Abstract
Background
Tumor location served as an important prognostic factor in glioma patients was considered to postulate molecular features according to cell origin theory. However, anatomic distribution of unique molecular subtypes was not widely investigated. The relationship between molecular phenotype and histological subgroup were also vague based on tumor location. Our group focuses on the study of glioma anatomic location of distinctive molecular subgroups and histology subtypes, and explores the possibility of their consistency based on clinical background.
Methods
We retrospectively reviewed 143 cases with both molecular information (IDH1/TERT/1p19q) and MRI images diagnosed as cerebral diffuse gliomas. The anatomic distribution was analyzed between distinctive molecular subgroups and its relationship with histological subtypes. The influence of tumor location, molecular stratification and histology diagnosis on survival outcome was investigated as well.
Results
Anatomic locations of cerebral diffuse glioma indicate varied clinical outcome. Based on that, it can be stratified into five principal molecular subgroups according to IDH1/TERT/1p19q status. Triple-positive (IDH1 and TERT mutation with 1p19q codeletion) glioma tended to be oligodendroglioma present with much better clinical outcome compared to TERT mutation only group who is glioblastoma inclined (median overall survival 39 months VS 18 months). Five molecular subgroups were demonstrated with distinctive locational distribution. This kind of anatomic feature is consistent with its corresponding histological subtypes.
Discussion
Each molecular subgroup in glioma has unique anatomic location which indicates distinctive clinical outcome. Molecular diagnosis can be served as perfect complementary tool for the precise diagnosis. Integration of histomolecular diagnosis will be much more helpful in routine clinical practice in the future.
Keywords: Glioma, Molecular diagnosis, Anatomic location
Background
Glioma is the most common malignant brain tumor with heterogeneous growth pattern which can be found in different cerebral lobes [1, 2]. This kind of locational variety has been demonstrated to be of great importance in patient diagnosis and prognosis which can reflect the tumor cells origin as well [3]. Many studies have been performed to prove relationship between molecular biomarkers and tumor location. [2, 4–6]. Recently, new WHO classification of cerebral diffuse gliomas was revised with complementary of three molecular biomarkers (IDH1/1p19q/H3F3A) integrated into comprehensive pathological diagnosis [7]. It demonstrated that glioma-related biomarkers have been playing much more important role in precise medicine. Robert B.Jenkins et al. has successfully used three major biomarkers to classify glioma into five principal molecular subsets represent distinctive clinical significance and germline variants. This finding hallmarked the development of molecular pathology in glioma which was published on New England Journal of Medicine [8]. In our study, we plan to use same stratification system in our patients cohort and delineate tumor locational tendency within different molecular subtypes. Furthermore, we will explore the consistency of tumor location between histological subtypes and their molecular counterparts to identify the complementary effect of molecular diagnosis in cerebral diffuse glioma, especially in outcome prediction.
Methods
Patients and tissue samples
We searched for Molecular Database and Image Bank in Department of Neurosurgery, Huashan Hospital and retrospectively selected 143 glioma samples for further study. H&E slides of all cases were reviewed by 2 individual neuropathologists to confirm the diagnosis of glioma according to WHO 2016 brain tumor guideline. Every patient has molecular diagnostic information. 140 out of 143 patients got complete follow-up. This research work was approved by Ethic Committee of Huashan Hospital and informed consents signed. Patients characteristic are listed in Table 1.
Table 1.
Characteristics of all patients
| Characteristic | Total number | Sex | Age | |||
|---|---|---|---|---|---|---|
| 143 | Male | Female | 0–35 | 36–60 | >60 | |
| Molecular subtype | ||||||
| Triple-positive | 41 (29%) | 23 | 18 | 9 | 32 | 0 |
| TERT and IDH mutation | 7 (5%) | 3 | 4 | 4 | 3 | 0 |
| IDH mutation only | 37 (26%) | 25 | 12 | 19 | 18 | 0 |
| Triple-negative | 27 (19%) | 14 | 13 | 7 | 17 | 3 |
| TERT mutation only | 30 (21%) | 21 | 9 | 5 | 13 | 12 |
| Other | 1 (1%) | 1 | 0 | 1 | 0 | 0 |
| WHO grade | ||||||
| Grade II | 82 (57%) | 45 | 37 | 34 | 45 | 3 |
| Grade III | 27 (19%) | 17 | 10 | 6 | 21 | 0 |
| Grade IV | 34 (24%) | 25 | 9 | 5 | 17 | 12 |
| Pathological Diagnosis | ||||||
| Astrocytoma | 69 (48%) | 43 | 26 | 29 | 37 | 3 |
| Oligodendroglioma | 40 (28%) | 19 | 21 | 11 | 29 | 0 |
| Glioblastoma | 34 (24%) | 25 | 9 | 5 | 17 | 12 |
Molecular profiles of IDH1/TERT/1p19q
Paraffin blocks of each case were prepared. Four 4um slides and six 10um slides were sectioned. DNA extraction was performed by commercial DNA extraction kit (Qiagen, Shanghai) using 10um slides. IDH1 and TERT mutational analysis were done by Sanger Sequencing with method reported previously [9]. The status of 1p19q was determined by FISH (fluorescence in situ hybridization). A case of 50% tumor cells present with reference probe signal ratio to target probe signal more than 2:1 was considered 1p19q codeletion, otherwise we called 1p19q intact [10]. Molecular information will be integrated into regular pathological diagnosis.
Tumor location analysis
Image segmentation is an important pre-processing step for location analysis. Convolutional neural network (CNN) was proved to be an effective method for medical image segmentation [11]. In our research, an approach based on CNN was adopted to extract brain tumors on MR images, which got satisfactory performance in the Brain Tumor Segmentation Challenge 2013 and 2015. (http://www.braintumorsegmentation.org/).
In order to study the location features in same coordinate system, the segmentation results were registered to MNI152 (Montreal Neurological Institute (MNI)) brain atlas [12]. A research platform also provided by MNI, SPM12, was used to accomplish this procedure. Both MNI152 and SPM12 were widely used in brain tumor registration [13].
Statistical analysis
Correlation coefficient was calculated to figure out the location relation between specific histology stratification and molecular phenotype. IBM SPSS statistic 20.0 software (SPSS, Chicago, IL, USA) was chosen as the analysis tool. A strong association would be found out with r value closed to 1. Median overall survival time (OS) was defined as the duration from the diagnosis and death or the last follow-up. Kaplan-Meier method was used to draw survival curve and analyzed by Log-rank test.
Results
The molecular combination of IDH1/TERT/1p19q has unique distribution among distinctive histological subtypes in glioma
The information of histology diagnosis and WHO grade among 143 glioma patients can been found in Table 1. Accordance with WHO 2016 instruction, oligoastrocytoma was subdivided into oligodendroglioma or astrocytoma according to 1p19q status [7]. By using panel of IDH1/TERT/1p19q, we divided the whole case cohort into 5 molecular subgroups according to NEJM paper [8]. There are 41 Triple-positive and 27 triple-negative tumors. Proportion of TERT and IDH1 mutation, IDH1 mutation only and TERT mutation only subgroup accounts for 26%, 19% and 21% respectively. Similar to previous studies, IDH1 mutation only tumors were more likely to be seen in astrocytoma with the ratio of 49.3%, while 82.5% triple-positive gliomas belong to oligodendroglioma. 64.7% glioblastomas have TERT mutation only. (Fig. 1).
Fig. 1.
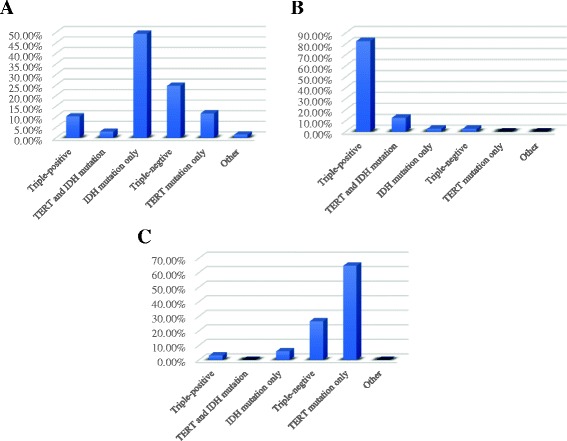
Distribution of IDH1/TERT/1p19q among distinctive histological subtypes. a astrocytoma, (b)oligodendroglioma, (c)glioblastoma
Different survival outcomes among distinctive molecular subgroups based on IDH1/TERT/1p19q classification system
We have completely follow-up in 140 patients, among whom 43 patients were dead and the other patients were still alive. Patients diagnosed as oligodendroglioma have the best clinical outcome with median overall survival 37.9 months (P < 0.01). Median overall survival are 33 months for astrocytoma and 20.5 months for glioblastoma (Fig. 2a). Regarding to molecular stratification, patients in triple-positive subgroup have the best survival outcome with 39 months of median overall survival compared to IDH1/TERT mutation subgroup (36.9 months), IDH1 mutation only subgroup (34 months), triple-negative subgroup (27.6 months) and TERT mutation only subgroup (19.9 months) (P < 0.0001) (Fig. 2b).
Fig. 2.
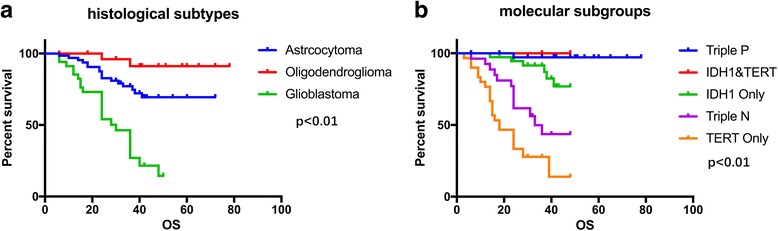
Median overall survival time for different histological subtypes (a) and molecular subgroups (b)
Patients with different anatomic position have unique survival outcome
Previous to studying anatomic preference to special molecular subgroups, we analyzed survival outcome between different tumor location. Herein, we found tumor located in frontal lobe indicated longer overall survival time of 66.1 months compared to tumors located in other cerebral regions (P < 0.01). Tumors located in hemisphere demonstrate better clinical outcome than those in central region even though no significance exists (Fig. 3).
Fig. 3.
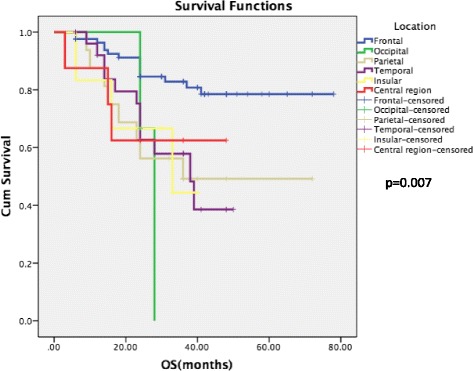
Survival outcome among different location subpopulations
Locational pattern is different among distinctive molecular subgroups
For triple-positive gliomas, tumor location tends to aggregate in bilateral frontal lobes. On the contrary, triple-negative tumors were more likely to locate in bilateral basal ganglia regions. In spite of that, IDH1/TERT mutation subgroup inclined to grow in left frontal lobe close to midline region. IDH1 mutation only subgroup was commonly seen in left frontal lobe and bilateral insular lobes. TERT mutation glioma apparently present with non-midline distribution while sitting in right frontal-insular lobe and left basal ganglia region. Meanwhile, TERT mutation only glioma has deep-seated location than triple-positive cases (Fig. 4).
Fig. 4.
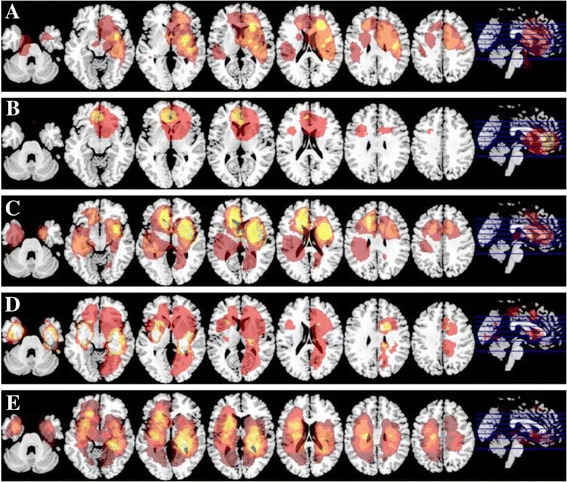
Distribution of tumor location according to IDH1/TERT/1p19q stratification regimen. a Triple-positive, (b) TERT and IDH mutation, (c) IDH mutation only, (d)Triple-negative, (e)TERT mutation only
Tumor location remains consistently between histological subtype and its corresponding molecular subgroup
Thus glioma histology stratification strongly associated with molecular phenotype, we investigate whether anatomic distribution remains consistent within these two classification systems. We compared triple-positive samples with oligodendroglioma, IDH1 mutation only subgroup with astrocytoma and TERT mutation only tumors with glioblastoma since these genetic events highly represent histological diagnosis. It’s interested to find out that the tumor location and growth pattern is quite similar to each other based on MR images. The location correlation coefficients are 0.97, 0.94 and 0.85 accordingly (Fig. 5a–c).
Fig. 5.
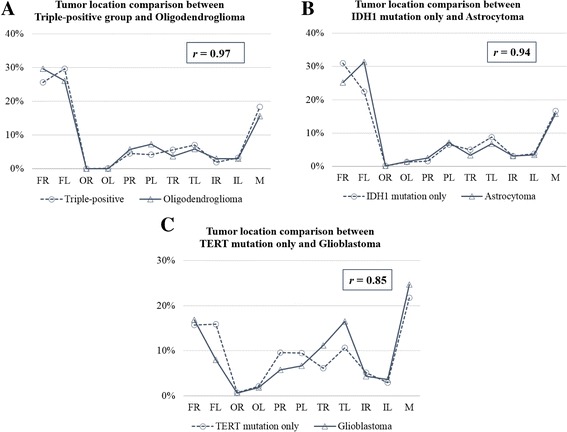
Tumor location correlation analysis between histological subtype and its corresponding molecular subgroup with (a) triple-positive group vs. oligodendroglioma, (b) IDH1 mutation only vs. astrocytoma, (c) TERT mutation only vs. glioblastoma
Dissimilarity in molecular background results in different tumor location within mixed diffuse glioma
In 2016 revised WHO diffuse glioma classification, the diagnosis of oligoastrocytoma was gone due to 1p19q can help clearly subdivide tumor into oligo-lineage or astroglial family. We have 14 oligoastrocytoma cases, 10 of them showed 1p19q codeletion with tumor likely to locate in bilateral frontal lobe which is similar to oligodendroglioma. On the other hand, tumor with 1p19q intact tends to grow in left insular lobe where is commonly seated by astrocytoma (Fig. 6).
Fig. 6.
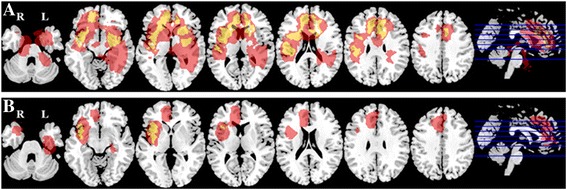
1p19q status of oligoastrocytoma decide tumor location, (a) Oligoastrocytoma with1p19q codeletion, (b) Oligoastrocytoma with 1p19q retain
Discussion
Cerebral diffuse glioma is a biological heterogeneous tumor [1]. Patient clinical outcome was affected by many factors including age, anatomic location, tumor size, extent of resection, genetic alteration [14]. Among them, anatomic location plays a crucial role not only for prognosis prediction but also for treatment strategy. It was widely acknowledged that prognosis is poor in midline glioma than non-midline tumor [10]. Regarding to hemispheric glioma, patient with tumor located in frontal lobe tends to be younger, IDH1 mutation and longer survival time [15]. This conclusion is accordance with our result. Furthermore, in our cohort, occipital lobe glioma implied negative impact on clinical outcome which is similar to Liu et al. [16]. The reason might be larger tumor size commonly seen in this area. Meanwhile, anatomic location somehow determines the extent of resection, for example, midline or deep-seated glioma is hard to get gross total resection due to preservation of functional structure or complex surgical corridor [17]. On the contrary, non-eloquent area tumor, especially superficial to the cortex is amendable to completely remove. For the same reason, longer survival time is strongly associated with gross total resection [18]. On the other hand, based on huge amounts of exploration of glioma genetic alteration in the recent years, a panel of classic biomarkers begins to exert great impact on glioma precise diagnosis and prognostic assessment [1]. IDH1/1p19q/H3F3A are the representatives introduced into newly revised 2016 WHO glioma guideline [7]. In our previous study, 4 biomarkers were used to stratify lower grade glioma into 4 subgroups predicting better clinical outcome than the roles of histological diagnosis and WHO grade [19]. The current findings firmly validate the great prognostic value of biomarkers in glioma. Similar to our findings, Jenkins et al. used a genetic combination of IDH1/1p19q/TERT to classify glioma into 5 subpopulations with unique clinical features and germline variants respectively which is highly recognized in the world [8]. We referred to this 3-biomarkers scheme in our study, and drew the same conclusion Triple-positive and IDH1/TERT double-mutation cases are more likely to be oligo-lineage. IDH1 mutation only cases are astrocytoma with maximal possibility. IDH1 wild type and TERT mutation tumors are commonly seen in glioblastoma. According to this scheme, survival outcome in our patient cohort is distinctive among all the subgroups. All these data demonstrate integration of molecular and histology diagnosis being helpful in prognostic and predictive value for glioma patients. Nevertheless, the perfect integration of these two systems still calls for huge efforts like large cohort clinical validation on many aspects, such as image features. Thus, we hypothesized anatomic location, genetic biomarkers and histology diagnosis are highly correlated and intertwined.
In order to verify our hypothesis, we tried to study the interconnection between anatomic location and genetic biomarkers in our patient cohort. Beforehand, many papers published have put forward the cell origin theory underlying possible relationship between these two factors [20, 21]. Many groups have successfully developed computational methods to predict glioma genetic alterations based on location features [18, 22, 23]. For example, IDH1 mutation was commonly seen in left frontal lobe, where TERT mutation only exists as well[2, 5, 23]. Such investigations were performed in the context of MGMT and TP53[24, 25]. In our study, we used a panel composed of IDH1/1p19q/TERT which is worldwide recognized in the precise diagnosis of glioma to demonstrate the anatomic distribution of different molecular subsets. Our data showed similar results to previous research works such as IDH1 mutation prefers to localize in left frontal lobe[2, 15]. Interestingly, we also found that triple-positive tumor located more superficial to cortex than TERT mutation only tumor. This finding may explain the differences in survival outcome and extent of resection. In spite of that,. highly consistency of location feature was observed between histological subpopulations and its corresponding molecular counterparts. For example, triple-positive tumor appears to have similar anatomic location with oligodendrogliomas. That means the axis of molecule-cell-tissue depicts the growth pattern of glioma which is additional evidence supporting the cell origin theory. Another interesting finding is that 1p19q codeletion oligoastrocytoma possessing different anatomic location with 1p19q intact oligoastrocytoma which is supportive to new 2016 WHO classification that the diagnosis of oligoastrocytoma is eliminated [7]. Since now, the diagnosis of oligoastrocytoma converts to either oligodendroglioma or astrocytoma according to molecular biomarkers [26]. These findings demonstrate definite molecular feature restricted to precise histological diagnosis. It strongly proved that molecular diagnosis can help clinicians make exactly right diagnostic decision facilitating to tailor personalized treatment.
On the other aspect, methods by using MR images to predict molecular biomarkers are popular recently, which was so-called Radiomics study. Ellingson et al. compared tumor volume ratio of T2 hyperintensity to contrast enhancement and central necrosis to differentiate mesechymal and non-mesenchymal molecular subtype in glioblastoma [27]. His research team also used perfusion and diffusion MRI signatures to successfully stratify lower grade glioma into three subpopulations as IDH1 mut/1p19q codel, IDH1 mut/1p19q non-condel and IDH1 wt [28]. MRS is another popular detectable technology to realize Radiomics study due to unique metabolic features inside glioma. It has been widely applied to predict IDH1 mutational status and medulloblastoma subgrouping [29, 30]. Compared to these methods, our team used anatomic location as basic tumor feature to predict biomarkers like IDH1/1p19q/TERT, which is more simple, cost effective and visualized. The raw materials we need are only T2 flair and T1 contrast MR images without sophisticated computation process. However, our method has its own limitations, like rough estimation accuracy. In general, our team illustrated a simple method to predict molecular biomarkers and reveal anatomic location among different molecular subgroups which offered an alternative in Radiomics study.
Conclusion
Although molecular biomarkers are getting involved in routine pathological diagnosis of cerebral diffuse gliomas, more evidence should be provided to validate perfect match between molecular subtypes and classic histological diagnosis. Our study showed distinct anatomic distribution among different molecular phenotypes which is consistent with corresponding histological subtypes. Integration of molecular biomarkers with histology diagnosis will not only contribute to precise diagnosis but also predict patient clinical outcome. This kind of pathological diagnosis system was highly recommended in future clinical practice. Moreover, we developed a simple and cost-effective method to predict biomarkers which was supposed to be widely used in Radiomics study in glioma.
Acknowledgements
We would like to thank graduate student Yusheng Tong, Zeju Li, Yuan Gao for collecting and preprocessing the data.
Funding
This work is supported by the National Basic Research Program of China (2015CB755500), Natural Science Foundation and Major Basic Research Program of Shanghai (No. 16JC1420100), Shanghai Sailing Program (16YF1415200).
Availability of data and materials
The data sets generated and analyzed during this study are available after the approval of the corresponding authors on reasonable request.
Abbreviations
- CNN
Convolutional neural network
- MNI
Montreal Neurological Institute
Authors’ contributions
QT, YL, JY and ZS conceived of the project, collected the data, performed the analysis of the data and wrote the paper. JY, ZS, WY, and LC provided expert guidance and revised the manuscript critically for important intellectual content. JY, YW, and ZS gave final approval of the version to be published. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the ethic committee of Huashan hospital, Fudan University. The reference number is: 2015–256-1. As this study is a retrospective study, most of participants are dead. Written informed consents were obtained from the participants who are alive. The ethic committee of Huashan hospital has been informed and also approved the situation of ‘consent of participate’ of this study.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qisheng Tang, Email: tqs5882@163.com.
Yuxi Lian, Email: 14210720155@fudan.edu.cn.
Jinhua Yu, Email: jhyu@fudan.edu.cn.
Yuanyuan Wang, Email: yywang@fudan.edu.cn.
Zhifeng Shi, Email: 13917793493@139.com.
Liang Chen, Email: chenlianghs@126.com.
References
- 1.Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J, Shi Z, Ji C, et al. Anatomical location differences between mutated and wild-type isocitrate dehydrogenase 1 in low-grade gliomas. Int J Neurosci. 2017;6:1–8. doi: 10.1080/00207454.2016.1270278. [DOI] [PubMed] [Google Scholar]
- 3.Jain R, Poisson L, Narang J, et al. Genomic mapping and survival prediction in glioblastoma: molecular subclassification strengthened by hemodynamic imaging biomarkers. Radiology. 2013;267(1):212–220. doi: 10.1148/radiol.12120846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellingson BM, Lai A, Harris RJ, Selfridge JM, et al. Probabilistic radiographic atlas of glioblastoma phenotypes. AJNR Am J Neuroradiol. 2013;34(3):533–540. doi: 10.3174/ajnr.A3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Zhang T, Li S, Fan X, Ma J, Wang L, Jiang T. Anatomical localization of isocitrate dehydrogenase 1 mutation: a voxel-based radiographic study of 146 low-grade gliomas. Eur J Neurol. 2015;22(2):348–354. doi: 10.1111/ene.12578. [DOI] [PubMed] [Google Scholar]
- 6.Sonoda Y, Shibahara I, Kawaguchi T, et al. Association between molecular alterations and tumor location and MRI characteristics in anaplastic gliomas. Brain Tumor Pathol. 2015;32(2):99–104. doi: 10.1007/s10014-014-0211-3. [DOI] [PubMed] [Google Scholar]
- 7.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 8.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang RQ, Shi Z, Chen H, et al. Biomarker-based prognostic stratification of young adult glioblastoma. Oncotarget. 2016;7(4):5030–5041. doi: 10.18632/oncotarget.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YX, Shi Z, Aibaidula A, et al. Not all 1p/19q non-codeleted oligodendroglial tumors are astrocytic. Oncotarget. 2016;7(40):64615–64630. doi: 10.18632/oncotarget.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira S, Pinto A, Alves V, Silva CA. Brain Tumor Segmentation using Convolutional Neural Networks in MRI Images. IEEE Trans Med Imaging. 2016[Epub ahead of print]. [DOI] [PubMed]
- 12.Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM) Philos Trans R Soc Lond Ser B Biol Sci. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellingson BM, Cloughesy TF, Pope WB, et al. Anatomic localization of O6-methylguanine DNA methyltransferase (MGMT) promoter methylated and unmethylated tumors: a radiographic study in 358 de novo human glioblastomas. NeuroImage. 2012;59(2):908–916. doi: 10.1016/j.neuroimage.2011.09.076. [DOI] [PubMed] [Google Scholar]
- 14.Reni M, Mazza E, Zanon S, Gatta G, Vecht CJ. Central nervous system gliomas. Crit Rev Oncol Hematol. 2017;113:213–234. doi: 10.1016/j.critrevonc.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Chen N, Yu T, Gong J, Nie L, Chen X, Zhang M, Xu M, Tan J, Su Z, Zhong J, Zhou Q. IDH1/2 Gene hotspot mutations in central nervous system tumours: analysis of 922 Chinese patients. Pathology. 2016;48(7):675–683. doi: 10.1016/j.pathol.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Liu TT, Achrol AS, Mitchell LA, Du WA, Loya JJ, Rodriguez SA, Feroze A, Westbroek EM, Yeom KW, Stuart JM, Chang SD, Harsh GR, 4th, Rubin DL. Computational identification of tumor anatomic location associated with survival in 2 large cohorts of human primary glioblastomas. AJNR Am J Neuroradiol. 2016;37(4):621–628. doi: 10.3174/ajnr.A4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenstat DD, Pollack IF, Demers A, Sapp MV, Lambert P, Weisfeld-Adams JD, Burger PC, Gilles F, Davis RL, Packer R, Boyett JM, Finlay JL. Impact of tumor location and pathological discordance on survival of children with midline high-grade gliomas treated on Children's cancer group high-grade glioma study CCG-945. J Neuro-Oncol. 2015;121(3):573–581. doi: 10.1007/s11060-014-1669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jungk C, Scherer M, Mock A, Capper D, Radbruch A, von Deimling A, Bendszus M, Herold-Mende C, Unterberg A. Prognostic value of the extent of resection in supratentorial WHO grade II astrocytomas stratified for IDH1 mutation status: a single-center volumetric analysis. J Neuro-Oncol. 2016;129(2):319–328. doi: 10.1007/s11060-016-2177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan AK, Yao Y, Zhang Z, Shi Z, Chen L, Chung NY, Liu JS, Li KK, Chan DT, Poon WS, Wang Y, Zhou L, Ng HK. Combination genetic signature stratifies lower-grade gliomas better than histological grade. Oncotarget. 2015;6(25):20885–20901. doi: 10.18632/oncotarget.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sreedharan S, Maturi NP, Xie Y, Sundström A, Jarvius M, Libard S, Alafuzoff I, Weishaupt H, Fryknäs M, Larsson R, Swartling FJ, Uhrbom L. Mouse models of pediatric Supratentorial high-grade glioma reveal how cell-of-origin influences tumor development and phenotype. Cancer Res. 2017;77(3):802–812. doi: 10.1158/0008-5472.CAN-16-2482. [DOI] [PubMed] [Google Scholar]
- 21.Alcantara Llaguno SR, Parada LF. Cell of origin of glioma: biological and clinical implications. Br J Cancer. 2016;115(12):1445–1450. doi: 10.1038/bjc.2016.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutman DA, Dunn WD, Jr, Grossmann P, Cooper LA, Holder CA, Ligon KL, Alexander BM, Aerts HJ. Somatic mutations associated with MRI-derived volumetric features in glioblastoma. Neuroradiology. 2015;57(12):1227–1237. doi: 10.1007/s00234-015-1576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang C, Zhang ZY, Chen LC, Sun Z, Zhang Y, Qin Z, Yao Y, Zhou LF. Subgroup characteristics of insular low-grade glioma based on clinical and molecular analysis of 42 cases. J Neuro-Oncol. 2016;126(3):499–507. doi: 10.1007/s11060-015-1989-5. [DOI] [PubMed] [Google Scholar]
- 24.Wang YY, Zhang T, Li SW, Qian TY, Fan X, Peng XX, Ma J, Wang L, Jiang T. Mapping p53 mutations in low-grade glioma: a voxel-based neuroimaging analysis. AJNR Am J Neuroradiol. 2015;36(1):70–76. doi: 10.3174/ajnr.A4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanas VG, Zacharaki EI, Thomas GA, Zinn PO, Megalooikonomou V, Colen RR. Learning MRI-based classification models for MGMT methylation status prediction in glioblastoma. Comput Methods Prog Biomed. 2017;140:249–257. doi: 10.1016/j.cmpb.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Sahm F, Reuss D, Koelsche C, Capper D, Schittenhelm J, Heim S, Jones DT, Pfister SM, Herold-Mende C, Wick W, Mueller W, Hartmann C, Paulus W, von Deimling A. Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol. 2014;128(4):551–559. doi: 10.1007/s00401-014-1326-7. [DOI] [PubMed] [Google Scholar]
- 27.Naeini KM, Pope WB, Cloughesy TF, Harris RJ, Lai A, Eskin A, Chowdhury R, Phillips HS, Nghiemphu PL, Behbahanian Y, Ellingson BM. Identifying the mesenchymal molecular subtype of glioblastoma using quantitative volumetric analysis of anatomic magnetic resonance images. Neuro-Oncology. 2013;15(5):626–634. doi: 10.1093/neuonc/not008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leu K, Ott GA, Lai A, Nghiemphu PL, Pope WB, Yong WH. Liau LM. Ellingson BM. Perfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II-III diffuse gliomas. J Neurooncol: Cloughesy TF; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blüml S, Margol AS, Sposto R, Kennedy RJ, Robison NJ, Vali M, Hung LT, Muthugounder S, Finlay JL, Erdreich-Epstein A, Gilles FH, Judkins AR, Krieger MD, Dhall G, Nelson MD, Asgharzadeh S. Molecular subgroups of medulloblastoma identification using noninvasive magnetic resonance spectroscopy. Neuro-Oncology. 2016;18(1):126–131. doi: 10.1093/neuonc/nov097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leather T, Jenkinson MD, Das K, Poptani H. Magnetic Resonance Spectroscopy for Detection of 2-Hydroxyglutarate as a Biomarker for IDH Mutation in Gliomas. Metabolites. 2017, 7(2). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analyzed during this study are available after the approval of the corresponding authors on reasonable request.


