Abstract
The function of most human long noncoding RNAs (lncRNAs) remains unclear. Our studies identified a highly up-regulated mammalian lncRNA, FOXD3-AS1, known as linc1623 in mice, in the setting of hyperoxia/reactive oxygen species (ROS)-induced lung injury. We found that ROS induced a robust expression of FOXD3-AS1 in mouse lung tissue. Functionally, FOXD3-AS1 promoted oxidative stress-induced lung epithelial cell death. In human lung epithelial cells, the microRNA-150 (miR-150) was identified to interact with FOXD3-AS1; this finding was confirmed using the luciferase reporter assays. Consistently, mutation on the miR-150 pairing sequence in FOXD3-AS1 abolished the interactions between FOXD3-AS1 and miR-150. Additionally, miR-150 mimics suppressed the level of FOXD3-AS1. The antisense oligos of FOXD3-AS1 significantly augmented the intracellular level of miR-150, supporting the theory of sponging effects of FOXD3-AS1 on miR-150. We further investigated the cellular function of miR-150 in our lung injury models. MiR-150 conferred a cytoprotective role in lung epithelial cells after oxidative stress, whereas FOXD3-AS1 promoted cell death. Taken together, our studies indicated that FOXD3-AS1 serves as a sponge or as a competing endogenous noncoding RNA for miR-150, restricting its capability to promote cell growth and thereby exaggerating hyperoxia-induced lung epithelial cell death.—Zhang, D., Lee, H., Haspel, J. A., Jin, Y. Long noncoding RNA FOXD3-AS1 regulates oxidative stress-induced apoptosis via sponging microRNA-150.
Keywords: epithelial cell, hyperoxia, lncRNA array, lung injury, epigenetic regulation
Lung alveolar epithelium has the second largest surface area in the body and acts as a first-line defense against noxious insult (1). Alveolar epithelial cells contribute to the integrity and function of the lungs (1, 2). In the pathogenesis of acute respiratory distress syndrome (ARDS) and its lesser form acute lung injury (ALI), alveolar epithelial cell death is one of the prominent features in response to oxidative stress and to the common noxious stimuli of ARDS/ALI (3, 4). Accumulating evidence demonstrates that epithelial cell death not only results in damage to the physical barrier against infectious or noninfectious insult but also severely attenuates the innate immune responses and epithelial cell-immunomodulatory cell crosstalk, which are triggered by lung epithelium (5–7).
Hyperoxia-induced ALI (HALI) is an established animal model to mimic human ARDS/ALI (8, 9). Hyperoxia is a well-documented source of reactive oxygen species (ROS), and a large influx of ROS is rapidly generated after exposure to excess oxygen. The imbalance between oxidants and antioxidants often leads to the disruption of homeostasis intracellularly or extracellularly and results in severe damage or death to cells and tissues (10). Lung endothelial and epithelial cells are common targets of ROS, resulting in profound pulmonary vascular leakage, alveolar flooding, and protein deposition (11). Alveolar epithelial cell death induced by hyperoxia has been well described (11). As an initial response to hyperoxic insult, cells respond with attempts to adapt and repair the oxidative stress using a variety of intracellular innate defenses, including autophagy. After prolonged exposure, cells die due to apoptosis, oncosis, autophagic cell death, or necrosis (12). Multiple signaling pathways have also been illustrated in hyperoxia-mediated cell death, such as the MAPK, TLR4, signal transducers and activators of transcription, and NF-κB pathways, which communicate between the receptor signal and the DNA (13). Furthermore, multiple survival genes and mediators have been well studied, such as antioxidant enzymes, Bcl-2, AKT, heme oxygenase, and heat shock proteins (13). Nonprotein mediators in the process of cell death [e.g., the noncoding RNA (ncRNA) molecules, which include, but are not limited to, microRNAs (miRNAs) and long ncRNAs (lncRNAs)] have gained recent attention.
Advanced sequencing technology has only recently uncovered the existence of a tremendous amount of ncRNA existing in almost all living organisms (14, 15). Furthermore, the widely abundant miRNAs, which are small ncRNAs, have been demonstrated to play crucial roles in gene regulation and expression (16). LncRNAs are defined as the non-protein-coding transcripts longer than 200 nt, distinguishing lncRNAs from their smaller counterparts, such as miRNAs (17). More than 200,000 noncoding transcripts have been identified, facilitated by next-generation deep sequencing (18). LncRNAs contribute to gene expression and regulation at multiple levels in a robustly versatile manner. In addition to regulating transcriptional machinery, lncRNAs control various aspects of post-transcriptional mRNA processing or epigenetic translation in a very similar way as the miRNAs and small nucleolar RNAs. The post-transcriptional regulations of ncRNAs frequently involve complementary base pairing with the target mRNA, subsequently masking key elements within the mRNA required to interact with trans-acting factors. Despite scattered reports on lung cancer and the increasing numbers of lncRNAs identified in the human genome, little is known about the functional roles of lncRNA in human lung diseases.
Our current studies investigated a novel lncRNA, FOXD3-AS1, which is highly expressed in human lung epithelial cells, and its functional roles in response to hyperoxia-induced oxidative stress. We further explored the underlying mechanisms by which the lncRNA FOXD3-AS1 conferred its functions. This study provides insights into the pathogenesis of lung injury involving noncoding RNAs, which potentially serve as novel targets for the development of diagnostic and therapeutic agents.
MATERIALS AND METHODS
Animals, cell culture, and isolation of primary lung epithelial cells
Wild-type C57BL/6 mice (6–8 wk of age) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). All the protocols involving animals in this study were approved by the institutional animal care and use committee of Boston University. Beas2B cells and A549 cells were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM and F12 medium, respectively. All media were supplemented with 10% fetal bovine serum. All cells were cultured at 37°C in a humidified atmosphere of 5% CO2/95% air. For hyperoxia treatment, cells were exposed to hyperoxia (95% oxygen with 5% CO2) in modular exposure chambers. For N-acetyl-l-cysteine (NAC) or H2O2 treatment, 5 mM NAC or 90 µM H2O2 was added to the culture medium. Primary lung epithelial cells were isolated from the wild-type (WT) mice as previously described (9). Briefly, mouse lung tissue was washed with PBS and incubated with dispase. Lung tissue was then dissociated in DMEM with 25 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) and 200 U/ml DNase. Isolated cells were plated on a dish precoated with CD45 and CD16/32. The pellets were centrifuged and resuspended in DMEM containing 10% FBS.
LncRNA array
A mouse lncRNA Profiler Quantitative PCR (qPCR) Array Kit (System Biosciences, Palo Alto, CA, USA) was used to determine lncRNA expression in mouse lung tissues under hyperoxic conditions.
Cloning, transfection, and dual-luciferase reporter assay
The fragment of human FOXD3-AS1 containing the miRNA-150 (miR-150) binding site was amplified from cDNA using Q5 High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA, USA) and inserted into pRL-TK vector (Promega, Madison, WI, USA) as previously described (19). The primers for FOXD3-AS1 cloning are as follows: forward primer (5′-TGCTCTAGAGGCTACTTGGAGTTGTTAAACG-3′), reverse primer (5′-AAGGAAAAAAGCGGCCGCGGGAATCAGAAGCACCACTG-3′). A QuikChange II Site-Directed Mutagenesis Kit was purchased from Agilent Technologies (Santa Clara, CA, USA). MiRNA mimic control and miR-150 mimic were purchased from Sigma-Aldrich (St. Louis, MO, USA). Scramble oligo and antisense oligo (ASO) for human FOXD3-AS1 and mouse linc1623 were from Integrated DNA Technologies (Coralville, IA, USA). Transfection was performed using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Dual-luciferase assay was performed 24 h after transfection using a Dual-Luciferase Reporter Assay System (Promega) per the manufacturer’s protocol.
RNA preparation, reverse transcription, and real-time qPCR
MiRNeasy Mini Kits (Qiagen, Valencia, CA, USA) were used for purification of total RNA from tissues and cells. Single-stranded cDNA was generated with a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). For miR-150 detection, real-time PCR was performed using TaqMan PCR kit (Thermo Fisher Scientific) and StepOnePlus Real-Time PCR Systems (Applied Biosystems, Foster City, CA, USA). For mouse linc1623 and human FOXD3-AS1 detection, SYBR green-based real-time PCR technique was used as previously described (20). 18S rRNA was used as a reference housekeeping gene. The primers are provided as the following: linc1623 (forward: 5′-GAAATGAGCTGGAAACGGAGC-3′; reverse: 5′-TTCCCAGTCTCTCCTACCAGAGC-3′), FOXD3-AS1 (forward: 5′-GGTGGAGGAGGCGAGGATG-3′; reverse: 5′-AGCGGACAGACAGGGATTGG-3′), and 18S (forward: 5′-ACCGCAGCTAGGAATAATGGA-3′; reverse: 5′-CAAATGCTTTCGCTCTGGTC-3′).
Western blot analysis
Western blot analysis was performed as described previously (9). In brief, cells were homogenized in RIPA lysis buffer supplemented with Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktail (Sigma-Aldrich). Protein lysates were resolved on SDS-PAGE gels before being transferred to the PVDF membrane. Anti-p53 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal anti-GAPDH (Thermo Fisher Scientific) was used as a loading control.
ASO delivery and immunohistochemistry
The scramble or ASO was administered using a tracheal instillation procedure as previously described (21). In short, the prepared oligos/Lipofectamine 2000 solution was delivered via the mouth to the lungs. After instillation, mice were exposed to hyperoxia for 2 d.
Immunohistochemistry was performed as previously reported (22). Briefly, lung sections were deparaffinized and hydrated through xylenes and graded alcohol series, followed by incubation with primary antibodies, washing, and dilution with biotinylated secondary antibody solution. Sections were developed with Vectastain ABC reagent per the manufacturer’s protocol. The Vectastain Elite ABC Kit was purchased from Vector Laboratories (Burlingame, CA, USA). Cleaved caspase-3 antibody was from Cell Signaling Technology (Danvers, MA, USA).
Cell viability assay, M30 immunofluorescence, and annexin V propidium iodide assay
A Cell Counting Kit-8 (Dojindo Molecular Technologies, Rockville, MD, USA) was used to determine the viability of Beas2B cells exposed to hyperoxia. For M30 immunofluorescence, fixed primary lung epithelial cells, Beas2B cells, and A549 cells were incubated with M30 CytoDeath fluorescein antibody (Roche, Basel, Switzerland) following the standard protocol specified by the manufacturer. Images were captured using a fluorescence microscope (original magnification, ×10; Eclipse TS100; Nikon, Tokyo, Japan). Annexin V-FITC Apoptosis Detection Kit was used (BioVision, Mountain View, CA, USA) according to the manufacturer’s protocol. Data were obtained and analyzed using FacsCanto flow cytometer (BD Biosciences, San Jose, CA, USA).
Statistical analysis
All data are presented as means ± sd. All the data from 3 independent experiments were averaged before normalization. For real-time qPCR, the same amounts of cDNAs were used, and all data were analyzed at the same time. Comparisons between 2 groups were performed using a 2-tailed, unpaired Student’s t test. Multiple groups were compared using a 1-way ANOVA with Tukey’s method. A value of P < 0.05 was considered statistically significant.
RESULTS
Hyperoxia robustly modified the lncRNA profiles in mouse lung tissue
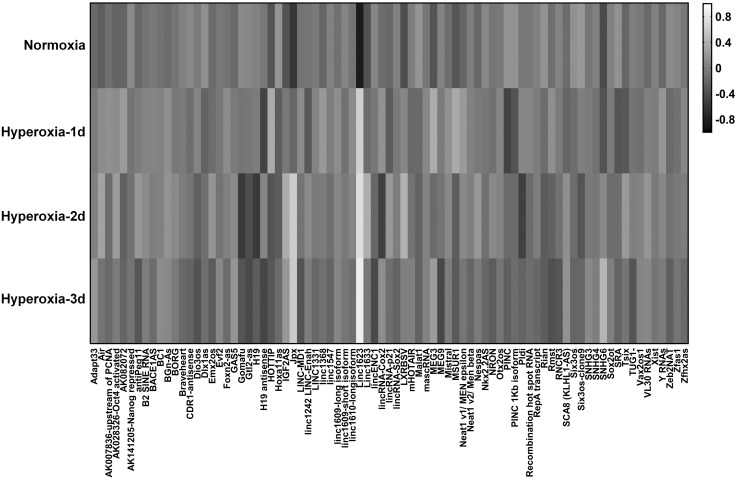
Initially, we profiled the lncRNAs in mouse lung tissue using HALI models. HALI is an established model mimicking human lung injury and ARDS. Alveolar epithelial cells compose the largest amount of surface area that is constantly exposed to oxidative stress, such as hyperoxia. Our results showed that lncRNA profiles were markedly altered after hyperoxia in a time-dependent manner (Fig. 1). We organized the data obtained from the heatmap in Fig. 1 and found that numerous lncRNAs were remarkably up-regulated (Table 1). Among all the up-regulated lncRNAs, FOXD3-AS1 (named linc 1623 in mice) was strikingly up-regulated by hyperoxia, up to more than 29-fold after d 1, more than 38-fold after d 2, and more than 51-fold after d 3 respectively (Table 1).
Figure 1.
LncRNA profile in mouse lung tissue during HALI. The heatmap was generated using hierarchical cluster analysis to show distinct lncRNA expression patterns in lung tissues between normoxia- and hyperoxia-treated mice. The intensity values were log10 transformed, centered by the mean of individual genes across all 4 groups, and subjected to cluster analysis for generating the heatmap. The bar was extracted to show the gray contrast level of the heatmap. White and black indicate high and low expression levels, respectively.
TABLE 1.
LncRNAs up-regulated by hyperoxia (>2-fold after 3 d hyperoxia exposure) in mouse lung tissue
| Name | Hyperoxia-1d | Hyperoxia-2d | Hyperoxia-3d |
|---|---|---|---|
| Linc1633 | 1.647 | 5.460 | 2.073 |
| GAS5 | 1.360 | 1.782 | 2.235 |
| Adapt33 | 1.484 | 1.585 | 2.416 |
| LXRBSV | 2.879 | 6.400 | 2.484 |
| IGF2AS | 2.037 | 5.272 | 3.533 |
| MEG3 | 5.072 | 1.528 | 3.795 |
| SNHG6 | 1.184 | 3.284 | 7.407 |
| Jpx | 2.555 | 16.035 | 15.581 |
| Linc1623 | 29.109 | 38.714 | 51.067 |
Oxidative stress–induced FOXD3-AS1 (linc1623) expression in lung epithelial cells
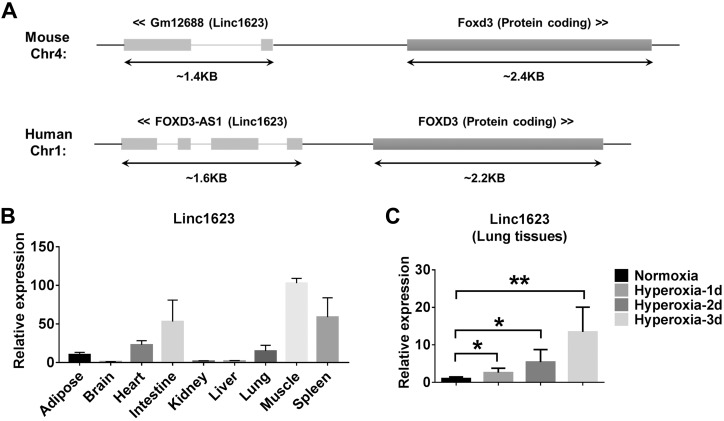
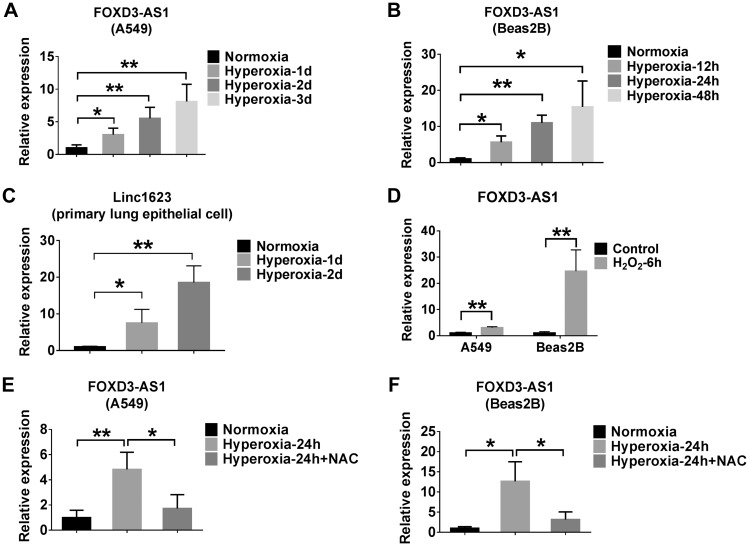
Similar genomic structure of mouse and human FOXD3-AS1 (linc1623) are found in genomes as shown in Fig. 2A. To determine the tissue types in which FOXD3-AS1 is expressed, we analyzed various mouse tissues using real-time PCR. In addition to lung tissue, abundant FOXD3-AS1 was found in muscle, spleen, intestine, heart, and adipose tissue in mice (Fig. 2B). Although the level of FOXD3-AS1 in lungs was not the most up-regulated at a steady state, FOXD3-AS1 was the most significantly up-regulated after exposure to hyperoxia (Table 1). We confirmed this observation in lung tissue using real-time PCR. Hyperoxia induced linc1623 (named FOXD3-AS1 in human) in mouse lung tissue in a time-dependent manner (Fig. 2C). Given that more than 95% of surface area is covered by epithelial cells, with which noxious stimuli can interact during the development of lung injury, we determined whether FOXD3-AS1 is up-regulated in lung epithelial cells after hyperoxia. Using the alveolar epithelial cell line A549 cells, lung bronchial epithelial Beas2B cells, and mouse primary lung epithelial cells, we demonstrated a similar pattern of FOXD3-AS1/linc1623 expression induced by hyperoxia in a time-dependent manner (Fig. 3A–C). Hyperoxia is a well-known source of ROS (12). Therefore, we directly treated the A549 and Beas2B cells with H2O2, a potent ROS donor. The FOXD3-AS1 levels in A549 and Beas2B cells were again highly induced (Fig. 3D). To analyze whether hyperoxia induced FOXD3-AS1 via hyperoxia-released ROS, we pretreated the cells with ROS inhibitor NAC and found that NAC abolished the effects of hyperoxia on the induction of FOXD3-AS1 (Fig. 3E, F).
Figure 2.
Genomic location and tissue distribution of linc1623. A) Genomic structure of mouse and human linc1623 in genomes are shown. B) Real-time PCR analysis of linc1623 in different mouse tissues (n = 4). C) Real-time PCR shows that a time course of hyperoxia induced the expression of linc1623 in mouse lung tissue (n = 4). *P < 0.05, **P < 0.01.
Figure 3.
Oxidative stress or hyperoxia induces FOXD3-AS1 expression in lung epithelial cells. A–C) A549, Beas2B, and primary lung epithelial cells were exposed to the hyperoxia for the indicated times. The expressions of FOXD3-AS1 in A549 (A), Beas2B (B), and primary lung epithelial (C) cells were determined using real-time PCR. D) A549 and Beas2B cells were treated with 90 µM H2O2 for 6 h. The expression level of FOXD3-AS1 was measured using real-time PCR. E, F) A549 (E) and Beas2B (F) cells were exposed to normoxia or hyperoxia for 24 h in the presence or absence of 5 mM NAC. Real-time PCR was performed to determine the level of FOXD3-AS1. Data represent 3 independent experiments. *P < 0.05, **P < 0.01.
FOXD3-AS1–mediated oxidative stress–induced apoptosis
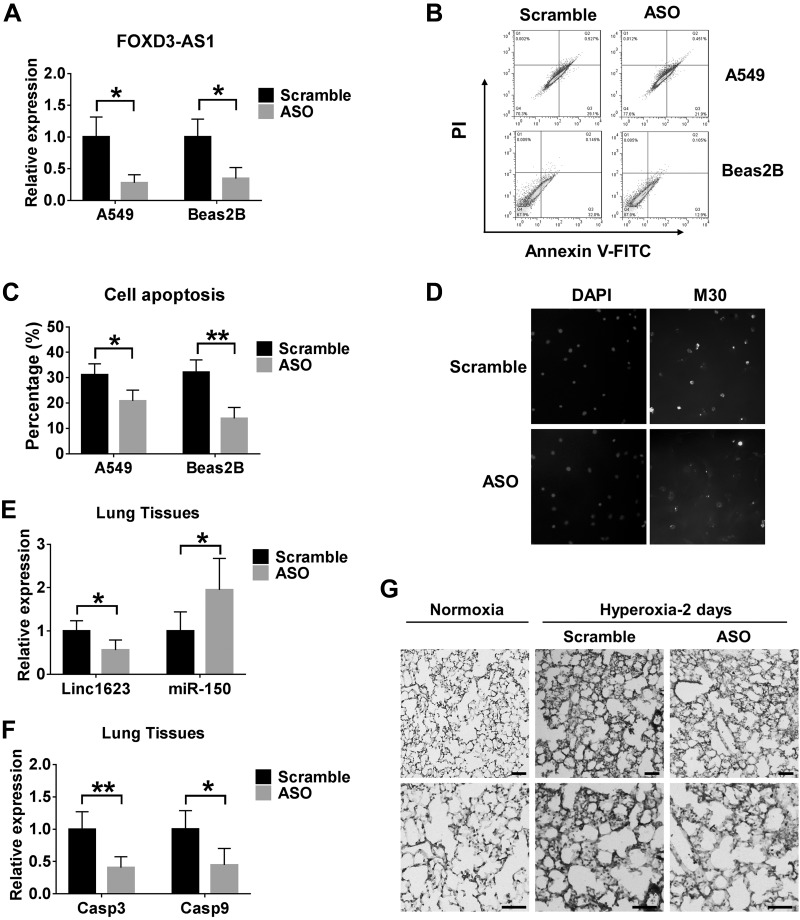
To determine the cellular function of FOXD3-AS1, we pretreated Beas2B or A549 cells with the scramble oligo (control) or ASO for FOXD3-AS1. We first confirmed the successful deletion of FOXD3-AS1 in A549 cells and Beas2B cells after pretreating them with ASO (Fig. 4A). Next, we exposed the cells to hyperoxia as previously described (9). Deletion of FOXD3-AS1 conferred a protective role in hyperoxia-induced apoptosis (Fig. 4B, C). Results produced by M30 staining using primary lung epithelial cells were similar to those obtained by flow cytometry analysis (Fig. 4D). We then performed in vivo studies using linc1623 ASO. The results showed that ASO significantly decrease the mRNA level of linc1623, caspase 3 and 9, and the protein level of cleaved caspase 3 in lung tissue under hyperoxia conditions (Fig. 4E–G).
Figure 4.
Knockdown of FOXD3-AS1 attenuates hyperoxia-induced cell death. A–C) A549 and Beas2B cells were transfected with scramble or ASO for FOXD3-AS1 and exposed to hyperoxia for 3 and 2 d, respectively. A) The relative levels of FOXD3-AS1 in A549 and Beas2B cells were measured using real-time PCR. B) Apoptosis was examined using the Annexin V-FITC apoptosis detection kit. A representative result of 3 independent experiments is shown. C) The levels of apoptosis presented as mean ± sd from 3 independent experiments (C). D) Primary lung epithelial cells were transfected with scramble or ASO and exposed to hyperoxia for 24 h. M30 immunofluorescence staining was used to detect apoptosis. E–G) Mice were administered scramble or ASO using a tracheal instillation followed by 2 d hyperoxia. E) Levels of linc1623 and miR-150 in the lung tissues. F) The relative mRNA levels of caspase-3 and -9 in lungs were measured using real-time PCR. G) Active caspase-3 was detected using immunohistochemistry staining.
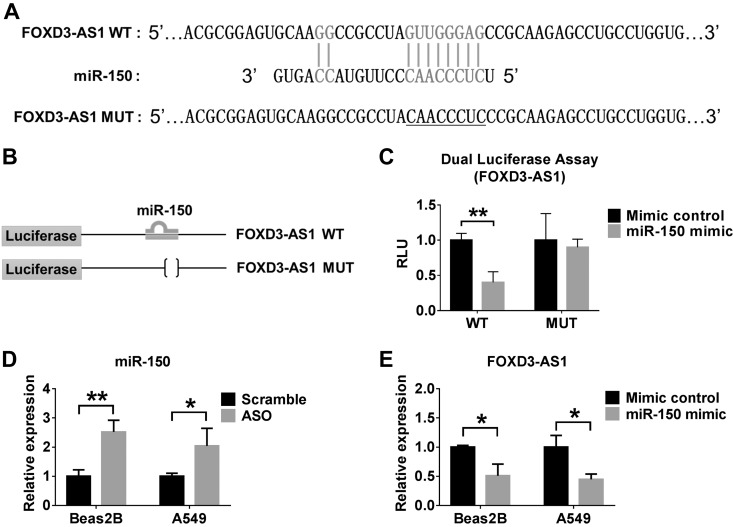
Deletion of FOXD3-AS1 protected cells against hyperoxia-induced apoptosis via regulation of miR-150 level
To explore the underlying mechanisms by which FOXD3-AS1 conferred a cytoprotective function in hyperoxia-induced apoptosis, we analyzed the structural sequence of FOXD3-AS1. We found a potential matching fragment with miR-150 on both the human and mouse FOXD3-AS1 (Fig. 5A). We next introduced a mutation into FOXD3-AS1 to disrupt base-pairing with the miR-150 seed sequence (Fig. 5A, underlined section). We constructed the luciferase report plasmids (Fig. 5B). The WT and mutant (Mut) forms of FOXD3-AS1 were cloned into the pRL-TK plasmid downstream of the luciferase gene. Consistent with our hypothesis, we found that miR-150 mimics significantly suppressed the FOXD3-AS1 transcriptional expression in WT FOXD3-AS1. On the other hand, in the mutant form of FOXD3-AS1, miR-150 mimics failed to affect its expression (Fig. 5C). We next transfected the Beas2B and/or A549 cells with scramble oligo (control) or ASO for FOXD3-AS1, followed by 2 and 3 d exposure to hyperoxia, respectively. Inhibition of FOXD3-AS1 using ASO resulted in markedly elevated miR-150 levels in both cells (Fig. 5D), suggesting that FOXD3-AS1 functions as a sponge for miR-150. We then transfected the Beas2B and A549 cells with the control mimics or with miR-150 mimics, followed by exposure to hyperoxia for 2 and 3 d, respectively. Consistently, the relative expression level of FOXD3-AS1 was robustly suppressed after exposure to miR-150 mimics (Fig. 5E).
Figure 5.
FOXD3-AS1 functions as a miRNA sponge for miR-150. A) The position of miR-150 regulatory element in FOXD3-AS1 is shown. Mutation (underlined) was introduced into FOXD3-AS1 to disrupt base-pairing with miR-150 seed sequence. B) Schematic diagram of the strategy for constructing luciferase report plasmids. The WT and Mut forms of FOXD3-AS1 were cloned into the pRL-TK plasmid downstream of the luciferase gene. C) Mimic control or miR-150 mimic was cotransfected with FOXD3-AS1 WT or Mut reporter into Beas2B cells. pGL3 luciferase plasmid was also cotransfected for normalization. D) Beas2B and A549 cells were transfected with scramble or ASO, followed by 2 and 3 d hyperoxia exposure, respectively. The relative level of miR-150 was measured using real-time PCR. E) Beas2B and A549 cells were transfected with mimic control or miR-150 mimic, followed by 2 and 3 d hyperoxia exposure, respectively. The relative expression level of FOXD3-AS1 was measured using real-time PCR. Data represent 3 independent experiments. *P < 0.05, **P < 0.01.
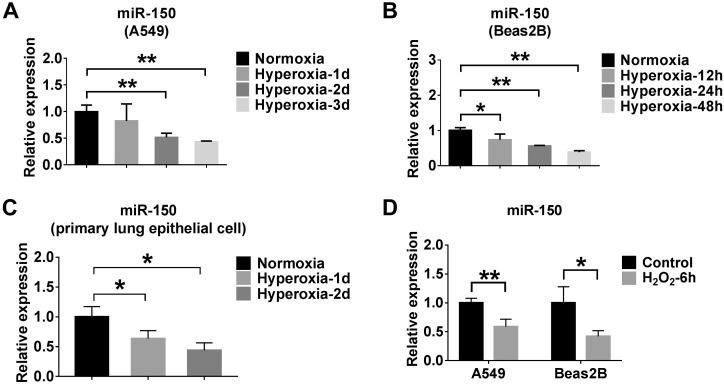
MiR-150 is negatively regulated by oxidative stress or hyperoxia
Next, we examined miR-150 levels in lung epithelial cells after hyperoxia exposure. We found that hyperoxia suppressed the level of miR-150 in a time-dependent manner in A549, Beas2B, and primary lung epithelial cells (Fig. 6A–C). To determine whether hyperoxia down-regulated miR-150 via ROS, we treated cells with H2O2, which markedly down-regulated the expression level of miR-150 (Fig. 6D).
Figure 6.
MiR-150 is negatively regulated by oxidative stress or hyperoxia. A–C) A549, Beas2B, and primary lung epithelial cells were exposed to the hyperoxia for the indicated times. The expressions of miR-150 in A549 (A), Beas2B (B), and primary lung epithelial (C) cells were determined using real-time PCR. D) A549 and Beas2B cells were treated with 90 µM H2O2 for 6 h. The expression level of miR-150 was measured using real-time PCR. Data represent 3 independent experiments. *P < 0.05, **P < 0.01.
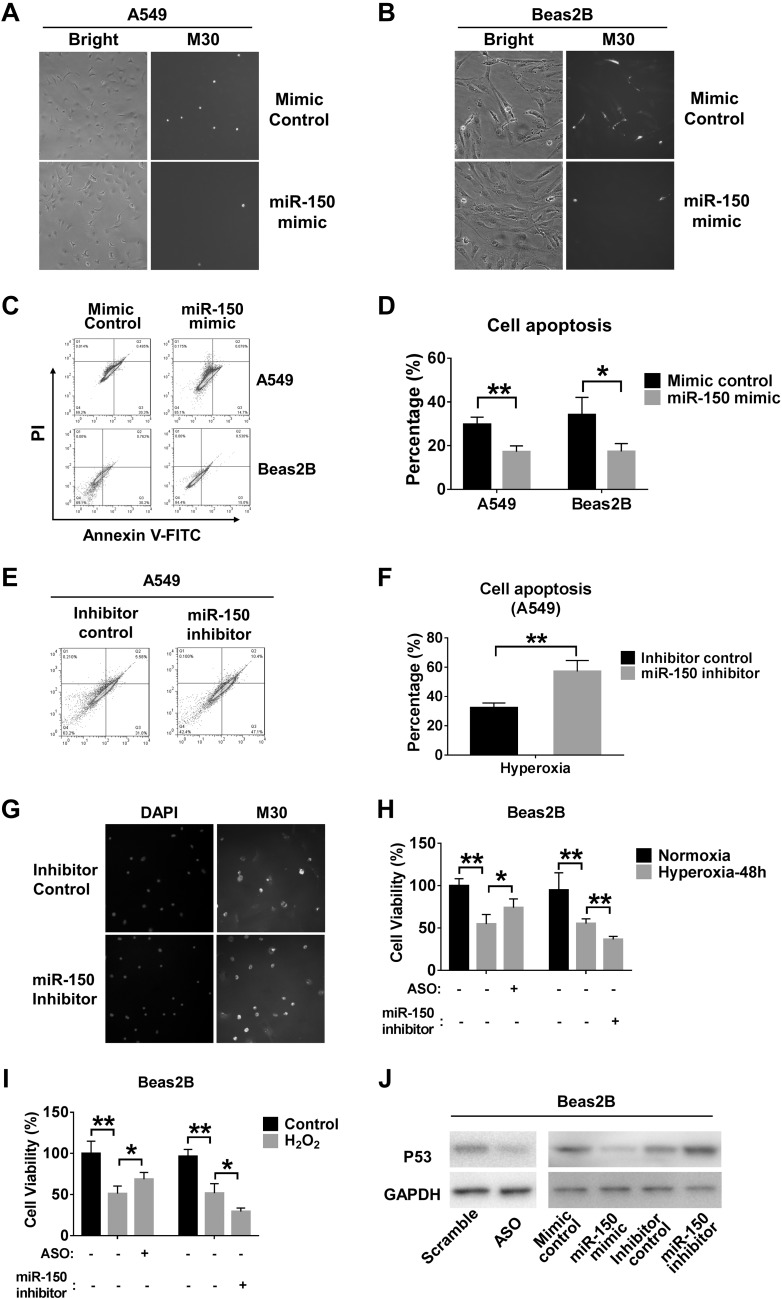
MiR-150 protects hyperoxia-induced apoptosis
To determine whether FOXD3-AS1 promoted hyperoxia-induced cell death via sponging miR-150, we confirmed the functional role of miR-150 in lung epithelial cells after hyperoxia. We found that miR-150 mimics significantly protected lung epithelial cells from hyperoxia-induced cell death, detected using M30 assays as described previously (9) (Fig. 7A, B). Using the Annexin-FITC assays, we further observed that miR-150 mimics conferred a cytoprotective role in hyperoxia-induced apoptosis (Fig. 7C, D). To confirm these observations, we transfected A549 cells with miR-150 inhibitors. Inhibition of miR-150 promotes apoptosis of A549 cells after hyperoxia (Fig. 7E, F). Using M30 cytodeath antibody, we observed consistent results in primary lung epithelial cells (Fig. 7G). Furthermore, miR-150 inhibition in Beas2B cells decreased cell viability, whereas ASO enhanced the survival of Beas2B cells exposed to hyperoxia or treated with H2O2 (Fig. 7H, I). We next explored the potential mechanisms by which FOXD3-AS1 controls apoptosis in the presence of hyperoxia. It has been reported that p53 is a direct target of mir-150 (23), which has a strong ability to activate apoptosis (24). Based on these studies, we proposed that FOXD3-AS1 and miR-150 affect epithelial cell apoptosis via p53. The protein level of p53 was determined using Western blot analysis in Beas2B cells exposed to hyperoxia. Our data show that knockdown of FOXD3-AS1 by ASO significantly depressed p53 expression in (Fig. 7J). This result was consistent with previous findings when we transfected Beas2B cells with miR-150 mimic or inhibitor (23). Taken together, these findings suggest that FOXD3-AS1 regulates hyperoxia-induced cell apoptosis via miR-150 and its target p53.
Figure 7.
MiR-150 protects hyperoxia-induced apoptosis via p53. A, B) A549 and Beas2B cells were transfected with mimic control or miR-150 mimic, followed by 24 h hyperoxia exposure. M30 immunofluorescence staining was used to detect apoptosis in A545 (A) and Beas2B (B) cells. Representative images were captured using a fluorescence microscope. Original magnification, ×20. C, D) A549 (C) and Beas2B (D) cells were transfected with mimic control or miR-150 mimic followed by 3 and 2 d hyperoxia exposure, respectively. Apoptosis was examined using the Annexin V-FITC apoptosis detection kit. A representative result of 3 independent experiments is shown. The levels of apoptosis are presented as mean ± sd from 3 independent experiments. E, F) A549 cells were transfected with inhibitor control or miR-150 inhibitor followed by 3 d hyperoxia exposure (E), and apoptosis was examined using the Annexin V-FITC apoptosis detection kit (F). A representative result of 3 independent experiments is shown. The levels of apoptosis are presented as mean ± sd from 3 independent experiments. G) Primary lung epithelial cells were transfected with inhibitor control or miR-150 inhibitor and exposed to hyperoxia for 24 h. M30 immunofluorescence staining was used to detect apoptosis. H, I) The effects of ASO and miR-150 inhibition on cell viability were detected after transfection of ASO or miR-150 inhibitor into Beas2B cells followed by hyperoxia exposure (H) or H2O2 treatment (I). J) P53 protein expressions were detected after transfection of ASO or miR-150 mimic/inhibitor in Beas2B cells followed by 48 h hyperoxia. *P < 0.05, **P < 0.01.
DISCUSSION
ARDS and ALI have high mortality and morbidity, reflecting the inefficacy of specific therapies despite decades of research (3, 4). Conventional research on ARDS/ALI often involves investigations on specific protein(s) or protein-related signaling pathways, gene expression and regulation, as well as post-translational modifications. The discovery of lncRNA reveals a novel insight into the pathogenesis of lung injury and potentially opens a new era for lung researchers and physicians to develop specific effective therapeutics and diagnostics for this devastating syndrome.
In the past decade, growing evidence has indicated that approximately 80% of transcriptions across the human genome are not associated with protein-coding genes. Kapranov et al. (25) reported that at least 4 times more lncRNA exists than protein-coding RNAs. Accumulating studies identified the strikingly large amount of noncoding transcripts with little or no open reading frame (26). More than 70% of lncRNAs are tissue specific (27). Furthermore, lncRNAs are developmental stage specific (28) and are cell subtype specific in heterogeneous tissues (29). Human lung tissue is composed of multiple cell types and a heterogeneous cell subtype in the epithelium. In the current report, we explored the expression of lncRNA profiles using mouse lung tissue in a well-established ARDS model (i.e., the HALI model). As shown in the heatmap (Fig. 1), we explored the lncRNAs that were significantly altered after hyperoxia. Several lncRNAs were up-regulated in the presence of hyperoxia-induced oxidative stress (white strip). Among them, Linc1623 (the counterpart of FOXD3-AS1 in mouse) is the most strikingly augmented lncRNA, reflected by its more than 10-fold up-regulation compared with other elevated lncRNAs. Moreover, linc1623 also responded to hyperoxia at the very early stage and was time dependent. On the other hand, although several other lncRNAs, (e.g., LXRBSV, IGF2AS, MEG3, and JDX) were up-regulated initially after hyperoxia, then subsided after a period of exposure. Lung epithelial cell death is a prominent feature in the hyperoxia-induced lung injury (2, 13). The persistent and robust elevation of linc1623 led us to further investigate the functional role of this lncRNA in both human and mouse lung epithelial cells. After examining its counterpart in human cells, we found that FOXD3-AS1 was essentially the same lncRNA in human cells as the linc1623 in mouse cells. We next confirmed its expression in human lung cells and determined its function on inducing lung epithelial cell apoptosis/cell death in the presence of hyperoxia-associated oxidative stress. Mechanistically, we identified a binding site of miR-150 in the last exon of FOXD3-AS1 (the fourth exon of transcription variant 1; the third exon of other 3 transcription variants). To the best of our knowledge, this is the first report showing that FOXD3-AS1 potentially serves as a sponge for miR-150. Moreover, our work is the first report that demonstrates an inducible lncRNA in response to hyperoxic stress, subsequently playing a functional role in the pathogenesis of ALI/ARDS.
In human epithelial cells, miR-150 mimics decreased luciferase activity and suppressed the FOXD3-AS1 transcriptional expression. We further confirmed the binding sites between FOXD3-AS1 and miR-150. These data suggest that FOXD3-AS1 serves as a sponge of miR-150. Using the HALI models, we found that miR-150 conferred cytoprotection after hyperoxia. Therefore, the sponging FOXD3-AS1 (or linc1623) exerted a proapoptotic effect after hyperoxia via interaction with miR-150.
Previous reports show that miR-150 promotes cervical cancer cell survival (30). It also induces the cell cycle progression from G1/G0 to S phase, leading to an enhancement of cell growth (30). Moreover, miR-150 was found significantly elevated in lung cancer clinical specimens (31, 32). MiR-150 represses SRCIN1 and consequently triggers the activation of the Src/focal adhesion kinase and Src/Ras/ERK pathway, which leads to the proliferation and migration of A549 cells (31). In support of this finding, miR-150 is also shown to suppress angiopoietin generation, protects against vascular injury, and reduces mortality resulting from sepsis (21). MiR-150 has a variety of target genes, including, but not limited, to small-molecule metabolism, transcriptional regulation, RNA metabolism, proteoglycan synthesis in cancer, mTOR signaling pathway, or Wnt signaling pathway (22). On the other hand, recent reports suggest that deletion of miR-150 confers a cytoprotective effect in Fas-induced apoptosis by enhancing the protective gene products Akt1, Akt2, total Akt, and p-Akt (Ser473) (23). Deletion of miR-150 attenuates the proapoptotic processes, characterized by less prominent poly (ADP-ribose) polymerase cleavage, less nuclear fragmentation, and less caspase activation. Mechanistic studies using luciferase assays suggest that the Akt1 and Akt2 are direct targets of miR-150 (23). Therefore, we believe that the cellular function of miR-150 is potentially cell type– and stimulus dependent.
Although more than 10,000 lncRNAs are encoded in the human genome, only a few have clearly identified functions. Accumulating evidence suggests that lncRNAs serve as miRNA sponges to compete with miRNA binding to mRNA transcripts. Recent studies on the lncRNA-mediated sponge regulatory network suggest that lncRNAs carry a subcellular localization-dependent function. These findings indicated the critical influence of cytoplasmic localization on the efficacy of a sponge lncRNA (24, 33). LncRNAs have widely varying subcellular distributions, including the nucleus, the cytoplasm, or both. Generally, nuclear lncRNAs were more effectively suppressed using ASOs, cytoplasmic lncRNAs were more effectively suppressed using RNAi, and dual-localized lncRNAs were suppressed using both methods (24, 33). Our data suggest that linc1623 may locate in cytoplasm or in both compartments. Both miRNA and lncRNA are essential gene regulators. Our report provided evidence of their cross-talk in the presence of oxidative stress. The sponge effect, in which an lncRNA acts by sequestering miRNAs, has been hypothesized in multiple cells. Currently, few approaches exist for the prediction of miRNA response elements in lncRNAs acting as sponges. It remains unclear how many copies of FOXD3-AS1 in each cell are required to effectively sponge the intracellular miR-150. Presumably, in addition to miR-150, there are many more miRNAs that can be sponged by FOXD3-AS1, and their influences on cellular events remain unknown at this moment. Moreover, due to the instability of the flanking sequences, a full-length WT and mutant FOXD3-AS1 have not been generated successfully. Furthermore, lncRNAs have significant cell subtype and tissue variability. Our observations in lung type I epithelial cells may not apply to other types of cells. Our further studies will investigate whether certain lncRNAs can serve as a signature of a specific cell type that undergoes a specific stimulation. Other directions include studies to confirm the subcellular locations and identification other binding molecules.
In summary, our study identified a novel lncRNA (FOXD3-AS1 in humans, linc1623 in mice) that can be highly up-regulated in lung epithelial cells after oxidative stress. We further identified a binding miRNA (miR-150) that can be sequestered by FOXD3-AS1 (linc1623). By sponging miR-150, FOXD3-AS1 exerted a proapoptotic effect in the presence of hyperoxic stress.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grant R01HL102076 (to Y.J.), NIH National Institute of Allergy and Infectious Diseases Grant R21AI121644 (to Y.J.), and NIH National Institute of General Medical Sciences Grant R01GM111313 (to Y.J.). Funding for open access is from the NIH.
Glossary
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- ASO
antisense oligo
- HALI
hyperoxia-induced acute lung injury
- lncRNA
long noncoding RNA
- miR/miRNA
microRNA
- Mut
mutant
- NAC
N-acetyl-l-cysteine
- ncRNA
noncoding RNA
- ROS
reactive oxygen species
- qPCR
quantitative PCR
- WT
wild type
AUTHOR CONTRIBUTIONS
D. Zhang, H. Lee, J. A. Haspel, and Y. Jin designed the research; D. Zhang and H. Lee performed the research; and D. Zhang, J. A. Haspel, and Y. Jin analyzed the data and wrote the paper.
REFERENCES
- 1.Thompson A. B., Robbins R. A., Romberger D. J., Sisson J. H., Spurzem J. R., Teschler H., Rennard S. I. (1995) Immunological functions of the pulmonary epithelium. Eur. Respir. J. 8, 127–149 [DOI] [PubMed] [Google Scholar]
- 2.Guillot L., Nathan N., Tabary O., Thouvenin G., Le Rouzic P., Corvol H., Amselem S., Clement A. (2013) Alveolar epithelial cells: master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 45, 2568–2573 [DOI] [PubMed] [Google Scholar]
- 3.Johnson E. R., Matthay M. A. (2010) Acute lung injury: epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 23, 243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gropper M. A., Wiener-Kronish J. (2008) The epithelium in acute lung injury/acute respiratory distress syndrome. Curr. Opin. Crit. Care 14, 11–15 [DOI] [PubMed] [Google Scholar]
- 5.Vir P., Gupta D., Agarwal R., Verma I. (2014) Immunomodulation of alveolar epithelial cells by Mycobacterium tuberculosis phosphatidylinositol mannosides results in apoptosis. APMIS 122, 268–282 [DOI] [PubMed] [Google Scholar]
- 6.Chuquimia O. D., Petursdottir D. H., Rahman M. J., Hartl K., Singh M., Fernández C. (2012) The role of alveolar epithelial cells in initiating and shaping pulmonary immune responses: communication between innate and adaptive immune systems. PLoS One 7, e32125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalessandri T., Strid J. (2014) Beneficial autoimmunity at body surfaces: immune surveillance and rapid type 2 immunity regulate tissue homeostasis and cancer. Front. Immunol. 5, 347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H., Zhang D., Zhu Z., Dela Cruz C. S., Jin Y. (2016) Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci. Rep. 6, 35250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D., Lee H., Cao Y., Dela Cruz C. S., Jin Y. (2016) miR-185 mediates lung epithelial cell death after oxidative stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 310, L700–L710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., Dhama K. (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Res. Int. 2014, 761264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mittal M., Siddiqui M. R., Tran K., Reddy S. P., Malik A. B. (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mach W. J., Thimmesch A. R., Pierce J. T., Pierce J. D. (2011) Consequences of hyperoxia and the toxicity of oxygen in the lung. Nurs. Res. Pract. 2011, 260482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaher T. E., Miller E. J., Morrow D. M., Javdan M., Mantell L. L. (2007) Hyperoxia-induced signal transduction pathways in pulmonary epithelial cells. Free Radic. Biol. Med. 42, 897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kung J. T., Colognori D., Lee J. T. (2013) Long noncoding RNAs: past, present, and future. Genetics 193, 651–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilusz J. E., Sunwoo H., Spector D. L. (2009) Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 23, 1494–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahid F., Shehzad A., Khan T., Kim Y. Y. (2010) MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta 1803, 1231–1243 [DOI] [PubMed] [Google Scholar]
- 17.Gomes A. Q., Nolasco S., Soares H. (2013) Non-coding RNAs: multi-tasking molecules in the cell. Int. J. Mol. Sci. 14, 16010–16039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isakov O., Ronen R., Kovarsky J., Gabay A., Gan I., Modai S., Shomron N. (2012) Novel insight into the non-coding repertoire through deep sequencing analysis. Nucleic Acids Res. 40, e86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D., Lee H., Zhu Z., Minhas J. K., Jin Y. (2017) Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 312, L110–L121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang D., Li Y., Yao X., Wang H., Zhao L., Jiang H., Yao X., Zhang S., Ye C., Liu W., Cao H., Yu S., Wang Y. C., Li Q., Jiang J., Liu Y., Zhang L., Liu Y., Iwai N., Wang H., Li J., Li J., Li X., Jin Z. B., Ying H. (2016) miR-182 regulates metabolic homeostasis by modulating glucose utilization in muscle. Cell Rep. 16, 757–768 [DOI] [PubMed] [Google Scholar]
- 21.Rajput C., Tauseef M., Farazuddin M., Yazbeck P., Amin M. R., Avin Br V., Sharma T., Mehta D. (2016) MicroRNA-150 suppression of angiopoetin-2 generation and signaling is crucial for resolving vascular injury. Arterioscler. Thromb. Vasc. Biol. 36, 380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang Z. H., Wang S. L., Zhao J. T., Lin Z. J., Chen L. Y., Su R., Xie S. T., Carter B. Z., Xu B. (2016) miR-150 exerts antileukemia activity in vitro and in vivo through regulating genes in multiple pathways. Cell Death Dis. 7, e2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W., Han C., Zhang J., Song K., Wang Y., Wu T. (2015) miR-150 deficiency protects against FAS-induced acute liver injury in mice through regulation of AKT. PLoS One 10, e0132734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lennox K. A., Behlke M. A. (2016) Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res. 44, 863–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapranov P., Cheng J., Dike S., Nix D. A., Duttagupta R., Willingham A. T., Stadler P. F., Hertel J., Hackermüller J., Hofacker I. L., Bell I., Cheung E., Drenkow J., Dumais E., Patel S., Helt G., Ganesh M., Ghosh S., Piccolboni A., Sementchenko V., Tammana H., Gingeras T. R. (2007) RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316, 1484–1488 [DOI] [PubMed] [Google Scholar]
- 26.Ulitsky I., Bartel D. P. (2013) lincRNAs: genomics, evolution, and mechanisms. Cell 154, 26–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabili M. N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A., Rinn J. L. (2011) Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25, 1915–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan L., Yang M., Guo H., Yang L., Wu J., Li R., Liu P., Lian Y., Zheng X., Yan J., Huang J., Li M., Wu X., Wen L., Lao K., Li R., Qiao J., Tang F. (2013) Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 20, 1131–1139 [DOI] [PubMed] [Google Scholar]
- 29.Liu S. J., Nowakowski T. J., Pollen A. A., Lui J. H., Horlbeck M. A., Attenello F. J., He D., Weissman J. S., Kriegstein A. R., Diaz A. A., Lim D. A. (2016) Single-cell analysis of long non-coding RNAs in the developing human neocortex. Genome Biol. 17, 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J., Hu L., Tian C., Lu F., Wu J., Liu L. (2015) microRNA-150 promotes cervical cancer cell growth and survival by targeting FOXO4. BMC Mol. Biol. 16, 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao M., Hou D., Liang H., Gong F., Wang Y., Yan X., Jiang X., Wang C., Zhang J., Zen K., Zhang C. Y., Chen X. (2014) miR-150 promotes the proliferation and migration of lung cancer cells by targeting SRC kinase signalling inhibitor 1. Eur. J. Cancer 50, 1013–1024 [DOI] [PubMed] [Google Scholar]
- 32.Yin Q. W., Sun X. F., Yang G. T., Li X. B., Wu M. S., Zhao J. (2015) Increased expression of microRNA-150 is associated with poor prognosis in non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 8, 842–846 [PMC free article] [PubMed] [Google Scholar]
- 33.Cabili M. N., Dunagin M. C., McClanahan P. D., Biaesch A., Padovan-Merhar O., Regev A., Rinn J. L., Raj A. (2015) Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 16, 20 [DOI] [PMC free article] [PubMed] [Google Scholar]