised resting potential (Table 1) in laryngeal C neurons, pointing to an increased neural excitation. Clearly, our data indicate that PNE aggravates the SLCF-mediated apnea and bradycardia by up-regulation of TRPV1 expression to augment the TRPV1 currents, raising the resting potential and depressing IKs to potentiate laryngeal C neurons.
The mechanisms by which PNE increases TRPV1 expression/current and depresses IKs are unclear. It is not surprising that PNE up-regulates TRPV1 expression widely in N/J neurons. This is consistent with our previous finding that PNE induces robust TRPV1 gene and protein expressions in the entire N/J ganglion (10). Several previous studies have shown that nerve growth factor (NGF), via binding to tropomyosin receptor kinase A (63, 64), up-regulates TRPV1 (65, 66) in primary sensory fibers. Laryngeal NGF may have the same modulatory effect on SLCFs, and its TRPV1 expression is probably increased by PNE. The functional enhancement of TRPV1 by PNE may also result from TRPV1 channel sensitization in addition to its expression up-regulation. Investigators have shown that PNE promotes IL-1β and substance P release in the airways, and these factors may contribute to the potentiation of TRPV1 sensitivity and excitability (32, 61). With respect to the depression of IKs, it may be due to the alteration of prostaglandin E2 level. PNE has been reported to increase cotinine (9), which is capable of enhancing prostaglandin E2 synthesis in humans (67). Prostaglandin E2 could decrease IKs in rat sensory neurons by cAMP transduction cascade (68). The direct evidence to show the causal role of the PNE-induced prostaglandin E2 up-regulation in depressing IKs needs further study.
CONCLUSIONS
Our finding in the present study is that PNE aggravates the SLCF-mediated apnea and bradycardia through up-regulating the expression of TRPV1, augmenting TRPV1 currents, and inhibiting IKs on laryngeal C neurons. Our findings broaden the toxicology of PNE in respiratory Ctrl and provide insight into the pathogenesis of some SIDS victims. There are 3 important questions awaiting further experiments to address: 1) It is important to define whether PNE increases laryngeal NGF levels that participate in the TRPV1 up-regulation in laryngeal C neurons; 2) although the population of laryngeal Aδ fibers is low in rat pups, they may be another factor that is affected by PNE and may contribute to the prolonged apneic response induced by PNE; 3) SLN afferents terminate dominantly onto the ipsilateral interstitial nucleus tractus solitaries, and local adenosine prolongs the SLN-mediated apnea via adenosine 2A receptor mechanism (69, 70). It would be interesting to determine whether PNE increases the adenosine 2A receptor expression that is also involved in the PNE-induced prolongation of SLCF-mediated apnea.
ACKNOWLEDGMENTS
The authors thank Ellen Blake (Lovelace Respiratory Research Institute) for editing the manuscript. This study was supported by U.S. National Institutes of Health, National Heart, Lung and Blood Institute Grants HL-107462 and HL-119683. The authors declare no conflicts of interest.
Glossary
- Cap
capsaicin
- Ctrl
control
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- fR
respiratory frequency
- HR
heart rate
- IKs
slow delayed rectifier potassium current
- MABP
mean arterial blood pressure
- NGF
nerve growth factor
- N/J
nodose/jugular
- PCF
bronchopulmonary C fiber
- PNE
prenatal nicotinic exposure
- SIDS
sudden infant death syndrome
- SLCF
superior laryngeal C fiber
- SLN
superior laryngeal nerve
- TE
expiratory duration
- TRPV1
transient receptor potential vanilloid 1
- TRPV1-IR
TRPV1 immunoreactivity
- VE
minute ventilation
- VT
tidal volume
AUTHOR CONTRIBUTIONS
F. Xu designed research; X. Gao and L. Zhao performed research; X. Gao and F. Xu wrote the paper; J. Zhuang developed figures and provided necessary help for experiments; and N. Zang analyzed data.
REFERENCES
- 1.Hafström O., Milerad J., and Sundell H. W. (2002) Prenatal nicotine exposure blunts the cardiorespiratory response to hypoxia in lambs. Am. J. Respir. Crit. Care Med. 166, 1544–1549 [DOI] [PubMed] [Google Scholar]
- 2.Harper R. M., Kinney H. C., Fleming P. J., and Thach B. T. (2000) Sleep influences on homeostatic functions: implications for sudden infant death syndrome. Respir. Physiol. 119, 123–132 [DOI] [PubMed] [Google Scholar]
- 3.Shea A. K., and Steiner M. (2008) Cigarette smoking during pregnancy. Nicotine Tob. Res. 10, 267–278 [DOI] [PubMed] [Google Scholar]
- 4.Wong-Riley M. T., Liu Q., and Gao X. P. (2013) Peripheral-central chemoreceptor interaction and the significance of a critical period in the development of respiratory control. Respir. Physiol. Neurobiol. 185, 156–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra R., Singh S. P., Kracko D., Matta S. G., Sharp B. M., and Sopori M. L. (2002) Chronic self-administration of nicotine in rats impairs T cell responsiveness. J. Pharmacol. Exp. Ther. 302, 935–939 [DOI] [PubMed] [Google Scholar]
- 6.Stéphan-Blanchard E., Bach V., Telliez F., and Chardon K. (2013) Perinatal nicotine/smoking exposure and carotid chemoreceptors during development. Respir. Physiol. Neurobiol. 185, 110–119 [DOI] [PubMed] [Google Scholar]
- 7.Campos M., Bravo E., and Eugenín J. (2009) Respiratory dysfunctions induced by prenatal nicotine exposure. Clin. Exp. Pharmacol. Physiol. 36, 1205–1217 [DOI] [PubMed] [Google Scholar]
- 8.Fregosi R. F., and Pilarski J. Q. (2008) Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respir. Physiol. Neurobiol. 164, 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuang J., Zhao L., and Xu F. (2014) Maternal nicotinic exposure produces a depressed hypoxic ventilatory response and subsequent death in postnatal rats. Physiol. Rep. 2, e12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang J., Zhao L., Zang N., and Xu F. (2015) Prenatal nicotinic exposure augments cardiorespiratory responses to activation of bronchopulmonary C-fibers. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L922–L930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hishida N., Tsubone H., and Sugano S. (1997) Fiber composition of the superior laryngeal nerve in rats and guinea pigs. J. Vet. Med. Sci. 59, 499–501 [DOI] [PubMed] [Google Scholar]
- 12.Yoshida Y., Tanaka Y., Hirano M., and Nakashima T. (2000) Sensory innervation of the pharynx and larynx. Am. J. Med. 108(Suppl 4a),51S–61S [DOI] [PubMed] [Google Scholar]
- 13.Abu-Shaweesh J. M., Dreshaj I. A., Haxhiu M. A., and Martin R. J. (2001) Central GABAergic mechanisms are involved in apnea induced by SLN stimulation in piglets. J. Appl. Physiol. (1985) 90, 1570–1576 [DOI] [PubMed] [Google Scholar]
- 14.Downing S. E., and Lee J. C. (1975) Laryngeal chemosensitivity: a possible mechanism for sudden infant death. Pediatrics 55, 640–649 [PubMed] [Google Scholar]
- 15.Lee J. C., Stoll B. J., and Downing S. E. (1977) Properties of the laryngeal chemoreflex in neonatal piglets. Am. J. Physiol. 233, R30–R36 [DOI] [PubMed] [Google Scholar]
- 16.Wennergren G., Hertzberg T., Milerad J., Bjure J., and Lagercrantz H. (1989) Hypoxia reinforces laryngeal reflex bradycardia in infants. Acta Paediatr. Scand. 78, 11–17 [DOI] [PubMed] [Google Scholar]
- 17.Sant’Ambrogio G., Tsubone H., and Sant’Ambrogio F. B. (1995) Sensory information from the upper airway: role in the control of breathing. Respir. Physiol. 102, 1–16 [DOI] [PubMed] [Google Scholar]
- 18.Mutoh T., Kanamaru A., Kojima K., Nishimura R., Sasaki N., and Tsubone H. (2000) Effects of perineural capsaicin treatment on cardiopulmonary reflexes elicited by laryngeal instillations of capsaicin and distilled water in sevoflurane-anesthetized dogs. J. Vet. Med. Sci. 62, 665–668 [DOI] [PubMed] [Google Scholar]
- 19.Liu B. Y., Tsai T. L., Ho C. Y., Lu S. H., Lai C. J., and Kou Y. R. (2013) Role of TRPA1 and TRPV1 in the ROS-dependent sensory irritation of superior laryngeal capsaicin-sensitive afferents by cigarette smoke in anesthetized rats. Pulm. Pharmacol. Ther. 26, 364–372 [DOI] [PubMed] [Google Scholar]
- 20.Lee L. Y., and Gu Q. (2009) Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr. Opin. Pharmacol. 9, 243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nisenbaum E. S., and Wilson C. J. (1995) Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J. Neurosci. 15, 4449–4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varro A., Baláti B., Iost N., Takács J., Virág L., Lathrop D. A., Csaba L., Tálosi L., and Papp J. G. (2000) The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J. Physiol. 523, 67–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jost N., Papp J. G., and Varró A. (2007) Slow delayed rectifier potassium current (IKs) and the repolarization reserve. Ann. Noninvasive Electrocardiol. 12, 64–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng J. H., and Kodama I. (2004) Two components of delayed rectifier K+ current in heart: molecular basis, functional diversity, and contribution to repolarization. Acta Pharmacol. Sin. 25, 137–145 [PubMed] [Google Scholar]
- 25.Chen H., Parker S. L., Matta S. G., and Sharp B. M. (2005) Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. Eur. J. Neurosci. 22, 380–388 [DOI] [PubMed] [Google Scholar]
- 26.Benowitz N. L., and Jacob P., III. (1984) Daily intake of nicotine during cigarette smoking. Clin. Pharmacol. Ther. 35, 499–504 [DOI] [PubMed] [Google Scholar]
- 27.Luck W., Nau H., Hansen R., and Steldinger R. (1985) Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev. Pharmacol. Ther. 8, 384–395 [DOI] [PubMed] [Google Scholar]
- 28.Adams E. J., Chavez G. F., Steen D., Shah R., Iyasu S., and Krous H. F. (1998) Changes in the epidemiologic profile of sudden infant death syndrome as rates decline among California infants: 1990-1995. Pediatrics 102, 1445–1451 [DOI] [PubMed] [Google Scholar]
- 29.Ballanyi K. (2004) Neuromodulation of the perinatal respiratory network. Curr. Neuropharmacol 2, 221–243 [DOI] [PubMed] [Google Scholar]
- 30.Stephenson R., Liao K. S., Hamrahi H., and Horner R. L. (2001) Circadian rhythms and sleep have additive effects on respiration in the rat. J. Physiol. 536, 225–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y., Gu Q., Lin R. L., Kryscio R., and Lee L. Y. (2010) Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-alpha in rat pulmonary sensory neurons. Am. J. Physiol. Lung Cell. Mol. Physiol. 299, L483–L492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L., Zhuang J., Zang N., Lin Y., Lee L. Y., and Xu F. (2016) Prenatal nicotinic exposure upregulates pulmonary C-fiber NK1R expression to prolong pulmonary C-fiber-mediated apneic response. Toxicol. Appl. Pharmacol. 290, 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyer S. M., Montgomery K. L., Towne C., Lee S. Y., Ramakrishnan C., Deisseroth K., and Delp S. L. (2014) Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat. Biotechnol. 32, 274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z., Zhuang J., Zhang C., and Xu F. (2013) Isoflurane depolarizes bronchopulmonary C neurons by inhibiting transient A-type and delayed rectifier potassium channels. Respir. Physiol. Neurobiol. 186, 164–172 [DOI] [PubMed] [Google Scholar]
- 35.Pendlebury J. D., Wilson R. J., Bano S., Lumb K. J., Schneider J. M., and Hasan S. U. (2008) Respiratory control in neonatal rats exposed to prenatal cigarette smoke. Am. J. Respir. Crit. Care Med. 177, 1255–1261 [DOI] [PubMed] [Google Scholar]
- 36.Xu F., Gu Q. H., Zhou T., and Lee L. Y. (2003) Acute hypoxia prolongs the apnea induced by right atrial injection of capsaicin. J. Appl. Physiol. (1985) 94, 1446–1454 [DOI] [PubMed] [Google Scholar]
- 37.Liu B. Y., Lin Y. J., Lee H. F., Ho C. Y., Ruan T., and Kou Y. R. (2015) Menthol suppresses laryngeal C-fiber hypersensitivity to cigarette smoke in a rat model of gastroesophageal reflux disease: the role of TRPM8. J. Appl. Physiol. (1985) 118, 635–645 [DOI] [PubMed] [Google Scholar]
- 38.Lin Y. J., Lin Y. S., Lai C. J., Yuan Z. F., Ruan T., and Kou Y. R. (2013) Perivagal antagonist treatment in rats selectively blocks the reflex and afferent responses of vagal lung C fibers to intravenous agonists. J. Appl. Physiol. (1985) 114, 361–370 [DOI] [PubMed] [Google Scholar]
- 39.Gu Q. D., Moss C. R., II., Kettelhut K. L., Gilbert C. A., and Hu H. (2016) Activation of TRPV4 regulates respiration through indirect activation of bronchopulmonary sensory neurons. Front. Physiol. 7, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamill O. P., Marty A., Neher E., Sakmann B., and Sigworth F. J. (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 [DOI] [PubMed] [Google Scholar]
- 41.Potenzieri C., Meeker S., and Undem B. J. (2012) Activation of mouse bronchopulmonary C-fibres by serotonin and allergen-ovalbumin challenge. J. Physiol. 590, 5449–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang R., Xu F., and Liu J. (2008) Prenatal hypoxia preconditioning improves hypoxic ventilatory response and reduces mortality in neonatal rats. J. Perinat. Med. 36, 161–167 [DOI] [PubMed] [Google Scholar]
- 43.Huang Y. H., Brown A. R., Costy-Bennett S., Luo Z., and Fregosi R. F. (2004) Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respir. Physiol. Neurobiol. 143, 1–8 [DOI] [PubMed] [Google Scholar]
- 44.St-John W. M., and Leiter J. C. (1999) Maternal nicotine depresses eupneic ventilation of neonatal rats. Neurosci. Lett. 267, 206–208 [DOI] [PubMed] [Google Scholar]
- 45.Hafström O., Milerad J., and Sundell H. W. (2002) Altered breathing pattern after prenatal nicotine exposure in the young lamb. Am. J. Respir. Crit. Care Med. 166, 92–97 [DOI] [PubMed] [Google Scholar]
- 46.Zhao L., Zhuang J., Gao X., Ye C., Lee L. Y., and Xu F. (2016) From the cover: prenatal nicotinic exposure attenuates respiratory chemoreflexes associated with downregulation of tyrosine hydroxylase and neurokinin 1 receptor in rat pup carotid body. Toxicol. Sci. 153, 103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanier B., Richardson M. A., and Cummings C. (1983) Effect of hypoxia on laryngeal reflex apnea: implications for sudden infant death. Otolaryngol. Head Neck Surg. 91, 597–604 [DOI] [PubMed] [Google Scholar]
- 48.Xia L., Leiter J. C., and Bartlett D., Jr. (2008) Laryngeal apnea in rat pups: effects of age and body temperature. J. Appl. Physiol. (1985) 104, 269–274 [DOI] [PubMed] [Google Scholar]
- 49.Fagenholz S. A., Lee J. C., and Downing S. E. (1979) Laryngeal reflex apnea in the chemodenervated newborn piglet. Am. J. Physiol. 237, R10–R14 [DOI] [PubMed] [Google Scholar]
- 50.Daley K. C. (2004) Update on sudden infant death syndrome. Curr. Opin. Pediatr. 16, 227–232 [DOI] [PubMed] [Google Scholar]
- 51.Guntheroth W. G., and Kawabori I. (1975) Hypoxic apnea and gasping. J. Clin. Invest. 56, 1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinney H. C., and Thach B. T. (2009) The sudden infant death syndrome. N. Engl. J. Med. 361, 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia L., Leiter J. C., and Bartlett D., Jr. (2013) Laryngeal reflex apnea in neonates: effects of CO2 and the complex influence of hypoxia. Respir. Physiol. Neurobiol. 186, 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guntheroth W. G., and Spiers P. S. (2001) Thermal stress in sudden infant death: is there an ambiguity with the rebreathing hypothesis? Pediatrics 107, 693–698 [DOI] [PubMed] [Google Scholar]
- 55.Leiter J. C., and Böhm I. (2007) Mechanisms of pathogenesis in the sudden infant death syndrome. Respir. Physiol. Neurobiol. 159, 127–138 [DOI] [PubMed] [Google Scholar]
- 56.Nilius B., Owsianik G., Voets T., and Peters J. A. (2007) Transient receptor potential cation channels in disease. Physiol. Rev. 87, 165–217 [DOI] [PubMed] [Google Scholar]
- 57.Lee L. Y., Gu Q., Xu F., and Hong J. L. (2013) Acid-sensing by airway afferent nerves. Pulm. Pharmacol. Ther. 26, 491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boggs D. F., and Bartlett D., Jr. (1982) Chemical specificity of a laryngeal apneic reflex in puppies. J. Appl. Physiol. 53, 455–462 [DOI] [PubMed] [Google Scholar]
- 59.St-Hilaire M., Samson N., Nsegbe E., Duvareille C., Moreau-Bussiere F., Micheau P., Lebon J., and Praud J. P. (2007) Postnatal maturation of laryngeal chemoreflexes in the preterm lamb. J. Appl. Physiol. (1985) 102, 1429–1438 [DOI] [PubMed] [Google Scholar]
- 60.Hayakawa T., Kuwahara-Otani S., Maeda S., Tanaka K., and Seki M. (2014) Calcitonin gene-related peptide immunoreactive sensory neurons in the vagal and glossopharyngeal ganglia innervating the larynx of the rat. J. Chem. Neuroanat. 55, 18–23 [DOI] [PubMed] [Google Scholar]
- 61.Zhao L., Zhuang J., and Xu F. (2016) Bronchopulmonary C-fibers’ IL1RI contributes to the prolonged apneic response to intra-atrial injection of capsaicin by prenatal nicotinic exposure in rat pups. Toxicol. Appl. Pharmacol. 303, 58–64 [DOI] [PubMed] [Google Scholar]
- 62.Jiang C., Cummins T. R., and Haddad G. G. (1994) Membrane ionic currents and properties of freshly dissociated rat brainstem neurons. Exp. Brain Res. 100, 407–420 [DOI] [PubMed] [Google Scholar]
- 63.Kashiba H., Noguchi K., Ueda Y., and Senba E. (1995) Coexpression of trk family members and low-affinity neurotrophin receptors in rat dorsal root ganglion neurons. Brain Res. Mol. Brain Res. 30, 158–164 [DOI] [PubMed] [Google Scholar]
- 64.Mu X., Silos-Santiago I., Carroll S. L., and Snider W. D. (1993) Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J. Neurosci. 13, 4029–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anand U., Otto W. R., Casula M. A., Day N. C., Davis J. B., Bountra C., Birch R., and Anand P. (2006) The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci. Lett. 399, 51–56 [DOI] [PubMed] [Google Scholar]
- 66.Zhang X., Huang J., and McNaughton P. A. (2005) NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 24, 4211–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saareks V., Riutta A., Alanko J., Ylitalo P., Parviainen M., Mucha I., Sievi E., and Vapaatalo H. (2000) Clinical pharmacology of eicosanoids, nicotine induced changes in man. J. Physiol. Pharmacol. 51, 631–642 [PubMed] [Google Scholar]
- 68.Evans A. R., Vasko M. R., and Nicol G. D. (1999) The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones. J. Physiol. 516, 163–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duy P. M., Xia L., Bartlett D., Jr., and Leiter J. C. (2010) An adenosine A(2A) agonist injected in the nucleus of the solitary tract prolongs the laryngeal chemoreflex by a GABAergic mechanism in decerebrate piglets. Exp. Physiol. 95, 774–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia L., Bartlett D., Jr., and Leiter J. C. (2008) An adenosine A(2A) antagonist injected in the NTS reverses thermal prolongation of the LCR in decerebrate piglets. Respir. Physiol. Neurobiol. 164, 358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]