Abstract
In the pathophysiologic setting of cerebral ischemia, excitotoxic levels of glutamate contribute to neuronal cell death. Our previous work demonstrated the ability of glutamate oxaloacetate transaminase (GOT) to metabolize neurotoxic glutamate in the stroke-affected brain. Here, we seek to identify small-molecule inducers of GOT expression to mitigate ischemic stroke injury. From a panel of phytoestrogen isoflavones, biochanin A (BCA) was identified as the most potent inducer of GOT gene expression in neural cells. BCA significantly increased GOT mRNA and protein expression at 24 h and protected against glutamate-induced cell death. Of note, this protection was lost when GOT was knocked down. To validate outcomes in vivo, C57BL/6 mice were intraperitoneally injected with BCA (5 and 10 mg/kg) for 4 wk and subjected to ischemic stroke. BCA levels were significantly increased in plasma and brain of mice. Immunohistochemistry demonstrated increased GOT protein expression in the brain. BCA attenuated stroke lesion volume as measured by 9.4T MRI and improved sensorimotor function—this protection was lost with GOT knockdown. BCA increased luciferase activity in cells that were transfected with the pERRE3tk-LUC plasmid, which demonstrated transactivation of GOT. This increase was lost when estrogen-related receptor response element sites were mutated. Taken together, BCA represents a natural phytoestrogen that mitigates stroke-induced injury by inducing GOT expression.—Khanna, S., Stewart, R., Gnyawali, S., Harris, H., Balch, M., Spieldenner, J., Sen, C. K., Rink, C. Phytoestrogen isoflavone intervention to engage the neuroprotective effect of glutamate oxaloacetate transaminase against stroke.
Keywords: phytonutrient, ischemia, brain
Under conditions of focal cerebral ischemia, neurotransmitter glutamate is released into the synaptic cleft by neurons and contributes to the pathophysiologic response of neurotoxicity and cell death in the brain (1). To that end, research efforts that have focused on the removal of pathologically elevated glutamate in the injured brain have been increasingly recognized for the therapeutic potential of glutamate removal to mitigate neurotoxicity (1–4). Our laboratory has recently demonstrated the therapeutic potential of inducible glutamate oxaloacetate transaminase (GOT), a glutamate metabolizing enzyme that can repurpose otherwise neurotoxic glutamate in the stroke-affected brain as energy substrate for glucose-starved neurons (5–7). Specifically, GOT enables glutamate metabolism and the anaplerotic refilling of tricarboxylic acid (TCA; or the Krebs) cycle intermediates via a truncated TCA cycle in the ischemic stroke–affected and, subsequently, hypoglycemic brain (6). Our observation identified that the correction of focal hypoxia during ischemic stroke induces GOT expression and rescues brain tissue from injury. In recognition of the clinical limitations of oxygen therapy for the treatment of patients affected by stroke (8), we sought to identify and test novel small-molecule inducers of GOT as a prophylactic intervention for at-risk stroke patients. As prophylactic stroke therapies—for example, low-dose aspirin—are increasingly recognized for their role in reducing the stroke burden in the United States by tens of thousands of patients each year (9), novel prophylactic therapies that are safe and that target protection against stroke-induced brain injury are desirable for the ∼800,000 new or recurring stroke events in the United States each year (10).
Screening of the 5′ promoter region of GOT has uncovered 2 estrogen-related receptor response element (ERRE) sequences (TCAAGGTCA) at positions −888 to −880 and −549 to −541 (11). ERRE sites are known to bind estrogen-related receptors (12) and other small-molecule agonists, including phytoestrogenic isoflavones (13), that induce the transcriptional activation of target genes. As a small, but growing, body of literature supports the therapeutic potential of phytoestrogen isoflavones against a range of diseases, including stroke (14), we sought to test whether phytoestrogen isoflavones induce GOT expression in neural cells and to determine the prophylactic efficacy of candidates to protect against ischemic stroke–induced injury in a GOT-dependent manner.
MATERIALS AND METHODS
All animal studies were performed in accordance with protocols approved by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University. Mice were maintained under standard conditions at 22 + 2°C with 12-h light/dark cycles and access to food and water ad libitum.
The following materials were obtained from the source indicated: BCA, calycosin, 5-O-methylgenistein, genistein, glycitin, formononetin, (+/−)-8-prenylnaringenin (flavaprenin), l-glutamic acid monosodium salt, DMSO, and propidium iodide were from Sigma-Aldrich (St. Louis, MO, USA); and calcein-AM was from Thermo Fisher Scientific (Waltham, MA, USA). For cell culture, DMEM, minimum essential medium, fetal calf serum, antibiotics (100 μg/ml streptomycin, 100 U/ml penicillin, and 0.25 μg/ml amphotericin), and culture dishes were also purchased from Thermo Fisher Scientific.
Cell culture
Mouse hippocampal HT4 neural cells were grown in DMEM that was supplemented with 10% fetal calf serum, 100 μg/ml streptomycin, 100 U/ml penicillin, and 0.25 μg/ml amphotericin at 37°C in a humidified atmosphere of 95% air and 5% CO2 as previously described (15–21).
Primary cortical neurons
Neurons were isolated from the cerebral cortex of rat fetuses (Sprague-Dawley, d 17 of gestation; Harlan Industries, Indianapolis, IN, USA) as described previously (6, 18, 19, 21, 22). In brief, cells were cultured in minimum essential medium that was supplemented with 10% heat-inactivated fetal bovine serum, 40 μM cystine, and antibiotics (100 μg/ml streptomycin, 100 U/ml penicillin, and 0.25 μg/ml amphotericin) and were maintained at 37°C in 5% CO2 and 95% air in a humidified incubator. Unless otherwise stated, all experiments were carried out 24 h after plating.
Phytoestrogen treatment
Stock solutions—103× working concentration—of phytoestrogens were prepared in DMSO. Phytoestrogens were added to culture dishes 24 h before glutamate. Respective controls were treated with an equal volume (0.1%, v/v) of DMSO.
Glutamate treatment
Glutamate was added to the cell culture medium as an aqueous solution. No change in the medium pH was observed in response to glutamate addition (23).
Determination of cell viability
The viability of cells was assessed by measuring lactate dehydrogenase (LDH) leakage from cells to medium 24 h after glutamate treatment. This protocol has been described in detail in previous reports (17, 18, 21, 22). In brief, cell viability was determined by using the following equation: viability = LDH activity of cells in monolayer/total LDH activity—that is, LDH activity of cells in the monolayer + LDH activity of detached cells + LDH activity in the cell culture medium. Cell viability was also measured by calcein-AM and propidium iodide solutions as previously described (5, 6). Cells were incubated with calcein-AM (3 μM) and propidium iodide (2.5 μM) in PBS for 15 min in cell culture incubators. After incubation, digital images were collected by using a Zeiss Observer.Z1 microscope and Zen 2012 (Blue Edition; Zeiss, Jena, Germany) suited for imaging cells growing in regular culture plates as previously described (6, 7, 22).
GOT small interfering RNA knockdown
HT4 neural cells (0.1 × 106 cells/well in 12-well plates) were cultured for 24 h before transfection. DharmaFect 1 transfection reagent (GE Dharmacon, Lafayette, CO, USA) was used to transfect cells with 100 nM small interfering RNA (siRNA) pool (GE Dharmacon) for 72 h according to the manufacturer's protocol (5, 6, 22). Cells were collected 72 h after transfection for the determination of GOT mRNA, protein, and enzyme activity.
Transient transfection and luciferase assay
HT4 cells were seeded in 24-well plates in antibiotic-free medium for 18–24 h before transfection. Lipofectamine LTX Reagent with Plus Reagent (Thermo Fisher Scientific) was used to transiently transfect cells with either 100 ng/well GOT(ERRE)-Luc or 300 ng/well pERRE3tk-LUC reporter plasmid according to the manufacturer's protocol. GOT(ERRE)-Luc promoter constructs were developed from a 1060-bp fragment of C57/BL6 mouse GOT promoter that contained wild-type (TCAAGGTCA) or mutated (CGCTATGCA) ERRE binding sites (–888 to –880 and –549 to –541, respectively) by synthesizing and cloning in pUCminusMCS and subcloning into commercial pGL3 vector via the KpnI and HindIII sites. pERRE3tk-LUC reporter plasmid was generously provided by Dr. Ronald M. Evans (The Salk Institute for Biological Studies, La Jolla, CA, USA). After 24 h of transfection, samples were treated with BCA. Normalization was achieved by cotransfection with Renilla plasmid (10 ng). Luciferase activity was determined by using the dual-luciferase reporter assay system (Promega, Madison, WI, USA). Data are presented as the ratio of firefly to Renilla luciferase assay as previously described (24–26).
RNA isolation and real-time quantitative PCR for mRNA
Total RNA was extracted by using the miRVana miRNA Isolation Kit according to the manufacturer’s protocol (Thermo Fisher Scientific). For gene expression studies, total cDNA synthesis was achieved by using the SuperScript III First Strand Synthesis System (Thermo Fisher Scientific). The abundance of mRNA for GOT was quantified by real-time PCR by using SYBR green-I and primers as previously described (5, 6, 22).
GOT activity assay
For GOT activity, samples were prepared as previously described (5), and GOT activity was measured by using an Aspartate Aminotransferase Activity Colorimetric Assay Kit (BioVision, Milpitas, CA, USA) according to the manufacturer’s instructions.
Western blot analysis
After protein extraction, protein concentrations were determined by using BCA protein assay. Samples (20 μg protein/lane) were separated on 10% SDS-PAGE and probed with anti-GOT Ab (1:1000; Abcam, Cambridge, MA, USA). To evaluate loading efficiency, membranes were probed with anti–β-actin or GAPDH Ab (15, 18, 22).
BCA extraction and analyses
To determine BCA uptake, HT4 cells were seeded in 6-well plates (0.3–0.5 × 106/well). After 24 h, cells were treated with BCA or DMSO vehicle. After 12- or 24-h time points, cells were collected as previously described for HPLC electrochemical detection (23). For plasma and brain, samples were collected as previously described (27, 28). To extract BCA, tert-butyl methyl ether was added in equal volumes to EDTA/SDS (aqueous phase). Samples were kept at 4°C during 15 min of vortexing, followed by centrifugation at 4300 g for 20 min. Of the upper-phase (tert-butyl methyl ether) volume, 75% was collected in glass, evaporated under nitrogen gas, and resuspended in 200 μl of mobile phase. Solutions were filtered and stored as previously described (23, 27, 28). HPLC was performed as previously described (29), with the following exceptions: mobile phase A consisted of 200 mM sodium acetate with glacial acetic acid (pH 4.8) in ddH2O:MeOH (80:20 v/v), and mobile phase B consisted of 200 mM sodium acetate with glacial acetic acid (pH 4.8) in ddH2O:MeOH:acetonitrile (40:40:20 v/v/v). Flow rate throughout the gradient program was maintained at 0.6 ml/min. The following gradient conditions were used: initial condition was 20% B, linearly changed to 80% B over 1 min, held at 80% B for 3 min, changed to 100% B for 6 min, held at 100% B for 3 min, then returned to 20% B for 7 min for a total run time of 20 min. Electrochemical cells were set at 440, 500, 560, 620, 680, 740, 800, and 860 mV. The column and cells were maintained at 37°C. Authentic standards of BCA (Sigma-Aldrich) were used. Concentration of BCA was quantified against calibration curves of authentic standards running CoulArray v.3.1 software (Thermo Fisher Scientific).
BCA supplementation
Male C57/BL6 (5 wk old; The Jackson Laboratory, Bar Harbor, ME, USA) mice were randomly divided into 3 groups, control group (75% DMSO), or test groups (BCA; 5 or 10 mg/kg body weight). BCA or vehicle was delivered by intraperitoneal injection for 5 d/wk for 4 wk.
In vivo GOT lentiviral delivery in the mouse brain
To knock down GOT in the mouse brain, iLenti-GFP scramble siRNA and iLenti-GFP GOT-1 siRNA lentiviral vector (8 μl of 1.0 × 108; Applied Biologic Materials, Richmond, BC, Canada) was delivered to S1 cortex of 8-wk-old C57BL/6 male mice by using stereotaxic injection as previously described (5, 6, 22, 30). This approach was effective in the knockdown of GOT protein in the brain 7 d after lentiviral delivery (5, 6). Stroke-affected S1 cortex was specifically targeted by using the following coordinates from bregma: –0.1 mm rostral, +2.0 mm lateral, –1.0 mm dorsal. Subsequent experiments were performed 1 wk after delivery.
Mouse stroke model
Focal cerebral ischemia was induced in 8- to 10-wk-old C57BL/6 male mice (The Jackson Laboratory) by using the intraluminal suture method of middle cerebral artery occlusion (MCAO) for 60 min as previously described (5, 6, 20, 22, 31). Successful MCAO was validated by laser Doppler flowmetry. MCAO was performed at 20–24 h after the last supplementation. After 48 h of MCAO, T2-weighted images were recorded. Mice that experienced surgical complications—for example, hemorrhage or death—during MCAO were excluded.
MRI
T2-weighted imaging was performed on stroke-affected rats or mice for infarct volume calculations using a 11.7-T (500-MHz) MR system comprised of a vertical bore magnet (Bruker Biospin, Ettlingen, Germany) as previously described (5, 6, 22, 31).
Sensorimotor assessment
At 24 h before MCAO (baseline) and 48 h poststroke, sensorimotor assessment was performed as described previously (6, 22). In brief, mice were placed in the center of a 1- × 1-m open field and allowed to freely move for 5 min while being recorded overhead using ANY-Maze video tracking software (v.4.5; Stoelting, Kiel, WI, USA). Software calculated distance, mean speed, and time mobile for baseline and 48 h poststroke open field tests.
Immunohistochemistry and microscopy
Immunostaining of GOT was performed on paraformaldehyde-fixed cryosections of brain samples. In brief, freshly dissected brain samples were fixed in 4% paraformaldehyde solution that was buffered in the physiologic pH range, cryopreserved in a 30% sucrose solution, and embedded in optimum cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA). OCT compound–embedded tissue was cryosectioned (12 μm), followed by pressure cooker antigen retrieval (citric acid–based antigen unmasking solution; Vector Laboratories, Burlingame, CA, USA), blocking in 10% normal serum, and incubation with rabbit anti-GOT mAb (1:100; Abcam). Signal was visualized by subsequent incubation with fluorescence-tagged secondary Ab (Alexa Fluor 568–tagged α-rabbit, 1:200) and counterstained with DAPI (Thermo Fisher Scientific). Images were captured on an Axio Observer.Z1 (Ziess), and mean fluorescent intensity from images was quantified by using Zen 2 (blue edition) software (Ziess) (31–33).
Statistical analyses
Data are reported as means ± sd/se. Before experimentation, mice were randomly assigned to treatment groups and coded for blinded data analyses. The sample size for each group was estimated on the basis of experience with the MCAO model. Differences in means were tested by using Student’s t test or 1-way ANOVA followed by Scheffé’s post hoc test. A value of P < 0.05 was considered statistically significant.
RESULTS
BCA is potent inducer of GOT expression and activity in neural cells
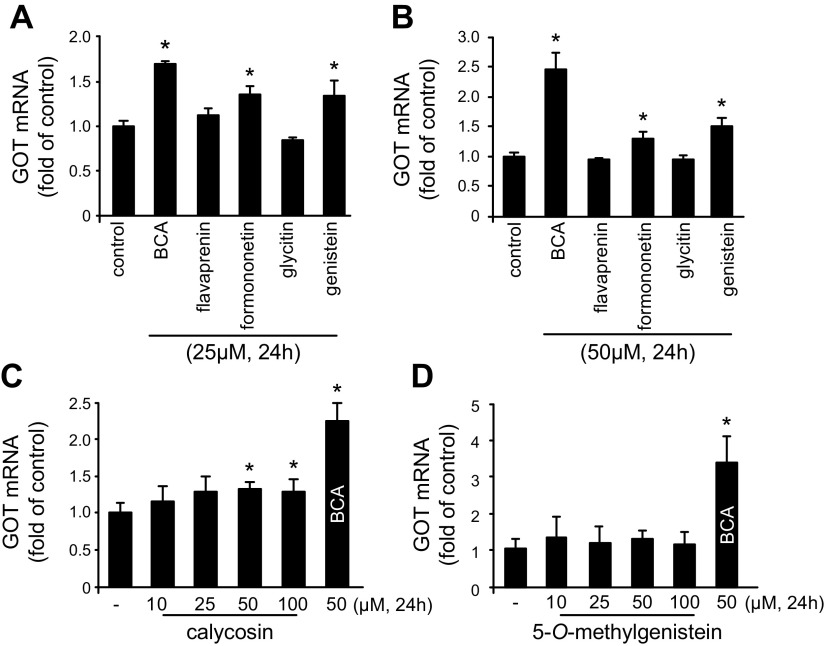
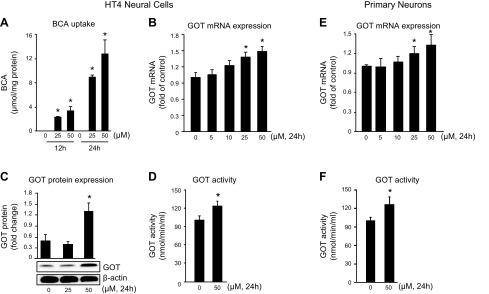
We have previously reported that GOT attenuates neurotoxicity by metabolizing glutamate in the stroke-affected brain (6). This observation led us to screen phytoestrogen isoflavones to identify a small-molecule inducer of GOT in HT4 neural cells. BCA (25 and 50 μM) was identified as the most potent inducer of GOT mRNA expression in HT4 neural cells compared with other phytoestrogen isoflavones and vehicle control DMSO (Fig. 1). To test the bioavailability of BCA, HT4 neural cells were treated with 25 or 50 μM BCA. Compared with DMSO vehicle, BCA treatment increased the intracellular concentration in a time- and dose-dependent manner (Fig. 2A). BCA treatment significantly induced GOT mRNA (Fig. 2B), protein (Fig. 2C), and activity (Fig. 2D) in HT4 neural cells. GOT mRNA expression and activity were significantly increased in primary cortical neurons that were treated with 50 μM BCA (Fig. 2E, F).
Figure 1.
BCA is a potent inducer of GOT mRNA expression in neural cells. A, B) HT4 cells were treated with 25 (A) or 50 μM (B) phytoestrogen isoflavones for 24 h. Phytoestrogen screening in HT4 neural cells identified BCA, an O-methylated isoflavone, as a potent inducer of GOT mRNA expression. C, D) GOT induction is specific to BCA compared with different O-methylated isoflavone family members calycosin (C) and 5-O-methylgenistein (D). Results are presented as means ± sd. *P < 0.05 vs. control.
Figure 2.
BCA induces GOT expression and activity in neural cells. A) Treatment of cells with BCA (25 and 50 μM) resulted in a time- and dose-dependent increase in cellular BCA content as measured by HPLC. B–D) HT4 neural cells that were treated with BCA showed a significant increase in GOT mRNA expression (B), protein expression (C), and activity (D). E, F) BCA significantly increased mRNA expression (E) and GOT activity (F) in primary cortical neurons. Results are presented as means ± sd. *P < 0.05 vs. control.
BCA protection against glutamate-induced toxicity is GOT dependent
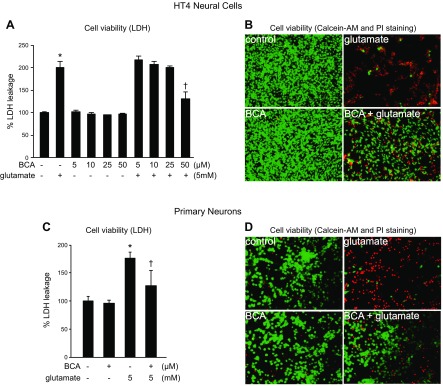
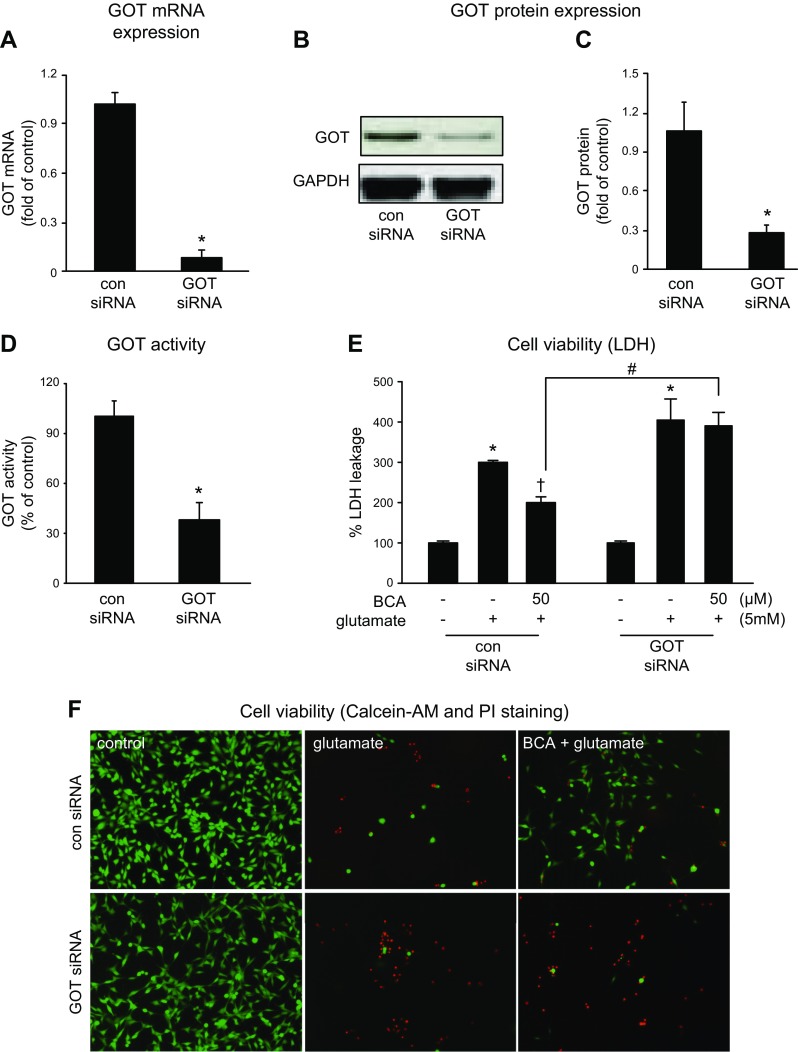
Treatment of HT4 cells (Fig. 3A, B) or primary cortical neurons (Fig. 3C, D) with 5 mM glutamate resulted in overt toxicity within 24 h of exposure. Pretreatment of cells with 50 μM BCA for 24 h significantly protected cells against glutamate-induced cell death as determined by LDH leakage (Fig. 3A, C) and visualization with calcein-AM and propidium iodide (Fig. 3B, D). To test the significance of GOT in BCA protection against glutamate-induced neural cell death, we adopted a knockdown approach using siRNA (Fig. 4). The siRNA approach successfully decreased GOT mRNA (93%; Fig. 4A), protein (74%; Fig. 4C), and activity (39%; Fig. 4D). This knockdown significantly abolished BCA neuroprotection against glutamate-induced toxicity (Fig. 4E, F).
Figure 3.
BCA protects against glutamate-induced toxicity. HT4 cells (A, B) or primary neurons (C, D) were treated with either BCA or vehicle control for 24 h and challenged with glutamate. BCA conferred protection against glutamate toxicity in neural cells. Cell viability was measured after 24 h of glutamate challenge by LDH leakage (A, C) or calcein-AM (green, live cells) and propidium iodide (PI; red, dead cells) staining (B, D). Original magnification: ×10 (B), ×20 (D). Results are presented as means ± sd. *P < 0.05 vs. no BCA and no glutamate; †P < 0.05 vs. no BCA with 5 mM glutamate.
Figure 4.
GOT knockdown attenuates the neuroprotection of BCA. A–D) Transfection with GOT siRNA decreased GOT mRNA expression (A), protein expression (B, C), and enzyme activity (D). Transfected cells were treated with BCA (24 h), then challenged with glutamate for 24 h. Cells with GOT knockdown were more susceptible to glutamate-induced toxicity. *P < 0.05. E, F) Cell viability was measured after 24 h of glutamate challenge by LDH leakage (E) or calcein-AM (green, live cells) and propidium iodide (PI; red, dead cells) staining (F). Original magnification, ×10. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Results are presented as means ± sd. *P < 0.05 for effect of glutamate; †P < 0.05 for effect of BCA; #P < 0.05 for effect of siRNA.
BCA attenuates ischemic stroke brain injury in a GOT-dependent manner
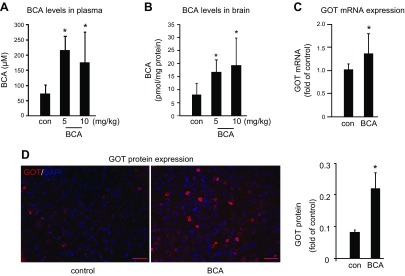
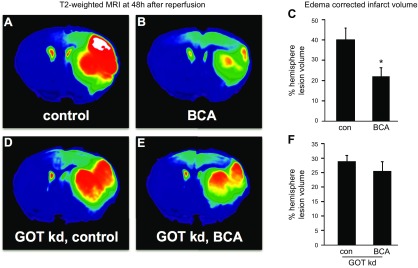
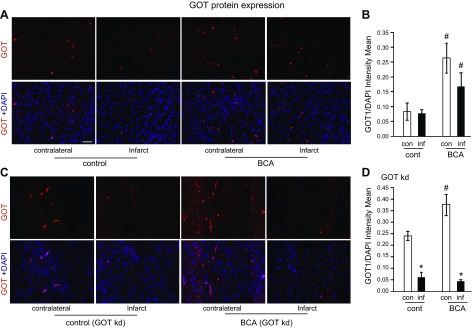
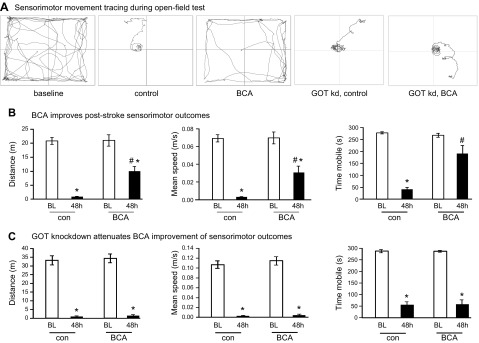
To develop the translational significance of BCA neuroprotection, we tested bioavailability in vivo. BCA is a safe dietary factor that is already under clinical investigation for other indications (34, 35). C57/BL6J mice received daily intraperitoneal injections of vehicle control or 5 or 10 mg/kg BCA for 4 wk. Both 5 and 10 mg/kg injections significantly increased BCA content in plasma and the brain (Fig. 5A, B). As the higher BCA dose (10 mg/kg) did not increase BCA levels in the brain more than the lower dose (5 mg/kg), the lower dose was used for all subsequent in vivo studies. Four weeks of daily BCA intraperitoneal injection at 5 mg/kg significantly increased GOT mRNA (Fig. 5C) and protein expression (Fig. 5D) in the mouse brain compared with vehicle control. After establishing that 4 wk of BCA supplementation was sufficient to increase GOT expression, transient focal cerebral ischemia was induced by MCAO in a separate cohort of mice. MRI analyses at 48 h after MCAO revealed that 4 wk of BCA supplementation was effective in reducing stroke-induced brain lesion volume by one half (Fig. 6A–C). We have previously published that GOT up-regulation in the mouse brain protects against stroke (5, 6). Here, we sought to test whether the protective effect of BCA against stroke was GOT dependent. To test this hypothesis, stereotaxic delivery of GOT lentiviral particles was performed after 3 wk of BCA supplementation and 7 d before stroke surgeries to allow sufficient GOT knockdown. As hypothesized, the protective effect of BCA supplementation on lesion volume was lost with GOT knockdown in the brain (Fig. 6D–F). Immunohistochemical analysis showed that the BCA-dependent induction of GOT protein expression was blunted with lentiviral knockdown in the stroke-affected hemisphere (Fig. 7A–D). Sensorimotor testing revealed that BCA supplementation significantly improved 48 h poststroke sensorimotor functions compared with controls (Fig. 8A, B). This functional improvement was lost with GOT knockdown (Fig. 8A, C).
Figure 5.
Intraperitoneally injected BCA circulates in the plasma and is delivered to brain tissue. C57/BL6J mice underwent daily intraperitoneal injection of vehicle control or 5 or 10 mg/kg BCA for 4 wk. A, B) Plasma (A) and brain (B) were collected 30 min after intraperitoneal injection on d 28 for HPLC determination of BCA levels. C, D) GOT mRNA (C) and protein (D) expression was increased in BCA-supplemented mouse brain. Scale bars, 50 µm. Data are presented as means ± se; n = 9. *P < 0.05 vs. placebo.
Figure 6.
BCA supplementation protects against stroke via GOT. C57/BL6 mice received daily intraperitoneal injection of vehicle control (75% DMSO in water) or BCA (5 mg/kg body weight) for 4 wk before 60 min of MCAO. A, B) BCA supplementation significantly reduced MCAO-induced brain injury (vehicle-supplemented mice, n = 7; BCA-supplemented mice, n = 6). D, E) This protection was lost with GOT knockdown (kd) (n = 6). A, B, D, E) Representative T2-weighted coronal slice from vehicle control (A), 5 mg/kg BCA (B), GOT kd + vehicle (D), GOT kd + 5 mg/kg BCA (E). C, F) Percentage hemisphere lesion volume calculated from T2-weighted MRI at 48 h after MCAO. Data are presented as means ± se. *P < 0.05 vs. vehicle.
Figure 7.
BCA increases GOT protein expression in stroke-affected and contralateral murine brain. A, C) Representative micrographs of GOT immunostaining (red) in contralateral and stroke-affected cortex of BCA-supplemented (n = 3), vehicle control (n = 4; A), and GOT-knockdown (kd) BCA-supplemented (n = 5) or vehicle control (n = 3; C). Sections were counterstained with DAPI. B, D) Expression of GOT quantified as mean fluorescent intensity normalized to DAPI. Scale bar, 50 µm. Data are presented as means ± se. #P < 0.05 compared with vehicle control; *P < 0.05 compared with contralateral hemisphere.
Figure 8.
BCA supplementation improves poststroke sensory motor function. A) Representative track plots from baseline (BL) and 48 h poststroke in control, BCA-supplemented, and GOT-knockdown (kd) groups. B,C) Distance (m), speed (m/s), and time mobile (s) were calculated by AnyMaze software at BL and 48 h poststroke for vehicle control (n = 7), BCA-supplemented (n = 6), and GOT-kd groups (n = 6). Results are presented as means ± se. *P < 0.05 compared with corresponding control; #P < 0.05 compared with infarct hemisphere of control group.
BCA induces GOT expression in an ERRE-dependent manner
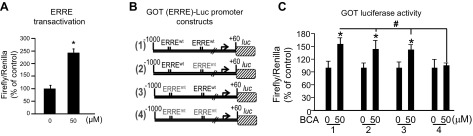
We queried the GOT promoter region to identify possible mechanisms of BCA induction. We subsequently identified 2 ERREs in the −888 to −880 and −549 to −541 regions of the GOT promoter. Phytoestrogens bind to ERRE sites in vitro and enhance transcriptional activation; thus, we tested whether BCA induced the transcriptional activation of ERRE in HT4 cells by using a dual luciferase reporter assay. Compared with DMSO control, BCA significantly increased luciferase activity in HT4 neural cells that were transfected with the pERRE3tk-LUC reporter plasmid (Fig. 9A). Next, to test whether BCA transcriptionally induces GOT in an ERRE-dependent manner, we mutated ERRE sites in a GOT-luciferase promoter construct. BCA-induced GOT-luciferase activity was abolished when both ERRE sites were mutated in the GOT promoter region (Fig. 9B, C).
Figure 9.
BCA induces GOT by transcriptional activation of ERRE. A) BCA-induced transcriptional activation of ERRE in HT4 cells. B) Schematized GOT (ERRE)-Luc promoter mutation constructs. C) BCA induced GOT-luciferase activity in the wild-type construct. This induction was lost when both ERRE sites were mutated within the GOT promoter. ERRE3-Luc or GOT-Luc constructs were transiently transfected in HT4 cells. After 48 h of transfection, cells were activated with BCA or vehicle control for 24 h. Results were normalized with Renilla luciferase activity. Data are presented as means ± sd. *P < 0.05 (BCA vs. vehicle control for a given construct); #P < 0.05 (construct 4 vs. construct 1 with BCA treatment).
DISCUSSION
In 2010, we demonstrated that the correction of stroke-induced hypoxia with supplemental oxygen (100% O2 at 2ATA) induced GOT expression and attenuated stroke injury (7). Such supplemental oxygen protection against stroke was GOT dependent (5). More recently, we identified that supplemental oxygen lowers glutamate levels at the stroke site and enables the anaplerotic refilling of TCA cycle intermediates in a GOT-dependent manner (6). The induction of GOT alone emerged as protective against stroke. Thus, the current work sought to identify alternative small-molecule inducers of GOT that may be considered for stroke therapies. Phytoestrogen isoflavones, such as BCA, are plant-derived, nonsteroidal compounds with the capability to bind to estrogen receptors because of their structural resemblance to estradiol (17-β-estradiol) (36). Soy and a variety of other food products—red clover, legumes, fruits, and flaxseed—are enriched with phytoestrogens (37). Recent efforts that have focused on uncovering the neuroprotective properties of phytoestrogen isoflavones have shown that these natural compounds have the potential to reduce neuronal damage and improve stroke outcomes (38). Daidzein protects neural cells against oxygen-glucose deprivation–induced cell death via activating peroxisome proliferator-activated receptor-γ (39) and improves poststroke sensorimotor outcomes in mice (40). Genistein is neuroprotective against H2O2-induced cell death (41, 42) and ischemic stroke in rats (43). BCA is a naturally occurring estrogenic isoflavone that is found primarily in the leaf of the red clover plant (Trifolium pratense) (44). A methyl derivative of the aforementioned isoflavone, genistein (45), a limited body of research has shown that BCA has potent phytochemical effects against a spectrum of diseases, including diabetes (46), Parkinson’s disease (47–49), and, recently found, focal cerebral ischemia (14). Regarding the latter, Wang et al. reported that BCA protects against stroke by blunting stroke-related neuroinflammation via a p38MAPK-dependent pathway. In the current study, we also observed BCA attenuation of stroke-induced inflammation as evidenced by reduced edema in T2-weighted MR images; however, this work recognizes that the phytoestrogen, BCA, protects against stroke by inducing neural GOT via transcriptional activation of ERREs. Whereas phytoestrogen isoflavone transcriptional activation of ERREs has been demonstrated in silico and in vitro (13), this work is the first to our knowledge to identify phytoestrogen transcriptional activation of ERRE in the GOT promoter region. The ERRE binding site sequence is conserved across promoter regions of genes that are associated with metabolic processes that include glutamate metabolism and energy sensing (50, 51). To that end, the transcriptional activation of functionally conserved metabolic networks via small-molecule binding to ERRE are increasingly recognized as therapeutic targets of interest for the treatment of disease (52) and injury (53). The current work is the first to uncover the significance of ERRE as a therapeutic target in the stroke-affected brain.
BCA is one of a number of phytoestrogen isoflavones that are enriched in red clover (54). Within the past 5 yr, other red clover isoflavone phytoestrogens have been shown to protect against ischemic stroke, including formononetin (55, 56), genistein (57–59), and daidzein (60, 61). In the current study, formononetin and genistein also induced GOT mRNA expression in vitro, albeit to a lesser degree than BCA in neuronal cells. Taken together, these outcomes warrant testing whether dietary red clover supplementation induces GOT expression and protects against stroke in a preclinical setting. As a forage legume with a long history of dietary consumption and ethnomedicinal use (62), red clover has a favorable safety profile and represents a nutraceutical product that could quickly translate to clinical testing.
Transaminases catalyze the amine group (NH2) transfer from an amino acid to an α-keto acid. As a transaminase, GOT transfers the amine group from 5-carbon glutamate to 4-carbon oxaloacetate such that glutamate becomes the 5-carbon TCA cycle intermediate, α-ketoglutarate, and oxaloacetate becomes the 4-carbon amino acid, aspartate. We have previously reported that this ability enables the anaplerotic flux of GOT-metabolized glutamate into a truncated TCA cycle in the stroke-affected and, subsequently, hypoglycemic brain (6). In the current work, this leads to the interesting possibility that BCA induction of GOT protects against stroke-induced injury by metabolizing otherwise excitotoxic glutamate to sustain cellular energetics in the absence of glucose. This conclusion is supported by an independent study that found that BCA was neuroprotective against glutamate-induced cytotoxicity in PC12 cells (63). In this study, we observed that BCA protection against stroke was lost with GOT knockdown. Specifically, both stroke-induced lesion volume and functional outcomes worsened with GOT knockdown, which demonstrates that GOT is essential for BCA protection. Taken together, the outcomes of this work show that BCA protection against ischemic stroke occurs via the GOT-mediated metabolism of glutamate in the stroke-affected brain (6).
ACKNOWLEDGMENTS
This work was funded by U.S. National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) Grant R01NS085272 (to C.R. and S.K.), and supported, in part, by NIH NINDS Grant R01NS42617 (to C.K.S.).
Glossary
- BCA
biochanin A
- ERRE
estrogen-related receptor response element
- GOT
glutamate oxaloacetate transaminase
- LDH
lactate dehydrogenase
- MCAO
middle cerebral artery occlusion
- siRNA
small interfering RNA
- TCA
tricarboxylic acid
AUTHOR CONTRIBUTIONS
S. Khanna, C. K. Sen, and C. Rink conceived of and designed the studies; S. Khanna, R. Stewart, S. Gnyawali, H. Harris, M. Balch, J. Spieldenner, and C. Rink acquired, analyzed, and interpreted data; S. Khanna, C. K. Sen, and C. Rink drafted the manuscript; and S. Khanna, S. Gnyawali, C. K. Sen, and C. Rink provided critical revision of the manuscript for important intellectual content.
REFERENCES
- 1.Khanna S., Briggs Z., Rink C. (2015) Inducible glutamate oxaloacetate transaminase as a therapeutic target against ischemic stroke. Antioxid. Redox Signal. 22, 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castillo J., Loza M. I., Mirelman D., Brea J., Blanco M., Sobrino T., Campos F. (2016) A novel mechanism of neuroprotection: blood glutamate grabber. J. Cereb. Blood Flow Metab. 36, 292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leibowitz A., Boyko M., Shapira Y., Zlotnik A. (2012) Blood glutamate scavenging: insight into neuroprotection. Int. J. Mol. Sci. 13, 10041–10066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhumadilov A., Boyko M., Gruenbaum S. E., Brotfain E., Bilotta F., Zlotnik A. (2015) Extracorporeal methods of blood glutamate scavenging: a novel therapeutic modality. Expert Rev. Neurother. 15, 501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rink C., Gnyawali S., Peterson L., Khanna S. (2011) Oxygen-inducible glutamate oxaloacetate transaminase as protective switch transforming neurotoxic glutamate to metabolic fuel during acute ischemic stroke. Antioxid. Redox Signal. 14, 1777–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rink C., Gnyawali S., Stewart R., Teplitsky S., Harris H., Roy S., Sen C. K., Khanna S. (2017) Glutamate oxaloacetate transaminase enables anaplerotic refilling of TCA cycle intermediates in stroke-affected brain. FASEB J. 31, 1709–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rink C., Roy S., Khan M., Ananth P., Kuppusamy P., Sen C. K., Khanna S. (2010) Oxygen-sensitive outcomes and gene expression in acute ischemic stroke. J. Cereb. Blood Flow Metab. 30, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rink C., Khanna S. (2011) Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid. Redox Signal. 14, 1889–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y., Rempe D. A. (2011) Prophylactic neuroprotection against stroke: low-dose, prolonged treatment with deferoxamine or deferasirox establishes prolonged neuroprotection independent of HIF-1 function. J. Cereb. Blood Flow Metab. 31, 1412–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin E. J., Blaha M. J., Chiuve S. E., Cushman M., Das S. R., Deo R., de Ferranti S. D., Floyd J., Fornage M., Gillespie C., Isasi C. R., Jiménez M. C., Jordan L. C., Judd S. E., Lackland D., Lichtman J. H., Lisabeth L., Liu S., Longenecker C. T., Mackey R. H., Matsushita K., Mozaffarian D., Mussolino M. E., Nasir K., Neumar R. W., Palaniappan L., Pandey D. K., Thiagarajan R. R., Reeves M. J., Ritchey M., Rodriguez C. J., Roth G. A., Rosamond W. D., Sasson C., Towfighi A., Tsao C. W., Turner M. B., Virani S. S., Voeks J. H., Willey J. Z., Wilkins J. T., Wu J. H., Alger H. M., Wong S. S., Muntner P.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2017) Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135, e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufour C. R., Wilson B. J., Huss J. M., Kelly D. P., Alaynick W. A., Downes M., Evans R. M., Blanchette M., Giguère V. (2007) Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 5, 345–356 [DOI] [PubMed] [Google Scholar]
- 12.Giguère V. (2002) To ERR in the estrogen pathway. Trends Endocrinol. Metab. 13, 220–225 [DOI] [PubMed] [Google Scholar]
- 13.Suetsugi M., Su L., Karlsberg K., Yuan Y. C., Chen S. (2003) Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol. Cancer Res. 1, 981–991 [PubMed] [Google Scholar]
- 14.Wang W., Tang L., Li Y., Wang Y. (2015) Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J. Neurol. Sci. 348, 121–125 [DOI] [PubMed] [Google Scholar]
- 15.Khanna S., Parinandi N. L., Kotha S. R., Roy S., Rink C., Bibus D., Sen C. K. (2010) Nanomolar vitamin E alpha-tocotrienol inhibits glutamate-induced activation of phospholipase A2 and causes neuroprotection. J. Neurochem. 112, 1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna S., Park H. A., Sen C. K., Golakoti T., Sengupta K., Venkateswarlu S., Roy S. (2009) Neuroprotective and antiinflammatory properties of a novel demethylated curcuminoid. Antioxid. Redox Signal. 11, 449–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna S., Roy S., Parinandi N. L., Maurer M., Sen C. K. (2006) Characterization of the potent neuroprotective properties of the natural vitamin E alpha-tocotrienol. J. Neurochem. 98, 1474–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna S., Roy S., Park H. A., Sen C. K. (2007) Regulation of c-Src activity in glutamate-induced neurodegeneration. J. Biol. Chem. 282, 23482–23490 [DOI] [PubMed] [Google Scholar]
- 19.Khanna S., Roy S., Ryu H., Bahadduri P., Swaan P. W., Ratan R. R., Sen C. K. (2003) Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J. Biol. Chem. 278, 43508–43515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanna S., Roy S., Slivka A., Craft T. K., Chaki S., Rink C., Notestine M. A., DeVries A. C., Parinandi N. L., Sen C. K. (2005) Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke 36, 2258–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park H. A., Kubicki N., Gnyawali S., Chan Y. C., Roy S., Khanna S., Sen C. K. (2011) Natural vitamin E α-tocotrienol protects against ischemic stroke by induction of multidrug resistance-associated protein 1. Stroke 42, 2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna S., Rink C., Ghoorkhanian R., Gnyawali S., Heigel M., Wijesinghe D. S., Chalfant C. E., Chan Y. C., Banerjee J., Huang Y., Roy S., Sen C. K. (2013) Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J. Cereb. Blood Flow Metab. 33, 1197–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sen C. K., Khanna S., Roy S., Packer L. (2000) Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J. Biol. Chem. 275, 13049–13055 [DOI] [PubMed] [Google Scholar]
- 24.Chan Y. C., Khanna S., Roy S., Sen C. K. (2011) miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J. Biol. Chem. 286, 2047–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan Y. C., Roy S., Huang Y., Khanna S., Sen C. K. (2012) The microRNA miR-199a-5p down-regulation switches on wound angiogenesis by derepressing the v-ets erythroblastosis virus E26 oncogene homolog 1-matrix metalloproteinase-1 pathway. J. Biol. Chem. 287, 41032–41043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan Y. C., Roy S., Khanna S., Sen C. K. (2012) Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler. Thromb. Vasc. Biol. 32, 1372–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanna S., Patel V., Rink C., Roy S., Sen C. K. (2005) Delivery of orally supplemented alpha-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic. Biol. Med. 39, 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel V., Rink C., Gordillo G. M., Khanna S., Gnyawali U., Roy S., Shneker B., Ganesh K., Phillips G., More J. L., Sarkar A., Kirkpatrick R., Elkhammas E. A., Klatte E., Miller M., Firstenberg M. S., Chiocca E. A., Nesaretnam K., Sen C. K. (2012) Oral tocotrienols are transported to human tissues and delay the progression of the model for end-stage liver disease score in patients. J. Nutr. 142, 513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy S., Venojarvi M., Khanna S., Sen C. K. (2002) Simultaneous detection of tocopherols and tocotrienols in biological samples using HPLC-coulometric electrode array. Methods Enzymol. 352, 326–332 [DOI] [PubMed] [Google Scholar]
- 30.Park H. A., Khanna S., Rink C., Gnyawali S., Roy S., Sen C. K. (2009) Glutathione disulfide induces neural cell death via a 12-lipoxygenase pathway. Cell Death Differ. 16, 1167–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khanna S., Heigel M., Weist J., Gnyawali S., Teplitsky S., Roy S., Sen C. K., Rink C. (2015) Excessive α-tocopherol exacerbates microglial activation and brain injury caused by acute ischemic stroke. FASEB J. 29, 828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghatak S., Li J., Chan Y. C., Gnyawali S. C., Steen E., Yung B. C., Khanna S., Roy S., Lee R. J., Sen C. K. (2016) AntihypoxamiR functionalized gramicidin lipid nanoparticles rescue against ischemic memory improving cutaneous wound healing. Nanomedicine (Lond.) 12, 1827–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen C. K., Khanna S., Harris H., Stewart R., Balch M., Heigel M., Teplitsky S., Gnyawali S., Rink C. (2017) Robot-assisted mechanical therapy attenuates stroke-induced limb skeletal muscle injury. FASEB J. 31, 927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nestel P., Cehun M., Chronopoulos A., DaSilva L., Teede H., McGrath B. (2004) A biochanin-enriched isoflavone from red clover lowers LDL cholesterol in men. Eur. J. Clin. Nutr. 58, 403–408 [DOI] [PubMed] [Google Scholar]
- 35.Teede H. J., McGrath B. P., DeSilva L., Cehun M., Fassoulakis A., Nestel P. J. (2003) Isoflavones reduce arterial stiffness: a placebo-controlled study in men and postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 23, 1066–1071 [DOI] [PubMed] [Google Scholar]
- 36.Turner J. V., Agatonovic-Kustrin S., Glass B. D. (2007) Molecular aspects of phytoestrogen selective binding at estrogen receptors. J. Pharm. Sci. 96, 1879–1885 [DOI] [PubMed] [Google Scholar]
- 37.Kurzer M. S., Xu X. (1997) Dietary phytoestrogens. Annu. Rev. Nutr. 17, 353–381 [DOI] [PubMed] [Google Scholar]
- 38.Burguete M. C., Torregrosa G., Pérez-Asensio F. J., Castelló-Ruiz M., Salom J. B., Gil J. V., Alborch E. (2006) Dietary phytoestrogens improve stroke outcome after transient focal cerebral ischemia in rats. Eur. J. Neurosci. 23, 703–710 [DOI] [PubMed] [Google Scholar]
- 39.Hurtado O., Ballesteros I., Cuartero M. I., Moraga A., Pradillo J. M., Ramírez-Franco J., Bartolomé-Martín D., Pascual D., Torres M., Sánchez-Prieto J., Salom J. B., Lizasoain I., Moro M. A. (2012) Daidzein has neuroprotective effects through ligand-binding-independent PPARγ activation. Neurochem. Int. 61, 119–127 [DOI] [PubMed] [Google Scholar]
- 40.Kim E., Woo M. S., Qin L., Ma T., Beltran C. D., Bao Y., Bailey J. A., Corbett D., Ratan R. R., Lahiri D. K., Cho S. (2015) Daidzein augments cholesterol homeostasis via ApoE to promote functional recovery in chronic stroke. J. Neurosci. 35, 15113–15126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morán J., Perez-Basterrechea M., Garrido P., Díaz E., Alonso A., Otero J., Colado E., González C. (2017) Effects of estrogen and phytoestrogen treatment on an in vitro model of recurrent stroke on HT22 neuronal cell line. Cell. Mol. Neurobiol. 37, 405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian Y., Cao L., Guan T., Chen L., Xin H., Li Y., Zheng R., Yu D. (2015) Protection by genistein on cortical neurons against oxidative stress injury via inhibition of NF-kappaB, JNK and ERK signaling pathway. Pharm. Biol. 53, 1124–1132 [DOI] [PubMed] [Google Scholar]
- 43.Cortina B., Torregrosa G., Castelló-Ruiz M., Burguete M. C., Moscardó A., Latorre A., Salom J. B., Vallés J., Santos M. T., Alborch E. (2013) Improvement of the circulatory function partially accounts for the neuroprotective action of the phytoestrogen genistein in experimental ischemic stroke. Eur. J. Pharmacol. 708, 88–94 [DOI] [PubMed] [Google Scholar]
- 44.De Rijke E., Zafra-Gómez A., Ariese F., Brinkman U. A., Gooije C. (2001) Determination of isoflavone glucoside malonates in Trifolium pratense L. (red clover) extracts: quantification and stability studies. J. Chromatogr. A 932, 55–64 [DOI] [PubMed] [Google Scholar]
- 45.Chan H. Y., Wang H., Leung L. K. (2003) The red clover (Trifolium pratense) isoflavone biochanin A modulates the biotransformation pathways of 7,12-dimethylbenz[a]anthracene. Br. J. Nutr. 90, 87–92 [DOI] [PubMed] [Google Scholar]
- 46.Harini R., Ezhumalai M., Pugalendi K. V. (2012) Antihyperglycemic effect of biochanin A, a soy isoflavone, on streptozotocin-diabetic rats. Eur. J. Pharmacol. 676, 89–94 [DOI] [PubMed] [Google Scholar]
- 47.Chen H. Q., Jin Z. Y., Li G. H. (2007) Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci. Lett. 417, 112–117 [DOI] [PubMed] [Google Scholar]
- 48.Wang J., He C., Wu W. Y., Chen F., Wu Y. Y., Li W. Z., Chen H. Q., Yin Y. Y. (2015) Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage and oxidative stress in a rat model of Parkinson’s disease. Pharmacol. Biochem. Behav. 138, 96–103 [DOI] [PubMed] [Google Scholar]
- 49.Wang J., Wu W. Y., Huang H., Li W. Z., Chen H. Q., Yin Y. Y. (2016) Biochanin A protects against lipopolysaccharide-induced damage of dopaminergic neurons both in vivo and in vitro via inhibition of microglial activation. Neurotox. Res. 30, 486–498 [DOI] [PubMed] [Google Scholar]
- 50.Audet-walsh É., Giguére V. (2015) The multiple universes of estrogen-related receptor α and γ in metabolic control and related diseases. Acta Pharmacol. Sin. 36, 51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eichner L. J., Giguère V. (2011) Estrogen related receptors (ERRs): a new dawn in transcriptional control of mitochondrial gene networks. Mitochondrion 11, 544–552 [DOI] [PubMed] [Google Scholar]
- 52.Stein R. A., McDonnell D. P. (2006) Estrogen-related receptor alpha as a therapeutic target in cancer. Endocr. Relat. Cancer 13 (Suppl 1), S25–S32 [DOI] [PubMed] [Google Scholar]
- 53.Hubbard W. J., Bland K. I., Chaudry I. H. (2015) The ERRor of our ways: estrogen-related receptors are about energy, not hormones, and are potential new targets for trauma and shock. Shock 44, 3–15 [DOI] [PubMed] [Google Scholar]
- 54.Spagnuolo P., Rasini E., Luini A., Legnaro M., Luzzani M., Casareto E., Carreri M., Paracchini S., Marino F., Cosentino M. (2014) Isoflavone content and estrogenic activity of different batches of red clover (Trifolium pratense L.) extracts: an in vitro study in MCF-7 cells. Fitoterapia 94, 62–69 [DOI] [PubMed] [Google Scholar]
- 55.Liang K., Ye Y., Wang Y., Zhang J., Li C. (2014) Formononetin mediates neuroprotection against cerebral ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2 ratio and upregulation PI3K/Akt signaling pathway. J. Neurol. Sci. 344, 100–104 [DOI] [PubMed] [Google Scholar]
- 56.Zhu H., Zou L., Tian J., Lin F., He J., Hou J. (2014) Protective effects of sulphonated formononetin in a rat model of cerebral ischemia and reperfusion injury. Planta Med. 80, 262–268 [DOI] [PubMed] [Google Scholar]
- 57.Aras A. B., Guven M., Akman T., Alacam H., Kalkan Y., Silan C., Cosar M. (2015) Genistein exerts neuroprotective effect on focal cerebral ischemia injury in rats. Inflammation 38, 1311–1321 [DOI] [PubMed] [Google Scholar]
- 58.Qian Y., Guan T., Huang M., Cao L., Li Y., Cheng H., Jin H., Yu D. (2012) Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem. Int. 60, 759–767 [DOI] [PubMed] [Google Scholar]
- 59.Wang S., Wei H., Cai M., Lu Y., Hou W., Yang Q., Dong H., Xiong L. (2014) Genistein attenuates brain damage induced by transient cerebral ischemia through up-regulation of ERK activity in ovariectomized mice. Int. J. Biol. Sci. 10, 457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aras A. B., Guven M., Akman T., Ozkan A., Sen H. M., Duz U., Kalkan Y., Silan C., Cosar M. (2015) Neuroprotective effects of daidzein on focal cerebral ischemia injury in rats. Neural Regen. Res. 10, 146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stout J. M., Knapp A. N., Banz W. J., Wallace D. G., Cheatwood J. L. (2013) Subcutaneous daidzein administration enhances recovery of skilled ladder rung walking performance following stroke in rats. Behav. Brain Res. 256, 428–431 [DOI] [PubMed] [Google Scholar]
- 62.Cornara L., Xiao J., Burlando B. (2016) Therapeutic potential of temperate forage legumes: a review. Crit. Rev. Food Sci. Nutr. 56 (Suppl 1), S149–S161 [DOI] [PubMed] [Google Scholar]
- 63.Tan J. W., Tham C. L., Israf D. A., Lee S. H., Kim M. K. (2013) Neuroprotective effects of biochanin A against glutamate-induced cytotoxicity in PC12 cells via apoptosis inhibition. Neurochem. Res. 38, 512–518 [DOI] [PubMed] [Google Scholar]