Abstract
Subclinical hypothyroidism is known to be associated with increased serum cholesterol. Since thyroid-stimulating hormone (TSH) exerts an inductor effect on cholesterol biosynthesis, we aimed to investigate the relationship between TSH mRNA and cholesterol metabolism in human adipose tissue (AT). Cross-sectionally, AT TSH-β (TSHB) mRNA was evaluated in 4 independent cohorts in association with serum total and LDL cholesterol, and AT lipidomics. Longitudinally, the effects of statins and of diet and exercise on AT TSHB mRNA were also examined. The bidirectional relationship between cholesterol and TSHB were studied in isolated human adipocytes. TSHB mRNA was consistently detected in AT from euthyroid subjects, and positively associated with serum total- and LDL-cholesterol, and with AT-specific cholesterol metabolism-associated lipids [arachidonoyl cholesteryl ester, C8-dihydroceramide, N-stearoyl-d-sphingosine, and GlcCer(18:0, 24:1)]. Reduction of cholesterol with statins and with diet and exercise interventions led to decreased TSHB mRNA in human AT, whereas excess cholesterol up-regulated TSHB mRNA in human adipocytes. In addition, recombinant human TSH α/β administration resulted in increased HMGCR mRNA levels in human adipocytes. In mice, subcutaneous AT Tshb expression levels correlated directly with circulating cholesterol levels. In summary, current results provide novel evidence of TSHB as a paracrine factor that is modulated in parallel with cholesterol metabolism in human AT.—Moreno-Navarrete, J. M., Moreno, M., Ortega, F., Xifra, G., Hong, S., Asara, J. M., Serrano, J. C. E., Jové, M., Pissios, P., Blüher, M., Ricart, W., Portero-Otin, M., Fernández-Real, J. M. TSHB mRNA is linked to cholesterol metabolism in adipose tissue.
Keywords: adipocyte, lipidomic, obesity, statins, HMGCR
A positive association between serum thyroid-stimulating hormone (TSH) levels and atherogenic lipid profile, including total cholesterol, LDL cholesterol, and fasting triglyceride levels, is well known (1–6). The effects of serum TSH on cholesterol biosynthesis have already been substantiated (7–9). Tian and collaborators (7) showed that TSH administration resulted in increased intracellular cAMP levels in parallel to increased expression of the key enzyme on cholesterol biosynthesis, 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), leading to raised intracellular and serum cholesterol in liver cells and mice, suggesting the presence of TSH/thyroid-stimulating hormone receptor (TSHR)/cholesterol axis in liver. TSH administration also inhibited the AMPK pathway, reducing AMPK activation/phosphorylation (8). Conversely, 5-aminoimidazole-4-carboxyamide ribonucleoside (an AMPK activator) administration resulted in attenuated TSH-induced cholesterol biosynthesis (9).
We hypothesized a relationship between adipose tissue (AT) TSHB mRNA and cholesterol metabolism. For this reason, we evaluated AT TSHB mRNA in 4 independent cohorts in association with serum total and LDL cholesterol. In one of these cohorts, the associations with AT lipidomics were also investigated. The bidirectional relationship between cholesterol biosynthesis and TSHB were also studied in human adipocytes and in mice, after a high-fat diet. Finally, mechanistic studies were performed to evaluate whether TSH directly modulates cholesterol biosynthesis in human adipocytes.
MATERIALS AND METHODS
Cross-sectional studies
Recruitment of patients from cross-sectional studies are described elsewhere [cohort 1 (10) and cohort 2 (11)]. In cohort 1, a group of participants with morbid obesity (BMI >35 kg/m2; n = 38) was studied. All these subjects were recruited at the Endocrinology Service of the Hospital of Girona “Dr. Josep Trueta,” were of Caucasian origin, and reported that their body weight had been stable for at least 3 mo before the study. Subjects were studied in the postabsorptive state. BMI was calculated as [weight (kg)]/[height (m)]2. The patients had no systemic disease other than obesity, and all were free of any infections in the month before the study. Liver diseases (specifically tumoral disease and hepatitis C virus infection) and thyroid dysfunction were specifically excluded by biochemical work-up. All subjects gave written informed consent, validated and approved by the Ethics Committee of the Hospital of Girona “Dr. Josep Trueta,” after the purpose of the study was explained to them. AT samples were obtained from subcutaneous AT (SAT) and visceral AT (VAT) depots during elective surgical procedures (cholecystectomy, surgery for abdominal hernia, and gastric bypass surgery). Samples of AT were immediately transported to the laboratory (5–10 min). The tissue was handled in strictly aseptic conditions. AT samples were washed in PBS, cut off with forceps and scalpel into small pieces (100 mg), and immediately flash frozen in liquid nitrogen before being stored at −80°C. In cohort 2, SAT from a group of participants (n = 73) with a wide range of obesity (age range, 25–75 yr; BMI, 19.5–85.2 kg/m2) was studied.
Intervention studies
In cohort 3, abdominal subcutaneous TSHB mRNA was studied before and after a 6-mo treatment with simvastatin (20 mg once daily) in 20 participants (statin cohort; mean age, 52.5 ± 6.9 yr; mean BMI, 42.8 ± 5.5 kg/m2; no type 2 diabetes, and concomitant antihypertensive medication with angiotensin-converting enzyme inhibitors and diuretics) (11). In addition, we measured subcutaneous TSHB mRNA before and 6 mo after a multimodal weight reduction program consisting of a restricted-calorie diet (with a caloric deficit of 800 kcal) combined with a structured (twice a week for 60 min) exercise program [cohort 4 (11), n = 15; mean age, 46.2 ± 2.5 yr; mean BMI, 34.5 ± 1.8 kg/m2; no type 2 diabetes; and no concomitant medication].
In cohorts 2, 3, and 4, all individuals were recruited at the University Hospital (Leipzig, Germany) and fulfilled the following inclusion criteria: 1) absence of any acute or chronic inflammatory disease, as determined by a leukocyte count <7000 Gpt/L, C-reactive protein <10.0 mg/dl, or clinical signs of infection; 2) undetectable antibodies against glutamic acid decarboxylase; 3) no medical history of hypertension (i.e., systolic blood pressure <140 mmHg and diastolic blood pressure <85 mmHg; 4) no clinical evidence of cardiovascular or peripheral artery disease; 5) no thyroid dysfunction; 6) no alcohol or drug abuse; and 7) no pregnancy. All anthropometric and biochemical measurements were performed as described elsewhere (11).
For cohorts 2–4, all study protocols have been approved by the Ethics Committee of the University of Leipzig. All participants gave written informed consent before taking part in the study.
AT cell separation
The isolation of adipocyte and stromal vascular fraction (SVF) cells was performed from SAT (n = 6) and VAT (n = 6) nonfrozen samples, which were washed 3–4 times with PBS and suspended in an equal volume of PBS supplemented with 1% penicillin-streptomycin and 0.1% collagenase type I warmed to 37°C. The tissue was placed in a shaking water bath at 37°C with continuous agitation for 60 min and centrifuged for 5 min at 300–500 g at room temperature. The supernatant, containing mature adipocytes, was recollected. The pellet was identified as the SVF. The CD14+ cell fraction was isolated from the SVF with magnetic immunobead technology with a column and magnetic separator, according to the manufacturer’s instructions (Miltenyi Biotec, Madrid, Spain). Isolated mature adipocytes and SVF cells were stored at −80°C for gene expression analysis.
Animal studies
To establish a direct relationship between obesity and TSH, we performed a diet-induced obesity study. Twelve male mice of 3 wk of age from an HCD/CD1 Swiss strain, obtained from Harlan Laboratories (Barcelona, Spain), were divided and individually caged in 2 groups and fed a normocaloric diet (n = 6) with 25% of calories from fat or a hypercaloric diet (n = 6) with 60% of calories from fat for 6 mo. The compositions of normocaloric and hypercaloric diets used are detailed in Supplemental Tables 1 and 2. Then, all animals were euthanized in the unfed condition, and SAT was extracted and immediately frozen under liquid nitrogen. The mice were obtained from Harlan Laboratories (Catalunya, Spain) and maintained at 23 ± 2°C under a 12:12-h light–dark cycle (lights on from 7 am to 7 pm). All mice were allowed unlimited access to their corresponding diet and water. All animal procedures followed the approved protocols from the Institutional Animal Care and Use Committee and the experiments were approved by the Ethics Committee of the University of Lleida (CEEA 18-01/12).
Human preadipocyte differentiation
Isolated human subcutaneous preadipocytes from subjects with obesity (BMI >30; Zen-Bio, Research Triangle Park, NC, USA) were cultured as previously described (12). In brief, isolated human subcutaneous preadipocytes were cultured (∼40,000 cells/cm2) in preadipocyte medium (Zen-Bio) composed of DMEM/nutrient mix F-12 (1:1, v/v), HEPES, fetal bovine serum, penicillin, and streptomycin in a humidified 37°C incubator with 5% CO2. Twenty-four hours after plating, the cells were checked for confluence (d 0), and differentiation was induced with differentiation medium (Zen-Bio) composed of preadipocyte medium, human insulin, dexamethasone, isobutylmethylxanthine, and peroxisome proliferator-activated receptor-γ agonist (rosiglitazone). After d 7, differentiation medium was replaced with fresh adipocyte medium (Zen-Bio) composed of DMEM/Nutrient Mix F-12 medium (1:1, v/v), HEPES, fetal bovine serum, biotin, pantothenate, human insulin, dexamethasone, penicillin, streptomycin, and amphotericin. Negative control (nondifferentiated cell) experiments were performed with preadipocyte medium during all differentiation processes. Fourteen days after the initiation of differentiation, cells appeared rounded with large lipid droplets apparent in the cytoplasm. Cells were then considered mature adipocytes and were harvested and stored at −80°C for RNA extraction, to study gene expression levels. The experiment was performed in triplicate for each sample. The differentiation was monitored with ADIPOQ (adiponectin, C1Q, and collagen domain containing) and FABP4 (fatty acid binding protein-4) gene expression.
Treatment of adipocytes with cholesterol
During the adipocyte differentiation experiment, cholesterol (1 and 10 μM) was added during adipocyte differentiation at each medium change (at d 0 and 7). The concentrations were chosen according to another study (12). Chloroform (0.1%) was used as the vehicle control in differentiated adipocytes.
Treatment of adipocytes with recombinant human TSH α/β
Fully differentiated subcutaneous adipocytes were treated with recombinant human TSH α/β (rhTSH, 1 and 10 mU/L) for 48 h.
RNA purification and gene expression measurements
RNA was prepared from these samples by using RNeasy Lipid Tissue Mini Kit (Qiagen, Izasa, Barcelona, Spain). For RNA purification from blood samples, whole blood was collected in PaxGene Blood RNA tubes (Qiagen). The integrity of each RNA sample was checked by the Agilent Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Total RNA was quantified by a spectrophotometer (GeneQuant; GE Health Care, Piscataway, NJ, USA) reverse transcribed to cDNA with the High Capacity cDNA Archive Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Gene expression was assessed by real-time PCR with a LightCycler 480 Real-Time PCR System (Roche Diagnostics, Barcelona, Spain), using TaqMan and SYBR green technology suitable for relative genetic expression quantification. The RT-PCR reaction was performed in a final volume of 12 μl. The cycle program consisted of an initial denaturing of 10 min at 95°C then 40 cycles of 15 s denaturizing phase at 95°C and 1 min annealing and extension phase at 60°C. A Ct value was obtained for each amplification curve and a ∆Ct value was first calculated by subtracting the Ct value for human cyclophilin A [peptidylprolyl isomerase A (PPIA)] RNA from the value for each sample. Fold changes compared with the endogenous control were then determined by calculating 2−∆∆Ct. The gene expression results are expressed as the expression ratio relative to PPIA gene expression according to the manufacturer’s guidelines.
The commercially available and prevalidated TaqMan primer and probe sets used were as follows: endogenous control cyclophilin (4333763; PPIA) and target genes such as TSHB (thyroid-stimulating hormone-β; Hs02759015_s1) and SMPD1 (sphingomyelin phosphodiesterase 1; acid lysosomal; Hs01086851_m1).
Protein preparation
To exclude blood vessel-derived TSH protein from AT samples, a piece of SAT was cleared of remaining connective tissue and blood vessels. Both crude and cleared AT proteins were extracted directly in RIPA buffer [0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40, 150 mM NaCl, and 50 mM Tris-HCl (pH 8.00)], supplemented with protease inhibitors (1 mM PMSF). Cellular debris and lipids were eliminated by centrifugation of the solubilized samples at 14,000 g for 10 min at 4°C, recovering the soluble fraction. Protein concentration was determined using the RC/DC Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA). TSHB protein was measured with a human TSH ELISA kit (ELH-TSH-1; RayBiotech, Norcross GA, USA).
Lipidomic analyses
Lipidomic analyses were performed as described in Jové et al. (12), in a subsample of consecutive subjects from cohort 1. In brief, 35–50 mg samples of AT (n = 23, either SAT or VAT) were homogenized in cold methanol (with 1 µM BHT; 1:20 w:v) at 4°C. Thereafter, we added 20 volumes of a buffer [containing 180 mM KCl, 5 mM 3-(N-morpholino) propanesulfonic acid, 2 mM EDTA, 1 mM diethylene triamine pentaacetic acid, and 1 mM butylated hydroxyl toluene (pH 7.3)] and 40 volumes of chloroform containing representative internal standard class lipids. The characteristics of these subjects did not differ significantly from those of the whole cohort.
Analytical methods
The Hitachi Cobas c711 instrument (Roche) was used to determine HDL cholesterol and total serum triglycerides. HDL cholesterol was quantified by a homogeneous enzymatic colorimetric assay through the cholesterol esterase/cholesterol oxidase/peroxidase reaction (Cobas HDLC3). Serum fasting triglycerides were measured by an enzymatic, colorimetric method with glycerol phosphate oxidase and peroxidase (Cobas TRIGL; Roche). LDL cholesterol was calculated with the Friedewald formula.
Statistical analyses
Statistical analyses were performed with SPSS 12.0 software (IBM, Armonk, NY, USA). Descriptive results of continuous variables are expressed as means ± sd for gaussian variables or median and interquartile range. Parameters that did not fulfill normal distribution criteria were log transformed to improve symmetry for subsequent analyses. The relation between variables was analyzed by simple correlation (Pearson’s test and Spearman’s test). A paired Student’s t test was used to compare the TSHB gene expression before and after intervention studies. A nonparametric test (Mann-Whitney U test) was used to analyze in vitro experiments. Values of P < 0.05 were considered statistically significant. The MassHunter Mass Profiler Professional Software (Agilent Technologies) was used to perform a nontargeted lipidomic analysis over the extracted features. For correlation analyses with TSHB expression and for partial least squares discriminant analysis of samples distributed by TSHB expression tertiles, we used the 50% more abundant feature, comprising a total of 720 molecular features, by using the Metaboanalyst v.3.0 platform (13).
RESULTS
Cross-sectional studies
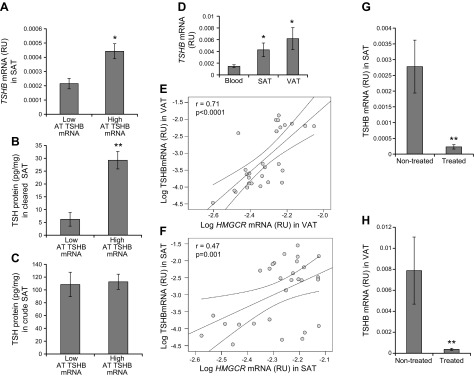
In cohort 1, AT TSHB gene expression was directly associated with total and LDL cholesterol (Table 1). AT TSHB gene expression was associated with TSH protein levels in cleared but not in crude AT (Fig. 1A–C). TSHB was significantly more expressed in both SAT and VAT compared to other white cell–enriched tissues, such as whole blood (Fig. 1D). Both VAT and SAT TSHB correlated positively with AT HMGCR mRNA levels (Fig. 1E, F), the key enzyme in cholesterol biosynthesis (7, 8). In fact, subjects taking statins from cohort 1 had decreased TSHB gene expression in SAT (11.2-fold decreased; Fig. 1G) and VAT (16.8-fold decreased; Fig. 1H) in parallel to decreased total cholesterol (4.56 ± 0.3 vs. 5.02 ± 0.8 mM; P = 0.04) and similar circulating TSH levels (P = 0.915). Otherwise, no correlations were found between both VAT and SAT TSHR gene expression and total (r = 0.23, P = 0.2 in VAT; r = 0.26, P = 0.1 in SAT) and LDL cholesterol (r = 0.27, P = 0.1 in VAT; r = 0.28, P = 0.08 in SAT) or TSHB (r = 0.14, P = 0.4 in VAT; r = 0.28, P = 0.08 in SAT) and HMGCR mRNA (r = 0.18, P = 0.2 in VAT; r = 0.06, P = 0.7 in SAT) levels.
TABLE 1.
Anthropometric and clinical parameters in all cohorts and bivariate correlations between AT TSHB gene expression and clinical parameters in all study cohorts
| Study cohort | n | Sex, M/F (n) | Age (yr) | BMI (kg/m2) | Fasting glucose (mg/dl) | Total cholesterol (mg/dl) | LDL cholesterol (mg/dl) | HDL cholesterol (mg/dl) | Fasting triglycerides (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 | |||||||||
| All subjects | 38 | 10/28 | 47.7 ± 8.9 | 45.1 ± 6.4 | 98.5 (90–105.2)a | 192.8 ± 32.8 | 122.3 ± 30.1 | 45.9 ± 10.3 | 106.5 (76.2–152.2)a |
| r | — | — | −0.09 | 0.19 | −0.26 | 0.36 | 0.43 | −0.16 | 0.11 |
| P | — | — | 0.6 | 0.2 | 0.1 | 0.02 | 0.006 | 0.3 | 0.5 |
| Cohort 2 | |||||||||
| All subjects | 73 | 28/45 | 52.2 ± 7.3 | 46.5 ± 8.1 | 100.4 ± 8.4 | 204 ± 38.1 | 128 ± 25.8 | 48.7 ± 9.8 | 129 ± 39 |
| r | — | — | −0.1 | 0.15 | −0.13 | 0.531 | 0.610 | −0.324 | 0.09 |
| P | — | — | 0.5 | 0.3 | 0.4 | <0.0001 | <0.0001 | <0.0001 | 0.7 |
| Cohort 3 | |||||||||
| Baseline | 20 | 12/8 | 52.5 ± 6.9 | 42.8 ± 5.5 | 94.3 ± 8 | 262 ± 43 | 147 ± 24 | 42 ± 10 | 156 ± 42 |
| 6 mo | 20 | 12/8 | — | 43.1 ± 5.7 | 97.2 ± 12 | 224 ± 37 | 133 ± 29 | 46 ± 11 | 136 ± 55 |
| P | — | — | — | 0.8 | 0.6 | 0.04 | 0.03 | 0.05 | 0.2 |
| Cohort 4 | |||||||||
| Before weight loss | 15 | 6/9 | 46.2 ± 2.5 | 34.5 ± 1.8 | 99.5 ± 6.2 | 193 ± 21 | 115 ± 26 | 43 ± 8.5 | 121 ± 27 |
| After weight loss | 15 | 6/9 | — | 32.8 ± 2.1 | 93.9 ± 9.5 | 199 ± 32 | 118 ± 28 | 49 ± 5.6 | 115 ± 24 |
| P | — | — | — | 0.03 | 0.14 | 0.4 | 0.7 | 0.04 | 0.2 |
Values are presented as means ± SD, or as indicated. Italic P values indicate statistical significance (P < 0.05). F, female; M, male. aValues are presented as median and interquartile range (in parentheses).
Figure 1.
A–C) SAT TSHB mRNA (A) and TSH protein levels in both cleared (B) and crude (C) SAT, according to TSH mRNA levels (n = 6). *P < 0.05 and **P < 0.01 vs. subjects with low AT TSHB mRNA. D) TSHB mRNA in blood, SAT, and VAT. *P < 0.05 vs. TSHB mRNA in blood. E, F) Bivariate correlation between TSHB and HMGCR gene expression in both VAT (E) and SAT (F). G, H) Effects of statins treatment on SAT (G) and VAT (H) TSHB mRNA levels in cohort 1. **P < 0.01 vs. nontreated subjects.
Confirming these associations, TSHB mRNA levels were consistently detected in AT from euthyroid subjects and were significantly and positively associated with total cholesterol (r = 0.531; P < 0.0001) and LDL cholesterol (r = 0.610; P < 0.0001) and inversely with HDL cholesterol (r = −0.324; P < 0.0001) in an independent cross-sectional study from Germany for validation purposes.
Nontargeted lipidomic analyses
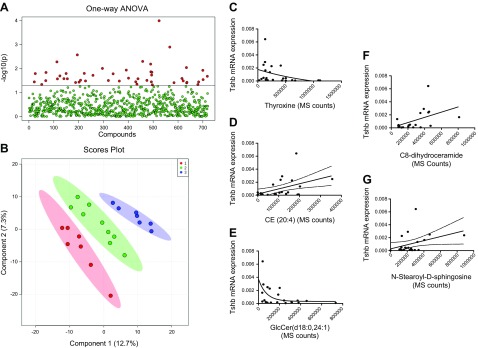
Nontargeted lipidomic analyses revealed that, when AT samples were considered in tertiles according to TSHB expression levels, a differential lipidomic signature was present, showing 48 differential molecules by ANOVA analyses (Fig. 2A and Table 2). A PLS-DA model was defined with high accuracy (Fig. 2B), disclosing a significant association between TSHB levels and changes in lipid composition across ATs. Correlation analyses of TSHB mRNA disclosed interesting associations with thyroxine (Fig. 2C) and with selected lipids (Fig. 2D–G), especially arachidonoyl cholesteryl ester (r = 0.46, P = 0.02), in line with the associations with circulating cholesterol levels. TSHB expression levels were also positively associated with sphingolipid family members such as C8-dihydroceramide (r = 0.47; P = 0.02) and N-stearoyl-d-sphingosine (r = 0.40; P = 0.04), consistent with the role of thyroid hormones in sphingolipid metabolism (14). In fact, SAT and VAT TSHβ were negatively linked to SMPD1 mRNA levels (r = −0.58 in SAT and r = −0.61 in VAT; P < 0.0001). Nonetheless, TSHB expression levels showed some negative relationships with other, structurally related lipids, such as GlcCer (18:0, 24:1) (r = −0.50; P < 0.01). In addition, SAT cholesterol levels were associated with TSHB (r = 0.51; P = 0.08) and HMGCR (r = 0.58; P = 0.04) mRNA levels.
Figure 2.
A, B) AT samples, distributed according TSHB expression tertiles, show a differential lipidomic profile, either by evaluating differential molecules by ANOVA, as shown by the number of lipids with a P < 0.05 (Fisher’s least significant difference) (A) and by a partial least significant discriminant analyses (with 1, 2, and 3 being the groups with increasing TSHB expression) (B). C–G) Bivariate correlation between TSHB gene expression and thyroxine (C) AT CE (20:4) (D), GlcCer (18:0, 24:1) (E), C8-dihydroceramide (F), and N-stearoyl-d-sphingosine (G) in cohort 1.
TABLE 2.
Differential molecules in AT according to tertiles of TSH expression abundance
| Potential ID | Retention time (min) | P | −log10(P) |
|---|---|---|---|
| 1423.3826 | 84.26165 | 0.00010047 | 3.998 |
| 10Z,13Z,16Z-nonadecatrienenitrile | 41.692455 | 0.0012567 | 2.9008 |
| GPEtn(25:0,26:0)[U] | 83.58068 | 0.0026608 | 2.575 |
| 32-Hydroxylanosterol | 66.44584 | 0.0049786 | 2.3029 |
| Pregn-4-ene-3a,20a-diol | 48.344715 | 0.0052011 | 2.2839 |
| 25-Azavitamin,D3 | 68.515366 | 0.0064387 | 2.1912 |
| 1-(9Z-octadecenoyl)-2-(5Z,8Z,11Z,14Z-eicosatetraenoyl)-sn-glycerol | 47.13082 | 0.0090018 | 2.0457 |
| C33,H69,N7,O2,S | 47.140064 | 0.0097668 | 2.0102 |
| 879.8339 | 84.15206 | 0.011458 | 1.9409 |
| 1,2,3-Trihexadecanoyl-sn-glycerol | 82.50817 | 0.011731 | 1.9307 |
| 25-Azacholesterol | 85.019714 | 0.01352 | 1.869 |
| 20:2,Cholesteryl,ester | 58.909252 | 0.014091 | 1.8511 |
| TG(17:0,18:0,20:5(5Z,8Z,11Z,14Z,17Z))[iso6] | 82.27365 | 0.014411 | 1.8413 |
Potential ID was attributed based on exact mass, isotope distribution, and chromatographic behavior in comparison to internal standard class lipids.
Intervention studies
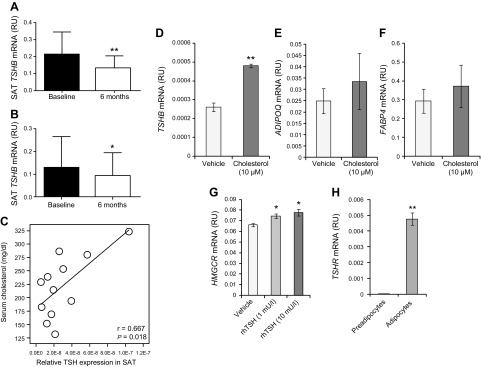
In cohort 3, the lowering of serum total and LDL cholesterol with statins (20 mg simvastatin) in humans resulted in a 35% reduction of TSHB gene expression in abdominal SAT compared with baseline (Fig. 3A). In cohort 4, diet- and exercise-induced weight loss resulted in lowering of TSHB gene expression in abdominal SAT (21% compared to baseline; Fig. 3B) in parallel to reduction of serum total and LDL cholesterol (data not shown).
Figure 3.
A, B) TSHB gene expression in abdominal SAT at baseline and 6 mo after treatment with statins (20 mg simvastatin) (A) and diet/exercise intervention (B). *P < 0.05 and **P < 0.01 vs. baseline. C) Linear regression analyses of the associations between serum cholesterol and Tshb mRNA levels in SAT from mice. D–F) Effects of cholesterol administration in excess (10 μM) on TSHB (D), ADIPOQ (E), and FABP4 (F). **P < 0.01. G) Effects of rhTSH (1 and 10 mU/L) administration on HMGCR mRNA levels, a key enzyme in cholesterol biosynthesis. *P < 0.05 vs. vehicle. Multiple comparisons were performed by Dunnett’s test. H) TSHR mRNA levels in fully differentiated adipocytes vs. preadipocytes. **P < 0.01 vs. preadipocytes.
Mouse results
SAT Tshb expression levels correlated directly with circulating cholesterol levels (Fig. 3C), even though no significant differences in SAT Tshb expression, and total HDL and LDL cholesterol levels were observed when comparing the high-fat diet and control diets (data not shown).
AT cell fractions
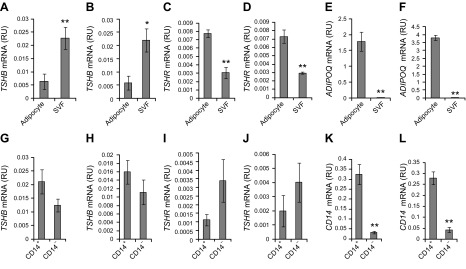
To identify the cells contributing AT TSHB gene expression, AT cell fractions were analyzed. In both VAT and SAT, TSHB mRNA was increased in cells from the SVF (Fig. 4A, B), whereas TSHR was increased in adipocytes (Fig. 4C, D). As expected, ADIPOQ mRNA was increased in adipocytes (Fig. 4E, F). In cells from the SVF, no significant differences were found on TSHB (Fig. 4G, H) and TSHR (Fig. 4I, J) mRNA comparing CD14+ and CD14− cells (Fig. 4K, L).
Figure 4.
A–F) TSHB (A, B), TSHR (C, D), and ADIPOQ (E, F), gene expression in adipocytes and SVF in both VAT (A, C, E) and SAT (B, D, F). *P < 0.05 and **P < 0.01 vs. adipocytes. G–L) TSHB (G, H), TSHR (I, J), and CD14 (K, L) gene expression in CD14+ and CD14− cells from the SVF in both VAT (G, I, K) and SAT (H, J, L). **P < 0.01 vs. CD14+.
In vitro experiments
The addition of excess cholesterol during differentiation of human adipocytes led to up-regulation of TSHB mRNA levels compared with differentiated cells from SAT in a standard protocol (1.8-fold increased, Fig. 3D), without significant effects on adipogenic mRNAs (ADIPOQ and FABP4; Fig. 3E, F). Otherwise, rhTSH (1 and 10 mU/L; 48 h) administration resulted in increased HMGCR mRNA levels in fully differentiated adipocytes (Fig. 3G), but not in preadipocytes. The expression of TSHR was 100-fold greater in adipocytes vs. preadipocytes (Fig. 3H).
DISCUSSION
To the best of our knowledge, this is the first study showing expression of TSHB (mRNA and protein) in human AT, specifically in the SVF. In line with previous studies (14, 15), TSHR gene expression was also demonstrated in human AT, being significantly increased in adipocytes.
The current study provides several points of in vivo and in vitro evidence that support a bidirectional relationship between AT TSHB gene expression and cholesterol metabolism. AT TSHB was associated with total and LDL cholesterol in 4 independent studies in humans. These associations were confirmed with AT levels of arachidonoyl cholesteryl ester, C8-dihydroceramide, N-stearoyl-d-sphingosine, and GlcCer (18:0, 24:1) (16). Supporting these observations, subjects taking statins had decreased TSHB gene expression in SAT and VAT in this small cross-sectional study in Spanish patients and in SAT in a longitudinal study in German subjects, paralleling the decreased in total and LDL cholesterol. Moreover, experiments in mouse and in human adipocytes in vitro confirmed the bidirectional enhancer effects of cholesterol on adipose TSHB gene expression and of TSH on adipocyte cholesterol biosynthesis. These findings suggest a contribution of SVF-derived TSH on adipocyte cholesterol biosynthesis.
It is well known that the liver is the main contributor to the circulating pool of cholesterol, whereas AT cholesterol is a minor contributor. In fact, HMGCR mRNA levels were increased 10-fold (P < 0.0001) in liver vs. AT (data not shown).
Serum TSH concentration is a well-known predictor of cardiovascular disease in patients with subclinical hypothyroidism (1, 17) and has been linked to increased risk of coronary atherosclerosis and increased arterial stiffness in euthyroid postmenopausal women (18, 19) and in the general population (20).
Circulating LDL, especially when oxidized, acts as a proinflammatory factor in adipocytes, by inducing endoplasmic reticulum (ER) stress associated with free cholesterol up-regulation (21). In fact, TSH acts as an inducer of endoplasmic reticulum stress, as shown in experimental subclinical hypothyroidism (22), closely linked with lipid disorders. Therefore, current findings showing the association between circulating cholesterol and TSHB expression in AT could indicate its role as a signal for ER stress. Of note, this cellular response has a physiologic aspect, trying to restore homeostasis. If the cause of the stress is not overcome, then ER stress becomes pathologic, being linked to inflammation.
The relationship between AT TSH levels and inflammatory response is supported indirectly by correlation with concentration of AT lipids related to inflammation, including those of the ceramide pathway. Thus, arachidonoyl cholesteryl ester is considered a precursor of cytotoxic oxidative byproducts (23), including some endoperoxides with signaling properties. Similarly, in line with the above-reported association with ER stress, it is known that in Mϕs, C8-dihydroceramide is an immunomodulator, by down-regulating TNF inflammatory response via ER stress (24). Increased sphingosine levels, associated with increased TSHB, could pave the way for increased ceramide concentration in AT. It is known that AT ceramide concentrations could be relevant mediators of fatty liver and insulin resistance, in that they are considered markers of AT inflammatory status (25).
The effects of serum TSH on cholesterol biosynthesis have already been demonstrated in liver cells (7–9), even though studies exploring the effects of cholesterol on TSHB expression are scarce. A high cholesterol diet in rats for 28 d resulted in significantly increased TSH biosynthesis in pituitary basophilic cells in parallel to increased serum LDL cholesterol and pituitary cholesterol content (26).
Our data support the potential occurrence of a TSH-mediated, local circuit of regulation of cholesterol synthesis in AT. Very early data supported the occurrence of AT cholesterol synthesis that runs in parallel with adipocyte size (27, 28). Starvation resulted in a marked loss of both cholesterol synthesis and cholesterol levels in AT (27, 28). Current data showing decreasing AT TSH with weight loss, and TSH induction of HMGCR in adipocytes support the notion that AT production of TSH could be a local mediator linking caloric intake to cholesterol synthesis. Serum cholesterol could serve as a signal of calorie abundance, which may be interpreted by AT as a need for increased size and local cholesterol synthesis, maybe via local TSH production. However, when a given limit is reached, serum cholesterol may act as a proinflammatory factor, as shown in nonhuman primates for visceral AT (29). This effect was specific for visceral AT, because SAT showed relative resistance toward cholesterol-induced inflammation. Our lipidomic data showing that relationships of AT cholesterol levels with TSHB and HMGCR expression was found in the subcutaneous depot, but not in the visceral depot, support a region-specific relationship of cholesterol synthetic pathways, TSH expression, and inflammation.
In the adenohypophysis, TSH production is in feedback equilibrium with T4 and T3 hormones. We found an inverse association between thyroxine and TSH mRNA in AT. Considering that thyroid hormones are up-regulated by cold exposure, it may be hypothesized that local TSH production related to low thyroid hormones is linked to an evolutionary response toward heat conservation by increasing the AT depot. Given the relationship between cholesterol content and AT size, this increased amount of AT would require increased local cholesterol synthesis. Conversely, thyroid hormones could inhibit cholesterol synthesis by decreasing TSH production (30). Of note, increased local cholesterol synthesis occurs with ongoing adipogenesis (28), and current data showed that cholesterol added exogenously works as stimulus for TSH production.
The possible molecular mechanisms that underlie the relationship between cholesterol and AT TSHB expression should be studied further, with the potential influences of peripheral thyroid hormones interacting with the obesity status. In this sense, it is known that the GATA2 transcription factor, an adipogenic modulator partially controlled by insulin (31), is a major ligand of the TSH promoter region (32). According to these mechanisms, insulin would enhance GATA2 transduction and TSHB expression. In fact, at the circulating level, there is an interplay among serum TSH levels, dyslipidemia, and insulin sensitivity (5). Whether this is the case in AT is still unknown.
In summary, current results provide novel evidence of TSHB as a factor modulated in parallel to cholesterol metabolism in human AT.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank A. Casañé (Institut de Recerca Biomèdica de Lleida) and E. Loshuertos and O. Rovira [Endocrinology, Institut d’Investigació Biomèdica de Girona (IdIBGi)] for technical help. We are indebted to the IdiBGi Biobank, integrated in the Spanish National Biobank Network, for the sample and data procurement. This work was partially supported by research grant PI15/01934 from the Instituto de Salud Carlos III from Spain, and was also supported by Fondo Europeo de Desarrollo Regional (FEDER). Centro de Investigación Biomédica en Red (CIBER), Fisiopatología de la Obesidad y Nutrición (OBN) is an initiative of the Instituto de Salud Carlos III of Spain. This study was also partially funded by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK083694 (to P.P.). The authors declare no conflicts of interest.
Glossary
- ADIPOQ
adiponectin, C1Q, and collagen domain containing
- AT
adipose tissue
- BMI
body mass index
- ER
endoplasmic reticulum
- FABP4
fatty acid binding protein 4
- HMGCR
hydroxy-3-methylglutaryl-CoA reductase
- PPIA
peptidylprolyl isomerase A
- SAT
subcutaneous adipose tissue
- SMPD1
sphingomyelin phosphodiesterase 1
- TSH
thyroid-stimulating hormone
- TSHR
thyroid-stimulating hormone receptor
- VAT
visceral adipose tissue
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. M. Moreno-Navarrete researched the data and wrote and edited the manuscript; M. Moreno, F. Ortega, G. Xifra, S. Hong, J. M. Asara, J. C. E. Serrano, and M. Jové researched the data and reviewed the manuscript; P. Pissios, M. Blüher, W. Ricart, and M. Portero-Otin researched the data, contributed to discussions, and reviewed the manuscript; and J. Manuel-Fernández-Real researched the data and wrote and reviewed the manuscript.
REFERENCES
- 1.Gao N., Zhang W., Zhang Y. Z., Yang Q., Chen S. H. (2013) Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis 227, 18–25 [DOI] [PubMed] [Google Scholar]
- 2.Witte T., Ittermann T., Thamm M., Riblet N. B., Völzke H. (2015) Association between serum thyroid-stimulating hormone levels and serum lipids in children and adolescents: a population-based study of german youth. J. Clin. Endocrinol. Metab. 100, 2090–2097 [DOI] [PubMed] [Google Scholar]
- 3.Zhao M., Yang T., Chen L., Tang X., Guan Q., Zhang B., Zhang X., Zhang H., Wang C., Xu J., Hou X., Li Q., Yu C., Zhao Y., Fang L., Yuan Z., Xue F., Ning G., Gao L., Xu C., Zhao J. (2015) Subclinical hypothyroidism might worsen the effects of aging on serum lipid profiles: a population-based case-control study. Thyroid 25, 485–493 [DOI] [PubMed] [Google Scholar]
- 4.Santos-Palacios S., Brugos-Larumbe A., Guillén-Grima F., Galofré J. C. (2013) A cross-sectional study of the association between circulating TSH level and lipid profile in a large Spanish population. Clin. Endocrinol. (Oxf.) 79, 874–881 [DOI] [PubMed] [Google Scholar]
- 5.Bakker S. J., ter Maaten J. C., Popp-Snijders C., Slaets J. P., Heine R. J., Gans R. O. (2001) The relationship between thyrotropin and low density lipoprotein cholesterol is modified by insulin sensitivity in healthy euthyroid subjects. J. Clin. Endocrinol. Metab. 86, 1206–1211 [DOI] [PubMed] [Google Scholar]
- 6.Wang F., Tan Y., Wang C., Zhang X., Zhao Y., Song X., Zhang B., Guan Q., Xu J., Zhang J., Zhang D., Lin H., Yu C., Zhao J. (2012) Thyroid-stimulating hormone levels within the reference range are associated with serum lipid profiles independent of thyroid hormones. J. Clin. Endocrinol. Metab. 97, 2724–2731 [DOI] [PubMed] [Google Scholar]
- 7.Tian L., Song Y., Xing M., Zhang W., Ning G., Li X., Yu C., Qin C., Liu J., Tian X., Sun X., Fu R., Zhang L., Zhang X., Lu Y., Zou J., Wang L., Guan Q., Gao L., Zhao J. (2010) A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology 52, 1401–1409 [DOI] [PubMed] [Google Scholar]
- 8.Zhang X., Song Y., Feng M., Zhou X., Lu Y., Gao L., Yu C., Jiang X., Zhao J. (2015) Thyroid-stimulating hormone decreases HMG-CoA reductase phosphorylation via AMP-activated protein kinase in the liver. J. Lipid Res. 56, 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S., Jing F., Yu C., Gao L., Qin Y., Zhao J. (2015) AICAR-induced activation of AMPK inhibits TSH/SREBP-2/HMGCR pathway in liver. PLoS One 10, e0124951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton N. M., Beltram J., Carter R. N., Michailidou Z., Gorjanc G., McFadden C., Barrios-Llerena M. E., Rodriguez-Cuenca S., Gibbins M. T., Aird R. E., Moreno-Navarrete J. M., Munger S. C., Svenson K. L., Gastaldello A., Ramage L., Naredo G., Zeyda M., Wang Z. V., Howie A. F., Saari A., Sipilä P., Stulnig T. M., Gudnason V., Kenyon C. J., Seckl J. R., Walker B. R., Webster S. P., Dunbar D. R., Churchill G. A., Vidal-Puig A., Fernandez-Real J. M., Emilsson V., Horvat S. (2016) Genetic identification of thiosulfate sulfurtransferase as an adipocyte-expressed antidiabetic target in mice selected for leanness. Nat. Med. 22, 771–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakaroun R., Raschpichler M., Klöting N., Oberbach A., Flehmig G., Kern M., Schön M. R., Shang E., Lohmann T., Dreßler M., Fasshauer M., Stumvoll M., Blüher M. (2012) Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 61, 706–714 [DOI] [PubMed] [Google Scholar]
- 12.Jové M., Moreno-Navarrete J. M., Pamplona R., Ricart W., Portero-Otín M., Fernández-Real J. M. (2014) Human omental and subcutaneous adipose tissue exhibit specific lipidomic signatures. FASEB J. 28, 1071–1081 [DOI] [PubMed] [Google Scholar]
- 13.Xia J., Sinelnikov I. V., Han B., Wishart D. S. (2015) MetaboAnalyst 3.0: making metabolomics more meaningful. Nucleic Acids Res. W1, 251–257https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25897128&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draman M. S., Stechman M., Scott-Coombes D., Dayan C. M., Rees D. A., Ludgate M., Zhang L. (2017) The role of thyrotropin receptor activation in adipogenesis and modulation of fat phenotype. Front. Endocrinol. (Lausanne) 8, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu S., Guan Q., Liu Y., Wang H., Xu W., Li X., Fu Y., Gao L., Zhao J., Wang X. (2012) Role of extrathyroidal TSHR expression in adipocyte differentiation and its association with obesity. Lipids Health Dis. 11, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babenko N. A., Natarova Y. A. (1999) Role of thyroid hormones in regulation of sphingomyelin metabolism in the liver. Biochemistry (Mosc.) 64, 912–915 [PubMed] [Google Scholar]
- 17.Delitala A. P., Fanciulli G., Maioli M., Delitala G. (2016). Subclinical hypothyroidism, lipid metabolism and cardiovascular disease. Eur. J. Intern. Med. 38, 17–24 [DOI] [PubMed] [Google Scholar]
- 18.Chon S. J., Heo J. Y., Yun B. H., Jung Y. S., Seo S. K. (2016) Serum thyroid stimulating hormone levels are associated with the presence of coronary atherosclerosis in healthy postmenopausal women. J. Menopausal Med. 22, 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambrinoudaki I., Armeni E., Rizos D., Georgiopoulos G., Kazani M., Alexandrou A., Deligeoroglou E., Livada A., Psychas C., Creatsa M., Bouboulis G., Alevizaki M., Stamatelopoulos K. (2012) High normal thyroid-stimulating hormone is associated with arterial stiffness in healthy postmenopausal women. J. Hypertens. 30, 592–599 [DOI] [PubMed] [Google Scholar]
- 20.Ittermann T., Lorbeer R., Dörr M., Schneider T., Quadrat A., Heßelbarth L., Wenzel M., Lehmphul I., Köhrle J., Mensel B., Völzke H. (2016) High levels of thyroid-stimulating hormone are associated with aortic wall thickness in the general population. Eur. Radiol. 26, 4490–4496 [DOI] [PubMed] [Google Scholar]
- 21.Song G., Wu X., Zhang P., Yu Y., Yang M., Jiao P., Wang N., Song H., Wu Y., Zhang X., Liu H., Qin S. (2016) High-density lipoprotein inhibits ox-LDL-induced adipokine secretion by upregulating SR-BI expression and suppressing ER stress pathway. Sci. Rep. 6, 30889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou L., Ding S., Li Y., Wang L., Chen W., Bo T., Wu K., Li C., Liu X., Zhao J., Xu C., Gao L. (2016) Endoplasmic reticulum stress may play a pivotal role in lipid metabolic disorders in a novel mouse model of subclinical hypothyroidism. Sci. Rep. 6, 31381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter N. A., Caldwell S. E., Mills K. A. (1995) Mechanisms of free radical oxidation of unsaturated lipids. Lipids 30, 277–290 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Park N. Y., Jang Y., Ma A., Jiang Q. (2015) Vitamin E γ-tocotrienol inhibits cytokine-stimulated NF-κB activation by induction of anti-inflammatory A20 via stress adaptive response due to modulation of sphingolipids. J. Immunol. 195, 126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolak M., Westerbacka J., Velagapudi V. R., Wågsäter D., Yetukuri L., Makkonen J., Rissanen A., Häkkinen A. M., Lindell M., Bergholm R., Hamsten A., Eriksson P., Fisher R. M., Oresic M., Yki-Järvinen H. (2007) Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 56, 1960–1968 [DOI] [PubMed] [Google Scholar]
- 26.Yang J., Zhang X., Liu Z., Yuan Z., Song Y., Shao S., Zhou X., Yan H., Guan Q., Gao L., Zhang H., Zhao J. (2016) High-cholesterol diet disrupts the levels of hormones derived from anterior pituitary basophilic cells. J. Neuroendocrinol. 28, 12369 [DOI] [PubMed] [Google Scholar]
- 27.Miettinen T. A., Tilvis R. S. (1981) Cholesterol synthesis and storage in adipose tissue. Int. J. Obes. 5, 613–618 [PubMed] [Google Scholar]
- 28.Kovanen P. T., Nikkilä E. A., Miettinen T. A. (1975) Regulation of cholesterol synthesis and storage in fat cells. J. Lipid Res. 16, 211–223 [PubMed] [Google Scholar]
- 29.Chung S., Cuffe H., Marshall S. M., McDaniel A. L., Ha J. H., Kavanagh K., Hong C., Tontonoz P., Temel R. E., Parks J. S. (2014) Dietary cholesterol promotes adipocyte hypertrophy and adipose tissue inflammation in visceral, but not in subcutaneous, fat in monkeys. Arterioscler. Thromb. Vasc. Biol. 34, 1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haluzik M., Nedvidkova J., Bartak V., Dostalova I., Vlcek P., Racek P., Taus M., Svacina S., Alesci S., Pacak K. (2003) Effects of hypo- and hyperthyroidism on noradrenergic activity and glycerol concentrations in human subcutaneous abdominal adipose tissue assessed with microdialysis. J. Clin. Endocrinol. Metab. 88, 5605–5608 [DOI] [PubMed] [Google Scholar]
- 31.Menghini R., Marchetti V., Cardellini M., Hribal M. L., Mauriello A., Lauro D., Sbraccia P., Lauro R., Federici M. (2005) Phosphorylation of GATA2 by Akt increases adipose tissue differentiation and reduces adipose tissue-related inflammation: a novel pathway linking obesity to atherosclerosis. Circulation 111, 1946–1953 [DOI] [PubMed] [Google Scholar]
- 32.Ohba K., Sasaki S., Matsushita A., Iwaki H., Matsunaga H., Suzuki S., Ishizuka K., Misawa H., Oki Y., Nakamura H. (2011) GATA2 mediates thyrotropin-releasing hormone-induced transcriptional activation of the thyrotropin β gene. PLoS One 6, e18667 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.