Abstract
The composition and fine structure of pectins found in plant cell walls are heterogeneous, with striking differences, depending on their source, and this eventually determines their functional and technological properties. The aim of this study was to extract and determine the chemical composition and physicochemical properties of pectins from different sources: passion fruit peel, orange pomace, and soy hull. Pectin extraction was performed with heated hydrochloric acid solution, followed by precipitation with 96% ethanol. Extraction yield, chemical composition, molar mass, physicochemical properties (fat absorption capacity, cation exchange capacity, water holding capacity, and antioxidant activity) of pectin were measured. Pectin extraction efficiency was higher for passion fruit peel and orange pomace (15.71 and 17.96%, respectively). Soy hull had low pectin extraction (5.66%). Galacturonic acid content was 23.21% for passion fruit peel pectin and 16.01% for orange pomace pectin. Water holding capacity, fat absorption capacity, and cation-binding capacity present in pectin extracted from passion fruit peel were higher, suggesting this poorly investigated product could be used as thickening and emulsifying agents in food preparations. Phenolic compounds with antioxidant capacity provide pectins with additional properties and expand their industrial use.
Keywords: Passion fruit peel, Orange pomace, Soy hull, Galacturonic acid, Functional properties
Introduction
Pectins are complex and heterogeneous macromolecules that, among other components, make up cell walls in plants. Their typical monosaccharides include galacturonic acid (dominant monosaccharide), rhamnose, galactose and arabinose. Pectin main chains are comprised of d-galacturonic acid units joined by α-1,4 glycosidic linkages (homogalacturonan) or by alternating units of α-1,4-linked d-galacturonic acid and α-1,2-linked L-rhamnose residues (rhamnogalacturonan-I). In the homogalacturonan, the units of galacturonic acid can be partially methyl-esterified at C6 and the units of rhamnose in rhamnogalacturonan-I can carry side chains of neutral monosaccharides (Martínez et al. 2010; Brouns et al. 2012). Their different composition and molecular arrangement expand their physicochemical properties and technological applications. They are used in the food industry as thickeners, emulsifiers, and stabilizers (Martínez et al. 2010; Dominiak et al. 2014; Cui and Chang 2014).
Pectins have shown some effects on health, including the reduction in the levels of cholesterol (6–7% of LDL-cholesterol) and glucose (decrease in glycemic response and insulin secretion), antioxidant activity (due to the presence of phenolic compounds), and inhibition of tumor growth and metastases (Gunness and Gidley 2010; Brouns et al. 2012).
The main commercial sources of pectins are citrus peels and apple pomace. However, other sources have been investigated, such as passion fruit peel, soy hull, pumpkin, kiwi fruit, pepper, banana peel, pineapple pulp, and olive pomace (Kalapathy and Proctor 2001; Canteri et al. 2010; Yapo 2010; Cui and Chang 2014; Yuliarti et al. 2015; Rubio-Senent et al. 2015).
Pectins are usually extracted in an acidic medium at 70–90 °C. The final extract is composed of pectins with different molar masses and degrees of esterification, and its quality is assessed based on galacturonic acid content. Commercial pectins have a galacturonic acid content greater than 65% in the ash-free dry weight, and molar masses vary from 100,000 to 200,000 Da (Joye and Luzio 2000). Depending on the degree of esterification, pectins will have different applications. Gel-forming capacity without the addition of sugar is inherent to low-methoxyl pectins (below 50%), making them suitable for use in low-calorie products. Notwithstanding, this reaction requires divalent cations (ex Ca+2) and pH between 2 and 7 for gelation (Yapo 2009a). On the other hand, high-methoxyl pectins require high concentrations of sugar for gelation.
It is known that different plant species, different tissues from the same plant, and different maturation stages lead to striking differences in the structure of pectin chains in terms of monosaccharide composition, molar mass, and degree of esterification (Yapo 2010; Yuliarti et al. 2015; Müller-Maatsch et al. 2016). Therefore, feedstock is believed to have a decisive influence on the quality and technological application of pectin extracts. So, it is very important that the molecular structure of pectins be investigated, once that their physicochemical and functional properties are very related to their structure and composition (Brouns et al. 2012; Cui and Chang 2014; Müller-Maatsch et al. 2016).
Accordingly, the aim of this study was to extract and determine the chemical composition and physicochemical characteristics of pectins from different sources (soy hull, passion fruit peel, and orange pomace).
Materials and methods
Materials
Orange pomace resulting of juice production was provided by Grupo Fischer (Fraiburgo, Santa Catarina, Brazil); passion fruit peel was obtained from commercially available fruits in Santa Maria, State of Rio Grande do Sul; and soy hull was provided by Camera Agroalimentos (Santa Maria, Rio Grande do Sul, Brazil).
Sample preparation
Orange pomace and passion fruit peel were provided in wet form and were immediately dried in a forced-air oven at 50 °C for 48 h. Soy hull was delivered dry. The samples were ground in a minimill (MA630/1, Marconi®) and stored under refrigeration (4 °C).
Feedstock characterization
The chemical composition of orange pomace, passion fruit peel, and soy hull was characterized. Moisture, protein and ash contents were measured using AACC (1995) methods 44-15A, 46-13, and 08-01, while lipid content was determined by the method proposed by Bligh and Dyer (1959). AOAC (1995) Method 991.43 was used to measure soluble and insoluble total dietary fiber. The concentration of uronic acid (acidic monosaccharides) was determined by the photometric method developed by Blumenkrantz and Asboe-Hansen (1973), and the content of reducing sugars followed the method proposed by Lane and Eynon (1934).
Pectin extraction
Orange pomace and soy hull pectins were extracted with 0.1N HCl at 90 °C under stirring for 45 min. Passion fruit peel pectins were extracted by the same procedure, but using 0.05N HCl. The extraction conditions for each feedstock were chosen based on the literature (Kalapathy and Proctor 2001). The ratios of sample and acid solution were different for each waste product, taking water hydration capacity into account. A total of 600 mL of HCl was used for 25 g of orange pomace; 350 mL for 50 g of soy hull; and 700 mL for 25 g of passion fruit peel. After extraction, centrifugation was performed at 3700 rpm for 15 min, the supernatant was collected, and 96% ethanol was added in an amount that was large enough to double the volume of the solution for pectin precipitation. The solution was kept under refrigeration for 12 h, and the precipitated pectin was collected, washed again with 96% ethanol, and allowed to stand for 30 min at room temperature after each addition of ethanol. This procedure was repeated once again for soy hull pectins and twice for the orange pomace and passion fruit peel pectins. Thereafter, the pectins were dried in a forced-air oven at 50 °C, ground in a minimill, and stored at 4 °C for later analysis.
Pectin yield, chemical composition, and molar mass
Pectin extraction yield was calculated by the ratio between the weight of the end product and the weight of feedstock submitted to extraction. Moisture, protein, and ash contents were measured using AACC methods 44-15A, 46-13, and 08-01, and lipid content was determined by the method of Bligh and Dyer (1959). The degree of esterification and content of methoxyl groups was determined by the method of Schultz (1965), modified by Yapo (2009b).
To determine monosaccharide composition, the samples were hydrolyzed with trifluoroacetic acid 1 M for 5 h at 100 °C. After hydrolysis, excess acid was removed by evaporation (Biermann 1989). After total acid hydrolysis, monosaccharides were solubilized in distilled water and reduced by the addition of approximately 10 mg of sodium borohydride for 16 h at 4 °C (Wolfrom and Thompson 1963b). Later on, strong acid cation exchange resin was added for removal of Na+ ions. The solutions were filtered, the solvent was evaporated in a vacuum, 1 mL of methanol was added for boric acid removal, and methyl borate was evaporated in a vacuum. This procedure was repeated three times. The alditols were acetylated by addition of 0.5 mL of acetic anhydride and 0.5 mL of pyridine, in sealed tubes, and stored for 12 h at room temperature (Wolfrom and Thompson 1963a). The reaction was interrupted by adding ice and the alditol acetate was extracted by the addition of chloroform and later elimination of pyridine in successive treatments with 5% copper sulfate and distilled water. After solvent evaporation, alditol acetates were submitted to gas–liquid chromatography (GLC) for determination of neutral monosaccharide composition.
The resulting alditol acetates were analyzed by GLC in a Trace GC Ultra chromatograph (Thermo Electron Corporation) equipped with a DB-225 (0.25 mm × 30 m) capillary column. The injector and flame ionization detector (FID) temperatures were 250 and 300 °C, respectively. The oven temperature was set at 100–215 °C at a heating rate of 40 °C/min. Helium was used as carrier gas at a flow of 1.0 mL/min. For determination of acid monosaccharide contents, uronic acid levels were measured by the method of Blumenkrantz and Asboe-Hansen (1973), using galacturonic acid as standard solution at 10–100 μg/mL and the absorbance was read at 520 nm.
Elution profile were made using a Waters 515 pump, injector, four ultrahydrogel columns—120, 250, 500, and 2000—with exclusion limits of 5.103, 8.104, 4.105, and 7.106, respectively, and a 2410 Waters differential refractometer. NaNO2 0.1 M containing 200 ppm of NaN3 was used as eluent. The samples were solubilized at 1.0 mg/mL in the eluent solution. Before the analyses, the samples were filtered in cellulose acetate membranes with a pore size of 0.22 μm.
Physicochemical properties of pectins
Fat absorption capacity of pectins was calculated using the method developed by Lin and Humbert (1974), and cation exchange capacity was determined by copper-binding capacity using the method of McBurney et al. (1983). Water holding capacity was measured by the method of McConnel et al. (1974). Phenolic compounds were extracted by the method proposed by Pérez-Jiménez et al. (2008). First, the compounds were extracted with methanol/water (50:50 v/v, pH 2), and then with acetone/water (70:30 v/v), yielding Extract 1. The residue from this aqueous/organic extraction was treated with methanol/H2SO4 at 85 °C for 20 h for the release of fiber-related polyphenols (Extract 2). Total phenolic compounds were obtained from the method of Folin–Ciocalteau (Singleton et al. 1999). After that, ferric reducing antioxidant power (FRAP), as proposed by Benzie and Strain (1996), was used. The results were calculated as gallic acid equivalent (GAE) and expressed in mg GAE/g.
Statistical analysis
Phenolic compounds and antioxidant activity were analyzed in triplicate, and the Pearson Correlation (p < 0.01) was performed using the Statistical Package for the Social Sciences (SPSS) version 18.0.
Results and discussion
The feedstocks showed high concentration of dietary fiber, especially of the insoluble type, for soy hull (Table 1). Passion fruit peel and soy hull had the highest uronic acid contents, greater than those of the orange pomace. Table 1 shows the highest content of soluble fiber for orange pomace, followed by passion fruit while soy hull has a soluble fiber content 2.6 times lower than orange pomace. This result suggested that, at least part of the uronic acid found in soy hull may have come from hemicelluloses which are insoluble fibers. Ouhida et al. (2002) detected uronic acid in water extractable and unextractable fractions. Recently, hot-compressed water extraction of polysaccharides from soy hulls afforded polysaccharides mainly composed by arabinose, 4-O-methyl-glucuronic acid and galactose (Liu et al. 2016).
Table 1.
Chemical composition of soy hull, passion fruit peel, and orange pomace
| Chemical composition (% dry basis) | Soy hull | Passion fruit peel | Orange pomace |
|---|---|---|---|
| Total dietary fiber | 83.73 | 62.64 | 54.62 |
| Soluble dietary fiber | 9.49 | 19.22 | 24.98 |
| Insoluble dietary fiber | 74.25 | 43.43 | 29.65 |
| Galacturonic acid | 18.85 | 23.21 | 16.01 |
| Reducing sugars | n.d. | 9.16 | 29.42 |
| Protein | 11.84 | 5.26 | 4.84 |
| Lipids | 1.67 | 1.26 | 4.29 |
| Ash | 4.09 | 6.04 | 3.26 |
n.d. not detected
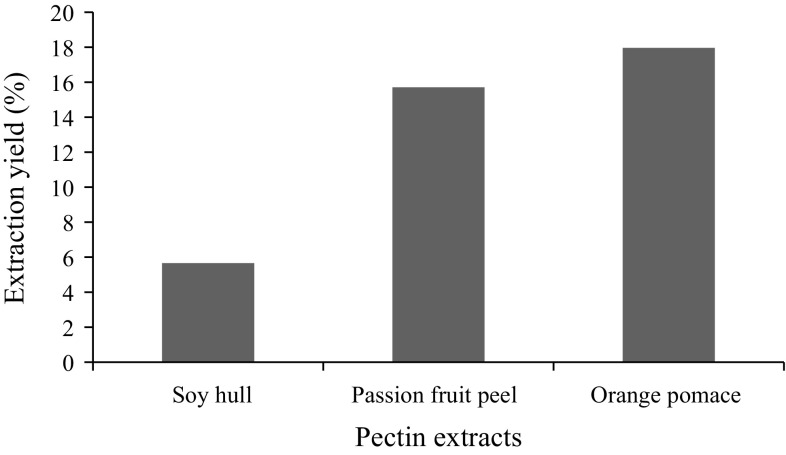
As suggested by the soluble fiber content, soy hull pectin had the lowest extraction yield whereas passion fruit peel and orange pomace pectins showed higher pectin levels (Fig. 1). Kalapathy and Proctor (2001) obtained extraction yields for soy hull pectin between 12 and 19% using different concentrations of acid for the extraction. By using hydrochloric acid 0.1N, the same authors found yields between 17 and 28%, which we could not reproduce in our study. The difference between the extraction methods lies in the fact that Kalapathy and Proctor (2001) used acidified 2-propanol for pectin precipitation while in our study we used ethyl alcohol. Other aspects that could affect the yield include, differences in the granulometry of soy hull flour, speed of stirring and processing of hulls. The extraction yield of passion fruit peel pectin was greater than the 13.9% obtained by Yapo (2009a) when they used nitric acid 0.3 M.
Fig. 1.
Extraction yield of soy hull, passion fruit peel, and orange pomace pectins
Table 2 shows the chemical composition of pectins extracted from soy hull, passion fruit peel, and orange pomace. The orange pomace pectin had the highest uronic acid content, as expected. The passion fruit peel pectin also showed high uronic acid content, albeit smaller than that obtained from pectins extracted from lemon rind (69.6%), passion fruit peel (68.4–71.9%), pumpkin (75–78%), and soy hull (68–72%) (Yapo 2009a; Kalapathy and Proctor 2001; Cui and Chang 2014). Note that the pectin extraction method used in this study can promote hydrolysis e release other polymers from cell wall consequently, other polysaccharide can also be solubilized. The high concentrations of arabinose, mannose, and galactose found in soy hull support this assumption and point out to the difference between the polysaccharides extracted from soy hull and those present in passion fruit peel and orange pomace. Mannose was the main monosaccharide found in soy hull fraction. Previous work from literature have also reported mannose as the main monosaccharide found in a water soluble fraction from soy hulls (Ouhida et al. 2002). Regarding the composition of the passion fruit peel pectin, the high content of glucose is due to the presence of cellulose, besides pectic substances (Yapo and Koffi 2008).
Table 2.
Chemical composition of pectins extracted from soy hull, passion fruit peel, and orange pomace
| Chemical composition (% dry basis) | Pectin extracts | ||
|---|---|---|---|
| Soy hull | Passion fruit peel | Orange pomace | |
| Rhamnose | 1.47 | 5.10 | 3.28 |
| Fucose | 0.51 | 0.00 | 0.00 |
| Arabinose | 7.41 | 2.01 | 2.48 |
| Xylose | 1.17 | 1.84 | 0.48 |
| Mannose | 31.47 | 0.84 | 0.64 |
| Galactose | 11.44 | 8.12 | 7.77 |
| Glucose | 1.47 | 14.48 | 4.96 |
| Uronic acid | 18.41 | 51.30 | 60.45 |
| Degree of esterification | 68.96 | 84.17 | 60.79 |
| Methoxyl groups | 3.05 | 9.08 | 8.08 |
| Protein | 13.70 | 2.93 | 4.15 |
| Lipids | 0.52 | 0.38 | 1.77 |
| Ashes | 4.25 | 4.13 | 3.65 |
| Phenolic compounds (mg GAE/g) | 11.53 | 16.85 | 13.19 |
On average, pectin accounts for only 30% of the sugar fraction in soy hull; the remainder consists of hemicelluloses (50%) and cellulose (20%) (Liu et al. 2013). Also, the large concentration of insoluble carbohydrates in soy hull hinders pectin extraction. Extraction with ammonium oxalate and microwave heating, as utilized by Liu et al. (2013), yielded polysaccharides made up mainly of galactose (47.45 mol%), xylose (25.09 mol%), and galacturonic acid content (15.07%) lower than that obtained in our study (18.41%).
The pectins from different sources had high degrees of esterification as shown in Table 2. The passion fruit peel had a degree of esterification greater than 70%, which is considered high for acid-extracted pectins (Dominiak et al. 2014). A high degree of esterification allows pectin to form gel quickly at high temperatures, having a more effective action on the lipid profile (Brouns et al. 2012; Dominiak et al. 2014). However, the degree of esterification represents only the ratio between methanol-esterified carboxyl groups and free carboxyl groups whereas the methoxyl rate refers to the amount of methoxyl groups in a sample (Gnanasambandam and Proctor 1999). Therefore, the degree of esterification should not be assessed separately, as it does not represent the actual amount of methyl esterifications, especially when the galacturonic acid content is low. Gnanasambandam and Proctor (1999) extracted pectin from soy hull using nitric acid 0.1N and the end product had 4.05% of methoxyl groups, a rate that is higher than that of soy hull pectin and lower than that of passion fruit peel and orange pomace pectins in our study.
Soy hull pectin showed a high protein level, due probably to the high hygroscopicity of this constituent, which was solubilized along with polysaccharides during extraction. The high concentration of phenolic compounds demonstrated that the extraction process did not succeed in ruling out the association of these constituents with complex polysaccharides in plant tissues.
Water holding capacity, fat absorption capacity, copper-binding capacity, and antioxidant activity were higher for passion fruit peel (Table 3). These findings indicate that these pectic substances are versatile enough to be used as food additives and also suggest their key role in human metabolism. Water holding capacity is relevant from a technological and physiological standpoint, as it can increase the volume and change the viscosity and texture of foods, in addition to reducing calories and changing fat digestion and uptake (Gunness and Gidley 2010; Rubio-Senent et al. 2015). Fat absorption capacity appears to be related to the degree of acetylation and esterification of the molecules, owing to the increase in their hydrophobicity (Rubio-Senent et al. 2015). The degree of acetylation was not measured in this study, but the degree of esterification was higher for passion fruit peel, which also had the highest fat absorption capacity. Moreover, high water holding capacity, combined with high fat absorption capacity, indicates good emulsifying properties, facilitating the solubilization or dispersion of two immiscible liquids. The water holding capacity of the passion fruit peel pectin was similar to that found by Rubio-Senent et al. (2015) for commercially available citrus fruit pectin (10.35 g of water/g). The same authors extracted pectins from olive pomace and obtained samples high fat absorption capacity (6.17 g of oil/g).
Table 3.
Physicochemical properties of pectins extracted from soy hull, passion fruit peel, and orange pomace
| Physicochemical property | Pectin extract | ||
|---|---|---|---|
| Soy hull | Passion fruit peel | Orange pomace | |
| Water holding capacity (g water/g sample) | 6.08 | 11.59 | 7.57 |
| Fat absorption capacity (g of oil/g of sample) | 2.73 | 4.05 | 2.97 |
| CBCa (mg Cu/g of sample) | 16.61 | 117.51 | 73.47 |
| Antioxidant activity (µM Trolox/g) | 29.68 | 63.24 | 45.92 |
aCopper-binding capacity
Polysaccharides such as pectin can act as chelating agents for metals, either because of their acid groups with high affinity for cations or because of the substitution of water molecules in cation solvation into hydroxyl groups (Chen et al. 2010). These two factors explain the high copper-binding capacity of passion fruit peel and orange pomace pectins. A good cation-binding capacity provides polysaccharides with properties that are important to human health and to food technology. In human health, a high cation-binding capacity increases the association of dietary fiber with bile salts in the gastrointestinal tract, reducing its participation in the digestion of fats and decreasing bile salt reabsorption (Lee et al. 2002). In food technology, a high cation-binding capacity helps stabilize emulsions as the metal polysaccharide complex prevents the metal from interacting with fatty acids, impeding its oxidation. Nevertheless, very high levels of metal polysaccharide complexes may lead to physical instability of the emulsion and flocculation (Chen et al. 2010).
Our study showed a positive correlation between the amount of phenolic compounds in the extracts and antioxidant activity (r = 0.99, p = 0.0002). The passion fruit peel pectin had the highest amount of phenolic compounds, with higher antioxidant activity. The ultimate goal of the extraction of pectins is not to use them in food products due to their antioxidant activity; this could be an additional goal, though. Other studies have already described antioxidant activity in polysaccharide samples due to the presence of phenolic compounds (Lai et al. 2010; Dalonso and Petckowicz 2012).
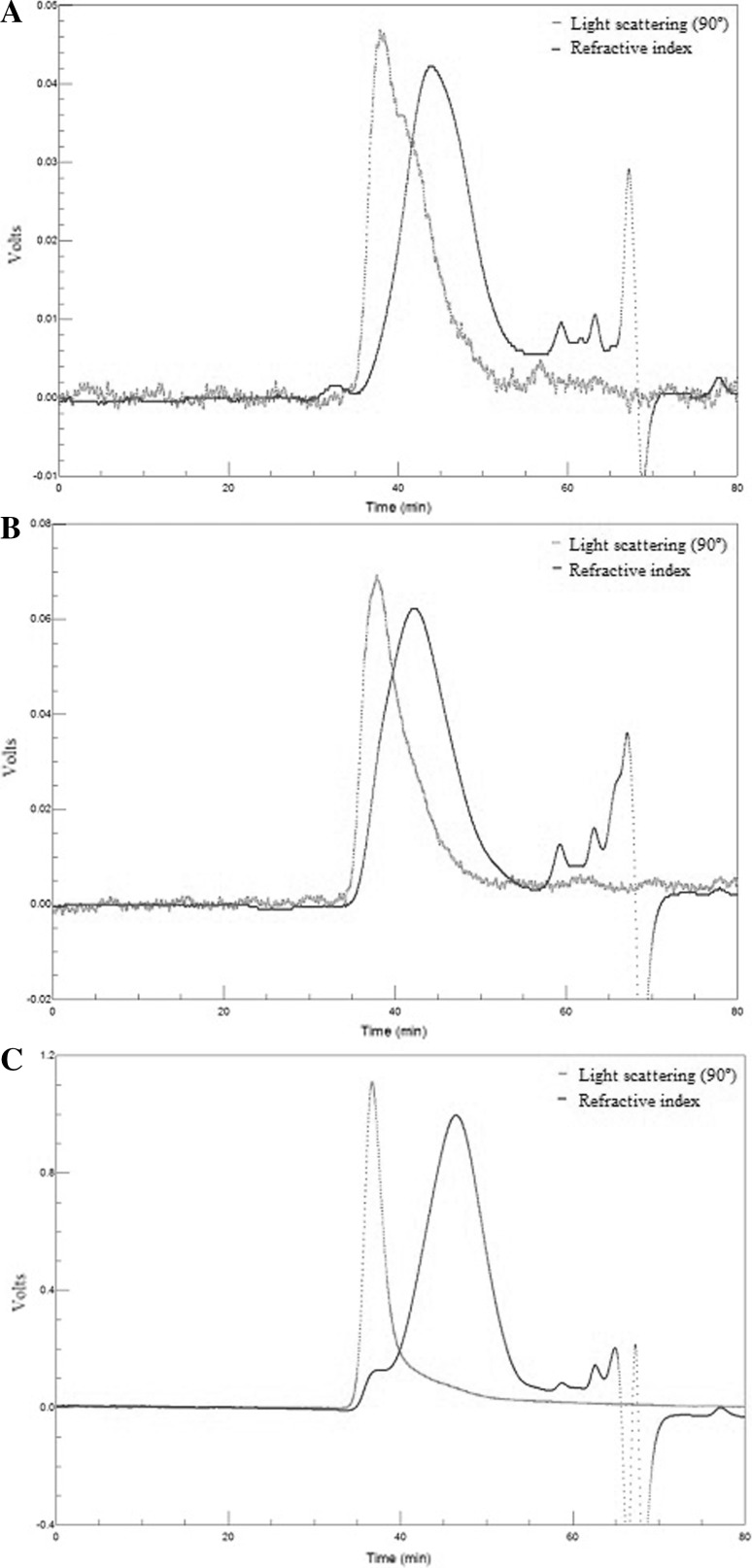
Figure 2 shows the elution of pectin extracts as detected by light scattering and refractive index, which allow gathering information about the molar mass of the sample. Static light scattering provides information about the size, molar mass, and concentration of the polymer, since the intensity of light polarized by a molecule is directly proportional to its hydrodynamic volume (Dominiak et al. 2014; Yuliarti et al. 2015). The light scattering detector revealed single peaks for each of the three analyzed samples, whose elution time ranged from 35 to 40 min, indicating the presence of a polymer with high molar mass.
Fig. 2.
Elution profile of pectin extracts: a soy hull, b passion fruit peel, c orange pomace
The refractive index is related to the concentration of the polymer solution (Yuliarti et al. 2015). Larger molecules, with a higher mass, are the first ones to undergo elution as they have a lower retention time whereas those with a smaller mass are found at the end of the process (Dominiak et al. 2014). According to the refractive index, there was a higher peak with elution between 40 and 55 min and lower peaks with longer elution, thus demonstrating the heterogeneity of the samples (Canteri et al. 2010; Dominiak et al. 2014). The high intensity of the signal detected by the refractive index does not coincide with the light scattering signal, indicating that compounds with high molar mass are found at low concentrations in the samples (Canteri et al. 2010). The washing with ethanol during pectin extraction can solubilize and cause some carbohydrates with a low molar mass, especially monosaccharides and disaccharides, to be lost. However, oligosaccharides and polysaccharides with lower molar masses remain in the middle, requiring other types of process for their total removal.
Conclusion
The efficiency in the extraction of pectins was greater than 15% for passion fruit peel and orange pomace, with a galacturonic acid content higher than 50% for both. A good-quality product from orange pomace is usually expected as most of the commercial pectin is extracted from citrus fruits. On the other hand, the high water holding capacity, high fat absorption, and high cation-binding capacity of passion fruit peel pectin indicate that this product can be exploited industrially as thickener and emulsifier in food preparations. The presence of phenolic compounds with antioxidant capacity provides additional properties to the pectin extract, broadening its industrial use.
References
- American Association of Cereal Chemists (1995) Official methods of analysis, vol 2, 9th edn. Saint Paul
- Association of Official Analytical Chemists (1995) Official methods of analysis, 16th edn. Washington
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Biermann CJ. Hydrolysis and the other cleavage of glycosidic linkages. In: Biermann CJ, Mcginnis GD, editors. Analysis of carbohydrates by GLC and MS. Boca Raton: CRC Press; 1989. pp. 27–41. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Brouns F, Theuwissen E, Adam A, Bell M, Berger A, Mensink RP. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur J Clin Nutr. 2012;66:591–599. doi: 10.1038/ejcn.2011.208. [DOI] [PubMed] [Google Scholar]
- Canteri MH, Scheer A, Petkowicz C, Ginies C, Renard C, Wosiacki G. Physicochemical composition of the yellow passion fruit pericarp fractions and respective pectic substances. J Food Nutr Res. 2010;49:113–122. [Google Scholar]
- Chen B, McClements DJ, Decker EA. Role of continuous phase anionic polysaccharides on the oxidative stability of menhaden oil-in-water emulsions. J Agr Food Chem. 2010;58:3779–3784. doi: 10.1021/jf9037166. [DOI] [PubMed] [Google Scholar]
- Cui SW, Chang YH. Emulsifying and structural properties of pectin enzymatically extracted from pumpkin. LWT Food Sci Technol. 2014;58:396–403. doi: 10.1016/j.lwt.2014.04.012. [DOI] [Google Scholar]
- Dalonso N, Petckowicz CLO. Guarana powder polysaccharides: characterisation and evaluation of the antioxidant activity of a pectic fraction. Food Chem. 2012;134:1804–1812. doi: 10.1016/j.foodchem.2012.03.088. [DOI] [PubMed] [Google Scholar]
- Dominiak M, Søndergaard KM, Wichmann J, Vidal-Melgosa S, Willats WGT, Meyer AS, Mikkelsen JD. Application of enzymes for efficient extraction, modification, and development of functional properties of lime pectin. Food Hydrocoll. 2014;40:273–282. doi: 10.1016/j.foodhyd.2014.03.009. [DOI] [Google Scholar]
- Gnanasambandam R, Proctor A. Preparation of soy hull pectin. Food Chem. 1999;65:461–467. doi: 10.1016/S0308-8146(98)00197-6. [DOI] [Google Scholar]
- Gunness P, Gidley MJ. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010;1:149–155. doi: 10.1039/c0fo00080a. [DOI] [PubMed] [Google Scholar]
- Joye DD, Luzio GA. Process for selective extraction of pectins from plant material by differential pH. Carbohydr Polym. 2000;43:337–342. doi: 10.1016/S0144-8617(00)00191-0. [DOI] [Google Scholar]
- Kalapathy U, Proctor A. Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy hull pectin. Food Chem. 2001;73:393–396. doi: 10.1016/S0308-8146(00)00307-1. [DOI] [Google Scholar]
- Lai F, Wen Q, Li L, Wu H, Li X. Antioxidant activities of water-soluble polysaccharide extracted from mung bean (Vigna radiata L.) hull with ultrasonic assisted treatment. Carbohydr Polym. 2010;81:323–329. doi: 10.1016/j.carbpol.2010.02.011. [DOI] [Google Scholar]
- Lane JH, Eynon L. Determination of reducing sugars by Fehling’s solutions with methylene blue indicator. London: Normam Rodge; 1934. pp. 1–8. [Google Scholar]
- Lee JK, Kim SY, Kim SU, Kim JH. Synthesis of cationic polysaccharide derivatives and their hypocholesterolaemic capacity. Biotechnol Appl Biochem. 2002;35:181–189. doi: 10.1042/BA20010088. [DOI] [PubMed] [Google Scholar]
- Lin MJY, Humbert ES. Certain functional properties of sunflower meal products. J Food Sci. 1974;39:368–370. doi: 10.1111/j.1365-2621.1974.tb02896.x. [DOI] [Google Scholar]
- Liu H, Guo X, Li J, Zhu D, Li J. The effects of MgSO4, d-glucono-d-lactone (GDL), sucrose, and urea on gelation properties of pectic polysaccharide from soy hull. Food Hydrocoll. 2013;31:137–145. doi: 10.1016/j.foodhyd.2012.10.013. [DOI] [Google Scholar]
- Liu HM, Wang FY, Liu YL. Hot-compressed water extraction of polysaccharides from soy hulls. Food Chem. 2016;202:104–109. doi: 10.1016/j.foodchem.2016.01.129. [DOI] [PubMed] [Google Scholar]
- Martínez M, Gullón B, Yáñez R, Alonso JL, Parajó JC. Kinetic assessment on the autohydrolysis of pectin-rich by-products. Chem Eng J. 2010;162:480–486. doi: 10.1016/j.cej.2010.05.048. [DOI] [Google Scholar]
- McBurney MI, Van Soest PJ, Chase LE. Cation exchange capacity and buffering capacity of neutral-detergent fibres. J Sci Food Agric. 1983;34:910–916. doi: 10.1002/jsfa.2740340903. [DOI] [Google Scholar]
- McConnel AA, Eastwood MA, Mitchell WD. Physical characteristics of vegetable foodstuffs that could influence bowel function. J Sci Food Agric. 1974;25:1457–1464. doi: 10.1002/jsfa.2740251205. [DOI] [PubMed] [Google Scholar]
- Müller-Maatsch J, Bencivenni M, Caligiani A, Tedeschi T, Bruggeman G, Bosch M, Petrusan J, Droogenbroeck BV, Elst K, Sforza S. Pectin content and composition from different food waste streams. Food Chem. 2016;201:37–45. doi: 10.1016/j.foodchem.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Ouhida I, Perez JF, Gasa J. Soybean (Glycine max) cell wall composition and availability to feed enzymes. J Agric Food Chem. 2002;50:1933–1938. doi: 10.1021/jf010686u. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez J, Arranz S, Tabernero M, Díaz-Rubio ME, Serrano J, Goñi I, Saura-Calixto F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res Int. 2008;41:274–285. doi: 10.1016/j.foodres.2007.12.004. [DOI] [Google Scholar]
- Rubio-Senent F, Rodríguez-Gutiérrez G, Lama-Muñoz A, Fernández-Bolaño J. Pectin extracted from thermally treated olive oil by-products: characterization, physico-chemical properties, in vitro bile acid and glucose binding. Food Hydrocoll. 2015;43:311–321. doi: 10.1016/j.foodhyd.2014.06.001. [DOI] [Google Scholar]
- Schultz TH (1965) Determination of the degree of esterification of pectin, determination of the ester methoxyl content of pectin by saponification and titration. Determination of the anhydro uronic acid content by decarboxylation and titration of the liberated carbon dioxide. In: Whistler RL, Wolfrom ML (eds) Methods in carbohydrate chemistry, vol 5. Academic Press, New York, pp 189–194
- Singleton VL, Orthofer R, Lamuelaraventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Wolfrom ML, Thompson A. Acetylation. Methods Carbohydr Chem. 1963;2:211–215. [Google Scholar]
- Wolfrom ML, Thompson A. Reduction with sodium borohydride. Methods Carbohydr Chem. 1963;2:65–68. [Google Scholar]
- Yapo BM. Biochemical characteristics and gelling capacity of pectin from yellow passion fruit rind as affected by acid extractant nature. J Agric Food Chem. 2009;57:1572–1578. doi: 10.1021/jf802969m. [DOI] [PubMed] [Google Scholar]
- Yapo BM. Pectin quantity, composition and physicochemical behaviour as influenced by the purification process. Food Res Int. 2009;42:1197–1202. doi: 10.1016/j.foodres.2009.06.002. [DOI] [Google Scholar]
- Yapo BM. Improvement of the compositional quality of monocot pectin extracts contaminated with glucuronic acid-containing components using a step-wise purification procedure. Food Bioprod Process. 2010;88:283–290. doi: 10.1016/j.fbp.2009.07.001. [DOI] [Google Scholar]
- Yapo MM, Koffi KL. The polysaccharide composition of yellow passion fruit rind cell wall: chemical and macromolecular features of extracted pectins and hemicellulosic polysaccharides. J Sci Food Agric. 2008;88:2125–2133. doi: 10.1002/jsfa.3323. [DOI] [Google Scholar]
- Yuliarti O, Matia-Merino L, Goh KKT, Mawson J, Williams MAK, Brennan C. Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 2015;166:479–485. doi: 10.1016/j.foodchem.2014.06.055. [DOI] [PubMed] [Google Scholar]