Abstract
Background
This field-based study examined the abundance and species complement of mosquitoes (Diptera: Culicidae) attracted to humans at four sites in the United Kingdom (UK). The study used a systematic approach to directly measure feeding by mosquitoes on humans at multiple sites and using multiple volunteers. Quantifying how frequently humans are bitten in the field by mosquitoes is a fundamental parameter in assessing arthropod-borne virus transmission.
Methods
Human landing catches were conducted using a standardised protocol by multiple volunteers at four rural sites between July and August 2013. Collections commenced two hours prior to sunset and lasted for a total of four hours. To reduce bias occurring due to collection point or to the individual attractiveness of the volunteer to mosquitoes, each collection was divided into eight collection periods, with volunteers rotated by randomised Latin square design between four sampling points per site. While the aim was to collect mosquitoes prior to feeding, the source of blood meals from any engorged specimens was also identified by DNA barcoding.
Results
Three of the four sites yielded human-biting mosquito populations for a total of 915 mosquitoes of fifteen species/species groups. Mosquito species composition and biting rates differed significantly between sites, with individual volunteers collecting between 0 and 89 mosquitoes (over 200 per hour) of up to six species per collection period. Coquillettidia richiardii (Ficalbi, 1889) was responsible for the highest recorded biting rates at any one site, reaching 161 bites per hour, whilst maximum biting rates of 55 bites per hour were recorded for Culex modestus (Ficalbi, 1889). Human-biting by Culex pipiens (L., 1758) form pipiens was also observed at two sites, but at much lower rates when compared to other species.
Conclusions
Several mosquito species are responsible for human nuisance biting pressure in southern England, although human exposure to biting may be largely limited to evening outdoor activities. This study indicates Cx. modestus can be a major human-biting species in the UK whilst Cx. pipiens f. pipiens may show greater opportunistic human-biting than indicated by earlier studies.
Electronic supplementary material
The online version of this article (10.1186/s13071-017-2360-9) contains supplementary material, which is available to authorized users.
Keywords: Mosquito, Biting rate, Human landing catch, Culex, Coquillettidia, Blood meal
Background
Direct studies of mosquitoes biting human populations are important in understanding the impact of biting nuisance and transmission rates for zoonotic and anthroponotic mosquito-borne pathogens globally [1]. In the UK, nuisance biting of humans by mosquitoes can be a significant and often overlooked problem in both rural and urban environments [2–4]. Furthermore, the emergence and re-emergence of mosquito-borne pathogens are considered significant threats to the UK [5, 6]. To date, at least 23 native mosquito species have been reported to bite humans in the UK (Table 1). This list includes several species that are proven or implicated vectors of important zoonotic and medical pathogens in Europe, including Plasmodium vivax [Anopheles maculipennis (sensu lato) (s.l.), comprised of three species: An. atroparvus (van Thiel, 1927), An. messeae (Falleroni, 1926) and An. daciae (Linton, Nicolescu & Harbach, 2004)], P. falciparum [Anopheles plumbeus (Stephens, 1828)] and several arthropod-borne viruses (arboviruses) [notably Culex and Aedes spp.]. No local human infection with mosquito-borne arboviruses has been reported in the UK for 150 years but indigenous mosquito species are competent vectors for some viruses [7–11], and the potential for emergence remains [5, 12].
Table 1.
Reported human-biting behaviour of mosquitoes within literature in the United Kingdom: published literature concerning human-biting mosquitoes, categorised according to biting nuisance reports, blood meal analysis studies and human-baited collections
| Mosquito species | Published evidence for human-biting behaviour in the UK | ||
|---|---|---|---|
| Biting nuisance reports | Blood meal analysis | Human-baited collections | |
| Aedes (Aedes) cinereus (Meigen, 1818)/Aedes (Aedes) geminus (Peus, 1970)a | Yes [75] | Yes [19, 20] | Yes [19, 20, 68] |
| Aedes (Aedimorphus) vexans (Meigen, 1830) | Yes [75] | – | – |
| Anopheles (Anopheles) algeriensis (Theobald, 1903) | Yes [76] | – | – |
| Anopheles (Anopheles) claviger (Meigen, 1804) | Yes [77, 78] | – | Yes [20] |
| Anopheles (Anopheles) maculipennis (s.l.)b | Yes [3, 75] | Yes [79]d | – |
| Anopheles (Anopheles) plumbeus (Stephens, 1828) | Yes [75] | Yes [19, 20] | Yes [19, 20, 68] |
| Coquillettidia (Coquillettidia) richiardii (Ficalbi, 1889) | Yes [3] | Yes [19, 20, 61] | Yes [19, 20, 68] |
| Culex (Barraudius) modestus (Ficalbi, 1889) | – | Yes [61] | Yes [60]e |
| Culex (Culex) pipiens (L., 1758)c | Yes [3, 77, 78] | Yes [19, 20] | – |
| Culiseta (Culiseta) annulata (Schrank, 1776) | Yes [3, 75, 77, 78] | Yes [19, 20] | Yes [19, 20] |
| Culiseta (Culicella) litorea (Shute, 1928) | – | Yes [19, 20, 80] | – |
| Culiseta (Culicella) morsitans (Theobald, 1901) | – | Yes [19, 20, 80] | – |
| Culiseta (Culiseta) subochrea (Edwards, 1921) | Yes [3, 75] | – | – |
| Aedes (Dahliana) geniculatus (Olivier, 1791) | Yes [75] | Yes [20] | Yes [19, 20, 68, 81] |
| Aedes (Ochlerotatus) annulipes (Meigen, 1830) | Yes [3] | – | Yes [19] |
| Aedes (Ochlerotatus) cantans (Meigen, 1818) | Yes [3, 77, 78] | Yes [19, 20, 61, 82] | Yes [19, 20, 68, 82, 83] |
| Aedes (Ochlerotatus) caspius (Pallas, 1771) | Yes [75, 77, 78] | – | Yes [19] |
| Aedes (Ochlerotatus) detritus (Haliday, 1833) | Yes [3, 75, 77, 78] | Yes [19, 20, 61] | Yes [19, 20, 68] |
| Aedes (Ochlerotatus) dorsalis (Meigen, 1830) | Yes [75] | Yes | – |
| Aedes (Ochlerotatus) flavescens (Müller, 1764) | Yes [75] | – | – |
| Aedes (Ochlerotatus) punctor (Kirby, 1837) | Yes [3, 75, 84] | Yes [19, 20] | Yes [19, 20, 68] |
| Aedes (Ochlerotatus) rusticus (Rossi, 1790) | Yes [77, 78] | – | Yes [19, 20, 68] |
aThese species were only recently separated in [39] and therefore are considered together
bStudies that did not delineate An. maculipennis (s.l.) to species level
cEcoforms of Culex pipiens (L., 1758) not separated
dThis study found evidence of human-biting in all three members of An. maculipennis (s.l.)
eNot a host-baited study per se, but an incidental collection of one specimen biting the collector
The number of mosquitoes biting a host is a key parameter in epidemiological models of vector-borne pathogen transmission, and reduction of this rate is an important target for disease control and prevention initiatives [13, 14]. Temporal and spatial variation in mosquito biting rates within an environment can significantly impact the risk of human infection with a mosquito-borne pathogen [15]. Although recent evidence suggests that some artificial trapping approaches are able to sample certain mosquito species at a comparable rate to that of human-baited collections [16–18], the standardised human landing catch remains a valuable method for assessing true biting rates on human hosts. The use of human landing catch techniques is particularly useful in areas where pathogen transmission is not considered to occur, as experiments can proceed without exposing personnel to an increased risk of infection [1].
Despite the importance of field data on human-biting mosquito populations, information on mosquitoes biting humans in the UK is largely based on studies conducted over 50 years ago and from a limited number of geographical locations [19, 20]. The studies by Service [19, 20] were also conducted by a lone worker and thus may have led to bias in frequency of landing and biting [21, 22]. In addition, the confounding influence of morphologically cryptic species, such as Aedes nigrinus (Eckstein, 1818) and Aedes sticticus (Meigen, 1838), may have significantly influenced the findings of early studies which were unable to harness the recently developed molecular methods for delineating these species [23]. The Pipiens complex, in particular, is a morphologically cryptic species group that has been reported to exhibit significant variation in bionomics and human-biting rates. Human-biting populations of Culex pipiens form pipiens have been detected in Portugal [24], despite this species being considered primarily ornithophilic [25]. Moreover, Cx. pipiens pipiens/molestus hybrid forms will feed on mammals [26–28], and may also act as bridge vectors of viruses to humans if they exhibit a more generalist feeding behaviour [29].
At present, active UK surveillance activities for adult mosquitoes are primarily based on trapping using Mosquito Magnet® (Woodstream Corporation, Lititz, Pennsylvania, USA) and BG Sentinel traps (Biogents, Regensburg, Germany) [30, 31]. Specimens are collected over extended (> 1 day) time periods, and therefore it is not possible to use these data to determine the biting rate of mosquitoes on humans at that location. Addressing this knowledge gap with field-based studies is vital to increase our understanding of current mosquito biting behaviour, and will allow future comparison with behavioural changes that could result from climate change [12], anthropogenic changes to the environment [32] or the establishment of an exotic species, such as Aedes albopictus (Skuse, 1895), in the UK [33].
This study therefore aimed to determine the human-biting rate of mosquito species assemblages present on four sites in the south of England during peak evening mosquito activity periods [19, 20] and during the summer months when mosquitoes are most abundant [34]. Mosquitoes were collected by human landing catch using multiple collectors to account for individual variations in attractiveness to biting, and meteorological data were collected to account for environmental variation. The sites chosen were biased towards livestock farms and nature reserves, which provide a setting in which humans, livestock and wildlife interact daily. Farm-related activities necessitate human presence outdoors, and may last for long periods of time which could overlap with periods of peak mosquito activity during the summer months.
Methods
Study sites
The study was conducted on four sites (denoted A, B, C and D), located in Oxfordshire, Kent, Hampshire and Surrey, respectively (see Fig. 1 and Additional file 1: Table S1 for further details). These were selected based on their location in the south of England, the presence of livestock on site and the presence of mosquito larvae in either natural or artificial habitats in preliminary site visits conducted between June and September 2012 (data not shown). Within each site, four sampling points were chosen at which to conduct human landing catches. Sampling points were situated a minimum of 50 m apart, located in areas frequented by humans, in logistically feasible positions and in locations that presented minimal chances of interference with farm activities. To facilitate future characterisation and comparison between sites and studies, sampling points at sites B, C, and D were documented in the form of a Google Photosphere, a 360° photograph, captured using a Google Nexus 5 mobile phone. These files (Additional files 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 13) can be uploaded and viewed online at http://photosphereviewer.net/.
Fig. 1.

Location of study farms in southern England. Map: Google
Human landing catch protocol
The study took place between July and August 2013 over a total of 24 collection evenings, six at each site. Four collectors from a pool of thirteen were assigned to each evening, and asked to refrain from applying scented products (soaps, deodorants) on their collection days. Each night of collection ran for four hours starting two hours prior to sunset. The four-hour period was split into eight collection periods of 25 min each at the four sampling points, with five minutes available after each period for movement of the volunteer to the next sampling point. Collectors were assigned to a sampling point for each collection period (1–8) by two sequential Latin square randomisations using a script in R v.3.2.0 [35]. Landing catches were conducted while sitting on a stool and a manual aspirator (John W. Hock, Gainsville, Florida, USA) was used to collect any mosquitoes alighting on one exposed lower leg (Fig. 2). Collected mosquitoes were placed in a 64 mm diameter cardboard pill pot (Watkins and Doncaster, Leominster, UK). Only specimens that landed on the leg were aspirated. A red-light head torch (Petzl, Crolles, France) was used by collectors when natural light intensity was insufficient to carry out collections. At the end of each evening, mosquitoes were transported to the laboratory in a cooler containing dry ice and stored at −20 °C prior to further analysis.
Fig. 2.

The human landing catch. A volunteer collector demonstrating a human landing catch at site C
Collection of meteorological data
Trials were limited to evenings of average forecasted wind speeds below 3 m/s and minimal rainfall (< 1 mm) as forecasted by www.xcweather.co.uk, following criteria used previously as optimal for mosquito activity [19]. Meteorological data were collected at one-minute intervals from each site using an automatic weather station with data logger model CR800 (Campbell Scientific, Loughborough, UK). Variables collected were air temperature (°C), relative humidity (%), wind speed (m/s), solar intensity (kJ/m2) and rainfall (mm), with data for each of these variables summarised prior to analysis to a mean value per hour. Due to unexpected failure of the weather station at site B, temperature, wind speed and rainfall data at hourly intervals were obtained from the nearest Met Office weather station located approximately 20 km away at Shoeburyness, Essex, UK. The temperature/relative humidity probe at site D failed shortly before collections commenced and therefore temperature data were collected using a TinyTag Plus2 datalogger (Gemini Data Loggers Ltd., West Sussex, UK) hung at the same height as the standard probe.
Identification of mosquitoes and blood meals
Mosquitoes were initially identified based on morphological characteristics using published keys [36, 37], according to the nomenclature of Wilkerson et al. [38]. Specimens difficult to separate morphologically and which display a broadly similar ecology [5, 36, 37, 39] were identified morphologically to species group only. Mosquitoes morphologically identified as An. maculipennis (s.l.) or Cx. pipiens/Culex torrentium (Martini, 1925) were subjected to molecular species delineation. DNA was extracted from the abdomens of the mosquitoes to facilitate both species delineation and blood meal analysis using previously established protocols [40, 41]. Specimens of An. maculipennis (s.l.) were identified to species-level by amplification of the 435-base pair (bp) region of the internal transcribed spacer 2 gene (ITS2) using primers 5.8S and 28S [42] in a polymerase chain reaction (PCR) assay, as described previously [40]. Sequences were assigned to a particular mosquito species when agreement was ≥ 98% to published sequences in GenBank, using the standard nucleotide BLAST tool [43]. To delineate Cx. pipiens/Cx. torrentium, specimens were subjected to two sequential duplex end-point PCR assays as described elsewhere [44]. To summarise, the first PCR assay separated Cx. pipiens/Cx. torrentium following the protocols of [44] and used the ACEtorr and ACEpip forward primers with the B1246s reverse primer, targeting the nuclear acetylcholinesterase-2 (ace-2) gene [45]. Specimens identified as Cx. pipiens were then subjected to a second duplex PCR assay to delineate between Cx. pipiens form pipiens and Cx. pipiens f. molestus. This PCR assay utilised the forward primer CQ11F and reverse primers molCQ11R and pipCQ11R targeting the CQ11 microsatellite locus [46, 47].
Blood meal host was determined using a six-primer cocktail (VF1_t1 + VF1d_t1 + VF1i_t1/VR1_t1 + VR1d_t1 + VR1i_t1) in an end-point PCR assay, targeting a 685 bp sequence of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene [48], following previously developed protocols [41]. Samples producing positive PCR results were sequenced uni-directionally with M13 primers [48] at 1 pmol/μl using the ABI PRISM® BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Warrington, UK), and sequences were assigned to a particular host when agreement was ≥ 98% to published sequences in GenBank using the standard nucleotide BLAST tool [43].
Data analysis
Comparisons between sites and the effect of meteorological variables on the overall biting rate were assessed using generalized linear mixed models (GLMMs) in package ‘glmmADMB’ [49, 50] in R v.3.2.0 [35]. Site was included as a fixed effect, with sampling point within each site included as a random factor nested within site. The collector was also included as a random factor. Time to sunset (transformed to a squared factor), temperature and wind speed were included as covariates with rainfall fitted as a fixed presence/absence factor. Models were fitted by maximum likelihood with the Laplace approximation, and model fit assessed by comparison of Akaike information criterion (AIC) [51] in R, with lower values indicating a better model fit. The final model was obtained by step-wise deletion of non-significant factors and variables until removal caused an increase in AIC value of greater than two units.
Results
Human-biting rates and species composition
A total of 915 mosquitoes of fourteen species, or morphologically indistinguishable species groups, were collected in the study (Table 2). The greatest number of specimens was collected from site B (n = 802), followed by site C (n = 72) and site D (n = 41). No mosquitoes were collected at site A throughout the study and therefore this farm was excluded from further analysis. Mean human-biting rates per site, including all mosquito species (mosquitoes/person/25 min/night), were 4.18 (range 0–89 mosquitoes) at site B, 0.38 (0–9) at site C and 0.21 (0–6) at site D. The highest mean biting rates per 25 min collection period were for Coquillettidia richiardii (Ficalbi, 1889) (2.59, range 0–67 mosquitoes), An. maculipennis (s.l.) (0.28, 0–29) and Cx. modestus (Ficalbi, 1889) (1.04, 0–23). Extrapolated hourly biting rates (biting rate ÷ 25 × 60) for Cq. richiardii, An. maculipennis (s.l.) and Cx. modestus were 6.2 (range 0–161), 0.67 (0–70) and 2.5 (0–55), respectively. Of these species, only Cq. richiardii was collected from three sites (B, C and D) with Cx. modestus collected from site B only.
Table 2.
Mosquitoes collected over the course of the study at the sites: the number (n), percentage composition (%), mean and range of biting rates at each farm. Mean values represent the number of bites per person, per 25 min, on an average night. The range represents the minimum and maximum number of specimens collected by one collector in any one 25 min period. Farm A is excluded as no mosquitoes were collected there during the study
| Species | Site B | Site C | Site D | Total | |||
|---|---|---|---|---|---|---|---|
| n (%) | Mean biting rate (range) | n (%) | Mean biting rate (range) | n (%) | Mean biting rate (range) | ||
| Aedes cinereus/geminus | 0 (0) | – | 0 (0) | – | 2 (4.9) | 0.01 (0–1) | 2 |
| Anopheles claviger | 3 (0.4) | 0.02 (0–1) | 1 (1.4) | 0.01 (0–1) | 0 (0) | – | 4 |
| Aedes geniculatus | 0 (0) | – | 0 (0) | – | 1 (2.4) | 0.01 (0–1) | 1 |
| Aedes cantans / annulipes | 0 (0) | – | 0 (0) | – | 10 (24.4) | 0.05 (0–2) | 10 |
| Aedes detritus | 3 (0.4) | 0.02 (0–1) | 68 (94.4) | 0.35 (0–9) | 0 (0) | – | 71 |
| Aedes flavescens | 26 (3.2) | 0.14 (0–6) | 0 (0) | – | 0 (0) | – | 26 |
| Aedes punctor | 0 (0) | – | 0 (0) | – | 3 (7.3) | 0.02 (0–1) | 3 |
| Aedes rusticus | 0 (0) | – | 0 (0) | – | 6 (14.6) | 0.03 (0–1) | 6 |
| Aedes spp. | 1 (0.1) | 0.01 (0–1) | 1 (1.4) | 0.01 (0–1) | 0 (0) | – | 2 |
| Anopheles maculipennis (s.l.)a | 54 (6.7) | 0.28 (0–29) | 0 (0) | – | 1 (2.4) | 0.01 (0–1) | 55 |
| Anopheles plumbeus | 0 (0) | – | 1 (1.4) | 0.01 (0–1) | 4 (9.8) | 0.02 (0–1) | 5 |
| Coquillettidia richiardii | 498 (62.1) | 2.59 (0–67) | 1 (1.4) | 0.01 (0–1) | 12 (29.3) | 0.06 (0–2) | 511 |
| Culex modestus | 199 (24.8) | 1.04 (0–23) | 0 (0) | – | 0 (0) | – | 199 |
| Culex pipiens b | 16 (2.0) | 0.08 (0–4) | 0 (0) | – | 2 (4.9) | 0.01 (0–1) | 18 |
| Culiseta annulata | 2 (0.3) | 0.01 (0–1) | 0 (0) | – | 0 (0) | – | 2 |
| Total | 802 | 4.18 (0–89) | 72 | 0.38 (0–9) | 41 | 0.21 (0–6) | 915 |
aIncludes both Anopheles atroparvus (van Thiel, 1927) and Anopheles messeae (Falleroni, 1926) / Anopheles daciae (Linton, Nicolescu & Harbach, 2004)
bIncludes Culex pipiens f. pipiens and two specimens not identified to ecoform
Generalized linear mixed models
A negative binomial GLMM with logit link function was the best fit to the data and indicated that site, time relative to sunset and wind speed significantly influenced the total number of mosquitoes collected. Relative to the biting rate at site C, the total number of mosquitoes collected was significantly higher at site B (P ≤ 0.001), whilst there was a non-significant difference in collections between sites C and D (Additional file 14: Table S2). An increase of 1 m/s in wind speed would be expected to lead to a 58% decrease in the total biting rate (P ≤ 0.001), whilst the number of mosquitoes collected would be expected to decrease by 29% for every 30-min period further from sunset (P ≤ 0.001) (Additional file 14: Table S2). Temporal trends relative to sunset were the most pronounced for species collected at site B. For example, Cq. richiardii and An. maculipennis (s.l.) showed peak biting activity in the collection period shortly after sunset, Cx. modestus and Aedes flavescens (Müller, 1764) showed peak activity one hour after sunset, whilst Cx. pipiens f. pipiens was collected only after sunset, at sites B and D (Fig. 3).
Fig. 3.
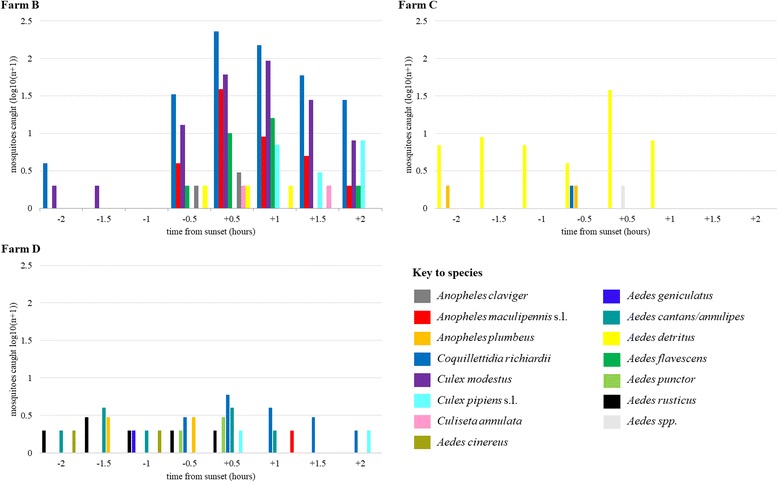
Mosquito biting activity relative to sunset. The log10 of the total mosquito species collected by human landing catch (all collectors) at farms B, C and D over the six visits to each farm. Farm A is excluded as no mosquitoes were collected there during the study
Only Cq. richiardii and Cx. modestus were collected in sufficient number (n > 100) to permit individual analysis of abundance. As 495/511 (96.9%) of Cq. richiardii were collected from site B, the remaining specimens were excluded from the analysis and site omitted as a factor from the model. The best-fit negative binomial model for Cq. richiardii indicated that time relative to sunset and wind speed significantly influenced the biting activity of this species, predicting that a 1 m/s increase in the wind speed would lead to an estimated 41% decrease in biting rate (P ≤ 0.001) and every 30-min movement away from sunset would be expected to lead to an 18% decrease in the biting rate (P ≤ 0.001) (Additional file 14: Table S3). Culex modestus was only collected from site B and thus site was also omitted from this model. The best-fit negative binomial model indicated that only time relative to sunset was a significant predictor of the biting rate for Cx. modestus; for every 30-min movement away from sunset a 21% decrease in the biting rate would be expected (P ≤ 0.001) (Additional file 14: Table S4).
Molecular species delineation and blood meal analysis
Species delineation by molecular methods of the 18 specimens identified morphologically as Cx. pipiens/Cx. torrentium indicated that only Cx. pipiens was present. Of these, 16/18 were identified as Cx. pipiens f. pipiens, whilst repeated attempts to identify the remaining two specimens proved unsuccessful. Of the 55 An. maculipennis (s.l.) collected, 50 were identified as An. atroparvus and five as An. daciae/An. messeae; the latter were collected only from site B and presented identical query results in BLAST searches, precluding their separation. Overall, therefore, sites B and D yielded nine species/species groups and site C five species.
Nineteen specimens comprising six species/species groups were found to contain blood in their abdomen (Table 3). Blood-feeding hosts were successfully identified in 12/19 specimens (63%). Ten blood meals were identified as being of human origin, with two blood meals, one from each of An. atroparvus and Cq. richiardii, identified as having originated from a cow (Bos taurus L.).
Table 3.
Results of blood-meal analysis of mosquitoes collected on humans. Blood meals identified from engorged specimens collected by human landing catch at farms B, C and D
| Species | Total blood-fed | Total (% positive for blood-meal host) | Blood-meal hosts (n) |
|---|---|---|---|
| Aedes cantans/annulipes | 1 | 1 (100) | Human, Homo sapiens (1) |
| Aedes detritus | 9 | 6 (67) | Human, Homo sapiens (6) |
| Aedes flavescens | 2 | 0 (0) | na |
| Anopheles atroparvus | 3 | 1 (33) | Cow, Bos taurus (1) |
| Coquillettidia richiardii | 3 | 3 (100) | Human, Homo sapiens (2); Cow, Bos taurus (1) |
| Culex modestus | 1 | 1 (100) | Human, Homo sapiens (1) |
| Total | 19 | 12 (63) |
Discussion
Quantifying mosquito biting rates on epidemiologically relevant hosts is a challenging undertaking, but is essential in order to understand the impact of nuisance-biting and the risk of mosquito-borne pathogen transmission. This study aimed to determine human-biting rates of native mosquitoes associated with four sites in southern England during the summertime evening peak of mosquito activity. Human-biting activity was identified on three of the four sites studied, despite the presence of alternative livestock and wildlife hosts in the area. Mosquito species assemblages differed between sites, but at least 15 species of seven genera were identified, including several proven or potential vectors of medically-important pathogens. Average biting rates differed significantly between farms: no human-biting was reported from site A in Oxfordshire, whilst biting rates at site B in Kent reached 89 mosquitoes in 25 min, or an extrapolated 214 bites per hour.
Biting rates at site B match those recorded at a site in Sandwich, Kent, some 40 miles south in 1981 [52] and match or exceed those reported from a wetland area in the Camargue, France, where West Nile virus (WNV) transmission has been reported [53]. The species composition of the biting population was significantly different to the earlier UK report, with the current study including Cx. modestus as the second most numerous species collected at the site. Feeding on both birds and mammals, Cx. modestus is an important bridge vector of WNV in mainland Europe [53–55]. Culex modestus is thought to have shown recent expansion in its distribution [56, 57] and has been targeted in WNV surveillance activities in the UK [30]. Although its establishment in the UK is considered a fairly recent event [58], this species has now been identified in several locations across the south of England [59]. To our knowledge, this study is the first to document the human-biting activity of Cx. modestus in the UK since an isolated report from the Portsmouth area in the 1940s [60].
Interestingly, the high abundance of human-biting mosquitoes identified at site B contrasts with parallel collections identifying only limited evidence of human feeding by mosquitoes at the same site [61] and an intensive study of blood-feeding behaviour conducted there in the subsequent year (2014), in which no human blood meals were identified [41]. The likely reasons for this are twofold. First, the mosquito behaviour post-feeding varies by species, with some being attracted to artificial resting traps [41], whilst others rest at other locations such as vegetation. This resulted in several of the major human-biting species collected in this study, such as Cx. modestus, being underrepresented in the resting collections, and others, such as Anopheles maculipennis (s.l.) and Culiseta annulata (Schrank, 1776), appearing under-represented in the human landing catch dataset. Secondly, the availability of humans to mosquitoes at their peak crepuscular biting times is normally relatively limited at the sites and, therefore, humans may not serve as a major blood meal source at these locations. This suggests that humans, although not necessarily a primary blood-feeding host, are readily fed upon when available by a range of mosquito species. Among these is the Pipiens ecoform of Cx. pipiens, collected from two sites in this study and which, despite being considered mainly ornithophilic, has also been reported to feed on humans and other mammals elsewhere [24]. Here, no blood-fed specimens were collected and therefore human feeding could not be confirmed by blood meal analysis. Nevertheless, collection by human landing catch indicates that this species may bite humans opportunistically. Taken together with experimental evidence for laboratory competence of mainland European Cx. pipiens f. pipiens for several arboviruses [62, 63], this species could therefore be considered a potential bridge, anthroponotic, and enzootic arbovirus vector in the UK.
As the aim of this study was to identify peak biting rates in ‘ideal’ flight conditions for mosquitoes, the range of meteorological data collected, and thus conclusions about the impact of each on biting activity, is limited. Nonetheless, wind speed was shown to be an important determinant of the total biting pressure experienced overall, and of the landing rates on humans of Cq. richiardii and Cx. modestus at site B. Although this result corresponds with existing findings demonstrating the importance of wind speed in influencing mosquito flight (e.g. [64, 65]), the requirement to use data from a station located 20 km away means this result should be viewed cautiously. Indeed, for future studies, we would advocate the assessment of meteorological variables at an even finer scale than attempted in this study, for example by collecting wind speed data from each sampling point, to capture within-site variation. Such fine-scale variation is an important factor to consider in rural environments as vegetation, in addition to artificial structures, can influence the meteorological conditions experienced within a small area [64].
The collections in this study targeted only the evening crepuscular biting period, in accordance with existing literature on the biting activity of UK mosquitoes [19] and the results of preliminary experiments. Peak mosquito biting occurred close to or shortly after sunset (Fig. 3), although this trend was more pronounced for certain species than others. Interestingly, Aedes detritus (Haliday, 1833) appeared to bite both before, during, and shortly after sunset, indicating that human exposure to biting by this arbovirus vector [7, 8] may begin earlier and persist for a longer period of time than with the other collected species. Studies conducted over a 24-h period (e.g. [53]) would be useful to provide a more complete picture of the mosquito biting risk posed to humans during other times of the day and night. For example, at least one of the sites (B) offers overnight stays to visitors and thus exposure to biting may occur beyond the evening period studied here. For example, species such as Aedes rusticus (Rossi, 1790) have been observed to bite during daylight hours in the UK (Nicholas Johnson personal communication). Further afield, 14-h landing catches conducted in the Ivory Coast to study the (normally daytime) biting activity of Aedes aegypti (L., 1762) found that biting by this species occurred throughout the night, with peak activity observed close to midnight [66].
One factor that was not considered in this study was the larval source of the species collected. This could be important as, particularly for smaller sites, the source of biting populations may be neighbouring habitats. Site D, for example, bordered an area of woodland larger than the farm itself. This area likely provided larval habitats for species such as Ae. cinereus (Meigen, 1818), Ae. punctor (Kirby, 1837) and Ae. rusticus which were collected exclusively from this site. Changes in species diversity and larval habitats could be determined by conducting human landing catches and larval sampling in a concentric radius, starting at the centre of each site and moving systematically into neighbouring habitat.
It is important from both a nuisance biting and pathogen transmission perspective to use a study design that most accurately reflects the true biting rate experienced by target hosts. The use of multiple volunteer collectors at four sampling points per site was designed to capture inter-individual differences in attractiveness to mosquito biting [21, 22], sampling efficiency [67] and within-site heterogeneity in exposure to biting [68]. No other European study has systematically assessed biting rate in this manner. In future, more representative data could be obtained by utilising moving, or roving, landing catches, which may better reflect the activity patterns of farm workers and visitors moving around on such sites. Human movement is an important but often overlooked component influencing biting and pathogen transmission risk [69]. Movement of a host may disrupt resting mosquitoes and provide an additional visual host stimulus causing mosquitoes to alight and feed. However, save for a few studies (e.g. [70–74]), moving catches remain a poorly explored method in studies of mosquitoes that bite humans.
Conclusions
Potential and proven native mosquito vectors are responsible for considerable levels of human nuisance biting in some rural areas of southern England. Coquillettidia richiardii, An. maculipennis (s.l.) and Cx. modestus were the most common human-biting species, with peak hourly biting rates reaching 161, 70 and 55 bites, respectively. In practice, human exposure to peak mosquito biting activity in these areas may be limited to workers undertaking seasonal outdoor farming activities. Were a mosquito-borne pathogen outbreak to occur at the sampled sites, these activities may need to be altered to conclude prior to sunset and workers advised to implement bite prevention measures. Mosquito species assemblages biting humans, livestock and wildlife hosts suggest that rural sites such as farms could be areas where both enzootic and zoonotic pathogen transmission could occur. Therefore, such sites should be the subject of greater attention for future field studies of biting behaviour and risk analyses for pathogen transmission.
Additional files
Table S1. Further information on each farm used in this study. (PDF) (PDF 334 kb)
Figure S1. Photosphere file, site B, sampling point 1. (JPG) (JPEG 2149 kb)
Figure S2. Photosphere file, site B, sampling point 2. (JPG) (JPEG 1922 kb)
Figure S3. Photosphere file, site B, sampling point 3. (JPG) (JPEG 1426 kb)
Figure S4. Photosphere file, site B, sampling point 4. (JPG) (JPEG 1302 kb)
Figure S5. Photosphere file, site C, sampling point 1. (JPG) (JPEG 1624 kb)
Figure S6. Photosphere file, site C, sampling point 2. (JPG) (JPEG 1066 kb)
Figure S7. Photosphere file, site C, sampling point 3. (JPG) (JPEG 2475 kb)
Figure S8. Photosphere file, site C, sampling point 4. (JPG) (JPEG 1454 kb)
Figure S9. Photosphere file, site D, sampling point 1. (JPG) (JPEG 1852 kb)
Figure S10. Photosphere file, site D, sampling point 2. (JPG) (JPEG 2606 kb)
Figure S11. Photosphere file, site D, sampling point 3. (JPG) (JPEG 1618 kb)
Figure S12. Photosphere file, site D, sampling point 4. (JPG) (JPEG 2726 kb)
Table S2. Regression coefficients, with Wald 95% confidence intervals and standard errors, for fixed effects of the best-fit negative binomial model used to describe total biting pressure. *** P ≤ 0.001. Table S3. Regression coefficients, with Wald 95% confidence intervals and standard errors, for fixed effects of the best-fit negative binomial model used to describe the biting activity of Coquillettidia richiardii (Ficalbi, 1889). *** P ≤ 0.001. Table S4. Regression coefficients, with Wald 95% confidence intervals and standard error, for fixed effects of the best-fit negative binomial model used to describe the biting activity of Culex modestus (Ficalbi 1889). *** P ≤ 0.001, * P ≤ 0.05. (PDF) (PDF 450 kb)
Table S5. Data used in this study. (XLSX) (XLSX 44 kb)
Acknowledgements
The authors would like to acknowledge the kind assistance of all the farms involved in the study, Luis Hernández-Triana for his assistance with the blood meal identifications, the support of all research groups involved and Noel Nelson (Met Office) for providing meteorological data from the weather station at Shoeburyness. The authors are very grateful to the following volunteers for contributing their time and efforts towards the data collection: Christopher J. Sanders, James Barber, John C. Schwartz, Claudia Rückert, Tamiko Brown-Joseph and Joana Ferrolho. We would also like to thank the two anonymous reviewers for their comments which helped to improve the manuscript.
Funding
The project was funded by the Biotechnology and Biological Sciences Research Council (BBSRC), grant BB/F016492/1. The Pirbright Institute receives grant-aided support from the BBSRC. This project received partial funding from Defra, the Scottish Government and Welsh Government through grants SE4112 and SV3045.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional file 15.
Abbreviations
- AIC
Akaike information criterion
- GLMM
generalized linear mixed model
- PCR
polymerase chain reaction
- WNV
West Nile virus
Authors’ contributions
VAB designed the study, conducted and analysed the field and laboratory work and wrote the manuscript. ARF, JM, AJW, JL, PPCM, NJ and SC contributed to the initial design of the study. MEE, JS, LT, LEH, NJ and SC conducted the human landing catches and contributed to the practical design, setup and logistics of the study. LEH provided the R code for the Latin Squares analysis and advice on experimental design. AJW contributed to data analysis and interpretation. ARF and SC obtained funding for the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Consent was obtained from all participants prior to their involvement in the study. Ethical approval was obtained from the London School of Hygiene and Tropical Medicine (LSHTM) ethics committee, reference number 6446.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13071-017-2360-9) contains supplementary material, which is available to authorized users.
Contributor Information
Victor A. Brugman, Email: vab@evolutionbiotech.com
Marion E. England, Email: marion.england@pirbright.ac.uk
Joanne Stoner, Email: jo.stoner@pirbright.ac.uk.
Laura Tugwell, Email: laura.tugwell@pirbright.ac.uk.
Lara E. Harrup, Email: lara.harrup@pirbright.ac.uk
Anthony J. Wilson, Email: anthony.wilson@pirbright.ac.uk
Jolyon M. Medlock, Email: jolyon.medlock@phe.gov.uk
James G. Logan, Email: james.logan@lshtm.ac.uk
Anthony R. Fooks, Email: tony.fooks@apha.gsi.gov.uk
Peter P.C. Mertens, Email: peter.mertens@nottingham.ac.uk
Nicholas Johnson, Email: nick.johnson@apha.gsi.gov.uk.
Simon Carpenter, Email: simon.carpenter@pirbright.ac.uk.
References
- 1.Silver JB. Mosquito ecology: field sampling methods. 3. Netherlands: Springer; 2007. [Google Scholar]
- 2.Malcolm CA. Public health issues posed by mosquitoes. An Independent Report. 2009;
- 3.Medlock JM, Hansford KM, Anderson M, Mayho R, Snow KR. Mosquito nuisance and control in the UK - a questionnaire-based survey of local authorities. Eur Mosq Bull. 2012;30:15–29. [Google Scholar]
- 4.Medlock JM, Vaux AGC. Colonization of UK coastal realignment sites by mosquitoes: implications for design, management, and public health. J Vector Ecol. 2013;38:53–62. doi: 10.1111/j.1948-7134.2013.12008.x. [DOI] [PubMed] [Google Scholar]
- 5.Medlock JM, Snow KR, Leach SA. Possible ecology and epidemiology of medically important mosquito-borne arboviruses in great Britain. Epidemiol Infect. 2007;135:466–482. doi: 10.1017/S0950268806007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould EA, Higgs S, Buckley A, Gritsun TS. Potential arbovirus emergence and implications for the United Kingdom. Emerg Infect Dis. 2006;12:549–555. doi: 10.3201/eid1204.051010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackenzie-Impoinvil L, Impoinvil DE, Galbraith SE, Dillon RJ, Ranson H, Johnson N, et al. Evaluation of a temperate climate mosquito, Ochlerotatus detritus (Aedes detritus), as a potential vector of Japanese encephalitis virus. Med Vet Entomol. 2015;29:1–9. doi: 10.1111/mve.12083. [DOI] [PubMed] [Google Scholar]
- 8.Blagrove MSC, Sherlock K, Chapman GE, Impoinvil DE, McCall PJ, Medlock JM, et al. Evaluation of the vector competence of a native UK mosquito Ochlerotatus detritus (Aedes detritus) for dengue, chikungunya and West Nile viruses. Parasit Vectors. 2016;9:452. doi: 10.1186/s13071-016-1739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffner F, Thiéry I, Kaufmann C, Zettor A, Lengeler C, Mathis A, Bourgouin C. Anopheles plumbeus (Diptera: Culicidae) in Europe: a mere nuisance mosquito or potential malaria vector? Malar J. 2012;11:393. doi: 10.1186/1475-2875-11-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsay SW, Hole DG, Hutchinson RA, Richards SA, Willis SG. Assessing the future threat from vivax malaria in the United Kingdom using two markedly different modelling approaches. Malar J. 2010;9:70. doi: 10.1186/1475-2875-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsdale CD, Gunn N. History of and prospects for mosquito-borne disease in Britain. J Eur Mosq Control Assoc. 2005;20:15–31. [Google Scholar]
- 12.Medlock JM, Leach SA. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect Dis. 2015;15:721–730. doi: 10.1016/S1473-3099(15)70091-5. [DOI] [PubMed] [Google Scholar]
- 13.Chitnis N, Hyman JM, Cushing JM. Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull Math Biol. 2008;70:1272–1296. doi: 10.1007/s11538-008-9299-0. [DOI] [PubMed] [Google Scholar]
- 14.Wonham MJ, De-Camino-Beck T, Lewis MA. An epidemiological model for West Nile virus: invasion analysis and control applications. Proc Biol Sci. 2004;271:501–507. doi: 10.1098/rspb.2003.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith DL, Dushoff J, McKenzie FE. The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol. 2004;2:e368. doi: 10.1371/journal.pbio.0020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govella NJ, Maliti DF, Mlwale AT, Masallu JP, Mirzai N, Johnson PCD, et al. An improved mosquito electrocuting trap that safely reproduces epidemiologically relevant metrics of mosquito human-feeding behaviours as determined by human landing catch. Malar J. 2016;15:465. doi: 10.1186/s12936-016-1513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krockel U, Rose A, Eiras ÁE, Geier M. New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in an urban environment. J Am Mosq Control Assoc. 2006;22:229–238. doi: 10.2987/8756-971X(2006)22[229:NTFSOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Tangena JAA, Thammavong P, Hiscox A, Lindsay SW, Brey PT. The human-baited double net trap: an alternative to human landing catches for collecting outdoor biting mosquitoes in Lao PDR. PLoS One. 2015;10:e0138735. doi: 10.1371/journal.pone.0138735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Service MW Observations on the ecology of some British mosquitoes. Bull Entomol Res. 1969;59:161–193. doi: 10.1017/S000748530000314X. [DOI] [Google Scholar]
- 20.Service MW Feeding behaviour and host preferences of British mosquitoes. Bull Entomol Res. 1971;60:653–661. doi: 10.1017/S0007485300042401. [DOI] [PubMed] [Google Scholar]
- 21.Knols BG, de Jong R, Takken W. Differential attractiveness of isolated humans to mosquitoes in Tanzania. Trans R Soc Trop Med Hyg. 1995;89:604–606. doi: 10.1016/0035-9203(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 22.Logan JG, Birkett MA, Clark SJ, Powers S, Seal NJ, Wadhams LJ, et al. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J Chem Ecol. 2008;34:308–322. doi: 10.1007/s10886-008-9436-0. [DOI] [PubMed] [Google Scholar]
- 23.Harbach RE, Dallimore T, Briscoe AG, Culverwell CL, Vaux AGC, Medlock JM. Aedes nigrinus (Eckstein, 1918) (Diptera, Culicidae), a new country record for England, contrasted with Aedes sticticus (Meigen, 1838). ZooKeys. 2017;671:119–30. [DOI] [PMC free article] [PubMed]
- 24.Gomes B, Sousa CA, Vicente JL, Pinho L, Calderón I, Arez E, et al. Feeding patterns of molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in a region of high hybridization. Parasit Vectors. 2013;6:93. doi: 10.1186/1756-3305-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harbach RE, Dahl C, White GB. Culex (Culex) pipiens Linnacus (Diptera: Culicidae): concepts, type designations, and description. Proc Entomol Soc Washingt. 1985;87:1–24.
- 26.Reusken CBEM, De Vries A, Buijs J, Braks MAH, Den Hartog W, Scholte EJ. First evidence for presence of Culex pipiens biotype molestus in the Netherlands, and of hybrid biotype pipiens and molestus in northern Europe. J Vector Ecol. 2010;35:210–212. doi: 10.1111/j.1948-7134.2010.00080.x. [DOI] [PubMed] [Google Scholar]
- 27.Gomes B, Sousa CA, Novo MT, Freitas FB, Alves R, Côrte-Real AR, et al. Asymmetric introgression between sympatric molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in the Comporta region. Portugal BMC Evol Biol. 2009;9:262. doi: 10.1186/1471-2148-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudolf M, Czajka C, Börstler J, Melaun C, Jöst H, von Thien H, et al. First nationwide surveillance of Culex pipiens Complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS One. 2013;8:e71832. doi: 10.1371/journal.pone.0071832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, et al. Emerging vectors in the Culex pipiens Complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- 30.Vaux AGC, Gibson G, Hernández-Triana LM, Cheke RA, McCracken F, Jeffries CL, et al. Enhanced West Nile virus surveillance in the North Kent marshes, UK. Parasit Vectors. 2015;8:91. [DOI] [PMC free article] [PubMed]
- 31.Vaux AGC, Murphy G, Baskerville M, Burden N, Convery N, Crossley L, et al. Monitoring for invasive and endemic mosquitoes at UK ports. Eur Mosq Bull. 2011;29:133–140. [Google Scholar]
- 32.Townroe S, Callaghan A. British container breeding mosquitoes: the impact of urbanisation and climate change on community composition and phenology. PLoS One. 2014;9:e95325. doi: 10.1371/journal.pone.0095325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medlock JM, Vaux AGC, Cull B, Schaffner F, Gillingham E, Pfluger V, Leach S. Detection of the invasive mosquito species Aedes albopictus in southern England. Lancet Infect Dis. 2017;17:140. doi: 10.1016/S1473-3099(17)30024-5. [DOI] [PubMed] [Google Scholar]
- 34.Medlock JM, Vaux AGC. Seasonal dynamics and habitat specificity of mosquitoes in an English wetland: implications for UK wetland management and restoration. J Vector Ecol. 2015;40:90–106. doi: 10.1111/jvec.12137. [DOI] [PubMed] [Google Scholar]
- 35.R Core Team: R. A language and environment for statistical computing; 2015.
- 36.Snow KR. Mosquitoes. Naturalists’ handbook 14. Slough: Richmond Publishing Co. Ltd.; 1990. [Google Scholar]
- 37.Cranston PS, Ramsdale CD, Snow KR, White GB. Adults, larvae and pupae of British mosquitoes (Culicidae) - a key. Cumbria: Freshwater Biological Association; 1987.
- 38.Wilkerson RC, Linton YM, Fonseca DM, Schultz TR, Price DC, Strickman DA. Making mosquito taxonomy useful: a stable classification of tribe Aedini that balances utility with current knowledge of evolutionary relationships. PLoS One. 2015;10:e0133602. doi: 10.1371/journal.pone.0133602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medlock JM, Vaux AGC. Aedes (Aedes) geminus Peus (Diptera, Culicidae) - an addition to the British mosquito fauna. Dipterists Dig. 2009;16:147–50.
- 40.Brugman VA, Hernández-Triana LM, Prosser SWJ, Weland C, Westcott DG, Fooks AR, Johnson N. Molecular species identification, host preference and detection of myxoma virus in the Anopheles maculipennis Complex (Diptera: Culicidae) in southern England, UK. Parasit Vectors. 2015;8:421. [DOI] [PMC free article] [PubMed]
- 41.Brugman VA, Hernández-Triana LM, England ME, Medlock JM, Mertens PPC, Logan JG, et al. Blood-feeding patterns of native mosquitoes and insights into their potential role as pathogen vectors in the Thames estuary region of the United Kingdom. Parasit Vectors. 2017;10:163. doi: 10.1186/s13071-017-2098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins FH, Paskewitz SM. A review of the use of ribosomal DNA (rDNA) to differentiate among cryptic Anopheles species. Insect Mol Biol. 1996;5:1–9. [DOI] [PubMed]
- 43.Madden T. The BLAST sequence analysis tool. National Center for Biotechnology Information: Bethesda; 2002. [Google Scholar]
- 44.Manley R, Harrup LE, Veronesi E, Stubbins F, Stoner J, Gubbins S, et al. Testing of UK populations of Culex pipiens L. for Schmallenberg virus vector competence and their colonization. PLoS One. 2015;10:e0134453. [DOI] [PMC free article] [PubMed]
- 45.Smith JL, Fonseca DM. Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae) Am J Trop Med Hyg. 2004;70:339–345. [PubMed] [Google Scholar]
- 46.Bahnck CM, Fonseca DM. Rapid assay to identify the two genetic forms of Culex (Culex) pipiens L. (Diptera: Culicidae) and hybrid populations. Am J Trop Med Hyg. 2006;75:251–255. doi: 10.4269/ajtmh.2006.75.251. [DOI] [PubMed] [Google Scholar]
- 47.Fonseca DM, Atkinson CT, Fleischer RC. Microsatellite primers for Culex pipiens quinquefasciatus, the vector of avian malaria in Hawaii. Mol Ecol. 1998;7:1617–1619. [PubMed] [Google Scholar]
- 48.Ivanova NV, Zemlak TS, Hanner RH, Hebert PDN. Universal primer cocktails for fish DNA barcoding. Mol Ecol Notes. 2007;7:544–548. doi: 10.1111/j.1471-8286.2007.01748.x. [DOI] [Google Scholar]
- 49.Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder MN, et al. AD model builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Methods Softw. 2012;27:233–239. doi: 10.1080/10556788.2011.597854. [DOI] [Google Scholar]
- 50.Skaug H, Fournier D, Bolker B, Magnusson A. Generalized linear mixed models using AD Model Builder. R package 0.8. 3.2; 2015.
- 51.Akaike H. Information theory and an extension of the maximum likelihood principle. In: International Symposium on Information Theory. New York: Springer; 1973. p. 267–81.
- 52.Ramsdale CD, Snow KR. Mosquito control in Britain. Dagenham: University of East London; 1995. [Google Scholar]
- 53.Balenghien T, Fouque F, Sabatier P, Bicout DJ. Horse-, bird-, and human-seeking behavior and seasonal abundance of mosquitoes in a West Nile virus focus of southern France. J Med Entomol. 2006;43:936–946. doi: 10.1093/jmedent/43.5.936. [DOI] [PubMed] [Google Scholar]
- 54.Balenghien T, Vazeille M, Grandadam M, Schaffner F, Zeller H, Reiter P, et al. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector-Borne Zoonotic Dis. 2008;8:589–595. doi: 10.1089/vbz.2007.0266. [DOI] [PubMed] [Google Scholar]
- 55.Balenghien T, Vazeille M, Reiter P, Schaffner F, Zeller H, Bicout DJ. Evidence of laboratory vector competence of Culex modestus for West Nile virus. J Am Mosq Control Assoc. 2007;23:233–236. doi: 10.2987/8756-971X(2007)23[233:EOLVCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 56.Votýpka J, Seblová V, Rádrová J. Spread of the West Nile virus vector Culex modestus and the potential malaria vector Anopheles hyrcanus in central Europe. J Vector Ecol. 2008;33:269–277. doi: 10.3376/1081-1710-33.2.269. [DOI] [PubMed] [Google Scholar]
- 57.Pradel JA, Martin T, Rey D, Foussadier R, Bicout DJ. Is Culex modestus (Diptera: Culicidae), vector of West Nile virus, spreading in the Dombes area, France? J Med Entomol. 2009;46:1269–1281. doi: 10.1603/033.046.0604. [DOI] [PubMed] [Google Scholar]
- 58.Golding N, Nunn MA, Medlock JM, Purse BV, Vaux AGC, Schäfer SM. West Nile virus vector Culex modestus established in southern England. Parasit Vectors. 2012;5:32. doi: 10.1186/1756-3305-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medlock JM, Vaux AGC. Distribution of West Nile virus vector, Culex modestus, in England. Vet Rec. 2012;171:278. doi: 10.1136/vr.e6123. [DOI] [PubMed] [Google Scholar]
- 60.Marshall JF. Records of Culex (Barraudius) modestus Ficalbi (Diptera: Culicidae) obtained in the south of England. Nature. 1945;156:172–173. doi: 10.1038/156172a0. [DOI] [Google Scholar]
- 61.Hernández-Triana LM, Brugman VA, Prosser SWJ, Weland C, Nikolova N, Thorne L, et al. Molecular approaches for blood meal analysis and species identification of mosquitoes (Insecta: Diptera: Culicidae) in rural locations in southern England, United Kingdom. Zootaxa. 2017;4250:67. [DOI] [PubMed]
- 62.de Wispelaere M, Desprès P, Choumet V. European Aedes albopictus and Culex pipiens are competent vectors for Japanese encephalitis virus. PLoS Negl Trop Dis. 2017;11:e0005294. doi: 10.1371/journal.pntd.0005294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vogels CBF, Fros JJ, Göertz GP, Pijlman GP, Koenraadt CJM. Vector competence of northern European Culex pipiens biotypes and hybrids for West Nile virus is differentially affected by temperature. Parasit Vectors. 2016;9:393. doi: 10.1186/s13071-016-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bidlingmayer WL. The measurement of adult mosquito population changes-some considerations. J Am Mosq Control Assoc. 1985;1:328–348. [PubMed] [Google Scholar]
- 65.Snow WF. Field estimates of the flight speed of some west African mosquitoes. Ann Trop Med Parasitol. 1980;74:239–242. doi: 10.1080/00034983.1980.11687334. [DOI] [PubMed] [Google Scholar]
- 66.Diarrassouba S, Dossou-Yovo J. [Atypical activity rhythm in Aedes aegypti in a sub-sudanian savannah zone of Côte d’Ivoire.] Bull la Société Pathol Exot. 1997;90:361–3. (In French). [PubMed]
- 67.Service MW A critical review of procedures from sampling populations of adult mosquitoes. Bull Entomol Res. 1977;67:343–382. doi: 10.1017/S0007485300011184. [DOI] [Google Scholar]
- 68.Service MW The daytime distribution of mosquitoes resting in vegetation. J Med Entomol. 1971;8:271–278. doi: 10.1093/jmedent/8.3.271. [DOI] [PubMed] [Google Scholar]
- 69.Stoddard ST, Morrison AC, Vazquez-Prokopec GM, Soldan VP, Kochel TJ, Kitron U, et al. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis. 2009;3:e481. doi: 10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Renshaw M. Population dynamics and ecology of Aedes cantans (Diptera: Culicidae) in England. PhD thesis. Liverpool: University of Liverpool; 1991.
- 71.Weaver SC, Fashing NJ. Dispersal behavior and vector potential of Aedes cantator (Diptera: Culicidae) in southern Maryland. J Med Entomol. 1981;18:317–323. doi: 10.1093/jmedent/18.4.317. [DOI] [PubMed] [Google Scholar]
- 72.Jupp PG, Kemp A. Studies on an outbreak of Wesselsbron virus in the free State Province, South Africa. J Am Mosq Control Assoc Assoc. 1998;14:40–45. [PubMed] [Google Scholar]
- 73.Marques GRAM, De Castro Gomes A. Comportamento antropofílico de Aedes albopictus (Skuse) (Diptera: Culicidae) na região do Vale do Paraíba, Sudeste do Brasil. Rev Saude Publica. 1997;31:125–30. [DOI] [PubMed]
- 74.Aitken THG, Worth CB, Jonkers AH, Tikasingh ES, Downs WG. Arbovirus studies in bush bush forest, Trinidad, WI, September 1959-December 1964. II. Field program and techniques. Am J Trop Med Hyg. 1968;17:237–252. doi: 10.4269/ajtmh.1968.17.237. [DOI] [PubMed] [Google Scholar]
- 75.Marshall JF. The British mosquitoes. London: British Museum; 1938. [Google Scholar]
- 76.Edwards FW. Anopheles algeriensis Theobald (Diptera: Culicidae) in Norfolk. J Entomol Soc South Engl. 1932;1:25–27. [Google Scholar]
- 77.Snow KR. Control of mosquito nuisance in Britain. J Am Mosq Control Assoc. 1987;3:271–275. [PubMed] [Google Scholar]
- 78.Snow KR. Mosquito nuisance and control in Britain: results of new research. Environ Health. 1996;104:294–297. [Google Scholar]
- 79.Danabalan R, Monaghan MT, Ponsonby DJ, Linton Y-M. Occurrence and host preferences of Anopheles maculipennis group mosquitoes in England and Wales. Med Vet Entomol. 2014;28:169–178. doi: 10.1111/mve.12023. [DOI] [PubMed] [Google Scholar]
- 80.Service MW The biology of Culiseta morsitans and Culiseta litorea (Diptera, Culicidae) in England. Bull Entomol Res. 1994;84:97–103. doi: 10.1017/S0007485300032260. [DOI] [Google Scholar]
- 81.Yates MG. The biology of the tree-hole breeding mosquito Aedes geniculatus (Olivier) (Diptera: Culicidae) in southern England. Bull Entomol Res. 1979;69:611–628. doi: 10.1017/S0007485300020162. [DOI] [Google Scholar]
- 82.Renshaw M, Service MW. Birley MH. Host finding, feeding patterns and evidence for a memorized home range of the mosquito Aedes cantans. Med Vet Entomol. 1994;8:187–193. doi: 10.1111/j.1365-2915.1994.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 83.Service MW Ecological and biological studies on Aedes cantans (Meig.) (Diptera: Culicidae) in southern England. J Appl Ecol. 1977;14:159–196. doi: 10.2307/2401833. [DOI] [Google Scholar]
- 84.Harold CHH. Studies on mosquito bionomics. J R Army Med Corps. 1926;47:81–94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Further information on each farm used in this study. (PDF) (PDF 334 kb)
Figure S1. Photosphere file, site B, sampling point 1. (JPG) (JPEG 2149 kb)
Figure S2. Photosphere file, site B, sampling point 2. (JPG) (JPEG 1922 kb)
Figure S3. Photosphere file, site B, sampling point 3. (JPG) (JPEG 1426 kb)
Figure S4. Photosphere file, site B, sampling point 4. (JPG) (JPEG 1302 kb)
Figure S5. Photosphere file, site C, sampling point 1. (JPG) (JPEG 1624 kb)
Figure S6. Photosphere file, site C, sampling point 2. (JPG) (JPEG 1066 kb)
Figure S7. Photosphere file, site C, sampling point 3. (JPG) (JPEG 2475 kb)
Figure S8. Photosphere file, site C, sampling point 4. (JPG) (JPEG 1454 kb)
Figure S9. Photosphere file, site D, sampling point 1. (JPG) (JPEG 1852 kb)
Figure S10. Photosphere file, site D, sampling point 2. (JPG) (JPEG 2606 kb)
Figure S11. Photosphere file, site D, sampling point 3. (JPG) (JPEG 1618 kb)
Figure S12. Photosphere file, site D, sampling point 4. (JPG) (JPEG 2726 kb)
Table S2. Regression coefficients, with Wald 95% confidence intervals and standard errors, for fixed effects of the best-fit negative binomial model used to describe total biting pressure. *** P ≤ 0.001. Table S3. Regression coefficients, with Wald 95% confidence intervals and standard errors, for fixed effects of the best-fit negative binomial model used to describe the biting activity of Coquillettidia richiardii (Ficalbi, 1889). *** P ≤ 0.001. Table S4. Regression coefficients, with Wald 95% confidence intervals and standard error, for fixed effects of the best-fit negative binomial model used to describe the biting activity of Culex modestus (Ficalbi 1889). *** P ≤ 0.001, * P ≤ 0.05. (PDF) (PDF 450 kb)
Table S5. Data used in this study. (XLSX) (XLSX 44 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional file 15.


