Abstract
A walnut protein hydrolysate (WPH) was prepared by using a mixture of pancreatin and viscozyme L from industrially available defatted walnut meal. The antioxidant effects of WPH were confirmed and quantified by reducing power, oxygen radical absorbance capacity, hydroxyl radical radical-scavenging activity and ABTS+· radical-scavenging activity assays. The protective effects of WPH on scopolamine-induced learning and memory deficits in mice were also evaluated based on in vivo behavioral tests. Results showed that WPH administration would lead to significantly decreased latencies while increased crossing times and target times in the spatial probe test, and increased escape latency and decreased error times in the step-down avoidance test for the scopolamine-induced dementia mice. Biochemical results indicated that the ameliorative effects of WPH on scopolamine-induced dementia mice could be attributed to the significantly increased amount of acetylcholine receptors. Therefore, WPH may be a potential therapeutic agent against Alzheimer’s disease.
Keywords: Walnut protein hydrolysate, Antioxidant, Scopolamine-induced dementia, Memory deficits, Acetylcholine receptors
Introduction
Alzheimer’s disease (AD) and memory deficits have drawn much attention worldwide because of their high prevalence, reoccurring effects and severe impacts on the daily lives of the elderly population and patients. Loss of neurons, fibers and synapses, decrease of acetylcholine level, increased inflammation and oxidative stress are closely associated with neurodegenerative dementias such as Alzheimer’s disease and aggressive brain aging (Nie et al. 2009).
A number of studies have been performed to examine the association between neuronal oxidative stress and Alzheimer’s disease (Nunomura et al. 2001). Free radicals accumulated in brain and other organs could damage proteins, lipids, nucleic acids and DNA inside the body, thereby increasing the susceptibility and incidence of various diseases (Valko et al. 2007). It is well known that neurodegenerative diseases involve loss of nerve cells from brain and spinal cord, resulting in functional reduction/inactivation (ataxia) and/or sensory dysfunction (dementia). For AD, damages induced by free radicals were found and identified through interfering the two normal pathways involving AD pathogenesis (Smith et al. 1991). Dietary intake of various antioxidant supplements can combat oxidative stress, offer upstream therapeutic barrier to damage on neuronal cells, prevent subsequent neuron inflammation and ameliorate neurological disorders via direct or indirect functions (Zandi et al. 2004). Several categories of antioxidants have shown such a positive function to alleviate AD progression and associated symptoms including peptides (Su et al. 2016) and polyphenols (Richard et al. 2011). Scopolamine, as a non-selective post-synaptic muscarinic blocker, can competitively antagonize against binding with acetylcholine and muscarinic receptors, even occupy post-synaptic receptor sites to increase acetylcholinesterase (AChE) activity in the cortex and hippocampus (Ebert and Kirch 1998). The cholinergic hypothesis states that degeneration or loss of cholinergic neurons and cholinergic neurotransmitter (such as acetylcholine) would cause deficits of cognitive function in AD patients (Vakalopoulos 2013). Therefore, scopolamine would decrease cerebral blood flow by cholinergic hypofunction (Tota et al. 2012) to induce memory and cognitive deficits in juvenilities in a way resembling elderly (Wesnes et al. 1988). Moreover, scopolamine could also promote degree of oxidative stress and inflammatory cytokines to trigger free radical injury and neuro-inflammation (Jang et al. 2013). Therefore, memory impairment mice induced by scopolamine have been used as an animal model of dementia or neurodegeneration to assess anti-amnesic activity by external stimulator, especially in the cases of that cholinergic system playing significant roles in the information storage and retrieval during acquiring new memories.
Walnut (Juglans regia L.) is the most widespread tree nut around the world. It is perceived as a nutrient-dense “brain food”, owing to its distinct profile of unsaturated fatty acids, proteins, vitamins and minerals (Chen et al. 2012). Defatted walnut meal is a by-product of oil industrial production, containing over 40% proteins with a large amount of essential amino acids (especially glutamine and arginine). At present, defatted walnut meal is mainly used as animal feed or fertilizer, however, some researchers have paid attention to the potential functional activities from walnut peptides, such as antioxidant and ACE inhibitory activity (Liu et al. 2013). Some literatures also have reported the memory-improving properties of walnut, but they always considered that omega-3 fatty acids from walnuts were the key contributors (Haider et al. 2011; Poulose et al. 2013), yet overlooking the roles of bioactive peptides. The objective of this work was to utilize defatted walnut meal to produce bioactive peptides for improving memory and learning. In vitro antioxidant activity assays, in vivo mouse behavioral tests and associated biochemical analyses were performed to assess the bioactivities of walnut protein hydrolysate (WPH), and the possible mechanisms underlying memory-improving effects was also elucidated.
Materials and methods
Materials and chemicals
Defatted walnut meal was provided by Hui Zhi Yuan Food Co., Ltd. (Yunnan, China). Pancreatin (240,000 U/g) was purchased from Guangzhou Geneshun Biotech Ltd. (Guangzhou, China). Viscozyme L was purchased from Novozymes (Beijing, China). Reduced glutathione (GSH) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Specific pathogen free (SPF) level Kunming mice (male and female; aged 3–4 weeks; body weight 20–22 g) were provided by Guangzhou University of Traditional Chinese Medicine. The scopolamine hydrobromide injection was purchased from Harvest Pharmaceutical Co., Ltd. (Shanghai, China). Piracetam was purchased from Wushan Pharmacy (Guangzhou, China). T-ChE testing cassette was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The Mouse-acetylcholine receptors (AchR) Elisa kit and Mouse-Ach Elisa kit were purchased from Wuhan Juntai Science and Technology Co., Ltd. (Wuhan, China). The BCA Protein Assay Kit (ML166920), revert aid first strand cDNA synthesis Kit (001704047) and SYBR (00174735) were purchased from Thermo Fisher Scientific Inc. TE buffer was purchased from Guangzhou Jinmei Biotechnology Co., Ltd. (Guangzhou, China). ChAT Primer DEPC (Diethylpyrocarbonate) was purchased from Sangon biological engineering Co., Ltd (Shanghai, China).
Preparation of walnut protein precipitate
The defatted walnut meal powder was mixed with NaOH solution (pH 9.0) at a ratio of 1:16 (w/w) under constant stirring for 2 h, before centrifugation at 8000×g for 30 min at 4°C (GL-21 M refrigerated centrifuge Xiangyi Instrument Co., Ltd. Changsha, China). The resultant supernatant was subject to pH adjustment (pH 4.0) and then centrifuged at 8000×g for 20 min at 4°C to yield the walnut protein precipitate, lyophilized (R2L-100KPS, Kyowa Vacuum Engineering, Tokyo, Japan) and stored at −20 °C.
Preparation of walnut protein hydrolysate (WPH)
Fifteen grams of walnut protein precipitate were dispersed in 240 mL of deionized water, and the pH of obtained mixture was adjusted to 7.0 with 1 M NaOH solution. Such a mixture was then hydrolyzed at 55 °C for 16 h with an enzyme mixture of pancreatin and viscozyme L (at a protease to substrate ratio of 0.8% w/w). The hydrolysate was heated up to 95 °C for 15 min to inactivate the enzymes, before being cooled to room temperature and centrifuged at 10,000×g for 20 min at 4 °C using GL-21 M refrigerated centrifuge. The supernatant was collected as walnut protein hydrolysate (WPH), lyophilized and stored at −18 °C till use.
Determination of protein recovery, degree of hydrolysis (DH) and peptide nitrogen percentage of WPH
The total nitrogen content was determined by Kjeldahl method with a conversion factor of 5.3 for walnut protein (Miller and Young 1977). Protein recovery was calculated as the mass percentage of the proteins present in the hydrolysate to that in the reaction mixture. Degree of hydrolysis (DH) was defined as the mass percentage of free amino groups released from proteins, and calculated based on the ratio of α-amino nitrogen to total nitrogen. The amino nitrogen content was determined by formaldehyde titration method (Nilsang et al. 2005). The peptide nitrogen percentage (PNP) was defined as the mass percentage of peptides in the whole hydrolysate, and determined by the method modified based on Kjeldahl method and formaldehyde titration method. To measure the total dissolved nitrogen in the protein hydrolysate, the hydrolysate (10 mL) was mixed thoroughly with trifluoroacetic acid (10 mL, 20%, w/v), before centrifugation at 10,000×g for 10 min at 4 °C. The supernatant was collected for determination of the total nitrogen content and α-amino nitrogen content. PNP of the sample was calculated using the following equation:
where Mmixture is the mass of the hydrolysate-trifluoroacetic acid mixture, Mhydrolysate is the mass of the hydrolysate, NT1 is the total nitrogen per unit hydrolysate, NT2 is the total nitrogen per unit mixture, and Na is the α-amino nitrogen per unit mixture.
Reducing power assay
The reducing power of WPH was determined using the method of Lu et al. (2010). An aliquot (2.0 mL) of WPH sample was mixed with 0.2 M sodium phosphate buffer (pH 6.6, 2.0 mL) and potassium ferricyanide (1% w/v, 2.0 mL) for the assay, and the absorbance was measured at 700 nm by Varioskan Flash Spectral Scan Multimode Plate Reader (Thermo Fisher Scientific, Waltham, MA). Higher absorbance indicated stronger reducing power. The blank was set up by using distilled water in the place of WPH sample.
Oxygen radical absorbance capacity (ORAC) assay
The ORAC antioxidant activity was determined according to the method of Dávalos et al. (2004). Briefly, the reaction was conducted in 75 mM sodium phosphate buffer (pH 7.4). The fluorescence was monitored using Varioskan Flash Spectral Scan Multimode Plate Reader (Thermo Fisher Scientific, Waltham, MA). Calibration was performed using Trolox solutions (final concentrations: 1–6 μM) for each assay. All the tests were at least performed in triplicate. The area under the fluorescence decay curve (AUC) was calculated as follows:
where f 0 is the initial fluorescence reading at 0 min, and f i is the fluorescence reading at time i. The net AUC corresponding to a sample was calculated as follows:
The linear regression equation between net AUC and antioxidant concentration was calculated. The final ORAC values were expressed as μmol Trolox equivalent (TE) per g or μmol antioxidant.
Hydroxyl radical scavenging activity assay
The hydroxyl radical scavenging activity was determined by 2-deoxyribose oxidation method (Chung et al. 1997) with a slight modification. The reaction mixture containing FeSO4-EDTA (0.2 mL, 10 mM), α-deoxyribose (0.5 mL, 10 mM), WPH solution (0.2 mL, 0.4 mg/mL), sodium phosphate buffer (pH 7.4, 0.9 mL, 0.2 M) and hydrogen peroxide (0.2 mL, 10 mM) was incubated at 37 °C for 1 h. There action was terminated by adding trichloroacetic acid and thiobarbituric acid, followed by boiling for 15 min and then cooling on ice. The absorbance at 532 nm was measured with UV–Visible Spectrophotometer (752 N, Analytical Instrumental, Shanghai, China). The control was set up via using distilled water. The hydroxyl radical scavenging activity as the inhibition rate of α-deoxyribose oxidation by hydroxyl radicals was calculated using the following equation:
where A 0 is the absorbance value of the control, and A s is the absorbance value of the test sample.
ABTS+· radical-scavenging activity
ABTS+· radical-scavenging activity was determined by decolorization assay (Ma and Xiong 2009), and expressed as Trolox equivalent antioxidant capacity (TEAC, μM TE/g). Briefly, phosphate buffer (0.2 M, pH 7.4) was used to dilute the test sample prior to absorbance measurement (at 734 nm). A standard curve was established using Trolox as standard (concentration ranged from 20 to 160 μM; absorbance monitored at 734 nm).
Assessment of learning and memory abilities in mice
NIH mice (20 ± 2 g, male, aged 6–8 weeks old) had been housed in a monitored environment (20 ± 2 °C, relative humidity 55 ± 5%, water and food supply ad libitum for 21 days, 12:12 h light–dark cycle), were randomly divided into 6 groups: (1) Control group (normal mice with oral administration of standard physiological saline in a same volume level as for WPH); (2) Model group (normal mice with intraperitoneal injection of 1.0 mg scopolamine/kg body weight); (3) Positive group (scopolamine-induced model mice with oral administration of Piracetam at 0.40 g/kg body weight); (4) WPH-L group (scopolamine-induced model mice with oral administration of low doge WPH at 0.20 g/kg body weight); (5) WPH-M group (scopolamine-induced model mice with oral administration of medium dose WPH at 0.33 g/kg body weight); (6) WPH-H group (scopolamine-induced model mice with oral administration of high dose WPH at 0.66 g/kg body weight). Clinical usage was used to calculate the equivalent dose, and this result was used as medium dose (0.33 g/kg). Low (0.20 g/kg) and high (0.66 g/kg) doses were defined as a half and double of the equivalent dose, respectively. All the mice of this study were approved by the Animal Care and Use Committee, Ministry of Science and Technology, China (Document 545, License No. SYXK-2002-001).
Morris water maze (MWM) test
This trial was performed according to the method of previous study (Su et al. 2016), involving two separate tests (Place Navigation and Probe Test). In Place Navigation test, the water maze was established as a circular pool (65 cm in diameter, 25 cm in depth) with four quadrants (northeast, northwest, southwest and southeast) filled with water (26 ± 1 °C; 20 cm in depth; opaque due to the added black ink). An escape platform submerged 1 cm below the water surface was set up in the northeast quadrant (target quadrant), which was located at the same position of the same quadrant for each trial. The escape latency for mice to find the platform within 60 s and stayed on the platform for 3 s was recorded.
In the subsequent Probe Test, the platform was removed and the escape latency (mice searching for the previous platform site within 60 s), the crossing times (the number of crossing over the target location), and the target time (the ratio of mice crossing the target quadrant) were measured.
Step-down avoidance test
The step-down avoidance test was performed on the day after the MWM using the passive avoidance task. The inhibitory avoidance apparatus was accommodated in a dark apartment. When the experiment began, an electric foot shock of 36 V charge was applied to the steel bars where the mice stood. Mice were trained for 30 min and their reactions were recorded. The numbers of correct response (jumping to the safe platform) and wrong response (jumping to the steel bars) were recorded.
Biochemistry analyses of acetylcholine (Ach), AchR and AchE contents and mRNA expression of choline acetyltransferase (ChAT) in mice.
The mice were anaesthetized and sacrificed after the behavioral trials. The head of mouse was removed rapidly. The brain tissues were harvested, separated, washed with saline, chilled on ice, homogenized with normal saline (at a brain tissue to saline mass ratio of 1:9), and centrifuged at 3500 × g for 10 min at 4 °C. The resultant supernatant was stored at −20 °C for the next detection of acetylcholine (Ach), acetylcholin esterase (AchE) and acetylcholine receptors (AchR). And expression of ChAT was analyzed by RT-PCR and immunofluorescence microscopy.
The analyses of Ach and AchE were based on the method of previous study (Su et al. 2016). The contents of Ach and AchR were calculated based on the calibration curves of the absorbance versus the concentrations of corresponding standard compounds. The experiments were performed following the instructions provided by the manufacturers of the Mouse-AchR Elisa kit and Mouse-Ach Elisa kit. The absorbance at 412 nm of the supernatant generated from mentioned method in the manuscript was determined following the AchE Activity Assay Kit (Colorimetric) instructions provided by the manufacturer Nanjing Jiancheng Bioengineering Institute. Bilateral hippocampus and cortex of mice were separated and placed on ice temporarily, and out of which a portion (50 mg) was weighed, transferred into the cryogenic vials, and snap-frozen in liquid nitrogen. These cryogenic vials were then stored at −80 °C ultra-low temperature freezer for biochemical analysis and detection of ChAT expression.
Statistical analysis
Data were expressed as “mean ± standard deviation” of six determinations (triplicate measurements for each of the duplicate test samples produced from at least two separate treatments or experiments). Statistical analysis was carried out using statistical package SPSS 17.0 (SPSS Inc., Chicago, IL) with one-way ANOVA. Significant difference (p < 0.05) between data was evaluated using Duncan’s multiple range tests and independent-sample T test.
Results and discussion
Protein recovery, degree of hydrolysis (DH) and peptide nitrogen percentage (PNP)
It is well known that protease could break down large molecular proteins into small molecule proteins, peptides or free amino acids, producing more functional products (antioxidants, anti-inflammatory etc.). The protein recovery of WPH was 84.51%, indicating high hydrolysis efficiency. Pancreatin as a mixture of enzymes such as trypsin, chymotrypsin, elastase and carboxypeptidases, could produce more complete cleavage of proteins/polypeptides into oligopeptides and typical amino acids due to their specificity activity. Furthermore, the multicomponent nature of Viscozyme L (co-occurrence of carbohydrases including arabinase, cellulase, hemicellulase and xylanase) also facilitated the cleavage of internal linkages with polysaccharide network (which was tightly associated with proteins) to catalyze polysaccharide into soluble monosaccharide, liberating more free proteins (Guan and Yao 2008). Therefore, a high protein recovery was anticipated upon the joint action of Pancreatin and Viscozyme L. Apart from protein recovery, DH and PNP were also crucial parameters for evaluating the hydrolysis efficacy. The DH and PNP herein were 6.6 and 69.17% for WPH, respectively. DH indicates the extent of cleaving peptide bonds (Zhang et al. 2016), and PNP reflects the proportion of soluble peptides in hydrolysate. The results indicated that almost 70.0% nitrogen in the current WPH came from peptides. This might suggest that the dominant role of these endonucleases. Additionally, various peptides with specific amino acid sequences, which also reflected the characteristics of the endonucleases, were released during hydrolysis.
Antioxidant activities of WPH
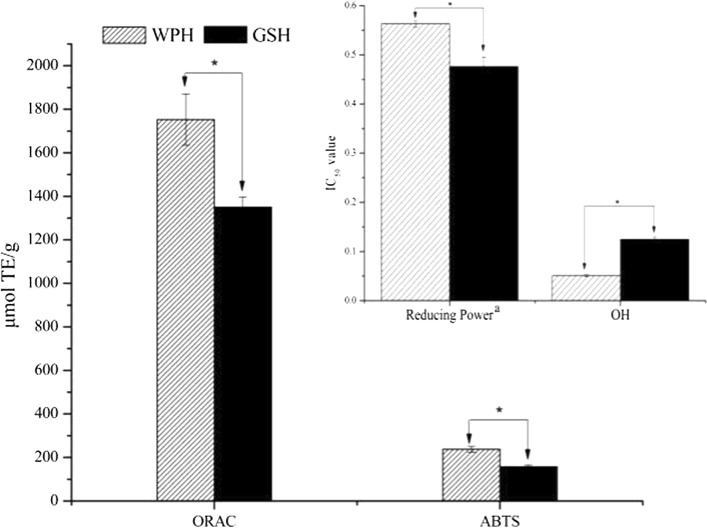
Different compositions of amino acids and peptides result into variation in antioxidant capacities. The WPH from multi-enzyme synergistic enzymatic hydrolysis of walnut protein could also release antioxidant substances. Results showed that WPH exhibited significantly higher (p < 0.05) antioxidant activities than GSH in ORAC, ABTS and reducing power assays, whereas reversed trend was observed in hydroxyl radical scavenging activity assay (Fig. 1). The ORAC value (1752.98 μmol TE/g) and IC50 value (0.051 mg/mL) for scavenging free hydroxyl radicals of WPH at 0.4 mg/mL were 1.30 and 2.45-fold higher than those of GSH, respectively. Meanwhile, WPH also exhibited 1.5-fold higher ABTS value (237.94 μmol TE/g) and 1.18-fold higher reducing power (0.563) than GSH, respectively. These indicated that WPH owned good antioxidant capacity. Additionally, the different antioxidant assays mentioned above were corresponded to different types of free radical species. Such as the hydrogen atom transfer reaction mechanism of ORAC is the most relevant to that in human body (Zheng et al. 2012). The hydroxyl radical scavenging assay might reflect severe and immediate damage on all types of molecules (including carbohydrates, nucleic acids, lipids, proteins and amino acids), which caused by highly destructive but short-lived free hydroxyl radicals generated naturally in the human body (Halliwell and Whiteman 2004; Halliwell 2001; Pihlanto 2006). Meanwhile, as a water-soluble free radical, ABTS+· could react readily with antioxidants in an aqueous system via the proton transfer mechanism. And reducing power assay measures the total electron donor ability of test samples. However, no matter which type of free radical species, excess free radicals would attack brain tissues and neuronal cells, causing oxidative stress in the brain, leading to cognitive impairment and ultimately memory dysfunction (Papandreou et al. 2011). In the present study, WPH exhibited high antioxidant activities in the four different assays, suggesting that WPH was an effective antioxidant for scavenging various free radicals through different scavenging ways. Relatively low detected value in the hydroxyl radical scavenging assay implied that WPH processed weak scavenging ability towards the short-lived hydroxyl radicals. Given the possible involvement of various free radicals in brain damage and memory impairment processes (Kumar et al. 2012), the all-round antioxidative abilities of WPH make it a potential therapeutic agent for alleviation of memory impairment. Zhang et al. (2016) indicated that different chemical mechanisms (i.e. hydrogen atom or electron transfer) or medium pH would influence antioxidant activity valued by different in vitro assays. In this study, only reducing power assay was different pH (pH 6.6) from the other three assays (pH 7.4). Therefore, different trends of antioxidant activities between hydroxyl radical scavenging activity and the other three types might be due to their different chemical mechanisms rather than medium pH.
Fig. 1.
Antioxidant activities of walnut protein hydrolysate (WPH) and reduced glutathione (GSH) solution. ORAC oxygen radical absorbance capacity, · OH hydroxyl radical scavenging activity, ABTS + · radical-scavenging activity. * indicates significant difference (p < 0.05). Sample concentration was 0.4 mg/mL
Learning and memory ability of mice in behavioral tests
Over 5 training days for place navigation in the Morris water maze (MWM) experiment, Model group exhibited much longer (p < 0.05) escape latency than Control group (Table 1), indicating that intraperitoneal injection of scopolamine would significantly (p < 0.05) induce learning and memory deficits in mice. Administration of Piracetam or WPH could significantly reduce (p < 0.05) escape latency of scopolamine-treated amnesia mice, and such a reduction became greater during the test. Therefore, both WPH and Piracetam could improve spatial memory capacity of amnesia of mice.
Table 1.
Effect of walnut protein hydrolysate (WPH) on scopolamine-induced amnesia mice in escape latency in place navigation trial
| Groups | Escape latency (s) | ||||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| Control | 30.43 ± 2.54 | 36.54 ± 3.77 | 30.33 ± 3.10 | 27.24 ± 2.74 | 23.64 ± 3.36 |
| Model | 44.12 ± 3.20# | 46.42 ± 2.61# | 40.29 ± 3.04# | 42.04 ± 3.30# | 42.04 ± 3.30# |
| Piracetam | 34.22 ± 3.00* | 34.22 ± 3.00* | 31.18 ± 2.90* | 30.55 ± 3.80* | 27.24 ± 3.34* |
| WPH | 34.23 ± 3.12* | 38.72 ± 3.11 | 35.24 ± 2.49 | 32.42 ± 3.09* | 27.89 ± 2.03* |
Different symbols in the same column: # versus Control, p < 0.05; * versus Model, p < 0.05. Escape latency represents the time of mice searching for the previous platform within 60 s
In the spatial probe test, Model group had a significantly (p < 0.05) longer escape latency, less crossing (the platform) times and lower target time, compared to the Control group (Table 2). It was interesting that Piracetam or WPH treatment could improve greatly the spatial memory of scopolamine-impaired mice, although WPH was less effective than Piracetam.
Table 2.
Effect of walnut protein hydrolysate (WPH) on scopolamine-induced amnesia mice in spatial probe test
| Groups | Escape latency (s) | Crossing-times (N) | Target-time (%) |
|---|---|---|---|
| Control | 14.45 ± 3.90 | 3.08 ± 0. 51 | 42.17 ± 2.22 |
| Model | 42.17 ± 2.22# | 0.86 ± 0.254# | 24.78 ± 3.62# |
| Piracetam | 15.17 ± 3.87* | 3.07 ± 0.430* | 39.14 ± 3.96* |
| WPH | 29.34 ± 6.65* | 1.92 ± 0.38* | 32.71 ± 2.70* |
Different symbols in the same column: # versus Control, p < 0.05; * versus Model, p < 0.05. In this section, the escape latency (mice searching for the previous platform within 60 s), the crossing times (the number of crossing over the target location), and the target time (the ratio of mice crossing the target quadrant) were recorded
After the MWM trials, test mice were examined for the step-down avoidance test. The scopolamine-induced amnesia mice (Model group) exhibited much shorter escape latency and much more error times (Table 3). However, both Piracetam and WPH treatment could alleviate the scopolamine-induced impairment, exhibiting shorter escape latency and less error responses. Additionally, the levels of escape latency and error times would be similar to ones of Control group.
Table 3.
Effect of walnut protein hydrolysate (WPH) on scopolamine-induced amnesia mice in step-down avoidance test
| Groups | Escape latency (s) | Error times (N) |
|---|---|---|
| Control | 244.64 ± 24.70 | 0.42 ± 0.15 |
| Model | 153.73 ± 35.46# | 1.40 ± 0.37# |
| Piracetam | 254.15 ± 26.76* | 0.46 ± 0.27* |
| WPH | 216.23 ± 28.24* | 0.54 ± 0.18* |
Different symbols in the same column: # versus Control, p < 0.05; * versus Model, p < 0.05. The step-down escape latency for mice to get down the platform and the number of mouse’s incorrect responses (error times) were recorded
In summary, WPH demonstrated greater potential function than Piracetam in place navigation, whereas the trends were reversed both in spatial probe and step-down avoidance tests. In terms of ameliorating scopolamine-induced memory deficits in mice, WPH could be an effective therapeutic agent for improving spatial memory (as in the MWM test) and short-term memory (as in the Step-down Avoidance Test).
The biochemical parameters of brain from mice
Biochemical analyses of brains from different mouse groups were performed to examine the effect of WPH on the brain cholinergic system (Table 4). A comparison between Control and Model groups revealed that scopolamine injection would significantly (p < 0.5) decrease Ach and AchR contents and mRNA expression of ChAT, while increased the AchE activity. Piracetam at the current dose could alleviate these symptoms. WPH treatments with all three doses (0.20, 0.33 or 0.66 g/kg) led to significantly up-regulation of AchR levels (p < 0.05) compared to Model group. In contrast, WPH might be able to up-regulate the expression of ChAT. It was notable that medium dose of WPH (0.33 g/kg) was significantly effective (p < 0.05) on promoting the expression of ChAT rather than the other two doses.
Table 4.
Effects of walnut protein hydrolysate (WPH) on the biochemical parameters of scopolamine-induced amnesia mice brain
| Group | Dose (g/Kg) | Ach (ng/mL) | AchR (pg/mL) | AchE (U/g.prot) | ChAT (2−ΔΔCt) |
|---|---|---|---|---|---|
| Control | – | 4.08 ± 0.25 | 1003.55 ± 42.40 | 233.96 ± 28.52 | 1.05 ± 0.18 |
| Model | – | 3.02 ± 0.10# | 670.53 ± 91.92# | 477.04 ± 82.84# | 0.60 ± 0.07# |
| Piracetam | 0.40 | 4.15 ± 0.29* | 903.96 ± 16.97* | 104.20 ± 51.27* | 1.82 ± 0.42* |
| WPH-L | 0.20 | 2.35 ± 0.03 | 1066.54 ± 40.48* | 380.32 ± 19.47 | 1.01 ± 0.21 |
| WPH-M | 0.33 | 2.23 ± 0.07 | 1051.41 ± 51.19* | 331.80 ± 27.49 | 3.92 ± 1.95* |
| WPH-H | 0.66 | 2.38 ± 0.18 | 1109.44 ± 52.44* | 413.46 ± 37.96 | 0.89 ± 0.15 |
Ach, AchR, AchE and ChAT represent acetylcholine, acetylcholine receptors, acetylcholin esterase and cholineacetyltransferase, respectively. WPH-L, WPH-M and WPH-H represent low, medium or high dose of WPH, respectively. Different symbols in the same column: # versus control, p < 0.05; *versus model: p < 0.05; n = 6 for Ach and AchR analyses, while n = 8 for analyses of AchE activity and mRNA expression of ChAT
Scopolamine treatment was applied to build the cholinergic dysfunctional mice model. In addition, the critical role of cholinergic system was to regulate neurotransmitter of brain, and repair memory deficits which might be induced by oxidative stress and free radical damage. Therefore, administration of antioxidant substances could be a better way to potentially prevent, alleviate and treat Alzheimer’s disease by regulating the cholinergic system (Su et al. 2016). In cholinergic system, Ach, AchE, AchR and ChAT were vital members which could maintain normal cholinergic system function. Acetylcholine (Ach), an important neurotransmitter of the brain cholinergic system, is reaction produce derived from acetyl coenzyme A (AcCoA) and choline. Additionally, Ach would also be catalyzed by AchE, leading to memory deficits (Vellom et al. 1993). Apart from that, Ach could bind to acetylcholine receptor (AchR) and then transmit signals across synapses. Moreover, ChAT is also involved in synthesis and transportation of Ach (Smith et al. 2006). Therefore, increase of AchR amount and ChAT expression could up-regulate efficiently the amount of Ach. It was reported that complex learning and memory deficits in AF64A-cholinotoxin-induced AD rat could be ameliorated, due to up-regulation of ChAT expression in the neural stem cells (Park et al. 2012). However, the level of ChAT expression did not increase as Ach content increasing after WPH administration in the present study. This might partially be due to relatively small reduction in AchE activity (as compared to the Model group). Another hypothesis was that increasing AchR was the dominant way of WPH administration on up-regulating the mount of Ach rather than enhancing ChAT expression.
The present biochemical analyses related to the cholinergic system revealed that WPH might exert its ameliorative effects on scopolamine-induced impaired mice through increasing amount of AchR.
Conclusion
The aim of this study was to investigate neuroprotective effects of WPH on scopolamine induced dementia model mice which was related to AD. It was both feasible and practical to produce antioxidative and neuroprotective hydrolysate from industrial by-product defatted walnut meal through enzymatic hydrolysis. The obtained WPH exhibited good free radical scavenging capacity and antioxidant activity. Additionally, WPH at three doses (0.20, 0.33 or 0.66 g/kg) also showed therapeutic potential function for scopolamine-induced memory deficits in mice with significantly increasing AchR level, while only medium dose of WPH (0.33 g/kg) could significantly promote the level of mRNA expression of ChAT. Therefore, WPH might ameliorate learning and memory deficits through regulating the cholinergic system (increasing AchR amount and up-regulating mRNA expression of ChAT) and protecting neurons in central nervous system from free radical damage (scavenging free radicals). The useful peptide fragments and their mechanism of action for ameliorating learning and memory deficits are still not clear. Future work on the compositional and neuroprotective profiles and mechanisms of key peptides is worthy to be carried out.
Acknowledgements
The authors gratefully acknowledge Strategic Emerging Industry Key Scientific and Technological Program of Guangdong Province (No. 2012A080800014), Guangzhou Science and Technology Plan Projects (No. 201604020122) and special funds for public welfare research and capacity building in Guangdong Province (NO. 2014B020204001) for their financial supports.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Chen N, Yang H, Sun Y, Niu J, Liu S. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides. 2012;38:344–349. doi: 10.1016/j.peptides.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Chung SK, Osawa T, Kawakishi S. Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra) Biosci Biotech Bioch. 1997;61:118–123. doi: 10.1271/bbb.61.118. [DOI] [Google Scholar]
- Dávalos A, Gómez-Cordovés C, Bartolomé B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem. 2004;52:48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- Ebert U, Kirch W. Scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur J Clin Invest. 1998;28:944–949. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- Guan X, Yao H. Optimization of Viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chem. 2008;106:345–351. doi: 10.1016/j.foodchem.2007.05.041. [DOI] [Google Scholar]
- Haider S, Batool Z, Tabassum S, Perveen T, Saleem S, Naqvi F, Javed H, Haleem DJ. Effects of walnuts (Juglans regia) on learning and memory functions. Plant Food Hum Nut. 2011;66:335–340. doi: 10.1007/s11130-011-0260-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases. Drug Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Brit J Pharmacl. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YJ, Kim J, Shim J, Kim C-Y, Jang J-H, Lee KW, Lee HJ. Decaffeinated coffee prevents scopolamine-induced memory impairment in rats. Behav Brain Res. 2013;245:113–119. doi: 10.1016/j.bbr.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Kumar H, More SV, Han SD, Choi JY, Choi DK. Promising therapeutics with natural bioactive compounds for improving learning and memory—a review of randomized trials. Molecules. 2012;17:10503–10539. doi: 10.3390/molecules170910503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Du M, Zhang Y, Xu W, Wang C, Wang K, Zhang L. Purification and identification of an ACE inhibitory peptide from walnut protein. J Agric Food Chem. 2013;61:4097–4100. doi: 10.1021/jf4001378. [DOI] [PubMed] [Google Scholar]
- Lu R-R, Qian P, Sun Z, Zhou X-H, Chen T-P, He J-F, Zhang H, Wu J. Hempseed protein derived antioxidative peptides: purification, identification and protection from hydrogen peroxide-induced apoptosis in PC12 cells. Food Chem. 2010;123:1210–1218. doi: 10.1016/j.foodchem.2010.05.089. [DOI] [Google Scholar]
- Ma Y, Xiong YL. Antioxidant and bile acid binding activity of buckwheat protein in vitro digests. J Agric Food Chem. 2009;57:4372–4380. doi: 10.1021/jf803670u. [DOI] [PubMed] [Google Scholar]
- Miller J, Young C. Protein nutritional quality of Florunner peanut meal as measured by rat bioassay. J Agric Food Chem. 1977;25:653–657. doi: 10.1021/jf60211a051. [DOI] [PubMed] [Google Scholar]
- Nie K, Yu JC, Fu Y, Cheng HY, Chen FY, Qu Y, Han JX. Age-related decrease in constructive activation of Akt/PKB in SAMP10 hippocampus. Biochem Bioph Res Co. 2009;378:103–107. doi: 10.1016/j.bbrc.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Nilsang S, Lertsiri S, Suphantharika M, Assavanig A. Optimization of enzymatic hydrolysis of fish soluble concentrate by commercial proteases. J Food Eng. 2005;70:571–578. doi: 10.1016/j.jfoodeng.2004.10.011. [DOI] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S. Oxidative damage is the earliest event in Alzheimer disease. J Neuropath Exp Neur. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res. 2011;219:197–204. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Park D, Lee HJ, Joo SS, Bae DK, Yang G, Yang YH, Lim I, Matsuo A, Tooyama I, Kim YB. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp Neurol. 2012;234:521–526. doi: 10.1016/j.expneurol.2011.12.040. [DOI] [PubMed] [Google Scholar]
- Pihlanto A. Antioxidative peptides derived from milk proteins. Int Dairy J. 2006;16:1306–1314. doi: 10.1016/j.idairyj.2006.06.005. [DOI] [Google Scholar]
- Poulose SM, Bielinski DF, Shukitt-Hale B. Walnut diet reduces accumulation of polyubiquitinated proteins and inflammation in the brain of aged rats. J Nutr Biochem. 2013;24:912–919. doi: 10.1016/j.jnutbio.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Richard T, Pawlus AD, Iglésias ML, Pedrot E, Waffo-Teguo P, Mérillon JM, Monti JP. Neuroprotective properties of resveratrol and derivatives. Ann N Y Acad Sci. 2011;1215:103–108. doi: 10.1111/j.1749-6632.2010.05865.x. [DOI] [PubMed] [Google Scholar]
- Smith C, Carney JM, Starke-Reed P, Oliver C, Stadtman E, Floyd R, Markesbery W. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Chung H, Rundquist S, Maat-Schieman ML, Colgan L, Englund E, Liu YJ, Roos RA, Faull RL, Brundin P. Cholinergic neuronal defect without cell loss in Huntington’s disease. Hum Mol Genet. 2006;15:3119–3131. doi: 10.1093/hmg/ddl252. [DOI] [PubMed] [Google Scholar]
- Su G, Zhao T, Zhao Y, Sun-Waterhouse D, Qiu C, Huang P, Zhao MM. Effect of anchovy (coilia mystus) protein hydrolysate and its maillard reaction product on combating memory-impairment in mice. Food Res Int. 2016;82:112–120. doi: 10.1016/j.foodres.2016.01.022. [DOI] [Google Scholar]
- Tota S, Nath C, Najmi AK, Shukla R, Hanif K. Inhibition of central angiotensin converting enzyme ameliorates scopolamine induced memory impairment in mice: role of cholinergic neurotransmission, cerebral blood flow and brain energy metabolism. Behav Brain Res. 2012;232:66–76. doi: 10.1016/j.bbr.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Vakalopoulos C. A cholinergic hypothesis of the unconscious in affective disorders. Front in Neurosci. 2013;7:220. doi: 10.3389/fnins.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vellom DC, Radić Z, Li Y, Pickering NA, Camp S, Taylor P. Amino acid residues controlling acetylcholinesterase and butyrylcholinesterase specificity. Biochem. 1993;32:12–17. doi: 10.1021/bi00052a003. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Simpson P, Kidd A. An investigation of the range of cognitive impairments induced by scopolamine 0.6 mg s.c. Hum Psychopharmacol Clin Exp. 1988;3:27–41. doi: 10.1002/hup.470030106. [DOI] [Google Scholar]
- Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, Norton MC, Welsh-Bohmer KA, Breitner JC. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu J, Liu L, Liu Y, Zhao T, Wu C, Sun-Waterhouse D, Zhao M, Su G. Physicochemical and sensory characteristics of soya protein isolate hydrolysates with added substrate-like amino acids. Int J Food Sci Tech. 2016;51:69–77. doi: 10.1111/ijfs.12943. [DOI] [Google Scholar]
- Zheng L, Su G, Ren J, Gu L, You L, Zhao M. Isolation and characterization of an oxygen radical absorbance activity peptide from defatted peanut meal hydrolysate and its antioxidant properties. J Agric Food Chem. 2012;60:5431–5437. doi: 10.1021/jf3017173. [DOI] [PubMed] [Google Scholar]