Abstract
Nanostructured lipid carriers (NLCs) offer many potential benefits for incorporating lipophilic molecules into clear/opaque food systems. In this study, the influence of pH, ionic strength, thermal treatment, simulated gastric juice (SGJ), and freeze–thawing on the stability of astaxanthin-loaded NLCs (Ax-NLCs) was examined. Ax-NLCs (containing α-tocopherol and ethylenediaminetetraacetic acid as antioxidants) were stabilized with Tween 80 and lecithin (Z-average size: 94 nm), and studied for the above mentioned purpose. The size of Ax-NLCs increased at low pH values (≤5), high NaCl concentrations (≥50 mM), and slightly at SGJ, mainly because of decreasing the ζ-potential. Moreover, thermal treatment at 80/90 °C led to an increase in Ax-NLCs size. Glycerol was found as an appropriate cryoprotectant for preventing aggregation of Ax-NLCs during freeze–thawing. pH, ionic strength, heat and SGJ had no drastic effects on the chemical stability of astaxanthin in NLCs. These results have valuable implications for the utilization of food-grade NLCs.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2749-7) contains supplementary material, which is available to authorized users.
Keywords: Nanostructured lipid carriers (NLCs), Astaxanthin, Physical stability, Delivery systems, Encapsulation, Functional foods
Introduction
Carotenoids, the most widespread class of lipophilic natural pigments, have been intensively investigated because many of them inhibit human health disorders and some also have pro-vitamin A activity (Amorim-Carrilho et al. 2014). Astaxanthin (Ax) is one of the most important carotenoids which mainly produced by fungi (Higuera-Ciapara et al. 2006). Ax has a high commercial value because exhibits exceptional antioxidant activity even higher than that of vitamin E and β-carotene (Naguib 2000), possesses important physiological functions (Higuera-Ciapara et al. 2006), and has an attractive pink color. However, the utilization of carotenoids as functional compounds (pigments and nutraceuticals) in many foods and beverages is currently limited owing to their poor water-solubility, high melting point, chemical instability, and low bioavailability (Qian et al. 2012). Therefore, they usually have to be inserted into aqueous-based formulations in the form of a colloidal delivery system (Tamjidi et al. 2013). Recently there is great interest in utilizing nanostructured lipid carriers (NLCs) to encapsulate lipophilic bioactive components for application in food and beverage products.
NLCs or oil-loaded solid lipid nanoparticles (SLNs) are oil-in-water (O/W) nano- or micro-emulsions in which a major portion (70–99.9%) of the lipid phase is constituted by solid lipids. NLCs have many of the advantages of polymeric particles, liposomes, emulsions and SLNs, and dispel some of their disadvantages (Tamjidi et al. 2013). In certain circumstances, NLCs may be a superior option compared with other colloidal carriers, and may improve the utilization and bioavailability of hydrophobic nutraceuticals (e.g., Ax) in aqueous-based foods and clear/opaque beverages. NLCs can be prepared from food-grade or GRAS ingredients on industrial scale, and hot homogenization method is the most feasible technique for production of them. Currently, NLCs containing lipophilic active compounds are increasingly developed and characterized for food applications (Aditya et al. 2013, 2014; Hejri et al. 2013; Hentschel et al. 2008; Liu and Wu 2010; Liu et al. 2012; Ni et al. 2015; Tamjidi et al. 2014a). Medium chain triglycerides and oleic acid (as oils), stearic acid, glycerol monostearate, glyceryl palmitostearate and glyceryl behenate (as fats), and Tween 80 and lecithin (as emulsifiers), are the most commonly used food-grade ingredients for NLCs (Tamjidi et al. 2013).
Previously, effect of ingredients on some characteristics of Ax-loaded NLCs (Ax-NLCs) has been examined, and a formulation of Ax-NLCs designed with suitable lipids, lipids ratio and Tween 80 content (Tamjidi et al. 2014a); then, to determine (a) suitable antioxidant(s) for further protection of Ax in NLCs, influence of different antioxidants on the stability of Ax-NLCs has been investigated (Tamjidi et al. 2014b). The objective of the current study was to investigate the effect of different environmental stresses, typically encountered by food products (with regard to pH, ionic strength, heat, simulated gastric juice and freeze–thawing), on the stability of Ax-NLCs stabilized with Tween 80 and lecithin. This study helps to gain a better understanding to identify which food processing, formulation, and conditions might be important to consider when developing functional foods containing Ax-NLCs.
Materials and methods
Materials
An Ax oleoresin (Ax content: 40%), extracted from the alga Haematococcus pluvialis, was purchased from Wuhan Ereli Import & Export Co. Ltd (Wuhan, China). Compritol® 888 ATO (glyceryl behenate) was purchased from Gattefossé Co. (Saint-Priest, France). Sodium azide, lecithin (L-α-phosphatidylcholine, Type IV–S ≥ 30%, TLC) and (±)-α-tocopherol were purchased from Sigma Co. (St. Louis, MO, USA). Oleic acid, Tween 80 and all other chemicals were purchased from Merck Co. (Darmstadt, Germany). Double distilled and deionized water was used.
Ax-NLCs preparation
A formulation of Ax-NLCs with optimum contents of Tween 80 and oil (Lipid phase: 757 mg glyceryl behenate, 218 mg oleic acid, 5 mg lecithin and 20 mg Ax; Aqueous phase: 555 mg Tween 80 in 18.445 g phosphate buffer solution (PBS: 5 mM; pH 7) containing 0.02 wt% sodium azide) was prepared by melt-emulsification and ultra-sonication technique, based on the previous study (Tamjidi et al. 2014a). Before homogenization, to increase the chemical stability of Ax, amounts of 5000 ppm α-tocopherol and 100 ppm EDTA were dissolved in the lipid and aqueous phases, respectively (Tamjidi et al. 2014b). The Ax-free NLCs formulation was produced in the same manner as above except that the Ax oleoresin was replaced with respective lipids.
Environmental stresses
Influence of pH
The pH of Ax-NLCs samples was adjusted to the desired final value (pH 3–8) using either NaOH or HCl solution (1 M). The samples were then transferred into glass tubes and stored in a dark place at ambient temperature (25 ± 2 °C) for 15 days.
Influence of ionic strength
Different amounts of NaCl crystals were dissolved in Ax-NLCs to a final volume of 5 mL to obtain concentrations of 0, 50, 100, 200, 350 or 500 mM NaCl. The samples were then transferred into glass tubes and stored in a dark place at ambient temperature for 15 days.
Influence of heat treatment
A 2 mL volume of Ax-NLCs was pipetted into a 5-mL screw-capped Pyrex glass tube and equilibrated in a water bath maintained at 50, 60, 70, 80 or 90 °C for 15 min. After, the samples were cooled down to 6 °C under continuous hand-shaking within an ice bath and then transferred into polypropylene microtubes and stored in a dark place at ambient temperature for 2 months.
Influence of simulated gastric juice (SGJ)
To investigate the effect of SGJ on physical and chemical stability of Ax-NLCs, briefly 1 or 2 mL of the freshly prepared Ax-NLCs was diluted with 0.1 M solution of HCl (pH 1.2) up to 50 mL. The samples flasks were then shaken using an orbital water bath shaker (KBLee2020 Daiki, South Korea) at 50 rpm for 4 h (37 °C), and analyzed for ζ-potential, particle size, and Ax content.
Influence of freeze–thawing
In order to select an appropriate cryoprotectant to improve the quality of freeze–thawed Ax-NLCs, the protective effect of type and concentration of some cryoprotectants on physical stability of Ax-NLCs during freeze–thawing was investigated. Different levels (0–20 wt%) of various cryoprotectants (sucrose, lactose, sorbitol, glycerol, mannitol or glucose) were added to the freshly prepared Ax-NLCs prior to freezing. A 2 mL volume of each sample was then pipetted into a polypropylene microtube, frozen by placing it in a −18 °C freezer for 24 h, and thawed by allowing it to stand at ambient temperature for 4 h.
Ax-NLCs characterization
Particle size and ζ-potential
Analysis of particle size (and polydispersity index; PDI) of Ax-NLCs was performed by photon correlation spectroscopy (PCS) using a Zetasizer (NanoSizer 3000, Malvern Instruments, Malvern, UK) at an angle of 90° in 0.01 m width cells at 25.0 ± 0.1 °C. The ζ-potential of Ax-NLCs was determined in a capillary cell using the same instrument. Prior to analysis, to avoid multiple scattering effects, the samples were diluted to a lipid phase content of 1 mg/mL with proper buffer solutions if required. The buffers used for dilution had the same pH and ionic composition as the samples being analyzed.
Extraction and quantification of Ax
The Ax of Ax-NLCs was extracted with a mixture of methanol and dichloromethane and measured spectrophotometrically (Secomam, Alès, France) at 484 nm (Tamjidi et al. 2014a). The degradation percentage of Ax was calculated according to the equation: Degradation (%) = (C 0 −C)*100/C 0; where C 0 is the content of Ax in NLCs after production, and C is the Ax content of NLCs at the desired time.
Statistical analysis
All measurements were carried out in triplicate and reported as means ± standard deviations. Statistical differences (P ≤ 0.05) between selected treatments were established using the general linear models method based on Fisher’s least significant difference (LSD) procedure by SAS ver. 9.0 (SAS Institute Inc., Cary, NC).
Results and discussion
Initial properties of Ax-NLCs
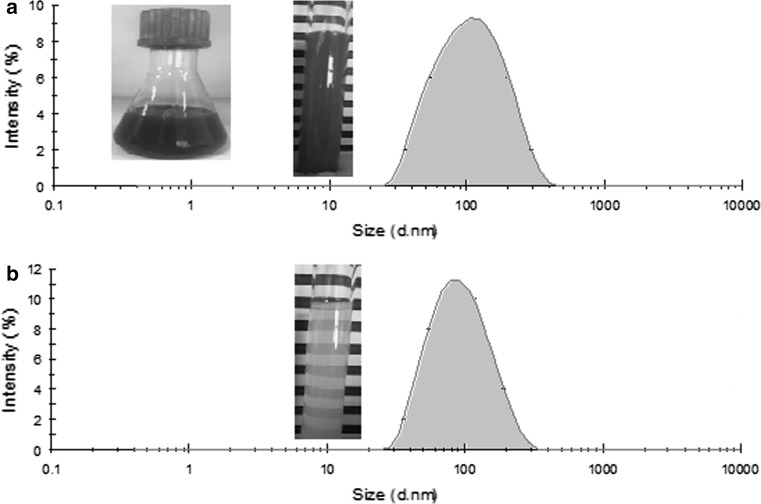
The initial properties of the freshly prepared Ax-NLCs containing 5 wt% lipid phase were characterized. The mean particles diameter (Z-average) of the Ax-NLCs was 94 nm. As Ax oleoresin, the Ax-NLCs formulation was deep red colored; but the Ax-free NLCs sample (Z-average size: 82 nm) was translucent (Fig. 1) indicating the fact that the particles are so small that they do not scatter light strongly (McClements 2002). The greater size of Ax-NLCs can be attributed to increasing interfacial tension and/or viscosity of the disperse phase in the presence of Ax, as was discussed previously (Tamjidi et al. 2014a). The PDI of the freshly prepared Ax-NLCs was well below 0.25 reflecting relatively homogeneous nanoparticles and narrow size distribution. The absolute ζ‐potential value of the fresh Ax-NLCs (around −24 mV) was satisfactorily higher than the required quantity to achieve a good physical stability (i.e., 20 mV) (Mitri et al. 2011). The Ax content of Ax‐NLCs was reduced by ≈10% during preparation, indicating that except for transition metal ions and free radicals, which, respectively, are prevented by EDTA and α-tocopherol, other factors (e.g., ultrasound waves) may also degrade Ax (Tamjidi et al. 2014b).
Fig. 1.
Particle size distribution of freshly-prepared Ax-NLCs (a) and astaxanthin-free NLCs (b) samples (Color version of the figure is available online)
Influence of pH on stability of Ax-NLCs
There are wide variations in the pH values of food and beverage products. Therefore, the influence of pH on the properties of Ax-NLCs was examined.
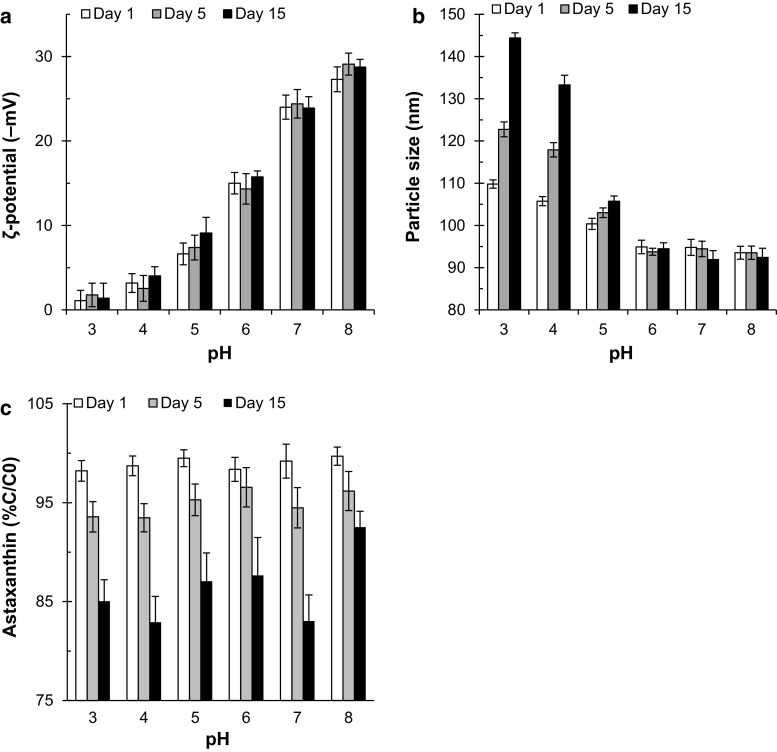
The pH dependence of the ζ-potential of Ax-NLCs, on 1st, 5th and 15st days, is shown in Fig. 2a. The nanoparticles became less negatively charged on 1st day when their pH was decreased from 8 to 3 (P < 0.05); the absolute ζ-potential decreased from ≈28 to ≈0 mV by decreasing pH from 8 to 3. This can be attributed to protonation of anionic groups of lecithin () and oleic acid (). Lecithin is a zwitterionic surfactant with an approximate isoelectric point (pI) at a pH of 3.5 (Cowell et al. 1982); the phospholipids in lecithin are positively charged below this pI and negatively charged above it. As pH decreases, the anionic groups of phospholipids protonate and the net charge of the interface decreases. On the other hand, the surface activity of oleic acid allows it to diffuse and concentrate at the water–lipid interface of the Ax-NLCs. Thus, it could potentially make the nanoparticles more negatively charged when pH values are above its pK a (≈5). Therefore, the surface charge of nanoparticles decreases dramatically as the pH falls in the range of 3–5. The ζ-potential of Ax-NLCs samples with different pH values did not change during 15 days of storage (P > 0.05).
Fig. 2.
Influence of pH on ζ-potential (a), particle size (b) and astaxanthin content (c) of Ax-NLCs dispersion during 15 days storage at 25 °C (n = 3)
pH value had a significant effect on the particle size of Ax-NLCs (P < 0.05). The particle size of Ax-NLCs samples with low pH values (≤5) was higher than the original Ax-NLCs sample’s on 1st day (P < 0.05). Moreover, the particle size of these samples (pH ≤ 5) further increased during 15 days of storage at 25 °C (P < 0.05); the lower pH values, the higher increment in particle size (Fig. 2b). However, the Ax-NLCs samples with higher pH values (6–8) were stable to size increment during the storage period (P > 0.05). This result is in agreement with the observation made on the ζ-potential values of Ax-NLCs. Previously, similar results were also reported by Choi et al. (2014), How et al. (2013) and Mahal et al. (2017). At high pH values the ζ-potential and thereby the electrostatic repulsion is still sufficiently strong to overcome attraction forces, but at low pH values it is no longer strong enough so that the attractive forces dominate, leading to nanoparticle aggregation (see Online Resource 1a). It was reported that the droplet aggregation of protein-stabilized nanoemulsion at the pH values close to the pI of protein is also because of the little net charge of droplets surfaces and thereby insufficient electrostatic repulsion among them (Qian et al. 2012). To achieve a good stability for a nanosuspension stabilized with a combination of electrostatic and steric forces (as in this study), a minimum absolute ζ-potential of 20 mV is required (Mitri et al. 2011). The above result suggests that Ax-NLCs may have no enough long-term stability in products with low viscosity and low pH value (pH 3–4) since nanoparticle aggregation may be problematic.
pH had no significant effect on the chemical stability of Ax in the NLCs on 1st and 5th days of the storage period; but the Ax content of Ax-NLCs with pH 8 was higher than other samples’ on 15st day (P < 0.05). In this study, because of the utilization of antioxidants, pH had no major effect on the Ax stability (Fig. 2c); however it was reported that the chemical stability of carotenoids in O/W emulsions decreases with pH (Boon et al. 2009; Qian et al. 2012), and the utilization of metal ions-chelating agents is very useful at retarding this degradation (Boon et al. 2009).
Influence of NaCl on stability of Ax-NLCs
The ionic strength of foods and beverages may vary considerably depending on their nature. Electrolyte concentration (and pH) have major effects on surface charge and physical stability of lipid nanoparticles (Heurtault et al. 2003). Therefore, the influence of ionic strength on the stability of Ax-NLCs was examined.
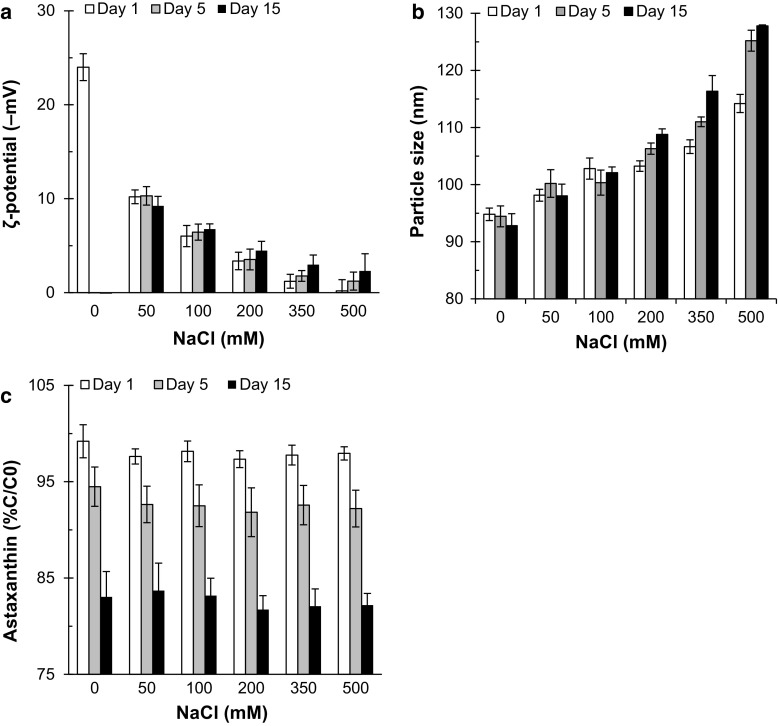
Figure 3a shows the effect of different concentrations of NaCl (0–500 mM) on the ζ-potential of Ax-NLCs stabilized by both Tween 80 and lecithin. The ζ-potential of Ax-NLCs remained negative at all NaCl concentrations. However, the magnitude of ζ-potential decreased from ≈24 to ≈0 mV by increasing NaCl concentration from 0 to 500 mM (P < 0.05). This phenomenon can be attributed to the electrostatic screening effect, which arises from the binding tendency of to the negatively charged groups of lecithin () and oleic acid () on the nanoparticles surfaces, thereby reducing the net negative charge. The electrostatic screening effect (of NaCl) has also been reported in previous studies (Qian et al. 2011, 2012; Mahal et al. 2017). No further changes were observed in the ζ-potential of Ax-NLCs samples during storage (P > 0.05).
Fig. 3.
Influence of ionic strength (0–500 mM NaCl) on ζ-potential (a), particle size (b) and astaxanthin content (c) of Ax-NLCs dispersion during 15 days storage at 25 °C (n = 3)
As shown in Fig. 3b, NaCl concentration had a significant effect on the particle size of Ax-NLCs; the particle size of samples containing 50–500 mM NaCl increased on 1st day in a salt concentration-dependent manner (P < 0.05). Moreover, the particle size of the samples with 200–500 mM NaCl further increased during the 15 days storage period (P < 0.05); however, the Ax-NLCs samples with lower NaCl contents (0–100 mM) were stable to size increment (P > 0.05). Generally, the increase in particle size can be mainly attributed to nanoparticles aggregation induced by the decrease of ζ-potential and thereby the electrostatic repulsion among them (see Online Resource 1b). As for pH effect, lower values of ζ-potential resulted in a further increase in particle size. At relatively low salt contents, the electrostatic repulsion is still sufficiently strong to overcome the attraction forces (e.g., van der Waals, hydrophobic and depletion), but above a critical salt content it is no longer strong enough so that the attractive forces dominate, leading to nanoparticle aggregation (Qian et al. 2012). Furthermore, NaCl may change the optimum curvature of the interfacial membrane, making the nanoparticles more prone to aggregation (Israelachvili 1992). The increase of particle size on 1st day may be attributed to aggregation induced by high collision frequency of nanoparticles at the interface of “salt crystal–continuous phase” during dispersion and dissolution of NaCl crystals.
It has been reported that adding NaCl to the aqueous phase decreases the ζ-potential, and increases the particle size of Tween 80-stabilized SLNs (Choi et al. 2014).
Ionic strength had no significant effect on the chemical stability of Ax in NLCs (Fig. 3c), probably due to the presence of antioxidants. However, it was also reported that NaCl has no important effect on the chemical stability of β-carotene in β-lactoglobulin-stabilized nanoemulsions (Qian et al. 2012).
Influence of heat treatment on stability of Ax-NLCs
Emulsion-based foods and beverages often experience variations in their temperature during manufacture, transport, storage, and utilization (Lee et al. 2011), therefore the influence of heat treatment on stability of Ax-NLCs was examined.
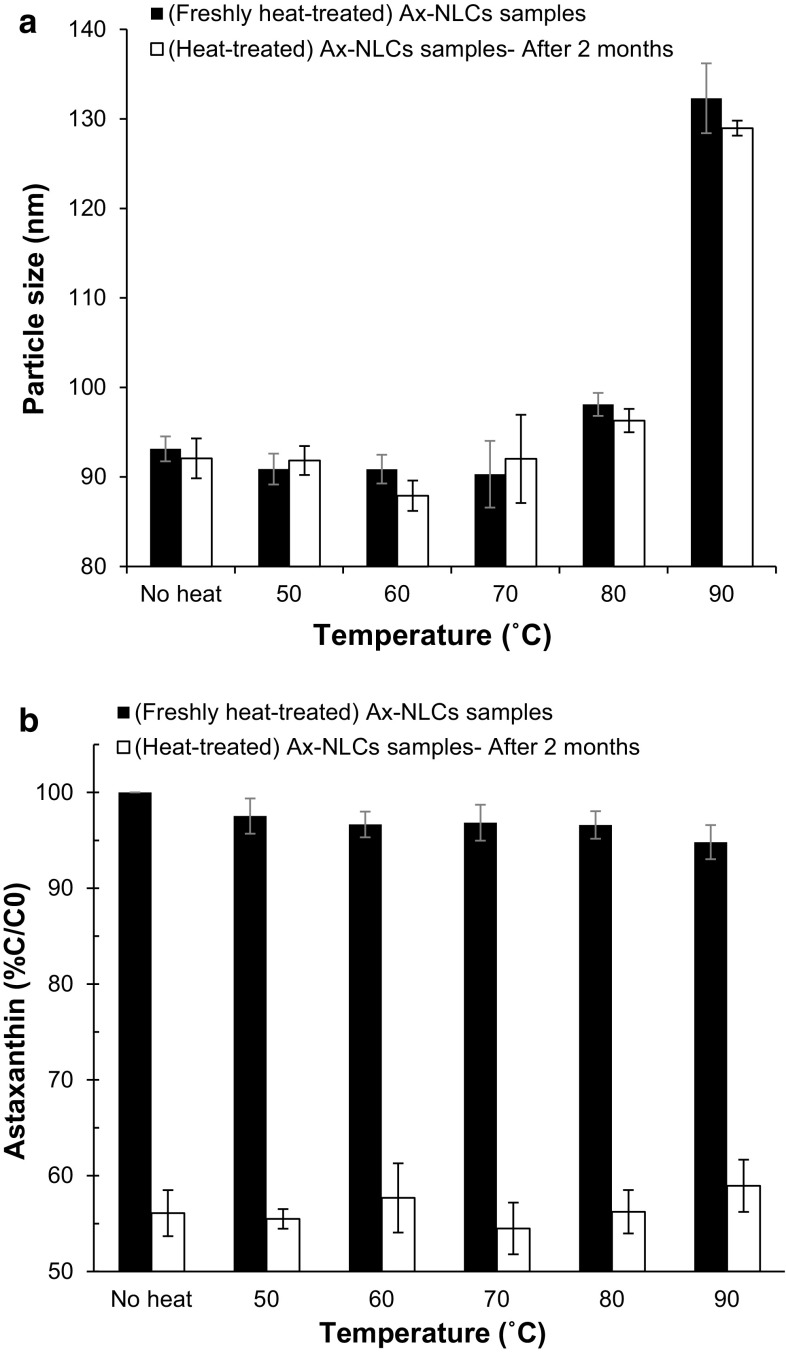
Heat treatment (50−90 °C/15 min) had a significant effect on the particle size of Ax-NLCs (Fig. 4a). The particle size of original Ax-NLCs sample (≈94 nm) increased to ≈98 and ≈132 nm after heating at 80 and 90 °C, respectively (P < 0.05). There are three possible reasons for this phenomenon. Firstly, elevated temperatures lead to dehydration of the hydrophilic surfactants head groups which alters the optimum curvature of the surfactant monolayer and decreases the steric repulsion between droplets (Israelachvili 1992); In other words, the hydrophilic-lipophilic balance (HLB) of surfactants changes at evaluated temperatures (Boyd et al. 1972). Secondly, the efficiency of Tween 80 decreases as the temperature approaches to its cloud point (≈93 °C). Thirdly, the increased kinetic energy at evaluated temperatures could accelerate the collisions of melted-nanoparticles, and the rate of coalescence (see Online Resource 1c). But, the particle size of Ax-NLCs did not change at low holding temperatures (≤70 °C) (P > 0.05). At low temperatures the lipid phase is still solid and the surfactant molecules form a rigid monolayer that is more resistant to coalescence, but at high temperatures they form a flexible monolayer, due to melting of the lipid phase and dehydrating of the polar head groups of surfactant molecules, that is more susceptible to coalescence (McClements et al. 2014). However, no significant increase in particle size was observed for the heat-treated and original Ax-NLCs samples after 2 months of storage at 25 °C (Fig. 4a).
Fig. 4.
Particle size (a) and astaxanthin content (b) of heat-treated (50–90 °C/15 min) Ax-NLCs dispersion after 0 or 60 days storage at 25 °C (n = 3)
No significant difference was observed among the ζ-potential values of original (−24 mV) and heat-treated Ax-NLCs formulations, and they were constant (P > 0.05) throughout the 2 months storage period (data not shown). It should be pointed out that the ζ-potential is independent of particle size, principally (Hiemenz and Rajagopalan 1997).
The Ax content of fresh Ax-NLCs sample reduced by 2.5−5.2% after heat treatment; no difference was observed among Ax contents of the freshly heat-treated NLCs samples (P > 0.05). The Ax contents of heat-treated and original Ax-NLCs samples reduced to ≈53% after 2 months storage at 25 °C (P < 0.05), but there was no significant difference among them (Fig. 4b).
The above results imply that Ax-NLCs can be thermally pasteurized and then added to pasteurized beverages and food products; moreover, they may be stable to thermal pasteurization within foods and beverages.
Influence of SGJ on stability of Ax-NLCs
In order to increase the bioaccessibility, the lipid nanocarriers should be stable in the stomach and maintain their integrity until they enter to small intestine (Aditya et al. 2014). Therefore, we examined the influence of SGJ on ζ-potential, particle size and Ax content of Ax-NLCs. The Ax-NLCs were diluted with SGJ to 2 or 4% (v/v), and shaken at 37 °C for 4 h.
SGJ had a drastic effect on the ζ-potential of Ax-NLCs. The absolute ζ-potential of Ax-NLCs decreased from 24 to 0 ± 1.5 mV after dilution (data not shown). This decrease is due to the protonation of anionic groups of lecithin and oleic acid at low pH values (see “Influence of pH on stability of Ax-NLCs” section). The time of shaking and the dilution ratio had no effect on the ζ-potential of Ax-NLCs in SGJ (P > 0.05).
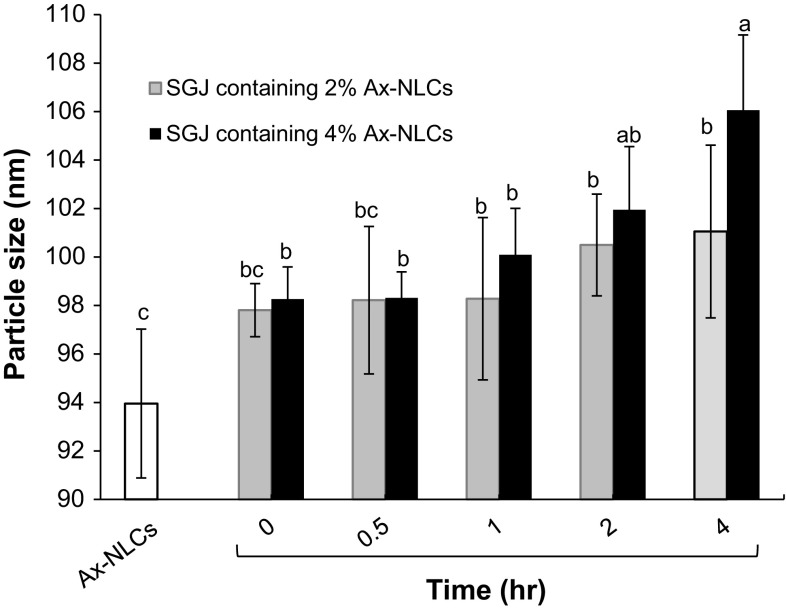
Figure 5 shows the effect of SGJ on the particle size of Ax-NLCs as a function of time and nanoparticles concentration. As compared to fresh Ax-NLCs, a slight increase in particle size was observed for the 2 freshly-diluted (i.e., at t = 0) samples; this increase was only significant for the sample containing 4% Ax-NLCs (P < 0.05) while became significant after 1 h for the sample containing 2% Ax-NLCs. From t = 0 to t = 4 h, no significant increase in particle size was observed for the sample containing 2% Ax-NLCs; but a significant increase in particle size was observed for the sample containing 4% Ax-NLCs at t = 4 h. Moreover, excepting for t = 4 h, no significant difference was observed between the 2 samples in the particle size at other sampling times (Fig. 5). The increase of particle size in SGJ is ascribed to nanoparticles aggregation owing to decreasing of ζ-potential and emulsifiers performance. The “instantaneous aggregation” at t = 0 may arise from high collision frequency of Ax-NLCs (ζ-potential ≈0) during dilution, when they are very close to each other, before uniform dispersion within SGJ. The fast and/or more increase in particle size of the sample containing 4% Ax-NLCs can be due to higher frequency and intensity of collisions of the denser nanoparticles and thereby more aggregation of them.
Fig. 5.
Particle size of simulated gastric juice (SGJ; pH 1.2, Temp. 37 °C) containing 2 or 4% Ax-NLCs during 4 h. Bars with different letters are significantly different at P < 0.05 (LSD test; n = 3)
It has been reported that after 2 h at SGJ, the size of NLCs stabilized with Tween 80-lecithin-polyvinyl alcohol or Tween 80-Span 20-lecithin does not increase (Aditya et al. 2013, 2014). The presence of non-ionic surface-active agents (e.g., Tween 80, polyvinyl alcohol and Span 20) at the interface leads to more stability of NLCs at acidic pH values via steric repulsion (Aditya et al. 2013). It was also reported that the emulsions stabilized with non-ionic surface-active agents are more stable to aggregation at the acidic condition of stomach (van Aken et al. 2011). This topic demonstrates the importance of interface engineering for targeted delivery of active compounds. Although the particle size of Ax-NLCs increased to some extent within SGJ, the both samples still showed a mean particle size in the nanoscale range.
After 4 h, SGJ had no significant effect on the Ax content in NLCs (P > 0.05; data not shown). Aditya et al. showed that the bioactive compounds (curcumin or genistein) within NLCs remain chemically stable for 2 h at SGJ, but 8 or 15% of them decreases after 6 h (Aditya et al. 2013).
The above results show that Ax-NLCs are chemically- and almost physically-stable within SGJ and they may increase the bioavailability of Ax at small intestine. Usually, with decreasing particle size below 500 nm, the bioavailability of encapsulated lipophilic compounds within them increases considerably (Acosta 2009).
Influence of freeze–thawing on stability of Ax-NLCs
Many emulsion-based foods or beverages may be frozen to extend their shelf life during storage or transport and then thawed before consumption or further processing. Nevertheless, with respect to extensive droplet aggregation during crystallization of oil and/or water phases, many food emulsions have relatively poor freeze–thaw stability (Thanasukarn et al. 2006). Various factors, including solid fat content, amount and type of emulsifiers, and size of emulsion droplets influence the freeze–thaw stability of emulsions (Lee et al. 2011). It is well known that hydrophilic solutes (such as sugars and polyols) can improve the freeze–thaw stability of O/W emulsions as cryoprotectant (Degner et al. 2014). Thus, we examined the influence of freeze–thawing on the physical stability of Ax-NLCs in the presence of different types and concentrations of cryoprotectants (Fig. 6).
Fig. 6.
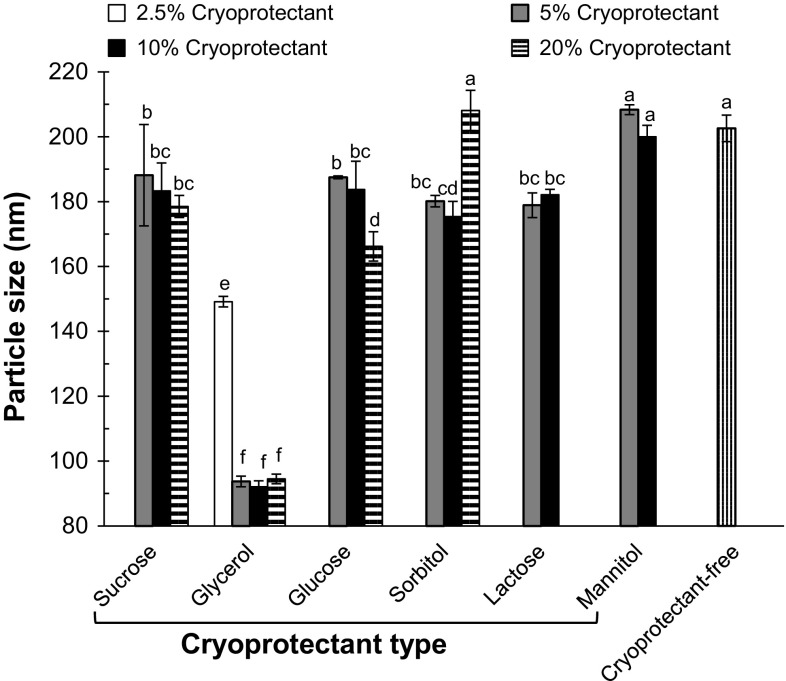
Particle size of freeze-thawed Ax-NLCs containing different types and concentrations of cryoprotectants. Bars with different letters are significantly different at P < 0.05 (LSD test; n = 3)
The Ax-NLCs were susceptible to aggregation during freeze–thawing in the absence of cryoprotectant; the mean particle diameter of fresh Ax-NLCs (94 nm) increased to 200 nm after the freeze–thaw treatment. Lipid droplets stabilized by small-molecule surfactants are typically less stable to freeze–thawing, because they only form very thin interfacial coatings around the lipid droplets (Thanasukarn et al. 2004). Elsewhere, a number of physicochemical mechanisms have been reported for the aggregation after freeze–thaw treatment (Thanasukarn et al. 2004). Type and concentration of cryoprotectant had a significant effect on the stability of Ax-NLCs to freeze–thawing; except glycerol, other employed cryoprotectants did not have a desirable protective effect on size increment of Ax-NLCs (Fig. 6). Low concentration of glycerol (2.5%) had a little protective effect, whereas size increment of Ax-NLCs was perfectly inhibited at a concentration of 5% glycerol. Glycerol has been approved as GRAS by the FDA (21CFR182.1320). Hsu et al. examined the effect of 0–10% sucrose, lactose or trehalose on the stability of waxy nanoparticles (stabilized with Brij 78 and Tween 20) during freeze–thaw cycle, and showed that 0.5–1% of these cryoprotectants are sufficient for prevention of particle aggregation (Hsu et al. 2003). Lee et al. showed that addition of 2.5% sucrose to the whey protein-stabilized nanoemulsions perfectly inhibits particle size increment during freeze–thaw cycle (Lee et al. 2011). These authors reported that sucrose increases the non-freezable water, makes hydrogen bonds with whey protein and protects it from dehydration, and prevents droplets aggregation; additionally sucrose increases the viscosity of continuous phase and thereby decreases the droplet–droplet interactions. Many factors including composition and physical properties of droplets/particles, and cooling condition affect the freeze–thaw stability of O/W emulsion (Degner et al. 2014).
Freez–thaw treatment, with or without cryoprotectant, had no significant effect on ζ-potential of Ax-NLCs (data not shown).
Conclusion
This study has investigated the effects of environmental stresses typically encountered by food products on the stability of Ax-NLCs stabilized with Tween 80 and lecithin. The increase of nanoparticle size in low pH values, high NaCl concentrations and SGJ is mainly due to decreasing of colloidal electrostatic repulsion induced by the decreased ζ-potential. The results obtained for effects of pH and ionic strength on characteristics of the Ax-NLCs formulation indicate that it may not have long-term physical stability in beverage and food products with low viscosity which also have low pH and/or high ionic strength. The increase of Ax-NLCs size at high temperatures can be attributed to the changes in interfacial characteristics, physical state of lipid phase, and kinetic energy. Although, the freshly produced Ax-NLCs formulation or its corresponding hot nanoemulsion (before cooling) can be added to target product after pasteurization at a sterile condition, it can also be thermally pasteurized thereafter. The Ax-NLCs formulation had a proper stability in SGJ; it therefore may improve the bioavailability of astaxanthin. Cryoprotectant type had a major effect on the freeze–thaw stability of Ax-NLCs, and glycerol (5%) was found as an appropriate choice. The above results provide useful information about stability of NLCs containing active molecules, thus having a practical implication in functional food development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the financial support of Isfahan University of Technology, and the technical support of Isfahan University of Medical Sciences.
Footnotes
Mohammad Shahedi and Jaleh Varshosaz were equally contributed to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2749-7) contains supplementary material, which is available to authorized users.
References
- Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr Opin Colloid In. 2009;14:3–15. doi: 10.1016/j.cocis.2008.01.002. [DOI] [Google Scholar]
- Aditya NP, Shim M, Lee I, Lee Y, Im M-H, Ko S. Curcumin and genistein coloaded nanostructured lipid carriers: in vitro digestion and antiprostate cancer activity. J Agric Food Chem. 2013;61:1878–1883. doi: 10.1021/jf305143k. [DOI] [PubMed] [Google Scholar]
- Aditya NP, Macedo AS, Doktorovova S, Souto EB, Kim S, Chang P-S, Ko S. Development and evaluation of lipid nanocarriers for quercetin delivery: a comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE) Lwt Food Sci Technol. 2014;59:115–121. doi: 10.1016/j.lwt.2014.04.058. [DOI] [Google Scholar]
- Amorim-Carrilho KT, Cepeda A, Fente C, Regal P. Review of methods for analysis of carotenoids. TrAC Trend Anal Chem. 2014;56:49–73. doi: 10.1016/j.trac.2013.12.011. [DOI] [Google Scholar]
- Boon CS, Mcclements DJ, Weiss J, Decker EA. Role of iron and hydroperoxides in the degradation of lycopene in oil-in-water emulsions. J Agric Food Chem. 2009;57:2993–2998. doi: 10.1021/jf803747j. [DOI] [PubMed] [Google Scholar]
- Boyd J, Parkinson C, Sherman P. Factors affecting emulsion stability, and the HLB concept. J Colloid Interface Sci. 1972;41:359–370. doi: 10.1016/0021-9797(72)90122-1. [DOI] [Google Scholar]
- Choi K-O, Aditya NP, Ko S. Effect of aqueous pH and electrolyte concentration on structure, stability and flow behavior of non-ionic surfactant based solid lipid nanoparticles. Food Chem. 2014;147:239–244. doi: 10.1016/j.foodchem.2013.09.095. [DOI] [PubMed] [Google Scholar]
- Cowell RD, Sullivan DR, Szuhaj BF. Lecithin and related phosphatides. In: Bluestein BR, Hilton LH, editors. Amphoteric surfactants. New York: Marcel Dekker; 1982. pp. 230–264. [Google Scholar]
- Degner BM, Chung C, Schlegel V, Hutkins R, McClements DJ. Factors influencing the freeze–thaw stability of emulsion-based foods. Compr Rev Food Sci. 2014;13:98–113. doi: 10.1111/1541-4337.12050. [DOI] [PubMed] [Google Scholar]
- Hejri A, Khosravi A, Gharanjig K, Hejazi M. Optimisation of the formulation of β-carotene loaded nanostructured lipid carriers prepared by solvent diffusion method. Food Chem. 2013;141:117–123. doi: 10.1016/j.foodchem.2013.02.080. [DOI] [PubMed] [Google Scholar]
- Hentschel A, Gramdorf S, Müller RH, Kurz T. β-carotene-loaded nanostructured lipid carriers. J Food Sci. 2008;73:N1–N6. doi: 10.1111/j.1750-3841.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- Heurtault B, Saulnier P, Pech B, Proust J-E, Benoit J-P. Physico-chemical stability of colloidal lipid particles. Biomater. 2003;24:4283–4300. doi: 10.1016/S0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- Hiemenz PC, Rajagopalan R. Principles of colloid and surface chemistry. New York: Marcel Dekker; 1997. [Google Scholar]
- Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- How CW, Rasedee A, Manickam S, Rosli R. Tamoxifen-loaded nanostructured lipid carrier as a drug delivery system: characterization, stability assessment and cytotoxicity. Colloid Surf B. 2013;112:393–399. doi: 10.1016/j.colsurfb.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Hsu C-H, Cui Z, Mumper R, Jay M. Preparation and characterization of novel coenzyme Q10 nanoparticles engineered from microemulsion precursors. AAPS PharmSciTech. 2003;4:24–35. doi: 10.1208/pt040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili JN. Intermolecular and surface forces. London: Academic Press; 1992. [Google Scholar]
- Lee SJ, Choi SJ, Li Y, Decker EA, Mcclements DJ. Protein-stabilized nanoemulsions and emulsions: comparison of physicochemical stability, lipid oxidation, and lipase digestibility. J Agric Food Chem. 2011;59:415–427. doi: 10.1021/jf103511v. [DOI] [PubMed] [Google Scholar]
- Liu C-H, Wu C-T. Optimization of nanostructured lipid carriers for lutein delivery. Colloid Surf A. 2010;353:149–156. doi: 10.1016/j.colsurfa.2009.11.006. [DOI] [Google Scholar]
- Liu GY, Wang JM, Xia Q. Application of nanostructured lipid carrier in food for the improved bioavailability. Eur Food Res Technol. 2012;234:391–398. doi: 10.1007/s00217-011-1645-z. [DOI] [Google Scholar]
- Mahal A, Tandon L, Khullar P, Ahluwalia GK, Bakshi MS. pH responsive bioactive lead sulfide nanomaterials: protein induced morphology control, bioapplicability, and bioextraction of nanomaterials. ACS Sustain Chem Eng. 2017;5:119–132. doi: 10.1021/acssuschemeng.6b00991. [DOI] [Google Scholar]
- McClements DJ. Theoretical prediction of emulsion color. Adv Colloid Interface Sci. 2002;97:63–89. doi: 10.1016/S0001-8686(01)00047-1. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Decker EA, Choi SJ. Impact of environmental stresses on orange oil-in-water emulsions stabilized by sucrose monopalmitate and lysolecithin. J Agric Food Chem. 2014;62:3257–3261. doi: 10.1021/jf404983p. [DOI] [PubMed] [Google Scholar]
- Mitri K, Shegokar R, Gohla S, Anselmi C, Müller RH. Lipid nanocarriers for dermal delivery of lutein: preparation, characterization, stability and performance. Int J Pharm. 2011;414:267–275. doi: 10.1016/j.ijpharm.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Naguib YMA. Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem. 2000;48:1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- Ni S, Sun R, Zhao G, Xia Q. Quercetin loaded nanostructured lipid carrier for food fortification: preparation, characterization and in vitro study. J Food Process Eng. 2015;38:93–106. doi: 10.1111/jfpe.12130. [DOI] [Google Scholar]
- Qian C, Decker EA, Xiao H, McClements DJ. Comparison of biopolymer emulsifier performance in formation and stabilization of orange oil-in-water emulsions. J Am Oil Chem Soc. 2011;88:47–55. doi: 10.1007/s11746-010-1658-y. [DOI] [Google Scholar]
- Qian C, Decker EA, Xiao H, McClements DJ. Physical and chemical stability of β-carotene-enriched nanoemulsions: influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012;132:1221–1229. doi: 10.1016/j.foodchem.2011.11.091. [DOI] [PubMed] [Google Scholar]
- Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Nanostructured lipid carriers (NLC): a potential delivery system for bioactive food molecules. Innov Food Sci Emerg Technol. 2013;19:29–43. doi: 10.1016/j.ifset.2013.03.002. [DOI] [Google Scholar]
- Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Design and characterization of astaxanthin-loaded nanostructured lipid carriers. Innov Food Sci Emerg Technol. 2014;26:366–374. doi: 10.1016/j.ifset.2014.06.012. [DOI] [Google Scholar]
- Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. EDTA and α-tocopherol improve the chemical stability of astaxanthin loaded into nanostructured lipid carriers. Eur J Lipid Sci Technol. 2014;116:968–977. doi: 10.1002/ejlt.201300509. [DOI] [Google Scholar]
- Thanasukarn P, Pongsawatmanit R, McClements DJ. Impact of fat and water crystallization on the stability of hydrogenated palm oil-in-water emulsions stabilized by whey protein isolate. Colloid Surf A. 2004;246:49–59. doi: 10.1016/j.colsurfa.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Thanasukarn P, Pongsawatmanit R, McClements DJ. Impact of fat and water crystallization on the stability of hydrogenated palm oil-in-water emulsions stabilized by a nonionic surfactant. J Agric Food Chem. 2006;54:3591–3597. doi: 10.1021/jf0524630. [DOI] [PubMed] [Google Scholar]
- van Aken GA, Bomhof E, Zoet FD, Verbeek M, Oosterveld A. Differences in in vitro gastric behaviour between homogenized milk and emulsions stabilised by Tween 80, whey protein, or whey protein and caseinate. Food Hydrocolloid. 2011;25:781–788. doi: 10.1016/j.foodhyd.2010.09.016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.