Abstract
During sepsis and shock states, mitochondrial dysfunction occurs. Consequently, adaptive mechanisms, such as fission, fusion, and mitophagy, are induced to eliminate damaged portions or entire dysfunctional mitochondria. The regulatory PINK1/Parkin and DJ-1 pathways are strongly induced by mitochondrial depolarization, although a direct link between loss of mitochondrial membrane potential (ΔΨ) and mitophagy has not been identified. Mitochondria also buffer Ca2+, and their buffering capacity is dependent on ΔΨ. Here, we characterize a role for calcium/calmodulin-dependent protein kinase (CaMK) I in the regulation of these mechanisms. Loss of ΔΨ with either pharmacologic depolarization or LPS leads to Ca2+-dependent mitochondrial recruitment and activation of CaMKI that precedes the colocalization of PINK1/Parkin and DJ-1. CaMKI is required and serves as both a PINK1 and Parkin kinase. The mechanisms operate in both immune and nonimmune cells and are induced in in vivo models of endotoxemia, sepsis, and hemorrhagic shock. These data support the idea that CaMKI links mitochondrial stress with the PINK1/Parkin and DJ-1 mechanisms of mitophagy.—Zhang, X., Yuan, D., Sun, Q., Xu, L., Lee, E., Lewis, A. J., Zuckerbraun, B. S., Rosengart, M. R. Calcium/calmodulin-dependent protein kinase regulates the PINK1/Parkin and DJ-1 pathways of mitophagy during sepsis.
Keywords: mitochondria, macrophage, autophagy, mitochondrial membrane potential
Preserving a critical threshold of energy (i.e., ATP) is essential for cell and, thus, organism survival to such a severe insult as sepsis. Mitochondria are the predominant source of oxidative metabolism (1); however, during sepsis and other shock states, mitochondrial dysfunction occurs that is characterized by a loss in mitochondrial membrane potential (ΔΨ), the elaboration of toxic reactive oxidant intermediates and, if progressive, the opening of the mitochondrial permeability transition pore, release of cytochrome C, and apoptosis (2–7). Not surprisingly, each cell is in possession of adaptive mechanisms that are engaged in such circumstances to prepare for and protect against this stress. These mechanisms include fission, fusion, and mitophagy—processes in which damaged portions of dysfunctional mitochondria are pinched off and sequestered for elimination (8–11).
Autophagy is an ancient cytoplasmic process that governs cellular biomass quantity and quality (12–16). It endows cells with the ability to recycle portions of their cytoplasm to support vital functions during periods of stress (i.e., starvation). Mitophagy—autophagy directed at the removal of damaged mitochondria—is considered to be of particular importance in the protection against organ injury (8–10, 17). Prototypically, PINK1 accumulation at the outer mitochondrial membrane selectively recruits Parkin, which, in turn, promotes the selective degradation of dysfunctional mitochondria by mitophagy (8, 9, 11, 17–21). Conditions that induce mitochondrial depolarization strongly activate this pathway, which is important for cell survival (8, 18, 19, 22, 23). In addition, mitochondria undergo fission and fragmentation in response to energetic stress, which isolates dysfunctional mitochondrial components from healthy functional mitochondrial components and targets the former for mitophagic elimination (10, 24–26). These processes are regulated, in part, by DJ-1, which has been shown to protect cells against reactive oxygen species (ROS)–mediated cell damage (27–31). Yet the mechanisms by which loss of ΔΨ leads to the selective targeting of dysfunctional mitochondria for mitophagy remains to be fully characterized.
Mitochondria also influence cellular Ca2+ signaling (32–36). By taking up and releasing Ca2+, mitochondria determine the spatiotemporal profile of cellular Ca2+ signals and the activity of Ca2+-regulated proteins. Mitochondria provide a high-capacity, low-affinity Ca2+-buffering system that sequesters Ca2+ predominantly via the negative electrochemical gradient that is generated by ΔΨ (32, 33, 37). Mitochondrial capacity to buffer Ca2+ is dependent on ΔΨ, and depolarization reduces buffering capacity, which leads to mitochondrial Ca2+ leak (33, 34, 37). Thus, Ca2+ may be an ideal candidate signal that, as a molecular flare, targets mitophagy to dysfunctional mitochondria.
Calcium/calmodulin-dependent protein kinases (CaMKs), a family of serine/threonine kinases that are responsive to intracellular calcium concentration ([Ca2+]i), are integral to the immune response, mediating Ca2+-dependent macrophage (Mϕ) function and regulating septic inflammation (38–44). We have recently shown that the predominantly cytoplasmic CaMKI regulates Mϕ autophagy in a model of LPS-induced acute neutrophilic lung inflammation (43). We now propose that CaMKI also regulates the protein machinery of mitophagy. Specifically, we hypothesize that sepsis perturbs mitochondrial integrity and ΔΨ, thereby generating a Ca2+ signal that recruits CaMKI to selectively target injured mitochondria for mitophagy.
MATERIALS AND METHODS
Reagents and Abs
Ultrapure LPS (Escherichia coli 0111:B4) was obtained from List Biologicals (Campbell, CA, USA). Carbonyl cyanide m-chlorophenyl hydrazine (CCCP) was obtained from Sigma-Aldrich (St. Louis, MO, USA). mAb for Thr177/180-phosphorylated CaMKI (pCaMKI) was a generous gift of Dr. Naohito Nozaki (Kagawa University, Kanagawa, Japan) (45). This mAb has been shown to recognize the active, threonine-phosphorylated species of CaMKIα (Thr177), CaMKIβ (Thr180), and CaMKIδ (Thr178) (42, 43, 45). Abs for CaMKI, autophagy-related protein 7 (ATG7), phospho-serine, ATP5a, cytochrome c oxidase subunit 4, tubulin, and actin were obtained from Abcam (Cambridge, MA, USA). Rabbit anti-PINK1 and anti-Parkin Abs were purchased from Novus Biologicals (Littleton, CO, USA). Mouse anti-Parkin Ab for immunoprecipitation and anti–DJ-1 Ab were obtained from Cell Signaling Technology (Danvers, MA, USA).
Animal experimentation
All animal experiments were performed in accordance with the U.S. National Institutes of Health guidelines under protocols approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. We randomly grouped 8- to 12-wk-old male C57BL/6J mice and assigned them to a specific experiment. Investigators who treated animals knew the treatment groups and collected samples, which were then analyzed by other investigators who were blinded to the specific treatment.
In vivo RNA inhibition
We performed in vivo RNA inhibition (RNAi) to selectively inhibit CaMKIα, as previously performed (42, 43). C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were administered CaMKIα or scrambled, nontarget (NT) small interfering RNA (siRNA; 6 mg/kg) delivered in (animal mass/10) ml lactated Ringer’s solution by hydrodynamic tail vein injection 72 h before experimentation. We and others have shown that this technique efficiently and specifically inhibits the expression of CaMKIα (42, 43). For all experiments, successful knockdown was assessed by immunoblot.
Endotoxemia
Mice were anesthetized by using a mixture of isoflurane and oxygen bled in at 3.5 L/min. Ultrapure LPS (E. coli 0111:B4) from List Biologicals was dissolved in DNase-/RNase-free sterile normal saline and intraperitoneally injected (5 mg/kg) (44, 46). At various time points after LPS, mice were euthanized, blood was isolated by cardiac puncture, and organs were harvested.
Cecal ligation and puncture
Mice underwent a 21-gauge double-puncture version of our model of cecal ligation and puncture (CLP) (41, 42). In brief, mice were anesthetized with isoflurane (2–4% induction) and ketamine/xylazine (75/6 mg/kg, i.p. injection). The surgical site was shaved and sterilely prepped and draped. A 1-cm midline laparotomy incision was made, and the cecum was identified, devascularized, and ligated tightly 10 mm from its tip with a 4.0 silk suture without obstructing the bowel. The cecum was punctured twice with a sterile 21-gauge needle on the antimesenteric border, and gentle pressure was applied to the cecum to extrude a small amount of feces (1 mm). The bowel was returned to the peritoneal cavity, and the abdominal incision was closed in 2 layers with 4.0 silk suture. Saline (3 cc/100 g) was then s.c. injected to resuscitate and prevent dehydration. After surgery, animals were i.m. injected with an analgesic (0.10 mg/kg buprenorphine) and every 6 h thereafter. Sham-surgery controls underwent laparotomy and bowel manipulation without CLP. Antibiotics were not administered.
Hemorrhage shock and resuscitation
Hemorrhage shock (HS) and resuscitation (HS/R) was performed as previously described (47). In brief, bilateral inguinal dissections were performed, small femoral arteriotomies were made, and femoral arteries were cannulated—one for bleeding and shed blood reinfusion, the other for continuous hemodynamic monitoring. Animals were hemorrhaged during a 15-min period to achieve a mean arterial pressure of 25 mmHg, which was maintained for 120 min by either reinfusion or withdrawal of shed blood. Animals were euthanized, and blood and tissues were collected for analysis. For the HS/R group, animals were transfused with 3 times the volume of maximal shed blood with lactated Ringer’s solution after 120 min of HS. Throughout all experiments, body temperature was maintained at 37°C. Animals were euthanized 4 h after HS/R and blood and tissues were collected for analysis. Control animals received cannulation only without hemorrhage.
Cells and treatment
Murine Mϕ RAW 264.7 and hepatocyte HuH-7 cell lines (American Type Culture Collection, Manassas, VA, USA) were grown in DMEM (BioWhittaker, Walkersville, MD, USA) that was supplemented with 10% fetal calf serum (Sigma-Aldrich), 50 U/ml penicillin, and 50 µg/ml streptomycin (Cellgro Mediatech, Kansas City, MO, USA). Selected cell populations were treated with LPS (100 ng/ml for RAW 264.7 and 1 μg/ml for HuH7) or CCCP (10 μM) for the time periods described in figure legends.
siRNA
RAW 264.7 Mϕs were plated in each well of a 24-well plate (immunofluorescence) or in a 10-cm dish (immunoprecipitation and mitochondrial isolation), which resulted in 30% confluence (42, 43). NT and CaMKI siRNA were obtained from Dharmacon (Lafayette, CO, USA). We used the Smartpool siRNA from Dharmacon that incorporates 4 separate siRNA sequences for each CaMKI. The following CaMKI sequences were used: CaMKIα: sense, 5′-GAACGAGAUUGCCGUCUUAUU-3′, antisense, 5′-UAAGACGGCAAUCUCGUUCUU-3′; sense, 5′-CGGAAGACAUUAGGGAUAUUU-3′, antisense, 5′-AUAUCCCUAAUGUCUUCCGUU-3′; sense, 5′-GGAGAGCUGUUUGACCAGGUU-3′, antisense, 5′-UUCGGUCAAACAGCUCUCCUU-3′; sense, 5′-AUACAGCUCUGGAUAAGAAUU-3′, antisense, 5′-UUCUUAUCCAGAGCUGUAUUU-3′.
In a separate tube, 3 µl HiPerfect (Qiagen, Valencia, CA, USA) was added in 100 µl Opti-MEM with 25 nM siRNA and incubated at room temperature for 10 min. This transfection mixture was applied to each well and incubated for 72 h. Volumes were optimized for experiments by using 10-cm dishes. The inhibition of each targeted protein was determined by immunoblot. All experiments and cell number determinations were performed in triplicate (42, 43).
Plasmid construction and transfection
Plasmids that encode a constitutively active CaMKI (CaMKI293) or a kinase-inactive CaMKI293K49A mutant were generous gifts of Dr. Marinna Picciotto (Yale University, New Haven, CT, USA). CaMKI293 contains a C-terminally truncated version of the CaMKI-encoding gene, truncated to 293. CaMKI293K49A was constructed by changing Lys49 to alanine in CaMKI293, which negatively affects ATP binding at the catalytic site (48, 49). For transient transfection, RAW 264.7 cells were seeded in a 10-cm dish plate. (42, 43) After 2 h of adhesion, Mϕs were transfected with 10 μg of plasmid CaMKI293 or CaMKI293K49A by using the Lipofectamine 2000 reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. After transfection, cells were handled as detailed in figure legends. The GFP-LC3 plasmid was the generous gift of Wentao Gao (University of Pittsburgh). RAW 264.7 cells were plated on glass coverslips and transfected with GFP-LC3. After 18 h, cells were exposed to either LPS (100 ng/ml) for 2 h or CCCP (10 μM) for 1 h. Cells were then fixed, permeabilized, and stained with anti-TOM20 Ab, which stains mitochondria independent of ΔΨ.
Cellular protein extraction
Total cellular protein was extracted at 4°C in 500 μl lysis buffer (42, 43). Protein concentration was determined by using a bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL, USA).
Mitochondrial isolation
Mitochondria were isolated from RAW 264.7 and HuH7 cells by using the Mitochondria Isolation Kit (Pierce Biotechnology) according to manufacturer instructions. Mitochondria were isolated from fresh mouse organ samples by differential centrifugation by using ice-cold isolation buffer 1 [225 mM mannitol, 75 mM sucrose, 10 mM HEPES, 1 mM EGTA, and 0.1% (w/v) fatty acid–free bovine serum albumin, pH 7.4] with both phosphatase (cocktail set II and IV; Calbiochem, San Diego, CA, USA) and protease (Sigma-Aldrich) inhibitors added. In brief, livers and spleens were cut into small pieces and homogenized by using a glass homogenizer and pestle, followed by 2 centrifugations at 1300 g for 10 min. Supernatant was then transferred into a new tube and centrifuged at 10,000 g for 10 min. The crude mitochondrial pellets were further fractionated by centrifugation via a discontinuous step gradient of 15, 22, and 50% Percoll in IBII (isolation buffer 1 without 0.1% bovine serum albumin) for 10 min at 31,000 g. After removal from the gradient, mitochondrial suspensions were washed twice with isolation buffer 2. Purified mitochondria were lysed with 2% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) in 1× cell lysis buffer (Cell Signaling Technology) with PMSF. Mitochondrial protein concentration was determined by using the Pierce BCA Protein Assay kit (Pierce Biotechnology).
Immunoprecipitation
Five microliters Ab was added to 500 μg isolated cellular protein within lysis buffer and incubated at 4°C overnight (43). Thirty microliters of 50% slurry of prewashed Protein A/G PLUS-Agarose (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was then added to each sample, followed by incubation for an additional 2 h at 4°C. Samples were spun at 14,000 rpm and washed 4 times in lysis buffer. Samples were then resuspended in 30 μl lysis buffer for future analysis.
Immunoblot
Total cellular lysate, mitochondrial lysate, or immunoprecipitated protein was electrophoresed in a 10 or 15% SDS-PAGE—on the basis of the protein of interest—and transferred to a Hybond-ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The membrane was blocked for 1 h at room temperature with 5% milk and incubated with primary Ab overnight at 4°C. Blots were then incubated in a horseradish peroxidase–conjugated secondary Ab against the primary Ab at room temperature for 1 h. The blot was developed by using the SuperSignal chemiluminescent substrate (Thermo Fisher Scientific) and exposed by using Gel Doc XR+ Imaging System (Bio-Rad, Hercules, CA, USA). All gels were reblotted for total protein to confirm equal loading or to assess knockdown after RNAi. Densitometry was performed by ImageJ64 (National Institutes of Health, Bethesda, MD, USA) (42–44).
Microscopy
Immunofluorescent microscopy was performed as previously described (43, 44, 50). Cells were plated on sterilized glass slides and allowed to adhere overnight at 37°C. For experiments with MitoTracker Red CMXROS (Thermo Fisher Scientific) staining, cells were stimulated with LPS for 4 h, then 100 nM MitoTracker Red CMXROS was added to the medium directly for 30 min of incubation before treatment stopped. Cells underwent 3 washes with PBS and were then fixed in 2% paraformaldehyde at 4°C. Samples were incubated with rabbit anti-CaMKI (Abcam), anti-Parkin (Abcam), or anti-TOM20 (Santa Cruz Biotechnology) for 60 min at room temperature. Coverslips or cover glass were adhered with gelvatol, a water-soluble mounting medium, and slides were placed at 4°C overnight before imaging. Samples were viewed at ×60 with either a 2- or 3-fold digital zoom with a numeric aperture of 0.8 on an Olympus Fluoview 1000 (Olympus, Tokyo, Japan). Imaging conditions were maintained at identical settings within each Ab-labeling experiment with original gating performed by using the negative control. Images were acquired digitally from a randomly selected pool of 10–15 fields for each experimental condition (50). Sample processing and imaging occurred at the Center for Biologic Imaging (University of Pittsburgh).
Electron microscopy
For in vitro experiments, RAW 264.7 cells were fixed with a glutaraldehyde mixture for 1 h, then switched to PBS and imaged with JEM 1011 Transmission Electron Microscope (Jeol, Tokyo, Japan) with ×12,000 magnification. Mitochondrial density and autophagosome density were assessed by using ImageJ by measuring the percentage surface area of mitochondria or autophagosomes, respectively, of the total non-nuclear area per micrograph. For each condition, 4 micrographs were read per sample and each condition was repeated in triplicate (6).
In vitro kinase assay
An in vitro kinase assay was performed as previous published (42, 43). In brief, 1 μg PINK1 or Parkin protein (ProSpec, East Brunswick, NJ, USA) was incubated in the presence or absence of 25 ng CaMKI for 10 min at 30°C with the following additions: 10 mM MgCl2, 0.2 mM ATP, 1 mM CaCl2, and 1 μM CaM in 50 μl reaction system. Reactions were terminated by boiling in SDS–2-ME dissociation solution and analyzed by immunoblot.
ΔΨ measurement
Tetramethylrhodamine methyl ester (TMRM) is a potentiometric, cell-permeable fluorescent indicator that accumulates in the highly negatively charged interior of mitochondria. Loss of ΔΨm causes TMRM to leak from mitochondria, which results in a loss of fluorescence intensity. RAW 264.7 cells were incubated with 200 nM TMRM and 1 μM MitoTracker Deep Red (color set as green) for 15 min, then exposed to either LPS (100 ng/ml) or CCCP (10 μM) for indicated times. Live imaging of Mϕs was then performed by using an inverted Nikon TIE fluorescent microscope (Nikon, Tokyo, Japan) that was equipped with a ×60 oil immersion optic (CFI PlanFluor, 1.43 numeric aperture; Nikon) and NIS Elements Software (Nikon), with the application of live time series TMRM fluorescence by illumination at 514 nm and detection at 570 nm (6, 7, 51).
Measurement of respiration
Mϕ respiration or mitochondrial function was determined by placing cells in XF24 cell culture plates (Seahorse Biosciences, North Billerica, MA, USA) in a final volume of 500 μl. In brief, oxygen consumption rates were measured at each time point at basal rates and after the addition of oligomycin (2.0 μM) or FCCP (5.0 μM), as previously described (52).
ATP quantification
ATP determination kit (Thermo Fisher Scientific) was used for the quantification of ATP content according to manufacturer instructions. Luminescence was measured by using the Soft-MaxPro ATPase Assay program on a Synergy Mx plate reader (BioTek, Winooski, VT, USA) (7).
Mitochondrial DNA extraction and real-time quantitative PCR
mtDNA from serum was isolated by using QiAmp DNA Blood Mini Kit (Qiagen) per manufacturer protocol. In brief, 200 μl serum was used for isolation and final mtDNA was eluted with 50 μl RNase- and DNase-free water. Real-time quantitative PCR amplification was performed on CFX Connect Real-Time PCR Detection System (Bio-Rad) by using the following thermal profile: 95°C for 30 min, 40 cycles of 95°C for 5 s, 60°C for 1 min. Mitochondrially-encoded NADH dehydrogenase 1 (mtNd1) was used to indicate the mtDNA level released into blood. The primer and primePCR template for mouse mtNd1 were purchased from Bio-Rad. Five microliters of mtDNA was used for each well mixed with iTaq Universal SYBR Green Supermix (Bio-Rad). The mtNd1 templet (20 million copies) was serial diluted ×10 (6).
Statistical analysis
Statistical analyses were performed by using STATA 12SE software (College Station, TX, USA). Values are expressed as means ± sem. Groups are compared by ANOVA. Values of P < 0.05 were considered statistically significant.
RESULTS
Mitochondrial depolarization induces the translocation of active pCaMKI, PINK1, Parkin, and DJ-1 to the mitochondrion
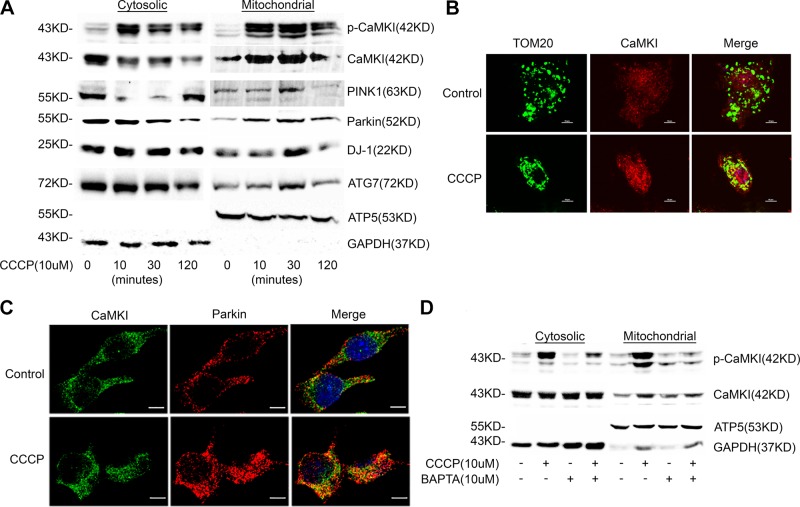
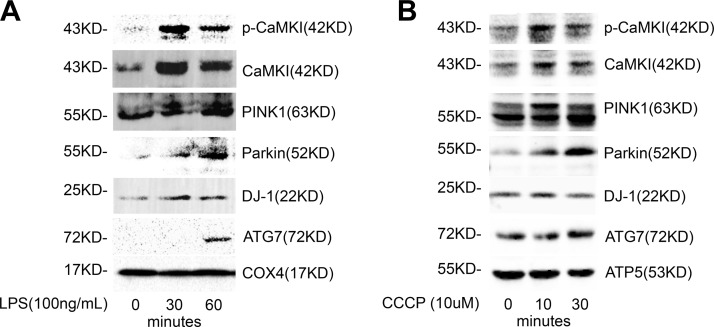
Mitochondrial depolarization is a strong stimulus for mitophagy (8, 9, 18, 19, 22, 23), and the capacity of mitochondria to buffer calcium is directly dependent on ΔΨ (32–34, 37). These data suggest that mitochondrial depolarization may alter calcium signaling and may be sufficient to induce the activation of CaMKI. Indeed, we observed that Mϕ exposure to the depolarizing agent, CCCP, induced a rapid accumulation of active p-Thr177-CaMKI in the mitochondrial fraction that correlated with increased mitochondrial expression of PINK1, Parkin, and the autophagy protein, ATG7 (Fig. 1A). In addition, we observed increased mitochondrial expression of DJ-1, a protein that has been shown to regulate mitochondrial fission during oxidative stress and to protect cells against oxidative damage (26–31, 53). Immunofluorescent microscopy demonstrated that CaMKI colocalized to the mitochondrial marker, TOM20 (Fig. 1B), and with Parkin (Fig. 1C). Activation and mitochondrial translocation of CaMKI was regulated by Ca2+, as Ca2+ chelation with BAPTA [1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid] was inhibitory (Fig. 1D).
Figure 1.
Mitochondrial depolarization induces the translocation of active pCaMKI, PINK1, and Parkin to the mitochondrion. A) RAW 264.7 cells were exposed to CCCP (10 μM) for indicated times. Total cell and mitochondria were isolated and lysed, and total cytosolic or mitochondrial protein were analyzed by immunoblot (n = 2 independent experiments). B) RAW 264.7 cells were exposed to CCCP (10 μM) for 1 h and analyzed by immunofluorescence (×60 with a 3-fold zoom) for CaMKI and TOM20 (mitochondrial marker; n = 2 independent experiments). C) RAW 264.7 cells were exposed to CCCP (10 μM) for 1 h and analyzed by immunofluorescence (×60 with a 2-fold zoom) for CaMKI and Parkin (n = 4 independent experiments). D) RAW 264.7 cells were exposed to CCCP (10 μM) for 1 h in either the presence or absence of BAPTA [1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; 10 μM]. Total cell and mitochondria were isolated and lysed, and total cytosolic or mitochondrial protein were analyzed by immunoblot (n = 2 independent experiments). GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Scale bars, 10 μm.
LPS induces the translocation of active pCaMKI, PINK1, Parkin, and DJ-1 to the mitochondrion
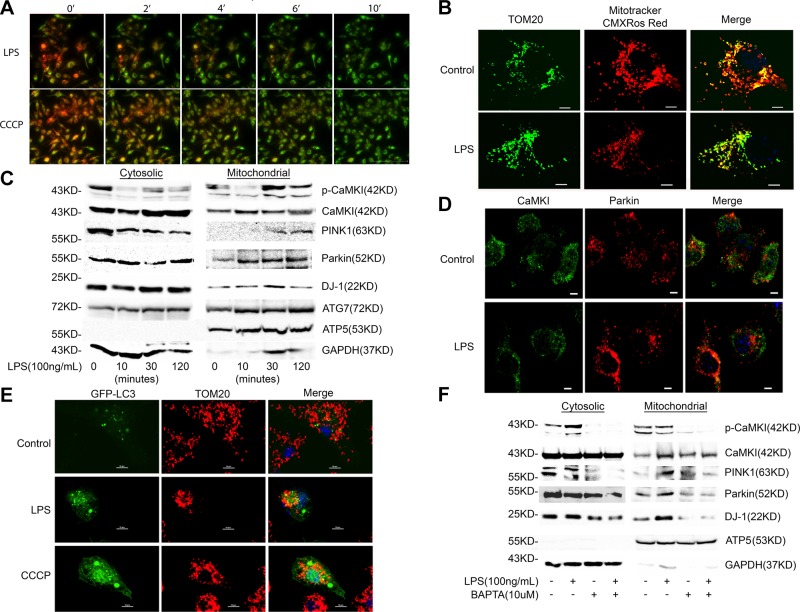
Mϕ or hepatocyte exposure to LPS causes a loss of ΔΨ (4, 6, 7, 54, 55). We too observed reduced ΔΨ when Mϕs were exposed to LPS, as evidenced by progressively less fluorescence of TMRM (Fig. 2A) and less avid concentration of CMXROS Red, the accumulation of which is directly dependent on ΔΨ (Fig. 2B) (56–58). We previously reported that LPS induces CaMKI activation and that CaMKI regulates Mϕ autophagy (42, 43). We now focused on CaMKI and mitophagy and initially explored whether, in Mϕs, LPS induces the translocation of CaMKI to mitochondria. As shown in Fig. 2C, LPS induced a time-dependent increase in mitochondrial CaMKI and active pCaMKI that peaked 30–60 min after LPS exposure (Fig. 2C). Mitochondrial recruitment of CaMKI preceded and correlated with an increase in mitochondrial expression of PINK1, Parkin, DJ-1, and the autophagy protein, ATG7 (Fig. 2C). Immunofluorescent microscopy revealed that LPS again induced colocalization of CaMKI with Parkin (Fig. 2D). In addition, both LPS and CCCP induced the colocalization of LC3 to the mitochondrion (Fig. 2E), and we have reported that CaMKI regulates LC3II and autophagy (43). These data support a regulatory role for CaMKI in mitophagy. Similar to what was observed with CCCP (Fig. 1D), the translocation and activation of CaMKI with LPS stimulation was Ca2+ dependent, as chelation with BAPTA [1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid] markedly attenuated the mitochondrial expression of active p-Thr177-CaMKI and total CaMKI (Fig. 2F). In addition, Ca2+ chelation inhibited the expression of PINK1 and DJ-1 at the mitochondrion.
Figure 2.
LPS induces a loss of ΔΨ and the translocation of CaMKI, PINK1, and Parkin to the mitochondrion. A) RAW 264.7 cells were stained with 200 nM TMRM and 1 μM Mitotracker Deep Red (color set to green) for 15 min. Cells were then exposed to LPS (100 ng/ml) or 10 μM CCCP for indicated times. Live imaging (514 nm excitation; 570 nm emission) of Mϕs was then performed by using an inverted Nikon TIE fluorescent microscope equipped with a ×60 oil immersion optic (CFI PlanFluor, 1.43 NA) and NIS Elements Software (n = 3 independent experiments). Scale bar, 100 μm. B) RAW 264.7 cells were plated on glass coverslips overnight, then exposed to LPS (100 ng/ml) for 4 h. Before treatment stopped, cells were stained with 100 nM Mitotracker CMXRos Red for 30 min, the accumulation of which is dependent on ΔΨ. After being fixed and permeabilized, cells were stained with anti-TOM20 Ab, which stains mitochondria independent of ΔΨ. Cells were imaged by immunofluorescence (×60 with a 3-fold zoom; n = 3 independent experiments). Scale bars, 10 μm. C) RAW 264.7 cells were exposed to LPS (100 ng/ml) for indicated times. Total cells and mitochondria were isolated and lysed, and total cytosolic or mitochondrial protein were analyzed by immunoblot (n = 2 independent experiments). D) RAW 264.7 cells were plated on glass coverslips, exposed to LPS (100 ng/ml) for 2 h, and then analyzed by immunofluorescence (×60 with a 2-fold zoom) for CaMKI and Parkin (n = 2 independent experiments). Scale bars, 10 μm. E) RAW 264.7 cells were plated on glass coverslips and transfected with GFP-LC3. After 18 h, cells were exposed to either LPS (100 ng/ml) for 2 h or CCCP (10 μM) for 1 h. Cells were then fixed, permeabilized, and stained with anti-TOM20 Ab. They were then analyzed by immunofluorescence (×60 with a 3-fold zoom; n = 4 independent experiments). Scale bars, 10 μm. F) RAW 264.7 cells were exposed to LPS (100 ng/ml) for 2 h either in the presence or absence of BAPTA [1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; 10 μM]. Total cell and mitochondria were isolated and lysed, and total cytosolic or mitochondrial protein were analyzed by immunoblot (n = 2 independent experiments). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
CaMKI regulates LPS-induced expression of PINK1, Parkin, and DJ-1 at the mitochondrion
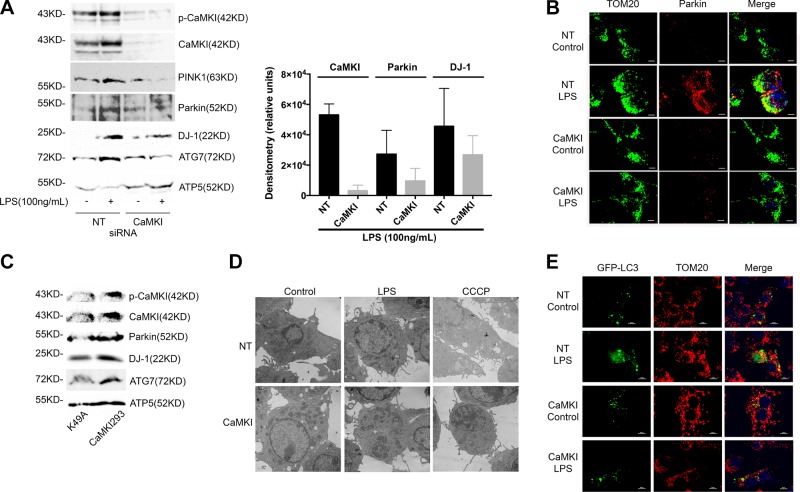
The canonical pathway for mitophagy involves Parkin recruitment to the mitochondrion, and depolarization is a strong stimulus for Parkin activation (8, 9, 17–19). The serine/threonine kinase, PINK1, functions upstream of Parkin to regulate its recruitment to the mitochondrion (8, 9, 17, 19, 20). Thus, we next explored whether CaMKI regulates the PINK1 and Parkin pathway in Mϕs. We observed that LPS increased the expression of both active p-Thr177-CaMKI, PINK1, and Parkin at the Mϕ mitochondrion, which was inhibited by RNAi of CaMKI (Fig. 3A). We also observed that the elevated expression of DJ-1 was inhibited by CaMKI siRNA (Fig. 3A). Immunofluorescent microscopy showed that LPS increased Parkin expression and localization at the mitochondrion, which was inhibited by CaMKI siRNA (Fig. 3B). Alternatively, the overexpression of a constitutively active species of CaMKI (CaMKI293) induced an elevated expression of Parkin, DJ-1, and ATG7 in the mitochondrial fraction compared with a kinase-negative (K49A) mutant (Fig. 3C). Electron microscopy showed reduced autophagosomes in Mϕs that were exposed to either LPS or CCCP after CaMKI siRNA (Fig. 3D), and loss of CaMKI inhibited the colocalization of punctate LC3 at the mitochondria (Fig. 3E). These data suggest that in Mϕs that are exposed to LPS, active CaMKI regulates PINK1 and Parkin and DJ-1 recruitment to the mitochondrion.
Figure 3.
CaMKI regulates LPS-induced PINK1 and Parkin to the mitochondrion. A) RAW 264.7 cells were transfected with either NT or CaMKI siRNA, and then exposed to LPS (100 ng/ml) for 2 h. Mitochondria were isolated and lysed, and mitochondrial protein was analyzed by immunoblot. Densitometry was performed on CaMKI, Parkin, and DJ-1 using ImageJ (n = 3 independent experiments). B) RAW 264.7 cells were plated on glass coverslips and transfected with either NT or CaMKI siRNA. Cells were exposed to LPS (100 ng/ml) for 4 h, then fixed, permeabilized, and stained with anti-TOM20 or anti-Parkin Ab and analyzed by immunofluorescence (×60 with a 3-fold zoom; n = 2 independent experiments). C) RAW 264.7 cells were transfected with a constitutively active CaMKI (CaMKI293) or a kinase-deficient mutant (K49A) for 8 h. Mitochondria were isolated and lysed, and mitochondrial protein was analyzed by immunoblot (n = 3 independent experiments). D) RAW 264.7 cells were plated on glass coverslips and transfected with either NT or CaMKI siRNA. Cells were then exposed to either LPS (100 ng/ml) for 2 h or CCCP (10 μM) for 1 h. Cells were fixed and analyzed by electron microscopy (n = 4 independent experiments). E) RAW 264.7 cells were plated on glass coverslips and transfected with either NT or CaMKI siRNA. Cells were transfected with GFP-LC3, then exposed to LPS (100 ng/ml) for 2 h. Cells were then fixed, permeabilized, and stained with anti-TOM20 Ab and analyzed by immunofluorescence (×60 with a 3-fold zoom; n = 2 independent experiments). Scale bars, 10 μm.
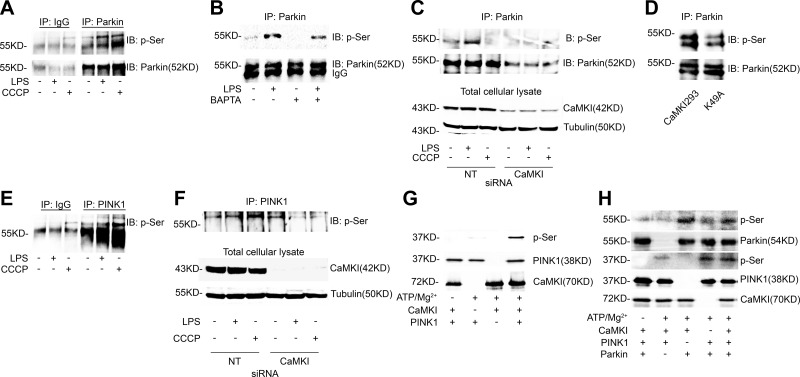
The activity of Parkin and its capacity to induce mitophagy is dependent on serine phosphorylation (8, 20, 59, 60). We observed that LPS induced serine phosphorylation of Parkin (pSer-Parkin; Fig. 4A), which was inhibited by either calcium chelation (Fig. 4B) or CaMKI siRNA (Fig. 4C). Transfection of Mϕs with constitutively active CaMKI increased pSer-Parkin compared with a kinase-negative CaMKI mutant (Fig. 4D). Prototypically, Parkin is serine phosphorylated by the upstream serine/threonine kinase, PINK1 (8, 19, 20, 30, 59, 60). Kinase catalytic activity of PINK1 is also regulated by serine phosphorylation (61). These data suggest that CaMKI may regulate pSer-Parkin by functioning as a PINK1 kinase. As shown in Fig. 4E, LPS induced the serine phosphorylation of PINK1, which—for both LPS (Fig. 4F, lane 2) and CCCP (Fig. 4F, lane 3)—was inhibited by CaMKI siRNA (Fig. 4F, lanes 5 and 6). By using an in vitro kinase assay, we observed that CaMKI directly phosphorylates PINK1 (Fig. 4G); however, we also observed that CaMKI directly phosphorylates Parkin (Fig. 4H). Thus, our data support that CaMKI regulates the PINK1 and Parkin pathway by functioning as a PINK1 and, potentially, Parkin kinase.
Figure 4.
CaMKI is a PINK1 kinase and regulates Parkin activation. A) RAW 264.7 cells were exposed to either LPS (100 ng/ml) for 2 h or CCCP (10 μM) for 1 h. Total cell lysate was isolated, immunoprecipitated (IP) with IgG control or anti-Parkin Ab, and analyzed by immunoblot (IB) for phosphoserine (p-Ser) or Parkin (n = 2 independent experiments). B) RAW 264.7 cells were exposed to LPS (100 ng/ml) for 2 h in either the presence or absence of BAPTA [1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; 10 μM]. Total cell lysate was isolated, IP with anti-Parkin Ab, and analyzed by IB for p-Ser or Parkin (n = 2 independent experiments). C) RAW 264.7 cells were subjected to RNAi by using either NT or CaMKI siRNA, then exposed to either LPS (100 ng/ml) for 2 h or CCCP (10 μM) for 1 h. Total cell lysate was isolated, IP for Parkin, and analyzed by IB (n = 2 independent experiments). D) RAW 264.7 cells were transfected with a constitutively active CaMKI (CaMKI293) or a kinase-deficient mutant (K49A) for 8 h. Total cellular lysate was isolated, IP for Parkin, and analyzed by IB (n = 2 independent experiments). E) RAW 264.7 cells were exposed to either LPS (100 ng/ml) for 2 h or CCCP (10 μM) for 1 h. Total cell lysate was isolated, IP with IgG control or anti-PINK1 Ab, and analyzed by IB for p-Ser (n = 2 independent experiments). F) RAW 264.7 cells were subjected to RNAi by using either NT or CaMKI siRNA, then exposed to either LPS (100 ng/ml) for 2 h or CCCP (10 μM) for 1 h. Total cell lysate was isolated, IP for PINK1, and analyzed by IB (n = 2 independent experiments). G, H) One microgram PINK1 (G; MW of recombinant protein, 37.9 kDa) or Parkin (H; MW of recombinant protein, 53.8 kDa) was incubated in the presence or absence of CaMKI (25 ng, MW of recombinant protein, 70 kDa) for 10 min at 30°C with the following additions: 10 mM MgCl2, 0.2 mM ATP, 1 mM CaCl2, and 1 μM CaM. Reactions were terminated by boiling in SDS–2-ME dissociation solution and analyzed by immunoblot (n = 4 independent experiments).
CaMKI regulates Mϕ bioenergetics
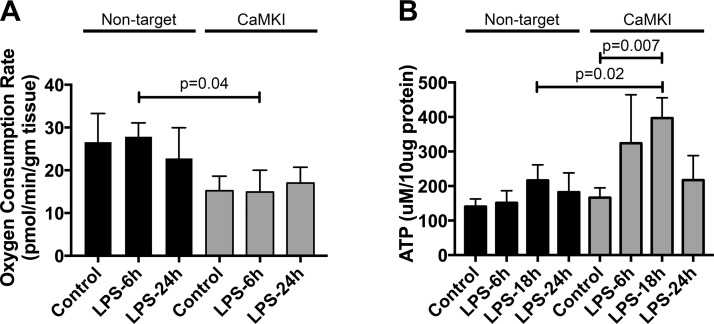
Many of these pathways regulate mitochondrial dynamics and, thus, may alter cellular bioenergetics. We observed that Mϕs that were subjected to CaMKI siRNA exhibited a reduced oxygen consumption rate at rest and after LPS stimulation compared with NT siRNA Mϕs (Fig. 5A). Both NT and CaMKI siRNA Mϕs had similar ATP concentrations at baseline; however, after LPS stimulation, CaMKI siRNA Mϕs had significantly more ATP than did NT siRNA cells (Fig. 5B).
Figure 5.
CaMKI regulates Mϕ bioenergetics. A) RAW 264.7 cells were plated and subjected to RNAi by using either NT or CaMKI siRNA. Cells were then exposed to LPS for the durations indicated and oxygen consumption rate determined by a Seahorse XFe24 analyzer. Data are presented as means ± sem (n = 4 independent experiments). B) RAW 264.7 cells were plated and subjected to RNAi by using either NT or CaMKI siRNA. Cells were then exposed to LPS for the durations indicated and ATP concentration quantified. Data are presented as means ± sem (n = 4 independent experiments).
CaMKI regulates hepatocyte mitochondrial PINK1/Parkin expression
We next investigated whether these mechanisms were induced in nonimmune cells that responded to septic insult. Hepatocytes express TLRs, are responsive to LPS stimulation, and mobilize [Ca2+]i after exposure to a variety of stresses (6, 62, 63). As shown in Fig. 6, LPS induced am increased expression of pCaMKI, CaMKI, PINK1, Parkin, ATG7, and DJ-1 at the mitochondria of hepatocytes (Fig. 6A). Similarly, hepatocyte mitochondria that were exposed to the depolarization agent, CCCP, exhibited higher expression of pCaMKI, CaMKI, PINK1, Parkin, and DJ-1 (Fig. 6B). Thus, it seems that both parenchymal cells as well as immune cells possess a CaMKI-dependent mechanism of regulating mitophagy that is induced by either depolarization or exposure to LPS.
Figure 6.
CaMKI localizes with PINK1/Parkin at the mitochondrion in hepatocytes. A) HuH7 cells were exposed to LPS (1 μg/ml) for indicated times. Mitochondria were isolated and lysed, and mitochondrial protein was analyzed by immunoblot (n = 2 independent experiments). B) Liver harvested from C57BL/6J mice underwent mitochondrial isolation, and isolated mitochondria were exposed to CCCP (10 μM) for the time points shown. Mitochondrial lysate was isolated and analyzed by immunoblot (n = 2 independent experiments).
CaMKI regulates the PINK1/Parkin and the DJ-1 pathways in the liver in vivo
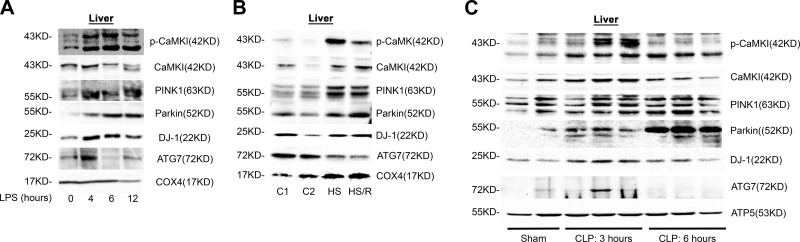
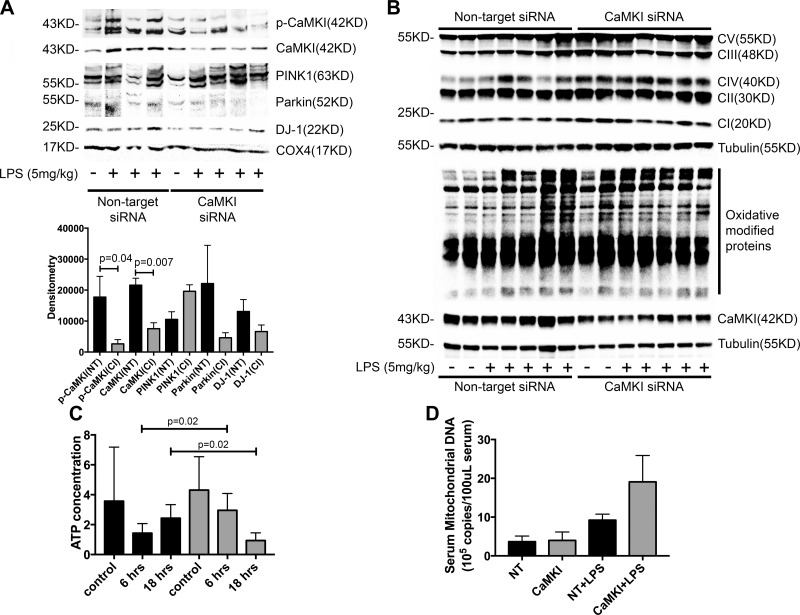
We next explored whether these mechanisms were operant in vivo, focusing on the liver, which is comprised of both hepatocytes and tissue-fixed Mϕs. As shown in Fig. 7A, the liver tissue of endotoxemic mice exhibited a steady increase in active pCaMKI and total CaMKI in the mitochondrial fraction that temporally correlated with increased mitochondrial expression of PINK1, Parkin, DJ-1, and ATG7. Similar results were obtained when mice were exposed to either hemorrhagic shock and reperfusion (Fig. 7B) or CLP sepsis (Fig. 7C). These data suggest that recruitment of active CaMKI to the mitochondrion and CaMKI-dependent induction of the PINK1 and Parkin and DJ-1 pathways of mitophagy and mitochondrial repair is a common adaptive response to mitochondrial injury, regardless of the specific inciting insult. To confirm this hypothesis, we used a technique of in vivo RNAi of CaMKI, as mice that are genetically deficient in CaMKI are not available because of embryologic lethality. We observed that mitochondria that were isolated from the liver tissue of mice that were administered CaMKI siRNA and subjected to endotoxemia had reduced expression of CaMKI compared with mice that received scrambled, NT siRNA. The reduced expression of CaMKI correlated with the reduced expression of mitochondrial Parkin and DJ-1 (Fig. 8A). Loss of these proteins correlated with an elevated expression of mitochondrial complexes II, III, IV, and V and heightened oxidatively modified proteins (Fig. 8B). The liver of mice that were administered NT siRNA and subjected to endotoxemia exhibited an early reduction in tissue ATP concentration, followed by a rebound that approximated baseline levels by 18 h after LPS (Fig. 8C). In contrast, mice that received CaMKI siRNA exhibited higher ATP concentrations at ATP that was followed by near complete loss of ATP at 18 h, a time point at which NT control mice were exhibiting resolution (Fig. 8C). The reduction in mitochondrial proteins elevated the expression of mitochondrial protein complexes that are correlated with heightened systemic release of mitochondrial DNA (Fig. 8D). These data support that in vivo CaMKI regulates the PINK1 and Parkin pathway of mitophagy.
Figure 7.
CaMKI mediates mitochondrial recruitment of PINK1, Parkin, and mitophagy in the liver in vivo. A) C57BL/6J mice were subjected to endotoxemia (LPS 5 mg/kg, i.p.). At the time points shown, mice were euthanized, livers harvested, and mitochondria isolated. Total mitochondrial protein lysate was analyzed by immunoblot (representative blot of n = 2 independent experiments, in which 1 mouse per group is shown; n = 2 mice total/group for all experiments combined). B) C57BL/6J mice were subjected to HS or HS/R. Mice were euthanized, the livers harvested, and mitochondria isolated. Total mitochondrial protein lysate was analyzed by immunoblot (representative blot of n = 2 independent experiments, in which 1 mouse per group is shown; n = 2 mice total/group for all experiments combined). C) C57BL/6J mice were subjected to CLP. At the time points shown, mice were euthanized, livers harvested, and mitochondria isolated. Total mitochondrial protein lysate was analyzed by immunoblot (representative blot of n = 2 independent experiments, in which 2–3 mice per group are shown; n = 4–6 mice total/group for all experiments combined).
Figure 8.
CaMKI regulates mitochondrial recruitment of Parkin and DJ-1, oxidative stress, and ATP concentrations during endotoxemia in vivo. C57BL/6J mice were subjected to in vivo RNAi by using either NT or CaMKI siRNA (6 mg/kg tail vein). After 72 h, mice were subjected to endotoxemia (5 mg/kg) for 6 or 18 h. Mice were euthanized, livers and blood harvested, and mitochondria isolated. A) Total mitochondrial protein lysate from 6 h endotoxemia was analyzed by immunoblot (representative blot of n = 2 independent experiments, in which 3–4 mice per group are shown; n = 6–8 mice total/group for all experiments combined). B) Total liver protein lysate from 18 h endotoxemia was analyzed by immunoblot for mitochondrial complexes I–V and oxidative modified proteins (representative blot of n = 2 independent experiments, in which 4–5 mice per group are shown; n = 8–10 mice total/group for all experiments combined). C) Liver tissues from 6 and 18 h endotoxemia were analyzed for ATP concentration. Data are presented as means ± sem (n = 6–10 mice total/group for all experiments combined). D) Serum was isolated from 18 h endotoxemia and analyzed for serum mitochondrial DNA concentration. Data are presented as means ± sem (n = 8–10 mice total/group for all experiments combined).
DISCUSSION
Preserving a critical threshold of energy is essential for cell survival, and the mitochondrion is the predominant source of cellular ATP; however, the energetic demands of sepsis can lead to mitochondrial depolarization, uncoupling of the electron transport chain with the release of toxic ROS, and insufficient ATP to meet the vital needs of the cell. If progressive, the mitochondrial permeability transition pore opens and cytochrome C is released, which heralds the induction of apoptosis and cell death (2, 5, 64). Thus, such mechanisms as mitophagy are in place to sequester these damaged mitochondria or mitochondrial parts into a double membrane autophagosome, thereby mitigating oxidant stress and cellular injury (8, 9, 11, 13, 15–17, 65, 66). The mechanisms that regulate these complex processes and the ramifications of any defects are still being elucidated. Here, we describe a novel mechanism by which the mitochondrion controls its own fate, capitalizing on the intimate and inverse relationship between its ΔΨ and Ca2+ signaling. We show that LPS induces mitochondrial depolarization, which leads to calcium signaling. CaMKI—responding to this Ca2+ flare—is recruited to the mitochondrion to directly activate PINK1 and Parkin and DJ-1, adaptive pathways that regulate mitophagy, mitochondrial fission/fusion and function, oxidative stress, and cellular bioenergetics. The process is equally relevant to immune and parenchymal cells and is rapidly induced in vivo during sepsis.
It is well established that depolarization itself is a strong inducer of Parkin expression and Parkin-dependent mitophagy (9, 18, 22, 23); however, the mechanistic link between depolarization and mitophagy has not been fully characterized. Recent evidence counters prior notions of the mitochondria as mere machinery under cellular control and highlights that they are central in the coordination of many cellular events (67). Mitochondria provide a high-capacity, low-affinity Ca2+ buffering system that sequesters Ca2+ predominantly via the negative electrochemical gradient that is generated by ΔΨ (32–34). Depolarization reduces buffering capacity and leads to mitochondrial Ca2+ leak (33, 34, 37). We now show that mitochondria participate in the regulation of mechanisms of quality control, in part, via this relationship between ΔΨ and Ca2+ regulation. We propose that a [Ca2+]i signal is induced by loss of ΔΨ. CaMKI serves as a keystone, transducing mitochondrial depolarization and the subsequent Ca2+ signal with activation of the DJ-1/PINK1/Parkin pathway. In doing so, CaMKI mediates adaptive mitophagic changes in response to LPS. Whether this Ca2+ signal originates from the mitochondrion and its loss of buffering capacity or some other [Ca2+]i store remains to be determined.
Our data complement prior investigations that describe mechanisms by which the family of multifunctional CaMK regulates autophagy and mitophagy. In Mϕs, LPS induces Ca2+ mobilization that, as we have shown, leads to the activation of all members of the CaMK cascade (39–44). Furthermore, we and others have shown that the family of CaMK integrates extensively with the machinery of autophagy (43, 44, 68). We recently characterized a CaMKIα-AMP kinase pathway that is rapidly induced in Mϕs that are exposed to LPS that regulates an early autophagic response (43). Data from these more recent experiments further support the regulatory importance of CaMKI in cellular responses to sepsis, but suggest a more specific autophagic process: mitophagy. Whether the CaMKI-dependent mitophagy studied herein also functions via an AMPK pathway in regulating PINK1/Parkin and DJ-1 is currently a focus of investigation, although recent studies suggest that DJ-1 functions upstream of AMPK (27, 69). In addition, the induction of autonomous CaMKI activity is prototypically dependent on upstream CaMKKα/β, and we are investigating whether CaMKK also serves an upstream regulatory role in these CaMKI-PINK1/Parkin pathways (39, 40, 42, 43, 70).
Mechanisms we initially characterized in Mϕs were observed to be equally relevant to the hepatocyte. Recent literature suggests a critical cytoprotective role for autophagy in both toxin-mediated and ischemia and reperfusion–induced injury in a variety of parenchymal cell types (6, 7, 71, 72). Autophagic mechanisms directed at the removal of damaged mitochondria—or mitophagy—are considered to be of particular importance in protecting against hepatocyte injury (73, 74). Hepatocytes express TLRs, are responsive to LPS stimulation, and mobilize [Ca2+]i with exposure to a variety of stresses (6, 62). Our data advance our understanding of these mechanisms to show that even in nonimmune cells, CaMKI links LPS signaling with the PINK1/Parkin machinery of mitophagy to protect against mitochondrial dysfunction and oxidant damage.
DJ-1 (PARK7) similarly relocalizes to mitochondria under conditions of oxidative stress and protects against cellular injury and death, in part, by regulating mitochondrial dynamics and function to mitigate ROS production (26, 28, 75, 76). DJ-1 deficiency increases cellular sensitivity to ROS and increases ROS levels (27, 28, 31, 76). Mitochondria of DJ-1–deficient cells exhibit loss of ΔΨ and a phenotype that is similar to PINK1 and Parkin mutants: an increased tendency to undergo fission and fragmentation (30, 31, 76). Up-regulation of DJ-1 can ameliorate PINK1, but not Parkin, deficiency and, collectively, these data suggest that DJ-1 functions upstream of Parkin but parallel to PINK1 (30, 77). Much of the phenotype of DJ-1–deficient cells is thought to be the consequence of ROS generation, as treatment with cell-permeable glutathione analogs rescues DJ-1–deficient cells (31, 76). This preservation of mitochondrial function may be achieved by a regulatory role in mitochondrial fission and fusion, events that sequester damaged mitochondrial components into mitochondria with lower ΔΨ that are then targeted for mitophagy (30). Furthermore, DJ-1 has been shown to directly bind to and induce sirtuin 1, a member of a conserved family of deacetylases that regulates autophagy, mitophagy, mitochondrial biogenesis, and a host of antioxidant protective mechanisms (29). We now show that CaMKI also regulates DJ-1 recruitment to the mitochondrion and again propose this is a result of a loss of ΔΨ and subsequent Ca2+ signal; however, members of the CaMK family have also been shown to be redox sensitive, exhibiting increased activity in response to oxidant stress (78, 79). Additional studies are needed to characterize the individual contributions of Ca2+ and ROS in CaMKI activation and the mechanistic link between CaMKI and DJ-1.
Ramifications of CaMKI-dependent mitophagy on organ function and physiology, particularly as it relates to developing novel therapeutics, was not a focus of these experiments. Current evidence creates a paradigm in which the early loss of cell function as a result of mitophagy and dampened mitochondrial function is adaptive and protective; however, if prolonged, irreversible cellular injury and organ failure ensue and contribute to host mortality. Previously, we observed that polymicrobial CLP sepsis elevated the serum concentrations of blood urea nitrogen and creatinine, parameters that are used in clinical medicine to quantify renal dysfunction (42). Inhibition of CaMKI, either biochemically or through siRNA, preserved organ function. We hypothesize that, early in sepsis, CaMKI regulates these mitophagic mechanisms to reduce oxidant stress and protect the cell; however, persistent CaMKI activation, a potential consequence of the high prevalence of perturbed Ca2+ regulation during sepsis, may be deleterious.
Mϕs pose a unique challenge in characterizing and interpreting these pathways as they relate to cell function—that is, balancing a protective loss of function with its phenotypic role of thwarting infection. We and others have shown that CaMKI is fundamental for Mϕ function, including cytokine production, phagocytosis, and autophagy, all of which serve as effector arms of Mϕs (42, 43). Thus, induction of CaMKI may serve to augment immune cell phenotype, of which autophagy is a component. Concomitant induction of mitophagy protects Mϕs from oxidant stress during a time of heightened metabolic demands, rather than induce a state of phenotypic hibernation. Consequently, it is difficult to distinguish the role of immune cell CaMKI function from that of nonimmune cell CaMKI function on the dynamic and integrated response of the host to sepsis, particularly as it relates to organ dysfunction. For instance, uncontrolled and systemic inflammation is thought to underlie the organ dysfunction of sepsis. Thus, CaMKI-dependent immune cell function and inflammation, CaMKI-dependent tissue-specific mitophagy (e.g., renal tubular cell, hepatocyte), or both may contribute to the loss of cellular function and organ failure. Subsequent experiments using tissue-specific inhibition of CaMKI will likely be necessary to determine the contributions of the aforementioned CaMKI-dependent mechanisms to the host physiology during sepsis and other inflammatory states.
In summary, LPS induces mitochondrial depolarization and a Ca2+ signal that increases the expression of CaMKI at the mitochondrion in both Mϕs and hepatocytes. Active CaMKI functions as a PINK1 kinase and regulates the PINK1/Parkin and DJ-1 pathways. These mechanisms are operant in vivo during endotoxemia and sepsis. The observation that CaMKI regulates these mechanisms in both immune and nonimmune cells underscores the need to further study these mechanisms in the context of tissue-specific injury and dysfunction in response to septic insult. Furthermore, additional studies are needed to translate these cellular events to organ physiology and host response in an attempt to identify the clinical utility of CaMKI modulation during inflammatory states.
ACKNOWLEDGMENTS
The authors thank Kevin P. Mollen and John E. Griepentrog (both from the University of Pittsburgh) for their assistance in interpreting the final collective data and reviewing the final manuscript. This work was supported by the U.S. National Institutes of Health, National Institute of General Medical Sciences Grant R01-GM082852 (to M.R.R.), and the Surgical Infection Society Junior Faculty Fellowship Award (to X.Z.). The authors declare no conflicts of interest.
Glossary
- ΔΨ
mitochondrial membrane potential
- ATG7
autophagy-related protein 7
- [Ca2+]i
intracellular calcium concentration
- CaMK
calcium/calmodulin-dependent protein kinase
- CCCP
carbonyl cyanide m-chlorophenyl hydrazine
- CLP
cecal ligation and puncture
- HS
hemorrhage shock
- HS/R
hemorrhage shock and resuscitation
- Mϕ
macrophage
- NT
nontargeting
- RNAi
RNA inhibition
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- TMRM
tetramethylrhodamine methyl ester
AUTHOR CONTRIBUTIONS
X. Zhang, B. S. Zuckerbraun, and M. R. Rosengart designed the research; X. Zhang, Y. Du, Q. Sun, L. Xu, E. Lee, and A. J. Lewis performed research and collected data; X. Zhang and M. R. Rosengart analyzed data; and X. Zhang, Y. Du, Q. Sun, L. Xu, E. Lee, A. J. Lewis, B. S. Zuckerbraun, and M. R. Rosengart wrote the manuscript.
REFERENCES
- 1.Weinberg S. E., Sena L. A., Chandel N. S. (2015) Mitochondria in the regulation of innate and adaptive immunity. Immunity 42, 406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M., Brealey D. (1999) Mitochondrial dysfunction in sepsis. Biochem. Soc. Symp. 66, 149–166 [DOI] [PubMed] [Google Scholar]
- 3.Singer M. (2007) Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit. Care Med. 35 (9 Suppl), S441–S448 [DOI] [PubMed] [Google Scholar]
- 4.Whelan S. P., Carchman E. H., Kautza B., Nassour I., Mollen K., Escobar D., Gomez H., Rosengart M. A., Shiva S., Zuckerbraun B. S. (2014) Polymicrobial sepsis is associated with decreased hepatic oxidative phosphorylation and an altered metabolic profile. J. Surg. Res. 186, 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crouser E. D. (2004) Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 4, 729–741 [DOI] [PubMed] [Google Scholar]
- 6.Carchman E. H., Whelan S., Loughran P., Mollen K., Stratamirovic S., Shiva S., Rosengart M. R., Zuckerbraun B. S. (2013) Experimental sepsis-induced mitochondrial biogenesis is dependent on autophagy, TLR4, and TLR9 signaling in liver. FASEB J. 27, 4703–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carchman E. H., Rao J., Loughran P. A., Rosengart M. R., Zuckerbraun B. S. (2011) Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology 53, 2053–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiyama A., Okamoto K. (2015) PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 33, 95–101 [DOI] [PubMed] [Google Scholar]
- 9.Ding W. X., Yin X. M. (2012) Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol. Chem. 393, 547–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youle R. J., van der Bliek A. M. (2012) Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunnari J., Suomalainen A. (2012) Mitochondria: in sickness and in health. Cell 148, 1145–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He C., Klionsky D. J. (2009) Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J., Klionsky D. J. (2007) Autophagy and human disease. Cell Cycle 6, 1837–1849 [DOI] [PubMed] [Google Scholar]
- 14.Huang W. P., Klionsky D. J. (2002) Autophagy in yeast: a review of the molecular machinery. Cell Struct. Funct. 27, 409–420 [DOI] [PubMed] [Google Scholar]
- 15.Klionsky D. J. (2005) Autophagy. Curr. Biol. 15, R282–R283 [DOI] [PubMed] [Google Scholar]
- 16.Klionsky D. J., Emr S. D. (2000) Autophagy as a regulated pathway of cellular degradation. Science 290, 1717–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youle R. J., Narendra D. P. (2011) Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Nartiss Y., Steipe B., McQuibban G. A., Kim P. K. (2012) ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy 8, 1462–1476 [DOI] [PubMed] [Google Scholar]
- 19.Springer W., Kahle P. J. (2011) Regulation of PINK1-Parkin-mediated mitophagy. Autophagy 7, 266–278 [DOI] [PubMed] [Google Scholar]
- 20.Kim Y., Park J., Kim S., Song S., Kwon S. K., Lee S. H., Kitada T., Kim J. M., Chung J. (2008) PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem. Biophys. Res. Commun. 377, 975–980 [DOI] [PubMed] [Google Scholar]
- 21.Durcan T. M., Fon E. A. (2015) The three ‘P’s of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev. 29, 989–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narendra D., Tanaka A., Suen D. F., Youle R. J. (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narendra D. P., Jin S. M., Tanaka A., Suen D. F., Gautier C. A., Shen J., Cookson M. R., Youle R. J. (2010) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan D. C. (2012) Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 46, 265–287 [DOI] [PubMed] [Google Scholar]
- 25.Van der Bliek A. M., Shen Q., Kawajiri S. (2013) Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 5, a011072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCoy M. K., Cookson M. R. (2012) Mitochondrial quality control and dynamics in Parkinson’s disease. Antioxid. Redox Signal. 16, 869–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi S. Y., Lu S. Y., Sivasubramaniyam T., Revelo X. S., Cai E. P., Luk C. T., Schroer S. A., Patel P., Kim R. H., Bombardier E., Quadrilatero J., Tupling A. R., Mak T. W., Winer D. A., Woo M. (2015) DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat. Commun. 6, 7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu Y., Lambert J. P., Nicholson C. K., Kim J. J., Wolfson D. W., Cho H. C., Husain A., Naqvi N., Chin L. S., Li L., Calvert J. W. (2016) DJ-1 protects the heart against ischemia-reperfusion injury by regulating mitochondrial fission. J. Mol. Cell. Cardiol. 97, 56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi-Niki K., Ganaha Y., Niki T., Nakagawa S., Kato-Ose I., Iguchi-Ariga S. M., Ariga H. (2016) DJ-1 activates SIRT1 through its direct binding to SIRT1. Biochem. Biophys. Res. Commun. 474, 131–136 [DOI] [PubMed] [Google Scholar]
- 30.Thomas K. J., McCoy M. K., Blackinton J., Beilina A., van der Brug M., Sandebring A., Miller D., Maric D., Cedazo-Minguez A., Cookson M. R. (2011) DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum. Mol. Genet. 20, 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCoy M. K., Cookson M. R. (2011) DJ-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy 7, 531–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santo-Domingo J., Demaurex N. (2010) Calcium uptake mechanisms of mitochondria. Biochim. Biophys. Acta 1797, 907–912 [DOI] [PubMed] [Google Scholar]
- 33.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. (2012) Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578 [DOI] [PubMed] [Google Scholar]
- 34.Walsh C., Barrow S., Voronina S., Chvanov M., Petersen O. H., Tepikin A. (2009) Modulation of calcium signalling by mitochondria. Biochim. Biophys. Acta 1787, 1374–1382 [DOI] [PubMed] [Google Scholar]
- 35.Kaftan E. J., Xu T., Abercrombie R. F., Hille B. (2000) Mitochondria shape hormonally induced cytoplasmic calcium oscillations and modulate exocytosis. J. Biol. Chem. 275, 25465–25470 [DOI] [PubMed] [Google Scholar]
- 36.Baughman J. M., Perocchi F., Girgis H. S., Plovanich M., Belcher-Timme C. A., Sancak Y., Bao X. R., Strittmatter L., Goldberger O., Bogorad R. L., Koteliansky V., Mootha V. K. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo Y., Bond J. D., Ingram V. M. (1997) Compromised mitochondrial function leads to increased cytosolic calcium and to activation of MAP kinases. Proc. Natl. Acad. Sci. USA 94, 9705–9710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosengart M. R., Arbabi S., Garcia I., Maier R. V. (2000) Interactions of calcium/calmodulin-dependent protein kinases (CaMK) and extracellular-regulated kinase (ERK) in monocyte adherence and TNFalpha production. Shock 13, 183–189 [DOI] [PubMed] [Google Scholar]
- 39.Zhang X., Wheeler D., Tang Y., Guo L., Shapiro R. A., Ribar T. J., Means A. R., Billiar T. R., Angus D. C., Rosengart M. R. (2008) Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J. Immunol. 181, 5015–5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Means A. R. (2008) The year in basic science: calmodulin kinase cascades. Mol. Endocrinol. 22, 2759–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collage R. D., Howell G. M., Zhang X., Stripay J. L., Lee J. S., Angus D. C., Rosengart M. R. (2013) Calcium supplementation during sepsis exacerbates organ failure and mortality via calcium/calmodulin-dependent protein kinase kinase signaling. Crit. Care Med. 41, e352–e360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X., Guo L., Collage R. D., Stripay J. L., Tsung A., Lee J. S., Rosengart M. R. (2011) Calcium/calmodulin-dependent protein kinase (CaMK) Ialpha mediates the macrophage inflammatory response to sepsis. J. Leukoc. Biol. 90, 249–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo L., Stripay J. L., Zhang X., Collage R. D., Hulver M., Carchman E. H., Howell G. M., Zuckerbraun B. S., Lee J. S., Rosengart M. R. (2013) CaMKIalpha regulates AMP kinase-dependent, TORC-1-independent autophagy during lipopolysaccharide-induced acute lung neutrophilic inflammation. J. Immunol. 190, 3620–3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X., Howell G. M., Guo L., Collage R. D., Loughran P. A., Zuckerbraun B. S., Rosengart M. R. (2014) CaMKIV-dependent preservation of mTOR expression is required for autophagy during LPS-induced inflammation and acute kidney injury. J. Immunol. 193, 2405–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitt J. M., Wayman G. A., Nozaki N., Soderling T. R. (2004) Calcium activation of ERK mediated by calmodulin kinase I. J. Biol. Chem. 279, 24064–24072 [DOI] [PubMed] [Google Scholar]
- 46.Howell G. M., Gomez H., Collage R. D., Loughran P., Zhang X., Escobar D. A., Billiar T. R., Zuckerbraun B. S., Rosengart M. R. (2013) Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS One 8, e69520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez H., Kautza B., Escobar D., Nassour I., Luciano J., Botero A. M., Gordon L., Martinez S., Holder A., Ogundele O., Loughran P., Rosengart M. R., Pinsky M., Shiva S., Zuckerbraun B. S. (2015) Inhaled carbon monoxide protects against the development of shock and mitochondrial injury following hemorrhage and resuscitation. PLoS One 10, e0135032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokokura H., Picciotto M. R., Nairn A. C., Hidaka H. (1995) The regulatory region of calcium/calmodulin-dependent protein kinase I contains closely associated autoinhibitory and calmodulin-binding domains. J. Biol. Chem. 270, 23851–23859 [DOI] [PubMed] [Google Scholar]
- 49.Stedman D. R., Uboha N. V., Stedman T. T., Nairn A. C., Picciotto M. R. (2004) Cytoplasmic localization of calcium/calmodulin-dependent protein kinase I-alpha depends on a nuclear export signal in its regulatory domain. FEBS Lett. 566, 275–280 [DOI] [PubMed] [Google Scholar]
- 50.Loughran P. A., Stolz D. B., Barrick S. R., Wheeler D. S., Friedman P. A., Rachubinski R. A., Watkins S. C., Billiar T. R. (2013) PEX7 and EBP50 target iNOS to the peroxisome in hepatocytes. Nitric Oxide 31, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji J., Baart S., Vikulina A. S., Clark R. S., Anthonymuthu T. S., Tyurin V. A., Du L., St Croix C. M., Tyurina Y. Y., Lewis J., Skoda E. M., Kline A. E., Kochanek P. M., Wipf P., Kagan V. E., Bayır H. (2015) Deciphering of mitochondrial cardiolipin oxidative signaling in cerebral ischemia-reperfusion. J. Cereb. Blood Flow Metab. 35, 319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardenes N., Corey C., Geary L., Jain S., Zharikov S., Barge S., Novelli E. M., Shiva S. (2014) Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood 123, 2864–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X., Petrie T. G., Liu Y., Liu J., Fujioka H., Zhu X. (2012) Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J. Neurochem. 121, 830–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauerfeld C. P., Rastogi R., Pirockinaite G., Lee I., Hüttemann M., Monks B., Birnbaum M. J., Franchi L., Nuñez G., Samavati L. (2012) TLR4-mediated AKT activation is MyD88/TRIF dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages. J. Immunol. 188, 2847–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tucsek Z., Radnai B., Racz B., Debreceni B., Priber J. K., Dolowschiak T., Palkovics T., Gallyas F. Jr., Sumegi B., Veres B. (2011) Suppressing LPS-induced early signal transduction in macrophages by a polyphenol degradation product: a critical role of MKP-1. J. Leukoc. Biol. 89, 105–111 [DOI] [PubMed] [Google Scholar]
- 56.Puleston D. (2015) Detection of mitochondrial mass, damage, and reactive oxygen species by flow cytometry. Cold Spring Harb. Protoc. 2015, pdb.prot086298. [DOI] [PubMed] [Google Scholar]
- 57.Pendergrass W., Wolf N., Poot M. (2004) Efficacy of MitoTracker green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A 61, 162–169 [DOI] [PubMed] [Google Scholar]
- 58.Solaini G., Sgarbi G., Lenaz G., Baracca A. (2007) Evaluating mitochondrial membrane potential in cells. Biosci. Rep. 27, 11–21 [DOI] [PubMed] [Google Scholar]
- 59.Shiba-Fukushima K., Imai Y., Yoshida S., Ishihama Y., Kanao T., Sato S., Hattori N. (2012) PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Sci. Rep. 2, 1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iguchi M., Kujuro Y., Okatsu K., Koyano F., Kosako H., Kimura M., Suzuki N., Uchiyama S., Tanaka K., Matsuda N. (2013) Parkin-catalyzed ubiquitin-ester transfer is triggered by PINK1-dependent phosphorylation. J. Biol. Chem. 288, 22019–22032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aerts L., Craessaerts K., De Strooper B., Morais V. A. (2015) PINK1 kinase catalytic activity is regulated by phosphorylation on serines 228 and 402. J. Biol. Chem. 290, 2798–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsung A., Klune J. R., Zhang X., Jeyabalan G., Cao Z., Peng X., Stolz D. B., Geller D. A., Rosengart M. R., Billiar T. R. (2007) HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 204, 2913–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nace G. W., Huang H., Klune J. R., Eid R. E., Rosborough B. R., Korff S., Li S., Shapiro R. A., Stolz D. B., Sodhi C. P., Hackam D. J., Geller D. A., Billiar T. R., Tsung A. (2013) Cellular-specific role of Toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice. Hepatology 58, 374–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singer M. (2005) Metabolic failure. Crit. Care Med. 33 (Suppl 12), S539–S542 [DOI] [PubMed] [Google Scholar]
- 65.Yang Z., Klionsky D. J. (2010) Mammalian autophagy: core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 22, 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huber T. B., Edelstein C. L., Hartleben B., Inoki K., Jiang M., Koya D., Kume S., Lieberthal W., Pallet N., Quiroga A., Ravichandran K., Susztak K., Yoshida S., Dong Z. (2012) Emerging role of autophagy in kidney function, diseases and aging. Autophagy 8, 1009–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Z., Butow R. A. (2006) Mitochondrial retrograde signaling. Annu. Rev. Genet. 40, 159–185 [DOI] [PubMed] [Google Scholar]
- 68.Høyer-Hansen M., Bastholm L., Szyniarowski P., Campanella M., Szabadkai G., Farkas T., Bianchi K., Fehrenbacher N., Elling F., Rizzuto R., Mathiasen I. S., Jäättelä M. (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell 25, 193–205 [DOI] [PubMed] [Google Scholar]
- 69.Vasseur S., Afzal S., Tardivel-Lacombe J., Park D. S., Iovanna J. L., Mak T. W. (2009) DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc. Natl. Acad. Sci. USA 106, 1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakayama Y., Endo M., Tsukano H., Mori M., Oike Y., Gotoh T. (2010) Molecular mechanisms of the LPS-induced non-apoptotic ER stress-CHOP pathway. J. Biochem. 147, 471–483 [DOI] [PubMed] [Google Scholar]
- 71.Kimura T., Takabatake Y., Takahashi A., Kaimori J. Y., Matsui I., Namba T., Kitamura H., Niimura F., Matsusaka T., Soga T., Rakugi H., Isaka Y. (2011) Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J. Am. Soc. Nephrol. 22, 902–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evankovich J., Zhang R., Cardinal J. S., Zhang L., Chen J., Huang H., Beer-Stolz D., Billiar T. R., Rosengart M. R., Tsung A. (2012) Calcium/calmodulin-dependent protein kinase IV limits organ damage in hepatic ischemia-reperfusion injury through induction of autophagy. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G189–G198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams J. A., Ni H. M., Ding Y., Ding W. X. (2015) Parkin regulates mitophagy and mitochondrial function to protect against alcohol-induced liver injury and steatosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G324–G340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eid N., Ito Y., Otsuki Y. (2016) Triggering of parkin mitochondrial translocation in mitophagy: implications for liver diseases. Front. Pharmacol. 7, 100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Canet-Avilés R. M., Wilson M. A., Miller D. W., Ahmad R., McLendon C., Bandyopadhyay S., Baptista M. J., Ringe D., Petsko G. A., Cookson M. R. (2004) The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA 101, 9103–9108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irrcher I., Aleyasin H., Seifert E. L., Hewitt S. J., Chhabra S., Phillips M., Lutz A. K., Rousseaux M. W., Bevilacqua L., Jahani-Asl A., Callaghan S., MacLaurin J. G., Winklhofer K. F., Rizzu P., Rippstein P., Kim R. H., Chen C. X., Fon E. A., Slack R. S., Harper M. E., McBride H. M., Mak T. W., Park D. S. (2010) Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 19, 3734–3746 [DOI] [PubMed] [Google Scholar]
- 77.Hao L. Y., Giasson B. I., Bonini N. M. (2010) DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc. Natl. Acad. Sci. USA 107, 9747–9752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Howe C. J., LaHair M. M., Maxwell J. A., Lee J. T., Robinson P. J., Rodriguez-Mora O., McCubrey J. A., Franklin R. A. (2002) Participation of the calcium/calmodulin-dependent kinases in hydrogen peroxide-induced Ikappa B phosphorylation in human T lymphocytes. J. Biol. Chem. 277, 30469–30476 [DOI] [PubMed] [Google Scholar]
- 79.Howe C. J., Lahair M. M., McCubrey J. A., Franklin R. A. (2004) Redox regulation of the calcium/calmodulin-dependent protein kinases. J. Biol. Chem. 279, 44573–44581 [DOI] [PubMed] [Google Scholar]