Abstract
Background
To address a clinical impact of atherosclerotic cardiovascular diseases (CVD) on cancer developments, we investigated an issue whether any difference in an incidence of cancers is present between patients with atherosclerotic CVD and those with non-atherosclerotic CVD.
Methods
Of a total of 32,095 consecutive patients with acquired CVD enrolled in the Sakakibara Health Integrative Profile cohort study, we segregated patients based on a presence of atherosclerotic or non-atherosclerotic CVD to investigate an incidence of cancers and mortality. We also evaluated an incidence of cancers in patients with a singular presence versus a plural presence of atherosclerotic CVD. Atherosclerotic CVD included coronary artery diseases, aortic diseases and peripheral artery diseases. Non-atherosclerotic CVD were any acquired CVD except atherosclerotic CVD.
Results
During a median follow-up of 1020 days (interquartile range, 665–1340 days), an incidence of cancers (5% vs. 2%, p = 0.0001) and overall mortality (6% vs. 3%, p = 0.0001) were more than two-fold higher in 10,592 patients with atherosclerotic CVD than in 21,503 patients with non-atherosclerotic CVD. A presence of atherosclerotic CVD (hazard ratio 1.372 with 95% confidence interval 1.199–1.569) was independently associated with an incidence of cancers. In patients with atherosclerotic CVD, 61 of 640 patients with a plural presence and 470 of 9932 patients with a singular presence developed cancers (9% vs. 5%, p = 0.0001). An incremental risk of death was found according to a presence of atherosclerotic CVD, cancers, and both of them (all p = 0.0001).
Conclusions
A presence of atherosclerotic CVD itself may have a potential risk for cancer developments.
Trial registration
ClinicalTrials.gov. number, NCT03005834
Keywords: Atherosclerotic cardiovascular diseases, Cancers, Mortality, The SHIP cohort study
Worldwide surveillance of health statistics [1], [2] and/or a large cohort study [3] has critically highlighted the indisputable fact that cardiovascular diseases (CVD), especially atherosclerotic CVD, and cancers are the two leading causes of death among non-communicable diseases irrespective of socioeconomic status. Recent epidemiological studies [4], [5], [6] also implied an incremental risk of death due to non-cardiac causes, especially attributable to cancer developments, during the long-term follow-up of patients with coronary artery diseases. Several scientific bodies addressing the pathogenesis of atherosclerotic CVD and/or cancers demonstrated a presence of common denominators such as the role of inflammation [7], [8], [9], [10], [11], neovascularity [12], [13], epigenetics [14], [15], [16], [17], [18], [19], and lifestyle behaviors [20], etc., to propose the possible association between both diseases. So far, however, the clinical impact of a presence of atherosclerotic CVD itself on cancer developments remains undefined.
To address this issue, we investigated the question whether any difference in an incidence of cancers is present between patients with atherosclerotic CVD and those with non-atherosclerotic CVD using longitudinal clinical outcomes in a total of 32,095 consecutive patients with acquired CVD enrolled in the Sakakibara Health Integrative Profile (SHIP) cohort study.
1. Methods
1.1. The SHIP cohort study
The SHIP cohort study was launched in 2006 for the purpose of improving healthy life expectancy in patients with any CVD who were admitted to the Sakakibara Heart Institute. The study used a continuous surveillance system to track all subsequent incidents of CVD and/or non-CVD including cancers, via direct contact in the outpatient department, hospital records, and a mailed questionnaire at least once a year. Organ sites of cancers were also identified whenever possible. The study sorted any diagnoses of patients with CVD into one or more of nine categories, namely coronary artery diseases, myocardial diseases, valvular heart diseases, any-cause heart failure, arrhythmias, aortic and/or peripheral vascular diseases, lifestyle related diseases, congenital CVD, and others. For example, if a patient had a couple of diseases, he or she had a plural category. Because congenital CVD must be occurred via Mendelian inheritance mainly as an underlying genetic disorder, and also related with the interactions between multifactorial genetic and environmental influences (e.g. maternal diabetes, alcohol, et al.), these backgrounds of those with congenital CVD are too complex to analyze the present hypothesis which focused on a clinical impact of atherosclerotic CVD on cancer developments. Furthermore, the age of subjects between atherosclerotic and congenital CVD was too different to analyze an incidence of cancers. Thus the present study focused on patients with acquired CVD after excluded those with congenital CVD. We defined atherosclerotic CVD when presented any of coronary artery diseases, aortic diseases and peripheral artery diseases [21], and then divided patients with acquired CVD into atherosclerotic CVD and non-atherosclerotic CVD in accordance with a presence or absence of atherosclerotic CVD. With regard to the detail of coronary artery diseases, there were acute coronary syndromes, previous myocardial infarction and stable ischemic heart diseases. Acute coronary syndromes were diagnosed according to the urgent algorithm composed of symptomatic abnormal ST segments in the 12-lead electrocardiogram with abnormal cardiac enzyme spillover, and urgent coronary angiogram et al. Previous myocardial infarction and stable ischemic heart diseases were also diagnosed by invasive and non-invasive cardiac examinations including myocardial ischemia and viability. A strategy for the treatment of those with coronary artery diseases (i.e. optimal medications with or without percutaneous coronary intervention and/or coronary bypass surgery) were determined by our heart team. Aortic diseases consisted of acute aortic syndromes and aortic aneurysms which were mainly diagnosed by computed tomogram with or without typical symptoms, and treated with medications, arterial bypass graft, and/or endovascular replacement, et al. Peripheral artery diseases were diagnosed when documented one or more of the following criteria: intermittent claudication with ankle-brachial index of < 0.9 or a history of intermittent claudication together with a previous and related intervention, such as angioplasty, stenting, atherectomy, peripheral arterial bypass graft, or other vascular intervention including amputation. Cerebrovascular diseases were included in the category of ‘others’ because the diseases included the mixed causes such as embolic strokes, lacunar infarction, cardio-embolic strokes, and hemorrhagic strokes, etc. So, it was possible that patients with atherosclerotic CVD also included other categories such as valvular heart diseases, heart failure, arrhythmia, and others, et al., while none of those with non-atherosclerotic CVD had coronary artery diseases, aortic diseases and peripheral artery diseases. As mentioned above, the SHIP cohort study handled to define lifestyle related diseases when confirmed a diagnosis of one or more of the presence of hypertension, dyslipidemia, diabetes mellitus and chronic kidney disease in accordance with the universal criteria [22]. The study also identified a history of tobacco smoking. The SHIP cohort study had complied with the Declaration of Helsinki, and was approved by the local ethics committee of our institute. All the patients gave written informed consent for study participation.
1.2. Flow diagram showing the study design (Fig. 1)
Fig. 1.
Flow diagram of the study design showing the first (A) and second (B) steps for analysis. CVD = cardiovascular diseases.
Among a total of 36,151 patients (58% male, 61 ± 23 years) with any CVD enrolled in the SHIP cohort study between June 2006 and July 2014, we excluded 245 patients who had already been diagnosed with any cancers at the time of enrollment in the SHIP cohort study, and 3811 patients with congenital CVD including Marfan syndrome. Thus, we analyzed a total of 32,095 consecutive patients with acquired CVD in the present study.
Our study was conducted in two steps to assess an incidence of cancers in patients with acquired CVD. The first step (analyses 1 and 2) consisted of a comparison between those with atherosclerotic CVD and non-atherosclerotic CVD with regard to an incidence of cancers. The second step (analyses 3 and 4) determined the association between a singular presence versus a plural presence of atherosclerotic CVD and an incidence of cancers.
At the first step (Fig. 1A), we classified the 32,095 patients into atherosclerotic and non-atherosclerotic CVD groups to evaluate an overall incidence of cancers in each group (analysis 1). Then, we compared an incidence of cancers between 8739 patients with atherosclerotic CVD and 8739 patients with non-atherosclerotic CVD under the well-matched baseline clinical characteristics of both groups (analysis 2). At the second step (Fig. 1B), we divided the 10,592 patients with atherosclerotic CVD into those with a singular disease or a plural disease, and studied an overall incidence of cancers in each group. We also identified an independent role of each disease in atherosclerotic CVD for cancer developments (analysis 3). Finally, we compared an incidence of cancers between 640 patients with a singular disease and 640 patients with a plural disease in atherosclerotic CVD under the well-matched baseline clinical characteristics of both groups (analysis 4). Cumulative mortality during follow-up was also evaluated in those with atherosclerotic CVD and non-atherosclerotic CVD.
1.3. Statistical analysis
Continuous variables are presented as mean ± standard deviation or median (interquartile range, ICR). Differences between the two groups (i.e. atherosclerotic CVD vs. non-atherosclerotic CVD, or a singular presence of atherosclerotic CVD vs. a plural presence of atherosclerotic CVD) were compared using the Student's t-test or Mann-Whitney U test for continuous variables, and Fisher's exact test or Pearson test for categorical variables. A cumulative incidence of cancers throughout follow-up was characterized using Kaplan-Meier curves with the log-rank test for comparison between the two groups. To assess a possibility of the role of atherosclerotic CVD itself and/or a number of atherosclerotic CVD (i.e. singular or plural disease) in an incidence of cancers, adjustment for differences in baseline clinical characteristics recorded in the SHIP cohort study (i.e. age, male sex, lifestyle related diseases, tobacco smoking, and follow-up periods) was performed using propensity score matching with a measurement of the C-statistic to evaluate the ability of control for confounding bias with a range of 0 to 1 and higher value indicating well-controlled. The Cox proportional hazards regression analysis was carried out to assess the independent associations of age, male sex, lifestyle related diseases, tobacco smoking, and the presence of atherosclerotic CVD with cancer developments, and to identify an independent role of each disease (i.e. coronary artery diseases, aortic diseases and peripheral artery diseases) in atherosclerotic CVD to develop cancers. An association of a presence of atherosclerotic CVD, cancers, and both of them with death during the follow-up periods was also analyzed by the Cox proportional hazard model. A two-tailed p value of < 0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using JMP version 11.2.1. (SAS Institute Japan Inc. Tokyo, Japan).
2. Results
2.1. Incidence of cancers in patients with atherosclerotic CVD and non-atherosclerotic CVD (analyses 1 and 2)
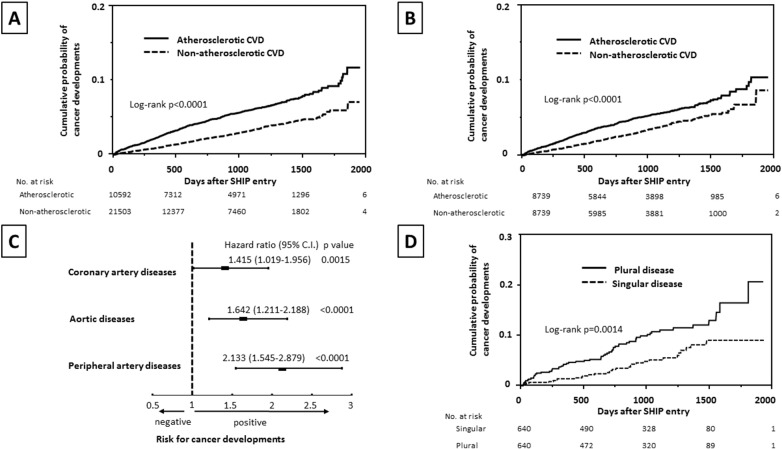
Of a total of 32,095 patients, 994 patients (3%) had cancer developments during the median follow-up of 1020 days (ICR, 665–1340 days). Cancers were found in 531 patients with atherosclerotic CVD (5% of atherosclerotic CVD) and 463 patients with non-atherosclerotic CVD (2% of non-atherosclerotic CVD, p < 0.0001 vs. atherosclerotic CVD). There was a significant difference in age, male sex, prevalence of lifestyle related diseases and tobacco smoking between those with atherosclerotic CVD and non-atherosclerotic CVD (Table 1). A cumulative probability of an overall incidence of cancers revealed a quite difference between patients with atherosclerotic CVD and non-atherosclerotic CVD (Fig. 2A). After a well-controlled propensity score matching for control of confounding factors between 8739 patients with atherosclerotic CVD and 8739 with non-atherosclerotic CVD (age, 69 ± 12 years vs. 69 ± 12 years, p = 0.3175, male sex, 68% vs. 68%, p = 0.9611, lifestyle related diseases, 69% vs. 68%, p = 0.2098, tobacco smoking, 38% vs. 38%, p = 0.9876, median duration of follow-up, 1068 days vs. 1049 days, p = 0.4228) with a C-statistics value of 0.7572, a considerably high probability of cancer developments was still found in those with atherosclerotic CVD (Fig. 2B). In the univariate Cox proportional hazards regression analysis, all of age (for each 1 year) (hazard ratio 1.048, 95% confidential intervals 1.042–10.55), male sex (1.909, 1.656–2.209), atherosclerotic CVD (1.887, 1.666–2.138), lifestyle related diseases (1.232, 1.079–1.408), and tobacco smoking (1.490, 1.312–1.690) were associated with an incidence of cancers, while the multivariate analysis demonstrated that only age (1.050, 1.044–1.057), male sex (1.949, 1.671–2.279), and a presence of atherosclerotic CVD (1.372, 1.199–1.569) were closely associated with cancer developments.
Table 1.
Baseline clinical characteristics of the study subjects.
| Characteristics | Overall CVD N = 32,095 |
Atherosclerotic CVD N = 10,592 |
Non-atherosclerotic CVD N = 21,503 |
p value |
|---|---|---|---|---|
| Age, year | 65 ± 16 | 70 ± 12 | 62 ± 17 | 0.0001 |
| Male sex, no. (%) | 18,978 (59) | 7765 (73) | 11,213 (52) | 0.0001 |
| Lifestyle related diseases, no. (%) | 18,089 (56) | 7769 (73) | 10,320 (48) | 0.0001 |
| Hypertension, no. (%) | 13,336 (42) | 5653 (53) | 7683 (36) | 0.0001 |
| Dyslipidemia, no. (%) | 10,306 (32) | 5360 (51) | 4946 (23) | 0.0001 |
| Diabetes mellitus, no. (%) | 4078 (13) | 2523 (24) | 1555 (7) | 0.0001 |
| Chronic kidney diseases, no. (%) | 1946 (6) | 1065 (10) | 881 (4) | 0.0001 |
| Tobacco smoking, no. (%) | 9443 (29) | 5140 (49) | 4303 (20) | 0.0001 |
| Duration of follow-up, median days (IQR) | 1020 (665–1340) | 1078 (721–1424) | 972 (609–1288) | 0.0001 |
| Category of acquired CVD, no. (%) | ||||
| Coronary artery diseases | 8980 (28) | 8980 (85) | 0 (0) | – |
| Aortic diseases | 1639 (5) | 1639 (16) | 0 (0) | – |
| Peripheral artery diseases | 654 (2) | 654 (6) | 0 (0) | – |
| Myocardial diseases | 2154 (7) | 197 (2) | 1957 (9) | 0.0001 |
| Unclassified or any-cause heart failure | 1826 (6) | 555 (5) | 1271 (6) | 0.0147 |
| Valvular heart diseases | 5265 (16) | 925 (9) | 4340 (20) | 0.0001 |
| Arrhythmias | 13,437 (42) | 1922 (18) | 11,515 (54) | 0.0001 |
| Others | 5736 (18) | 1237 (12) | 4499 (21) | 0.0001 |
Plus-minus values are mean ± SD. CVD = cardiovascular diseases, IQR = interquartile range.
Fig. 2.
Cumulative probability of cancer developments in all the patients (A) and following adjustment for baseline characteristics (B), according to patients with atherosclerotic CVD or non-atherosclerotic CVD. The multivariate Cox proportional hazards regression analysis for cancer developments in each disease in atherosclerotic CVD (C). Cumulative probability of cancer developments in patients with a singular presence and a plural presence of atherosclerotic CVD after adjustment for baseline clinical characteristics (D). CVD = cardiovascular diseases, 95% C.I. = 95% confidence intervals.
2.2. Incidence of cancers in terms of a singular presence or a plural presence of atherosclerotic CVD (analyses 3 and 4)
Of a total of 10,592 patients with atherosclerotic CVD, 9932 patients with a singular presence of atherosclerotic CVD and 660 patients with a plural presence of atherosclerotic CVD were identified. As to the detail of each group, 9932 patients with a singular presence of atherosclerotic CVD consisted of 8348 patients with coronary artery diseases, 1222 patients with aortic diseases, and 362 patients with peripheral artery diseases, while 660 patients with a plural presence of atherosclerotic CVD included 368 patients with coronary artery diseases and aortic diseases, 243 patients with coronary artery diseases and peripheral artery diseases, 28 patients with aortic diseases and peripheral artery diseases, and 21 patients with all of three diseases. In the Cox proportional hazards regression model, all the three diseases in atherosclerotic CVD showed an independent association with cancer developments (Fig. 2C). An incidence of cancers was markedly different between patients with a singular presence and a plural presence of atherosclerotic CVD regardless of whether propensity score matching for baseline clinical characteristics with a C-statistics value of 0.6997 (Table 2 and Fig. 2D).
Table 2.
Comparison between patients with a singular and a plural presence of atherosclerotic CVD.
| Characteristics | Before propensity-score matching |
After propensity-score matching |
||||
|---|---|---|---|---|---|---|
| Singular N = 9932 |
Plural N = 660 |
p value | Singular N = 640 |
Plural N = 640 |
p value | |
| Age, year | 69 ± 12 | 74 ± 8 | 0.0001 | 74 ± 9 | 74 ± 8 | 0.9538 |
| Male sex, no. (%) | 7220 (73) | 545 (83) | 0.0001 | 526 (82) | 526 (82) | 1.0000 |
| Lifestyle related diseases, no. (%) | 7234 (73) | 535 (81) | 0.0001 | 515 (80) | 517 (81) | 0.9436 |
| Tobacco smoking, no. (%) | 4693 (47) | 447 (68) | 0.0001 | 430 (67) | 429 (67) | 0.9526 |
| Duration of follow-up, median days (IQR) | 1078 (721–1421) |
1085 (721–1447) |
0.7069 | 1077 (737–1430) |
1085 (721–1447) |
0.9219 |
| Cancer developments, no. (%) | 470 (5) | 61 (9) | 0.0001 | 31 (5) | 61 (10) | 0.0012 |
Plus-minus values are mean ± SD. CVD = cardiovascular diseases, IQR = interquartile range.
2.3. Mortality during follow-up
A total of 1157 patients were dead during follow-up (mortality, 4%). In detail, 611 patients with atherosclerotic CVD and 546 patients with non-atherosclerotic CVD were dead (mortality, 6% vs. 3%, p = 0.0001). Regarding the mortality in atherosclerotic CVD, 94 of 660 patients with a plural presence were dead as compared with 517 of 9932 patients with a singular presence (mortality, 14% vs. 5%, p = 0.0001). An incremental risk of death was found in the order of a presence of atherosclerotic CVD (hazard ratio 1.436, 95% confidential intervals, 1.246–1.658), cancers (5.209, 4.344–6.205), and both of them (5.463, 4.395–6.715).
2.4. Organ sites of cancers in patients with atherosclerotic and non-atherosclerotic CVD
Organ sites could be identified in 92% of patients with cancers in the present study. As to the distribution of organ sites of cancers, a difference in the prevalence of colorectal, breast, and lymphoid cancers was identified between patients with atherosclerotic CVD and those with non-atherosclerotic CVD (Supplementary Table).
3. Discussion
In the present study, we found more than two-fold higher incidence of cancers in patients with atherosclerotic CVD than those with non-atherosclerotic CVD, and identified a remarkably high incidence of cancers in those with a plural presence of atherosclerotic CVD compared to those with a singular presence of atherosclerotic CVD. We also confirmed high mortality in patients with atherosclerotic CVD, especially in those with a plural presence of atherosclerotic CVD. These findings provide noteworthy insights into a presence of atherosclerotic CVD as the incremental risk for cancer developments which may have a role in a poor prognosis.
With the dramatic improvements in acute cardiac care in patients with atherosclerotic CVD in the modern era, the mission of long-term healthy cardiovascular care may focus on the prevention of non-cardiac diseases as well as the circumvention of adverse events of atherosclerotic CVD [23]. Spoon DB, et al. [5] reported an obvious change in the causes of death from cardiac to non-cardiac causes such as cancers, in patients successfully treated with coronary angioplasty for coronary artery diseases. The study by Hasin et al. [6] also speculated that heart failure complicated by myocardial infarction is a possible risk for cancers even with a limitation regarding an irregular medical surveillance. Because the SHIP cohort study holds a continuous health survey system for all patients with CVD, we could serve a certain amount of confidence in our observations that a high incidence of cancers is present in patients with atherosclerotic CVD when compared with those with non-atherosclerotic CVD.
Why do patients with atherosclerotic CVD, especially with a plural presence of atherosclerotic CVD, have a high incidence of cancers as compared to those with non-atherosclerotic CVD? Using the univariate Cox proportional hazards regression analysis, we confirmed that lifestyle related diseases and tobacco smoking have a statistically significant effect on cancer developments. The Atherosclerotic Risk In Communities (ARIC) study elegantly reported a significant low incidence of cancers in normal subjects when adhered to lifestyle guidelines for the prevention of atherosclerotic CVD [4]. This revealed a presence of common lifestyle risk factors for both atherosclerotic CVD and cancer developments. Interestingly, when focused on the first 3 years (i.e. similar periods to the SHIP cohort study) in the ARIC study, a cumulative incidence of cancers in the worst sub-population who did not adhere none of the healthy lifestyle behaviors was still low as compared with 5% incidence in those with atherosclerotic CVD in the SHIP cohort study. Furthermore, after adjusting for all these co-factors in the present study, we found a high incidence of cancers in patients with atherosclerotic CVD compared to those with non-atherosclerotic CVD. The multivariate Cox proportional hazards regression analysis identified a close association of a presence of atherosclerotic CVD itself with cancer developments. From the point of these views, we could consider the possible role of a presence of atherosclerotic CVD itself in cancer developments. A high incidence of cancers in patients with a plural presence of atherosclerotic CVD rather than a singular presence of atherosclerotic CVD also provides us the fundamental assumption of athero-carcinogenesis that the intensity of atherosclerosis itself would augment the risk for cancer developments.
Cancers are well-characterized by the uncontrolled growing and spreading phenotypes, which may be the result of complex interactions between external causes, for instance habitual behaviors, infections, and/or environmental co-factors, and internal determinants such as inherited mutations, endocrine and/or immune conditions (9). In view of the common intrinsic denominators between atherosclerotic CVD and cancers, the principal pathogenesis for a development of atherosclerosis such as inflammatory reactions (8,10,11), microRNAs [16], [17], [19], and angiogenesis [12], etc. may also play a considerable role in cancer developments via epigenetic modifications, [14], [15], [18] and/or affecting tumor microenvironments [13].
Clear differences in the proportion of organ sites of cancers in patients with atherosclerotic CVD and non-atherosclerotic CVD would suspect the biodiversity of the underlying mechanisms of cancer developments. In addition to a simple possibility that a higher incidence of breast cancer and lymphoma is caused by a higher prevalence of females and younger age in the non-atherosclerotic CVD group, a possible effect of aspirin, which is a standard prescription for atherosclerotic CVD, on preventing colorectal cancer [24], [25], a characteristics of a deleterious mutation of the specific genes with a development of breast cancers [26], and a specific chromosomal abnormalities as the primary cause for lymphoid cancers [27] may take part in a difference in a proportion of organ sites of cancers between patients with atherosclerotic CVD and non-atherosclerotic CVD.
In the field of cardio-oncology [28], cardiovascular effects of cancer therapies that target various kinase receptors and immuno-proteasome modulations reveal the pivotal issues in the health of cancer survivors. These issues also led us to question whether cancers themselves adversely affect the development of atherosclerotic CVD. Thus, we exactly expect the tremendous future science to address the pivotal issues whether and how a bidirectional link between atherosclerosis and carcinogenesis might be presented [29].
Our study has several limitations. First, we could not deny any possibility about a presence of subclinical cancers at the time of enrollment in the SHIP cohort study. However, this probability seems to be equal between patients with atherosclerotic CVD and non-atherosclerotic CVD, and hence, this may not affect the reliability of our observations. Second, because of insufficient data available about medications, a degree of radiation exposure to manage CVD, and detailed causes of death during the follow-up in the SHIP cohort study, we could not confirm an exact role of aspirin in a lower incidence of colorectal cancers in patients with atherosclerotic CVD, whether a dose of radiation confounded cancer developments, and how subsequent cardiovascular events could contribute the competing risk of deaths. Third, because the SHIP cohort study has a limited number of information regarding stages of cancers, we could not produce the stratified analysis according to cancer stages. As the next step, we will conduct a detailed analysis to elucidate the timeline between severity of atherosclerotic CVD and cancer developments. Fourth, although excluded patients with Marfan syndrome for the analysis of the present study, it remained a possibility of aortic diseases complicated with bicuspid aortic diseases and/or autoimmune inflammatory aortic diseases such as Takayasu arteritis in the category of “aortic diseases.” As to cerebrovascular diseases in the category of ‘others’, a total of 594 patients with cerebrovascular diseases were included and were composed of 259 with atherosclerotic CVD and 335 with non-atherosclerotic CVD (3% vs. 2%, p = 0.0001). Even when excluded all patients with cerebrovascular diseases from both groups to omit a possibility of atherosclerotic cerebrovascular diseases, an incidence of cancers was still significantly higher in those with atherosclerotic CVD than with non-atherosclerotic CVD (5% vs. 2%, p = 0.0001), and this incidence was not different from the original incidence. Finally, although a well-matched propensity score analysis provided us a constant result in both analysis of the first and second steps in the present study, this analysis could not eliminate an influence of unknown confounding variables regarding hidden background characteristics such as a presence of atherosclerotic cerebrovascular diseases, behaviors of an alcohol intake, an amount of X-ray exposure, and the medications on the present findings. In addition, we must recognize the utmost caution regarding a quite difference in baseline clinical characteristics of the study population before and after adjustment by the propensity score analysis to convince a definite conclusion. The next step of the SHIP cohort study will be set up to trace more detailed characteristics of the diseases and also medications for these issues.
Even though the SHIP cohort study was carefully constructed in accordance with Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidance [30], the quality of this clinical evidence needs to be confirmed by the nationwide and/or worldwide prospective cohort trial in future.
4. Conclusion
In the SHIP cohort study, patients with atherosclerotic CVD exhibit two-fold higher incidence of cancer developments than those with non-atherosclerotic CVD, which may provide a pivotal role in poor prognosis in patients with atherosclerotic CVD.
Conflict of interest
All of us have nothing to disclose for this study.
Funding
None.
Authors' contributions
MS designed this study, analyzed the data, and wrote the report. HT, TH and TS with an advice from SI contributed to prove the statistical analysis. YN, YI and TY interpreted all data and results, and contributed to the writing report. TS, MS, and SH took charge of the overall management of the SHIP cohort study. All authors contributed to implementation of the study, data acquisition, and approved the report for publication.
The following is the supplementary data related to this article.
Organ sites of cancers in patients with atherosclerotic and non-atherosclerotic CVD.
Acknowledgments
Acknowledgements
We are sincerely grateful to Mr. Toshiaki Masuda, Mr. Masataka Yoshitomi, and Ms. Ikuko Niimura for their profound contributions in managing the data in the SHIP cohort study, and are appreciative of all the staff of Sakakibara Heart Institute. We highly appreciate Dr. Tetsuji Kaneko, who is a member of Teikyo Academic Research Center, for his advice regarding the statistical analysis in the present study.
References
- 1.Di Cesare M., Khang Y.H., Asaria P., Blakely T., Cowan M.J., Farzadfar F. Inequalities in non-communicable diseases and effective responses. Lancet. 2013;381:585–597. doi: 10.1016/S0140-6736(12)61851-0. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Monitoring Health for the Sustainable Development Goals. 2016. World Health Statistics 2016; pp. 1–121. [Google Scholar]
- 3.Yusuf S., Rangarajan S., Teo K., Islam S., Li W., Liu L. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N. Engl. J. Med. 2014;371:818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen-Torvik L.J., Shay C.M., Abramson J.G., Friedrich C.A., Nettleton J.A., Prizment A.E. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spoon D.B., Psaltis P.J., Singh M., Holmes D.R., Jr., Gersh B.J., Rihal C.S. Trends in cause of death after percutaneous coronary intervention. Circulation. 2014;129:1286–1294. doi: 10.1161/CIRCULATIONAHA.113.006518. [DOI] [PubMed] [Google Scholar]
- 6.Hasin T., Gerber Y., Weston S.A., Jiang R., Killian J.M., Manemann S.M. Heart failure after myocardial infarction is associated with increased risk of cancer. J. Am. Coll. Cardiol. 2016;68:265–271. doi: 10.1016/j.jacc.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 8.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M. Acute coronary syndromes as auto-inflammatory disorders. Curr. Pharm. Des. 2012;18:4370–4384. doi: 10.2174/138161212802481228. [DOI] [PubMed] [Google Scholar]
- 11.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 12.Doyle B., Caplice N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J. Am. Coll. Cardiol. 2007;49:2073–2080. doi: 10.1016/j.jacc.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 13.Friedl P., Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Esteller M. Epigenetics in cancer. N. Engl. J. Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 15.Slack F.J., Weidhaas J.B. MicroRNA in cancer prognosis. N. Engl. J. Med. 2008;359:2720–2722. doi: 10.1056/NEJMe0808667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small E.M., Olson E.N. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creemers E.E., Tijsen A.J., Pinto Y.M. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 18.Dawson M.A., Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Boon R.A., Dimmeler S. MicroRNAs in myocardial infarction. Nat. Rev. Cardiol. 2015;12:135–142. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 20.Reicher-Reiss H., Jonas M., Goldbourt U., Boyko V., Modan B. Selectively increased risk of cancer in men with coronary heart disease. Am. J. Cardiol. 2001;87:459–462. doi: 10.1016/s0002-9149(00)01405-3. [DOI] [PubMed] [Google Scholar]
- 21.Mann Douglas L., Zipes Douglas P., Libby Peter, Bonow Robert O., Braunwald Eugene., editors. Braunwald's Heart Disease. A Textbook of Cardiovascular Medicine. 10th edition. Elsevier Saunders; 2015. Part VII. Atherosclerotic cardiovascular disease; pp. 1029–1386. [Google Scholar]
- 22.Fihn S.D., Gardin J.M., Abrams J., Berra K., Blankenship J.C., Dallas A.P. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Steg P.G., Cheong A.P. Death (after percutaneous coronary intervention) is no longer what it used to be. Circulation. 2014;129:1267–1269. doi: 10.1161/CIRCULATIONAHA.114.008492. [DOI] [PubMed] [Google Scholar]
- 24.Flossmann E., Rothwell P.M. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomized and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 25.Nan H., Hutter C.M., Lin Y., Jacobs E.J., Ulrich C.M., White E. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA. 2015;313:1133–1142. doi: 10.1001/jama.2015.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien K.M., Cole S.R., Tse C.K., Perou C.M., Carey L.A., Foulkes W.D. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin. Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nussenzweig A., Nussenzweig M.C. Origin of chromosomal translocations in lymphoid cancer. Cell. 2010;141:27–38. doi: 10.1016/j.cell.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moslehi J.J. Cardiovascular toxic effects of targeted cancer therapies. N. Engl. J. Med. 2016;375:1457–1467. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- 29.Opie L.H., Lopaschuk G.D. What is good for the circulation also lessens cancer risk. Eur. Heart J. 2015;36:1157–1162. doi: 10.1093/eurheartj/ehu457. [DOI] [PubMed] [Google Scholar]
- 30.Vandenbroucke J.P., von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann. Intern. Med. 2007;147:W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Organ sites of cancers in patients with atherosclerotic and non-atherosclerotic CVD.